Abstract
Human adenoviruses (HAdVs) cause severe inflammatory respiratory infections, but previous epidemiological studies lacked analysis of the characteristics of the inflammation. Consecutive patients <13 years old with acute febrile illness during a 2‐year period were tested. HAdV strains were isolated from nasopharyngeal swabs, and molecular identification was performed by hexon, fiber, and species‐specific PCR methods. Blood inflammatory markers, including the white blood cell (WBC) count, CRP, and 29 cytokines, were measured. A total of 187 patients were enrolled, and HAdV types were identified from 175 patients (93.5%). Species C (types 2, 1, 5, and 6, in order of frequency) was most common at 37.1%, followed by B (type 3) at 30.9% and E (type 4) at 26.9%. Species C was detected predominantly in 1‐year‐old, whereas B and E were in older ages. Species C and B had seasonal circulation patterns, but E was found in only one season during the 2‐year study period. The WBC count was highest in patients with species C. Eleven of the 29 tested serum cytokines were detected. Seven kinds, including G‐CSF, IL‐6, and TNF‐α, were elevated in species C infections, whereas IL‐10 was lowest in species C. Species differences in inflammatory responses, especially regarding serum cytokines were described in common pediatric HAdV infections. Species C causes the strongest inflammatory responses in young children.
Keywords: adenovirus, cytokine/chemokine, epidemiology, inflammation, seasonal incidence
1. INTRODUCTION
Family Adenoviridae, genus Adenovirus, human adenovirus types (HAdVs) are known to cause strong inflammatory responses, such as elevation of the white blood cell (WBC) count and C‐reactive protein (CRP), which sometimes leads to overuse of antibiotics.1 Differences in the immune responses to the various species of HAdV have not been well studied. Links between epidemiology and immune responses may provide clues to understanding the mechanisms underlying HAdVs' circulation patterns.
Although HAdVs are one of the most common pathogens in young children, accounting for 10‐29% of upper respiratory infections,2, 3 the diseases they cause are believed to be mild and self‐limiting.4 However, HAdVs sometimes cause outbreaks of severe disease not only in children,5, 6, 7, 8 but also in adults.9 Understanding the circulation patterns of and inflammatory responses to HAdV in healthy children might have epidemiological significance since virus circulating in the normal population may serve as a reservoir10 for outbreaks. There have been, however, no reports that investigated community‐based epidemiology, including inflammatory testing for common HAdV respiratory disease in children.
To address the above issue, we conducted a prospective study aimed at clarifying the circulation patterns and clinical/laboratory features of respiratory HAdV infections in children at a primary care clinic in a small community. Their inflammatory responses were studied in detail, including production of cytokines.
2. METHODS
2.1. Study design and participants
A prospective cross‐sectional study was performed. The target population was pediatric outpatients <13 years old with HAdV respiratory infection confirmed by a rapid test for adenovirus antigen (ImmunoAce Adeno, Tauns Laboratories Co., Shizuoka, Japan). To avoid selection bias, the eligible patients were surveyed consecutively at a pediatric clinic that looked after the health care of most of the children in a small community. Adenovirus rapid tests were routinely performed using an immunochromatography kit (ImmunoAce Adeno) with a nasopharyngeal swab for patients with febrile respiratory illness. Laboratory tests were also routinely performed to measure the WBC count and CRP. For this study, we obtained all nasopharyngeal swabs that gave a positive rapid test result as well as the residual sera from the routine blood testing performed during the study period, that is October 1st, 2013 to September 30th, 2015. Consecutive nasopharyngeal samples from patients with laboratory‐defined common HAdV respiratory infections were thus surveyed for 2 years. Nasopharyngeal swabs were placed in 5‐mL tubes containing 1‐2 mL of normal saline and frozen at −80°C until use. The residual sera were similarly stored.
2.2. Ethical approval
The study protocol was approved by the Ethics Committee of Mie National Hospital (#25‐16). Informed consent was obtained from all the parents or caregivers of the enrolled patients.
2.3. Adenovirus detection by the immunochromatography kit
The manufacturer of the kit states that it detects HAdV‐1, 2, 3, 4, 5, 6, 7, 8, 11, 19, and 37. We confirmed that it also detected HAdV‐14, 16, 21, 34, 35, and 57 from clinical isolates with similar sensitivity at 105 to 107 copies, as for the other types. Thus, the kit covered all respiratory HAdV types except for HAdV‐50, which is very rare and has never been detected in Japan.
