Summary
We conducted an infrared thermal imaging‐based genetic screen to identify Arabidopsis mutants displaying aberrant stomatal behavior in response to elevated concentrations of CO 2.
This approach resulted in the isolation of a novel allele of the Arabidopsis BIG locus (At3g02260) that we have called CO 2 insensitive 1 (cis1).
BIG mutants are compromised in elevated CO 2‐induced stomatal closure and bicarbonate activation of S‐type anion channel currents. In contrast with the wild‐type, they fail to exhibit reductions in stomatal density and index when grown in elevated CO 2. However, like the wild‐type, BIG mutants display inhibition of stomatal opening when exposed to elevated CO 2. BIG mutants also display wild‐type stomatal aperture responses to the closure‐inducing stimulus abscisic acid (ABA).
Our results indicate that BIG is a signaling component involved in the elevated CO 2‐mediated control of stomatal development. In the control of stomatal aperture by CO 2, BIG is only required in elevated CO 2‐induced closure and not in the inhibition of stomatal opening by this environmental signal. These data show that, at the molecular level, the CO 2‐mediated inhibition of opening and promotion of stomatal closure signaling pathways are separable and BIG represents a distinguishing element in these two CO 2‐mediated responses.
Keywords: abscisic acid (ABA), Arabidopsis thaliana, BIG gene, CO2 signaling, stomatal function, S‐type anion channel
Introduction
Stomata consist of a pair of guard cells that surround a central pore and serve to regulate water loss and the uptake of CO2. Both the aperture of the stomatal pore and the number of stomata that develop on the leaf surface are controlled by environmental signals. By integrating external signals and local cues, stomata ‘set’ gas exchange to suit the prevailing environmental conditions (Hetherington & Woodward, 2003). One of the signals that controls stomatal aperture and influences stomatal development, in both the short and long term, is the atmospheric concentration of carbon dioxide ([CO2]) (Kim et al., 2010; Franks et al., 2012). In response to an increase in [CO2], stomatal aperture reduces, as, in general, do the number of stomata that develop on the surface of the leaves (Vavasseur & Raghavendra, 2005; Kim et al., 2010; Franks et al., 2012). Understanding how the plant perceives changes in [CO2] and integrates this information with other internal and external signals, resulting in the adjustments of stomatal aperture and density, is of key importance in the context of understanding the impact of global environmental change on plants (Assmann & Jegla, 2016).
Recently, we have begun to understand more about the underlying cellular mechanisms responsible for coupling increased [CO2] to reduced stomatal conductance (Kim et al., 2010; Assmann & Jegla, 2016; Engineer et al., 2016). In this context, it is important to recognize that elevated CO2‐induced reductions in stomatal conductance are the net result of two processes: the promotion of stomatal closure and the inhibition of stomatal opening (Assmann, 1993). These processes are separable; abscisic acid (ABA)‐induced stomatal closure is distinct from ABA‐inhibited stomatal opening (Allen et al., 1999; Wang et al., 2001; Mishra et al., 2006). However, before the current work, it was not known whether this also applied to [CO2]‐induced changes in stomatal aperture.
There is evidence that the guard cell ABA and CO2 signaling responsible for the inhibition of light‐induced stomatal opening pathways converge (Webb & Hetherington, 1997). It has been suggested that elevated [CO2] brings about its effects on stomatal aperture and development by accessing the ABA signaling pathway, because there is a requirement for both ABA and the ABA receptors of the PYR/RCAR family in these responses (Chater et al., 2015). There are other data suggesting that the early steps in CO2‐mediated closure converge with ABA signaling downstream of ABA receptors and the two pathways influence each other on convergence (Xue et al., 2011; Merilo et al., 2013; Hõrak et al., 2016; Jakobson et al., 2016; Yamamoto et al., 2016). Obviously, these processes are not mutually exclusive. Although the mechanism(s) through which the guard cell ABA signaling pathway is accessed is not fully understood, it has been possible to distinguish, on a genetic basis, components that function in CO2‐mediated closure, but not in guard cell ABA signaling. In Arabidopsis, these include β‐carbonic anhydrases which are encoded by the CA1 and CA4 genes (Hu et al., 2010), the protein kinase HT1 (HIGH LEAF TEMPERATURE 1) (Hashimoto et al., 2006), RHC1, a MATE transporter (Tian et al., 2015), and the MAP kinase MPK4 (Hõrak et al., 2016; Jakobson et al., 2016). Loss of the CAs, RHC1 and MPK4 impairs CO2‐induced closure (Hashimoto et al., 2006; Hu et al., 2010; Tian et al., 2015; Hõrak et al., 2016; Jakobson et al., 2016), whereas recessive ht1 alleles show a constitutive high CO2 response (Hashimoto et al., 2006; Hashimoto‐Sugimoto et al., 2016).
In 1987, Woodward discovered an inverse relationship between atmospheric [CO2] and stomatal density (Woodward, 1987). We know less about the operation of this developmental signaling pathway, but the putative β‐keto acyl CoA synthase encoded by the HIC gene is involved, as are the CO2 Response Secreted Protease (CRSP), the β‐carbonic anhydrases CA1 and CA4, and the peptide Epidermal Patterning Factor 2 (EPF2) (Gray et al., 2000; Doheny‐Adams et al., 2012; Engineer et al., 2014). Most recently, it has been shown that the activity of the reactive oxygen species (ROS)‐producing NADPH oxidases, encoded by the RBOHD and RBOHF genes, is involved in the CO2‐mediated reduction in stomatal density, as is ABA and the ABA receptors encoded by the PYR/RCAR family (Chater et al., 2015).
During an infrared thermal imaging genetic screen in Arabidopsis (Wang et al., 2004), we isolated a novel allele of the BIG locus (At3g02260) that we name CO 2 insensitive 1 (cis1), which is compromised in both elevated [CO2]‐induced closure and reduction in stomatal density. However, when challenged with ABA, cis1 displays reductions in stomatal aperture that are indistinguishable from the wild‐type (WT), suggesting that BIG (CIS1) functions upstream of ABA or in an ABA‐independent signaling pathway responsible for the control of stomatal aperture by CO2. We also found that the activation of the guard cell S‐type anion channel by bicarbonate is compromised by the loss of BIG function. Furthermore, in contrast with elevated [CO2]‐mediated closure, the ability of elevated [CO2] to inhibit stomatal opening was not affected in this mutant. In summary, we have identified BIG as a new component in the signaling pathway responsible for the control of stomatal development by elevated [CO2]. We also show that BIG features in the signaling pathway through which elevated [CO2] controls stomatal aperture. Importantly, we show that BIG is only involved in elevated [CO2]‐induced stomatal closure and is not involved in the inhibition of stomatal opening by this environmental signal or in stomatal responses to ABA. These results show that, at the molecular level, these pathways are separable, with BIG representing a component that distinguishes these two CO2‐mediated responses.
