Abstract
Background
We previously reported that serotonergic transmission plays an important role in antidepressant effects of ketamine. However, detailed mechanisms have not been elucidated. Among the serotonin receptor subtypes, the serotonin1A receptor in the medial prefrontal cortex has an important role in depression. Here, we investigated the role of the medial prefrontal cortex serotonin1A receptor and its signaling mechanism in the antidepressant effects of ketamine.
Methods
The role of serotonin1A receptor-mediated signaling mechanism (phosphoinositide-3 kinase/Akt) in the medial prefrontal cortex was examined in the mouse forced swimming test and western blotting.
Results
Ketamine exerted antidepressant effects that lasted for 24 hours, and the sustained antidepressant effects were attenuated by intra-medial prefrontal cortex injection of a serotonin1A receptor antagonist, WAY100635. The sustained antidepressant effects were mimicked by intra- medial prefrontal cortex, but not systemic, administration of a serotonin1A receptor agonist, (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT). The sustained antidepressant effects of ketamine and 8-OH-DPAT were abrogated by intra- medial prefrontal cortex injection of a phosphoinositide-3 kinase inhibitor. Ketamine increased the phosphorylation of Akt in the medial prefrontal cortex at 60 minutes after administration, which was blocked by a serotonin1A receptor antagonist and a phosphoinositide-3 kinase inhibitor. Furthermore, the sustained antidepressant effects of ketamine and 8-OH-DPAT were attenuated by pretreatment of intra-medial prefrontal cortex injection of a mechanistic target of rapamycin complex-1 inhibitor.
Conclusions
These results indicate that selective stimulation of the medial prefrontal cortex serotonin1A receptor and subsequent activation of the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin complex-1 pathway may be necessary for ketamine to exert the sustained antidepressant effects, and that this mechanism could be targeted to develop a novel and effective approach for treating depression.
Keywords: ketamine, 5-HT1A receptor, antidepressant, phosphoinositide-3 kinase, Akt
Significance Statement
Discovery of the antidepressant effects of ketamine opened up a new opportunity for the treatment of depression. However, mechanisms underlying the antidepressant effects of ketamine still remain largely unexplored. Recent evidence has indicated that serotonergic transmission mediates the antidepressant effects of ketamine. The present study demonstrated the importance of selective stimulation of the postsynaptic 5-HT1A receptor in the medial prefrontal cortex and subsequent activation of phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin complex-1 signaling in the sustained antidepressant effects of ketamine. These findings show the important role of a particular 5-HT1A receptor-mediated signaling cascade in the antidepressant actions of ketamine, which could facilitate the development of more effective antidepressants.
Introduction
Major depressive disorder (MDD) represents a major social problem, with an estimated lifetime prevalence rate in the United States of approximately 17% (Kessler et al., 2003). With the currently available antidepressants, all of which act on the monoaminergic system, it takes several weeks before the antidepressant effects are manifested, and more than 30% of patients remain resistant to a series of treatments (Rush et al., 2006). Therefore, there is a strong need to develop newer antidepressants with mechanisms of action distinct from those of the currently available medications.
Glutamatergic systems have emerged as an attractive target for the development of drugs to treat depression. Indeed, a noncompetitive N-methyl-D-aspartate receptor antagonist, ketamine, has been demonstrated to show potent and rapid antidepressant effects in patients with MDD, including those with treatment-resistant depression (Aan Het Rot et al., 2012; Berman et al., 2000; Zarate et al., 2006). Moreover, ketamine has also been reported to rapidly reduce suicidal ideation (Price and Mathew, 2015; Ionescu et al., 2016), indicating the antisuicide potential of ketamine. Collectively, the discovery of the antidepressant effects of ketamine is regarded as the most outstanding discovery in the field of depression research in over 60 years. However, ketamine also has undesirable side effects, such as psychotomimetic/dissociative symptoms and abuse potential (Krystal et al., 2013). Therefore, to develop agents that are as potent and rapid-acting as ketamine against MDD, but with better safety profiles, intensive investigations on elucidation of mechanisms underlying the antidepressant effects of ketamine are being conducted.
