Abstract
Background
EGALITY was a phase III confirmatory efficacy and safety study conducted in patients with plaque‐type psoriasis as a part of totality of evidence gathered during the development of GP2015, an etanercept biosimilar.
Objective
To demonstrate equivalent efficacy and comparable safety and immunogenicity of GP2015 and the etanercept originator product (ETN, Enbrel®) and evaluate effects of repeated switching between GP2015 and ETN. Results for efficacy, safety and immunogenicity during treatment period (TP) 2 (TP2) are presented pooling the two continued treatment arms (pooled continued) versus the two treatment arms with repeated switches (pooled switched).
Methods
Patients (n = 531) were randomized 1:1 to self‐administer GP2015 or ETN twice‐weekly subcutaneously during TP1. Patients with a ≥50% improvement in Psoriasis Area and Severity Index (PASI 50) at week 12 were re‐randomized for TP2 to continue the same treatment at once‐weekly dosing or to undergo three consecutive treatment switches between GP2015 and ETN until week 30. Patients continued the last‐assigned treatment during TP2, until week 52.
Results
Mean (standard deviation [SD]) PASI scores at baseline were similar in patients who underwent multiple switches compared to those with continued treatments during TP2. During TP2, PASI 50, PASI 75 and PASI 90 response rates, percent change from baseline in PASI scores and all other efficacy parameters were similar between the pooled switched and pooled continued treatment groups at all time points. The incidence of treatment‐emergent adverse events including injection site reactions was comparable between the pooled switched (36.7%) and pooled continued (34.9%) groups. None of the patients in either treatment group were positive for binding anti‐drug antibodies in TP2.
Conclusion
Treatment efficacy, safety and immunogenicity were similar between the pooled continued and pooled switched treatments during TP2, indicating that there are no effects in the short term on clinical data of multiple switches between GP2015 and ETN.
Introduction
Biosimilars are biological medicines that are highly similar to the originator product.1, 2, 3 Availability of biosimilars for biological products which go off patent is expected to decrease per‐patient treatment costs and provide greater patient access, contributing to the reduction in disability, morbidity and mortality associated with inflammatory diseases.4, 5
There are well‐defined regulatory guidelines in the United States (US) and the European Union (EU) on scientific considerations for demonstration of biosimilarity; the EU guidelines are considered the gold standard for authorization of biosimilar medicines.2, 3 From the perspective of interchangeability between an originator and a biosimilar, the US Food and Drug Administration (US FDA) has recently issued a draft guidance on considerations in demonstrating interchangeability of a biosimilar with a reference product (originator).6 In the EU, there is no separate designation of interchangeability, rather biosimilars can be viewed as interchangeable,7 and the decision is left to individual member states for framing the rules.1, 8, 9 Apart from the regulatory perspective, the evaluation of the effect of multiple switches between originator and biosimilar with regard to efficacy, safety and immunogenicity is of great interest to physicians and patients.
GP2015 (Erelzi™) is an etanercept biosimilar approved by the US FDA for treatment of rheumatoid arthritis and polyarticular juvenile idiopathic arthritis in patients aged ≥2 years, psoriatic arthritis, ankylosing spondylitis and plaque psoriasis. The EGALITY study was conducted as a part of the totality of the evidence to demonstrate biosimilarity of GP2015 to its reference, and contributed key confirmatory clinical data, in the sensitive indication of psoriasis. The purpose of the EGALITY study was to demonstrate equivalence in efficacy and to compare safety and immunogenicity of GP2015 and the etanercept originator product (ETN, Enbrel® [EU‐authorised]) in patients with moderate‐to‐severe chronic plaque‐type psoriasis, as well as to evaluate the effects of repeated switching between GP2015 and ETN on efficacy, overall safety and immunogenicity.
The main results of the EGALITY study and the efficacy and safety of GP2015 versus ETN treatment for up to 52 weeks were reported previously.10 The primary efficacy endpoint to show equivalence in PASI 75 response rates at week 12 was achieved. The innovative study design (Fig. 1), which included multiple switches between ETN and GP2015, allows the analysis of the effects of these switches compared to continued treatments with ETN and GP2015. Here, we describe the results of the multiple‐switch period.
Figure 1.
