Summary
This study aimed to estimate the diagnostic utility of biomarkers for suspected venous thromboembolism (VTE) in pregnancy and the puerperium. Research nurses/midwives collected blood samples from 310 pregnant/postpartum women with suspected pulmonary emboli (PE) and 18 with diagnosed deep vein thrombosis (DVT). VTE was diagnosed using imaging, treatment and adverse outcome data. Primary analysis was limited to women with conclusive imaging (36 with VTE, 247 without). The area under the curve (AUC) for each biomarker was: activated partial thromboplastin time 0·669 (95% confidence interval 0·570–0·768), B‐type natriuretic peptide 0·549 (0·453–0·645), C‐reactive protein 0·542 (0·445–0·639), Clauss fibrinogen 0·589 (0·476–0·701), D‐Dimer (by enzyme‐linked immunosorbent assay) 0·668 (0·561–0·776), near‐patient D‐Dimer 0·651 (0·545–0·758), mid‐regional pro‐atrial natriuretic peptide 0·524 (0·418–0·630), prothrombin fragment 1 + 2 0·562 (0·462–0·661), plasmin‐antiplasmin complexes 0·639 (0·536–0·742), prothombin time 0·613 (0·508–0·718), thrombin generation lag time 0·702 (0·598–0·806), thrombin generation endogenous potential 0·559 (0·437–0·681), thrombin generation peak 0·596 (0·478–0·715), thrombin generation time to peak 0·655 (0·541–0·769), soluble tissue factor 0·531 (0·424–0·638) and serum troponin 0·597 (0·499–0·695). No diagnostically useful threshold for diagnosing or ruling out VTE was identified. In pregnancy and the puerperium, conventional and candidate biomarkers have no utility either for their negative or positive predictive value in the diagnosis of VTE.
Keywords: pulmonary embolism, pregnancy, postpartum, biomarkers, D‐dimer, diagnosis
Pulmonary embolism (PE) is the leading direct cause of death in pregnancy and postpartum (Knight et al, 2016) but symptoms suggesting PE are common in pregnancy and puerperium, and recent studies (Goodacre et al, 2015) have reported a low positive yield from imaging, with only 5% of scans confirming a PE. This suggests that many women are undergoing imaging involving potentially harmful radiation to exclude PE.
A number of conventional and candidate biomarkers could be used in women during pregnancy and the puerperium with suspected venous thromboembolism (VTE) to either exclude VTE or to positively identify it. For example, D‐dimer is currently used in the non‐pregnant population with suspected PE for its negative predictive value to safely withhold imaging from those with a low clinical risk and negative D‐dimer (Wells et al, 2001; Stein et al, 2003; Crawford et al, 2016; van der Hulle et al, 2017), while troponin and brain natriuretic peptide (BNP) are used for their positive predictive value to grade extent of PE. Guidelines from the Royal College of Obstetricians and Gynaecologists (2015) and American Thoracic Society (Leung et al, 2011) currently recommend that all pregnant and postpartum women with suspected PE should receive diagnostic imaging, whereas guidelines from the European Society of Cardiology (Konstantinides et al, 2014) suggest a possible role for D‐dimer in selecting patients.
A recent review (Goodacre et al, 2015) found insufficient data to support using normal values of D‐dimer in pregnant and postpartum women to exclude VTE. The main limitation in previous studies was methodological; the low prevalence of PE in cohorts with suspected PE and consequent lack of precision in estimates of diagnostic sensitivity. Low prevalence means that the ideal study design to estimate diagnostic accuracy, a cohort study, provides an imprecise estimate of sensitivity unless it is extremely large. A case‐control study could provide a more precise estimate, albeit with a higher risk of bias (Lijmer et al, 1999).
A compromise between these designs is a cohort study augmented with additional cases of confirmed disease to increase the precision of estimates of sensitivity. We used this design in the Diagnosis of PE in Pregnancy (DiPEP) study to evaluate clinical features, decision rules, D‐dimer and chest x‐ray (http://www.isrctn.com/ISRCTN21245595). A cohort of pregnant or postpartum women presenting with suspected PE to eleven prospectively recruiting hospitals was augmented with cases of diagnosed PE, identified across all hospitals participating in the United Kingdom Obstetric Surveillance System (UKOSS) research platform. This design was unable to increase the number of cases for biomarker analysis, however, because it was impractical to seek consent to additional blood sampling from the cases identified through UKOSS. We therefore used an alternative strategy to increase the number of cases and identified women diagnosed with deep vein thrombosis (DVT) during the study at any of the prospectively recruiting sites. Because PE are a consequence of a DVT, there are good pathophysiological reasons for expecting that biomarkers will have the same sensitivity in PE and DVT and empirical studies of D‐dimer have shown similar sensitivity in DVT and PE (Stein et al, 2003).
We therefore undertook a cohort study augmented with additional cases to estimate the diagnostic utility of classical and alternative biomarkers in pregnant and puerperium women with suspected VTE.
Methods
Study population
We prospectively recruited pregnant and postpartum women with suspected PE or diagnosed DVT through emergency departments and maternity units at eleven hospitals. Suspected PE was defined on the basis of a clinician deciding that imaging for PE would be required, although not all women ultimately received imaging for PE. In a proportion of cases the woman declined imaging or the decision that imaging was required was reversed by a more senior clinician. We excluded women who required life support on arrival at hospital, who had been diagnosed with PE in the current pregnancy before the start of the study, were unable or unwilling to provide informed consent, aged less than 16 years or previously recruited to the study. Clinicians in the participating hospitals prospectively identified pregnant or postpartum woman with suspected PE considered to require diagnostic imaging or with diagnosed DVT. They contacted the research nurse or recruiting clinician, who provided women with study information and checked eligibility criteria. Informed consent to participate was then sought.
Data collection
Data relating to demographics, presenting features, physiology, previous medical and obstetric problems, any problems in the current pregnancy, results of investigations, treatments given and adverse events were collected on a Case Report Form. Women with suspected PE were followed up at 30 days after recruitment by hospital record review and questionnaire survey to record any additional adverse events or health care. Where insufficient information was obtained to verify status at 30 days the woman's primary care physician was contacted and asked to provide details of additional investigations or events using primary care records.
Classification of the reference standard
Women with suspected PE were classified as having VTE by two independent assessors, blind to biomarker measurements, who used a structured process to classify diagnostic imaging results, adverse events and treatments, and thus classify all women as having VTE or not. This process was also used to determine whether they were included in the primary analysis or secondary analysis. Primary analysis was limited to women who had VTE diagnosed or ruled out through imaging, surgery or post mortem. Secondary analyses were planned involving: (i) Inclusion of women with clinically diagnosed PE; (ii) Inclusion of women with clinically excluded PE; (iii) Exclusion of women with sub‐segmental PE.