Materials and Methods
Plant growth
All Arabidopsis (Arabidopsis thaliana L.) lines used were in the Columbia background (Col‐0). Seeds of doc1‐1 and big‐1 were obtained from NASC (the European Arabidopsis Stock Centre, http://arabidopsis.org.uk). Seed germination and plant growth were performed as described previously (Liang et al., 2010).
Mutant screen
To identify genes required for stomatal CO2 responses, we screened 20 000 seeds from an Arabidopsis EMS (ethyl methanesulfonate) M2 population representing 40 independent pools (each pool corresponding to c. 1000 M1 plants) by infrared thermal imaging (Wang et al., 2004; Xie et al., 2006). Screening was carried out on 3–4‐wk‐old plants in a purpose‐built chamber (84 × 68 × 20 cm3), located inside a controlled environment room. The CO2 concentration inside the chamber was controlled externally from CO2 cylinders. Air flow in the chamber was maintained at 0.03 m s−1 using fans. Relative humidity inside the chamber was c. 60%, temperature was 22°C and light intensity was 120 μmol m−2 s−1. Plants were placed in the chamber and exposed to 360 ppm [CO2] (360 ppm [CO2] cylinder (balanced air mixture)). After 40 min, thermal images were captured and the plants were then exposed to 1500 ppm [CO2] (1500 ppm [CO2] cylinder (balanced air mixture)) for a further 40 min, and thermal images were captured. Pairs of images were compared to identify putative CO2 response mutants. Infrared thermal imaging was performed using an Inframetrics middle infrared (3.4–5 μm) camera model SC1000E (FLIR Systems Inc., Wilsonville, OR, USA). Images were stored in a ThermaCam Image file format (IMG) and analysed with the ThermaCam™ Researcher 2001 software (FLIR Systems). Mutants exhibiting altered leaf thermal profiles compared with WT were selected, self‐pollinated, and seeds (M3) were collected for further investigation. Backcross seeds (F1s) were obtained using mutant lines as female and Col‐0 as male. F2 was used for segregation analysis. Mutants segregating in F2 were backcrossed to WT Col‐0 for another two generations before being used for fine mapping and phenotyping.
Map‐based mutant gene cloning
cis1 mutants were outcrossed to WT plants in the Landberg erecta background (Ler) and the segregating F2 seedlings were screened using infrared thermography. A total of 868 cis1 mutants were used for mapping. Twenty‐two simple sequence length polymorphism (SSLP) markers were used for bulked segregant analysis as described previously (Lukowitz et al., 2000). The Arabidopsis single nucleotide polymorphism (SNP) collections (http://www.arabidopsis.org/) were used to design SSLP, cleaved amplified polymorphic sequences (CAPS) and derived CAPS (dCAPS) markers for fine mapping. The mutation was narrowed down to a c. 100‐kb region at the top arm of chromosome III between SSLP marker nga172 and CAPS marker CA1, and is adjacent to SSLP marker nga32. T‐DNA insertion lines representing all the annotated genes within this region were obtained from NASC and screened using infrared thermal imaging. A T‐DNA insertion line (SALK_105495) of At3g02260 which also showed morphological similarity to the mutant ‘cis1’ was identified. We performed an allelism test using the F1 progeny of the cis1 and big‐1 (SALK_105495) cross using thermal imaging. This confirmed that cis1 and big‐1 are allelic to each other. We used PCR‐based genotyping and gene sequencing to confirm the presence of a T‐DNA insertion in gene At3g02260 of the SALK_105495 line and a single point mutation in gene At3g02260 of the cis1 mutant.
Measurements of stomatal density, index, aperture and cell viability
Stomatal density and index were measured on leaf abaxial surfaces as described previously (Chater et al., 2015). The effect of CO2 on stomatal aperture was measured using the isolated epidermal strip bioassay technique as described previously (Chater et al., 2015). Forty stomatal pores were measured per treatment in three separate replicated tests. To avoid experimenter bias, all the aperture measurements were performed blind. Cell viability was assessed as described in Chater et al. (2015). Experiments on independently grown plant material were carried out three times and data were analysed by SigmaPlot 10 (Systat Software Inc., San Jose, CA, USA).
Gas exchange measurements
Time‐resolved stomatal conductance analyses of intact leaves of 5‐wk‐old plants were conducted using a Li‐6400 gas exchange analyzer with a fluorometer chamber (Li‐Cor, Lincoln, NE, USA), as described by Hu et al. (2010). The photon flux density was set at 150 μmol m−2 s−1; temperature and relative humidity were held at 21°C and c. 60–70%, respectively. Stomatal conductance was stabilized at 400 ppm CO2 (as ambient concentration) for 30 min and then shifted to 800 ppm for another 30 min before being shifted to 100 ppm for 1.5 h. Data shown are the means ± SE, n = 4 leaves for each genotype.
Patch clamp experiments
Arabidopsis guard cell protoplasts were isolated according to the procedure described previously (Siegel et al., 2009). The whole‐cell currents were recorded using a patch clamp amplifier (Axopatch 200B) and a digitizer (Digidata 1550) (Molecular Devices LLC, Sunnyvale, CA, USA). CO2/bicarbonate‐activated S‐type anion currents were recorded as described previously (Xue et al., 2011). The bath solution contained 30 mM CsCl, 2 mM MgCl2, 1 mM CaCl2 and 10 mM Mes/Tris, pH 5.6. The pipette solution contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM ethyleneglycol‐bis(β‐aminoethylether)‐N,N′‐tetraacetic acid (EGTA), 6.03 mM CaCl2 (2 μM free Ca2+), 5 mM Mg‐ATP, 10 mM HEPES/Tris, pH 7.1. Bicarbonate was freshly added to the pipette solution before patching the protoplasts each day. At pH 7.1, 11.5 mM free bicarbonate was balanced with 2 mM free CO2 in the pipette solution. For more details, please consult Xue et al. (2011).
