Abstract
Background:
Nephrotoxicity is one of the side effects of cisplatin (CP) therapy which is gender related. CP disturbs renal function through glomerular filtration rate and electrolytes transport disturbances. This study was designed to compare some markers related to renal function in two protocols of CP treatment in rats.
Materials and Methods:
Male and female rats were subjected to receive single (treat 1; 7.5 mg/kg) and continues doses (treat 2; 3 mg/kg/day for 5 days) of CP, and the measurements were compared with control animals.
Results:
The serum level of blood urea nitrogen (BUN) and creatinine (Cr), and Cr-clearance, kidney tissue damage score, kidney weight, body weight change, and Na excretion was altered significantly (P < 0.05) in animals treated with continuous dose of CP (treat 2), while alteration of BUN and Cr was gender related. The kidney levels of malondialdehyde and nitrite were significantly different between male and female in two protocols of treatments.
Conclusion:
Renal function after CP therapy alters in rats’ gender dependently, and it is related to protocol of treatment.
Keywords: Cisplatin, gender, rats, renal function
Introduction
Cisplatin (CP) is one of the most popular and potent drugs for solid tumor therapy.[1,2] CP kills cancer cells by destroying their DNA.[3] One of the side effects of CP is nephrotoxicity[4] which is related to platinum based[5] and CP accumulation in the kidney.[6] CP reduces the antioxidants levels,[7] and causes inflammation, endoplasmic reticulum stress, and oxidative stress[8,9] contribute to its induced kidney toxicity.[10] CP also increases lipid peroxidation,[11,12] and its side effects are gender related.[13,14,15,16] It is reported that CP enhanced urinary sodium excretion in male but not in female rats.[17,18,19] CP also decreases glomerular filtration rate and performs electrolyte disturbances largely due to the acute cytotoxic properties of CP on distal and proximal tubules.[1,20] This short study was designed to compare some renal markers in two protocols of CP treatment in male and female rats.
Materials and Methods
Forty-four adult female (n = 22, 175.18 ± 2.70 g) and male (n = 22, 246.27 ± 4.01 g) Wistar rats (three groups in each gender) were housed at the temperature of 23°C–25°C.
All experiments were approved by the Isfahan University of Medical Sciences Ethics Committee.
The male rats in Group 1 (vehicle, n = 6), Group 2 (treat 1, n = 8), and Group 3 (treat 2, n = 6) respectively received saline, single dose of CP (7.5 mg/kg ip) at the 1st day of the experiment, and CP (3 mg/kg/day, ip) for 5 days. The female rats in the Groups 4, 5, and 6 received the same regimen as Groups 1, 2, and 3, respectively. At day 8, all rats were housed in metabolic cages to collect the output urine for 6 h. Then, the blood samples were obtained, and the animals were sacrificed. The kidney and uterus were excised and weighed immediately. The left kidney was used for histopathological investigations through hematoxylin and eosin staining. The kidney tissue damage score (KTDS) was graded by a pathologist from 1 to 4, while zero score was assigned to normal tissue without damage. The right kidney was homogenized and centrifuged. The serum levels of creatinine (Cr) and blood urea nitrogen (BUN) were determined using quantitative diagnostic kits (pars Azmoon, Iran) by autoanalyzer (Technicon, Ireland LTD). The serum and urine levels of sodium (Na) were measured by flame photometer, and the level of malondialdehyde (MDA) was measured manually.
Statistical analysis
The data are presented as mean ± standard error of mean. The quantitative data were compared by ANOVA using LSD. The Mann–Whitney test was applied to compare the KTDS among the groups. P ≤ 0.05 was considered as statistically significant.