Sample size calculation
We aimed to recruit 250 women with suspected PE, resulting in about five women with PE and 245 without, assuming a prevalence of 2%. The incidence of DVT in pregnancy and postpartum is around four times that of PE (Kane et al, 2013) so we anticipated recruiting around 20 women with DVT during the study. Thus, the sample for the biomarker study was expected to include 245 women with suspected PE but negative diagnostic imaging, five women with diagnosed PE and 20 women with diagnosed DVT (i.e. 25 with confirmed VTE). This would allow estimation of sensitivity or specificity of 90% with a standard error of about 8% and 2% respectively.
Blood sample collection, handling and storage and analysis
Serum and citrate blood samples were collected by a member of the clinical team or research nurse/midwife using venepuncture, ideally whilst obtaining routine blood samples for standard clinical assessment in diagnostic workup. Samples were collected as soon as possible but this was often after anticoagulation had been given, as a consequence of guidelines recommending immediate interim parenteral anticoagulant therapy if a diagnostic imaging cannot be carried out immediately (National Institute for Health and Care Excellence, 2012). Sample preparation was conducted by the research nurse/midwife or a member of the hospital laboratory staff. The samples were centrifuged at 2000 g for 15 mins at room temperature within four hours of being obtained. Citrate samples were further processed to obtain platelet‐free plasma.
Plasma and serum samples were stored in aliquots in −70°C freezers at each participating hospital (with the exception of one location where a −40°C freezer was used) for the duration on the study until all samples were transported for analysis to Guy's St Thomas Trust (GSTT), London, UK.
Biomarker analysis
The biomarkers selected for analysis are outlined in Table 1. GSTT established normal ranges for the assays using 20 normal plasma/serum samples (depending on the assay), with the 99th percentile used as the top of the normal range. The resulting normal ranges are also shown in Table 1.
Table 1.
Biomarkers selected for analysis
| Biomarker | Description | Reference range |
|---|---|---|
| D‐Dimers (ELISA) | A fibrin degradation product ‐ a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. Measured ELISA and a highly sensitive assay. | 0–400 ng/ml |
| D‐dimers (Innovance) | As above, but near‐patient testing and fast turn‐around time allows for day to‐day use. This point of care test was used by many routine laboratories in the UK in 2016. | 0–1.13 mg/l |
| Plasmin‐antiplasmin complexes | An ELISA assay that measures the level of plasmin‐antiplasmin complexes and thus is a very sensitive assay of plasmin activation. | 150–800 μg/l |
| Prothrombin fragment 1 + 2 | A small molecule cleaved from prothrombin when thrombin is generated. It is thus a sensitive marker of thrombin generation i.e. coagulation turnover. It is an ELISA assay | 200–1200 pmol/l |
| Thrombin Generation | Thrombin generation can be measured dynamically using the ETP, a term introduced by Hemker in 1986 that refers to the total amount of thrombin generated during the test. Commonly measured variables when analysing thrombin generation include the Lag Time, the Time to Peak Thrombin Generation, the ETP ‐ the area under the curve. |
Lag Time: 0.9–3.4 min ETP: 696–1533 nmol/l*min Peak: 103–475 nmol/l Time to Peak: 1.4–7.7 min |
| Prothrombin time | A routine measure of the extrinsic pathway of coagulation, used to determine the clotting tendency of blood. | 11.7–15.9 s |
| Activated partial thromboplastin time | A routine measure of the intrinsic and common coagulation pathways, used to detect abnormalities in blood clotting. | 27–52 s |
| Clauss fibrinogen | A functional measure of fibrinogen | 2.03–4.11 g/l |
| Soluble Tissue Factor | A marker of tissue factor activation ‐ when tissue factor is upregulated part of the molecule may be cleaved and enters the systemic circulation. | 40–300 pg/ml |
| Troponin I | Part of the troponin complex in cardiac muscle tissue, used to detect myocardial damage resulting from myocardial ischaemia or non‐cardiac causes, such as PE. | 0.91–2.63 ng/ml |
| B‐type natriuretic peptide | A polypeptide secreted by the ventricles of the heart in response to excessive stretching of heart muscle cells, used to measure heart strain resulting from primary heart disease or noncardiac causes such as PE. | 107–523 pg/ml |
| C‐ reactive protein | CRP is an acute‐phase protein, the levels of which rise in response to inflammation. Elevation of CRP has been shown to be associated with a diagnosis of PE. | 0–3104 ng/ml |
| MRproAMP | MRproANP is an emerging measure of right ventricular strain which occurs as a consequence of pulmonary embolism. | 0–954 pmol/l |
CRP, C reactive protein; ELISA, enzyme‐linked immunosorbent assay; ETP, endogenous thrombin potential; MRproANP, mid‐regional pro‐atrial natriuretic peptide; PE, pulmonary embolism.
Analytic techniques
Citrated plasma was utilised for all the haemostatic assays. Serum was utilised for Troponin‐1, natriuretic peptide B (NPPB), mid‐regional pro‐atrial natriuretic peptide (MRproANP) and C‐reactive protein (CRP) assays.
Thrombin generation (TG) was measured by the Thrombinoscope (ThermoElectron Corporation, Cambridge, UK). The samples were tested in batches to minimize variability. The frozen plasma aliquots were placed in a water bath at 37°C to thaw for 5 min. PPP‐reagent low, which consists of 1 pmol/l tissue factor with 4 μmol/l phospholipids, was used because of expected hypercoagulability. Aliquots (20 μl) of PPP‐reagent was added to each thrombin generation well together with 80 μl of platelet‐free plasma and 20 μl of fluorogenic substrate and calcium (FluCa). The fluorogenic substrate consisted of amino‐methyl‐coumarin (AMC). The calibrator wells consisted of 80 μl platelet‐free plasma, 20 μl calibrator and 20 μl FluCa. All the reagents were from Diagnostica Stago, Theale, UK. The analysis was conducted in an enzyme‐linked immunosorbent assay (ELISA) plate (Diagnostica Stago Theale, UK) that enables the thrombin formation to be followed in a Fluoroscan. The coefficient of variation was 2·2% to 3·2% intra‐assay and 5·1% to 16·7% interassay for lag time, endogenous thrombin potential (ETP), Peak and time to Peak.