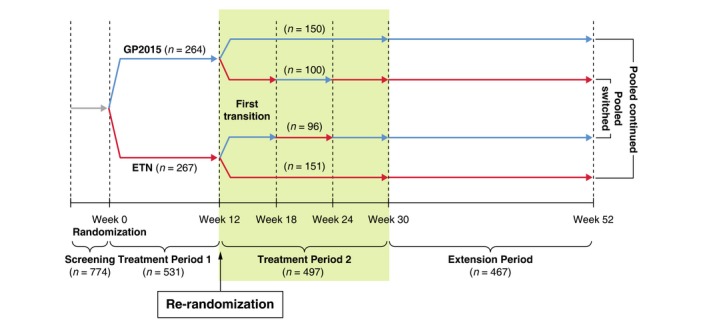
Study design. Patients who had achieved at least a 50% improvement in PASI 50 from baseline at week 12 were re‐randomized during treatment period 2 to either continue the same treatment on a once‐weekly dosing schedule, or to undergo a sequence of 3 treatment switches between GP2015 and ETN at 6‐week intervals until week 30. Of the 14 patients who did not enter treatment period 2, 5 patients did not achieve PASI 50 at week 12 (3 others who also did not achieve PASI 50 continued erroneously during treatment period 2); 7 patients discontinued immediately after week 12 (3 patients were not re‐randomized; 4 were re‐randomized but did not take any study drug during treatment period 2); 2 patients achieved PASI 50 at week 12 but had no data beyond week 12. ETN=etanercept originator product
Methods
The EGALITY study (NCT01891864) was a multicentre, randomized, double‐blind, phase 3, confirmatory efficacy and safety study conducted from 24 June, 2013, to 30 March, 2015, across 74 centres in 11 European countries and South Africa. Patients aged ≥18 years, with active but clinically stable chronic plaque‐type psoriasis diagnosed ≥6 months before baseline, who had previously received phototherapy or systemic psoriasis therapy at least once or who were candidates for such therapies in the opinion of the investigator were included in the study.10
The study design is shown in Fig. 1. In brief, following a 2–4‐week screening period, eligible patients were randomized 1:1 to receive self‐administered 50 mg subcutaneous injection of GP2015 or ETN twice‐weekly until week 12 (treatment period 1 [TP1]). Patients who had achieved at least a 50% improvement in PASI scores from baseline (PASI 50) at week 12 were re‐randomized to either continue the same treatment on a once‐weekly dosing schedule (‘continued GP2015’ and ‘continued ETN’ groups respectively), or undergo a sequence of three treatment switches between GP2015 and ETN at 6‐week intervals until week 30 (‘switched GP2015’ and ‘switched ETN’ groups, respectively [TP2, Fig. 1]).
This study was conducted in accordance with the ethical principles derived from the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practices and in compliance with local regulatory requirements and was reviewed and approved by the Independent Ethics Committee or Institutional Review Board at each centre. All patients provided written informed consent before entering the study.
Assessments
Efficacy assessments included the absolute and percent change from baseline in PASI score versus time; PASI 50, PASI 75 and PASI 90 response rates (proportion of patients showing at least a 50%/75%/90% improvement in PASI score from baseline visit) and proportion of investigator's global assessment (IGA) mod 2011 responders (defined as a patient who achieved a score of 0 [‘clear’] or 1 [‘almost clear’] and improved by at least 2 points of the IGA scale compared with baseline); the IGA mod 2011 scores were assessed using a 5‐point rating scale.11
Other assessments included the Dermatology Life Quality Index (DLQI), a 10‐item general dermatology disability index designed to assess health‐related quality of life in adult patients with skin diseases such as psoriasis.12 Each item on the scale has four response categories ranging from 0 (not at all) to 3 (very much). The DLQI total score is a sum of the 10 questions and ranges from 0 to 30, with higher scores indicating more severe health‐related quality of life impairment.
Safety was assessed by evaluating treatment‐emergent adverse events (AEs) including injection site reactions (ISRs) as well as laboratory assessments and physical examinations.
Immunogenicity assessment included analysis of anti‐drug antibodies (ADA) using a screening assay, followed by a confirmatory specificity assay and a competitive ligand binding assay to assess neutralizing capacity of ADAs.
Statistical analysis
A prespecified analysis was performed to evaluate the effect of multiple switches in comparison to continued treatments. To this end, the two continued treatment arms of ETN and GP2015 during TP2 (week 12 to week 30) were pooled and compared to the pooled TP2 switched treatment arms (‘pooled continued treatment group’ vs. ‘pooled switched treatment group’).