2.4. Adenovirus culture
To isolate infective HAdV from the nasopharyngeal samples, we performed adenovirus culture in a respiratory epithelial cell line, A549 (ATCC CCL‐185). Each frozen tube of saline extract was thawed. The contents were filtered using a disposable syringe filter having a pore size of 0.20 µm (Asahi Glass Co., Ltd., Tokyo, Japan). Each filtrate was tested for presence of virus in the A549 cell line. A549 cells were cultured in minimum essential medium (MEM) supplemented with 10% inactivated fetal bovine serum (FBS) in 50‐mL Falcon® tissue culture flasks (Corning, Corning, NY) and grown to 80% confluence. The culture medium was then removed, and 1 mL of each filtrate was inoculated into a flask. After 1 h of incubation, the inoculated sample solution was removed, MEM containing 1‐2% inactivated FBS was added to the flask, and culture was continued at 37°C in 5% CO2 for 7‐21 days.11 The cells were observed daily with an inverted microscope for signs of cytopathic effects (CPE), and adenovirus culture was considered positive when characteristic, round, “cluster of grapes” CPE was observed for over ≥25% of the grown area.12 The cells and medium were then collected and freeze‐thawed at least three times. After centrifugation at 1300 rpm for 10 min, the supernatant was collected and stored at −80°C until PCR assay.
2.5. Identification of adenovirus type and species
Molecular identification of isolated HAdVs was performed using multiple PCR methods.13, 14, 15, 16 The primers for hexon, fiber, and species PCR are listed in Tables 1, 2, and 3, respectively. The PCR products in hexon‐PCR were sequenced directly using a kit (BigDye Terminator Cycle Sequencing Kit; Applied Biosystems, Foster City, CA) and a genetic analyzer (3100‐Avant; Applied Biosystems). The nucleotide sequences of reference strains of each HAdV type were obtained from the GenBank nucleotide database.
Table 1.
Primers for hexon PCR
| Primer name | Sequence (5′ → 3′) | Target | Amplicon size (bp) |
|---|---|---|---|
| Adhex‐GT3F Adhex‐GT2R | CSGGNCAGGAYGCYTCGGRGTA CACCCATGTTRCCWGTNCTGTT | HAdV hexon | 1029 (1st PCR) |
| Adhex‐GT2F Adhex‐GT1R | AAYAARTTTAGRAAYCCCAC TTRTCYCTRAADSCAATGTARTT | HAdV hexon | 905 (2nd PCR) |
Table 2.
primers for fiber PCR
| Primer name | Sequence (5‘ → 3‘) | Target | Amplicon size (bp) |
|---|---|---|---|
| AdCF | TGCTTGCGCTHAAAATGGGCA | HAdV C fiber | a |
| Ad1R | CGAGTATAAGACGCCTATTTACA | HAdV‐1 fiber | 630 |
| Ad2R | CGCTAAGAGCGCCGCTAGTA | HAdV‐2 fiber | 204 |
| Ad5R | ATGCAAAGGAGCCCCGTAC | HAdV‐5 fiber | 455 |
| Ad6R | CTTGCAGTCTTTATCTGAAGCA | HAdV‐6 fiber | 929 |
| AdnB1 | TACCCYTATGAAGATGAAAGC | HAdV B fiber | a |
| Adn3t | TGTTTTTAAATAAGGTGTTAACG | HAdV‐3 fiber | 517 |
| Adn7t | GCCATTATTTGACAGTTGGCAGT | HAdV‐7 fiber | 404 |
| Adn4F | ACCAGTAGTACAGAAACAGGAG | HAdV‐4 fiber | a |
| Adn4t | CTTGAGCACTGCTTACTGTGCC | HAdV‐4 fiber | 295 |
| Ad57F1 | ATCCGCGCCCCTTAAAGTTGT | HAdV‐57 fiber | 808 a |
Forward primer reference 19, 20
Table 3.