The prothrombin time (PT), activated partial thromboplastin time (APTT), and Clauss fibrinogen were measured on the ACL300R (Werfen Ltd., Warrington, UK) using PT High Sensitivity (HS) Plus reagent for the PT, HemosIL APTT SP liquid for the APTT, and Fibrinogen C for the Clauss fibrinogen. All reagents were purchased from Werfen Ltd. The tests were measured according to the manufacturer's instructions for the ACL300R analyser. The coefficient of variation was 3·2–3·5% intra‐assay and 3·6–4·2% interassay for PT, APTT and Clauss fibrinogen.
The latex‐based D‐Dimer was measured on the CA660 analyser from Sysmex UK (Milton Keynes, UK) and Innovance D‐Dimer reagent (Sysmex UK, Milton Keynes, UK) according to the manufacturer's instructions. The coefficient of variation was 6·0% intra‐assay and 12% interassay for the Innovance D‐Dimer.
The Zymutest D‐Dimer ELISA assay (Quadratech Diagnostics Ltd., Epsom, UK) was used to measure the D‐Dimer levels according to the manufacturer's instructions. The coefficient of variation was 4·6% intra‐assay and 10·8% interassay for the Zymutest D‐Dimer.
The PAP ELISA (Immunodiagnostics Systems Ltd., Tyne & Wear, UK), was used to measure the PAP according to the manufacturer's instructions. The coefficient of variation was 4·2% intra‐assay and 7·3% interassay for the PAP.
The PF 1 + 2 Micro (Sysmex, Milton Keynes, UK) was used to measure prothrombin fragment (PF) 1 + 2 according to the manufacturer's instructions. The coefficient of variation was 6·0% intra‐assay and 9·0% interassay.
The Immubind soluble TF (Invitech Ltd, Cambridgeshire, UK), was used according to the manufacturer's instructions. The coefficient of variation was 6·0% intra‐assay and 5·0% interassay.
The Troponin 1 Type 3 ELISA (Bio Techne, Abingdon, UK) was used to measure the Troponin 1 levels, according to the manufacturer's instructions. The coefficient of variation was 4·0% intra‐assay and 4·6% interassay for Troponin 1.
The BNP ELISA (Bio Techne, Abingdon, UK) was used according to manufacturer's instructions. The coefficient of variation was 10% intra‐assay and 15% interassay for the NPPB assay.
The Human MRproANP ELISA (2B Scientific, Oxfordshire, UK), was used to measure the MRproANP levels, according to manufacturer's instructions. The coefficient of variation was 8% intra‐assay and 10% interassay for the MRproANP.
The human CRP Quantikine assay (Bio Techne, Abingdon, UK) was used to measure the CRP levels, according to manufacturer's instructions. The coefficient of variation was 5·5% intra‐assay and 6·5% inteassay for the CRP assay.
Statistical analysis
We included all women with suspected PE or diagnosed DVT who provided consent and an analysable blood sample. Blood samples were analysed at GSTT, where the staff were blinded to the clinical outcome of the patient, and the results sent to the Sheffield Clinical Trials Research Unit (CTRU). The area under the curve (AUC) was calculated for each biomarker, together with the sensitivity and specificity at the upper limit of the normal range. We then examined the receiver operator characteristic (ROC) curve to determine whether there was an optimal threshold for clinical practice, where sensitivity exceeds 95% but specificity still allows a meaningful proportion of women without PE to have the diagnosis excluded. Primary and secondary analyses were undertaken on the basis of the classification of the reference standard, as outlined above.
Results
We recruited 324 women with suspected PE between 15 February 2015 and 31 August 2016. Screening identified 35 women who were unable or unwilling to give consent and 95 who were eligible but not approached to participate. The recruited women had a mean age of 29·3 years, mean body mass index (BMI) 28·0 kg/m2, 204 (63%) were white British, 21 (6·5%) were in the first trimester, 110 (34·0%) in the second, 138 (42·6%) in the third and 55 (17·0%) were postpartum.
Blood samples were taken from 312/324. The reasons for failure to take a blood sample were: inability to draw blood (7), patient refused (2), unavailability of blood handling services (1), patient discharged before venepuncture (1) and unknown (1). Two samples were not labelled correctly (one from a woman in whom PE was clinically ruled out without imaging and one with PE ruled out by negative imaging) and were therefore not analysed, leaving 310 samples for analysis.
We recruited 18 women with diagnosed DVT, nine of whom were recruited at Guy's and St Thomas's Hospital Obstetric Unit (a specialist centre). A further six were eligible for recruitment but declined to participate. The women with diagnosed DVT had a mean age of 28·3 years, mean BMI 26·3 kg/m2, nine (50%) were white, one was in the first trimester, one in the second trimester, nine in the third trimester and seven were postpartum.
Adding the 18 samples from women with DVT to the 310 from women with suspected PE totalled 328 samples for analysis. The 310 women recruited with suspected PE consisted of 18 with PE confirmed by imaging (including 1 sub‐segmental), 5 with clinically diagnosed PE (3 with equivocal imaging and 2 with no imaging; all treated), 247 with PE ruled out after imaging (242 with negative imaging and 5 untreated after equivocal imaging) and 40 with PE clinically ruled out without imaging (none treated). Thus 36 with VTE and 247 without were included in the primary analysis; 41 with VTE and 247 without in the secondary analysis including clinically diagnosed PE; 36 with VTE and 287 without in the secondary analysis including clinically ruled out PE; and 35 with PE and 247 without in the secondary analysis excluding sub‐segmental PE.
Table 2 compares the mean biomarker levels between women with and without VTE in the primary analysis. The mean levels of D‐dimer (both assays), thrombin generation (lag time and time to peak), Clauss fibrinogen and plasmin‐antiplasmin were significantly higher in the women with VTE than those without. Mean levels of the other biomarkers did not significantly differ between the groups.
Table 2.
Mean (SD) biomarker levels for the patient groups in the primary analysis
| Biomarker | Mean (SD) in women with no VTE N = 247 | Mean (SD) in women with VTE N = 36 | P‐value |
|---|---|---|---|
| APTT | 39.7 (22.07) | 41.4 (13.24) | 0.660 |
| Clauss fibrinogen | 5.37 (1.69) | 6.30 (2.73) | 0.007 |
| C‐reactive protein | 5348 (1705) | 5603 (1646) | 0.401 |
| Prothombin time | 16.2 (5.39) | 18.7 (13.16) | 0.089 |
| D‐Dimer (ELISA) | 1247 (1474) | 2401 (2642) | 0.001 |
| D‐Dimer (Innovance) | 1.147 (1.269) | 2.282 (3.388) | 0.004 |
| Thrombin Generation (Lag Time) | 8.70 (4.84) | 13.85 (8.30) | <0.001 |
| Thrombin Generation (Endogenous Potential) | 1217 (558) | 1081 (561) | 0.241 |
| Thrombin Generation (Time to Peak) | 14.8 (9.06) | 21.5 (13.61) | 0.001 |
| Thrombin Generation (Peak) | 162 (116) | 130 (124) | 0.160 |
| Plasmin –antiplasmin complexes | 688 (251) | 915 (647) | 0.004 |
| BNP | 372 (900) | 385 (731) | 0.932 |
| MRproANP | 603 (1016) | 753 (1159) | 0.415 |
| Soluble Tissue Factor | 291 (319.6) | 488 (1067.3) | 0.065 |
| Prothrombin fragment 1 + 2 | 623 (408) | 550 (333) | 0.298 |
| Troponin | 1.328 (2.458) | 0.762 (0.968) | 0.105 |
APTT, activated partial thromboplastin time; BNP, brain natriuretic peptide; ELISA, enzyme‐linked immunosorbent assay; MRproANP; mid‐regional pro‐atrial natriuretic peptide; SD, standard deviation; VTE, venous thromboembolism.