Summary statistics were presented for observed and percentage change from baseline in PASI scores, IGA modified 2011 scores and DLQI scores during TP2. The PASI 50, 75 and 90 response rates were analyzed using a logistic regression model adjusting for the stratification factors (bodyweight category and prior systemic therapy). Covariate‐adjusted difference in proportions and corresponding 95% confidence intervals (CIs) for the difference was presented. Safety and immunogenicity results were summarized descriptively.
The per‐protocol set during TP2 (TP2 PPS) comprised all patients who had completed the study until week 30 without major protocol deviations. TP2 full analysis set (TP2 FAS) included all patients who underwent re‐randomization at week 12 and who received at least one dose of study treatment during TP2. For the analysis based on the FAS, missing values with respect to PASI response variables were imputed with non‐response regardless of the reason for missing data. The TP2 safety set included all patients who received at least 1 dose of study treatment during TP2.
Results
Of the 531 randomized patients, 497 patients were treated in TP2 (Fig. 1) and included in both the TP2 FAS and the safety set. 472 patients completed TP2. Discontinuations were infrequent and common reasons for discontinuation during TP2 were ‘patient decision’ (n = 9, 1.8%) and ‘adverse events’ (n = 7, 1.4%). Due to a re‐randomization ratio of 2:3, the pooled switched treatment group contained 196 patients, while the pooled continued treatment group contained 301 patients. TP2 PPS included 446 patients (pooled switched treatment group [n = 179] and pooled continued treatment group [n = 267]). Baseline (before first drug administration at the start of the study) demographic and disease characteristics of patients who underwent multiple switches compared with those who continued treatment during TP2 were well balanced (Table 1).
Table 1.
Baseline demographic and disease characteristics of patients re‐assigned to switched/continued treatment groups for treatment period 2 (full analysis set)
| Demographic and disease characteristics | Pooled switched treatment group | Pooled continued treatment group |
|---|---|---|
| N = 196 | N = 301 | |
| Age (years), mean (SD) | 41.7 (12.4) | 42.8 (12.6) |
| Sex, n (%) | ||
| Male | 117 (59.7) | 192 (63.8) |
| Race, n (%) | ||
| Caucasian | 194 (99.0) | 300 (99.7) |
| Asian | 0 | 1 (0.3) |
| Unknown | 1 (0.5) | 0 |
| Other | 1 (0.5) | 0 |
| Bodyweight (kg), mean (SD) | 86.7 (20.9) | 87.0 (19.6) |
| Weight group, n (%) | ||
| <90 kg | 115 (58.7) | 176 (58.5) |
| ≥90 kg | 81 (41.3) | 125 (41.5) |
| BMI (kg/m2), mean (SD) | 28.6 (5.9) | 28.8 (5.8) |
| Duration since initial diagnosis of plaque‐type psoriasis (years), mean (SD) | 17.0 (11.5) | 17.7 (11.4) |
| IGA mod 2011, n (%) | ||
| 3 = Moderate | 139 (70.9) | 211 (70.1) |
| 4 = Severe | 57 (29.1) | 90 (29.9) |
| PASI score, mean (SD) | 22.5 (9.5) | 22.6 (9.0) |
| Presence of psoriatic arthritis, n (%) | 29 (14.8) | 68 (22.6) |
| Prior systemic therapy, n (%) | ||
| No | 135 (68.9) | 201 (66.8) |
| Any | 59 (30.1) | 97 (32.2) |
| TNF antagonist | 2 (1.0) | 3 (1.0) |
| BSA affected (%), mean (SD) | 30.8 (14.2) | 30.7 (14.5) |
Baseline refers to the start of the study before first drug administration.
BMI, body mass index; BSA, body surface area; IGA, investigator's global assessment; PASI, psoriasis area and severity index; SD, standard deviation; TNF, tumour necrosis factor.
Efficacy
Mean (standard deviation [SD]) PASI scores at baseline (before first drug administration) were comparable between patients who underwent multiple switches and those with continued treatments during TP2 (22.60 [9.540] for pooled switched and 22.29 [8.622] for pooled continued treatment groups). The mean (SD) PASI score and mean percent change from baseline in PASI score were also comparable between pooled switched and pooled continued treatment groups at all time points during TP2 (Fig. 2a, b).
Figure 2.