Reverse transcription‐polymerase chain reaction (RT‐PCR) and quantitative RT‐PCR analysis
Total RNA from aerial parts of the plants was prepared using an RNeasy total RNA mini kit (Qiagen, Hilden, Germany) and digested with RNase‐free DNase I (Thermo Fisher Scientific Inc. Waltham, MA, USA); the absence of genomic DNA contamination was confirmed by PCR using RNA as template without reverse transcription. First‐strand cDNA was synthesized using Superscript II® reverse transcriptase (Invitrogen, Thermo Fisher Scientific) and oligo d(T)15–18 (Promega (Beijing) Biotech Co. Ltd, Beijing, China) mRNA primer with 1 μg of total RNA as the template. cDNA corresponding to 20 ng of total RNA and 300 nM of each primer were used in PCRs. The primers for RT‐PCR amplification of BIG fragments were: primer pair1, F1 (5′‐CAGCAAGCTCTATACCTTCAG‐3′) and R1 (5′‐TCCATCC ATCCACTCAACTC‐3′); primer pair 2, F2 (5′‐GTCTTCT ACTTCACTGACCAACTCC‐3′) and R2 (5′‐TCCATCTTC TTCTTCCTCTACATCC‐3′); Actin7 was amplified with forward primer (5′‐TGTTCCCAAGTATTGTTGGTCGTC‐3′) and reverse primer (5′‐TGCTGAGGGATGCAAGGA TTGATC‐3′) as a loading control. The PCR conditions were as follows: one cycle (94°C, 5 min), 35 cycles (94°C, 30 s; 62°C, 30 s; 72°C, 1 min), one cycle (72°C, 7 min). Quantitative PCR was carried out on an Mx3005P (Stratagene, La Jolla, CA, USA) or an ECO (Illumina Inc., San Diego, CA, USA) real‐time PCR thermal cycler in a total reaction volume of 20 μl using the SYBR green dye PCR Master Mix (Thermo Fisher Scientific) and the conditions 95°C for 10 min, 40 two‐step cycles at 95°C for 15 s and 60°C for 1 min, followed by dissociation melting curve analysis to determine the PCR specificity. The gene‐specific primers used for BIG were: F, 5′‐GAATGGGAAGGAGCTATGTTG‐3′; R, 5′‐GATACTGTG CTAAGGGAACTG‐3′; for Actin3 (At3g53750), the primers were: F, 5′‐GGCAGAATATGATGAGTCAGG‐3′; R, 5′‐AAAGAAGAGCAGAGAACGAAG‐3′. The relative RNA levels were calculated from cycle threshold (C T) values according to the ΔC T method, and relative target mRNA levels were normalized to Actin3 mRNA levels. Reactions were repeated independently three times with similar results.
Results
The cis1 mutant is involved in the response of stomatal conductance to elevated CO2
To understand the underlying cellular basis of the effect of elevated CO2 on stomatal development and function, we carried out a forward genetic screen using infrared thermography. We reasoned that mutants failing to exhibit reductions in aperture, in this case induced by exposure to elevated [CO2], would be visible because they would exhibit reduced leaf temperature as a result of increased leaf evapotranspiration relative to WT (Darwin, 1904). Infrared thermography has been used previously to isolate mutants carrying lesions in stomatal responses to ABA (Raskin & Ladyman, 1988; Merlot et al., 2002), reduced atmospheric relative humidity (Xie et al., 2006; Liang et al., 2010) and CO2 (Hashimoto et al., 2006; Negi et al., 2008). Using this approach, we screened M2 plants from an EMS‐mutagenized population of Arabidopsis and identified cis1 that displayed significantly lower leaf surface temperature (0.68°C) relative to WT when challenged for 40 min with 1500 ppm [CO2] (Fig. 1a,b). Genetic analysis revealed that this phenotype was caused by a single recessive Mendelian mutation (data not shown). To investigate the lesion in the cis1 mutant further, we measured the stomatal conductance (g s). Figure 1(c,d) shows that, in WT, challenge with 800 ppm CO2 results in a reduction in g s, whereas the response is attenuated in cis1. By contrast, both cis1 and WT display an increase in g s when exposed to low (100 ppm) CO2. We confirmed this response in big‐1, a second independent allele of cis1 (Supporting Information Fig. S1). These data suggest that the cis1 mutant is compromised in the stomatal response to elevated [CO2].
Figure 1.
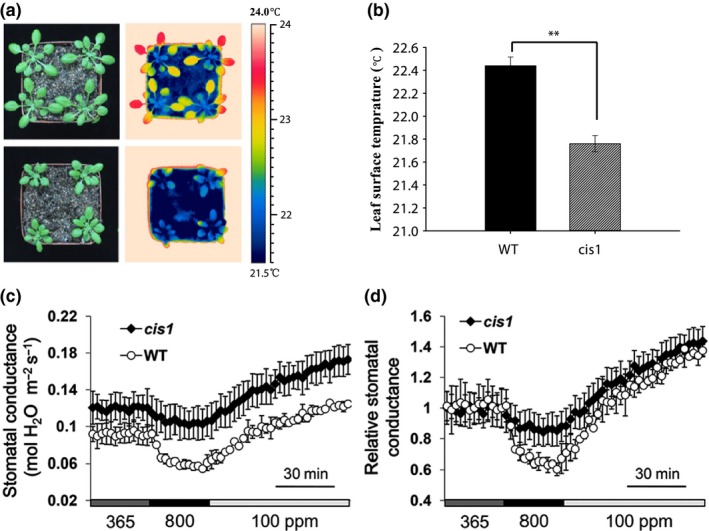
The CO 2 insensitive 1 (cis1) mutant displays a lower leaf surface temperature under elevated CO 2 than wild‐type (WT) Arabidopsis. (a) Infrared thermograms showing that the leaf surface temperature of the cis1 mutant is lower than that of WT when the plants are exposed to 1500 ppm CO 2. (b) The average leaf surface temperature of the cis1 mutant is c. 0.68°C lower than that of the WT plant when both are exposed to 1500 ppm CO 2. Bars are mean ± SE (Student's t‐test, **, P ≤ 0.001, n = 20). (c) In contrast with WT, the cis1 mutant fails to display elevated (800 ppm) CO 2‐induced reduction in stomatal conductance, but exhibits a WT response when exposed to low (100 ppm) CO 2. Bars are mean ± SE (representative data, n = 4). (d) Relative stomatal conductance in (c). Bars are mean ± SE (representative data are presented, n = 4).
Identification of the CIS1 gene locus
We performed map‐based gene cloning to identify the CIS1 locus, and mapped the mutation to a 107‐kb region of chromosome III close to the doc1 mutations (data not shown; Gil et al., 2001). Seeds for T‐DNA insertion lines of all annotated genes within this region were obtained from NASC and screened using infrared thermal imaging. A T‐DNA insertion line (SALK_105495) of At3g02260 was identified that displayed similar thermal behavior to the cis1 mutant. Sequencing of cis1 revealed a single point mutation (G to A substitution) in locus At3g02260 localized at a splicing acceptor site at position +8542 (GT…AG to GT…AA) (Fig. 2a), which resulted in alternative spliced mRNAs as shown in Fig. S2. Real‐time quantitative PCR revealed that, compared with WT, cis1 (At3g02260) gene transcript abundance was reduced to a third (Fig. 2b).
Figure 2.
