Abstract
Background
N‐cadherin is an important molecular in epithelial‐mesenchymal transition (EMT) and has been reported to be associated with aggressive behaviours of tumours. However, prognostic value of N‐cadherin in solid malignancies remains controversially.
Materials and Methods
The Pubmed/MELINE and EMBASE databases were used for a comprehensive literature searching. Pooled risk ratio (RR) and hazard ratio (HR) with their corresponding 95% confidence intervals (CIs) were employed to quantify the prognostic role.
Results
Involving 36 studies with 5705 patients were performed to investigate relationships between N‐cadherin upregulation and clinicopathological features, survival. Results suggested upregulated N‐cadherin was associated with lymph node metastasis (RR = 1.16, 95% CI [1.00, 1.35]), higher histological grade (RR = 1.36, 95%CI [1.14, 1.62]), angiolymphatic invasion (RR = 1.19, 95% CI [1.06, 1.34]) and advanced clinical stage (RR = 1.32, 95% CI [1.06, 1.64]), while upregulated N‐cadherin was apt to be associated with distant metastasis (RR = 1.43, 95% CI [0.99, 2.05]). Moreover, N‐cadherin was correlated with poor prognosis of 3‐year survival (HR = 1.78, 95% CI [1.51, 2.10]), 5‐year survival (HR = 1.57, 95% CI [1.17, 2.10]) and overall survival (OS) (HR = 1.32, 95% CI [1.20, 1.44]). Subgroup analyses according to cancer types were also conducted for applying these conclusions to some tumours more properly. No publication bias was found except subgroup analysis of distant metastasis (P = .652 for Begg's test and 0.023 for Egger's test).
Conclusions
Taken together, upregulation of N‐cadherin is associated with more aggressive behaviours of epithelial‐derived solid malignancies and can be regarded as a predictor of poor survival.
Keywords: clinicopathological features, epithelial‐derived solid tumours, N‐cadherin, prognosis
1. INTRODUCTION
As a life‐threatening disease, cancer has increasingly been a heavy burden of healthcare system.1, 2 The failure of cancer therapy and poor outcomes are mostly owing to the development of local invasion and distant metastasis. Activating of invasion and metastasis has been demonstrated as one of the most important hallmark capabilities of cancer.1 To date, researchers have paid tremendous attention to the biological behaviour of cancer cells, aiming at finding potential therapeutic targets.
Invasion and metastasis are considered as complex processes involving detachment of cancer cell from primary tumour site and formation of new tumour foci in distant organs.3, 4, 5 Epithelial‐mesenchymal transition (EMT), a developmental regulatory programme, identified as transformed epithelial cells loss epithelial constraints and then acquire the abilities to invade, resist apoptosis and metastasis, which is believed to play a vital role in broadly regulating invasion and metastasis.2, 6, 7, 8, 9 Cadherins are a family of calcium‐dependent glycoproteins, which responsible for Ca2+‐dependent cell‐cell adhesions. Of the members of this family, N‐cadherin (neuronal cadherin or cadherin‐2), a transmembrane glycoprotein, which is normally expressed in neuroectodermal and mesenchymal‐derived tissues, plays crucial role in lots of processes, such as cell‐cell adhesion, embryogenesis, gastrulation, neurulation, migration and invasion.4, 10, 11, 12, 13 Today, N‐cadherin is regarded as a vital marker of EMT.14 Neoexpression or upregulated expression of N‐cadherin has been reported to accelerate migration and invasion of cancer cells, which showed a contrary function to those of E‐cadherin.15, 16, 17, 18 This process of reciprocally downregulating E‐cadherin and upregulating N‐cadherin is known as cadherin switching that characterizes EMT.19 Cadherin switching is a necessary procedure during the course of development, while it will lead to an aggressive tumour cell phenotype and enable tumour cells obtain ability of migration from primary site and metastasis to distant tissues.20 Study reveals that N‐cadherin‐positive cancer cells are capable of building cell‐cell adhesion with stromal cells that express N‐cadherin, thus facilitating invasion process.21 Some studies indicate that N‐cadherin boosts the combination of fibroblast growth factor (FGF) with the receptor to initiate FGFR signal transduction, thereby inducing signalling cascades that promote migration and invasion of cancer cells.22 Furthermore, studies have shown that N‐cadherin promotes cells survival and protects cancer cells from apoptosis by activating the phosphatidylinositol 3‐kinase (PI3K)‐AKT (also known as protein kinase B) pathway.17, 23, 24 Therefore, N‐cadherin presents distinct functions from its adhesion activity.
Nevertheless, the prognostic role of N‐cadherin in tumours still remains controversially. Some studies suggested that expression of N‐cadherin was generally upregulated in prostate, breast cancer and urothelial tumours, and was associated with poorer clinical outcomes.10, 11, 25 In addition, the upregulation of N‐cadherin was significantly related with postoperative recurrence in hepatocellular carcinoma (HCC) patients.26 However, it was also reported that the expression of N‐cadherin was decreased in glioblastoma, ovarian carcinoma, osteosarcoma and HCC, which may provide a hypothesis that N‐cadherin serves as a tumour suppressor and its downregulation links to poor surgical outcomes.15, 27, 28, 29 Therefore, this meta‐analysis was conducted to assess the relationship of N‐cadherin expression with clinicopathological features and prognosis of different carcinomas, especially in epithelial‐derived solid tumours.
2. MATERIALS AND METHODS
2.1. Primary search strategy
The Pubmed/MELINE and EMBASE databases were used for related publications search by variably combining the terms “cancer [Title/Abstract],” “tumour [Title/Abstract],” “carcinoma [Title/Abstract],” “neoplasm [Title/Abstract]” and “N‐cadherin [Title/Abstract],” without any limitation of publication date. The most recent search update was conducted on 23 February 2017. To avoid missing additional studies, the references of all eligible studies were further explored in these databases. PRISMA and the broader EQUATOR guidelines were referred to in the reporting of our study to comply with the standard of meta‐analysis.30
2.2. Inclusion and exclusion criteria
The included studies were required to meet the following criteria: (i) the patients with solid tumours included in these studies were diagnosed histopathologically; (ii) the correlation of N‐cadherin expression and solid tumours was evaluated; (iii) clinicopathological features, 3/5‐year survival, OS or Kaplan‐Meier survival curves were investigated; and (iv) full text was written in English. Studies were excluded if (i) the study was published in conference abstract, letters, editorials, reviews, expert opinion or case reports; (ii) studies of animals or cell lines; (iii) the relevant clinicopathological features or outcomes of patients were not able to be acquired for necessary analysis; (iv) overlapped or duplicated studies.
2.3. Data extraction
Data were carefully retrieved independently by 2 reviewers from all the eligible studies, using a standardized form with the following characteristics: author, year, country, study design, number of patients, mean age, method used for evaluating expression of N‐cadherin, antibody source, definition of N‐cadherin positive, expression rate of N‐cadherin, median follow‐up time, clinicopathological features and survival data. Discrepancies between 2 reviewers were solved by discussing until agreement was reached with the third reviewer. Quality assessment of included studies was conducted on the basis of the Newcastle‐Ottawa quality assessment scale (NOS scale). Six points or higher were regarded as high quality.