Recently, we and others showed that serotonergic (5-HTergic) transmission is involved in the antidepressant actions of ketamine, as demonstrated by the following. First, the antidepressant effects of ketamine were no longer observed in serotonin (5-HT)-depleted animals (Gigliucci et al., 2013; Fukumoto et al., 2014, 2016; du Jardin et al., 2016). Second, the antidepressant effects of ketamine were attenuated by 5-HT1A receptor blockade (Fukumoto et al., 2014). Third, ketamine increased 5-HT release in the medial prefrontal cortex (mPFC), likely via activation of the neurons of the dorsal raphe nucleus (DRN) (Nishitani et al., 2014; Pham et al., 2017). Therefore, it is conceivable that stimulation of the postsynaptic 5-HT1A receptor, presumably by the increased 5-HT release, may be involved in the antidepressant effects of ketamine. It has been reported that stimulation of the postsynaptic 5-HT1A receptor in the mPFC and limbic system is involved in the antidepressant response (Blier and de Montigny, 1994; Haddjeri et al., 1998;). Furthermore, the levels of 5-HT1A receptor protein and 5-HT1A receptor binding are decreased in the PFC of patients with depression (Szewczyk et al., 2009; Wang et al., 2016a). The suppression of 5-HT1A heteroreceptors in the mPFC is reported to cause depression-related phenotype (Garcia-Garcia et al., 2017). These findings suggest that postsynaptic 5-HT1A receptor in the mPFC, the brain region responsible for antidepressant actions of ketamine (Fuchikami et al., 2015; Fukumoto et al., 2016), has a critical role in depression and antidepressant actions. However, the involvement of postsynaptic 5-HT1A receptor stimulation in the mPFC in the antidepressant effects of ketamine, particularly in the sustained antidepressant effects, the effects at 24 hours after administration, has not yet been elucidated.
In the present study, we investigated the role of the mPFC 5-HT1A receptor and its downstream signaling in the antidepressant effects of ketamine. Among 5-HT1A receptor-mediated signaling mechanisms, we particularly focused on the involvement of 5-HT1A receptor-mediated phosphoinositide-3 kinase (PI3K)/Akt signaling in the mPFC, because not only is this pathway known to be a critical signaling cascade for neural plasticity (Chilmonczyk et al., 2015), but also changes in neural plasticity have been suggested to play important roles in the antidepressant actions of ketamine (Li et al., 2010; Gerhard et al., 2016; Dong et al., 2017). Moreover, because PI3K/Akt signaling activates mechanistic target of rapamycin complex 1 (mTORC1) signaling, which has also been strongly implicated in synaptogenesis (Hoeffer and Klann, 2010; Duman and Voleti, 2012; Duman et al., 2016) and antidepressant actions of ketamine (Li et al., 2010; Koike et al., 2011), we additionally investigated the involvement of mTORC1 signaling in the mPFC in the antidepressant actions of ketamine.
Materials and Methods
Animals and Housing
Eight- or 9-week-old male C57BL/6J mice (Charles River Labora tories) were used for all the experiments. The animals were maintained under controlled temperature (23°C ±3°C) and humidity (50%±20%) conditions under a 12-h-light/-dark cycle (lights on at 7:00 am). Food and water were provided ad libitum. All the studies were performed according to the guidelines of the Taisho Pharmaceutical Co., Ltd. Animal Care Committee, and met the Japanese Experimental Animal Research Association standards, as defined in the Guidelines for Animal Experiments.