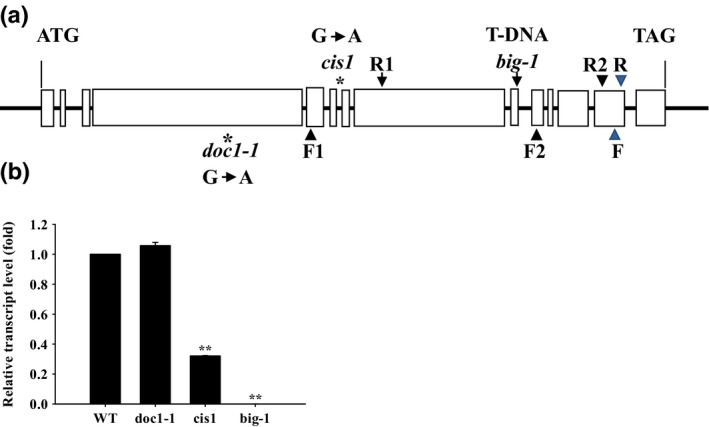
CO 2 insensitive 1 (cis1) is a new allele of the BIG gene in Arabidopsis. (a) Schematic structure of the BIG gene. The intron and exon organization of the BIG gene shown was determined by comparison of the cDNAs obtained by reverse transcription‐polymerase chain reaction (RT‐PCR) and genomic sequences from the Arabidopsis wild‐type (WT) Col‐0. Boxes indicate exons and lines between boxes indicate introns. The locations of the single base mutations and T‐DNA insertion of cis1, doc1‐1 and big‐1 are indicated. Diagram not to scale. * Indicates the site of point mutation. (b) The relative mRNA levels of BIG mutant alleles obtained by quantitative PCR with a pair of primers (F and R) with binding sites shown in (a). Values are mean ± SE, n = 3.** Indicates the difference is statistically significant , P ≤ 0.001.
At3g02260 has previously been named BIG and is annotated as encoding a large protein of 5098 amino acids, containing multiple conserved functional domains including three putative Zn‐finger domains (Kanyuka et al., 2003; Kasajima et al., 2007). Our sequencing revealed that the original annotation is incorrect, as the open reading frame of BIG is 63 bp shorter than predicted, because 30 bp of the sequence of intron 1, 21 bp of intron 5 and 12 bp of intron 7 had been annotated as part of the respective neighboring exons (Notes S1). Hence, the BIG open reading frame (ORF) is 15 234 bp long, encoding a putative 5077‐amino‐acid peptide, as predicted by Gil et al. (2001).
Many alleles of big mutants, for example ga6, tir3, doc1, asr1, lpr1, elk1, asa1, umb1, crm1 and rao3, have been independently isolated. All mutants are characterized by deficient organ elongation (dwarfism) and have altered root architecture, reduced apical dominance, defects in light responses and aberrant auxin transport. They also show altered sensitivities to GA, cytokinin, ethylene, low phosphate and water withholding treatments (Li et al., 1994; Ruegger et al., 1997; Sponsel et al., 1997; Gil et al., 2001; Lease et al., 2001; Kanyuka et al., 2003; López‐Bucio et al., 2005; Kasajima et al., 2007; Yamaguchi et al., 2007; Ivanova et al., 2014). Interestingly, insects and mammals possess homologs of the BIG protein and these are involved in signaling. Calossin/Pushover in Drosophila melanogaster and mammalian p600/UBR4 are homologs of BIG, both of which have a calmodulin (CaM) ‐binding domain and are probably involved in Ca2+ signaling (Xu et al., 1998; Parsons et al., 2015).
To confirm the identity of cis1, we obtained two additional mutant alleles of BIG. doc1‐1 was originally isolated in a genetic screen for components of light signaling and harbors a single base change from G to A at position +5514 (Fig. 2a), resulting in a change from a conserved cysteine (Cys) residue to tyrosine (Tyr). This missense BIG mutation perturbs auxin transport and plant growth (Gil et al., 2001) but, in our quantitative PCR analysis, no change to the transcript abundance of BIG was detected (Fig. 2b). big‐1 harbors a T‐DNA insertion in exon 9 before position +13 617 of the BIG gene (Kasajima et al., 2007) (Fig. 2a). We detected no BIG transcript in this mutant by quantitative PCR (Fig. 2b).
BIG is also involved in the control of stomatal development by elevated CO2
The data in Fig. 3(a) show that stomatal and epidermal pavement cell densities are greater in the BIG mutant alleles than in WT (P ≤ 0.001). This reflects the fact that both guard cells and epidermal cells were significantly smaller than in WT (data not shown). Stomatal development is controlled by CO2, with stomatal density and index typically reduced in plants grown under elevated [CO2] (Woodward, 1987; Woodward & Kelly, 1995). We next investigated whether BIG has a role to play in the control of stomatal development by elevated [CO2]. In WT, growth at elevated [CO2] resulted in a decrease in stomatal density and index (Fig. 3b,c). In marked contrast, under the same conditions, growth at elevated [CO2] resulted in significant increases in both stomatal density and index in the BIG mutants (Fig. 3b,c). These data suggest that, in addition to controlling stomatal aperture, BIG is also required for the reduction in stomatal density and index caused by higher than ambient [CO2].
Figure 3.
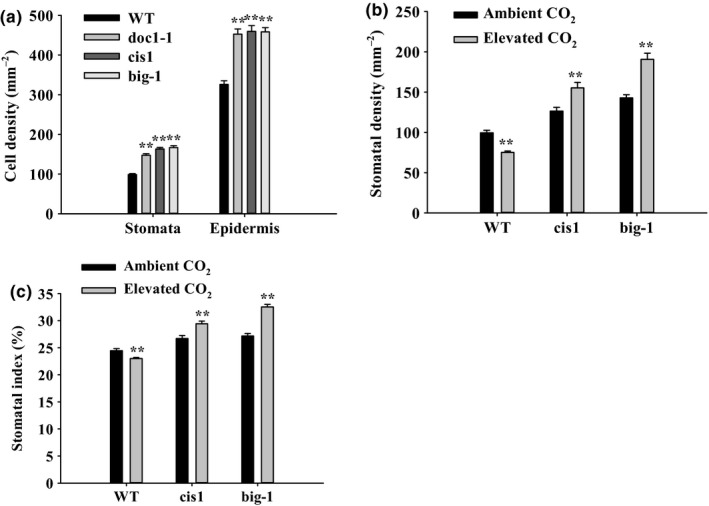
BIG gene mutants have higher stomatal density than wild‐type (WT) Arabidopsis. (a) Compared with WT,BIG mutants exhibit increased stomata and epidermal pavement cells (‘Epidermis’) density when grown at ambient [CO 2]. Error bars represent ± SE (Mann–Whitney rank sum test, **, P ≤ 0.001, n = 72). (b) Stomatal density of WT and BIG mutant seedlings grown at ambient (450 ppm) and elevated (1000 ppm) [CO 2]. When grown at 1000 ppm [CO 2], the mean stomatal density of WT was significantly reduced compared with growth at ambient [CO 2] (error bars represent ± SE (Mann–Whitney rank sum test, **, P ≤ 0.001, n > 20)), whereas, in the BIG gene alleles, stomatal density increased in these conditions (error bars represent ± SE (Student's t‐test, **, P ≤ 0.001, n > 20)). (c) Stomatal index of WT and BIG mutant seedlings grown at 450 ppm and 1000 ppm [CO 2]. When grown at 1000 ppm, the mean stomatal index of WT was significantly reduced compared with growth at ambient [CO 2] (error bars represent ± SE (Student's t‐test, **, P ≤ 0.001, n > 20)), whereas, in the BIG gene mutants, the stomatal index increased in these conditions (error bars represent ± SE (Student's t‐test, or Mann–Whitney rank sum test, **, P ≤ 0.001, n > 20)).