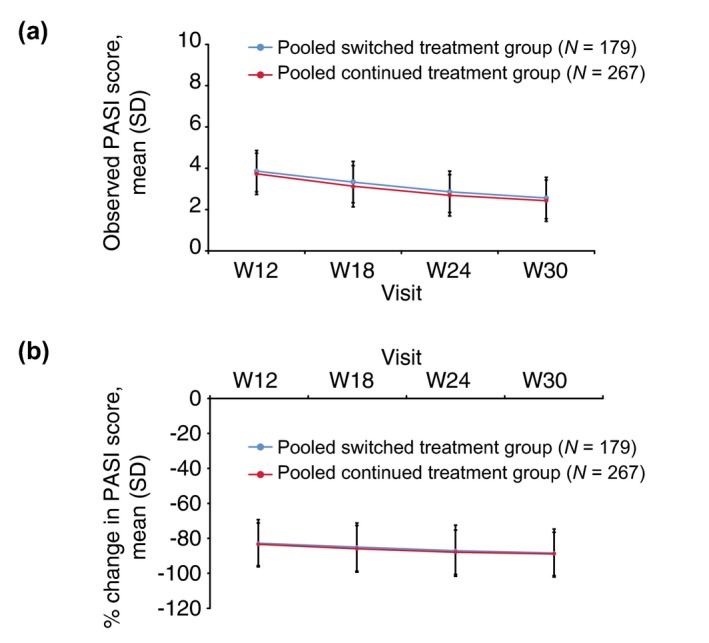
Observed and percent change from baseline in PASI score during TP2 (TP2 per‐protocol set). (a) Observed PASI scores, (b) Percent change in PASI scores. Pooled switched treatment group included patients who switched from treatment with either GP2015 or ETN to treatment sequences ETN>GP2015 > ETN or GP2015 > ETN>GP2015 during treatment period 2, respectively. The pooled continued treatment group included patients who received GP2015 or ETN continuously from treatment period 1. ETN=etanercept originator product; PASI, psoriasis area and severity index; TP2, treatment period 2; SD, standard deviation; W, week.
In addition, PASI 50, PASI 75 and PASI 90 adjusted response rates were comparable between the pooled switched and pooled continued treatment groups during TP2: the adjusted PASI 50 response rates remained steady, whereas the adjusted PASI 75 and PASI 90 response rates gradually increased, from week 12 up to week 30 (Fig. 3).
Figure 3.
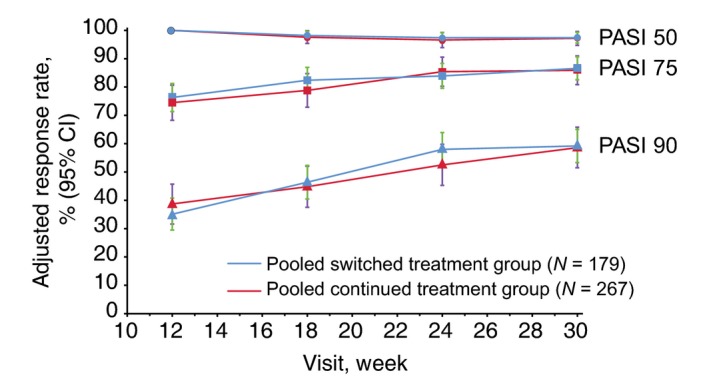
Adjusted PASI 50, 75 and 90 response rates during TP2 (TP2 per‐protocol set). Pooled switched treatment group included patients who switched from treatment with either GP2015 or ETN to treatment sequences ETN>GP2015 > ETN or GP2015 > ETN>GP2015 during treatment period 2, respectively. The pooled continued treatment group included patients who received GP2015 or ETN continuously from treatment period 1. CI, confidence interval; ETN, etanercept originator product; PASI, psoriasis area and severity index; PPS, per‐protocol set; TP2, treatment period 2; W, week.
At baseline, a majority of patients in the TP2 PPS pooled switched (69.8%) and pooled continued (71.5%) treatment groups had an IGA mod 2011 score of 3; the remaining patients had a score of 4. The proportion of IGA mod 2011 responders (patients who achieved a score of 0 or 1 and improved by at least 2 points of the IGA scale compared with baseline) gradually increased from week 12 up to week 30 in both groups in a comparable fashion (Fig. 4).
Figure 4.