Drug Administration
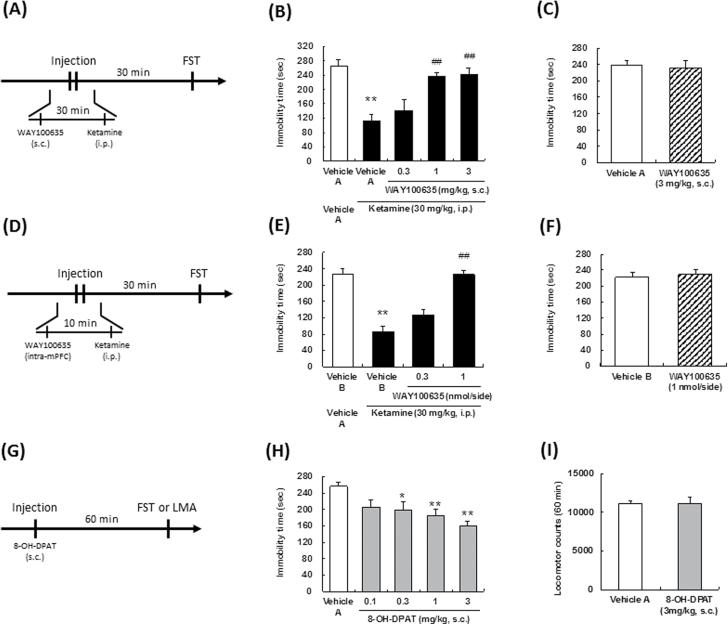
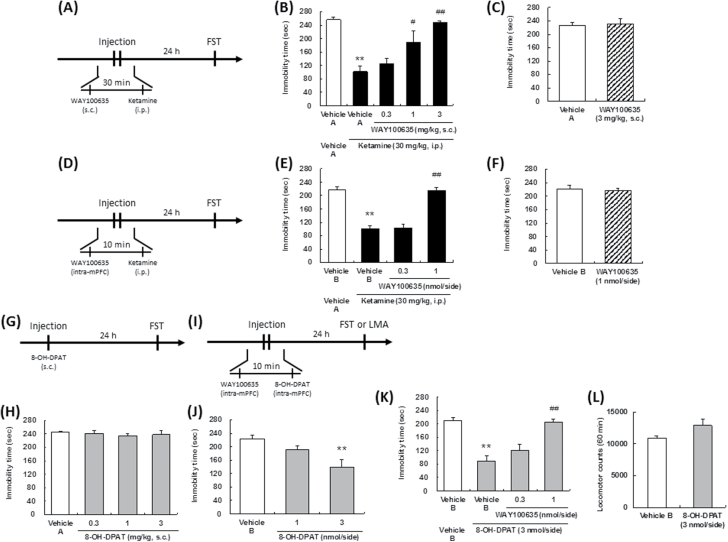
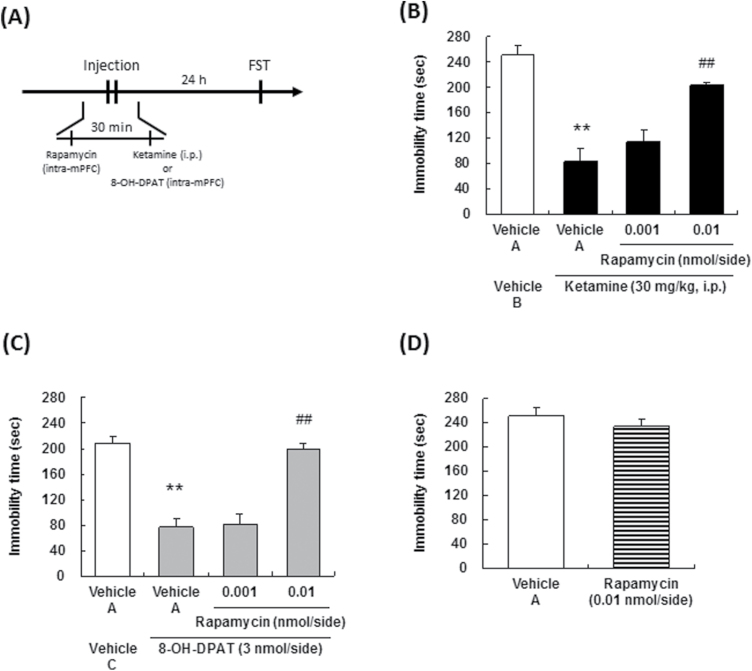
Ketamine (Veterinary Ketalar 50; Sankyo Yell Pharmaceutical Co., Ltd) was diluted with saline. N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane carboxamide (WAY100635, a 5-HT1A receptor antagonist) maleate (Sigma-Aldrich Co) and (±)-8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT, a 5-HT1A receptor agonist) (Sigma-Aldrich Co.) were dissolved in saline or Ringer’s solution (147 mM NaCl, 4 mM KCl, and 1.2 mM CaCl2). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002, a PI3K inhibitor) (Focus Biomolecules) and rapamycin, an mTORC inhibitor (Sigma-Aldrich Co), were dissolved in 6.2% dimethylsulfoxide (DMSO)/Ringer’s solution and 1% DMSO/Ringer’s solution, respectively. All these solutions were diluted with Ringer’s solution prior to being used for the intracerebral microinjections. The schedules of administration of each compound are illustrated in Figure 1 (A, D, G), Figure 2 (A, D, G, I), Figure 3 (A), Figure 4 (A, C), and Figure 5 (A). In the case of ketamine administration, the effects at 30 minutes after administration are defined as acute effects, while those at 24 hours after administration are defined as sustained effects. Doses of 8-OH-DPAT (0.1–3 mg/kg for s.c. administration, 1 and 3 nmol/side for intra-mPFC injection) and WAY100635 (0.3–3 mg/kg for s.c. administration, 0.3 and 1 nmol/side for intra-mPFC injection) were selected by previous reports (Matsuda et al.,1995).
