Abstract
Several phenomena in contemporary perinatology create challenges for analyzing pregnancy outcomes. These include recent increases in iatrogenic delivery at late preterm and early term gestation, which are incongruent with the belief that stillbirth and neonatal death risks decrease exponentially with advancing gestational age. Perinatal epidemiologists have also puzzled over the paradox of intersecting birthweight‐specific and gestational age‐specific perinatal mortality curves for decades. For example, neonatal mortality rates among preterm infants of women who smoke are substantially lower than neonatal mortality rates among preterm infants of non‐smoking women, whereas the reverse pattern occurs at term gestation. This mortality crossover is observed across several contrasts (for example, women with hypertensive disorders of pregnancy vs. normotensive women, older vs. younger women, twins vs. singletons) and outcomes (stillbirth, neonatal death, sudden infant death syndrome and cerebral palsy), and irrespective of how advancing “maturity” is defined (birthweight or gestational age). One approach proposed to address and explain these unexpected phenomena is the fetuses‐at‐risk model. This formulation involves a reconceptualization of the denominator for perinatal outcome rates from births to surviving fetuses. In this overview of the fetuses‐at‐risk model, we discuss the central tenets of the births‐based and the fetuses‐based formulations. We also describe the extension of the fetuses‐at‐risk approach to outcomes into and beyond the neonatal period and to a multivariable adaptation. Finally, we provide a substantive context by discussing biological mechanisms underlying the fetuses‐at‐risk model and contemporary obstetric phenomena that are better understood from that model than from one based on births.
Keywords: Fetuses‐at‐risk, gestational age, neonatal death, perinatal, stillbirth, survival analysis
Abbreviations
- SGA
small‐for‐gestational‐age
- SIDS
sudden infant death syndrome
Key message.
The fetuses‐at‐risk approach, which treats gestational age as survival time, enables the estimation of the incidence of birth, growth‐restriction and perinatal death, and so provides insights into important perinatal phenomena.
Enigmatic phenomena in perinatology
The contemporary perinatal landscape reveals several puzzling phenomena. One is the increase in labor induction and elective cesarean delivery in recent decades, which has led to a significant shortening of gestation. The increase in iatrogenic late preterm and early term delivery appears to conflict with the belief that stillbirth and neonatal death rates decrease exponentially with increasing gestational age 1. In fact, recent increases in iatrogenic, medically indicated early delivery, which have shortened gestational duration while reducing perinatal mortality, are congruent with perinatal death rates increasing with advancing gestation (on average).
Another puzzle is the paradox of intersecting birthweight‐specific and gestational age‐specific perinatal mortality curves 2. The paradox was first described in 1971 by Yerushalmy 3, who showed that neonatal mortality rates among low birthweight infants of smokers are substantially lower than neonatal mortality rates among low birthweight infants of non‐smokers (with the reverse being true at higher birthweight). This enigmatic effect of smoking was subsequently recognized as a general phenomenon 2, 4, 5 involving diverse exposures [twins vs. singletons 6, 7, infants of older vs. younger mothers 8, 9, African American vs. White women 10, 11, 12, women with vs. without hypertensive disorders 13] and outcomes [stillbirth and neonatal death 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, sudden infant death syndrome (SIDS) 14, 15 and cerebral palsy 16] and irrespective of how advancing “maturity” is defined (birthweight or gestational age (4–13 weeks)]. Figure 1(a) and Table 1 show rates of gestational age‐specific perinatal death among all births at and beyond 28 weeks of gestation in the USA from 2011 to 2013. At preterm gestation, women with hypertensive disorders of pregnancy have lower perinatal mortality than women without hypertension, whereas at 38 weeks of gestation and beyond, the mortality advantage favors women without hypertension. Several explanations and models have been proposed to address this intriguing crossover in perinatal mortality curves. One such approach involves a reformulation of risk during fetal and infant life. This reformulation, the fetuses‐at risk approach 17, 18, 19, 20, 21, changes the denominator for perinatal outcome rates from births to surviving fetuses (Figure 1b and Table 1). In this paper, we provide an overview of the fetuses‐at‐risk model and contrast the births‐based and fetuses‐based formulations. We also describe the extension of the fetuses‐at‐risk approach to outcomes into and beyond the neonatal period and to a multivariable adaptation. We provide a substantive context by discussing biological mechanisms underlying the fetuses‐at‐risk model and contemporary obstetric phenomena that are better understood from that model than from one based on births. In the final section, we include a brief discussion of some of the criticisms directed at the fetuses‐at‐risk approach.
Figure 1.
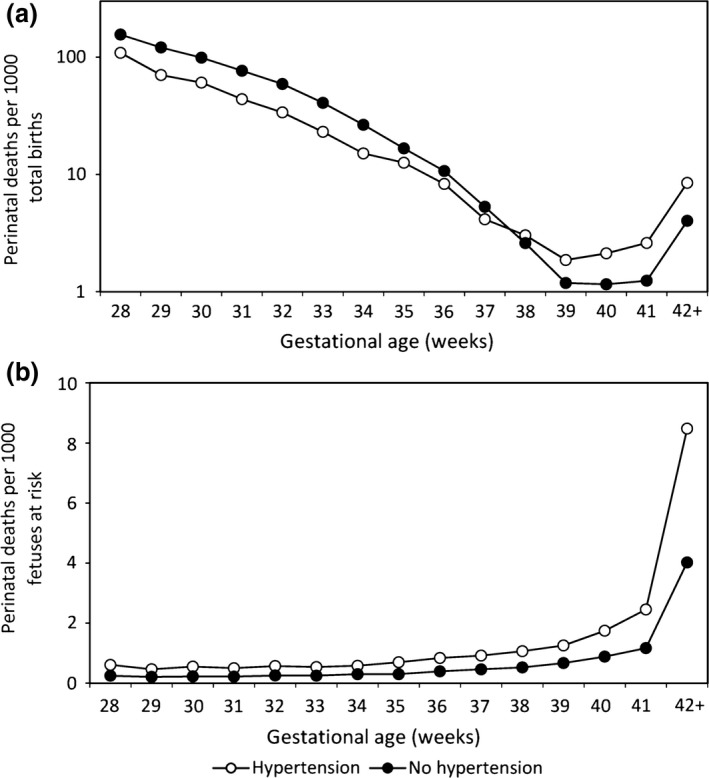
Gestational age‐specific perinatal mortality rates among women with and without hypertensive disorders of pregnancy with (a) rates calculated using the traditional perinatal formulation (per 1000 total births) and (b) rates calculated using the fetuses‐at‐risk approach (per 1000 fetuses at risk), USA, 2011–2013.
Table 1.