2.4. Statistical analysis
Included studies were divided into 7 groups for analysing the prognostic value of N‐cadherin expression in solid tumour patients regarding histological grade, lymph node status, angiolymphatic invasion, distant metastasis, clinical stage, 3‐year survival, 5‐year survival and OS. Clinicopathological features were synthesized using the risk ratio (RR) as the calculating measure, while the survival outcome data using the hazard ratio (HR) as calculating measure. RR/HR with 95% confidence interval (CI) value was performed to quantitatively evaluate how increased N‐cadherin expression impacts the prognosis of solid tumour patients. Reported RR/HR and their 95% CI were directly extracted from included studies. If RR/HR and 95% CIs were not available in the original articles, the methods demonstrated by Parmer et al30 and Tierney et al31 were applied to calculate RR/HR and 95% CIs. Moreover, when the exploitable data were presented in the figure form, Engauge Digitizer version 4.1 (free software downloading from http://sourceforge.net) was applied to extract data from Kaplan‐Meier curves thereby obtaining HR and 95% CIs. Heterogeneities of included studies were assessed using I 2 metric and Q statistic. If heterogeneity was significantly obvious (I 2 > 50% or P < .1), a random effect model was used. Otherwise, a fixed effect model would be chosen (I 2 < 50% or P > .1).The pooled RRs/HRs and 95% CIs were presented in forest plots. If the 95% CIs of RR/HR did not cover the value 1 with P < .05, the difference between groups would be regarded as statistically significant. In addition, funnel plots (Begg's test) and Egger's linear regression method (Egger's test) were performed to evaluate the publication bias through assessing the asymmetry of funnel plots (if P < .05, considering to be statistically significant).32 All statistical analyses in this meta‐analysis were performed using STATA version 12.0 (STATA corporation, College Station, TS, USA).
3. RESULTS
3.1. Study selection and characteristics
According to the initial search algorithm, total 4476 studies were screened out, and 389 candidate studies were read in full. Eventually, a total of 36 publications were included in this meta‐analysis, and the other 353 studies were out of scope, as shown in Figure 1. A total of 5705 patients were included in this study, ranging from 38 to 1035 patients per study, the main characteristics and NOS scale of eligible studies were presented in Table 1. Most of studies were prospective cohort studies (n = 25), and 11 studies were retrospective cohort studies. The median follow‐up time varied from 10.1 months to 125 months.
Figure 1.
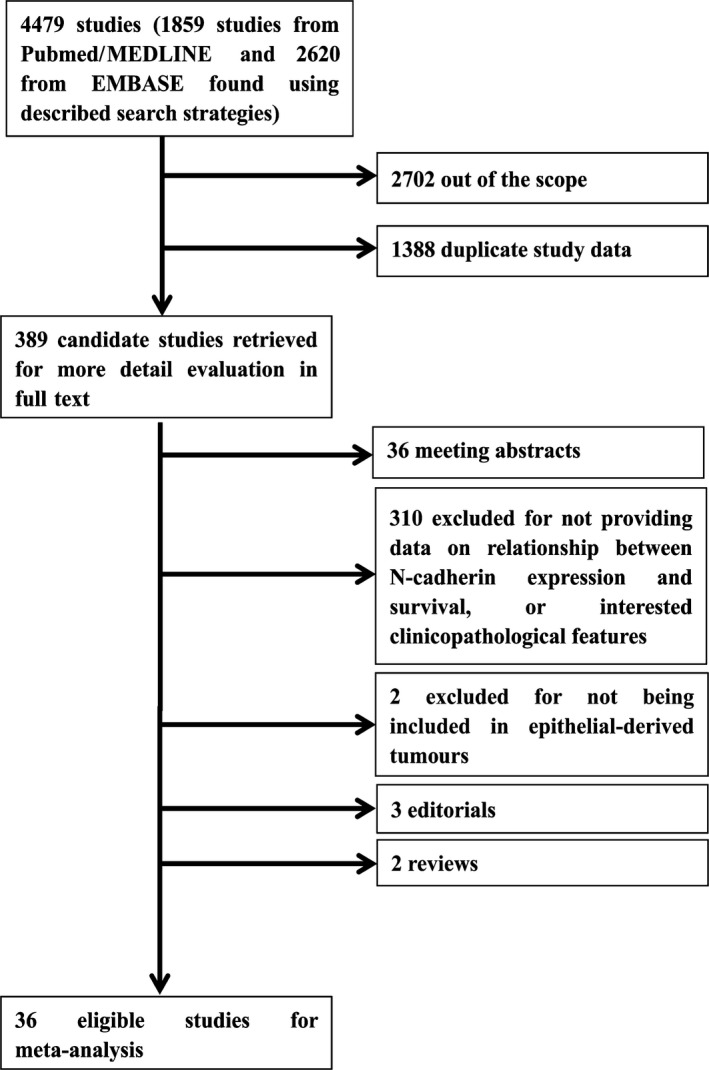
Flow chart of the literature search and selection of included studies
Table 1.
Characteristics of the eligible studies
| First author (ref) | Year | Country | Study design | Number (M/F) | Mean age | Method | Antibody source | Definition of N‐cadherin positive | Expression rate (%) | Median follow‐up (m) | Quality stars (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HNC (n = 6) | |||||||||||
| Nguyen, P.T. 39 | 2011 | Japan | Retro. | 80 (NR) | NR | IHC | BD Biosciences | ≥20% | NR | NR | 7 |
| Gasparotto, D. 41 | 2011 | Italy | Pro. | 69 (65/4) | NR | IHC | Novocastra | ≥Median expression level | NR | 45 | 8 |
| Ding, L. 43 | 2014 | China | Pro. | 50 (25/25) | 53.5 | IHC | Zymed | ≥20% | NR | 60.34 | 9 |
| Mohan, A. 56 | 2006 | India | Retro. | 62 (38/24) | 2.67 | IHC | Upstate Cell Signaling Solutions | ≥5% | 93.5 | NR | 8 |
| Luo, W.R. 58 | 2011 | China | Pro. | 122 (92/30) | 47.6 | IHC | Zymed | IRS ≥ 6a | NR | 51.9 | 9 |
| Greco, A. 67 | 2015 | Italy | Pro. | 82 (70/12) | 61 | IHC | Santa Cruz Biotechnology | NR | NR | NR | 7 |
| Breast cancer (n = 7) | |||||||||||
| Aleskandarany, M.A. 32 | 2014 | England | Pro. | 1035 (NR) | 54 | IHC | Sigma | NR | NR | 125 | 8 |
| Choi, Y. 33 | 2013 | Korea | Retro. | 389 (NR) | 50.6 | IHC | Invitrogen | ≥10% | NR | NR | 7 |
| Markiewicz, A. 34 | 2014 | Poland | Pro. | 108 (NR) | 60 | IHC | Dako | ≥10% | NR | 28.8 | 8 |
| ElMoneim, H.M.A. 35 | 2011 | Egypt | Retro. | 132 (NR) | 52.67 | IHC | Dako | ≥10% | NR | NR | 7 |
| Kovács, A. 37 | 2003 | England | Pro. | 100 (NR) | NR | IHC | Zymed | ≥20% | NR | NR | 7 |
| Cao, Y.W. 38 | 2014 | China | Retro. | 200 (NR) | NR | IHC | Abcam | IRS > 3 | NR | NR | 6 |
| Carvalho, S.T. 64 | 2011 | Brazil | Pro. | 82 (NR) | NR | IHC | BD Biosciences | ≥10% | NR | 120 | 8 |
| Lung cancer (n = 7) | |||||||||||
| Li, X.X. 36 | 2015 | China | Pro. | 65 (44/21) | 59 | IHC | Abcam | IRS ≥ 3 | 35.4 | 30.17 | 8 |
| Nakashima, T. 40 | 2003 | Japan | Pro. | 150 (NR) | NR | IHC | BD Bioscience | ≥20% | 30.6 | 41.4 | 7 |
| Hui, L. 42 | 2013 | China | Pro. | 120 (81/39) | 60 | IHC | Santa Cruz Biotechnology | IRS ≥ 2 | 30.7 | 30.8 | 8 |
| Zhou, Y. 57 | 2016 | China | Pro. | 153 (141/12) | NR | IHC | Abcam | IRS > 4 | 79.08 | 57 | 7 |
| Grinberg‐Rashi, H. 59 | 2009 | Israel | Pro. | 107 (76/31) | 61.06 | IHC | Dako | Mesothelioma as positive control | NR | 34 | 8 |
| Liu, S. 63 | 2013 | China | Pro. | 113 (61/52) | 60.3 | IHC | Santa Cruz Biotechnology | ≥10% | 40.7 | NR | 8 |
| Miao, Y. 66 | 2012 | China | Pro. | 105 (63/42) | 60.4 | IHC | Abcam | ≥10% | 44.8 | NR | 8 |
| Cancer of digestive system (n = 7) | |||||||||||
| Kamikihara, T. 44 | 2012 | Japan | Pro. | 146 (99/47) | 63 | IHC | Dako | ≥5% | 21.2 | 43.2 | 9 |
| Araki, K. 45 | 2011 | Japan | Pro. | 38 (NR) | NR | IHC | Invitrogen Corp. | NR | 23.7 | NR | 7 |