Appendix S1 provide further details for each biomarker, with a box‐whisker plot showing the distribution for those with DVT, PE, no PE and excluded from the analysis. The distributions of all biomarkers overlapped substantially between those with and without VTE.
The ROC curves for D‐dimer biomarkers are shown in Figure 1; the ROC curves for APTT, PF1 and 2, prothrombin and thrombin generation biomarkers are shown in Figure 2; and those for the other biomarkers are shown in Figure 3. It was not possible to identify a threshold for any biomarker that would optimise sensitivity (>98%) while maintaining meaningful specificity.
Figure 1.
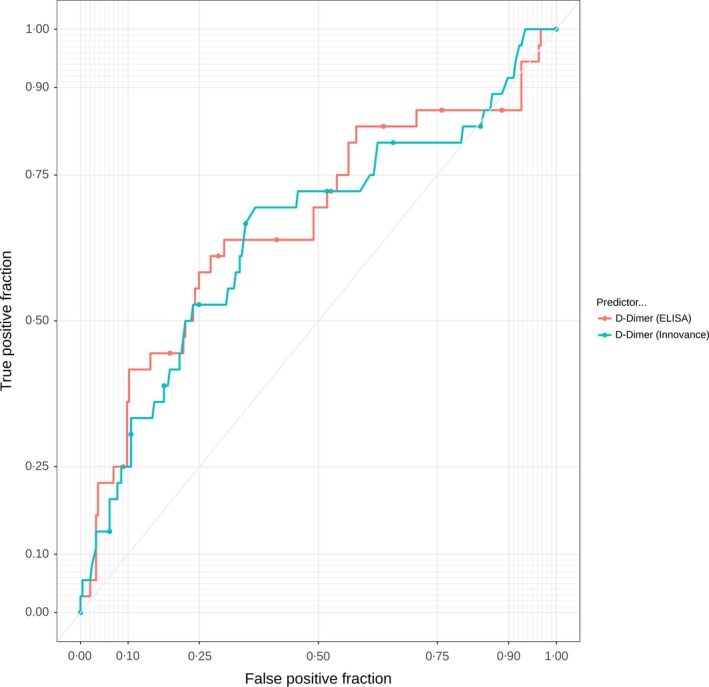
Receiver operator characteristic curves for D‐dimer biomarkers. ELISA, enzyme‐linked immunosorbent assay.
Figure 2.
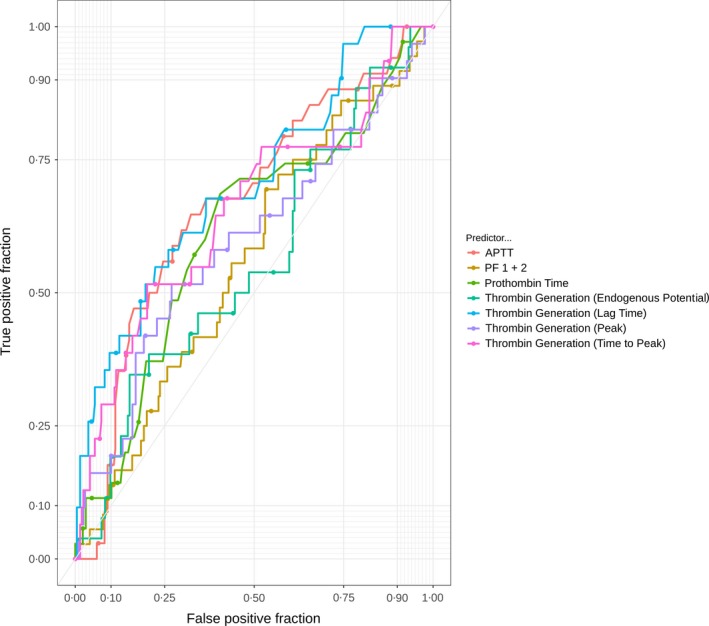
Receiver operator characteristic curves for thrombin‐related biomarkers. APTT, activated partial thromboplastin time; PF 1 + 2, Prothrombin fragment 1 + 2.
Figure 3.
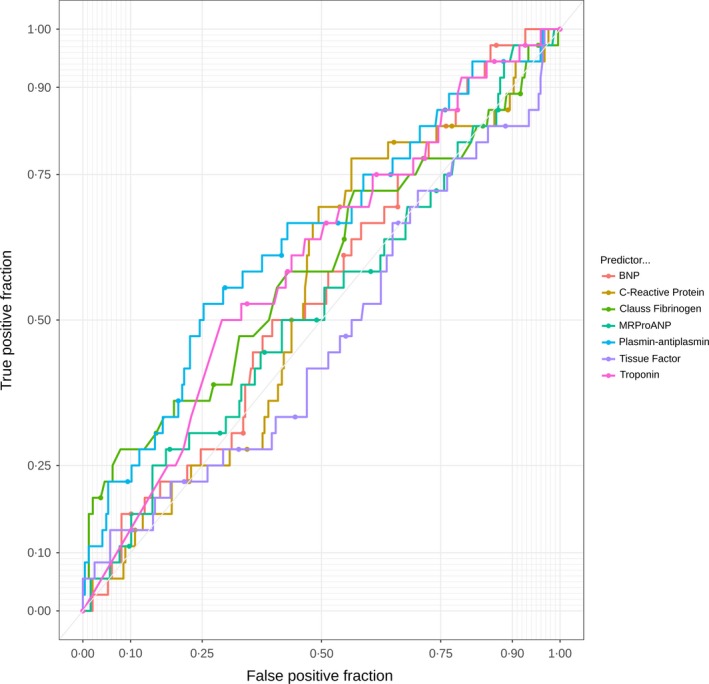
Receiver operator characteristic curves for other biomarkers. BNP, brain natriuretic peptide; MRproANP, mid‐regional pro‐atrial natriuretic peptide.
