Abstract
Hepatocyte transplantation is a promising therapeutic approach for various liver diseases. Despite the liver's tolerogenic potential, early immune‐mediated loss of transplanted cells is observed, and longterm acceptance has not been achieved yet. Patients deemed tolerant after liver transplantation presented an increased frequency of regulatory T cells (Tregs), which therefore also might enable reduction of posttransplant cell loss and enhance longterm allograft acceptance. We hence characterized hepatocyte‐induced immune reactions and evaluated the immunomodulatory potential of Tregs applying mixed lymphocyte cultures and mixed lymphocyte hepatocyte cultures. These were set up using peripheral blood mononuclear cells and primary human hepatocytes, respectively. Polyclonally expanded CD4+CD25highCD127low Tregs were added to cocultures in single‐/trans‐well setups with/without supplementation of anti‐interferon γ (IFNγ) antibodies. Hepatocyte‐induced alloresponses were then analyzed by multicolor flow cytometry. Measurements indicated that T cell response upon stimulation was associated with IFNγ‐induced major histocompatibility complex (MHC) class II up‐regulation on hepatocytes and mediated by CD4+ T cells. An indirect route of antigen presentation could be ruled out by use of fragmented hepatocytes and culture supernatants of hepatocytes. Allospecific proliferation was accompanied by inflammatory cytokine secretion. CD8+ T cells showed early up‐regulation of CD69 despite lack of cell proliferation in the course of coculture. Supplementation of Tregs effectively abrogated hepatocyte‐induced alloresponses and was primarily cell contact dependent. In conclusion, human hepatocytes induce a CD4+ T cell alloresponse in vitro, which is associated with MHC class II up‐regulation on hepatocytes and is susceptible to suppression by Tregs. Liver Transplantation 24 407–419 2018 AASLD.
Abbreviations
- FasL
Fas Ligand
- FOXP3
forkhead box P3
- HLA
human leukocyte antigen
- HT
hepatocyte transplantation
- IFNγ
interferon γ
- IL
interleukin
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- MLC
mixed lymphocyte culture
- MLHC
mixed lymphocyte hepatocyte culture
- PBMC
peripheral blood mononuclear cell
- PHH
primary human hepatocyte
- SEM
standard error of the mean
- sCD40L
soluble CD40 ligand
- Th
T helper
- TNF‐α
tumor necrosis factor α
- Treg
regulatory T cell
Hepatocyte transplantation (HT) is a promising therapeutic approach as treatment for various liver diseases.1 Primary human hepatocytes (PHHs) may be cryopreserved for use of HT in emergencies2 and genetically modified extracorporally prior to transplantation.3 In animal experiments, HT leads to hepatic remodeling with histologically indistinguishable engrafted hepatocytes.4 These achievements could not yet be transferred into clinical practice, where HT only resulted in transient amelioration of liver function5 prolonging survival for up to 52 days, before patients require orthotopic liver transplantation.6 Reasons for the limited cell survival might be competition with tissue‐resident cells in a nonpreconditioned environment7 and rejection by the recipient's immune system.8
Rare occurrence of hyperacute rejection and immunomodulating effects in combined hepatorenal grafting9 highlight the liver's immunoprivileged status with indications that allograft survival is independent of aggressiveness of immunosuppressive medication or human leukocyte antigen (HLA) matching.10 Experiments in mice demonstrated induction of strong cell‐mediated immune responses independently by both CD4+ and CD8+ T cells in hepatocyte rejection.11 Contribution of humoral responses is also suggested with alloantibody‐mediated reactions increased in CD8+‐deficient recipient mice.12 Alterations induced during cell isolation and removal of other immunocompetent cells may also augment hepatocytes' immunogenicity.13 Instant blood‐mediated inflammatory reaction after hepatocyte infusion was lately described to induce cell losses up to 70%.14
Tolerated liver allografts showed higher fractions of regulatory T cells (Tregs), and acute rejection was induced upon Treg depletion.15 Tregs are known to suppress T and B cell–mediated immune responses, modify effectors of innate immunity, or activate effector functions in target tissues and are thus considered key players in transplantation tolerance.16 Treg therapy in transplantation is currently moving to the clinic.17
The future clinical goal is clear: reduction of postoperative immune‐mediated cell loss after HT to enable more effective engraftment with donor hepatocytes and to improve longterm allograft acceptance. In an in vitro model based on coculture of peripheral blood mononuclear cells (PBMCs) with PHH, the aims of this project are further characterization of immune responses induced by isolated allogeneic hepatocytes and analysis of the immunomodulatory potential of Tregs.
Patients and Methods
HEPATOCYTE ISOLATION AND CULTURE
Liver tissue was obtained from patients undergoing partial hepatectomy and upon written informed consent (approved by the ethic commission of Hannover Medical School, #252‐2008). Hepatocytes were isolated by modified 2‐step collagenase perfusion as previously reported18 and cultured using collagen‐precoated 6‐well plates. After 16‐18 hours, culture medium was changed to remove dead cells to ensure formation of a confluent monolayer.
ISOLATION AND EXPANSION OF TREG
Tregs were isolated as reported previously19 and sorted for CD4+CD25highCD127low phenotype. Purity of the isolated Tregs was verified by intracellular staining with anti–forkhead box P3 (FOXP3)–Alexa647 according to the manufacturer's instructions (BioLegend, San Diego, CA). Polyclonal expansion ensued by culture in 96‐well round bottom plates with 1‐2 × 105 cells/well in supplemented RPMI1640 with CD3/CD28‐expanderbeads (Dynabeads, Invitrogen, Carlsbad, CA; initial ratio to cells 4:1) and 300 U/mL interleukin (IL) 2. Bead:cell ratio was reduced to 1:1 on day 10 and further to 1:4 on day 20. Medium was changed every second day, and populations split after 5‐7 days. Following expansion for 14‐28 days, Tregs were used in mixed lymphocyte culture (MLC)/mixed lymphocyte hepatocyte culture (MLHC) experiments. The purity of the expanded Tregs was ascertained by intracellular FOXP3 staining as described above.
Mixed Lymphocyte Culture
MLCs were set up as previously reported,20 but they were modified for analysis of cell proliferation by flow cytometry: responder cells were stained with PKH‐26 according to the manufacturer's instructions (Sigma‐Aldrich, St. Louis, MO) and as reported elsewhere.21 Allogeneic stimulator cells were irradiated with 30 Gy (GammaCell 2000) and dyed with CellVue Maroon (Polysciences, Warrington, PA). Expanded Tregs were also stained with CellVue Maroon (both according to the manufacturer's instructions). Cells were cocultured with 1 × 106 cells/well each in 2 mL of supplemented Williams Medium E on 24‐well plates for up to 10 days. Tregs were added at a 1:1 ratio on day 0 where applicable. Experimental groups were as follows: stimulator + naïve responder PBMC; stimulator + responder PBMC + Treg; stimulator control; responder control. Medium was changed in part (0.5 mL) daily.