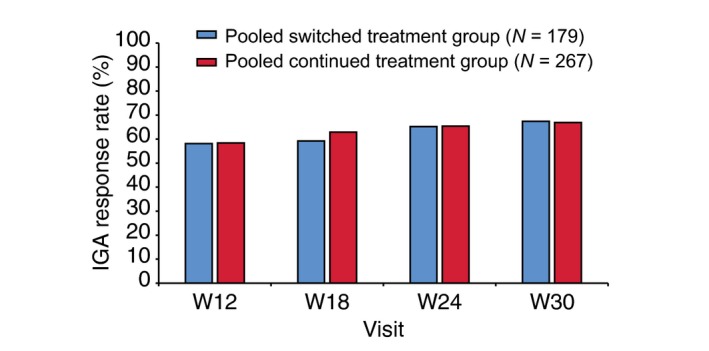
Proportion of IGA responders during TP2 (TP2 per‐protocol set). An IGA responder was defined as a patient who achieved a score of 0 (‘clear’) or 1 (‘almost clear’) and improved by at least 2 points on the IGA scale compared with baseline IGA, Investigator's Global Assessment; TP2, treatment period 2; W, week.
Comparable efficacy results were observed in TP2 FAS, corroborating the results observed in TP2 PPS. Also, no relevant differences were identified in any efficacy parameter when considering the four treatment arms (two continued and two switched) separately during TP2.
DLQI scores
The mean DLQI total scores in TP2 PPS were comparable between the pooled switched and pooled continued treatment groups at baseline (13.4 vs. 13.9), week 12 (4.2 vs. 4.4) and week 30 (3.5 vs. 3.7). Moreover, the mean percent change from baseline in DLQI total scores (improvement) was similar between pooled switched and pooled continued treatment groups at each time point during TP2 (Fig. 5). The mean percent change was also similar between groups for each of the six subscales (Table 2). In general, the proportion of patients achieving a DLQI score of 0 or 1 overall and for each subscale was comparable between pooled switched and pooled continued treatment groups at each time point during TP2.
Figure 5.
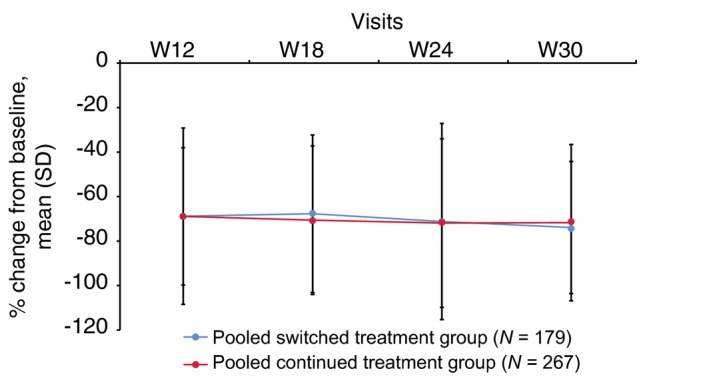
Percent change in DLQI overall scores during TP2 (TP2 per‐protocol set). Pooled switched treatment group included patients who switched from treatment with either GP2015 or ETN to treatment sequences ETN>GP2015 > ETN or GP2015 > ETN>GP2015 during treatment period 2, respectively. Pooled continued treatment group included patients who received GP2015 or ETN continuously from treatment period 1. DLQI, Dermatology Life Quality Index; ETN, etanercept originator product; TP2, treatment period 2; W, week.
Table 2.
Percent change in DLQI subscale scores during TP2 (TP2 per‐protocol set)
| DLQI subscales | Baseline | Week 12 | Week 18 | Week 24 | Week 30 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled switched treatment group | Pooled continued treatment group | Pooled switched treatment group | Pooled continued treatment group | Pooled switched treatment group | Pooled continued treatment group | Pooled switched treatment group | Pooled continued treatment group | Pooled switched treatment group | Pooled continued treatment group | |
| Symptoms and feelings | 3.6 (1.44) | 3.6 (1.49) | −65.55 (33.289) | −66.39 (33.318) | −63.49 (35.045) | −63.99 (34.385) | −68.38 (33.851) | −66.39 (35.623) | −69.48 (32.122) | −66.83 (34.675) |
| Daily activities | 2.8 (1.68) | 2.7 (1.61) | −72.68 (35.119) | −68.43 (37.373) | −71.94 (35.829) | −71.10 (45.073) | −73.75 (39.972) | −74.04 (37.371) | −75.58 (37.279) | −72.52 (42.472) |
| Leisure | 2.5 (1.73) | 2.6 (1.70) | −69.36 (43.131) | −67.75 (40.801) | −69.32 (51.953) | −73.32 (37.275) | −72.98 (58.990) | −72.49 (45.037) | −73.53 (42.471) | −75.20 (41.905) |
| Work/school | 1.2 (1.09) | 1.2 (1.17) | −78.93 (39.895) | −67.05 (54.489) | −74.52 (44.880) | −75.89 (46.845) | −81.25 (37.892) | −75.14 (46.820) | −75.00 (50.626) | −78.31 (46.769) |
| Personal relationships | 2.0 (1.73) | 2.4 (1.84) | −71.38 (38.392) | −69.42 (42.864) | −70.52 (45.623) | −74.53 (41.282) | −76.77 (41.984) | −78.29 (38.758) | −79.18 (38.659) | −77.72 (38.889) |
| Treatment | 1.3 (0.96) | 1.4 (0.94) | −72.02 (40.115) | −77.16 (37.430) | −75.06 (38.514) | −75.73 (42.671) | −76.84 (41.035) | −78.24 (36.085) | −74.76 (39.388) | −75.39 (39.634) |
Values shown are mean (SD). Baseline refers to the start of the study before first drug administration.