| Nakajima, S. 49 | 2004 | Japan | Pro. | 40 (NR) | 66.3 | IHC | Zymed | ≥10% | 32.5 | 10.1 | 7 |
| Guo, S. 50 | 2013 | China | Retro. | 76 (47/29) | 65 | IHC | Santa Cruz Biotechnology | IRS ≥ 5 | 32.9 | NR | 7 |
| Cho, S.B. 53 | 2008 | Korea | Pro. | 68 (55/13) | 60 | IHC | Dako | ≥30% | NR | 30 | 6 |
| Jie, D. 54 | 2013 | China | Pro. | 108 (65/43) | 54 | IHC | R&D Systems | IRS ≥ 1 | 41.7 | NR | 7 |
| Fu, H. 65 | 2016 | China | Retro. | 74 (63/11) | NR | IHC | NR | IRS ≥ 2 | 36.48 | NR | 7 |
| Urothelial carcinoma (n = 3) | |||||||||||
| Abufaraj, M. 51 | 2016 | Italy | Pro. | 678 (380/298) | NR | IHC | Dako | Higher than normal bladder and prostate tissues | 43.1 | 37.5 | 9 |
| Fondrevelle, M.E. 60 | 2009 | France | Pro. | 70 (52/18) | 69 | IHC | Zymed | Higher than normal myocardium tissue | 17.14 | 30 | 8 |
| Baumgart, E. 61 | 2007 | America | Pro. | 572 (NR) | NR | IHC | Zymed | NR | 8.2 | 61.2 | 8 |
| Prostate cancer (n = 2) | |||||||||||
| Liu, GL. 46 | 2014 | China | Retro. | 59 (NR) | 68 | IHC | Bioss Biotechnology | ≥5% | NR | NR | 7 |
| Drivalos, A. 47 | 2015 | Greece | Retro. | 157 (NR) | 66 | IHC | Dako | IRS > 3 | NR | NR | 7 |
| Gynecologic Cancer (n = 4) | |||||||||||
| Li, B. 48 | 2016 | China | Retro. | 127 (NR) | NR | IHC | Santa Cruz Biotechnology | IRS ≥ 3 | 3.1 | 72 | 8 |
| Ma, Y. 52 | 2015 | China | Retro. | 81 (NR) | 48.1 | IHC | Beijing Zhong Shan Biotechnology | ≥10% | 28.4 | NR | 7 |
| Marques, F.R. 56 | 2004 | Brazil | Pro. | 47 (NR) | 51 | IHC | Zymed | ≥10% | NR | 48 | 7 |
| Do, TV. 62 | 2008 | America | Pro. | 40 (NR) | 62.4 | IHC | Invitrogen | IRS ≥ 1.5 | NR | NR | 6 |
Abbreviations: F, female; IRS, immuno‐reactive score; IHC, immunohistochemistry; m, month; M, male; NOS, Newcastle‐Ottawa quality assessment scale; NR, not reported; Pro., prospective study; Retro., retrospective study; ref, reference.
IRS = percentage score × intensity score. Percentage score of stained tumour cells is considered as follows: no staining (score = 0), <10% stained (score = 1), 10%‐50% stained (score = 2), 51%‐80% stained (score = 3) and 81%‐100% stained (score = 4). Intensity score is estimated as no staining (score = 0), weak staining (score = 1), moderate staining (score = 2) and strong staining (score = 3).
Among the eligible studies, 36 studies compared the correlation of clinicopathological features and N‐cadherin expression, including lymph node metastasis (n = 21), histological grade (n = 21), angiolymphatic invasion (n = 8), distant metastasis (n = 7) and clinical stage (n = 14). In addition, 10 studies assessed the association of 3‐year survival and N‐cadherin positivity, 8 studies for 5‐year survival and 22 studies for OS. In terms of NOS scale, 3 articles scored 6 points, 16 articles scored 7 points, 13 articles scored 8 points and 4 articles scored 9 points, hence, all of these inclusive studies were regarded as qualified enough for meta‐analysis.
3.2. Lymph node metastasis
Lymph node metastasis was evaluated in 21 studies with 3900 patients (positive lymph node metastasis versus negative lymph node metastasis).33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 Pooled RR indicated that increased N‐cadherin apt to cause lymph node metastasis (RR = 1.16, 95%CI [1.00, 1.35], I 2 = 57.6%, P = .806; Figure 2A). Thus, a random effect mode was employed due to obvious heterogeneity. According to the subgroup analyses of different tumour types, N‐cadherin overexpression in prostate cancer contributed to a significantly positive lymph node metastasis (RR = 2.94, 95%CI [1.29, 6.70]) in 2 studies with 216 patients.47, 48
Figure 2.
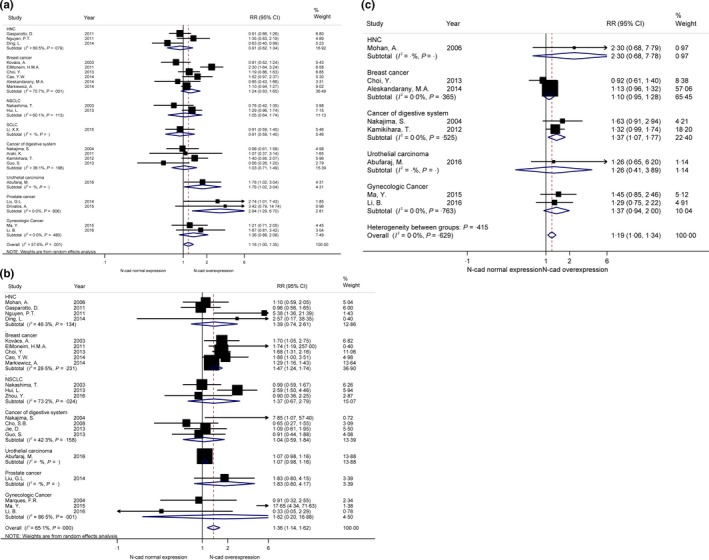
Forrest plots of risk ratios (RRs) for correlation between N‐cadherin upregulation and clinicopathological features. A, Lymph node metastasis; B, histological grade; C, angiolymphatic invasion
3.3. Histological grade
A total of 21 studies with 2995 patients assessed the impact of N‐cadherin expression and histological grade (poor differentiation vs well/moderate differentiation).34, 35, 36, 38, 39, 40, 41, 42, 43, 44, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 Pooled RR showed that increased N‐cadherin expression was significantly associated with worse differentiation (RR = 1.36, 95% CI [1.14, 1.62]) (Figure 2B). Because of obvious heterogeneity, random effect model was performed (I 2 = 65.1%, P = .000). Subgroup analyses of different tumour types showed N‐cadherin upregulation was significantly associated with worse differentiation of breast cancer (RR = 1.47, 95% CI [1.24, 1.74]), while no statistically significant relationships were found between upregulation of N‐cadherin and oestrogen receptor (ER), progesterone receptor (PR) or HER2 expression in breast cancer in 5 studies with 821 patients (RR = 0.79, 95% CI [0.61, 1.02]; RR = 0.88, 95% CI [0.73, 1.06]; RR = 1.04, 95% CI [0.80, 1.34]; respectively) (Figure S1A).33, 34, 36, 38, 39
3.4. Angiolymphatic invasion
Pooled RR of 8 inclusive studies with 2239 patients showed the upregulation of N‐cadherin was significantly correlated with angiolymphatic invasion (RR = 1.19, 95%CI [1.06, 1.34]) (Figure 2C).34, 36, 45, 49, 50, 52, 53, 57 A subgroup analysis suggested that upregulation of N‐cadherin was significantly associated with angiolymphatic invasion in cancers of digestive system in 2 studies with 186 patients (RR = 1.37, 95%CI [1.07, 1.77]).45, 50 No obvious heterogeneity was observed, hence a fixed effect model was employed (I 2 = 0.0%, P = .629; Table 2).