Results
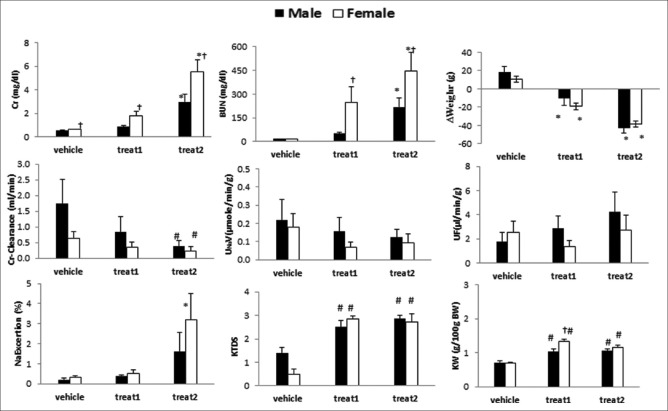
The data are presented in Figure 1. The results indicated the markers such as BUN, Cr, Cr-clearance, KTDS, kidney weight (KW), body weight change, and Na excretion were altered significantly (P < 0.05) in animals treated with continuous dose of CP (3 mg/kg/day, ip) when compared with vehicle group. However, the responses of the above-mentioned makers to the single dose of CP were different [Figure 1]. The kidney levels of MDA and nitrite were significantly different between male and female in two protocols of treatments [Table 1]. The sample images of kidney tissues are shown in Figure 2.
Figure 1.
The serum level of creatinine and blood urea nitrogen, body weight change (Δweight) creatinine-clearance, sodium urine load (UNaV), urine flow, percentage of sodium excretion, kidney tissue damage score and kidney weight in male and female rats treated with vehicle, single dose (7.5 mg/kg) of cisplatin (treat 1), and continuous dose (3 mg/kg/day for 5 days) of cisplatin (treat 2). *Significant difference from vehicle and treat1 in similar gender (P ≤ 0.05), #Significant difference from vehicle in similar gender (P ≤ 0.05), †Significant difference from male in same treated group (P ≤ 0.05)
Table 1.
The serum and kidney levels of malondialdehyde and nitrite, and the weights of uterus and testis
Figure 2.
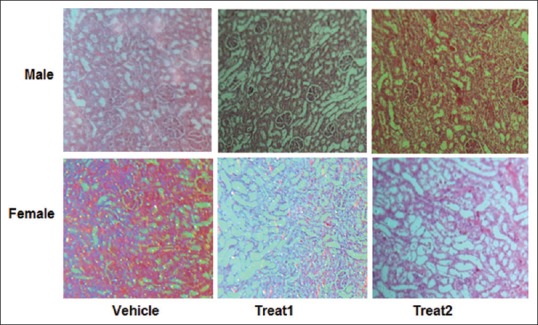
The samples images of kidney tissues in male and female rats treated with vehicle, single dose (7.5 mg/kg) of cisplatin (treat 1), and continuous dose (3 mg/kg/day for 5 days) of cisplatin (treat 2). More damage was seen in the “treat 1” and “treat 2” groups
Discussion
Gender and CP usage doses are influenced CP-induced nephrotoxicity. The role of gender in CP-induced nephrotoxicity was reported before.[13,14,15,18,21,22,23] In addition, Stakisaitis et al. reported that CP increases urinary Na excretion gender dependently in rats.[24] This short study indicated that the gender difference also depended on the protocol treatment. The result of this study showed that CP decreased body weight because CP causes gastrointestinal disturbance.[25,26] In addition, the alteration in KW by CP may be related to retention of water and salt.[27] Although the alteration of kidney damage and KW was not influenced by drug usage doses the markers related to kidney function was related to CP usage doses. It seems that is related to platinum based[5] and CP accumulation in the kidney.[6]
Conclusion
Renal function after CP therapy alters in rats’ gender dependently, and it is also related to protocol of CP administration.
Financial support and sponsorship
This research was supported by Isfahan University of Medical Sciences (Grant #294243).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors appreciate Ms. Bahar Mazaheri for her technical assistance.