Mixed Lymphocyte Hepatocyte Culture
On the basis of the in vitro model by Bumgardner et al.,22 a modified approach of MLHC was developed: experiments were performed analogous to MLC described above, with allogeneic PHH monolayer cultures as stimulator. Again, responder PBMCs were stained with PKH‐26, and Tregs were stained with CellVue Maroon. MLHC was performed in 6‐well plates with 2 mL supplemented Williams Medium E with daily change of 0.5 mL. PHHs were seeded at 1.5 × 106/well. The 5 × 106 naïve responder PBMC with/without expanded Tregs at a 1:1 ratio were added on day 0 where applicable. Experimental groups were as follows: PHH + PBMC; PHH + PBMC + Treg; PHH control; PBMC control; and Treg control. Culture supernatants were stored at −80°C for cytokine analysis in batch.
INDIRECT ROUTE OF ANTIGEN PRESENTATION
PKH‐26–marked PBMCs were cultured in Williams Medium E supplemented with PHH lysates prepared by repeated snap‐freezing/thawing of 1 × 106 PHHs or daily supplementation of 0.5 mL of culture supernatant of PHH cultured as monolayers (cross presentation of proteins from dead PHH).
TRANS‐WELL EXPERIMENTS AND BLOCKADE OF IL10/INTERFERON γ
Trans‐well experiments were set up analogous to MLHCs described above with Tregs added in trans‐well inlets (pore diameter 0.5 μm; Greiner bio‐one, Kremsmünster, Austria) prohibiting direct cell contact with PHH or PBMC. Experiments were performed in the presence/absence of anti‐IL10 (1 μg/mL; BioLegend) or anti‐interferon γ (0.5 μg/mL; Invitrogen) antibodies, respectively. Medium change and sample collection were performed as described above.
FLOW CYTOMETRY
For analysis of early stimulation on day 3, unlabeled PBMCs were retrieved from culture and stained with monoclonal antibodies as indicated: fluorescein isothiocyanate–conjugated CD4 and CD8 (Immuno Tools, Friesoythe, Germany), phycoerythrin‐conjugated CD25 and CD69 (BD Bioscience, San Jose, CA). For late proliferation control, PKH‐26–stained PBMCs were analyzed on day 10. Additional staining for CD4 and CD8 was performed to distinguish T cell subpopulations. Expanded Tregs were excluded during gating for analysis by CellVue‐labeling (Supporting Fig. 1) to prevent confounding. Flow cytometric measurements were performed using a FACSCalibur (BD Biosciences), and results were analyzed by FACSDiva software.
Hepatocytes retrieved from MLHCs on day 10 were stained with allophycocyanin‐conjugated anti–HLA‐DR for detection of major histocompatibility complex (MHC) class II expression. MHC class II expression also was examined on PHH monocultures stimulated with interferon γ (IFNγ) (100 ng/mL; Peprotech, Rocky Hill, NJ) for 3 days, with nonstimulated cells serving as control. Flow cytometric analyses were performed using a FACSCanto II (BD Biosciences).
CYTOKINE ANALYSIS
Cytokine profiles from culture supernatants were determined using the Luminex‐based multiplex technology according to the manufacturer's instructions. Cytokines were quantified in culture supernatants using the human Th17‐Plex (Bio‐Rad, Chicago, IL). Standard curves and cytokine concentrations were calculated with Bio‐Plex Manager 6.0 software; the detection limit of all proteins was between 1 and 10 pg/mL.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS statistics, version 24.0 (IBM, Armonk, NY). Mann‐Whitney U and Wilcoxon signed‐rank tests were applied as appropriate. Differences were regarded statistically significant with P < 0.05. Results were expressed as mean ± standard error of the mean (SEM) unless otherwise indicated.
Results
MLC VERSUS MLHC
To characterize immune responses against allogeneic PHH, we established a novel coculture system based on the principle of conventional MLC: MLHC (Fig. 1A). Alloresponses induced by PHHs in vitro were compared with patterns in conventional MLCs, and the results were used for definition of optimal time points for further sample acquisition. Read‐out of proliferative responses by flow cytometry ensued as shown in Fig. 1B. Gating strategy is displayed in Supporting Fig. 1.
Figure 1.
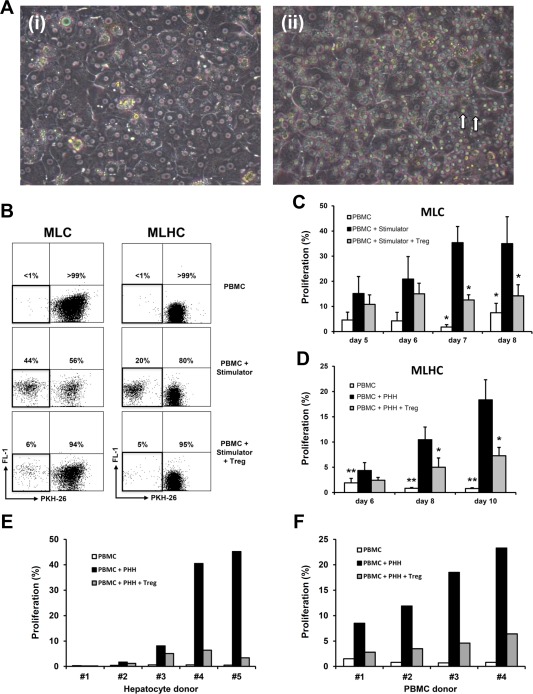
Comparison of proliferative alloresponses determined by MLC and MLHC. (A) Phase‐contrast microscopic images (magnification ×200) of cell cultures 48 hours after setup; (i) PHH as adhesive monolayers, (ii) PHH cocultured with PBMC (marked ↑) as MLHC. (B) Representative dot‐plots of flow cytometric analyses depicting proliferative alloresponses of MLCs and MLHC for different experimental groups. (C, D) Bar charts summarizing proliferative alloresponses in the course (MLC, days 5‐6 n ≥ 3, days 7‐8 n ≥ 8; MLHC, n ≥ 8; each represented as mean ± SEM; *P < 0.05 and **P < 0.01 compared with PBMC + stimulator group). (E, F) Bar charts demonstrating alloproliferation variance on day 10 of MLHC applying PHH from different donors on the same responder PBMC and vice versa.
In conventional MLCs (Fig. 1B,C), proliferative alloresponse upon stimulation with irradiated allogeneic PBMCs reached a plateau on day 7 of coculture. Because of prospective clinical use of immunomodulatory Tregs, we analyzed polyclonal Treg supplementation within MLCs that resulted in significant suppression of proliferation. In comparison, alloresponses within MLHCs resulted in timely delayed and less intense proliferation of responder PBMCs (Fig. 1B,D). No plateau was reached during coculture of 10 days (no prolonged culture performed due to dedifferentiation of PHHs in longterm culture). Nonetheless, these alloresponses could again be significantly reduced upon coculture with Tregs.