Stillbirth, neonatal and perinatal mortality rates among women with hypertensive disorders of pregnancy and women without hypertension, USA, 2011–2013
| Gestational age (weeks) | Live births | Stillbirths | Neonatal deaths | Fetuses at riska | Traditional (per 1000) | Fetuses at risk (per 1000) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBRa per 1000 | NMRa per 1000 | PMRa per 1000 | SBRa per 1000 | NMRa per 1000 | PMRa per 1000 | |||||
| Women with hypertensive disorders | ||||||||||
| 28 | 3834 | 286 | 162 | 734 483 | 69.4 | 42.3 | 108.7 | 0.4 | 0.2 | 0.6 |
| 29 | 4589 | 221 | 117 | 730 363 | 45.9 | 25.5 | 70.3 | 0.3 | 0.2 | 0.5 |
| 30 | 6308 | 282 | 117 | 725 553 | 42.8 | 18.5 | 60.5 | 0.4 | 0.2 | 0.5 |
| 31 | 7941 | 257 | 102 | 718 963 | 31.3 | 12.8 | 43.8 | 0.4 | 0.1 | 0.5 |
| 32 | 11 672 | 306 | 98 | 710 765 | 25.5 | 8.4 | 33.7 | 0.4 | 0.1 | 0.6 |
| 33 | 16 003 | 279 | 96 | 698 787 | 17.1 | 6.0 | 23.0 | 0.4 | 0.1 | 0.5 |
| 34 | 25 927 | 268 | 127 | 682 505 | 10.2 | 4.9 | 15.1 | 0.4 | 0.2 | 0.6 |
| 35 | 35 926 | 327 | 129 | 656 310 | 9.0 | 3.6 | 12.6 | 0.5 | 0.2 | 0.7 |
| 36 | 62 343 | 375 | 145 | 620 057 | 6.0 | 2.3 | 8.3 | 0.6 | 0.2 | 0.8 |
| 37 | 123 209 | 350 | 162 | 557 339 | 2.8 | 1.3 | 4.1 | 0.6 | 0.3 | 0.9 |
| 38 | 151 979 | 321 | 140 | 433 780 | 2.1 | 0.9 | 3.0 | 0.7 | 0.3 | 1.1 |
| 39 | 189 058 | 216 | 137 | 281 480 | 1.1 | 0.7 | 1.9 | 0.8 | 0.5 | 1.3 |
| 40 | 75 786 | 113 | 48 | 92 206 | 1.5 | 0.6 | 2.1 | 1.2 | 0.5 | 1.7 |
| 41 | 15 337 | 26 | 14 | 16 307 | 1.7 | 0.9 | 2.6 | 1.6 | 0.9 | 2.5 |
| ≥42 | 937 | 7 | 1 | 944 | 7.4 | 1.1 | 8.5 | 7.4 | 1.1 | 8.5 |
| Total | 730 849 | 3634 | 1595 | 734 483 | 4.9 | 2.2 | 7.1 | 4.9 | 2.2 | 7.1 |
| Women without hypertensive disorders | ||||||||||
| 28 | 15 767 | 1795 | 933 | 11 016 885 | 102.2 | 59.2 | 155.3 | 0.2 | 0.1 | 0.2 |
| 29 | 17 463 | 1536 | 757 | 10 999 323 | 80.8 | 43.3 | 120.7 | 0.1 | 0.1 | 0.2 |
| 30 | 23 444 | 1667 | 814 | 10 980 324 | 66.4 | 34.7 | 98.8 | 0.2 | 0.1 | 0.2 |
| 31 | 29 760 | 1597 | 801 | 10 955 213 | 50.9 | 26.9 | 76.5 | 0.1 | 0.1 | 0.2 |
| 32 | 45 291 | 1892 | 890 | 10 923 856 | 40.1 | 19.7 | 59.0 | 0.2 | 0.1 | 0.3 |
| 33 | 65 168 | 1758 | 966 | 10 876 673 | 26.3 | 14.8 | 40.7 | 0.2 | 0.1 | 0.3 |
| 34 | 120 468 | 2054 | 1190 | 10 809 747 | 16.8 | 9.9 | 26.5 | 0.2 | 0.1 | 0.3 |
| 35 | 189 664 | 2068 | 1127 | 10 687 225 | 10.8 | 5.9 | 16.7 | 0.2 | 0.1 | 0.3 |
| 36 | 383 134 | 2671 | 1452 | 10 495 493 | 6.9 | 3.8 | 10.7 | 0.3 | 0.1 | 0.4 |
| 37 | 875 405 | 2836 | 1816 | 10 109 688 | 3.2 | 2.1 | 5.3 | 0.3 | 0.2 | 0.5 |
| 38 | 1 855 937 | 2991 | 1834 | 9 231 447 | 1.6 | 1.0 | 2.6 | 0.3 | 0.2 | 0.5 |
| 39 | 4 154 044 | 2522 | 2412 | 7 372 519 | 0.6 | 0.6 | 1.2 | 0.3 | 0.3 | 0.7 |
| 40 | 2 456 048 | 1560 | 1284 | 3 215 953 | 0.6 | 0.5 | 1.2 | 0.5 | 0.4 | 0.9 |
| 41 | 714 125 | 445 | 441 | 758 345 | 0.6 | 0.6 | 1.2 | 0.6 | 0.6 | 1.2 |
| ≥42 | 43 686 | 89 | 87 | 43 775 | 2.0 | 2.0 | 4.0 | 2.0 | 2.0 | 4.0 |
| Total | 10 989 404 | 27 481 | 16 804 | 11 026 885 | 2.5 | 1.5 | 4.0 | 2.5 | 1.5 | 4.0 |
NMR, neonatal mortality rate; PMR, perinatal mortality rate; SBR, stillbirth rate. Fetuses‐at‐risk mortality rates provided in this Table are cumulative incidence rates.
The number of fetuses at risk is calculated by summing all live births and stillbirths at that gestational age and beyond. Traditional birth‐based mortality rates are expressed as deaths per 1000 total births (or live births), while fetuses‐at‐risk rates are expressed per 1000 fetuses at risk.
The births‐based approach
Although the perinatal period embraces both fetal and infant life, a clear distinction is usually made between events occurring before and after birth 19. This distinction is particularly evident in the separate and overlapping time scales used to measure the duration of life, namely, gestational age and chronological age. Birth is the end of the gestational age scale and the beginning of the chronological age scale. The overlapping nature of the two scales can lead to incongruent situations unless gestational age differences are recognized when evaluating chronological age‐related issues. Neonatologists caring for preterm infants integrate the two time scales into a single “corrected gestational age” scale, which they refer to as “postmenstrual age”. Nevertheless, the widespread use of dual time scales and the primacy accorded to chronological age discounts the continuum with pregnancy.
For stillbirth and neonatal death, the births‐based approach naturally leads to estimation of gestational age‐specific and birthweight‐specific rates. Gestational age‐specific stillbirth rates are calculated by dividing the number of stillbirths at any gestational week by the number of total births (live births plus stillbirths) at that gestational week. Gestational age‐specific neonatal death rates are similarly calculated by dividing the number of neonatal deaths at a particular gestational week by the number of live births at that gestational week. Birthweight‐specific stillbirth (and neonatal death) rates are calculated in analogous fashion, with the number of deaths in a given birthweight category as the numerator and the number of total births (or live births) in the same birthweight category as the denominator. Under this formulation, rates of stillbirth and neonatal death decline exponentially as gestational age and birthweight increase (Figure 2a,b and Table 1). Although most perinatologists have abandoned the births‐based approach for estimating stillbirth risk across pregnancy, commitment to this model occurs when analyses are stratified by gestational age or when gestational age (or preterm birth) is included as an independent variable in logistic regression analyses 22, 23, 24, 25.