References
- 1.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334:115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 2.Thomson D. Cisplatin-based therapy: A neurological and neuropsychological review. Psychooncology. 2000;9:29–39. doi: 10.1002/(sici)1099-1611(200001/02)9:1<29::aid-pon428>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Lieb JD, Sancar A, Adar S. Cisplatin DNA damage and repair maps of the human genome at single-nucleotide resolution. Proc Natl Acad Sci U S A. 2016;113:11507–12. doi: 10.1073/pnas.1614430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 6.Esteban-Fernández D, Verdaguer JM, Ramírez-Camacho R, Palacios MA, Gómez-Gómez MM. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J Anal Toxicol. 2008;32:140–6. doi: 10.1093/jat/32.2.140. [DOI] [PubMed] [Google Scholar]
- 7.Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: Antioxidant system. Basic Clin Pharm Toxicol. 1995;76:386–94. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 8.Rjiba-Touati K, Boussema IA, Belarbia A, Achour A, Bacha H. Protective effect of recombinant human erythropoietin against cisplatin-induced oxidative stress and nephrotoxicity in rat kidney. Int J Toxicol. 2011;30:510–7. doi: 10.1177/1091581810411931. [DOI] [PubMed] [Google Scholar]
- 9.Ateşşahín A, Ceríbaşi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:121–6. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 10.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol. 2009;112:e1–9. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 11.Burits M, Bucar F. Antioxidant activity of nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Türk G, Ateşşahin A, Sönmez M, Ceribaşi AO, Yüce A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril. 2008;89:1474–81. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Eshraghi-Jazi F, Nematbakhsh M, Pezeshki Z, Nasri H, Talebi A, Safari T, et al. Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2013;7:383–9. [PubMed] [Google Scholar]
- 14.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F, et al. Gender difference in cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wongtawatchai T, Agthong S, Kaewsema A, Chentanez V. Sex-related differences in cisplatin-induced neuropathy in rats. J Med Assoc Thai. 2009;92:1485–91. [PubMed] [Google Scholar]
- 16.Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol. 2005;25:491–9. doi: 10.1159/000088171. [DOI] [PubMed] [Google Scholar]
- 17.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: Gender-related differences. Ren Fail. 2012;34:1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 19.Mansoori A, Oryan S, Nematbakhsh M. Role of mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J Physiol Pharmacol. 2014;65:633–9. [PubMed] [Google Scholar]
- 20.Arany I, Sa firstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–4. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 21.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Jilanchi S, Nematbakhsh M, Bahadorani M, Talebi A, Eshraghi-Jazi F, Mansouri A, et al. Vitamin e is a nephroprotectant agent in male but not in female in a model of cisplatin-induced nephrotoxicity. ISRN Nephrol. 2013;2013:280395. doi: 10.5402/2013/280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nematbakhsh M, Pezeshki Z. Sex-related difference in nitric oxide metabolites levels after nephroprotectant supplementation administration against cisplatin-induced nephrotoxicity in wistar rat model: The role of vitamin E, erythropoietin, or N-acetylcysteine. ISRN Nephrol. 2013;2013:612675. doi: 10.5402/2013/612675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stakisaitis D, Dudeniene G, Jankūnas RJ, Grazeliene G, Didziapetriene J, Pundziene B, et al. Cisplatin increases urinary sodium excretion in rats: Gender-related differences. Medicina (Kaunas) 2010;46:45–50. [PubMed] [Google Scholar]
- 25.Bradner WT, Schurig JE. Toxicology screening in small animals. Cancer Treat Rev. 1981;8:93–102. doi: 10.1016/s0305-7372(81)80029-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Aggarwal SK. Effects of cisplatin and taxol on inducible nitric oxide synthase, gastrin and somatostatin in gastrointestinal toxicity. Anticancer Drugs. 1997;8:853–8. doi: 10.1097/00001813-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Pratibha Ravindra DA, Kulkarni SS, Padmanabh V, Rataboli Chitra Y, Dhume KU. Cisplatin induced histological changes in renal tissue of rat. J Cell Anim Biol. 2010;4:108–11. [Google Scholar]