Table 2.
Summary of the outcomes presented in this meta‐analysis
| Group | No. of studies | No. of total patients | RR/HR (95% CI) (N‐cad overexpression vs N‐cad normal expression) | P for heterogeneity | I 2 | References |
|---|---|---|---|---|---|---|
| Lymph node metastasis | 21 | 3900 | 1.16 (1.00, 1.35) | .001 | 57.6% | 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 |
| Histological grade | 21 | 2995 | 1.36 (1.14, 1.62) | .000 | 65.1% | 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 |
| Angiolymphatic invasion | 8 | 2239 | 1.19 (1.06, 1.34) | .629 | 0.0% | 33, 35, 44, 48, 49, 51, 52, 56 |
| Distant metastasis | 7 | 434 | 1.43 (0.99, 2.05) | .382 | 5.9% | 36, 44, 46, 49, 50, 54, 58 |
| Clinical stage | 14 | 1479 | 1.32 (1.06, 1.64) | .000 | 68.9% | 35, 36, 38, 39, 42, 43, 44, 45, 47, 49, 53, 54, 57, 58 |
| 3‐year survival | 10 | 2370 | 1.78 (1.51, 2.10) | .174 | 29.4% | 32, 42, 43, 44, 54, 58, 59, 60, 61, 62 |
| 5‐year survival | 8 | 2180 | 1.57 (1.17, 2.10) | .019 | 58.2% | 32, 43, 44, 54, 58, 59, 61, 62 |
| OS | 22 | 4081 | 1.32 (1.20, 1.44) | .007 | 48.0% | 32, 40, 41, 42, 43, 44, 45, 48, 49, 51, 54, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 |
HR, hazard ratio; RR, risk ratio; OS, overall survival.
3.5. Distant metastasis
Distant metastasis was investigated in 7 studies with 434 patients.37, 45, 47, 50, 51, 55, 59 Pooled RR showed a no statistically significant relationship between N‐cadherin upregulation and distant metastasis (RR = 1.43, 95%CI [0.99, 2.05]) (Figure 3A). A fixed effect model was used for observing no obvious heterogeneity (I 2 = 5.9%, P = .382). Subgroup analysis in cancers of digestive system demonstrated significant association between N‐cadherin upregulation and distant metastasis, in 4 studies with 370 patients (RR = 1.90, 95%CI [1.01, 3.59]).45, 50, 51, 55
Figure 3.
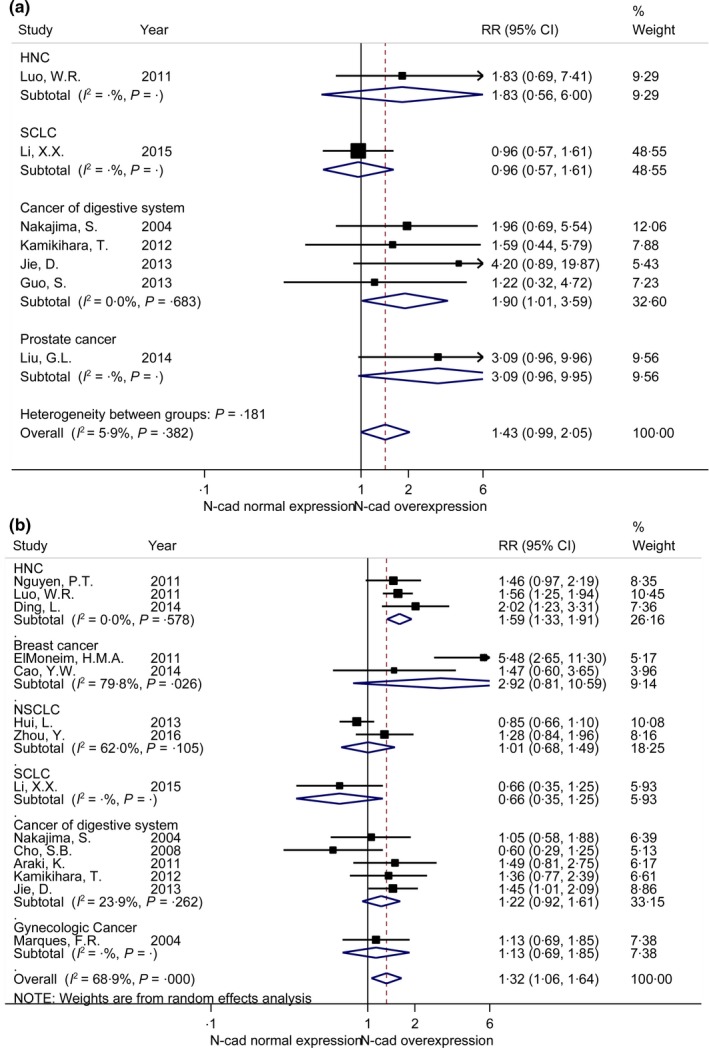
Forrest plots of risk ratio (RRs) for correlation between N‐cadherin upregulation and clinicopathological features. A, Distant metastasis; B, clinical stage
3.6. Clinical Stage
A total of 14 studies with 1479 patients discussed the relationship between N‐cadherin upregulation and clinical stage (III/IV vs I/II).36, 37, 39, 40, 43, 44, 45, 46, 48, 50, 54, 55, 58, 59 Pooled RR showed higher N‐cadherin expression was related to advanced clinical stage (RR = 1.32, 95% CI [1.06, 1.64]) (Figure 3B). A random effect model was performed for obvious heterogeneity (I 2 = 68.9%, P = .000).Subgroup analysis of head and neck cancers (HNC) indicated an association between N‐cadherin upregulation and advanced clinical stages, in 3 studies with 252 patients (RR = 1.59, 95% CI [1.33, 1.91]).40, 44, 59
3.7. 3‐year and 5‐year survival
Quantitative analysis of pooled RR confirmed that upregulation of N‐cadherin was shown to be an unfavourable prognostic marker of 3‐year survival (HR = 1.78, 95%CI [1.51, 2.10]) with no obvious heterogeneity in 10 studies with 2370 patients (Figure 4A).33, 43, 44, 45, 55, 59, 60, 61, 62, 63 Subgroup analysis showed N‐cadherin overexpression was related to worse 3‐year survival in HNC (HR = 2.24, 95%CI [1.31, 3.81]), nonsmall cell lung cancer (NSCLC) (HR = 2.02, 95%CI [1.52, 2.70]) and cancer of digestive system (HR = 2.19, 95%CI [1.35, 3.56]). Similar result was also found in 5‐year survival (HR = 1.57, 95%CI [1.17, 2.10]) in 8 studies with 2180 patients (Figure 4B).33, 44, 45, 55, 59, 60, 62, 63 Subgroup analysis indicated the upregulation of N‐cadherin was associated with worse 5‐year survival in HNC (HR = 1.87, 95%CI [1.05, 3.33]) and cancer of digestive system (HR = 1.66, 95%CI [1.13, 2.44]). Considering the heterogeneity, a fixed effect model and a random effect model were performed in 3‐year (I 2 = 29.4%, P = .174) and 5‐year survival analysis (I 2 = 58.2%, P = .019), respectively.