Figure 2.
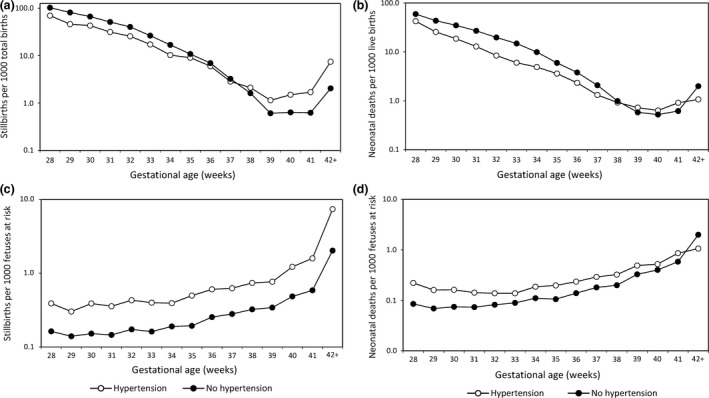
Gestational age‐specific stillbirth and gestational age‐specific neonatal mortality rates among women with and without hypertensive disorders of pregnancy with rates calculated using the traditional perinatal formulation (per 1000 total births; (a) and (b), respectively) and rates calculated using the fetuses‐at‐risk approach (per 1000 fetuses at risk; (c) and (d), respectively), USA, 2011–2013.
The fetuses‐at‐risk approach for stillbirth
The births‐based calculation of gestational age‐specific stillbirth rates was challenged in 1987 by Yudkin et al. 17, who argued that rates of stillbirth at any gestation should be estimated based on the fetuses at risk of stillbirth at that gestation, i.e. delivered and undelivered fetuses surviving to that gestation. Figure 3 provides a schematic depiction of the course of a cohort of pregnancies and the fetuses‐based calculation of gestational age‐specific stillbirth rates. Unlike the births‐based formulation, the fetuses‐based approach treats gestational age as survival time and allows the estimation of the incidence of stillbirth among a population of surviving fetuses. Rates of stillbirth now increase as gestational age advances. The increase is relatively small on an arithmetic scale (Figure 2c), however – unlike the exponential decline in stillbirth rates observed under the births‐based model (Figure 2a and Table 1).
Figure 3.
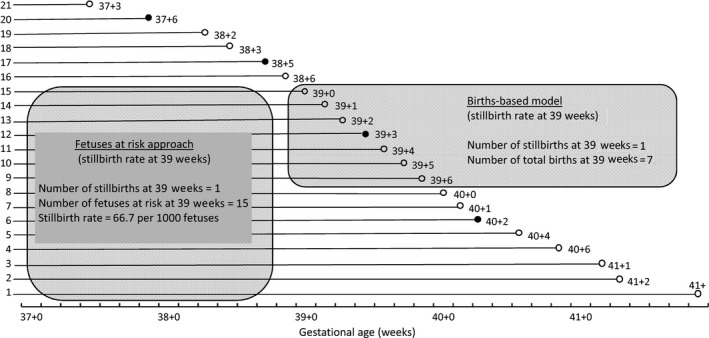
Schematic depiction of a cohort of pregnancies showing the calculation of the traditional gestational age‐specific stillbirth rate and the calculation of the stillbirth rate (cumulative incidence) under the fetuses‐at‐risk formulation.
The Yudkin et al. formulation of stillbirth risk was a revolutionary change from the births‐based treatment of gestational age and birthweight as prognostic marker variables. In epidemiological terms, Yudkin et al.'s formulation measured the cumulative incidence of stillbirth, with stillbirths occurring within a given gestational week counted as incident cases (numerator) and all the fetuses surviving to the beginning of that gestational week (denominator) considered at risk of stillbirth. Recognition of gestational age as survival time resulted in additional applications of the fetuses‐at‐risk approach, including studies on labor induction for women with prolonged pregnancy 26 and modifications to the original proposition by Yudkin et al. 26, 27, 28, 29, 30, 31, 32.
Cumulative incidence vs. incidence density of stillbirth
Calculating the incidence of stillbirth using the number of surviving fetuses entering the gestational week of interest as the denominator estimates the cumulative incidence of stillbirth. Cumulative incidence is particularly useful, because it estimates the risk (probability) of stillbirth over a specified time period: one gestational week in this instance. Incidence density is an alternative measure of risk widely used in epidemiological studies. It accounts for time at risk, since in many situations, subjects may end their contribution to follow up (and therefore should be “censored”) for reasons other than occurrence of the outcome 33. One modification to the Yudkin et al. formulation involves estimation of incidence density, obtained by quantifying the number of stillbirths that occur per unit of fetal‐time (for example, in one fetal‐week). An approximate estimate of the incidence density of stillbirth at any gestational week can be obtained by averaging the number of fetuses at the beginning and the end of the gestational week of interest 30, 32. This assumes that losses (through live birth or stillbirth) are distributed uniformly across the gestational week. A more accurate estimate of incidence density would require censoring fetuses on the day of death in utero or delivery and thereby calculating the exact number of days that fetuses spend in the gestational week at issue. Figure 4 shows the course of a pregnancy cohort and the calculations of different measures of stillbirth incidence. Although the numbers of fetuses, stillbirths and live births in Figure 4 are fictitious, the example illustrates the fact that births are not distributed evenly through each gestational week.
Figure 4.
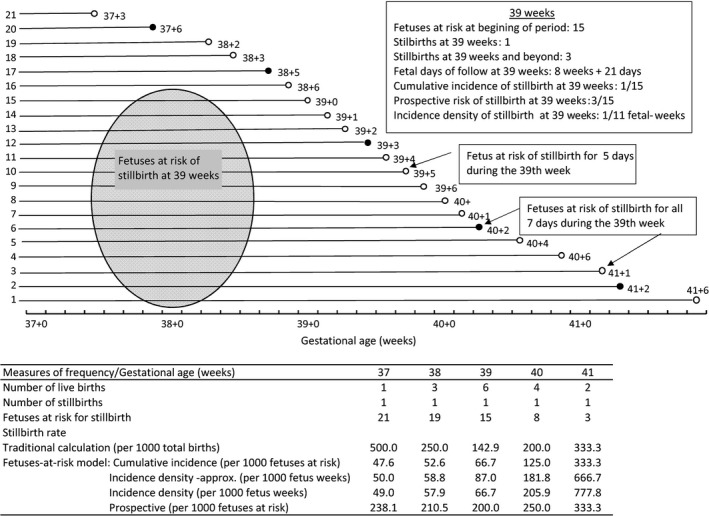
Schematic depiction of a cohort of pregnancies showing the calculation of the traditional gestational age‐specific stillbirth rates and different subtypes of stillbirth incidence calculated under the fetuses‐at‐risk formulation.