For analysis of interindividual stimulatory potentials of allogeneic hepatocytes on PBMCs, immune responses induced by PHHs from different donors on the same responder PBMC were compared and vice versa. Donors of PHHs, PBMCs, and Tregs were not screened for immunological parameters such as HLA. Not only did the same responder PBMC react with significant variance in proliferation upon stimulation with various allogeneic donor PHHs, but also response intensities varied greatly for different PBMC responders with PHHs of the same donor (Fig. 1E,F), which does not seem to result from different levels of MHC mismatches (Supporting Fig. 2). Nonetheless, proliferative responses could be suppressed effectively by Treg coculture.
CHARACTERIZATION OF PROLIFERATIVE ALLORESPONSES IN MLHC
For further characterization, involvement of CD4+ and CD8+ T cell subpopulations in proliferative alloresponses on day 10 of MLHC was investigated. Fluorescence‐activated cell sorting analysis showed distinct proliferation of CD4+ T cells and efficient suppression upon Treg supplementation. Only limited proliferation was observed for CD8+ T cells; Treg coculture delivered no mentionable effect (Fig. 2A,B).
Figure 2.
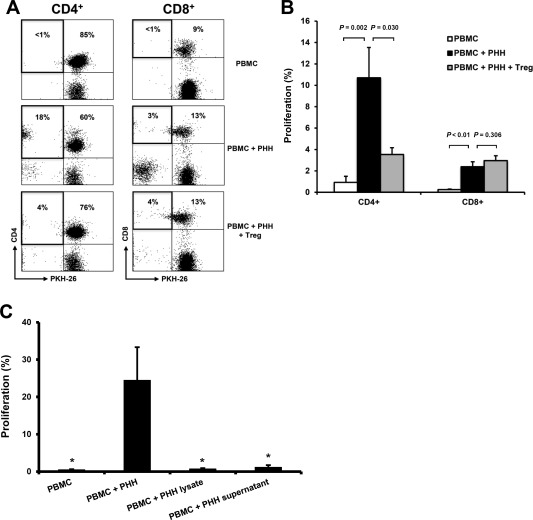
Flow cytometric characterization of hepatocyte‐induced T cell alloresponse in MLHC. (A) Representative dot‐plots of flow cytometric analyses depicting proliferative alloresponses on day 10 of MLHC for different experimental groups. (B) Bar chart summarizing proliferative alloresponses for CD4+ and CD8+ T cell subpopulations, respectively (n = 19, represented as mean ± SEM). (C) Bar charts demonstrating alloproliferation following stimulation of PBMC with PHH lysates as well as culture supernatant of PHH (n = 5, each represented as mean ± SEM; *P < 0.05 compared with PBMC + PHH group).
PHHs constitutively express HLA class I, predicting a CD8+ T cell–driven immune response, whereas CD4+ T cell proliferation is triggered by antigen‐presenting cells expressing MHC class II. Indirect antigen presentation routes were ruled out by stimulating PBMCs with PHH lysates and culture supernatants of PHH (Fig. 2C). Consequently, we hypothesized that PHHs up‐regulate expression of MHC class II as part of the immune reaction and investigated its expression on hepatocytes used within MLHC. Suitably, HLA‐DR was induced in PHHs stimulated by PBMC coculture (Fig. 3A,B) but not in PBMC‐free PHH cultures. For consolidation, we added IFNγ to PBMC‐free PHH cultures. IFNγ is known to induce HLA class II expression on antigen‐presenting or stromal cells.23, 24 Indeed, supplementation of IFNγ caused significant up‐regulation of HLA class II expression on PHH (Fig. 3C). To further elucidate the role of IFNγ on a functional level in this setting, blocking anti‐IFNγ antibody was applied in MLHC: dose‐dependent reduction of MHC class II on PHH was observed with a consecutive reduction of induced alloproliferation (Fig. 3D,E; for titrations see Supporting Fig. 3), confirming involvement of IFNγ in this process.
Figure 3.
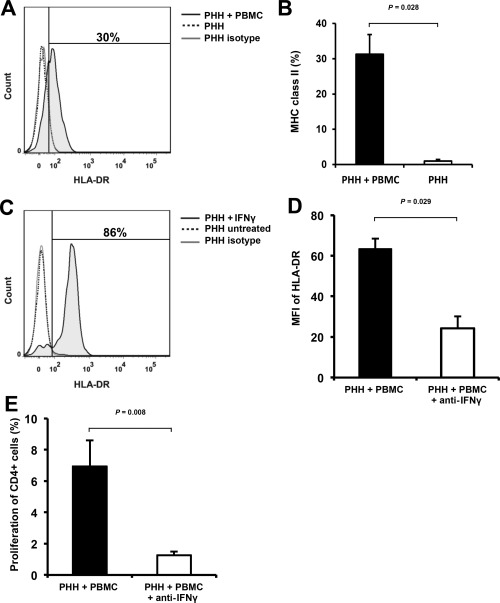
Evaluation of the role of IFNγ for hepatocyte‐induced proliferative alloresponses in MLHC. (A) Representative histogram of MHC class II surface receptor expression (HLA‐DR) on PHH retrieved from MLHC after 10 days (indicated as percentage expression) versus controls. (B) Bar chart summarizing MHC class II expression on PHH (n = 6, represented as mean ± SEM). (C) Representative histogram depicting the effect of IFNγ treatment on MHC class II expression on PHH (indicated as percentage expression). (D) Bar chart summarizing the effect of blocking anti‐IFNγ antibody (0.5 μg/mL) on MHC class II expression (indicated as MFI) on PHH in MLHC (n = 4, represented as mean ± SEM). (E) Bar chart summarizing proliferative responses of CD4+ cells in MLHC with/without supplementation of 0.5 μg/mL anti‐IFNγ antibody (n = 5, represented as mean ± SEM).
Measurement of cytokine levels on day 10 in supernatants of MLHC was performed to further characterize immune reactions in this setting. Generally, cytokine levels were highest in the stimulation group (PHH + PBMC) suggesting an inflammatory milieu with significant reduction upon the addition of Tregs (Fig. 4A). IFNγ levels were especially elevated in MLHCs, matching our hypothesis above. In addition, T helper (Th) 2–associated cytokines IL10, IL21, and IL31 were induced upon PHH/PBMC coculture. Likewise, soluble CD40 ligand (sCD40L) levels as a marker of CD4+ T cell activation were highest in this group corresponding to elevated CD40L concentrations in acute allograft rejection.25 Treg supplementation significantly reduced this cytokine induction, but it could not significantly suppress secretion of proinflammatory cytokine IL6. Th17 and Th22 cytokines seem to play a minor role because these signature cytokines were poorly secreted. In supernatants of PBMC monocultures only low cytokine amounts were detected, whereas for some, slightly elevated levels were found in PHH monocultures, indicating that allogeneic stimulation generally seemed mandatory for significant cytokine induction (Fig. 4A).