Figure 4.
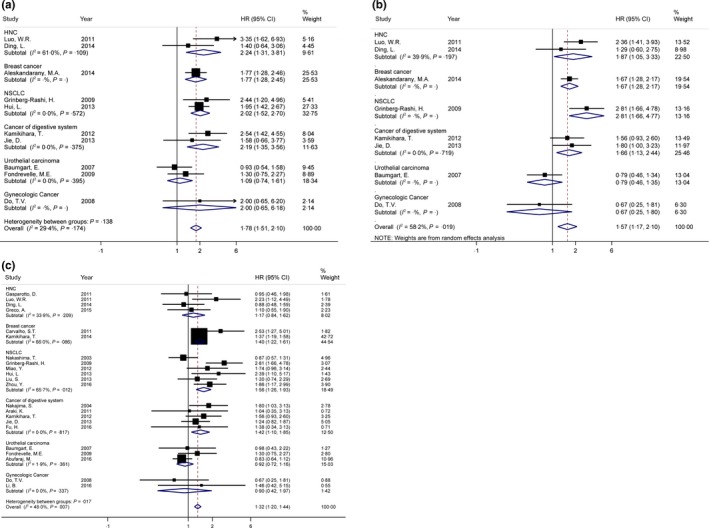
Forrest plots of hazard ratios (HRs) for correlation between N‐cadherin upregulation and survival outcomes. A, 3‐year survival; B, 5‐year survival; C, overall survival (OS)
3.8. Overall survival
N‐cadherin upregulation was proved to have a significant association with worse OS, with pooled HR = 1.32 (95% CI [1.20, 1.44]), in 22 studies with 4081 patients (Figure 4C).33, 41, 42, 43, 44, 45, 46, 49, 50, 52, 55, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 According to subgroup analyses of different cancer types, N‐cadherin upregulation in breast cancer (HR = 1.40, 95% CI [1.22, 1.61]), NSCLC (HR = 1.56, 95% CI [1.26, 1.93]) (n = 6, with 748 patients) and cancers of digestive system (HR = 1.42, 95% CI [1.10, 1.85]) (n = 5, with 406 patients) acted as an unfavourable indicator of OS. Due to no obvious heterogeneity was observed, a fixed effect model was performed (I 2 = 48.0%).
3.9. Publication bias
Both Begg's funnel plot and Egger's test were employed to evaluate the publication bias in all studies, including lymph node metastasis, histological grade, angiolymphatic invasion, distant metastasis, clinical stage, 3‐year and 5‐year survival and OS, respectively (Figure 5). In addition, Begg's funnel plot of molecular markers of breast cancer (ER, PR and HER2) was shown in Figure S1B. Begg's funnel plot showed no statistically significant asymmetry in this meta‐analysis, involving lymph node metastasis (P = .629), histological grade (P = .763), angiolymphatic invasion (P = .322), distant metastasis (P = .652), clinical stage (P = .477), 3‐year and 5‐year survival and OS (P = .655, P = .216, P = .933, respectively). Furthermore, no evidence of publication bias was observed in Egger's test of lymph node metastasis (P = .735), histological grade (P = .115), angiolymphatic invasion (P = .159), clinical stage (P = .772), 3‐year and 5‐year survival and OS (P = .920, P = .492, P = .634, respectively), and molecular makers of breast cancer (P = .741). Egger's test showed potential publication bias in analysis of distant metastasis (P = .023).
Figure 5.
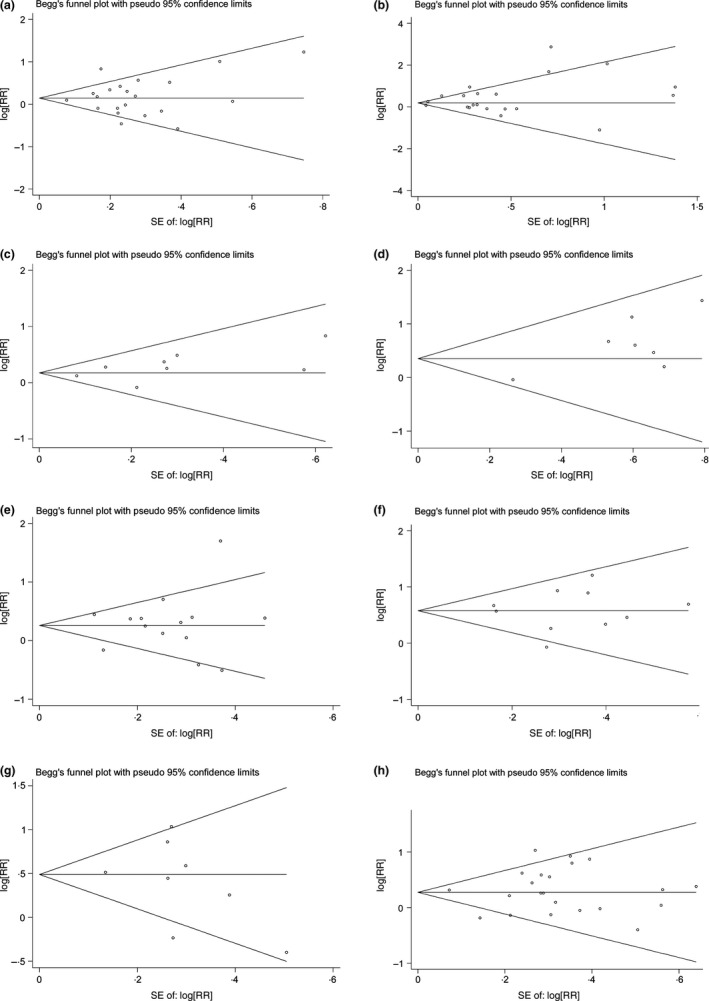
Funnel graph for assessing the potential publication bias of this meta‐analysis. A, Lymph node metastasis; B, histological grade; C, angiolymphatic invasion; D, distant metastasis; E, clinical stage; F, 3‐year and 5‐year survivals; G, overall survival (OS)
4. DISCUSSION
Many laboratorial evidences have revealed that N‐cadherin could drive tumour cells to move into surrounding stroma, thereby promoting tumour cells migration, invasion and metastasis.15, 18, 20, 69 In addition, the upregulation of N‐cadherin was also noticed in different cancer types and was also hinted to link to higher histological grade and metastatic tendency.69, 70, 71 Hence, N‐cadherin has been implicated as potential target for anticancer therapy.72 Monoclonal antibody targeting of N‐cadherin has been reported to be effective in inhibiting proliferation, metastasis and castration resistance of prostate cancer.10 Moreover, a kind of N‐cadherin antagonist has been developed by researchers, a synthetic cyclic pentapeptide (known as ADH‐1). In an animal model of pancreatic cancer, ADH‐1 showed the inhibitory effect against tumour growth and metastasis.73 Importantly, a phase I/II clinical trial has shown combination of ADH‐1 and melphalan could suppress tumour growth in patients with locally advanced melanoma.74 Furthermore, ADH‐1 was evaluated to be well tolerable both in animal models and humans.75, 76 However, according to current clinical evidences, the clinical significance of N‐cadherin still was unclear. The majority of studies demonstrated N‐cadherin as an unfavourable prognostic marker in a variety of tumours, while some studies showed the tumour suppressor role of N‐cadherin. Therefore, an integrated study is urgently needed to answer this question.