Cumulative incidence and incidence density are widely used in clinical and epidemiological studies to quantify disease (or other outcome) incidence, and their relative advantages and disadvantages vary with context. Cumulative incidence approximates incidence density when the time interval over which cumulative incidence is calculated is small 33. Thus, Yudkin et al.'s calculation of the cumulative incidence of stillbirth over a 1‐week period approximates incidence density, especially at early gestation when few births occur. However, at gestation ages between 38 and 40 weeks, when large numbers of births occur, the two measures diverge. But in most situations, the patterns of stillbirth incidence are likely to be similar, irrespective of whether cumulative incidence or incidence density is estimated.
Prospective risk of stillbirth
A second modification proposed to Yudkin et al.'s model of stillbirth estimates the incidence of stillbirth at and beyond the gestational period in question: the prospective risk of stillbirth 26, 30. This risk estimates the cumulative incidence over the remainder of the pregnancy, without specifying the absolute duration over which the incidence is estimated. Hence, the prospective risk of stillbirth at 37 weeks of gestation involves several weeks of follow‐up time, whereas the duration of follow‐up time for estimating the prospective risk of stillbirth at 41 weeks is much shorter (Figure 4). The prospective stillbirth risk quantifies stillbirths that would be averted if all pregnancies were delivered at the beginning of the specified gestational week. One problem with this measure of risk is the challenge of comparing prospective risks estimated over varying periods of time. Whereas cumulative incidence and incidence density calculations show slowly rising stillbirth risk with increasing pregnancy duration, the variable follow‐up periods used in calculating the prospective risk of stillbirth lead to a higher risk at 38 weeks than at 42 weeks and a doubling of stillbirth risk from 24 weeks to 43 weeks, gestation 26. Also, contrasting the hypothetical reductions in stillbirth risk associated with delivering all pregnancies at a specific gestational age works well for prolonged pregnancy but needs to be set against the complications of immaturity at preterm and early term gestation 29. The prospective risk of stillbirth can nonetheless be of some value in the clinical setting, especially at late gestation, both to prognosticate for individual women and to help healthcare providers balance the risks of intervention with those of watchful waiting.
Implications of the fetuses‐at‐risk model for stillbirth
Whereas the births‐based model of stillbirth shows a relatively lower stillbirth risk among women with hypertensive disorders of pregnancy (versus those without hypertensive disorders) at early gestation and a higher stillbirth risk at later gestation (Figure 2a), the risk of stillbirth under the fetuses‐at‐risk model is consistently higher among women with hypertensive disorders at all gestational ages (Figure 2c). Under the fetuses‐at‐risk model, stillbirth risks are also consistently higher at all gestational ages in other vulnerable groups (such as smokers and twins), in contrast to the crossover pattern seen under the births‐based approach. However, this phenomenon of a consistently higher stillbirth risk among vulnerable groups under the fetuses‐at‐risk approach was not appreciated until recently 7, as Yerushalmy's paradox was mostly viewed as a postnatal phenomenon involving neonatal or infant deaths.
The extended fetuses‐at‐risk model for pregnancy‐related phenomena
More recently, the fetuses‐at‐risk approach has been extended beyond stillbirth to encompass other outcomes that have their origins in pregnancy 7, 19. Fetuses are considered candidates for estimating risks of outcomes, even those manifesting in the postnatal period, provided the pathogenesis of the outcomes of the interest was established during gestation. The simplest outcome relevant to the extended fetus‐at‐risk approach is the occurrence of birth itself. The risk of birth at any gestation is calculated as the number of fetuses delivered at that gestation divided by the number of fetuses at that gestation 19. The rate of preterm birth (<37 weeks) can be viewed as the cumulative incidence of birth <37 weeks of gestation.
The extension of the fetuses‐at‐risk approach to pregnancy‐related outcomes such as fetal growth restriction, neonatal death and cerebral palsy has been the source of some controversy 34, 35, 36, 37, 38, 39, 40. Fetal growth restriction has been traditionally inferred from measurements of birthweight‐for‐gestational age, based on norms created by quantifying centiles of birthweight at each gestational age. The 10th or 3rd centiles of birthweight‐for‐gestational age are the cut‐offs typically used for identifying small‐for‐gestational‐age (SGA) infants, whereas the 90th or 97th centile identifies large‐for‐gestational‐age infants. The higher morbidity and mortality rates observed among SGA and large‐for‐gestational‐age infants confirm the utility of these measurements, although implicit in these indices is the assumption that fetal growth rates are constant across gestation (foe example, 10% with “true” growth restriction at each gestational week). The incongruence between the constancy of growth restriction rates across pregnancy and the exponentially declining stillbirth rates (under the births‐based formulation) has been overshadowed by the clinical utility of this framework for inferring growth restriction in utero.
Under the fetuses‐at‐risk formulation, however, the rate of gestational age‐specific growth restriction is ideally calculated among fetuses in utero, but challenges in accurately estimating fetal weight have led to the use of a proxy measure: “revealed” SGA 19. This index is calculated by dividing the number of SGA live births at any gestation by the number of surviving fetuses at that gestation. Although the utility of this index is somewhat compromised by its dependence on the birth rate, revealed SGA rates show that growth restriction increases with advancing gestation – congruent with the rise in stillbirth rates seen under the fetuses‐at‐risk model.
The fetuses‐at‐risk approach has also been used for estimating gestational age‐specific rates of neonatal death 7, 19, 28, SIDS 14, 15 and cerebral palsy 16, 41. It is hardly surprising that events like neonatal death have their origins in utero, as a majority occur during the first day or even the first hour after birth. In Canada, for example, 1430 neonatal deaths were recorded in 2013, of which 957 (67%) occurred on the first day after birth 42. Many early neonatal deaths follow severe fetal compromise in utero due to pregnancy complications (such as severe preeclampsia). The gestational age‐specific patterns of stillbirths and neonatal deaths are also very similar (Figure 2a–d and Table 1), suggesting a common or overlapping etiology. Similarly, a substantial majority of cerebral palsy cases appear to have their origins in utero, thereby justifying the use of fetuses as the denominator for estimating gestational age‐specific cerebral palsy rates 43, 44, 45, 46, 47. Some experts disagree, either because of uncertainties about the in utero pathogenesis of cerebral palsy 40 or because its diagnosis depends on a live infant or older child 34. In fact, SIDS and cerebral palsy show the same crossover paradox as that of intersecting perinatal mortality curves 14, 15, 16, 41; SIDS and cerebral palsy risks are relatively lower among vulnerable populations (such as mothers with hypertensive disorders or twins) at early gestation and higher at later gestation. However, under the fetuses‐at‐risk formulation, the risks of neonatal death, SIDS and cerebral palsy are consistently higher among women with hypertension (vs. those without hypertension) and among twins (vs. singletons) at all gestational ages. In these fetuses‐at‐risk calculations, the latent period between birth and neonatal death, SIDS or the diagnosis of cerebral palsy is discounted under the assumption that the pathological process or neurological injury responsible for death or cerebral palsy occurred before birth and was present at birth.