Table 3 reports the area under the ROC curve (AUROC) for the continuous biomarker and diagnostic parameters for the biomarkers at the pre‐defined threshold for positivity and the threshold that optimised sensitivity (>95%) at the expense of specificity. No biomarker had sufficient sensitivity to rule out VTE while achieving meaningful specificity, with the possible exception of thrombin generation (lag time) with an AUC of 0·702 and sensitivity of 97% and specificity of 25% at the threshold that optimised sensitivity at the expense of specificity.
Table 3.
AUROC, sensitivity and specificity for each biomarker
| Biomarker | AUC 95% CI | Sensitivity at predefined threshold 95% CI | Specificity at predefined threshold 95% CI | Sensitivity at threshold with optimal sensitivity 95% CI | Specificity at threshold with optimal sensitivity 95% CI |
|---|---|---|---|---|---|
| APTT |
0·669 0·570 to 0·768 |
0·088 0·019 to 0·237 |
0·914 0·870 to 0·947 |
0·971 0·847 to 0·999 |
0·086 0·053 to 0·130 |
| BNP |
0·549 0·453 to 0·645 |
0·167 0·064 to 0·328 |
0·879 0·831 to 0·917 |
0·972 0·855 to 0·999 |
0·146 0·104 to 0·196 |
| C‐Reactive protein |
0·542 0·445 to 0·639 |
0·861 0·705 to 0·953 |
0·121 0·083 to 0·169 |
0·972 0·855 to 0·999 |
0·032 0·014 to 0·063 |
| Clauss fibrinogen |
0·589 0·476 to 0·701 |
0·778 0·608 to 0·899 |
0·228 0·177 to 0·286 |
0·972 0·855 to 0·999 |
0·066 0·038 to 0·106 |
| D‐Dimer (ELISA) |
0·668 0·561 to 0·776 |
0·861 0·705 to 0·953 |
0·196 0·148 to 0·251 |
0·972 0·855 to 0·999 |
0·037 0·017 to 0·069 |
| D‐Dimer (Innovance) |
0·651 0·545 to 0·758 |
0·528 0·355 to 0·696 |
0·727 0·666 to 0·781 |
0·972 0·855 to 0·999 |
0·078 0·047 to 0·118 |
| MRproANP |
0·524 0·418 to 0·630 |
0·278 0·142 to 0·452 |
0·785 0·729 to 0·835 |
0·972 0·855 to 0·999 |
0·097 0·063 to 0·141 |
| Prothrombin fragment 1 + 2 |
0·562 0·462 to 0·661 |
0·056 0·007 to 0·187 |
0·935 0·896 to 0·962 |
0·972 0·855 to 0·999 |
0·045 0·023 to 0·079 |
| Plasmin‐antiplasmin complexes |
0·639 0·536 to 0·742 |
0·472 0·304 to 0·645 |
0·763 0·705 to 0·815 |
0·972 0·855 to 0·999 |
0·041 0·020 to 0·074 |
| Prothombin Time |
0·613 0·508 to 0·718 |
0·486 0·314 to 0·660 |
0·730 0·669 to 0·785 |
0·971 0·851 to 0·999 |
0·084 0·052 to 0·127 |
| Thrombin Generation (Lag Time) |
0·702 0·598 to 0·806 |
1·000 0·888 to 1·000 |
0·000 0·000 to 0·017 |
0·968 0·833 to 0·999 |
0·251 0·195 to 0·314 |
| Thrombin Generation (Endogenous Potential) |
0·559 0·437 to 0·681 |
0·167 0·064 to 0·328 |
0·755 0·696 to 0·808 |
0·962 0·804 to 0·999 |
0·069 0·038 to 0·112 |
| Thrombin Generation (Peak) |
0·596 0·478 to 0·715 |
0·000 0·000 to 0·097 |
0·996 0·977 to 1·000 |
0·968 0·833 to 0·999 |
0·059 0·032 to 0·099 |
| Thrombin Generation (Time to Peak) |
0·655 0·541 to 0·769 |
0·861 0·705 to 0·953 |
0·204 0·155 to 0·260 |
1·000 0·888 to 1·000 |
0·114 0·075 to 0·164 |
| Soluble Tissue Factor |
0·531 0·424 to 0·638 |
0·222 0·101 to 0·392 |
0·771 0·714 to 0·822 |
0·972 0·855 to 0·999 |
0·037 0·017 to 0·069 |
| Troponin |
0·597 0·499 to 0·695 |
0·056 0·007 to 0·187 |
0·887 0·840 to 0·923 |
0·972 0·855 to 0·999 |
0·085 0·053 to 0·127 |
95% CI, 95% confidence interval; APTT, activated partial thromboplastin time; AUC, area under the curve; AUROC, area under the receiver operator characteristic curve; BNP, brain natriuretic peptide; ELISA, enzyme‐linked immunosorbent assay; MRproANP, mid‐regional pro‐atrial natriuretic peptide.
Secondary analysis
Table S1 shows the results of secondary analysis. The AUC (with 95% confidence interval [CI]) is compared between the primary and secondary analyses. There were no meaningful differences.
Analysis excluding women who had received anticoagulant
Anticoagulation with heparin is known to interfere with many of the biomarker assays studied here. Unfractionated heparin will prolong the APTT and thrombin time and low molecular weight heparin may cause a slight prolongation of the APTT. But both, by suppressing activation of activated Factor X (FXa) and thrombin, will affect all parameters of the thrombin generation assay and PF1 + 2 will be decreased. Furthermore, by decreasing the generation of thrombin, which is a major stimulator of fibrinolysis, the D‐dimer and plasmin‐antiplasmin values maybe reduced.
We found that 240/328 women had received anticoagulation prior to blood sampling. We therefore undertook an unplanned analysis in which these samples were excluded. The primary analysis involved only 66 women, of whom only 4 had VTE, so the findings are limited by small numbers. Table 4 compares the mean biomarker values between women with and without PE. The differences observed in the main analysis between women with and without PE disappeared or even reversed when those receiving anticoagulation were removed, but this probably reflects the small numbers. Table 5 shows the AUROC for each biomarker and the sensitivity and specificity at pre‐defined and optimal thresholds. BNP (AUC 0·774; 95% CI 0·670–0·878), PF1 + 2 (0·795; 0·644–0·947), thrombin generation lag time (0·735; 0·531–0·940) and troponin (0·742; 0·453–1·000) may have some potential to rule out VTE with acceptable sensitivity but confidence intervals were wide and estimates would need to be validated in a larger cohort with VTE.
Table 4.