Figure 4.
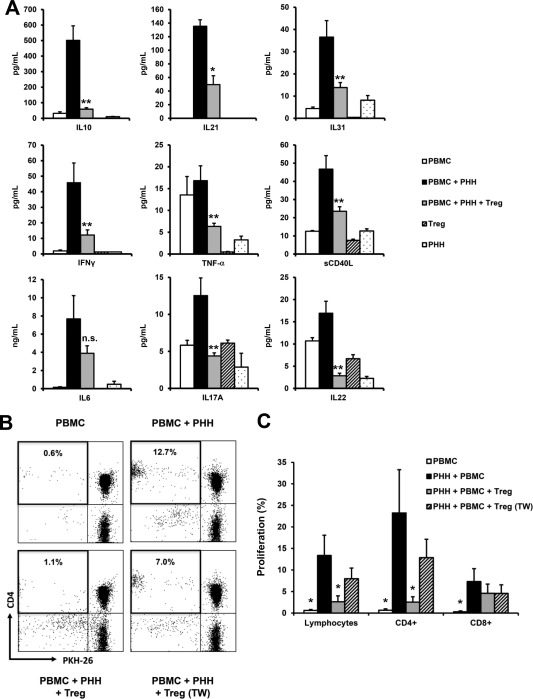
Determination of hepatocyte‐induced cytokine responses and characterization of the suppressive effect of Treg in MLHC. (A) Bar charts depicting results of cytokine analysis from culture supernatants on day 10 of MLHC. Expression levels of selected cytokines for respective experimental groups are represented as mean ± SEM for PBMC (n = 5), PBMC + PHH (n = 8), PBMC + PHH + Treg (n = 12), Treg (n = 3), and PHH (n = 2), respectively; *P < 0.05, **P < 0.01 and n.s. = not significant for comparison of PBMC + PHH versus PBMC + PHH + Treg. (B) Representative dot‐plots of flow cytometric analyses depicting proliferative alloresponses on day 10 of MLHC for different experimental groups with Treg in single‐ and trans‐well setup, respectively. (C) Bar chart summarizing proliferative alloresponses of lymphocytes, CD4+ and CD8+ T cells comparing immunomodulatory effects of Treg (n = 8, represented as mean ± SEM; *P < 0.05 compared with PHH + PBMC group).
High levels of the anti‐inflammatory cytokine IL10 were expected to be reversed: Tregs typically exert suppressive effects by IL10 secretion.26 For further assessment, T cells and Tregs were spatially separated by trans‐wells, which significantly reduced modulatory potential of Tregs (Fig. 4B,C), indicating an inferior role of IL10 secretion for Treg‐mediated, mainly cell‐contact–dependent suppression in MLHC. Results of in vitro blockade of IL10 using appropriate antibodies support this because proliferative alloresponse is enhanced in MLHC without Treg coculture or Treg coculture in the trans‐well setting. The suppressive effect of Tregs could not be reversed when direct cell contact was enabled. Results were not regarded statistically significant due to the small number of cases (Supporting Fig. 4).
ROLE OF EARLY ACTIVATION MARKERS IN MLHC
Several authors have described mediation of rejection reactions by both CD4+ and CD8+ T cells. Because no proliferative reaction of CD8+ T cells was found in MLHC, other possibilities like rapid responses27 with expression of early activation marker CD69 were considered.28 Early activated CD8+ T cells die prematurely after transplantation due to sparse IL2 production and diminished expression of CD25 (IL2R a‐chain) leading to “passive death.”29, 30 Assuming the same occurrence in vitro, this could explain low proliferation detected on day 10 of MLHC. To monitor rapid CD8+ T cell response and its relation to CD4+ T cells, stimulated PBMCs were analyzed on day 3 for activation markers CD25 and CD69. Indeed, up‐regulation of CD69 on CD8+ T cells could be observed: overall expression of CD69 (Fig. 5A,B) as well as its cell surface density (P = 0.017 for PBMC versus PBMC + PHH) were increased and significantly suppressed upon Treg supplementation. Hardly any correlation with up‐regulation of CD25 was detected for CD8+ T cells (data not shown).
Figure 5.
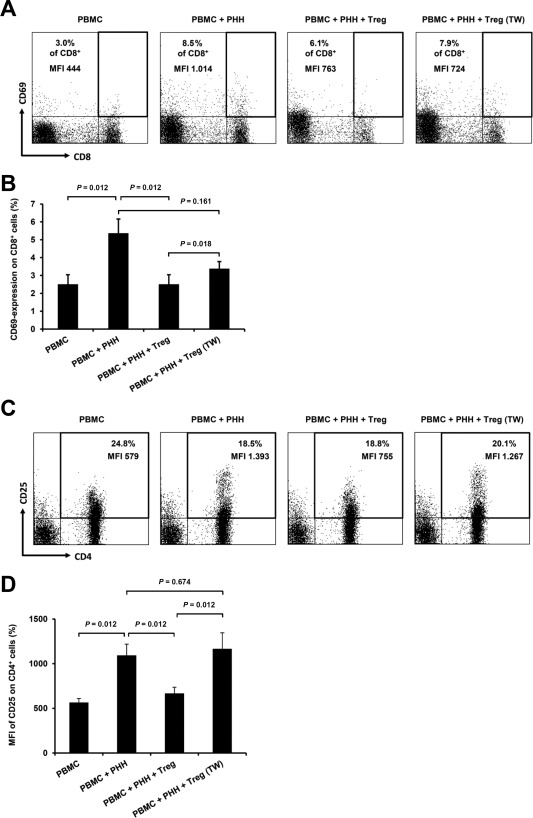
Flow cytometric analyses of early activation markers CD69 and CD25 on T cells in the early phase of MLHC. (A) Representative dot‐plots of flow cytometric analyses depicting CD69 surface expression on CD8+ T cells on day 3 of MLHC for different experimental groups (percentage of marker expression and MFI are indicated). (B) Bar chart summarizing percentage of CD69 expression on CD8+ T cells for different experimental groups (n ≥ 20, represented as mean ± SEM). (C) Representative dot‐plots of flow cytometric analyses depicting CD25 surface expression on CD4+ T cells on day 3 of MLHC for different experimental groups (percentage of marker expression and MFI are indicated). (D) Bar chart summarizing MFI of CD25 expression on CD4+ T cells for different experimental groups (n ≥ 20, represented as mean ± SEM).