Postnatal outcomes such as neonatal death can result from a variety of pathological processes. For instance, early‐onset preeclampsia can cause delivery at 24 weeks of gestation, and the newborn infant is likely to die irrespective of whether or not it was compromised in utero. On the other hand, severe preeclampsia at 37 weeks of gestation can result in the live birth and neonatal death of a growth‐restricted and compromised newborn, irrespective of whether the birth was spontaneous or iatrogenic. In either case, the neonatal death would be attributable to preeclampsia: preeclampsia leading to very early delivery would be the pathogenic mechanism responsible for death in the former instance, whereas preeclampsia leading to growth restriction and compromise would be responsible for the death at 37 weeks.
The fetuses‐at‐risk approach can include maternal outcomes such as labor induction, cesarean delivery, preeclampsia and chorioamnionitis, with pregnancies‐at‐risk replacing the fetuses‐at‐risk denominator (denominator counts are the same whether pregnancies or fetuses are counted, except in the case of multi‐fetal pregnancies). Hence the risks of maternal complications such as pre‐eclampsia and chorioamnionitis increase as gestation advances 48, 49, 50, as do the risks of labor induction and cesarean delivery used to address these complications 1.
Competing risks in the extended fetuses‐at‐risk model
Numerous competing risks can affect pregnancy outcomes. Examples include the competing risks of spontaneous preterm birth vs. iatrogenic preterm birth, antepartum vs. intrapartum stillbirth and stillbirth vs. neonatal death. Causal questions affected by such competing risks are perhaps best addressed (as in state‐of‐the‐art perinatal randomized trials) by combining the two outcomes as a composite. Hence, studying perinatal death (as opposed to stillbirth and neonatal death separately) avoids the competing risks problem and is appropriate as both entities are equally relevant from a social and clinical perspective 18. Other reasons for treating stillbirth and neonatal death as a composite outcome include similar severity and similar gestational age‐specific patterns – which suggests a common or overlapping etiology (Figure 2). On the other hand, the study of etiological factors not shared by stillbirths and neonatal deaths, and evaluation of obstetric services that may have a different effect on stillbirth and neonatal death rates, would require separate analysis and reporting of the two mortality subtypes.
Left truncation and restricted cohorts
Left truncation refers to the fact that events occurring before cohort assembly can alter the experience of the study cohort. For example, many perinatal databases of live births and stillbirths are based on vital statistics and perinatal registries that begin at 20 weeks of gestation. It is therefore possible that a reproductive toxicant (for example, smoking) that increases the risk of miscarriage (before 20 weeks of gestation) among women already at higher risk (for instance, fetuses of women with abnormal placentation) leads to an artefactual protective association between maternal smoking and preeclampsia 51.
Restriction of cohorts to specific subpopulations can also lead to biased inferences and paradoxical results. For example, analyses restricted to very preterm infants from neonatal intensive care units represent the left (early gestation) part of the intersecting mortality curves paradox. Such analyses show that infants of older mothers have lower neonatal mortality rates than infants of younger mothers 8, and that infants of mothers with hypertension have lower rates of neonatal death than infants of mothers without hypertension 52.
Multivariable adaptation of the fetuses‐at‐risk model
The extended fetuses‐at‐risk model with gestational age treated as survival time lends itself to a multivariable regression analysis. The Cox (proportional hazards) model has been adapted for this purpose to incorporate the non‐proportional hazards that characterize harmful exposures with different effects in early vs. late gestation 21, 53. One excellent feature of this model is that it allows birth to be modeled as a time‐varying covariate that can be included or excluded from the model to assess how the effect of any factor is mediated through its effect on (preterm) birth 21, 53.
In this context, it is worth highlighting the consequence of introducing gestational age or birthweight as a covariate into a logistic regression model evaluating the effect of any determinant on stillbirth or neonatal death. Modeling gestational age (or birthweight) as an independent variable in a logistic model converts the analysis to the births‐based formulation (i.e. stillbirths at any gestation are assessed as a fraction of live births plus stillbirths at that gestation) and leads to the paradox of intersecting mortality curves. Logistic models that include all births at and beyond any arbitrary gestational age will provide a fetuses‐at‐risk‐type of analysis (with non‐proportional hazards averaged), provided that gestational age and birthweight are not “adjusted for” in the model.
Biological mechanisms supporting the fetuses‐at‐risk approach
Substantial animal and human biomedical research supports the fetuses‐at‐risk approach 54, 55, 56, 57, 58, 59, 60, 61, 62, 63. Studies on sheep, horses, cows and humans all show that uterine and umbilical blood flow to the fetus increases as pregnancy advances. However, blood flow normalized for fetal weight declines in the latter part of pregnancy 55, 56, 57, 58, 59, resulting in reduced oxygen and nutritional availability and higher carbon dioxide levels 58, 59, 60. Although absolute fetal size increases even in late pregnancy, the rate of growth (as a fraction of existing size) falls off considerably 61, 62, 63. Interesting fetal adaptations occur as a result of the declining support provided by the maternal and placental unit, including a decrease in fetal movements caused by neuropeptides released in response to decreasing oxygen availability 64. A large body of biomedical literature 54, 57, 63 supports the pattern of increasing fetal growth restriction, maternal complications and perinatal death with advancing gestation under the extended fetuses‐at‐risk model.
A recent study of preterm infants born to women who experienced unintentional injury during pregnancy showed a higher risk of neonatal death and serious morbidity than that observed among infants born at the same gestational age to non‐injured women 65. These findings suggest that the chronic stress associated with a suboptimal intrauterine environment can lead to fetal adaptations that protect infants born alive at preterm gestation and could represent the biological mechanism underlying the paradox by which preterm infants born to vulnerable women experience lower risks of adverse neonatal outcomes.
Randomized trial evidence supporting the fetuses‐at‐risk approach
Meta‐analysis 66 of 21 randomized trials involving 8749 women shows that cesarean delivery rates are reduced with early delivery by labor induction (rate ratio 0.89, 95% CI 0.81–0.97), whereas 17 randomized trials 66 involving 7407 women show lower perinatal mortality rates following early delivery at term gestation and beyond (rate ratio 0.31, 95% CI 0.12–0.88). Such reductions in both cesarean delivery and perinatal death achieved through early delivery are consistent with extended fetuses‐at‐risk models – which show that cesarean delivery and perinatal death risks increase as gestation advances 7, 19.
Similar support for the fetuses‐at risk model is evident in the findings of the Disproportionate Intrauterine Growth Intervention Trial At Term (DIGITAT) trial 67, which compared labor induction with expectant management among 650 pregnant women with suspected intrauterine growth restriction at term. Women assigned to the labor induction group were delivered 10 days earlier and had infants who weighed 130 g less than those born to women in the expectant management arm. Although the primary outcome of composite neonatal morbidity and mortality was not significantly different in the two groups, a large difference was observed in SGA infants <3rd centile: 12.5% in the labor induction arm vs. 30.6% in the expectant management group (p < 0.001). The finding of higher rates of growth restriction with increasing pregnancy duration is consistent with fetuses‐at‐risk models, which also show that revealed SGA rates increase as gestation advances.