Pooled switched treatment group (N = 179) included patients who switched from treatment with either GP2015 or ETN to treatment sequences ETN>GP2015 > ETN or GP2015 > ETN>GP2015 during treatment period 2, respectively.
Pooled continued treatment group (N = 267) included patients who received GP2015 or ETN continuously from treatment period 1.
DLQI, Dermatology Life Quality Index; ETN, etanercept originator product; SD, standard deviation; TP2, treatment period 2.
Safety
A majority of patients from the pooled switched (91.8%) and the pooled continued (90.4%) treatment groups did not miss any study drug dose during TP2. The median duration of drug exposure was similar in both treatment groups (120 days).
The proportion of patients with at least 1 treatment‐emergent AE during TP2 was similar between pooled switched (36.7%) and pooled continued (34.9%) treatment groups. ISRs were treatment‐emergent AEs with the highest incidence in pooled switched and pooled continued treatment groups (4.6% vs 4.3%) followed by pharyngitis (2.6% vs. 3.3%), nasopharyngitis (2.0% vs. 2.7%), headache (2.6% vs. 1.7%) and viral upper respiratory tract infection (2.6% vs. 1.3%, Table 3). The majority of ISRs were mild in severity in both groups (4.1% vs. 4.0%), and none were severe. Treatment‐related TEAEs were also observed in a similar proportion of patients from the pooled switched (13.3%) and pooled continued (12.3%) treatment groups during TP2.
Table 3.
Treatment‐emergent adverse events during TP2, by pooled treatment groups (TP2 safety set)
| Pooled switched treatment group N = 196 n (%) | Pooled continued treatment group N = 301 n (%) | |
|---|---|---|
| Treatment‐emergent AEs (total, n ) | 116 | 168 |
| Any treatment‐emergent AE | 72 (36.7) | 105 (34.9) |
| Any SAE | 6 (3.1) | 3 (1.0) |
| Any treatment‐related treatment‐emergent AE | 26 (13.3) | 37 (12.3) |
| Discontinuations due to treatment‐emergent AE | 6 (3.1) | 3 (1.0) |
| Deaths | 0 | 0 |
| TEAEs (Preferred term) with a ≥2% incidence in any treatment group | ||
| Injection site reaction | 9 (4.6) | 13 (4.3) |
| Headache | 5 (2.6) | 5 (1.7) |
| Pharyngitis | 5 (2.6) | 10 (3.3) |
| Viral upper respiratory tract infection | 5 (2.6) | 4 (1.3) |
| Back pain | 4 (2.0) | 4 (1.3) |
| Nasopharyngitis | 4 (2.0) | 8 (2.7) |
| Psoriasis | 4 (2.0) | 4 (1.3) |
Patients experiencing multiple events were counted only once within each treatment group.
Events occurring with an incidence of ≥2% in any treatment group during treatment period 2 (safety set) are presented and sorted by descending order of frequency within the pooled switched treatment group column.
AE terms are coded using MedDRA version 17.0.
Total number of treatment‐emergent AEs is inclusive of injection site reactions.
MedDRA, medical dictionary for regulatory activities; SAE, serious adverse event; TEAE, treatment‐emergent adverse event; TP2, treatment period 2.