As far as we know, this study is the first comprehensive and most full‐scale meta‐analysis to investigate the prognostic value of N‐cadherin upregulation in epithelial‐derived solid tumours. At the beginning, 38 studies were involved in this meta‐analysis, while 2 studies were about nonepithelial solid tumours, including melanoma and sarcoma.77, 78 Thus, we excluded these 2 studies for making our conclusions more applicable to epithelia‐derived solid tumours. In summary, upregulation of N‐cadherin was shown to be significantly associated with lymph node metastasis, higher histological grade, angiolymphatic invasion and advanced clinical stage. According to different cancer types, subgroup analyses further explored relationships between some special cancer types and clinicopathological features. Results suggested upregulation of N‐cadherin was associated with lymph node metastasis in prostate cancer, higher histological grade in breast cancer, advanced clinical stage in HNC, positive angiolymphatic invasion and distant metastasis in cancers of digestive system. Importantly, pooled HRs showed upregulation of N‐cadherin was an obvious unfavourably prognostic factor in analyses of 3‐year survival, 5‐year survival and OS, especially for NSCLC and cancers of digestive system according to results from subgroup analyses of OS. Therefore, patients with higher N‐cadherin expression may need more aggressive treatment, especially for NSCLC and cancers of digestive system.
In spite of the inspiring outcomes, some limitations could not be ignored. First, although all of the included studies used IHC method to measure expression level of N‐cadherin, different antibody sources or various cut‐off values could lead to potential bias. However, limited by insufficient number of studies, the subgroup analyses of antibody sources or cut‐off values were unable to be carried out. Second, obvious heterogeneity was existed in analyses of lymph node metastasis, histological grade, clinical stage, 5‐year survival and OS, which may be derived from different cancer types, cancer stages, follow‐up time, etc. Random effect model was employed to reduce the effect of heterogeneity on outcomes. In addition, corresponding subgroup analyses were also conducted to make outcomes more reliable and decrease potential bias. Finally, it would be better if we could conduct a comprehensive analysis about prognostic value of cadherin switching in tumours. Loss of E‐cadherin always accompanied with upregulation of N‐cadherin in tumours. Besides, loss of E‐cadherin has been proved to be associated with unfavourable outcomes in various tumours.79, 80, 81, 82 The analysis in combination of E‐cadherin and N‐cadherin might be more valuable in prognostic evaluation of some special tumours, while only a few studies assessed the impact of cadherin switching.83
In conclusion, our study suggests that positivity of N‐cadherin is associated with more aggressive behaviour of epithelial‐derived solid malignancies, linked to lymph node metastasis, higher histological grade, angiolymphatic invasion and advanced clinical stage. Moreover, N‐cadherin could be regarded as a useful predictor for unfavourable prognosis of 3‐year survival, 5‐year survival and OS in epithelial‐derived solid malignancies. The expression status of N‐cadherin can provide a reference for treatment strategy and prognostic prediction of epithelial‐derived solid malignancies.
DISCLOSURE OF POTENTIAL CONFLICT OF INTERESTS
No potential conflict of interests were disclosed.
AUTHOR CONTRIBUTIONS
Y Luo, T Yu and HS Shi contributed to the design of the study and manuscript writing. T Yu, QW Zhang, QY Fu, MM xiang, HN peng and Li LU contributed to the data extraction and analysis process of this study. YZ Hu and TY Zheng contributed to the revision of the manuscript. QW Zhang and HS Shi contributed to the financial support of this study.
Supporting information
ACKNOWLEDGEMENTS
We thank Tagir Taipov (Bashkir State Medical University, Russia) for revising our manuscript. This work was funded by grant from National Natural Science Foundation of China (81300320).
Luo Y, Yu T, Zhang Q, et al. Upregulated N‐cadherin expression is associated with poor prognosis in epithelial‐derived solid tumours: A meta‐analysis. Eur J Clin Invest. 2018;48:e12903 https://doi.org/10.1111/eci.12903
Yong Luo and Ting Yu contributed equally to this work.
REFERENCES
- 1. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 3. Shenoy AK, Jin Y, Luo H, et al. Epithelial‐to‐mesenchymal transition confers pericyte properties on cancer cells. J Clin Invest. 2016;126:4174‐4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu YA, Liang BY, Guan Y, et al. Loss of N‐cadherin is associated with loss of E‐cadherin expression and poor outcomes of liver resection in hepatocellular carcinoma. J Surg Res. 2015;194:167‐176. [DOI] [PubMed] [Google Scholar]
- 5. Li X, Jiang Z, Ma Q. YAP is critical mediator of TGF‐β1 induced EMT and cell invasion in pancreatic cancer. Pancreas. 2016;45:1519. [Google Scholar]
- 6. Dai X, Thongchot S, Dokduang H, et al. Potential of selenium compounds as new anticancer agents for cholangiocarcinoma. Anticancer Res. 2016;36:5981‐5988. [DOI] [PubMed] [Google Scholar]
- 7. Mao W, Sun Y, Zhang H, Cao L, Wang J, He P. A combined modality of carboplatin and photodynamic therapy suppresses epithelial‐mesenchymal transition and matrix metalloproteinase‐2 (MMP‐2)/MMP‐9 expression in HEp‐2 human laryngeal cancer cells via ROS‐mediated inhibition of MEK/ERK signalling pathway. Lasers Med Sci. 2016;31:1697‐1705. [DOI] [PubMed] [Google Scholar]
- 8. Urayama S, Habib A, Pan W, Alzofon N, Wang S. Potential targets and role of Ezh2 in pancreatic cancer. Pancreas. 2016;45:1543‐1544. [Google Scholar]
- 9. Nurwidya F, Takahashi F, Tajima K, et al. Knockdown of Oct4 reverses epithelial‐mesenchymal transition induced by insulin‐like growth factor 1 in lung cancer. Respirology. 2016;21:15. [Google Scholar]
- 10. Tanaka H, Kono E, Tran CP, et al. Monoclonal antibody targeting of N‐cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang H, Svoboda RA, Lazenby AJ, et al. Up‐Regulation of N‐Cadherin by Collagen I‐activated discoidin domain receptor 1 in pancreatic cancer requires the adaptor molecule Shc. J Biol Chem. 2016;291:23208‐23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Derycke LD, Bracke ME. N‐cadherin in the spotlight of cell‐cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463‐476. [DOI] [PubMed] [Google Scholar]
- 13. Stemmler MP. Cadherins in development and cancer. Mol BioSyst. 2008;4:835‐850. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N‐cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N‐cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell‐cell adhesion. J Cell Biol. 1996;135:1643‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kou Y, Li L, Li H, et al. Berberine suppressed epithelial mesenchymal transition through cross‐talk regulation of PI3K/AKT and RARα/RARβ in melanoma cells. Biochem Biophys Res Comm. 2016;479:290‐296. [DOI] [PubMed] [Google Scholar]