Frequency of CD4+ T cells expressing CD25 was not increased within 3 days of PHH‐allostimulation but higher densities of expressed CD25 were apparent (Fig. 5C,D) comprising different subpopulations: activated helper T cells, polyclonal supplemented Tregs, and constitutional responder Tregs. Supplemented Tregs could not influence measurements as CellVue labeling was used for gating (Supporting Fig. 1). Constitutional responder Tregs are not distinguishable via CellVue staining in this setting and appear in monitored subpopulations. However, supplemented polyclonal Tregs could suppress increased surface marker expression, indicating successful prevention of T cell activation, rendering presence of constitutional responder Tregs neglectable. No equivalent findings were observed for CD69 on CD4+ T cells (data not shown).
Discussion
HT was simulated in a novel in vitro experimental transplant setting (MLHC) using PHH modeling donor cells and cocultured human PBMCs representing the recipient immune system. Tregs were cocultured to evaluate their potential use for immunomodulation in this transplant scenario.
Proliferative response of PBMCs on allogeneic PHHs was delayed and reduced compared with conventional MLC. Indirect routes of antigen presentation were ruled out. Proliferative response on day 10 is mainly mediated by CD4+ T cells, with barely any proliferative reaction of CD8+ T cells. However, CD8+ T cells were activated on day 3 with significant up‐regulation of CD69. In MLHCs as well as MLCs, Tregs suppressed all immune reactions. Reduced effects in the trans‐well setup indicate cell contact–dependent mechanisms for interaction with PHHs and PBMCs.
Activation with allogeneic PHHs revealed reduced proliferation of responder PBMCs due to hepatocytes not being typical antigen‐presenting cells. Freshly isolated PHHs are known as HLA class I+, class II–, and can thereby stimulate allospecific cytotoxic T cells.22 However, observed T cell reactions were predominantly CD4+ mediated, an activation mainly triggered by HLA class II. Hepatocytes can up‐regulate HLA class II in inflammation and immune‐mediated liver disease.29, 30 Consequently, inflammatory milieus in rejection reactions could induce MHC class II presentation by PHHs and explain helper T cell activation. Observed up‐regulation of HLA‐DR on hepatocytes upon stimulation by PBMC supports this hypothesis. Furthermore, addition of IFNγ to culture medium induced the same effect, as similarly reported for other cell types,23, 24 which could be reversed by appropriate blocking antibodies.
Generally, ABO‐mismatch and disparity in MHC surface antigens are well‐known to influence outcomes of transplanted tissues resulting in their use as predictors for allograft survival.31, 32, 33 In liver transplantation, however, positive cross‐matches appear more relevant than MHC or even ABO compatibility, highlighting the organ's tolerogenic properties.34 Despite this behavior in solid organ transplantation, our experiments with different allogeneic responder PBMCs in coculture with the same PHHs or vice versa led to broad variation of proliferative outcomes, although correlation with the degree of HLA matching was still not observed. The absence of other resident, immunocompetent cells typically present in solid organs could explain these differences. Furthermore, proposed exposure of intercellular surface molecules on hepatocytes during cell isolation might change their immunogenicity.35
Generally, acute rejection is considered mainly CD4+ helper T cell–dependent with generation of a Th1 cytokine profile including secretion of IFNγ.36 This reaction is susceptible to immunoregulation and assumed responsible for longterm allograft survival. However, only total T cell depletion results in significant prolongation of allograft survival, as CD4+‐ and CD8+‐dependent rejection mechanisms are codominant.37 Cytotoxicity seems alloantibody mediated in acute CD4+‐dependent rejection reactions, especially when CD8+ or Th1 pathways are deficient.37 CD8‐deficient recipients present IL4‐producing CD4+ T cells, triggering increased alloantibody production. Down‐regulating effects of CD8+ T cells on posttransplant antibody production depend on IFNγ and Fas Ligand (FasL) or perforin.38 Clinically, induction of donor‐specific antibody formation following HT is discussed controversially.39, 40
Thus, reduced alloproliferation of CD8+ T cells following stimulation with PHHs is surprising, especially considering MHC class I expression prevailing on hepatocytes. However, more rapid CD8+ T cell response with increased expression of CD69 matches our findings for CD69 expression on day 3. Furthermore, expression of CD69 on peripheral CD8+ cells after kidney transplantation in vivo correlates closely with acute rejection of allografts,41 and alloreactive CD8+ T cells are considered responsible for late rejection reactions, resulting in diminished longterm survival.42
T cell–mediated immune response on allogeneic hepatocytes is reported as B cell–dependent through interactions with the CD40/CD40L pathway.43 Our model delivered very high levels of sCD40L in supernatants of stimulated PBMC, compatible to increased CD40L expression in acute allograft rejection,25 which induces CD4+ and CD8+ T cell activation and hepatocyte apoptosis.44 CD40 interaction is necessary for CD4+ T cell activation, but CD8+ response is independent of this pathway.45
Already in the PHH control, cytokine analyses revealed baseline production of proinflammatory tumor necrosis factor α (TNF‐α) which is known for its role in liver diseases.46 Likewise increased levels of IL22 and IL17A were observed. IL22 is mainly produced by group 3 innate lymphoid cells and has protective, mitogenic, and antifibrotic properties, suitable to trigger proliferation of hepatocyte progenitor cells upon inflammation.47 The levels measured might therefore indicate the induction of endogenic repair mechanisms in isolated PHHs, especially because IL22 cooperates with IL17A in inflammation control.48 The unexpected presence of sCD40L in the control group could be a result of hepatocyte ischemia intraoperatively or during cell culture: inflammation as well as hypoxia are known to trigger the CD40/CD40L pathway.49, 50
Furthermore, high levels of anti‐inflammatory IL10 following allostimulation with PHH seem interesting considering the inflammatory environment. Activated CD8+ T cells are known to stimulate hepatocytes to produce anti‐inflammatory cytokines (eg, IL4, IL10)51 and Th2 CD4+ T cells produce IL10. High IL10 levels in allografts reduce production of Th1 cell cytokines, regulating proliferation and differentiation of T, B, and natural killer cells, resulting in cell engraftment prolonging allograft survival.52 IL10 is also instrumental for Treg function,53 suggesting an explanation of IL10 as an activation marker for constitutional Tregs in recipient PBMCs, whereas being consumed in the PHH + PBMC + Treg group. High IL10 levels could be regarded as an attempt of counter‐regulation against inflammation by responder T cells themselves.
Trans‐well experiments, resulting in reduced suppressive potential of Tregs, indicate mainly cell contact–mediated modulatory effects of Treg in MLHC. This aligns with observations by others53 that Treg effects depend on IL35 and IL10 secretion as well as cell contact. These effects are bidirectional because T cells can also increase the modulatory potential of Tregs. Our trans‐well experiments and in vitro blockade of IL10 support this hypothesis of cell contact–dependent mechanisms of action.