Primers for species PCR
| Primer name | Sequence (5‘→3‘) | Target | Amplicon size (bp) |
|---|---|---|---|
| AdA1 AdA2 | GCTGAAGAAMCWGAAGAAAATGA CRTTTGGTCTAGGGTAAGCAC | HAdV A fiber | 1444‐1537 |
| AdB1 AdB2 | TSTACCCYTATGAAGATGAAAGC GGATAAGCTGTAGTRCTKGGCAT | HAdV B fiber | 670‐772 |
| AdC1 AdC2 | TATTCAGCATCACCTCCTTTCC AAGCTATGTGGTGGTGGGGC | HAdV C fiber | 1988‐2000 |
| AdD1 AdD2 | GATGTCAAATTCCTGGTCCAC TACCCGTGCTGGTGTAAAAATC | HAdV D fiber | 1205‐1221 |
| AdE1 AdE2 | TCCCTACGATGCAGACAACG AGTGCCATCTATGCTATCTCC | HAdV E fiber | 967 |
| AdF1 AdF2 | ACTTAATGCTGACACGGGCAC TAATGTTTGTGTTACTCCGCTC | HAdV F fiber | 541‐586 |
2.6. Measurement of serum cytokine concentrations
A MILLIPLEX MAP Human Cytokine/Chemokine Kit (Millipore, Billerica, MA) was used on a Luminex 100™ analyzer (Luminex, Tokyo, Japan) to measure the serum levels of 29 cytokines: interferon α2 (IFN‐α2), IFN‐γ, interleukin 2 (IL‐2), IL‐3, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐10, IL‐12p40, IL‐12p70, IL‐13, IL‐15, IL‐17, IFN‐γ‐inducible protein 10 (IP‐10), tumor necrosis factor α (TNF‐α), TNF‐β, IL‐1 receptor antagonist (IL‐1ra), IL‐1α, IL‐1β, eotaxin, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), granulocyte‐colony stimulating factor (G‐CSF), granulocyte‐macrophage colony stimulating factor (GM‐CSF), monocyte chemoattractant protein‐1 (MCP‐1), macrophage inflammatory protein 1α (MIP‐1α), and MIP‐1β.
2.7. Statistical analysis
Descriptive statistics were generated. Continuous variables were presented as mean values with standard deviations (SDs), and categorical variables were presented as numbers and percentages. Comparisons between groups were analyzed using one‐way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA). P < 0.05 were considered significant.
3. RESULTS
3.1. Frequency of the isolated HAdVs
Within the 2‐year study period, 187 children were enrolled, and 177 (94.7%) viral strains were isolated. The HAdV species and types were identified for 175 of the 177 isolates. They included three species and six types, that is species B (type 3), species C (types 2, 1, 5, and 6, in the order of frequency), and species E (type 4). Species C was the most common (37.1%), followed by species B (30.9%) and species E (26.9%) (Figure 1). Multiple types/species were co‐detected in 13 samples (5.1%) (Supplementary Table S1).
Figure 1.
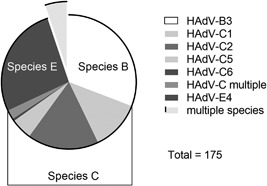
Frequency of species and types of isolated HAdVs. Of 177 virus isolates, HAdV species and types were identified for 175 samples. Identified types are listed in the right column and the pie chart indicates proportion of the types and species
3.2. Temporal distribution of the isolated HAdVs
The time trends of virus detection by species (Figure 2A‐C) and types (Supplementary Table S2) during the 2‐year study period showed that HAdV circulated from early spring to early fall, less frequently in winter. Multiple types/species circulated concurrently, but the circulation pattern appeared to differ with the species. The incidence of species B was high in summer and early fall (Figure 2A), whereas species C circulated year‐round, but more in late spring and summer, less in winter (Figure 2B). In contrast to the yearly circulation patterns seen for species B and C, species E circulated in only one season, that is early spring in 2013, but in only one case each in 2014 and 2015 (Figure 2C).
Figure 2.