There were no deaths during TP2. In general, the incidence of serious treatment‐emergent AEs and discontinuations due to treatment‐emergent AEs was lower during TP2 than during the first 12 weeks of treatment (3.1% in the pooled switched and 1.0% in the pooled continued treatment group). Serious treatment‐emergent AEs during TP2 included diverticulitis, tonsillitis, umbilical hernia, cholelithiasis, psoriasis, psoriatic arthropathy and pulmonary sarcoidosis (1 patient [0.5%] each) in the pooled switched group; and pneumonia, meniscus injury and upper limb fracture (1 patient [0.3%] each) in the pooled continued group. None of the serious treatment‐emergent AEs in either treatment group were considered treatment‐related. No treatment‐emergent AEs leading to study discontinuation or study drug interruption were reported in more than one patient in either treatment group.
Clinical laboratory parameters, vital signs, physical examination and ECG findings were all comparable for pooled continued and pooled switched groups; no patterns were evident that would suggest a relation to treatment or a potential safety concern.
Immunogenicity
None of the patients from both treatment groups were positive for ADAs during TP2.
Discussion
The EGALITY study confirmed biosimilarity of GP2015 and ETN during the first 12 weeks of treatment.10 The results observed during TP2 are of importance considering that EGALITY is the first study (and the only study to our knowledge), which actually demonstrates that multiple switches between a biosimilar (GP2015) and its originator (ETN) have no impact on efficacy, safety and immunogenicity, across the reported period. All efficacy parameters based on PASI scores as well as the DLQI demonstrated comparable outcomes between pooled switched and pooled continued treatment groups.
Overall, the incidence and types of treatment‐emergent AEs, treatment‐related treatment‐emergent AEs and serious adverse events were comparable between the pooled switched and pooled continued treatment groups. Patients switching between ETN and GP2015 are not expected to experience differences in ISRs, thereby alleviating potential concerns.
Immunogenicity is an important concern for biologicals, considering the serious safety issues associated with them.13 In addition, immunogenicity is crucial for biosimilars because posttranslational modifications can increase the immunogenic potential of a protein.1 In general, etanercept has a lower incidence of immunogenicity compared with other TNFα inhibitors.14, 15 Importantly, psoriasis is an indication which allows for an unbiased and sensitive detection of potential differences in immunogenicity, without concomitant immunosuppressive therapy as a confounding factor.
Of note, no ADAs were reported during TP2 of the EGALITY study, suggesting that multiple switches between GP2015 and ETN do not adversely impact immunogenicity. The 6‐week interval for each switch during TP2 was considered of sufficient duration for assessing immunogenicity response, because all ADA responses during treatment period 1 were observed within 4 weeks. In the EGALITY study, ADAs were evaluated using a validated state‐of‐the‐art technique implying a high assay sensitivity according to FDA's draft guideline16 and high drug tolerance above the highest measured drug concentration of the study. The bioanalytical strategy for immunogenicity assessment followed a tiered approach, and included a screening assay, a confirmatory specificity assay and a competitive ligand binding assay to assess the neutralizing capacity of ADAs.17
Switching between an originator and a biosimilar is expected to become common practice in real‐life situations; the innovative design of the EGALITY study has shown that frequent switches at short intervals between GP2015 and ETN had no negative impact on efficacy, immunogenicity and safety during the reported period. Patient registries will play an important role in further establishing the long‐term efficacy and safety of switching.
Conclusion
Similar efficacy was observed between continued treatment and alternating treatment between GP2015 and ETN. Moreover, no clinically relevant differences were noted in safety or immunogenicity between the two groups, indicating no impact of repeated switches between GP2015 and ETN.
Acknowledgments
The authors thank all investigators (Clinicaltrials.gov [NCT01891864]) and participating patients who contributed to the successful conduct of this study, and Lakshmi Venkatraman (Product Lifecycle Services‐NBS, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for medical writing and editorial assistance. Professor Griffiths is a National Institute for Health Research Senior Investigator.