Clinical insights provided by the fetuses‐at‐risk model
Many clinicians believe that labor induction leads to an increased rate of cesarean delivery. However, randomized trials robustly demonstrate that labor induction reduces, rather than increases, the risk of cesarean delivery 61. The false impression created by observations in routine obstetric practice occurs because clinicians tend to informally compare rates of cesarean delivery among women who have labor induction at any particular gestation and those with spontaneous labor at that same gestation. Such a comparison is flawed, however, because women in spontaneous labor are not an appropriate comparison group for assessing the effect of labor induction on cesarean delivery. The correct comparison group is women at the same gestation who are managed expectantly, as in randomized trials 68. The delayed delivery in the expectant management group ensures that the labor induction group will have lower rates of cesarean delivery, since rates of fetal and maternal complications (and consequently cesarean delivery) increase with advancing gestation, as seen in extended fetuses‐at‐risk models.
Another puzzling clinical phenomenon explained by the fetuses‐at‐risk model is the temporal trend in pre‐eclampsia rates in many high‐income countries. Temporal increases in age at childbirth, nulliparity, prepregnancy weight and diabetes mellitus all suggest that preeclampsia rates should increase in conjunction with the increases in these risk factors. However, most high‐income countries have recorded a fall in rates of pregnancy hypertension and preeclampsia 69. A study from New South Wales, Australia 70, reported that rates of pregnancy hypertension decreased from 9.9% in 2001 to 7.7% in 2012, with rates of preeclampsia declining from 3.3% to 2.4% over the same period (p‐value for trend <0.0001). This study estimated that the observed reduction in pregnancy hypertension was mostly due to increases in early delivery through labor induction and elective cesarean delivery. The reduction in pregnancy hypertension and preeclampsia is explained by the fetuses‐at‐risk model, which shows that pregnancy complications such as preeclampsia increase with advancing gestation 48, 49, 50.
Another temporal trend that may be explained by the extended fetuses‐at‐risk model is the fall in SGA live births observed in Canada and the USA in recent decades. Temporal trends in SGA in the USA have followed a non‐linear pattern, with substantial declines from 10.1% in 1990–92 to 8.9% in 2002–04, followed by a small increase to 9.1% in 2008–10 71. These changes do not cohere with temporal trends in maternal smoking (which decreased steadily from 1990 to 2010) but are more consistent with changes in the gestational age distribution caused by increases in early delivery.
Criticisms of the fetuses at risk model
Critics of the fetuses‐at‐risk approach argue that fetuses at a given gestation cannot serve as the appropriate denominator for postnatal outcomes at that gestation, because postnatal outcomes can occur only among fetuses born alive at that gestation. Such arguments question the assumption that postnatal outcomes (such as cerebral palsy) have their origins in utero 40, besides making the case that most fetuses at any gestation (especially at an early gestation such as 26 weeks) cannot possibly experience a postnatal outcome as they will not be born at that gestation 34.
Proponents of the fetuses‐at‐risk formulation respond that the approach is a valid epidemiological model that allows causal inferences (for example, about the effects of smoking or hypertension on perinatal death), because it treats gestational age as survival time, respects the fetus–infant continuum, incorporates latent periods, and models perinatal phenomena through the estimation of key indices such as the incidence of birth, growth restriction and perinatal death 19. On the other hand, the births‐based model represents an excellent prognostic (albeit non‐causal) model. It begins with live birth and casts survival time in utero (i.e. gestational age or a correlate, namely, birthweight) as a predictor of postnatal outcomes. The argument that only live‐born infants (but not fetuses) at a given gestation can serve as the denominator for postnatal events at that gestation is flawed, however, because all fetuses at any gestation are at risk of live birth and subsequent postnatal outcomes at that gestation. That risk is low in low‐risk women at early gestation (for example, 26 weeks) but can be better appreciated in women with severe, early‐onset pre‐eclampsia at the same gestation. In fact, it is entirely legitimate for a woman at 26 weeks of gestation to enquire about her baby's risk for an adverse outcome such as stillbirth, neonatal death or cerebral palsy. Quantification of such risk would require the follow up of a cohort of fetuses and estimation of outcome rates using fetuses as the denominator. Fetuses‐at‐risk calculations of postnatal outcome risk are analogous to estimates of age‐specific mortality from breast cancer or coronary heart disease. Such rates have been estimated by epidemiologists for decades. Arguing that such mortality rates should be calculated only among people with breast cancer or coronary heart disease essentially changes cause‐specific mortality rates to case‐fatality rates.
Another criticism of the fetuses‐at‐risk model is that it avoids but does not explain the paradox of intersecting perinatal mortality curves. The fact that the fetuses‐at‐risk approach avoids the crossover is not a weakness but a strength from the standpoint of scientific parsimony. The crossing of perinatal mortality curves observed under the births‐based formulation is problematic because the findings are biologically implausible from a causal perspective and because the births‐based model reduces parsimony by requiring an additional (interaction) term to represent the phenomenon.
The fetuses‐at‐risk model has also been criticized because innocuous factors that do not influence birth rates, growth or survival will show an increasing postnatal incidence with advancing gestation under the fetuses‐at‐risk calculation but a constant rate under the births‐based approach. However, the fetuses‐at‐risk model for postnatal outcomes is only intended for those outcomes whose pathogenesis occurs in utero (and can therefore be expected to influence the risk of birth, growth restriction or survival).
Conclusions
The fetuses‐at‐risk approach provides a survival analysis perspective for pregnancy outcomes. The approach involves a shift in emphasis from a births‐based approach to a fetuses‐based perspective. Some aspects of the approach, especially its extension to postnatal phenomena that have their origins in pregnancy, remain controversial. Yet this formulation is supported by biomedical studies (both animal and human), accounts for the fetus–infant continuum, addresses several puzzling paradoxes and provides insight into clinical phenomena that have otherwise defied explanation.
Funding
KSJ's work is supported by the BC Children's Hospital Research Institute and a Chair award (APR‐126338) from the Canadian Institutes of Health Research (CIHR). This work is supported by a CIHR grant (MOP‐142368).