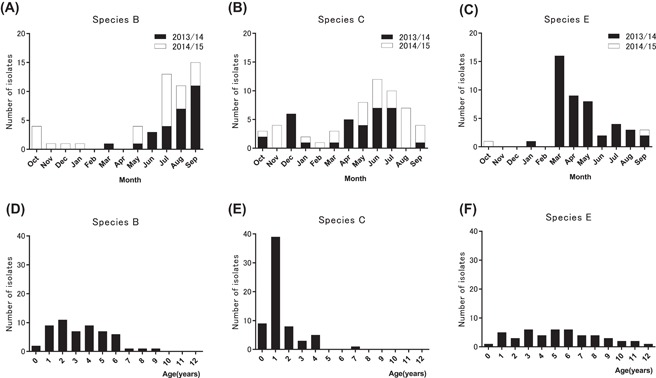
Temporal and age distribution of the isolated HAdVs during the 2‐year study period. Sample collection in this study started in October 2013 and ended in September 2015. Number of isolates identified in each month for species (B), species (C), and species (E) are shown in (2A), (2B), and (2C), respectively. Data for the first year (from October 2013 to September 2014) are depicted as solid columns and the second year (from October 2014 to September 2015) as open columns. Number of isolates by age for species (B), species (C) and species E are depicted in (2D), (2E) and (2F), respectively
3.3. Gender and age distributions of the isolated HAdVs
The male/female ratios of the patients infected with species B, C, and E were 27/27, 37/24, and 25/23, respectively, indicating no gender preference by species (chi‐square test, P = 0.48). The mean age of all patients was 3.2 ± 2.7 y. Patients infected with species C were significantly younger than those infected with species B and E: 1.4 ± 1.3, 3.3 ± 2.1, and 5.3 ± 3.0 y, respectively (one way ANOVA; P < 0.0001, followed by Tukey's multiple comparisons test; P < 0.0001). It is of note that the age distribution of patients infected with species C showed a sharp peak at age 1 (Figure 2E), in contrast to the broad age distributions of patients infected with species B (0‐9 years) and species E (0‐12 years) (Figures 2D and 2F). Supplementary Table S3 shows the age distributions of the patients with each type.
3.4. Clinical and laboratory findings
The clinical and laboratory data were compared among the species. The recorded clinical symptoms were similar among all the patients, and none of the children were severe enough to be hospitalized. The mean body temperature (BT) of the subjects was 39.2°C, with no difference in BT among the species (Table 4).
Table 4.
Body temperature, white blood cell count and CRP of patients infected with each species
| Species B | Species C | Species E | P value* | |
|---|---|---|---|---|
| Body temperature (°C) a | 39.2 ± 0.5 (38.0‐40.2) | 39.2 ± 0.6 (37.8‐40.4) | 39.4 ± 0.6 (38.2‐40.8) | 0.2782 |
| WBC (/µL) a | 9975 ± 3163 (4200‐16500) | 14219 ± 4707** (7900‐28300) | 10024 ± 2884 (4400‐18000) | <0.0001 |
| CRP (mg/dL) a | 2.9 ± 2.3 (0.2‐10.3) | 3.7 ± 3.3 (0.2‐12.4) | 4.3 ± 2.9 (0.7‐12.1) | 0.134 |
Mean ± SD (range), *ANOVA, **Tukey's multiple comparisons test P < 0.0001
The WBC count and CRP concentration were determined for 148 (89.7%) and 150 (90.9%) of the children, respectively. The WBC count in patients infected with species C was significantly higher than in those with species B and E (Table 4, Supplementary Figure S1A). Because the normal WBC count range is higher in infancy, we performed age‐matched comparison for subgroups of patients less than 36 months of age (Supplementary Figure S1B) and at 36 months or more (Supplementary Figure S1C). The WBC count with species C was still higher than the counts with species B and E in both subgroups. Since the WBC count changes rapidly during the course of infection, the sampling date may have influenced the WBC data. However, there was no significant difference in the mean number of days from onset to sampling among the species (data not shown). There was no significant difference in the CRP concentration among the species (Table 4), even when age‐matched comparison was performed for the subgroups of less than 36 months of age and 36 months or older (data not shown).
3.5. Serum cytokine concentrations
The residual serum was obtained from 139 patients (74.3%), and 29 cytokines were measured. Eleven kinds of cytokines were detected in more than 70% of the samples (Table 5). Among them, seven cytokines, namely, G‐CSF, IFN‐γ, IL‐6, TNF‐α, MCP‐1, MIP‐1β, and eotaxin, were significantly elevated in patients with species C (Figure 3A‐G), whereas IL‐10 was significantly lower in those with species C (Figure 3H), than the other species. The serum levels of three cytokines, that is IL‐1ra, IL‐8, and IP‐10, did not differ among the species groups (Supplementary Figure S2).