Figure 1.
Effect of a serotonin (5-HT)1A receptor antagonist on the acute antidepressant effects of ketamine. The drug administration schedules are illustrated in A, D, and G. Vehicle A, saline; vehicle B, Ringer’s solution. Values indicate the mean±SEM. (B), n=6–7; (C), n=8; (E), n=8; (F), n=6; (H), n=7–9; (I), n=6. *P<.05, **P<.01 compared with vehicle A-treated vehicle A (B), vehicle B-treated vehicle A (E), and vehicle A (H), ##P<.01 compared with vehicle A-treated ketamine (B), vehicle B-treated ketamine (E).
Figure 2.
Effect of a serotonin (5-HT)1A receptor antagonist on the sustained antidepressant effects ketamine and 8-OH-DPAT. The drug administration schedules are illustrated in A, D, G, and I. Vehicle A, saline; vehicle B, Ringer’s solution. Values indicate the mean±SEM. (B), n=6–7; (C), n=8; (E), n=8; (F), n=8; (H), n=8; (J), n=7–8; (K), n=7–8; (L), n=6. *P<.05, **P<.01 compared with vehicle A-treated vehicle A (B), vehicle B-treated vehicle A (E), vehicle B-treated vehicle B (K), and vehicle B (J); #P<.05, ##P<.01 compared with vehicle A-treated ketamine (B), vehicle B-treated ketamine (E), vehicle B-treated 8-OH-DPAT (K).
Figure 3.