To date there are only a few clinical studies on the use of Tregs for immunomodulatory therapy in solid organ transplantation.17 Two interesting ongoing studies are the multicentric phase 1/2 ONE Study, which focuses on the safety and efficacy of Tregs in renal transplantation (NCT02129881) and the phase 1 ThRIL study examining the application of Tregs in liver transplantation (NCT02166177).
All in all, allogeneic PHHs induce a T cell–mediated immune reaction of PBMCs in vitro as a primarily proliferative alloresponse of CD4+ T cells with concomitant up‐regulation of MHC class II on hepatocytes. Although CD8+ T cells show no comparable proliferative response, expression of early activation marker CD69 is significantly up‐regulated following stimulation with allogeneic hepatocytes. All these effects in the inflammatory milieu, indicated by cytokine levels in culture supernatants, can be effectively suppressed by coculture with polyclonal Tregs. The immunomodulatory potential of these Tregs seems primarily mediated by cell contact.
Supporting information
Additional supporting information may be found in the online version of this article.
Supplementary Figure 1: Gating strategy for flow‐cytometric detection of proliferative responses in mixed lymphocyte cultures and mixed lymphocyte hepatocyte cultures.
Representative examples for gating strategy for flow‐cytometric determination of cell proliferation on day 10 of culture for conventional mixed lymphocyte cultures (MLC) and mixed lymphocyte hepatocyte cultures (MLHC). (A) For MLC the following three different experimental groups are compared: (i) PKH‐26 stained PBMC alone, (ii) PKH‐26 stained PBMC co‐cultured with irradiated stimulator PBMC marked by CellVue and (iii) PKH‐26 stained PBMC co‐cultured with irradiated stimulator PBMC and expanded polyclonal Tregs, both marked by CellVue. (B) For MLHC the following three different experimental groups are compared: (i) PKH‐26 stained PBMC alone, (ii) PKH‐26 stained PBMC co‐cultured with adherent primary human hepatocytes (PHH) and (iii) PKH‐26 stained PBMC co‐cultured with adherent PHH and expanded polyclonal Treg marked by CellVue.
PBMC are detected in forward and side scatters (gate P1) (left panels, respectively). In a next step only CellVue negative cells were considered for further analysis (gate P2) (middle panels, respectively). Finally, proliferative responses of PKH‐26 stained cells were detected as loss of intensity of intracellular staining (right panels, respectively), with possibility for co‐staining for CD4 or CD8 via FL‐1 (not shown). Subpopulations were assessed as percentage of whole lymphocyte population.
Supplementary Figure 2: Evaluation of the effect of HLA mismatch on the alloproliferative response induced by primary human hepatocytes in vitro.
Mixed lymphocyte hepatocyte cultures were set up as described above but with some modifications: PBMC and liver donors were typed for HLA expression (HLA A/B/DR) according to ASHI standards. Furthermore, typed PBMC were frozen in supplemented RPMI 1640 (20% FCS, 10% DMSO) and stored in liquid nitrogen until PHH from typed donors were available to perform MLHC. MLHC were set up with PKH‐26 labelled PBMC from 11 to 12 donors simultaneously and proliferative responses were detected on day 10 by flow‐cytometry as reported above.
Bar chart summarising proliferative alloresponses in accordance to the total number of HLA mismatches and number of HLA‐DR mismatches between PHH from 3 different donors and appropriated PBMC donors used for MLHC, respectively (n = number of cases for the specified group of mismatches present; data presented as Mean ± SEM).
Supplementary Figure 3: Titration of anti‐interferon‐gamma antibody to block hepatocyte‐induced alloresponses in MLHC.
(A) Representative titration curve depicting the effect of anti‐IFNγ antibody treatment to block upregulation of MHC class II expression (HLA‐DR) on PHH during MLHC determined by flow‐cytometry on day 10 of culture. (B) Representative titration curve depicting the effect of anti‐IFNγ treatment to block hepatocyte‐induced proliferative alloresponse determined on day 10 of MLHC (presented as CD4+ proliferation of responder PBMC).
Supplementary Figure 4: Evaluation of the role of interleukin‐10 for Treg‐mediated suppression of hepatocyte‐induced alloproliferation in MLHC.
Bar chart summarising proliferative alloresponses with/without additional supplementation of 1μg/ml anti‐interleukin‐10 (IL‐10) antibodies in MLHC with/without co‐culture of Treg and/or use of trans‐well inlets (n = 3, represented as Mean ± SEM; n.s. = not significant).
Acknowledgments
The authors thank Sonja Kollrich, Corinna Löbbert, and Ingrid Meder for their excellent technical support. Assistance of the Cell Sorting Core Facility of Hannover Medical School supported in part by Braukmann‐Wittenberg‐Herz‐Stiftung and DFG is also acknowledged.
This study was partially supported by “Hochschulinterne Leistungsförderung” of Hannover Medical School, Else Kröner‐Fresenius‐Stiftung (2010_A49) (both for Florian W. R. Vondran), “StrucMed Programme” of Hannover Biomedical Research Schools, and the German Research Foundation (SFB 738; Christine S. Falk, Florian W. R. Vondran, and Michael Bock; projects B3, C11).