The BIG protein is involved in the signaling pathway by which elevated [CO2] induces stomatal closure, but not in the pathway through which elevated [CO2] inhibits stomatal opening.
The results from the gas exchange experiments (Fig. 1c,d) prompted us to make direct measurements of stomatal responsiveness by quantifying changes in stomatal aperture (Chater et al., 2015). Figure 4(a) shows that, in contrast with WT, the stomata of cis1, big‐1 and doc1‐1 mutants failed to close when subjected to 700 ppm CO2. These data indicate that BIG is required for elevated CO2‐induced stomatal closure. Elevated [CO2] is also known to inhibit light‐induced stomatal opening (Mansfield et al., 1990). In contrast with CO2‐induced stomatal closure, the inhibition of light‐induced stomatal opening of the BIG mutants was similar to that of WT (Fig. 4b). The specific role of the BIG gene in the pathway by which elevated [CO2] brings about stomatal closure is highlighted by our observation that the series of allelic mutants all display WT behavior in response to ABA. This holds for both ABA‐induced stomatal closure and the inhibition by ABA of light‐induced stomatal opening (Fig. 4c,d). The intact stomatal ABA response as well as the impaired CO2 response were both observed in more than one of our laboratories, underlining the robustness of the CO2 specificity of the stomatal phenotype in big mutant alleles.
Figure 4.
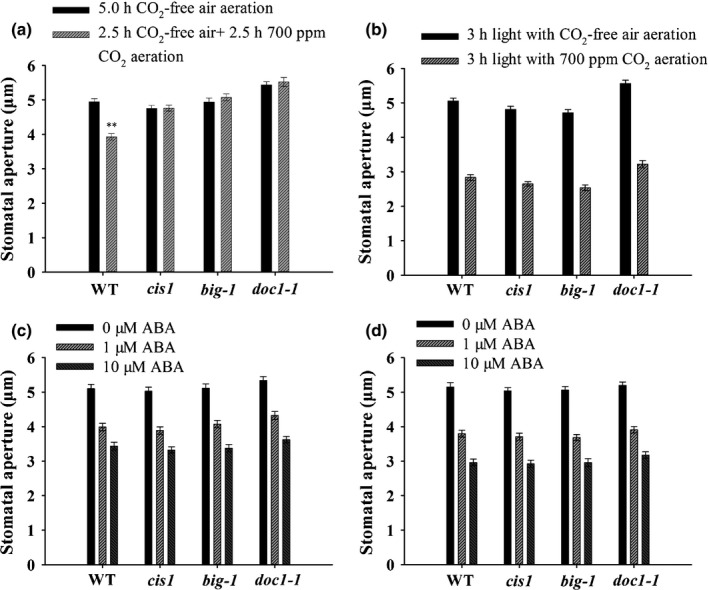
Stomatal responses of BIG gene mutants to elevated CO 2 or exogenous abscisic acid (ABA) in Arabidopsis. (a) Elevated CO 2‐induced stomatal closure is impaired in BIG gene mutants. Values are mean ± SE (Mann–Whitney rank sum test, **, P ≤ 0.001, n = 40). (b) Elevated CO 2‐induced inhibition of stomatal opening is not compromised in BIG gene mutants. Error bars represent SE (n = 40). (c) ABA‐induced stomatal closure is not compromised in BIG gene mutants. Bars are mean ± SE (n = 40). (d) The inhibition of light‐induced stomatal opening by ABA is not compromised in BIG gene mutants. Values are mean ± SE (n = 40). WT, wild‐type.
BIG is required for the activation of S‐type anion channels by elevated bicarbonates
S‐type anion channels are recognized as one of the main players in guard cell signaling. They mediate the release of anions from guard cells and promote stomatal closure in response to diverse stimuli, including increased [CO2] (Kollist et al., 2011; Wang et al., 2016). An increase in the cytoplasmic bicarbonate concentration activates S‐type anion channels in guard cells and correlates with elevated [CO2]‐induced stomatal closure in diverse mutant backgrounds (Vahisalu et al., 2008; Xue et al., 2011; Merilo et al., 2013). To understand the role of BIG in guard cell signaling further, we investigated whether the activation of S‐type anion channels by applied bicarbonate was impaired by mutations in BIG. In WT guard cell protoplasts, large anion currents were recorded when the pipette solution contained 11.5 mM free bicarbonate (Fig. 5b). However, in guard cell protoplasts of the doc1‐1 and big‐1 mutant alleles, lower anion currents were activated by the same concentration of bicarbonate in the pipette solution (Fig. 5e,h). At a voltage of −145 mV, the average activated currents were −39.7 ± 4.6 pA for WT (Fig. 5c), −20.0 ± 2.0 pA for the doc1‐1 mutant (Fig. 5f) and −16.8 ± 1.8 pA for the big‐1 mutant (Fig. 5i). The differences between WT and each mutant allele of BIG were statistically significant (P ≤ 0.01). These results demonstrate that the BIG protein is required for elevated intracellular bicarbonate‐induced activation of guard cell plasma membrane S‐type anion channel currents that function in CO2‐induced stomatal closure and further reinforce the importance of BIG in stomatal closure.
Figure 5.