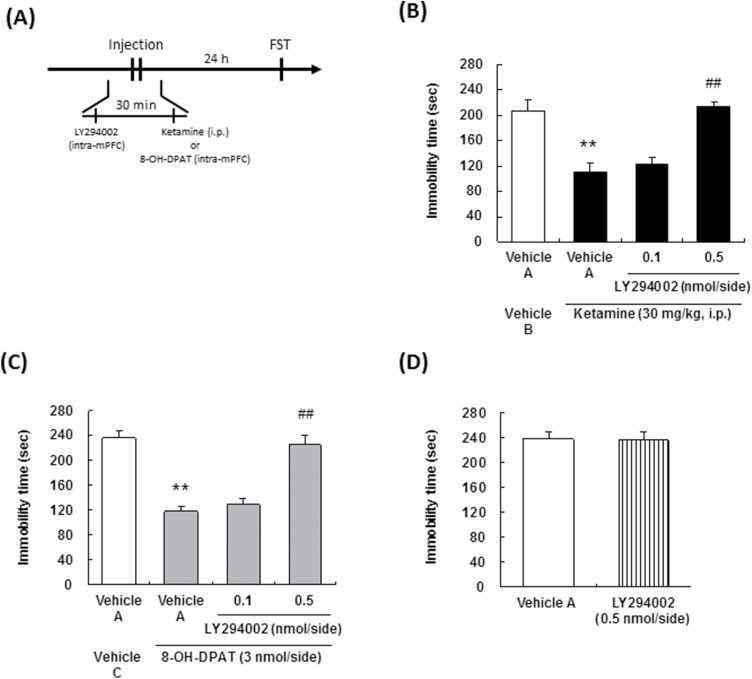
Effect of a phosphoinositide-3 kinase (PI3K) inhibitor on the sustained antidepressant effects of ketamine and 8-OH-DPAT. The drug administration schedules are illustrated in A. Vehicle A, 6.2 % DMSO/Ringer’s solution; vehicle B, saline; vehicle C, Ringer’s solution. Values indicate the mean±SEM. (B), n=8; (C), n=8; (D), n=5–6. **P<.01 compared with vehicle A-treated vehicle B (B), vehicle A-treated vehicle C (C); ##P<.01 compared with vehicle A-treated ketamine (B), vehicle A-treated 8-OH-DPAT (C).
Figure 4.
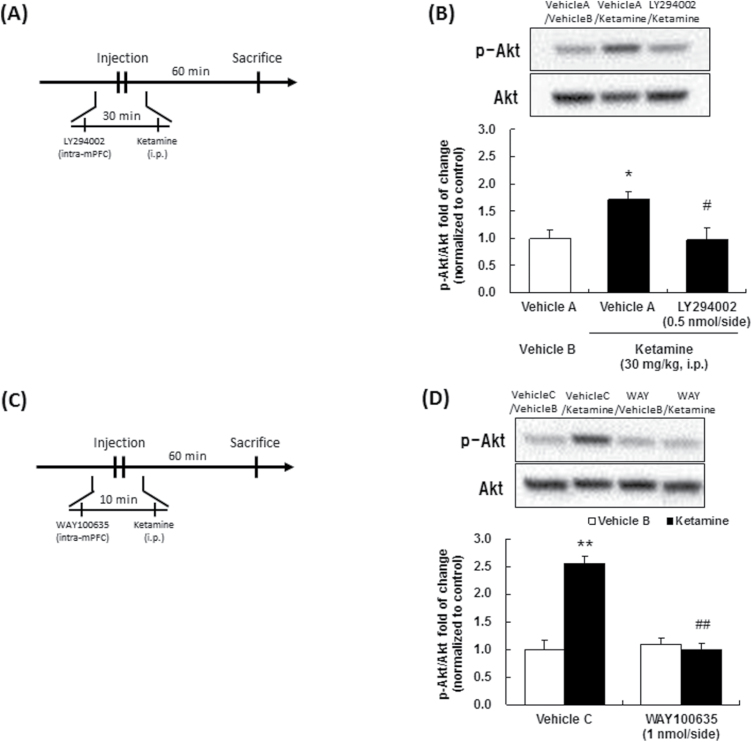
Effect of ketamine on phosphorylation of Akt in the medial prefrontal cortex (mPFC). The drug administration schedules are illustrated in A and C. Representative western-blot images of phosphorylated-Akt (Ser-473) and Akt total protein are shown in the upper figures. Quantitative analyses of phosphorylated Akt (Ser-473) relative to Akt total protein are shown in the bar graph. Vehicle A, 6.2 % DMSO/Ringer’s solution; vehicle B, saline; vehicle C, Ringer’s solution. Values are expressed as percent of control (vehicle treated) and indicate the mean±SEM. (B), n=3; (D), n=3–4. *P<.05, **P<.01 compared with vehicle A-treated vehicle B (B), vehicle C-treated vehicle B (D); #P<.05, ##P<.01 compared with vehicle A-treated ketamine (B), vehicle C-treated ketamine (D).
Figure 5.