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg 2009;16:97‐100. [DOI] [PubMed] [Google Scholar]
- 2. Hewitt NJ, Li AP. Cryopreservation of hepatocytes. Methods Mol Biol 2015;1250:13‐26. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen TH, Mainot S, Lainas P, Groyer‐Picard MT, Franco D, Dagher I, Weber A. Ex vivo liver‐directed gene therapy for the treatment of metabolic diseases: advances in hepatocyte transplantation and retroviral vectors. Curr Gene Ther 2009;9:136‐149. [DOI] [PubMed] [Google Scholar]
- 4. Weber A, Groyer‐Picard MT, Franco D, Dagher I. Hepatocyte transplantation in animal models. Liver Transpl 2009;15:7‐14. [DOI] [PubMed] [Google Scholar]
- 5. Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler‐Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998;338:1422‐1426. [DOI] [PubMed] [Google Scholar]
- 6. Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver Transpl 2000;6:32‐40. [DOI] [PubMed] [Google Scholar]
- 7. Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol 2013;3:485‐513. [DOI] [PubMed] [Google Scholar]
- 8. Han B, Lu Y, Meng B, Qu . Cellular loss after allogenic hepatocyte transplantation. Transplantation 2009;87:1‐5. [DOI] [PubMed] [Google Scholar]
- 9. Lerut J, Sanchez‐Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant 2006;6:1774‐1780. [DOI] [PubMed] [Google Scholar]
- 10. Oldhafer F, Bock M, Falk CS, Vondran FW. Immunological aspects of liver cell transplantation. World J Transplant 2016;6:42‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bumgardner GL, Li J, Prologo JD, Heininger M, Orosz CG. Patterns of immune responses evoked by allogeneic hepatocytes: evidence for independent co‐dominant roles for CD4+ and CD8+ T‐cell responses in acute rejection. Transplantation 1999;68:555‐562. [DOI] [PubMed] [Google Scholar]
- 12. Bumgardner GL, Gao D, Li J, Baskin JH, Heininger M, Orosz CG. Rejection responses to allogeneic hepatocytes by reconstituted SCID mice, CD4, KO, and CD8 KO mice. Transplantation 2000;70:1771‐1780. [DOI] [PubMed] [Google Scholar]
- 13. Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell‐mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl 2008;14:688‐694. [DOI] [PubMed] [Google Scholar]
- 14. Lee CA, Dhawan A, Smith RA, Mitry RR, Fitzpatrick E. Instant blood‐mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant 2016;25:1227‐1236. [DOI] [PubMed] [Google Scholar]
- 15. Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, Perkins JD. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3‐expressing CD25+CD4+ regulatory T cells. Am J Transplant 2008;8:1639‐1651. [DOI] [PubMed] [Google Scholar]
- 16. Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T‐cell suppression ‐ a diverse arsenal for a moving target. Immunology 2008;124:13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T‐cell‐based cell therapy in living donor liver transplantation. Hepatology 2016;64:632‐643. [DOI] [PubMed] [Google Scholar]
- 18. Kleine M, Riemer M, Krech T, DeTemple D, Jäger MD, Lehner F, et al. Explanted diseased livers ‐ a possible source of metabolic competent primary human hepatocytes. PloS One 2014;9:e101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vondran FW, Timrott K, Tross J, Kollrich S, Schwarz A, Lehner F, et al. Impact of basiliximab on regulatory T‐cells early after kidney transplantation: down‐regulation of CD25 by receptor modulation. Transpl Int 2010;23:514‐523. [DOI] [PubMed] [Google Scholar]
- 20. Vondran FW, Timrott K, Tross J, Kollrich S, Gwinner W, Lehner F, et al. Association of high anti‐donor alloreactivity and low frequency of FoxP3‐expressing cells prior to kidney transplantation with acute graft rejection. Clin Transplant 2011;25:905‐914. [DOI] [PubMed] [Google Scholar]
- 21. Wallace PK, Tario JD Jr, Fisher JL, Wallace SS, Ernstoff MS, Muirhead KA. Tracking antigen‐driven responses by flow cytometry: monitoring proliferation by dye dilution. Cytometry A 2008;73:1019‐1034. [DOI] [PubMed] [Google Scholar]
- 22. Bumgardner GL, Matas AJ, Chen S, Cahill D, Cunningham TR, Payne WD, et al. Comparison of in vivo and in vitro immune response to purified hepatocytes. Transplantation 1990;49:429‐436. [DOI] [PubMed] [Google Scholar]
- 23. Giroux M, Schmidt M, Descoteaux A. IFN‐gamma‐induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor‐1 is regulated by protein kinase C‐alpha. J Immunol 2003;171:4187‐4194. [DOI] [PubMed] [Google Scholar]
- 24. Romieu‐Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN‐gamma, TGF‐beta, and cell density. J Immunol 2007;179:1549‐1558. [DOI] [PubMed] [Google Scholar]
- 25. Shulzhenko N, Morgun A, Franco M, Souza MM, Almeida DR, Diniz RV, et al. Expression of CD40 ligand, interferon‐gamma and Fas ligand genes in endomyocardial biopsies of human cardiac allografts: correlation with acute rejection. Braz J Med Biol Res 2001;34:779‐784. [DOI] [PubMed] [Google Scholar]
- 26. Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin‐10. Front Immunol 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao D, Li J, Orosz CG, Bumgardner GL. Different costimulation signals used by CD4(+) and CD8(+) cells that independently initiate rejection of allogenic hepatocytes in mice. Hepatology 2000;32:1018‐1028. [DOI] [PubMed] [Google Scholar]
- 28. Holz LE, Warren A, Le Couteur DG, Bowen DG, Bertolino P. CD8+ T cell tolerance following antigen recognition on hepatocytes. J Autoimmun 2010;34:15‐22. [DOI] [PubMed] [Google Scholar]
- 29. Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology 1988;8:449‐454. [DOI] [PubMed] [Google Scholar]
- 30. Lobo‐Yeo A, Senaldi G, Portmann B, Mowat AP, Mieli‐Vergani G, Vergani D. Class I and class II major histocompatibility complex antigen expression on hepatocytes: a study in children with liver disease. Hepatology 1990;12:224‐232. [DOI] [PubMed] [Google Scholar]
- 31. Gordon RD, Fung JJ, Iwatsuki S, Duquesnoy RJ, Starzl TE. Immunological factors influencing liver graft survival. Gastroenterol Clin North Am 1988;17:53‐59. [PubMed] [Google Scholar]
- 32. Sanfilippo F. The influence of HLA and ABO antigens on graft rejection and survival. Clin Lab Med 1991;11:537‐550. [PubMed] [Google Scholar]
- 33. Nikaein A, Backman L, Jennings L, Levy MF, Goldstein R, Gonwa T, et al. HLA compatibility and liver transplant outcome. improved patient survival by HLA and cross‐matching. Transplantation 1994;58:786‐792. [PubMed] [Google Scholar]
- 34. Adams DH, Sanchez‐Fueyo A, Samuel D. From immunosuppression to tolerance. J Hepatol 2015;62(suppl):S170‐S185. [DOI] [PubMed] [Google Scholar]
- 35. Olszewski WL, Hiwot H, Interewicz B, Rudowska A, Szyper E, Mecner B. Hepatocyte transplantation‐‐in vitro cytotoxic reaction of autologous granulocytes and mononuclears to isolated hepatocytes. Ann Transplant 1999;4:11‐16. [PubMed] [Google Scholar]
- 36. Obata F, Yoshida K, Ohkubo M, Ikeda Y, Taoka Y, Takeuchi Y, et al. Contribution of CD4+ and CD8+ T cells and interferon‐gamma to the progress of chronic rejection of kidney allografts: the Th1 response mediates both acute and chronic rejection. Transpl Immunol 2005;14:21‐25. [DOI] [PubMed] [Google Scholar]