Table 5.
Number (percentage) of patients with detected serum cytokines (>3 pg/mL)
| Infected HAdV species | Species | Total | ||
|---|---|---|---|---|
| Cytokine | B | C | E | |
| n = 54 | n = 65 | n = 47 | n = 166 | |
| EGF | 27 (50) | 39 (60) | 27 (57.4) | 93 (56) |
| Eotaxin a | 45 (83.3) | 54 (83.1) | 35 (74.5) | 134 (80.7) |
| G‐CSF a | 46 (85.2) | 53 (81.5) | 39 (83) | 138 (83.1) |
| GM‐CSF | 13 (24.1) | 28 (43.1) | 10 (21.3) | 51 (30.7) |
| IFN‐α2 | 9 (16.7) | 18 (27.7) | 3 (6.4) | 30 (18.1) |
| IFN‐γ a | 47 (87) | 54 (83.1) | 34 (72.3) | 135 (81.3) |
| IL‐10 a | 45 (83.3) | 51 (78.5) | 34 (72.3) | 130 (78.3) |
| IL‐12p40 | 15 (27.8) | 33 (50.8) | 6 (12.8) | 54 (32.5) |
| IL‐12p70 | 6 (11.1) | 10 (15.4) | 5 (10.6) | 21 (12.7) |
| IL‐13 | 4 (7.4) | 9 (13.8) | 6 (12.8) | 19 (11.4) |
| IL‐15 | 5 (9.3) | 10 (15.4) | 6 (12.8) | 21 (12.7) |
| IL‐17 | 11 (20.4) | 14 (21.5) | 9 (19.1) | 34 (20.5) |
| IL‐1ra a | 46 (85.2) | 54 (83.1) | 38 (80.9) | 138 (83.1) |
| IL‐1α | 13 (24.1) | 21 (32.3) | 9 (19.1) | 43 (25.9) |
| IL‐1β | 1 (1.9) | 5 (7.7) | 3 (6.4) | 9 (5.4) |
| IL‐2 | 1 (1.9) | 5 (7.7) | 2 (4.3) | 8 (4.8) |
| IL‐3 | 0 (0) | 2 (3.1) | 0 (0) | 2 (1.2) |
| IL‐4 | 10 (18.5) | 14 (21.5) | 10 (21.3) | 34 (20.5) |
| IL‐5 | 1 (1.9) | 2 (3.1) | 0 (0) | 3 (1.8) |
| IL‐6 a | 42 (77.8) | 53 (81.5) | 39 (83) | 134 (80.7) |
| IL‐7 | 13 (24.1) | 27 (41.5) | 7 (14.9) | 47 (28.3) |
| IL‐8 a | 45 (83.3) | 54 (83.1) | 35 (74.5) | 134 (80.7) |
| IP‐10 a | 47 (87) | 54 (83.1) | 40 (85.1) | 141 (84.9) |
| MCP‐1 a | 47 (87) | 52 (80) | 36 (76.6) | 135 (81.3) |
| MIP‐1α | 9 (16.7) | 21 (32.3) | 2 (4.3) | 32 (19.3) |
| MIP‐1β a | 44 (81.5) | 52 (80) | 33 (70.2) | 129 (77.7) |
| TNF‐α a | 45 (83.3) | 52 (80) | 38 (80.9) | 135 (81.3) |
| TNF‐β | 5 (9.3) | 9 (13.8) | 5 (10.6) | 19 (11.4) |
| VEGF | 17 (31.5) | 34 (52.3) | 12 (25.5) | 63 (38) |
N, number of patients tested for serum cytokines.
Cytokine was detected in more than 70% of patients.
Figure 3.