Effect of an mechanistic target of rapamycin complex 1 (mTORC1) inhibitor on the sustained antidepressant effects of ketamine and 8-OH-DPAT in the forced swim test (FST). The drug administration schedules are illustrated in A. Vehicle A, 1 % DMSO/Ringer’s solution; vehicle B, saline; vehicle C, Ringer’s solution. Values indicate the mean±SEM. (B) n=7–8, (C) n=8, (D) n=7–8. **P<.01 compared with vehicle A-treated vehicle B (B), vehicle A-treated vehicle C (C); ##P<.01 compared with vehicle A-treated ketamine (B), vehicle A-treated 8-OH-DPAT (C).
Microinjection
Each mouse was anesthetized with pentobarbital (50 mg/kg, i.p.) and fixed to a brain stereotaxic apparatus (Narishige Instruments). For the injection into the mPFC, the brains were implanted with guide cannulas (Eicom) bilaterally, so that the tips were positioned near the mPFC (anteroposterior, 2.0 mm from bregma; lateral, ±1.4 mm; ventral, -2.3 mm; angle, 20°). The cannulas were held in place with dental cement. A dummy cannula was inserted into the guide cannula to prevent clogging. Microinjection of the compounds was performed on day 2 or 3 after the surgery. Before the microinjections, the dummy cannulas were removed from the guide cannula, and a 28-gauge injection cannula, extending 0.5 mm from the tip of the guide cannula, was inserted. The injection cannula was connected via a Teflon tubing to a micro syringe (Hamilton Co) driven by a micro infusion pump (Harvard Apparatus, Inc). Injections of the compounds were performed for 2 minutes at the rate of 0.05 μL/min. The injection cannulas were left in position for an additional 2 minutes before being withdrawn. After the behavioral test, Evans blue was infused, followed by preparation of coronal sections to confirm the locations of the cannula tips. The locations of the cannula tips are shown in supplementary Figures 1 to 12.
Forced Swimming Test (FST)
The FST was performed by a previously reported method (Fukumoto et al., 2016), a modified method reported by Dulawa et al. (2004) that includes 2 swimming sessions at day 1 and day 2. In brief, the mice were placed in a swim tank for 6 minutes on day 1. By doing this procedure, a state of helplessness is induced to prolong immobility in a swimming session on day 2. On day 2, they were placed again in the swim tank for 6 minutes to measure the immobility time. The swimming session on day 2 was regarded as the test session, and drugs were administered after swimming session on day 1. The swimming sessions were conducted by placing the mice in cylinders (24 cm height×17 cm diameter) containing water (25ºC ±1ºC) up to a height of 13 cm, so that the mice could not support themselves by touching the bottom of the tank with their paws. The FST was conducted between 8:00 am and 5:00 pm. The test sessions were videotaped from the front of the cylinders for later scoring. The total duration of immobility during a 6-minute test session was measured by an observer blinded to the treatment conditions. Immobility was defined as floating in the water without struggling and only making movements that were necessary to keep the head above the water. Different from the original method (Porsort et al., 1977), we measured immobility for 6 minutes to evaluate the effects of drugs. In the present method, mice showed immobility soon after placing them in a swim tank in the test session, as shown in representative groups (supplementary Figure 13).
Measurement of the Spontaneous Locomotor Activity
Mice were housed individually in transparent acrylic cages (φ30 cm×30 cm). The animals were injected with each of the compounds at the designated time points, and the spontaneous locomotor activities were recorded for 60 minutes after the injections using the SCANET apparatus (Neuroscience Inc) placed in a sound-proof box.