Mean (SD) biomarker levels for the patient groups with those having received anticoagulation excluded
| Biomarker | Mean (SD) in women with no VTE N = 62 | Mean (SD) in women with VTE N = 4 | P‐value |
|---|---|---|---|
| APTT | 33·4 (16·67) | 33·4 (6·57) | 0·993 |
| Prothombin time | 14·8 (2·108) | 14·2 (0·772) | 0·610 |
| Clauss fibrinogen | 5·41 (1·81) | 6·61 (2·61) | 0·219 |
| D‐Dimer (ELISA) | 1114 (848) | 832 (667) | 0·517 |
| D‐Dimer (Innovance) | 1·126 (0·826) | 0·797 (0·420) | 0·432 |
| Thrombin Generation (Lag Time) | 6·20 (1·646) | 6·98 (0·919) | 0·354 |
| Thrombin Generation (Endogenous Potential) | 1501 (389) | 1575 (351) | 0·711 |
| Thrombin Generation (Time to Peak) | 10·03 (2·57) | 10·31 (1·40) | 0·823 |
| Thrombin Generation (Peak) | 235 (100·3) | 248 (71·0) | 0·798 |
| Plasmin –antiplasmin complexes | 678 (205) | 821 (276) | 0·204 |
| BNP | 256 (586·31) | 29 (5·47) | 0·205 |
| MRproANP | 478 (904) | 1371 (1358) | 0·095 |
| Soluble Tissue Factor | 222 (157·8) | 164 (37·4) | 0·428 |
| Prothrombin fragment 1 + 2 | 711 (386) | 373 (161) | 0·095 |
| Troponin | 1·03 (1·24) | 2·12 (1·65) | 0·122 |
| C‐reactive protein | 5410 (1596) | 5884 (1734) | 0·564 |
APTT, activated partial thromboplastin time; BNP, brain natriuretic peptide; ELISA, enzyme‐linked immunosorbent assay; MRproANP; mid‐regional pro‐atrial natriuretic peptide; SD, standard deviation; VTE, venous thromboembolism.
Table 5.
AUROC, sensitivity and specificity for each biomarker, excluding those who had received anticoagulation
| Biomarker | AUC 95% CI | Sensitivity at predefined threshold 95% CI | Specificity at predefined threshold 95% CI | Sensitivity at threshold with optimal sensitivity 95% CI | Specificity at threshold with optimal sensitivity 95% CI |
|---|---|---|---|---|---|
| APTT |
0·581 0·244 to 0·919 |
0·00 0·000 to 0·602 |
0·967 0·885 to 0·996 |
1 0·398 to 1 |
0·217 0·121 to 0·342 |
| BNP |
0·774 0·670 to 0·878 |
0·00 0·000 to 0·602 |
0·935 0·843 to 0·982 |
1 0·398 to 1 |
0·742 0·615 to 0·845 |
| C‐Reactive protein |
0·609 0·250 to 0·968 |
1·00 0·398 to 1·000 |
0·097 0·036 to 0·199 |
1 0·398 to 1 |
0·113 0·047 to 0·219 |
| Clauss fibrinogen |
0·648 0·259 to 1·000 |
0·75 0·194 to 0·994 |
0·250 0·147 to 0·379 |
1 0·398 to 1 |
0·117 0·048 to 0·226 |
| D‐Dimer (ELISA) |
0·615 0·210 to 1·000 |
0·50 0·068 to 0·932 |
0·148 0·070 to 0·262 |
1 0·398 to 1 |
0·213 0·119 to 0·337 |
| D‐Dimer (Innovance) |
0·613 0·299 to 0·926 |
0·25 0·006 to 0·806 |
0·672 0·540 to 0·787 |
1 0·398 to 1 |
0·262 0·158 to 0·391 |
| MRproANP |
0·698 0·357 to 1·000 |
0·50 0·068 to 0·932 |
0·823 0·705 to 0·908 |
1 0·398 to 1 |
0·210 0·117 to 0·332 |
| Prothrombin fragment 1 + 2 |
0·795 0·644 to 0·947 |
0·00 0·000 to 0·602 |
0·918 0·819 to 0·973 |
1 0·398 to 1 |
0·639 0·506 to 0·758 |
| Plasmin‐antiplasmin complexes |
0·684 0·335 to 1·000 |
0·50 0·068 to 0·932 |
0·770 0·645 to 0·868 |
1 0·398 to 1 |
0·180 0·094 to 0·300 |
| Prothombin time |
0·572 0·306 to 0·838 |
0·00 0·000 to 0·602 |
0·831 0·710 to 0·916 |
1 0·398 to 1 |
0·220 0·123 to 0·347 |
| Thrombin generation (Lag time) |
0·735 0·531 to 0·940 |
1·00 0·398 to 1·000 |
0·000 0·000 to 0·060 |
1 0·398 to 1 |
0·450 0·321 to 0·584 |
| Thrombin generation (Endogenous potential) |
0·454 0·155 to 0·753 |
0·50 0·068 to 0·932 |
0·525 0·393 to 0·654 |
1 0·398 to 1 |
0·233 0·134 to 0·360 |
| Thrombin generation (Peak) |
0·462 0·229 to 0·696 |
0·00 0·000 to 0·602 |
0·852 0·738 to 0·930 |
1 0·398 to 1 |
0·317 0·203 to 0·450 |
| Thrombin generation (Time to Peak) |
0·577 0·320 to 0·834 |
1·00 0·398 to 1·000 |
0·213 0·119 to 0·337 |
1 0·398 to 1 |
0·350 0·231 to 0·484 |
| Soluble tissue factor |
0·422 0·159 to 0·686 |
0·00 0·000 to 0·602 |
0·885 0·778 to 0·953 |
1 0·398 to 1 |
0 0·286 to 0·543 |
| Troponin |
0·742 0·453 to 1·000 |
0·25 0·006 to 0·806 |
0·903 0·801 to 0·964 |
1 0·398 to 1 |
0·306 0·196 to 0·437 |
95% CI, 95% confidence interval; APTT, activated partial thromboplastin time; AUC, area under the curve; AUROC, area under the receiver operator characteristic curve; BNP, brain natriuretic peptide; ELISA, enzyme‐linked immunosorbent assay; MRproANP, mid‐regional pro‐atrial natriuretic peptide.