- 37. Horne PH, Lunsford KE, Eiring AM, Wang Y, Gao D, Bumgardner GL. CD4+ T‐cell‐dependent immune damage of liver parenchymal cells is mediated by alloantibody. Transplantation 2005;80:514‐521. [DOI] [PubMed] [Google Scholar]
- 38. Zimmerer JM, Pham TA, Wright CL, Tobin KJ, Sanghavi PB, Elzein SM, et al. Alloprimed CD8(+) T cells regulate alloantibody and eliminate alloprimed B cells through perforin‐ and FasL‐dependent mechanisms. Am J Transplant 2014;14:295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zimmerer JM, Bumgardner GL. Hepatocyte transplantation and humoral alloimmunity. Am J Transplant 2016;16:1940. [DOI] [PubMed] [Google Scholar]
- 40. Jorns C, Nowak G, Nemeth A, Zemack H, Mörk LM, Johansson H, et al. De novo donor‐specific hla antibody formation in two patients with Crigler‐Najjar syndrome type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplant 2016;16:1021‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Posselt AM, Vincenti F, Bedolli M, Lantz M, Roberts JP, Hirose R. CD69 expression on peripheral CD8 T cells correlates with acute rejection in renal transplant recipients. Transplantation 2003;76:190‐195. [DOI] [PubMed] [Google Scholar]
- 42. Lunsford KE, Jayanshankar K, Eiring AM, Horne PH, Koester MA, Gao D, Bumgardner GL. Alloreactive (CD4‐Independent) CD8+ T cells jeopardize long‐term survival of intrahepatic islet allografts. Am J Transplant 2008;8:1113‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bumgardner GL, Li J, Heininger M, Orosz CG. Costimulation pathways in host immune responses to allogeneic hepatocytes. Transplantation 1998;66:1841‐1845. [DOI] [PubMed] [Google Scholar]
- 44. Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas‐mediated hepatocyte death during allograft rejection. J Exp Med 1999;189:441‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ, et al. CD40‐CD40 ligand‐independent activation of CD8+ T cells can trigger allograft rejection. J Immunol 2000;165:1111‐1118. [DOI] [PubMed] [Google Scholar]
- 46. Tilg H. Cytokines and liver diseases. Can J Gastroenterol 2001;15:661‐668. [DOI] [PubMed] [Google Scholar]
- 47. Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti‐fibrotic functions of interleukin‐22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 2013;28(suppl 1):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guabiraba R, Besnard AG, Marques RE, Maillet I, Fagundes CT, Conceição TM, et al. IL‐22 modulates IL‐17A production and controls inflammation and tissue damage in experimental dengue infection. Eur J Immunol 2013;43:1529‐1544. [DOI] [PubMed] [Google Scholar]
- 49. Rau SJ, Hildt E, Himmelsbach K, Thimme R, Wakita T, Blum HE, Fischer R. CD40 inhibits replication of hepatitis C virus in primary human hepatocytes by c‐Jun N terminal kinase activation independent from the interferon pathway. Hepatology 2013;57:23‐36. [DOI] [PubMed] [Google Scholar]
- 50. Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Activation of CD40 with platelet derived CD154 promotes reactive oxygen species dependent death of human hepatocytes during hypoxia and reoxygenation. PloS One 2012;7:e30867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dikopoulos N, Wegenka U, Kröger A, Hauser H, Schirmbeck R, Reimann J. Recently primed CD8+ T cells entering the liver induce hepatocytes to interact with naive CD8+ T cells in the mouse. Hepatology 2004;39:1256‐1266. [DOI] [PubMed] [Google Scholar]
- 52. Sembeil R, Sanhadji K, Vivier G, Chargui J, Touraine JL. Prolonged survival of mouse skin allografts after transplantation of fetal liver cells transduced with hIL‐10 gene. Transpl Immunol 2004;13:1‐8. [DOI] [PubMed] [Google Scholar]
- 53. Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL‐35‐ and IL‐10‐dependent manner. J Immunol 2009;182:6121‐6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supplementary Figure 1: Gating strategy for flow‐cytometric detection of proliferative responses in mixed lymphocyte cultures and mixed lymphocyte hepatocyte cultures.
Representative examples for gating strategy for flow‐cytometric determination of cell proliferation on day 10 of culture for conventional mixed lymphocyte cultures (MLC) and mixed lymphocyte hepatocyte cultures (MLHC). (A) For MLC the following three different experimental groups are compared: (i) PKH‐26 stained PBMC alone, (ii) PKH‐26 stained PBMC co‐cultured with irradiated stimulator PBMC marked by CellVue and (iii) PKH‐26 stained PBMC co‐cultured with irradiated stimulator PBMC and expanded polyclonal Tregs, both marked by CellVue. (B) For MLHC the following three different experimental groups are compared: (i) PKH‐26 stained PBMC alone, (ii) PKH‐26 stained PBMC co‐cultured with adherent primary human hepatocytes (PHH) and (iii) PKH‐26 stained PBMC co‐cultured with adherent PHH and expanded polyclonal Treg marked by CellVue.
PBMC are detected in forward and side scatters (gate P1) (left panels, respectively). In a next step only CellVue negative cells were considered for further analysis (gate P2) (middle panels, respectively). Finally, proliferative responses of PKH‐26 stained cells were detected as loss of intensity of intracellular staining (right panels, respectively), with possibility for co‐staining for CD4 or CD8 via FL‐1 (not shown). Subpopulations were assessed as percentage of whole lymphocyte population.
Supplementary Figure 2: Evaluation of the effect of HLA mismatch on the alloproliferative response induced by primary human hepatocytes in vitro.
Mixed lymphocyte hepatocyte cultures were set up as described above but with some modifications: PBMC and liver donors were typed for HLA expression (HLA A/B/DR) according to ASHI standards. Furthermore, typed PBMC were frozen in supplemented RPMI 1640 (20% FCS, 10% DMSO) and stored in liquid nitrogen until PHH from typed donors were available to perform MLHC. MLHC were set up with PKH‐26 labelled PBMC from 11 to 12 donors simultaneously and proliferative responses were detected on day 10 by flow‐cytometry as reported above.
Bar chart summarising proliferative alloresponses in accordance to the total number of HLA mismatches and number of HLA‐DR mismatches between PHH from 3 different donors and appropriated PBMC donors used for MLHC, respectively (n = number of cases for the specified group of mismatches present; data presented as Mean ± SEM).
Supplementary Figure 3: Titration of anti‐interferon‐gamma antibody to block hepatocyte‐induced alloresponses in MLHC.
(A) Representative titration curve depicting the effect of anti‐IFNγ antibody treatment to block upregulation of MHC class II expression (HLA‐DR) on PHH during MLHC determined by flow‐cytometry on day 10 of culture. (B) Representative titration curve depicting the effect of anti‐IFNγ treatment to block hepatocyte‐induced proliferative alloresponse determined on day 10 of MLHC (presented as CD4+ proliferation of responder PBMC).
Supplementary Figure 4: Evaluation of the role of interleukin‐10 for Treg‐mediated suppression of hepatocyte‐induced alloproliferation in MLHC.
Bar chart summarising proliferative alloresponses with/without additional supplementation of 1μg/ml anti‐interleukin‐10 (IL‐10) antibodies in MLHC with/without co‐culture of Treg and/or use of trans‐well inlets (n = 3, represented as Mean ± SEM; n.s. = not significant).