Western Blotting
According to the report that ketamine transiently increased phosphorylation of Akt in the PFC, which may trigger the subsequent events (Li et al., 2010), we measured phospho-Akt at 60 minutes after ketamine administration. Medial prefrontal cortical tissue specimens were homogenized and sonicated in cell lysis buffer (50 mM Tris-HCl buffer, pH 7.5, containing 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 10 mM NaF, and protease inhibitor cocktail (Sigma-Aldrich Co). For western blotting, equal amounts of protein (20 μg) from each sample were separated by SDS-PAGE. The proteins were transferred to PVDF membranes (PerkinElmer), and the membranes were blocked in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% bovine serum albumin for 60 minutes and incubated with the primary antibodies (phospho-Akt (Ser473), 1:1000; Cell Signaling Technology) overnight at 4°C. The following day, the blots were washed 3 times in TBS containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:10,000; GE Healthcare) for 60 minutes at room temperature. After 3 final washes with TBS containing 0.1% Tween 20, the immunoreactive bands were detected using enhanced chemiluminescence. The blots were then incubated in stripping buffer for 60 minutes at room temperature, followed by 3 washes with TBS containing 0.1% Tween 20. The stripped blots were blocked for 60 minutes and incubated with the primary antibody (Akt, 1:1000; Cell Signaling Technology) as the loading control.
Statistical Analysis
The results were expressed as the mean±SEM. Statistical significance was determined by a 1-way ANOVA or a 2-way ANOVA, followed by the Dunnett, Tukey, or LSD posthoc test for comparing the treated group with the control group and multi-group comparisons, respectively. Statistical differences between any 2 groups were determined using the Student’s t test; P<.05 was considered to indicate statistically significant difference.
Results
Role of the 5-HT1A Receptor in the mPFC in the Acute Antidepressant Effects of Ketamine
Following systemic administration, ketamine (30 mg/kg, i.p.) significantly reduced the immobility time in the FST at 30 minutes after the drug administration (acute antidepressant effect) (Figure 1B, 1E). We previously confirmed that ketamine did not significantly increase locomotor activity at the time of the FST in the present condition (Fukumoto et al., 2016). Subcutaneous administration of the 5-HT1A receptor antagonist, WAY100635, abrogated the reduction of the immobility time induced by ketamine [F(4,27)=11.4, P<.01] (Figure 1B). Involvement of the 5-HT1A receptor in the actions of ketamine was also reported from a previous study conducted using a novelty-suppressed feeding test (Fukumoto et al., 2014). Then, we investigated the involvement of the 5-HT1A receptor in the mPFC in the acute antidepressant effects of ketamine. Microinjection of WAY100635 into the mPFC attenuated the reduction of the immobility time induced by ketamine [F(3,28)=35.03, P<.01] (Figure 1E). On the other hand, s.c. [F(1,14)=0.08, P=0.79] (Figure 1C) or intra-mPFC [F(1,19)=0.13, P=0.73] (Figure 1F) administration of WAY100635 per se had no effect on the immobility time in the FST. Therefore, the 5-HT1A receptor stimulation in the mPFC is involved in the acute antidepressant effects of ketamine. Systemic administration of the 5-HT1A receptor agonist, 8-OH-DPAT (0.3–3 mg/kg, s.c.), significantly reduced the immobility time in the FST at 60 minutes after administration [F(4,35)=6.00, P<.01] (Figure 1H), while it did not affect the spontaneous locomotor activity [F(1,10)=0.00, P=0.97] (Figure 1I), confirming that 5-HT1A receptor stimulation exerts the acute antidepressant effects.