Joseph KS, Kramer MS. The fetuses‐at‐risk approach: survival analysis from a fetal perspective. Acta Obstet Gynecol Scand 2018;97:454–465
Conflict of interest
KSJ and MSK have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1. Joseph KS. Theory of obstetrics: an epidemiologic framework for justifying medically indicated early delivery. BMC Pregnancy Childbirth. 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lie RT. Invited commentary: intersecting perinatal mortality curves by gestational age‐are appearances deceiving? Am J Epidemiol. 2000;152:1117–9. [DOI] [PubMed] [Google Scholar]
- 3. Yerushalmy J. The relationship of parents’ cigarette smoking to outcome of pregnancy – implications as to the problem of inferring causation from observed associations. Am J Epidemiol. 1971;93:443–56. [DOI] [PubMed] [Google Scholar]
- 4. Meyer MB, Comstock GW. Maternal cigarette smoking and perinatal mortality. Am J Epidemiol. 1972;96:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Buekens P, Wilcox AJ. Why do small twins have a lower mortality than small singletons? Am J Obstet Gynecol. 1993;168:937–41. [DOI] [PubMed] [Google Scholar]
- 6. Joseph KS. Exegesis of effect modification: biologic or spurious? Paediatr Perinat Epidemiol. 2009;23:417–20. [DOI] [PubMed] [Google Scholar]
- 7. Joseph KS, Liu S, Demissie K, Wen SW, Platt RW, Ananth CV, et al. A parsimonious explanation for intersecting perinatal mortality curves: understanding the effects of plurality and parity. BMC Pregnancy Childbirth. 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanungo J, James A, McMillan D, Lodha A, Faucher D, Lee SK, et al.; Canadian Neonatal Network . Advanced maternal age and the outcomes of preterm neonates: a social paradox? Obstet Gynecol. 2011;118:872–7. [DOI] [PubMed] [Google Scholar]
- 9. Lisonkova S, Paré E, Joseph KS. Does advanced maternal age confer a survival advantage to infants born at early gestation? BMC Pregnancy Childbirth. 2013;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilcox AJ, Russell IT. Birthweight and perinatal mortality: III. Towards a new method of analysis. Int J Epidemiol. 1986;15:188–96. [DOI] [PubMed] [Google Scholar]
- 11. Mittendorf R, Williams MA, Kennedy JL Jr, Berry RE, Herschel M, Aronson MP, et al. A hypothesis to explain paradoxical racial differences in neonatal mortality. Am J Prev Med. 1993;9:327–30. [PubMed] [Google Scholar]
- 12. Joseph KS, Demissie K, Platt RW, Ananth CV, McCarthy BJ, Kramer MS; for the Canadian Perinatal Surveillance System . A parsimonious explanation for intersecting perinatal mortality curves: understanding the effects of race and of maternal smoking. BMC Pregnancy Childbirth. 2004;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutcheon JA, Lisonkova S, Joseph KS. The epidemiology of preeclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 14. Pharoah PO, Platt MJ. Sudden infant death syndrome in twins and singletons. Twin Res Hum Genet. 2007;10:644–8. [DOI] [PubMed] [Google Scholar]
- 15. Lisonkova S, Hutcheon JA, Joseph KS. Sudden Infant Death Syndrome: a re‐examination of temporal trends. BMC Pregnancy Childbirth. 2012;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pharoah PO, Cooke T. Cerebral palsy and multiple births. Arch Dis Child Fetal Neonatal Ed. 1996;75:F174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1:1192–4. [DOI] [PubMed] [Google Scholar]
- 18. Kramer MS, Liu S, Luo Z, Yuan H, Platt RW, Joseph KS. Analysis of perinatal mortality and its components: time for a change? Am J Epidemiol. 2002;156:493–7. [DOI] [PubMed] [Google Scholar]
- 19. Joseph KS. Incidence‐based measures of birth, growth restriction and death can free perinatal epidemiology from erroneous concepts of risk. J Clin Epidemiol. 2004;57:889–97. [DOI] [PubMed] [Google Scholar]
- 20. Joseph KS. The fetuses‐at‐risk approach: causal and non‐causal models In: Louis GB, Platt RW. (eds). Reproductive and perinatal epidemiology. Oxford: Oxford University Press, 2011. pp. 243–61. [Google Scholar]
- 21. Platt RW. The fetuses‐at‐risk approach: an evolving paradigm In: Louis GB, Platt RW. (eds). Reproductive and perinatal epidemiology. Oxford: Oxford University Press, 2011. pp. 262–74. [Google Scholar]
- 22. Ota E, Ganchimeg T, Morisaki N, Vogel JP, Pileggi C, Ortiz‐Panozo E, et al. ; WHO Multi‐Country Survey on Maternal and Newborn Health Research Network . Risk factors and adverse perinatal outcomes among term and preterm infants born small‐for‐gestational‐age: secondary analyses of the WHO Multi‐Country Survey on Maternal and Newborn Health. PLoS ONE. 2014;9:e105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnesen L, Martínez G, Mainero L, Serruya S, Durán P. Gestational syphilis and stillbirth in Latin America and the Caribbean. Int J Gynaecol Obstet. 2015;128:241–5. [DOI] [PubMed] [Google Scholar]
- 24. McClure EM, Saleem S, Goudar SS, Moore JL, Garces A, Esamai F, et al. Stillbirth rates in low‐middle income countries 2010–2013: a population‐based, multi‐country study from the Global Network. Reprod Health. 2015;12(Suppl 2):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viswanath K, Rakesh PS, Chakraborty A, Prasad JH, Minz S, George K. A community based case control study on determinants of perinatal mortality in a tribal population of southern India. Rural Remote Health. 2015;15:3388. [PubMed] [Google Scholar]
- 26. Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: evaluating gestation‐specific risks of fetal and infant mortality. Br J Obstet Gynaecol. 1998;105:169–73. [DOI] [PubMed] [Google Scholar]
- 27. Feldman GB. Prospective risk of stillbirth. Obstet Gynecol. 1992;79:547–53. [PubMed] [Google Scholar]
- 28. Cotzias CS, Paterson‐Brown S, Fisk NM. Prospective risk of unexplained stillbirth in singleton pregnancies at term: population based analysis. BMJ. 1999;319:287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hilder L, Costeloe K, Thilaganathan B. Prospective risk of stillbirth: study results are flawed by reliance on cumulative prospective risk. Br Med J. 2000;320:444. [PMC free article] [PubMed] [Google Scholar]
- 30. Boulvain M, Faltin D, Ibecheole V, Irion O. Prospective risk of stillbirth: randomized trials of early induction of labor are needed. Br Med J. 2000;320:445. [PubMed] [Google Scholar]
- 31. Yudkin P, Redman C. Prospective risk of stillbirth. Impending fetal death must be identified and pre‐empted. BMJ. 2000;320:445–6. [PubMed] [Google Scholar]
- 32. Smith GC. Life‐table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol. 2001;184:489–96. [DOI] [PubMed] [Google Scholar]
- 33. Greenland S, Rothman KJ. Measures of occurrence In: Rothman KJ, Greenland S, Lash TL. (eds). Modern epidemiology, 3rd edn New York: Wolters Kluwer – Lippincott Williams and Wilkins, 2008. pp. 32–50. [Google Scholar]
- 34. Basso O. Implications of using a fetuses‐at‐risk approach when fetuses are not at risk. Paediatr Perinat Epidemiol. 2016;30:3–10. [DOI] [PubMed] [Google Scholar]
- 35. Joseph KS. A consilience of inductions supports the extended fetuses‐at‐risk model. Paediatr Perinat Epidemiol. 2016;30:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith GC. Quantifying the risk of different types of perinatal death in relation to gestational age: researchers at risk of causing confusion. Paediatr Perinat Epidemiol. 2016;30:18–9. [DOI] [PubMed] [Google Scholar]
- 37. Schisterman EF, Sjaarda LA. No right answers without knowing your question. Paediatr Perinat Epidemiol. 2016;30:20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caughey AB, Snowden JM. Measuring perinatal complications: different approaches depending on who is at risk. Paediatr Perinat Epidemiol. 2016;30:23–4. [DOI] [PubMed] [Google Scholar]
- 39. Basso O. No rates were harmed in the making of this paper: response to critiques. Paediatr Perinat Epidemiol. 2016;30:25–7. [DOI] [PubMed] [Google Scholar]
- 40. Paneth N. Candidates at risk for postnatal outcomes. Epidemiology. 2012;23:939. [DOI] [PubMed] [Google Scholar]
- 41. Joseph KS, Allen AC, Lutfi S, Murphy‐Kaulbeck L, Vincer MJ, Wood E. Does the risk of cerebral palsy increase or decrease with increasing gestational age? BMC Pregnancy Childbirth. 2003;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Infant deaths and mortality rates by age group and sex, Canada, annual. Cansim table 1020506. Cansim Multidimensional@ CHASS. Statistics Canada. Ottawa. 2017. (http://datacenter2.chass.utoronto.ca/cgi-bin/cansimdim/c2_arrays.pl).