Conflicts of interest disclosures
Dr Gerdes has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: Abbott/AbbVie, Almirall‐Hermal, Amgen, Bayer HealthCare, Biogen Idec, Bioskin, Boehringer‐Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Isotechnika, Janssen‐Cilag, Leo Pharma, Medac, Merck Serono, Mitsubishi Tanabe, MSD, Novartis, Pfizer, Sandoz Biopharmaceuticals, Schering‐Plough, Takeda, Teva, UCB Pharma, VBL therapeutics and Wyeth Pharma. Professor Thaçi has received research support from Abbvie, Almiral, Amgen, Astellas, Biogen‐Idec, Boehringer‐Ingelheim, Celgene, Dignity, Elli‐Lilly, Forward‐Pharma, GlaxoSmithKline, Leo, Janssen‐Cilag, Maruho, MSD, Mitsubishi Pharma, Novartis, Pfizer, Roche and Sandoz and honoraria from AbbVie, Biogen‐Idec, Celgene, Janssen, Leo, Mundipharma, Novartis, Pfizer and Roche‐Possay. Professor Thaci has acted as a consultant for Abbvie, Biogen‐Idec, Celgene, Dignity, Galapagos, Maruho, Mitsubishi, Novartis, Pfizer and Xenoport and been part of scientific advisory boards for AbbVie, Amgen, Biogen‐Idec, Celgene, Eli‐Lilly, GlaxoSmithKline, Janssen, Leo‐Pharma, Mundipharma, Novartis, Pfizer and Sandoz. Professor Griffiths has received consultancy/honoraria and/or research funding from Abbvie, Galderma, Janssen, LEO‐Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, Sun Pharmaceuticals and UCB Pharma. Professor Arenberger has received grants from Novartis. J Poetzl and H Woehling are employees of Hexal AG. G Wuerth and M Afonso were employees of Hexal AG at the time of the study.
Study funding
The study was funded by Hexal AG, a Sandoz company. The funder had a role in the study design, data collection, data analysis and manuscript preparation.
References
- 1. Daller J. Biosimilars: a consideration of the regulations in the United States and European union. Regul Toxicol Pharmacol 2016; 76: 199–208. [DOI] [PubMed] [Google Scholar]
- 2. USFDA . Scientific considerations in demonstrating biosimilarity to a reference product ‐ Guidance for industry. Last updated on 2015. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm537135.pdf (last accessed on Feb 06, 2017).
- 3. European Medicines Agency . Guideline on similar biological medicinal products containing biotechnology‐derived proteins as active substance: non‐clinical and clinical issues. 2014. Last updated on December 18, 2014. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf (last accessed: Feb 06, 2017).
- 4. Kay J. Editorial: biosimilars: new or Deja Vu? Arthritis Rheumatol 2016; 68: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 5. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits 2013; 6: 469–478. [PMC free article] [PubMed] [Google Scholar]
- 6. USFDA . Considerations in demonstrating interchangeability with a reference product‐ Guidance for industry. Last updated on 2017. URL http://www.fda.gov/ucm/groups/fdagov‐public/@fdagov‐drugs‐gen/documents/document/ucm537135.pdf (last accessed on Apr 04, 2017).
- 7. Kurki P, van Aerts L, Wolff‐Holz E et al Interchangeability of biosimilars: a European perspective. BioDrugs 2017; 31: 83–91. [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Chow SC. On the regulatory approval pathway of biosimilar products. Pharmaceuticals (Basel) 2012; 5: 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li EC, Abbas R, Jacobs IA, Yin D. Considerations in the early development of biosimilar products. Drug Discov Today 2015; 20(Suppl 2): 1–9. [DOI] [PubMed] [Google Scholar]
- 10. Griffiths CEM, Thaci D, Gerdes S et al The EGALITY study: a confirmatory, randomized, double‐blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate‐to‐severe chronic plaque‐type psoriasis. Br J Dermatol 2017; 176: 928–938. [DOI] [PubMed] [Google Scholar]
- 11. Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5‐point Investigator's Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat 2015; 26: 23–31. [DOI] [PubMed] [Google Scholar]
- 12. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 13. Weise M, Bielsky MC, De Smet K et al Biosimilars: what clinicians should know. Blood 2012; 120: 5111–5117. [DOI] [PubMed] [Google Scholar]
- 14. de Vries MK, van der Horst‐Bruinsma IE, Nurmohamed MT et al Immunogenicity does not influence treatment with etanercept in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68: 531–535. [DOI] [PubMed] [Google Scholar]
- 15. Vincent FB, Morand EF, Murphy K et al Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)‐specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72: 165–178. [DOI] [PubMed] [Google Scholar]
- 16. USFDA . Assay development and validation for immunogenicity testing of therapeutic protein products ‐ Guidance for industry. Last updated on 2016. https://www.fda.gov/downloads/Drugs/Guidances/UCM192750.pdf (last accessed on Apr 05, 2017).
- 17. Poetzl J, Arlt I, von Richter O, Wöhling H, Afonso M and Schaffar G. State‐of‐the‐art immunogenicity evaluation in phase 3 confirmatory study (EGALITY) with etanercept biosimilar GP2015. J Eur Acad Dermatol Venereol 2017. http://doi.org/10.1111/jdv.14632. [DOI] [PubMed]