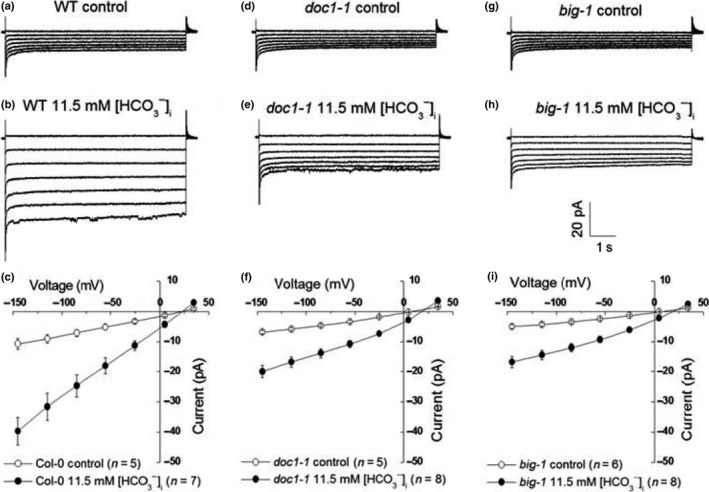
Bicarbonate‐activated S‐type anion currents were suppressed in BIG mutant guard cell protoplasts. (a) Typical recording in wild‐type (WT) guard cell protoplasts without bicarbonate. (b) Typical recording of 11.5 mM [HCO 3 −]i‐activated S‐type anion currents in WT guard cell protoplasts. (c) Average current–voltage relationships of whole‐cell currents as recording in (a) (open circles, n = 5) and (b) (closed circles, n = 7). Error bars represent ± SE. (d) Representative recording in doc1‐1 mutant guard cell protoplasts without bicarbonate added to the pipette solution. (e) Representative whole‐cell current recording in doc1‐1 mutant guard cell protoplasts with 11.5 mM [HCO 3 −]i added to the pipette solution. (f) Average current–voltage relationships of whole‐cell currents as recording in (d) (open circles, n = 5) and (e) (closed circles, n = 8). Error bars represent ± SE. (g) Representative recording in big‐1 mutant guard cell protoplasts without bicarbonate added to the pipette solution. (h) Representative whole‐cell current recording in big‐1 mutant guard cell protoplasts with 11.5 mM [HCO 3 −]i bicarbonate added to the pipette solution. (i) Average current–voltage relationships of whole‐cell currents as recording in (g) (open circles, n = 6) and (h) (closed circles, n = 8). Error bars represent ± SE.
Discussion
BIG is involved in stomatal closure induced by elevated CO2, but not in elevated CO2‐induced inhibition of stomatal opening
We conducted a genetic screen that resulted in the identification of a novel allele of the BIG gene that we call CIS1, which plays a regulatory role in stomatal function and development. Our phenotypic analyses revealed that CIS1 is involved in the reduction in stomatal conductance induced by elevated CO2 (Figs 1b,c, S1). On the surface of a leaf, during the day, stomata are exposed to frequently conflicting signals from the environment. Guard cells integrate these signals and the overall result is the optimization of gas exchange under the prevailing environmental conditions. Looking at this more closely, in the case of stomatal closure, it is necessary to stimulate the processes associated with the loss of guard cell turgor, whilst simultaneously inhibiting the cellular reactions involved in solute accumulation and stomatal opening. The opening and closure responses are physiologically distinct and are not the reverse of each other (Assmann, 1993; Li et al., 2000). When we investigated the role of BIG in these processes, we found, intriguingly, that it was only involved in elevated CO2‐induced stomatal closure. In marked contrast, all of the BIG mutants exhibited WT behavior in our bioassay of CO2 inhibition of light‐stimulated stomatal opening (Fig. 4a,b). To extend our investigation of the role of BIG in the regulation of stomatal aperture, we also investigated whether it played a role in stomatal closure induced by ABA. The data in Fig. 4(c,d) clearly indicate that BIG is not involved in ABA‐promoted closure or in ABA‐inhibited light‐induced opening. Because BIG encodes a protein which, in guard cells, is only involved in CO2‐induced closure and not CO2‐inhibited opening, this makes it possible, at the molecular level, to distinguish, and to start to define, these different processes. In this sense, these data fit well with the observation that, in molecular terms, ABA‐induced stomatal closure is distinct from the inhibition of opening by ABA. Examples include GPA1, which is involved in ABA inhibition of opening, but not in closure (Wang et al., 2001), a sphingosine‐1‐phosphate phosphatase, long‐chain base phosphate lyase double mutant (sppasedpl1), which displays WT behavior during ABA‐induced closure, but is slightly impaired in the ABA inhibition of stomatal opening response (Worrall et al., 2008), PI‐phospholipase C, which is involved in the ABA‐inhibition of opening, but not closure (Mills et al., 2004), and the observation that some members of the PYR/PYL ABA receptor family involved in stomatal opening inhibition are different from those involved in stomatal closure induction (Yin et al., 2013). The second striking result to emerge from these experiments is that BIG is not involved in ABA‐induced reductions in stomatal aperture (Fig. 4c,d). This suggests that the BIG protein lies upstream of the point of convergence of the guard cell CO2 and ABA signaling pathways (Webb & Hetherington, 1997; Xue et al., 2011; Merilo et al., 2013; Chater et al., 2015; Jakobson et al., 2016; Yamamoto et al., 2016). Looking downstream of the point of convergence, it is well known that both ABA‐ and CO2‐induced stomatal closure involve the activation of slow anion channels (Kim et al., 2010; Assmann & Jegla, 2016; Engineer et al., 2016). Our data reveal that mutations in BIG depressed the activation of S‐type anion channels by bicarbonate (Fig. 5), in line with the impaired elevated [CO2]‐induced stomatal closure. A recent study by Yamamoto et al. (2016) has provided evidence that different parts of SLAC1 are separately responsible for sensing ABA and CO2 signals. It is the transmembrane domain of SLAC1 channels that perceives CO2 signals, in contrast with the N‐ and C‐terminal ends of SLAC1 which are responsible for ABA signaling in Arabidopsis (Brandt et al., 2015; Yamamoto et al., 2016). Further investigation is needed to determine whether the activation of S‐type anion channels by ABA is affected by the loss of BIG gene function.
BIG is also involved in the control of stomatal development by elevated CO2
Figure 3(a) shows that mutations in BIG result in significant increases in guard and epidermal pavement cell densities, consistent with the findings of Guo et al. (2013). Growth at elevated [CO2] typically results in a reduction in stomatal index and density (Hetherington & Woodward, 2003; Assmann & Jegla, 2016; Engineer et al., 2016). The results in Fig. 3(b,c) clearly show that, in marked contrast with WT, the stomatal indices and density of BIG mutants increased when the plants were grown at 1000 ppm CO2. The epidermal cell densities in the mutants remained significantly higher than those of WT at this elevated [CO2] (Fig. S3). It is likely, as with ßca1ca4, epf2 and hic mutants (Gray et al., 2000; Engineer et al., 2014), that loss of BIG function relieves the elevated [CO2]‐mediated repression of stomatal development. How might BIG bring about an effect on CO2‐mediated stomatal development? One possibility that would merit future investigation is that this is an auxin‐related response. The BIG gene has been reported to encode a protein associated with auxin transport (Gil et al., 2001; Kanyuka et al., 2003) and is specifically required in the process by which auxin inhibits endocytosis and promotes its own efflux from cells (Paciorek et al., 2005). In this context, it is worth noting that evidence is emerging that auxin inhibits stomatal development. Mutants disrupted in the TAA1/TAR auxin biosynthesis or polar auxin transport and auxin signaling, as observed in multiple tir1/afb auxin receptor mutants, cause stomatal clustering (Balcerowicz et al., 2014; Le et al., 2014; Zhang et al., 2014). However, we observed no stomatal clustering in the cis1 and related mutants. Further work will be required to reveal whether disruptions to auxin signaling underlie the BIG stomatal mutant phenotype.