Discussion
The present study did not identify any biomarker that would be clinically useful to either exclude or confirm suspected PE in pregnancy and the puerperium. This was despite the fact that women with VTE had significantly higher levels of Clauss fibrinogen, both D‐Dimer assays, plasmin‐antiplasmin complexes and thrombin generation measures (lag time and time to peak) when all samples (including those taken after anticoagulation) were included in analysis. There was considerable overlap in biomarker values between those with VTE and those without, which meant that only thrombin generation (lag time) had an AUC greater than 0·7 in the main analysis. The ROC curves showed that there was no threshold for positivity that would provide acceptable sensitivity while maintaining useful specificity, with the possible exception of thrombin generation (lag time), with sensitivity of 97% and specificity of 25% at a threshold that optimised sensitivity at the expense of specificity.
Over two‐thirds of the women (240/330) had received anticoagulation prior to blood sampling. Most of the haemostatic biomarkers were therefore potentially affected because all forms of heparin used to treat these women (unfractionated and low molecular weight) suppress coagulation activation and as a result the fibrinolytic response. Thus, thrombin generation and PF1 + 2 are suppressed and APTT and TT prolonged. Furthermore, thrombin is a major stimulator of fibrinolysis, so when thrombin is reduced there will be less increment in plasmin‐antiplasmin complexes and D‐dimer levels.
We repeated the analysis excluding those who had received anticoagulation but this reduced the sample size markedly and included only four women with VTE. No biomarker showed any association with VTE, probably reflecting lack of statistical power. BNP, PF1 + 2, thrombin generation (lag time) and troponin may have some potential to rule out VTE with acceptable sensitivity but confidence intervals were wide, being based on only four cases with VTE.
This is the largest study to date of biomarkers for PE in pregnancy and puerperium. Previous studies have shown that the negative predictive value of D‐dimer is useful when used in combination with a low Wells score to exclude PE in the non‐pregnant population (Wells et al, 2001; Stein et al, 2003; Crawford et al, 2016; van der Hulle et al, 2017). There is substantial evidence that D‐dimer levels increase continuously during a normal pregnancy across all gestations and that the “normal range” outside of pregnancy cannot be applied to pregnant women (Browne et al, 2014; Hedengran et al, 2016). Hedengran et al (2016) also showed that the D‐dimer values in individual healthy pregnant women fluctuated by more than 50% and concluded that they may not be of value in the diagnosis of VTE during pregnancy. Thus, although there is some evidence that, as biologically expected, D‐dimer levels are increased in women with PE in pregnancy (Kovac et al, 2010), discrimination between ‘normal’ D‐dimer levels in pregnancy and increased D‐dimers due to VTE is difficult. Furthermore, we found that several women with PE had normal D‐dimer values, so using D‐dimer for its negative predictive value would result in ‘missed’ VTE.
Previous studies of D‐dimer in cohorts of women with suspected PE in pregnancy or postpartum have been limited by small numbers. Damodaram et al (2009) (N = 37, 24 with intermediate or high probability VQ scan) reported sensitivity and specificity of 73% and 15% respectively. O'Connor et al (2011) [N = 125, 5 with PE on computed tomography (CT) pulmonary angiography] reported values of 0% and 74%. Hassanin et al (2011) (N = 60, 4 with PE on CT pulmonary angiography) reported that all women had a positive D‐dimer, and Cutts et al (2014) (N = 183, 4 with high probability VQ scan) reported that 48 out of 51 with D‐dimer measurements were positive.
This analysis has a number of limitations that need to be considered. The DiPEP study included women from all hospitals in the UK who were identified retrospectively with diagnosed PE and women from eleven prospectively recruiting hospitals who presented with suspected PE. We could only collect blood samples from women who were prospectively recruited with suspected PE so we had to supplement the anticipated low prevalence of PE among these women by including women with diagnosed DVT. Women with DVT are likely to have a lower thrombotic load than women with PE and are less likely to have cardiac strain, so biomarkers may be less sensitive in DVT than PE. Furthermore, most of the blood samples were taken after anticoagulation was given, which we considered (especially from the effect on thrombin generation) to have interfered with the biomarker assays and reduced their diagnostic value. This is a potential consequence of current guidelines (National Institute for Health and Care Excellence, 2012), which state that patients with suspected PE should be given anticoagulation before diagnostic testing if any delay is anticipated. We repeated the analysis having excluded women who had received anticoagulation but this resulted in a small sample size with little statistical power to draw reliable conclusions. It is therefore possible that biomarkers could have a potential role in diagnosing PE in pregnancy and postpartum, but the practical difficulties involved in taking samples before anticoagulation (both in routine practice and in any future research studies) is likely to limit their practical potential.
Conclusion
Biomarkers cannot currently be recommended as a way of selecting women with suspected PE in pregnancy or postpartum for imaging. In particular, D‐dimer should not be recommended for use in the diagnostic work‐up of PE in pregnancy. Future research would ideally test biomarkers on a large cohort, including a substantial number with VTE, and involve blood sampling before anticoagulation is given, but current guidance makes this very difficult to achieve. Our study is reflective of this: we were only able to recruit four women with VTE who had not received anticoagulation before blood sampling despite recruiting from eleven sites over 18 months.
Author contribution
SG was Chief Investigator for the DiPEP study, KH the Project Manager and BJH led the biomarker study. BJH and SG designed the biomarker study. KH was responsible for recruitment, data collection and blood sample collection. KP undertook laboratory analysis. NS undertook statistical analysis. All named authors contributed to management of the project and interpretation of the data. All named authors contributed to redrafting and approved the final draft of the paper. SG is guarantor for the paper.
Competing interests
The authors have no competing interests other than the grant support received by their respective institutions to deliver the study, as outlined in sources of funding below.
Supporting information
Table S1. AUROC with 95% CI for each biomarker in primary and secondary analysis.
Appendix S1. Details of biomarker analysis (including women who had received anticoagulation).
Appendix S2. The DiPEP research group.
Acknowledgements
We thank all members of the DiPEP research group for their invaluable assistance in delivering this project and who are detailed in Appendix S2.