- 43. Blair E, Stanley FJ. Intrapartum asphyxia: a rare cause of cerebral palsy. J Pediatr. 1988;112:515–9. [DOI] [PubMed] [Google Scholar]
- 44. Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487–91. [DOI] [PubMed] [Google Scholar]
- 45. Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–9. [DOI] [PubMed] [Google Scholar]
- 46. Nelson KB, Ellenberg JH. Antecedents of cerebral palsy: multivariate analysis of risk. N Engl J Med. 1986;315:81–6. [DOI] [PubMed] [Google Scholar]
- 47. Croen LA, Grether JK, Curry CJ, Nelson KB. Congenital abnormalities among children with cerebral palsy: more evidence for prenatal antecedents. J Pediatr. 2001;138:804–10. [DOI] [PubMed] [Google Scholar]
- 48. Caughey AB, Stotland NE, Escobar GJ. What is the best measure of maternal complications of term pregnancy: ongoing pregnancies or pregnancies delivered? Am J Obstet Gynecol. 2003;189:1047–52. [DOI] [PubMed] [Google Scholar]
- 49. Caughey AB, Musci TJ. Complications of term pregnancies beyond 37 weeks of gestation. Obstet Gynecol. 2004;103:57–62. [DOI] [PubMed] [Google Scholar]
- 50. Lisonkova S, Joseph KS. Incidence of pre‐eclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. Am J Obstet Gynecol 2013;209:544.e1–12. [DOI] [PubMed] [Google Scholar]
- 51. Lisonkova S, Joseph KS. Left truncation bias as a potential explanation for the protective effect of smoking on preeclampsia. Epidemiology. 2015;26:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gemmell L, Martin L, Murphy KE, Modi N, Håkansson S, Reichman B, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks’ gestation. J Perinatol. 2016;36:1067–72. [DOI] [PubMed] [Google Scholar]
- 53. Platt RW, Joseph KS, Ananth CV, Grondines J, Abrahamowicz M, Kramer MSA. proportional hazards model with time‐dependent covariates and time‐varying effects for analysis of fetal and infant death. Am J Epidemiol. 2004;160:199–206. [DOI] [PubMed] [Google Scholar]
- 54. Rurak D, Joseph KS. Deterioration in Fetal Lamb Blood Gas and Acid‐Base Status with Advancing Gestation. In: Society for Gynecologic Investigation 59th Annual Scientific Meeting, San Diego, March 2012 (Abstract F‐102).
- 55. Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor‐Tritsch I, et al. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121–5. [DOI] [PubMed] [Google Scholar]
- 56. Konje JC, Kaufmann P, Bell SC, Taylor DJ. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol. 2001;185:608–13. [DOI] [PubMed] [Google Scholar]
- 57. Link G, Clark KE, Lang U. Umbilical blood flow during pregnancy: evidence for decreasing placental perfusion. Am J Obstet Gynecol. 2007;196:489.e1–7. [DOI] [PubMed] [Google Scholar]
- 58. Bell AW, Kennaugh JM, Battaglia FC, Makowski EL, Meschia G. Metabolic and circulatory studies of fetal lamb at midgestation. Am J Physiol. 1986;250(5 Pt 1):E538–44. [DOI] [PubMed] [Google Scholar]
- 59. Bell AW, Battaglia FC, Meschia G. Relation between metabolic rate and body size in the ovine fetus. J Nutr. 1987;117:1181–6. [DOI] [PubMed] [Google Scholar]
- 60. Rurak DW, Richardson BS, Patrick JE, Carmichael L, Homan J. Oxygen consumption in the fetal lamb during sustained hypoxemia with progressive acidemia. Am J Physiol. 1990;258(5 Pt 2):R1108–15. [DOI] [PubMed] [Google Scholar]
- 61. Todros T, Ferrazzi E, Groli C, Nicolini U, Parodi L, Pavoni M, et al. Fitting growth curves to head and abdomen measurements of the fetus: a multicentric study. J Clin Ultrasound. 1987;15:95–105. [DOI] [PubMed] [Google Scholar]
- 62. Rossavik IK, Deter RL. Mathematical modeling of fetal growth: I. basic principles. J Clin Ultrasound. 1984;12:529–33. [DOI] [PubMed] [Google Scholar]
- 63. Bertino E, Di Battista E, Bossi A, Pagliano M, Fabris C, Aicardi G, et al. Fetal growth velocity: kinetic, clinical, and biological aspects. Arch Dis Child Fetal Neonatal Ed. 1996;74:F10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rurak D, Wittman B. Real‐time ultrasound assessment of body and breathing movements and abdominal diameter in fetal lambs from 55 days of gestation to term. Reprod Sci. 2013;20:414–25. [DOI] [PubMed] [Google Scholar]
- 65. Liu S, Basso O, Kramer MS. Association between unintentional injury during pregnancy and excess risk of preterm birth and its neonatal sequelae. Am J Epidemiol. 2015;182:750–8. [DOI] [PubMed] [Google Scholar]
- 66. Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labor for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2012;6:CD004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Boers KE, Vijgen SM, Bijlenga D, van der Post JA, Bekedam DJ, Kwee A, et al. ; DIGITAT study group . Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ. 2010;21:341:c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Caughey AB, Nicholson JM, Cheng YW, Lyell DJ, Washington AE. Induction of labor and cesarean delivery by gestational age. Am J Obstet Gynecol. 2006;195:700–5. [DOI] [PubMed] [Google Scholar]
- 69. Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population‐based trends in pregnancy hypertension and pre‐eclampsia: an international comparative study. BMJ Open. 2011;1:e000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roberts CL, Algert CS, Morris JM, Ford JB. Increased planned delivery contributes to declining rates of pregnancy hypertension in Australia: a population‐based record linkage study. BMJ Open. 2015;5:e009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Metcalfe A, Lisonkova S, Joseph KS. The association between temporal changes in the use of obstetrical intervention and small‐for‐gestational age live births. BMC Pregnancy Childbirth. 2015;15:233. [DOI] [PMC free article] [PubMed] [Google Scholar]