- 18. Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N‐cadherin promotes motility in human breast cancer cells regardless of their E‐cadherin expression. J Cell Biol. 1999;147:631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727‐735. [DOI] [PubMed] [Google Scholar]
- 20. Zheng X, Hu H, Li S. High expression of lncRNA PVT1 promotes invasion by inducing epithelial‐to‐mesenchymal transition in esophageal cancer. Oncol Lett. 2016;12:2357‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christofori G. New signals from the invasive front. Nature. 2006;441:444‐450. [DOI] [PubMed] [Google Scholar]
- 22. Chen YH, Zhou BY, Wu XJ, et al. CCL22 and IL‐37 inhibit the proliferation and epithelial‐mesenchymal transition process of NSCLC A549 cells. Oncol Rep. 2016;36:2017‐2024. [DOI] [PubMed] [Google Scholar]
- 23. Tan H, Wang X, Yang X, Li H, Liu B, Pan P. Oncogenic role of epithelial cell transforming sequence 2 in lung adenocarcinoma cells. Exp Ther Med. 2016;12:2088‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Tran NL, Adams DG, Vaillancourt RR, Heimark RL. Signal transduction from N‐cadherin increases Bcl‐2. Regulation of the phosphatidylinositol 3‐kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905‐32914. [DOI] [PubMed] [Google Scholar]
- 25. Ota Y, Takahashi K, Otake S, et al. Extracellular vesicle‐mediated transfer of microRNA‐30e modulates epithelial‐mesenchymal transition in human cholangiocarcinoma. Hepatology (Baltimore, MD) 2016;63:225A‐226A. [Google Scholar]
- 26. Seo DD, Lee HC, Kim HJ, et al. Neural cadherin overexpression is a predictive marker for early postoperative recurrence in hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2008;23:1112‐1118. [DOI] [PubMed] [Google Scholar]
- 27. Kashima T, Kawaguchi J, Takeshita S, et al. Anomalous cadherin expression in osteosarcoma. Possible relationships to metastasis and morphogenesis. Am J Pathol. 1999;155:1549‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhan DQ, Wei S, Liu C, et al. Reduced N‐cadherin expression is associated with metastatic potential and poor surgical outcomes of hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:173‐180. [DOI] [PubMed] [Google Scholar]
- 29. Utsuki S, Sato Y, Oka H, Tsuchiya B, Suzuki S, Fujii K. Relationship between the expression of E‐, N‐cadherins and beta‐catenin and tumor grade in astrocytomas. J Neurooncol. 2002;57:187‐192. [DOI] [PubMed] [Google Scholar]
- 30. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35‐53. [DOI] [PubMed] [Google Scholar]
- 31. Lustri AM, Di Matteo S, Fraveto A, et al. TGF‐β signaling is an effective target to block proliferation and induce apoptosis of human cholangiocarcinoma (CCA) cells: a study on human primary cell cultures. Hepatology (Baltimore, MD) 2016;63:236A. [Google Scholar]
- 32. Zhang H, Sun JD, Yan LJ, Zhao XP. PDGF‐D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem Biophys Res Comm. 2016;478:845‐851. [DOI] [PubMed] [Google Scholar]
- 33. Aleskandarany MA, Negm OH, Rakha EA, et al. Epithelial mesenchymal transition in early invasive breast cancer: further evidence using reverse phase protein array. J Pathol. 2013;231:S19. [DOI] [PubMed] [Google Scholar]
- 34. Choi Y, Lee HJ, Jang MH, et al. Epithelial‐mesenchymal transition increases during the progression of in situ to invasive basal‐like breast cancer. Hum Pathol. 2013;44:2581‐2589. [DOI] [PubMed] [Google Scholar]
- 35. Markiewicz A, Wełnicka‐Jaśkiewicz M, Seroczyńska B, et al. Epithelial‐mesenchymal transition markers in lymph node metastases and primary breast tumors ‐ Relation to dissemination and proliferation. Am J Transl Res. 2014;6:793‐808. [PMC free article] [PubMed] [Google Scholar]
- 36. ElMoneim HMA, Zaghloul NM. Expression of E‐cadherin, N‐cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics. 2011;66:1765‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li XX, Li RJ, Zhao LJ, Liu NB, Wang P. Expression of molecular factors correlated with metastasis in small cell lung cancer and their significance. Int J Clin Exp Pathol. 2015;8:14676‐14684. [PMC free article] [PubMed] [Google Scholar]
- 38. Kovács A, Dhillon J, Walker RA. Expression of P‐cadherin, but not E‐cadherin or N‐cadherin, relates to pathological and functional differentiation of breast carcinomas. J Clin Pathol. 2003;56:318‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao YW, Wan GX, Sun JP, et al. Implications of the Notch1‐Snail/Slug‐epithelial to mesenchymal transition axis for lymph node metastasis in infiltrating ductal carcinoma. Kaohsiung J Med Sci. 2015;31:70‐76. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N‐cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial‐mesenchymal transition. Histol Histopathol. 2011;26:147‐156. [DOI] [PubMed] [Google Scholar]
- 41. Nakashima T, Huang C, Liu D, et al. Neural‐cadherin expression associated with angiogenesis in non‐small‐cell lung cancer patients. Br J Cancer. 2003;88:1727‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gasparotto D, Polesel J, Marzotto A, et al. Overexpression of TWIST2 correlates with poor prognosis in head and neck squamous cell carcinomas. Oncotarget. 2011;2:1165‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hui L, Zhang S, Dong X, Tian D, Cui Z, Qiu X. Prognostic significance of twist and N‐cadherin expression in NSCLC. PLoS ONE 2013;8:e62171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ding L, Zhang Z, Shang D, et al. α‐Smooth muscle actin‐positive myofibroblasts, in association with epithelial‐mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43:335‐343. [DOI] [PubMed] [Google Scholar]
- 45. Kamikihara T, Ishigami S, Arigami T, et al. Clinical implications of N‐cadherin expression in gastric cancer. Pathol Int. 2012;62:161‐166. [DOI] [PubMed] [Google Scholar]
- 46. Araki K, Shimura T, Suzuki H, et al. E/N‐cadherin switch mediates cancer progression via TGF‐β‐induced epithelial‐to‐mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105:1885‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu GL, Yang HJ, Liu T, Lin YZ. Expression and significance of E‐cadherin, N‐cadherin, transforming growth factor‐β1 and Twist in prostate cancer. Asian Pac J Trop Med. 2014;7:76‐82. [DOI] [PubMed] [Google Scholar]
- 48. Drivalos A, Chrisofos M, Efstathiou E, et al. Expression of alpha5‐integrin, alpha7‐integrin, Epsilon‐cadherin, and N‐cadherin in localized prostate cancer. Urol Oncol 2015;34:165.e11‐165.e18. [DOI] [PubMed] [Google Scholar]
- 49. Rastogi I, Rajanna S, Webb A, et al. Mechanism of c‐Met and EGFR tyrosine kinase inhibitor resistance through epithelial mesenchymal transition in non‐small cell lung cancer. Biochem Biophys Res Comm. 2016;477:937‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakajima S, Doi R, Toyoda E, et al. N‐cadherin expression and epithelial‐mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 2004;10:4125‐4133. [DOI] [PubMed] [Google Scholar]
- 51. Guo S, Xu J, Xue R, Liu Y, Yu H. Overexpression of AIB1 correlates inversely with E‐cadherin expression in pancreatic adenocarcinoma and may promote lymph node metastasis. Int J Clin Oncol. 2014;19:319‐324. [DOI] [PubMed] [Google Scholar]