In conclusion, we demonstrate that, in Arabidopsis, the BIG protein is involved in the elevated [CO2]‐mediated control of stomatal closure and density. Our results reveal that we have identified a component which is involved in the signaling pathway by which elevated CO2 promotes stomatal closure. However, BIG is not involved in the elevated [CO2]‐mediated inhibition of light‐induced opening or in stomatal closure initiated by ABA. These data indicate that elevated [CO2]‐mediated closure and inhibition of opening are, in molecular terms, distinguishable. Our data suggest that BIG lies upstream of the point of convergence of ABA and CO2, or resides in an as yet undefined parallel signaling pathway that converges at or above the SLAC1 ion channel.
Author contributions
A.M.H. conceived the study. Y‐K.L. and A.M.H. designed the research. Y‐K.L., J.H., R‐X.Z., K.P., C.T., S.L., S.X., A.L., H.H., J.Z., K.E.H. and K.H. conducted the experiments. J.H., J.K., M.R.M., J.E.G., J.I.S., Y‐K.L. and A.M.H. analyzed the data. A.M.H., Y‐K.L. and J.E.G. wrote the manuscript. All authors read and approved the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 The big‐1 mutant fails to display elevated (800 ppm) CO2‐induced reduction in stomatal conductance.
Fig. S2 PCR amplification of the BIG fragment from cDNAs of wild‐type (WT) and mutant plants.
Fig. S3 Epidermal cell density of wild‐type (WT) and BIG gene mutant seedlings grown at elevated (1000 ppm) [CO2].
Notes S1 Determination of the intron–exon structure of BIG by DNA sequencing.
Acknowledgements
The authors are grateful to Professor H.M.O. Leyser (University of Cambridge, UK) for the gift of the EMS‐mutagenized Arabidopsis population. Y‐K.L. acknowledges the National Key Research and Development Program (2016YFD0100600) and the National Natural Science Foundation of China (31171356, 31470360) for providing research funding. A.M.H. and J.E.G. acknowledge the support of the UK Biotechnological and Biological Sciences Research Council (BB/J002364/1). Research in J.I.S.'s laboratory was supported by the National Science Foundation (MCB‐16162360 and National Institutes of Health (NIH) (GM060396) grants. Research in J.K.'s laboratory was supported by grants from the Deutsche Forschungsgemeinschaft (DFG). S.X. was supported by the National Natural Science Foundation of China (31670267, 31770283). J.K., A.M.H. and J.I.S. acknowledge support from Human Frontier Science Program (HFSP). H.H. received support from the 1000‐talents Plan for young researchers from China and the Fundamental Research Funds for the Central Universities (2662017PY034).
Contributor Information
Yun‐Kuan Liang, Email: ykliang@whu.edu.cn.
Alistair M. Hetherington, Email: Alistair.Hetherington@bristol.ac.uk.
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. 1999. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant Journal 19: 735–747. [DOI] [PubMed] [Google Scholar]
- Assmann SM. 1993. Signal transduction in guard cells. Annual Review of Cell Biology 9: 345–375. [DOI] [PubMed] [Google Scholar]
- Assmann SM, Jegla,T . 2016. Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2 . Current Opinion in Plant Biology 33: 157–167. [DOI] [PubMed] [Google Scholar]
- Balcerowicz M, Ranjan A, Rupprecht L, Fiene G, Hoecker U. 2014. Auxin represses stomatal development in dark‐grown seedlings via Aux/IAA proteins. Development 141: 3165–3176. [DOI] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG, Poretsky E, Belknap TF, Waadt R, Aleman F et al 2015. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: 03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang Y‐K, McLachlan DH, Casson S, Isner JC, Wilson I et al 2015. Elevated CO2‐induced responses in stomata require ABA and ABA signaling. Current Biology 25: 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin F. 1904. On a self‐recording method applied to the movements of stomata. Botanical Gazette 37: 81–105. [Google Scholar]
- Doheny‐Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE. 2012. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society of London B: Biological Sciences 367: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CB, Ghassemian M, Anderson JC, Peck SC, Hu H, Schroeder JI. 2014. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 513: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CB, Hashimoto‐Sugimoto M, Negi J, Israelsson‐Nordström M, Azoulay‐Shemer T, Rappel WJ, Iba K, Schroeder JI. 2016. CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends in Plant Science 21: 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Leitch IL, Ruszala EM, Hetherington AM, Beerling DJ. 2012. Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. 2001. BIG: a calossin‐like protein required for polar auxin transport in Arabidopsis . Genes Development 15: 1985–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JE, Holroyd GH, Van Der Lee FM, Baharmi AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM. 2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716. [DOI] [PubMed] [Google Scholar]
- Guo X, Lu W, Ma Y, Qin Q, Hou S. 2013. The BIG gene is required for auxin‐mediated organ growth in Arabidopsis. Planta 237: 1135–1147. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K. 2006. Arabidopsis HT1 kinase controls stomatal movements in response to CO2 . Nature Cell Biology 8: 391–397. [DOI] [PubMed] [Google Scholar]
- Hashimoto‐Sugimoto M, Negi J, Monda K, Higaki T, Isogai Y, Nakano T, Hasezawa S, Iba K. 2016. Dominant and recessive mutations in the Raf‐like kinase HT1 gene completely disrupt stomatal responses to CO2 in Arabidopsis . Journal of Experimental Botany 67: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424: 901–908. [DOI] [PubMed] [Google Scholar]
- Hõrak H, Sierla M, Tõldsepp K, Wang C, Wang Y‐S, Nuhkat M, Valk E, Pechter P, Merilo E, Salojärvi J et al 2016. A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2‐induced stomatal closure. Plant Cell 28: 2493–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HH, Boisson‐Dernier A, Israelsson‐Nordstrom M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. 2010. Carbonic anhydrases are upstream regulators of CO2‐controlled stomatal movements in guard cells. Nature Cell Biology 12: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Law SR, Narsai R, Duncan O, Lee JH, Zhang B, Van Aken O, Radomiljac JD, Van Der Merwe M, Yi K et al 2014. A functional antagonistic relationship between auxin and mitochondrial retrograde signaling regulates ALTERNATIVE OXIDASE1a expression in Arabidopsis thaliana . Plant Physiology 165: 1233–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson L, Vaahtera L, Tõldsepp K, Nuhkat M, Wang C, Wan YS, Hõrak H, Valk E, Pechter P, Sindarovska Y et al 2016. Natural variation in Arabidopsis Cvi‐0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biology 14: e2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ. 2003. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant Journal 35: 57–70. [DOI] [PubMed] [Google Scholar]
- Kasajima I, Ohkama‐Ohtsun N, Ide Y, Hayashi H, Yoneyama T, Suzuki Y, Naito S, Fujiwara T. 2007. The BIG gene is involved in regulation of sulfur deficiency‐responsive genes in Arabidopsis thaliana . Physiologia Plantarum 129: 351–363. [Google Scholar]