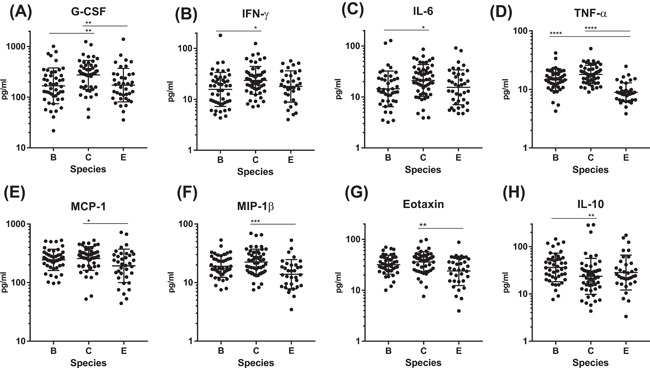
Serum cytokine/chemokine levels by HAdV species. Of 11 cytokines detected in more than 70% of the samples, 7 cytokines (A‐G), were significantly elevated and IL‐10 (H) was significantly lower in patients with species C than those with the other species. Dunn's multiple comparisons test; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001
4. DISCUSSION
This study aimed to investigate the circulation patterns and clinical/laboratory features of respiratory HAdV infections, particularly the inflammatory responses to HAdV, which have not been reported in previous studies, especially in regard to serum cytokines.
We determined the epidemiology and laboratory characteristics of HAdVs causing common respiratory infections in otherwise healthy children without any underlying disease. We found that species C (types 2, 1, 6, and 5, by order of frequency) was most common at 37.1%, followed by species B (type 3) at 30.9% and species E (type 4) at 26.9 %. Species C predominated at young age, especially at 1 year. It induced stronger inflammatory responses, as evidenced by higher WBC, higher proinflammatory cytokines such as G‐CSF and TNF‐α, and lower anti‐inflammatory IL‐10 in the serum, compared with the other two species. In contrast, species B and E were widely distributed up to age 9 years and 12 years, respectively, and caused weaker inflammatory responses compared with species C.
There have many reports on outbreaks and severe infections caused by HAdV,8, 9, 17, 18, 19, 20 but few have investigated the epidemiology of common respiratory HAdVs that cause mild disease and circulate regularly. We performed a systematic review of the epidemiology of HAdVs. We used “adenovirus”, “epidemiology“ and “children” as keywords to conduct a PubMed search of original research articles in English, published from 1957 to 2017. This search yielded a total of 1015 articles. We then excluded 303 studies dealing with gastroenteritis or conjunctivitis and 315 studies that dealt with patients with underlying conditions such as immunocompromised states and post‐transplantation. Next, we excluded 103 papers that studied severe HAdV infections and outbreak settings. We also excluded 231 studies of hospitalized children and lower respiratory adenovirus infections, and 45 studies of other viral infections. Finally, we selected 18 articles on the epidemiology of upper respiratory adenovirus infections in normal children, and the main findings are summarized in Supplementary Table S4. Briefly, the prevalent HAdV species were C and B. Species E occurred sporadically. Young children of <6 years of age were infected, and species C affected younger children than species B. Seasonality varied depending on the area of the globe, generally from spring to summer in temperate zones and in winter and spring in tropical zones. Our present results correspond well with those earlier epidemiological findings. In terms of inflammatory responses, only WBC and CRP had been compared between species B and C in previous reports.18, 21 Species differences in serum cytokine levels have not been studied.
An outbreak of HAdV‐4 was reported in military trainees, but not in young children.9 The reason why young, otherwise healthy, adults were susceptible to the virus was unclear. Here, we found that HAdV‐4 circulated in only one season during the 2‐year study period. In two other epidemiological studies in children, that type was identified only once during a 12‐year period.21, 22 These results suggest that HAdV‐4 circulates only in a restricted time period, meaning that it induces little herd immunity in children and leaves young adults “susceptible”. In contrast, we and others4, 23, 24, 25 found that species C circulated every year and infected only young children, not older children (Supplementary Table S4). This suggests that species C readily generates herd immunity in children, and thus older children, possibly even young adults, may be protected.
Our current findings that the WBC count and several inflammatory cytokines were elevated in patients infected with species C may be related to the above‐mentioned phenomenon. Higher inflammatory markers may indicate higher innate immune responses leading to protective acquired immunity.26 However, the species showed no differences in CRP. This may be explained by the differential cytokine responses found here. That is, the patients infected with species C showed significantly elevated levels of G‐CSF, a hematopoietic cytokine for granulocytes; TNF‐α and IL‐6, inflammatory cytokines that activate vascular endothelium to recruit leukocytes; and MCP‐1, MIP‐1β, and eotaxin, chemokines for monocytes and eosinophils. Those responses may explain the higher WBC count seen with species C. Meanwhile, among the cytokines that induce CRP production in the liver, only IL‐6, not IL‐1β, was elevated with species C.