- 52. Abufaraj M, Moschini M, Soria F, et al. Prognostic role of expression of N‐cadherin in patients with upper tract urothelial carcinoma: A multi‐institutional study. World J Urol 2016;35:1073‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma Y, Zheng X, Zhou J, Zhang Y, Chen K. ZEB1 promotes the progression and metastasis of cervical squamous cell carcinoma via the promotion of epithelial‐mesenchymal transition. Int J Clin Exp Pathol. 2015;8:11258‐11267. [PMC free article] [PubMed] [Google Scholar]
- 54. Cho SB, Lee KH, Lee JH, et al. Expression of E‐ and N‐cadherin and clinicopathology in hepatocellular carcinoma. Pathol Int. 2008;58:635‐642. [DOI] [PubMed] [Google Scholar]
- 55. Jie D, Zhongmin Z, Guoqing L, et al. Positive expression of LSD1 and negative expression of E‐cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58:1581‐1589. [DOI] [PubMed] [Google Scholar]
- 56. Marques FR, Fonsechi‐Carvasan GA, De Angelo Andrade LA, Bottcher‐Luiz F. Immunohistochemical patterns for alpha‐ and beta‐catenin, E‐ and N‐cadherin expression in ovarian epithelial tumors. Gynecol Oncol. 2004;94:16‐24. [DOI] [PubMed] [Google Scholar]
- 57. Mohan A, Nalini V, Mallikarjuna K, Jyotirmay B, Krishnakumar S. Expression of motility‐related protein MRP1/CD9, N‐cadherin, E‐cadherin, α‐catenin and β‐catenin in retinoblastoma. Exp Eye Res. 2007;84:781‐789. [DOI] [PubMed] [Google Scholar]
- 58. Zhou Y, Liao Q, Han Y, et al. Rac1 overexpression is correlated with epithelial mesenchymal transition and predicts poor prognosis in non‐small cell lung cancer. J Cancer. 2016;7:2100‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luo WR, Wu AB, Fang WY, Li SY, Yao KT. Nuclear expression of N‐cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology. 2012;61:237‐246. [DOI] [PubMed] [Google Scholar]
- 60. Grinberg‐Rashi H, Ofek E, Perelman M, et al. The expression of three genes in primary non‐small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15:1755‐1761. [DOI] [PubMed] [Google Scholar]
- 61. Fondrevelle ME, Kantelip B, Reiter RE, et al. The expression of Twist has an impact on survival in human bladder cancer and is influenced by the smoking status. Urol Oncol. 2009;27:268‐276. [DOI] [PubMed] [Google Scholar]
- 62. Baumgart E, Cohen MS, Neto BS, et al. Identification and prognostic significance of an epithelial‐mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13:1685‐1694. [DOI] [PubMed] [Google Scholar]
- 63. Do TV, Kubba LA, Du H, Sturgis CD, Woodruff TK. Transforming growth factor‐beta1, transforming growth factor‐beta2, and transforming growth factor‐beta3 enhance ovarian cancer metastatic potential by inducing a Smad3‐dependent epithelial‐to‐mesenchymal transition. Mol Cancer Res. 2008;6:695‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu S, Miao Y, Fan C, et al. Clinicopathologic correlations of liver kinase B1, E‐Cadherin, and N‐Cadherin expression in non‐small cell lung cancer. Appl Immunohistochem Mol Morphol. 2013;21:334‐340. [DOI] [PubMed] [Google Scholar]
- 65. Carvalho ST, Stiepcich MM, Fregnani JH, Nonogaki S, Rocha R, Soares FA. Evaluation of prognostic factors in stage IIA breast tumors and their correlation with mortality risk. Clinics. 2011;66:607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tashiro E, Henmi S, Odake H, Ino S, Imoto M. Involvement of the MEK/ERK pathway in EGF‐induced E‐cadherin down‐regulation. Biochem Biophys Res Comm. 2016;477:801‐806. [DOI] [PubMed] [Google Scholar]
- 67. Miao Y, Li AL, Wang L, et al. Overexpression of NEDD9 is associated with altered expression of E‐cadherin, β‐catenin and N‐cadherin and predictive of poor prognosis in non‐small cell lung cancer. Pathol Oncol Res. 2013;19:281‐286. [DOI] [PubMed] [Google Scholar]
- 68. Greco A, De Virgilio A, Rizzo MI, Pandolfi F, Rosati D, De Vincentiis M. The prognostic role of E‐cadherin and β‐catenin overexpression in laryngeal squamous cell carcinoma. Laryngoscope 2015;126:E148‐E155. [DOI] [PubMed] [Google Scholar]
- 69. Han P, Liu JM, Li DX, et al. Netrin‐1 promotes liver cancer cell collective invasion in a 3D cell culture model. J Dig Dis. 2016;17:99. [Google Scholar]
- 70. Jaggi M, Nazemi T, Abrahams NA, et al. N‐cadherin switching occurs in high Gleason grade prostate cancer. Prostate. 2006;66:193‐199. [DOI] [PubMed] [Google Scholar]
- 71. Zou H, Cao X, Xiao Q, et al. Synergistic inhibition of characteristics of liver cancer stem‐like cells with a combination of sorafenib and 8‐bromo‐7‐methoxychrysin in SMMC‐7721 cell line. Oncol Rep. 2016;36:1731‐1738. [DOI] [PubMed] [Google Scholar]
- 72. Blaschuk OW, Devemy E. Cadherins as novel targets for anti‐cancer therapy. Eur J Pharmacol. 2009;625:195‐198. [DOI] [PubMed] [Google Scholar]
- 73. Shintani Y, Fukumoto Y, Chaika N, et al. ADH‐1 suppresses N‐cadherin‐dependent pancreatic cancer progression. Int J Cancer 2008;122:71‐77. [DOI] [PubMed] [Google Scholar]
- 74. Könnecke M, Burmeister M, Pries R, et al. Epithelial–Mesenchymal transition in chronic rhinosinusitis: differences revealed between epithelial cells from nasal polyps and inferior turbinates. Arch Immunol Ther Exp 2016;65:1‐17. [DOI] [PubMed] [Google Scholar]
- 75. Zhi L, Gao Y, Yu C, et al. N‐Cadherin aided in maintaining the characteristics of leukemic stem cells. Anat Rec. 2016;299:990‐998. [DOI] [PubMed] [Google Scholar]
- 76. Augustine CK, Yoshimoto Y, Gupta M, et al. Targeting N‐cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Can Res. 2008;68:3777‐3784. [DOI] [PubMed] [Google Scholar]
- 77. Yan S, Holderness BM, Li Z, et al. Epithelial‐Mesenchymal expression phenotype of primary melanoma and matched metastases and relationship with overall survival. Anticancer Res. 2016;36:6449‐6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han K, Zhao T, Chen X, et al. MicroRNA‐194 suppresses osteosarcoma cell proliferation and metastasis in vitro and in vivo by targeting CDH2 and IGF1R. Int J Oncol. 2014;45:1437‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang N, He YL, Pang LJ, et al. Down‐regulated E‐cadherin expression is associated with poor five‐year overall survival in bone and soft tissue sarcoma: results of a meta‐analysis. PLoS ONE. 2015;10:e0121448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng J, Qi S, Wang P, et al. Meta‐analysis of downregulated E‐cadherin as a poor prognostic biomarker for cervical cancer. Future Oncol 2016;12:715‐726. [DOI] [PubMed] [Google Scholar]
- 81. Hou H, Chen L, Zha Z, et al. Long form collapsin response mediator protein‐1 promotes the migration and invasion of osteosarcoma cells. Oncol Lett. 2016;12:23‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yan B, Zhang W, Jiang LY, Qin WX, Wang X. Reduced E‐Cadherin expression is a prognostic biomarker of non‐small cell lung cancer: a meta‐analysis based on 2395 subjects. Int J Clin Exp Med. 2014;7:4352‐4356. [PMC free article] [PubMed] [Google Scholar]
- 83. Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E‐cadherin to N‐cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003‐7011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