- Kim TH, Böhmer M, Hu HH, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Jossier M, Laanemets K, Thomine S. 2011. Anion channels in plant cells. FEBS Journal 278: 4277–4292. [DOI] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M et al 2014. Auxin transport and activity regulate stomatal patterning and development. Nature Communications 5: 3090. [DOI] [PubMed] [Google Scholar]
- Lease KA, Wen JQ, Li J, Doke JT, Liscum E, Walker JC. 2001. A mutant Arabidopsis heterotrimeric G‐protein β subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H‐M, Altschmied L, Chory J. 1994. Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Development 8: 339–349. [DOI] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM. 2000. Regulation of abscisic acid‐induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287: 300–303. [DOI] [PubMed] [Google Scholar]
- Liang Y‐K, Xie XD, Lindsay SE, Wang YB, Masle J, Williamson L, Leyser O, Hetherington AM. 2010. Cell wall composition contributes to the control of transpiration efficiency in Arabidopsis thaliana . Plant Journal 64: 679–686. [DOI] [PubMed] [Google Scholar]
- López‐Bucio J, Hernández‐Abreu E, Sánchez‐Calderón L, Pérez‐Torres A, Rampey RA, Bartel B, Herrera‐Estrella L. 2005. An auxin transport independent pathway is involved in phosphate stress‐induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiology 137: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheibel W‐R. 2000. Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiology 123: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield TA, Hetherington AM, Atkinson CJ. 1990. Some current aspects of stomatal physiology. Annual Review of Plant Physiology and Plant Molecular Biology 41: 55–75. [Google Scholar]
- Merilo E, Laanemets K, Hu HH, Xue S, Jakobson L, Tulva I, Gonzalez‐Guzman M, Rodriguez PL, Schroeder JI, Broschè M et al 2013. PYR/RCAR receptors contribute to ozone‐, reduced air humidity‐, darkness‐, and CO2‐induced stomatal regulation. Plant Physiology 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli AC, Gently B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J. 2002. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant Journal 30: 601–609. [DOI] [PubMed] [Google Scholar]
- Mills LN, Hunt L, Leckie CP, Aitken FL, Wentworth M, McAinsh MR, Gray JE, Hetherington AM. 2004. The effects of manipulating phospholipase C on guard cell ABA‐signalling. Journal of Experimental Botany 55: 199–204. [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang W, Deng F, Zhao J, Wang X. 2006. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312: 264–266. [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai‐Yamada M, Uchimiya H, Hashimoto M, Iba K. 2008. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrášek J, Stierhof Y‐D, Kleine‐Vehn J, Morris DA, Emans N, Jürgens G, Geldner N et al 2005. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256. [DOI] [PubMed] [Google Scholar]
- Parsons K, Nakatani Y, Nguyen MD. 2015. p600/UBR4 in the central nervous system. Cellular and Molecular Life Sciences 72: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Ladyman JA. 1988. Isolation and characterization of a barley mutant with abscisic‐acid‐insensitive stomata. Planta 173: 73–78. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. 1997. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. 2009. Calcium elevation‐dependent and attenuated resting calcium‐dependent abscisic acid induction of stomatal closure and abscisic acid‐induced enhancement of calcium sensitivities of S‐type anion and inward‐rectifying K+ channels in Arabidopsis guard cells. Plant Journal 59: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponsel VM, Schmidt FW, Porter SG, Nakayama M, Kohlstruk S, Estelle M. 1997. Characterization of new gibberellin‐responsive semidwarf mutants of Arabidopsis. Plant Physiology 115: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Pan Y, Jia J, Zhang H, Bai F, Zhang P, Zhu H, He Y et al 2015. A molecular pathway for CO2 response in Arabidopsis guard cells. Nature Communications 6: 6057. [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R et al 2008. SLAC1 is required for plant guard cell S‐type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS. 2005. Guard cell metabolism and CO2 sensing. New Physiologist 165: 665–682. [DOI] [PubMed] [Google Scholar]
- Wang C, Hu H, Qin X, Zeise B, Xu D, Rappel WJ, Boron WF, Schroeder JI. 2016. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2‐permeable PIP2; 1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell 28: 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM. 2001. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072. [DOI] [PubMed] [Google Scholar]
- Wang YB, Holroyd G, Hetherington AM, Ng CKY. 2004. Seeing ‘cool’ and ‘hot’‐infrared thermography as a tool for non‐invasive, high‐throughput screening of Arabidopsis guard cell signalling mutants. Journal of Experimental Botany 55: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Webb AAR, Hetherington AM. 1997. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiology 114: 1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from pre‐industrial levels. Nature 327: 617–618. [Google Scholar]
- Woodward FI, Kelly CK. 1995. The influence of CO2 concentration on stomatal density. New Phytologist 131: 311–327. [Google Scholar]
- Worrall D, Liang Y‐K, Alvarez S, Holroyd GH, Spiegel S, Panagopulos M, Gray JE, Hetherington AM. 2008. Involvement of sphingosine kinase in plant cell signalling. Plant Journal 56: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XD, Wang YB, William L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HM et al 2006. The identification of genes involved in stomatal response to reduced atmospheric relative humidity. Current Biology 16: 882–887. [DOI] [PubMed] [Google Scholar]
- Xu X‐ZS, Wes PD, Chen H, Li HS, Yu M, Morgan S, Liu Y, Montell C. 1998. Retinal targets for calmodulin include proteins implicated in synaptic transmission. Journal of Biological Chemistry 273: 31297–31307. [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. 2011. Central function of bicarbonate in S‐type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO Journal 30: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Suzuki M, Fukaki H, Morita‐Terao M, Tasaka M, Komeda Y. 2007. CRM1/BIG‐mediated auxin action regulates Arabidopsis inflorescence development. Plant Cell Physiology 48: 1275–1290. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Negi J, Wang C, Isogai Y, Schroeder JI, Iba K. 2016. The transmembrane region of guard cell SLAC1 channels perceives CO2 signals via an ABA‐independent pathway in Arabidopsis . Plant Cell 28: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Ye W, Hayashi M, Nakamura Y, Kinoshita T, Mori IC, Murata Y. 2013. Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiology 163: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ. 2014. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proceedings of the National Academy of Sciences, USA 111: E3015–E3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 The big‐1 mutant fails to display elevated (800 ppm) CO2‐induced reduction in stomatal conductance.
Fig. S2 PCR amplification of the BIG fragment from cDNAs of wild‐type (WT) and mutant plants.
Fig. S3 Epidermal cell density of wild‐type (WT) and BIG gene mutant seedlings grown at elevated (1000 ppm) [CO2].
Notes S1 Determination of the intron–exon structure of BIG by DNA sequencing.