We believe several points characterize our study. First, we identified HAdV types by multiple methods, that is screening with an immunochromatography kit, virus culture and three PCR methods (fiber, hexon, and species). Especially, we confirmed that the immunochromatography kit (ImmunoAce Adeno) detected all the respiratory HAdVs with sufficient sensitivity except for HAdV‐50, which is very rare and has never been detected in Japan. Second, we employed a prospective “near” population‐based method by surveying consecutive children diagnosed with febrile respiratory illnesses at a single pediatric clinic that looked after the health care of most children living in a small community. In addition, we employed laboratory‐defined criteria for inclusion of patients using the rapid ImmunoAce Adeno test. This serial inclusion based on definitive criteria minimized selection bias.
Some limitations should also be mentioned. The study population was recruited at only one clinic. A multi‐center population‐based study would better delineate the epidemiology. However, such a study might not be feasible due to the logistics of investigating the large number of patients contracting this very infectious disease. Thus, we intentionally used a single site that provided care to most of the children in the small community. We think that the present results accurately reflect the actual HAdV epidemic patterns in that community.
In conclusion, this study elucidated the temporal and age‐related epidemiology and inflammatory responses of HAdV respiratory infections in children. A detailed understanding of the epidemiology may help in planning future strategies for prevention of common, sometimes lethal, HAdV infections.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. White blood cell count by HAdV species in all subjects (A), patients less than 36 months of age (B) and patients at 36 monthsor more (C).
FIgure S2.Serum cytokine/chemokine levels by HAdV species.
Table S1. List of isolates co‐detected by different PCR methods
Table S2. Temporal distribution of viral types
Table S3. Viral types detected at each patient age
Table S4. Summary of findings for respiratory HAdV epidemiology in healthy children
ACKNOWLEDGMENTS
This research was supported by a grant‐in‐aid from the Japan Agency for Medical Research and Development (AMED) and a medical research grant from the Association of Pediatricians in Mie. The authors would like to thank Ms. Manami Negoro for her excellent technical assistance, and Dr. Toshiaki Ihara for his advice regarding the research and valuable comments on this study.
Nakamura H, Fujisawa T, Suga S, et al. Species differences in circulation and inflammatory responses in children with common respiratory adenovirus infections. J Med Virol. 2018;90: 873–880. https://doi.org/10.1002/jmv.25032
REFERENCES
- 1. Van Lierde S, Corbeel L, Eggermont E. Clinical and laboratory findings in children with adenovirus infections. Eur J Pediatr. 1989; 148:423–425. [DOI] [PubMed] [Google Scholar]
- 2. Kim JK, Jeon JS, Kim JW, Rheem I. Epidemiology of respiratory viral infection using multiplex rt‐PCR in Cheonan, Korea (2006‐2010). J Microbiol Biotechnol. 2013; 23:267–273. [DOI] [PubMed] [Google Scholar]
- 3. Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008; 46:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakamoto M, Yazaki N, Katsushima N, Mizuta K, Suzuki H, Numazaki Y. Longitudinal investigation of epidemiologic feature of adenovirus infections in acute respiratory illnesses among children in Yamagata, Japan (1986–1991). Tohoku J Exp Med. 1995; 175:185–193. [DOI] [PubMed] [Google Scholar]
- 5. Moura PO, Roberto AF, Hein N, et al. Molecular epidemiology of human adenovirus isolated from children hospitalized with acute respiratory infection in Sao Paulo. Brazil. J Med Virol. 2007; 79:174–181. [DOI] [PubMed] [Google Scholar]
- 6. Lee WJ, Jung HD, Cheong HM, Kim K. Molecular epidemiology of a post‐influenza pandemic outbreak of acute respiratory infections in Korea caused by human adenovirus type 3. J Med Virol. 2015; 87:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Zhou W, Zhao Y, et al. Molecular typing and epidemiology profiles of human adenovirus infection among paediatric patients with severe acute respiratory infection in China. PLoS ONE. 2015; 10:0123234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin YC, Lu PL, Lin KH, et al. Molecular Epidemiology and Phylogenetic Analysis of Human Adenovirus Caused an Outbreak in Taiwan during 2011. PLoS ONE. 2015; 10:e027377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barraza EM, Ludwig SL, Gaydos JC, Brundage JF. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J Infect Dis. 1999; 179:1531–1533. [DOI] [PubMed] [Google Scholar]