Funding: This work was supported by a grant from the United Kingdom National Institute for Health Research Health Technology Assessment programme (project reference 13/21/01). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Browne, A.M. , Cronin, C.G. , NiMhuircheartaigh, J. , Donagh, C. , Morrison, J.J. , Lohan, D.G. & Murphy, J.M. (2014) Evaluation of imaging quality of pulmonary 64‐MDCT angiography in pregnancy and puerperium. American Journal of Roentgenology, 202, 60–64. [DOI] [PubMed] [Google Scholar]
- Crawford, F. , Andras, A. , Welch, K. , Sheares, K. , Keeling, D. & Chappell, F. . D‐dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Systematic Review. 2016;(8). CD010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts, B.A. , Tran, H.A. , Merriman, E. , Nandurkar, D. , Soo, G. , DasGupta, D. , Prassannan, N. & Hunt, B.J. (2014) The utility of the wells clinical prediction model and ventilation‐perfusion scanning for pulmonary embolism in pregnancy. Blood Coagulation and Fibrinolysis, 25, 375–378. [DOI] [PubMed] [Google Scholar]
- Damodaram, M. , Kaladindi, M. , Luckit, J. & Yoong, W. (2009) D‐dimers as a screening test for venous thromboembolism in pregnancy: is it of any use? Journal of Obstetrics and Gynaecology, 29, 101–103. [DOI] [PubMed] [Google Scholar]
- Goodacre, S. , Nelson‐Piercy, C. , Hunt, B. & Chan, W.‐S. (2015) When should we use diagnostic imaging to investigate for pulmonary embolism in pregnant and postpartum women? Emergency Medical Journal, 32, 78–82. [DOI] [PubMed] [Google Scholar]
- Hassanin, I.M.A. , Shahin, A.Y. , Badawy, M.S. & Karam, K. (2011) D‐dimer testing versus multislice computed tomography in the diagnosis of postpartum pulmonary embolism in symptomatic high‐risk women. International Journal of Gynecology and Obstetrics, 115, 200–201. [DOI] [PubMed] [Google Scholar]
- Hedengran, K.K. , Andersen, M.R. , Stender, S. & Szecsi, P.B. Large D‐Dimer fluctuation in normal pregnancy: a longitudinal cohort study of 4,117 Samples from 714 Healthy Danish Women. Obstetrics and Gynecology International 2016; 2016: 3561675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hulle, T. , Cheung, W.Y. , Kooij, S. , Beenen, L.F.M. , van Bemmel, T. , van Es, J. , Faber, L.M. , Hazelaar, G.M. , Heringhaus, C. , Hofstee, H. , Hovens, M.M.C. , Kaasjager, K.A.H. , van Klink, R.C.J. , Kruip, M.J.H.A. , Loeffen, R.F. , Mairuhu, A.T.A. , Middeldorp, S. , Nijkeuter, M. , van der Pol, L.M. , Schol‐Gelok, S. , Ten Wolde, M. , Klok, F.A. & Huisman, M.V. (2017) Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet, 390, 289–297. [DOI] [PubMed] [Google Scholar]
- Kane, E.V. , Calderwood, C. , Dobbie, R. , Morris, C. , Roman, E. & Greer, I.A. (2013) A population‐based study of venous thrombosis in pregnancy in Scotland 1980–2005. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 169, 223–229. [DOI] [PubMed] [Google Scholar]
- Knight, M. , Nair, M. , Tuffnell, D. , Kenyon, S. , Shakespeare, J. , Brocklehurst, P. & Kurinczuk, J.J. on behalf of MBRRACE‐UK . (2016)Saving Lives, Improving Mothers’ Care ‐ Surveillance of Maternal Deaths in the UK 2012‐14 and Lessons Learned to Inform Maternity Care From the UK and Ireland Confidential Enquiries Into Maternal Deaths and Morbidity 2009‐14. National Perinatal Epidemiology Unit, University of Oxford, Oxford. [Google Scholar]
- Konstantinides, S.V. , Torbicki, A. , Agnelli, G. , Danchin, N. , Fitzmaurice, D. , Galiè, N. , Gibbs, J.S. , Huisman, M.V. , Humbert, M. , Kucher, N. , Lang, I. , Lankeit, M. , Lekakis, J. , Maack, C. , Mayer, E. , Meneveau, N. , Perrier, A. , Pruszczyk, P. , Rasmussen, L.H. , Schindler, T.H. , Svitil, P. , Vonk Noordegraaf, A. , Zamorano, J.L. & Zompatori, M. ; on behalf of theTask Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) . (2014) ESC guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal, 2014, 3033–3069. [Google Scholar]
- Kovac, M. , Mikovic, Z. , Rakicevic, L. , Srzentic, S. , Mandic, V. , Djordjevic, V. , Radojkovic, D. & Elezovic, I. (2010) The use of D‐dimer with new cutoff can be useful in diagnosis of venous thromboembolism in pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 148, 27–30. [DOI] [PubMed] [Google Scholar]
- Leung, A.N. , Bull, T.M. , Jaeschke, R. , Lockwood, C.J. , Boiselle, P.M. , Hurwitz, L.M. , James, A.H. , McCullough, L.B. , Menda, Y. , Paidas, M.J. , Royal, H.D. , Tapson, V.F. , Winer‐Muram, H.T. , Chervenak, F.A. , Cody, D.D. , McNitt‐Gray, M.F. , Stave, C.D. & Tuttle, B.D. ; on behalf of the ATS/STR Committee on Pulmonary Embolism in Pregnancy . (2011) An Official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline: evaluation of suspected pulmonary embolism in pregnancy. American Journal of Respiratory and Critical Care Medicine, 184, 1200–1208. [DOI] [PubMed] [Google Scholar]
- Lijmer, J.G. , Mol, B.W. , Heisterkamp, S. , Bonsel, G.J. , Prins, M.H. , van der Meulen, J.H. & Bossuyt, P.M. (1999) Empirical evidence of design‐related bias in studies of diagnostic tests. JAMA, 282, 1061–1066. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . NICE clinical guideline 144: Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. 2012. Available at: https://www.nice.org.uk/guidance/CG144 (accessed 06/11/2017) [PubMed]
- O'Connor, C. , Moriarty, J. , Walsh, J. , Murray, J. , Coulter‐Smith, S. & Boyd, W. (2011) The application of a clinical risk stratification score may reduce unnecessary investigations for pulmonary embolism in pregnancy. The Journal of Maternal‐Fetal & Neonatal Medicine, 24, 1461–1464. [DOI] [PubMed] [Google Scholar]
- Royal College of Obstetricians & Gynaecologists . (2015) Thromboembolic Disease in Pregnancy and the Puerperium: Acute Management. Green‐top Guideline No 37b. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37b.pdf (accessed 21/03/2016)
- Stein, P.D. , Hull, R.D. , Patel, K.C. , Olson, R.E. , Ghali, W.A. , Brant, R. , Biel, R.K. , Bharadia, V. & Kalra, N.K. (2003) D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Annals of Internal Medicine, 140, 589–602. [DOI] [PubMed] [Google Scholar]
- Wells, P.S. , Anderson, D.R. , Rodger, M. , Stiell, I. , Dreyer, J.F. , Barnes, D. , Forgie, M. , Kovacs, G. , Ward, J. & Kovacs, M.J. (2001) Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer. Annals of Internal Medicine, 135, 98–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. AUROC with 95% CI for each biomarker in primary and secondary analysis.
Appendix S1. Details of biomarker analysis (including women who had received anticoagulation).
Appendix S2. The DiPEP research group.