- 10. Ashford RW. When is a reservoir not a reservoir? Emerg Infect Dis. 2003; 9:1495–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enomoto M, Fujimoto T, Konagaya M, et al. Cultivation for 21 days should be considered to isolate respiratory adenoviruses from samples containing small numbers of adenoviral genomes. Jpn J Infect Dis. 2010; 63:338–341. [PubMed] [Google Scholar]
- 12. Ahluwalia GS, Scott‐Taylor TH, Klisko B, Hammond GW. Comparison of detection methods for adenovirus from enteric clinical specimens. Diagn Microbiol Infect Dis. 1994; 18:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adhikary AK, Inada T, Banik U, Numaga J, Okabe N. Identification of subgenus C adenoviruses by fiber‐based multiplex PCR. J Clin Microbiol. 2004; 42:670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banik U, Adhikary AK, Suzuki E, Inada T, Okabe N. Multiplex PCR assay for rapid identification of oculopathogenic adenoviruses by amplification of the fiber and hexon genes. J Clin Microbiol. 2005; 43:1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okada M, Ogawa T, Kubonoya H, Yoshizumi H, Shinozaki K. Detection and sequence‐based typing of human adenoviruses using sensitive universal primer sets for the hexon gene. Arch Virol. 2007; 152:1–9. [DOI] [PubMed] [Google Scholar]
- 16. Xu W, McDonough MC, Erdman DD. Species‐specific identification of human adenoviruses by a multiplex PCR assay. J Clin Microbiol. 2000; 38:4114–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell LS, Taylor B, Reimels W, Barrett FF, Devincenzo JP. Adenovirus 7a: a community‐acquired outbreak in a children's hospital. Pediatr Infect Dis J. 2000; 19:996–1000. [DOI] [PubMed] [Google Scholar]
- 18. Cheng CC, Huang LM, Kao CL, et al. Molecular and clinical characteristics of adenoviral infections in Taiwanese children in 2004–2005. Eur J Pediatr. 2008; 167:633–640. [DOI] [PubMed] [Google Scholar]
- 19. Lewis PF, Schmidt MA, Lu X, et al. A community‐based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis. 2009; 199:1427–1434. [DOI] [PubMed] [Google Scholar]
- 20. Ghanaiem H, Averbuch D, Koplewitz BZ, et al. An outbreak of adenovirus type 7 in a residential facility for severely disabled children. Pediatr Infect Dis J. 2011; 30:948–952. [DOI] [PubMed] [Google Scholar]
- 21. Wang YF, Shen FC, Wang SL, et al. Molecular epidemiology and clinical manifestations of adenovirus respiratory infections in taiwanese children. Medicine (Baltimore). 2016; 95:e3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang SL, Chi CY, Kuo PH, et al. High‐incidence of human adenoviral co‐infections in taiwan. PLoS ONE. 2013; 8:e75208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adrian T, Wigand R, Knocke KW, Schafer G, Grundmann M. Genome type analysis of adenoviruses: isolates from one year from the Hannover area. Arch Virol. 1989; 105:89–101. [DOI] [PubMed] [Google Scholar]
- 24. Moro MR, Bonville CA, Suryadevara M, et al. Clinical features, adenovirus types, and local production of inflammatory mediators in adenovirus infections. Pediatr Infect Dis J. 2009; 28:376–380. [DOI] [PubMed] [Google Scholar]
- 25. Rojas LJ, Jaramillo CA, Mojica MF, Escalante MP, Delgado P. Molecular typing of adenovirus circulating in a Colombian paediatric population with acute respiratory infection. Epidemiol Infect. 2012; 140:818–822. [DOI] [PubMed] [Google Scholar]
- 26. Zheng S, De BP, Choudhary S, et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003; 18:619–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. White blood cell count by HAdV species in all subjects (A), patients less than 36 months of age (B) and patients at 36 monthsor more (C).
FIgure S2.Serum cytokine/chemokine levels by HAdV species.
Table S1. List of isolates co‐detected by different PCR methods
Table S2. Temporal distribution of viral types
Table S3. Viral types detected at each patient age
Table S4. Summary of findings for respiratory HAdV epidemiology in healthy children