Role of the 5-HT1A Receptor in the mPFC in The Sustained Antidepressant Effects of Ketamine
Following systemic administration, ketamine (30 mg/kg, i.p.) exhibited a sustained antidepressant effect (effect lasting for at least 24 hours after the drug administration) (Figure 2B, 2E). Subcutaneous administration of WAY100635 abrogated the reduction of the immobility time induced by ketamine [F(4,27)=12.65, P<.01] (Figure 2B). Microinjection of WAY100635 into the mPFC abrogated the reduction of the immobility time in the FST induced by ketamine [F(3,28)=52.09, P<.01] (Figure 2E). Subcutaneous [F(1,14)=0.00, P=.98] (Figure 2C) and intra-mPFC [F(1,14)=0.00, P=.98] (Figure 2F) injections of WAY100635 per se had no effect on the immobility time in the FST. Therefore, the sustained antidepressant effects of ketamine are mediated through 5-HT1A receptor stimulation in the mPFC. To confirm that stimulation of the 5-HT1A receptor in the mPFC is associated with sustained antidepressant effects, the effect of 8-OH-DPAT was investigated. Systemic administration of 8-OH-DPAT did not result in sustained antidepressant effects in the FST [F(3,28)=0.21, P=.89] (Figure 2H). Importantly, in contrast to the systemic administration of 8-OH-DPAT, local injection of 8-OH-DPAT into the mPFC was associated with sustained antidepressant effects [F(2,20)=6.11, P<.01] (Figure 2J), with no effect on the spontaneous locomotor activity [F(1,10)=4.26, P=.71] (Figure 2L). Microinjection of WAY100635 into the mPFC abrogated the reduction of the immobility time in the FST induced by 8-OH-DPAT [F(3,27)=23.01, P<.01] (Figure 2K).
Role of PI3K/Akt Signaling in the mPFC in the Sustained Antidepressant Effects of Ketamine and 8-OH-DPAT
The sustained antidepressant effects of ketamine have been reported to be mediated via changes in synaptic plasticity (Li et al., 2010; Dong et al., 2017), and PI3K/Akt signaling is a critical signaling cascade involved in synaptic plasticity induced by 5-HT1A receptor activation (Chilmonczyk et al., 2015). Therefore, we investigated the involvement of PI3K/Akt signaling in the mPFC in the sustained antidepressant effects of ketamine. Microinjection of a PI3K inhibitor, LY294002, into the mPFC significantly abrogated the reduction of the immobility time in the FST induced by ketamine [F(3,28)=17.07, P<.01] (Figure 3B) and 8-OH-DPAT [F(3,28)=29.36, P<.01] (Figure 3C), only at the highest dose. Intra-mPFC injection of LY294002 per se had no effect on the immobility time in the FST [F(1,9)=0.00, P=.95] (Figure 3D). Moreover, significant increase in the phosphorylation of Akt was observed in the mPFC at 60 minutes after systemic ketamine (30 mg/kg, i.p.) administration (Figure 4B, 4D). Importantly, the increase in Akt phosphorylation induced by ketamine was blocked by intra-mPFC injection of not only LY294002 (Figure 4B), but also that of WAY100635 (Figure 4D). These results indicate that PI3K activation is involved in the sustained antidepressant effects of ketamine and 8-OH-DPAT, and that ketamine activates Akt signaling via stimulation of the PI3K and 5-HT1A receptor in the mPFC.
Role of mTORC1 in the mPFC in the Sustained Antidepressant Effects of Ketamine and 8-OH-DPAT
We next investigated if mTORC1, which is downstream of PI3K/Akt, is involved in the antidepressant actions of ketamine. Microinjection of an mTORC1 inhibitor, rapamycin, into the mPFC abrogated the sustained antidepressant effects of ketamine [F(3,27)=23.26, P<.01] (Figure 5B) and 8-OH-DPAT [F(3,28)=35.13, P<.01] (Figure 5C), only at the highest dose. On the other hand, local injection of rapamycin into the mPFC per se had no effect on immobility time in the FST [F(1,13)=0.86, P=.37] (Figure 5D). These results suggest not only that stimulation of mTORC1 signaling in the mPFC mediates the sustained antidepressant effects of ketamine, but also that mTORC1 signaling is necessary to exert the mPFC 5-HT1A receptor stimulation-mediated antidepressant actions.
Discussion
The present results indicated that stimulation of 5-HT1A receptor and subsequent activation of PI3K/Akt/mTORC1 signaling in the mPFC may play an important role in the sustained antidepressant effects of ketamine.
In the present study, both the acute and sustained antidepressant effects of ketamine observed in the FST were attenuated by local injection of WAY100635, a 5-HT1A receptor antagonist, into the mPFC, indicating that stimulation of the mPFC 5-HT1A receptor plays an important role in both acute and sustained antidepressant actions of ketamine. Interestingly, acute, but not sustained, antidepressant effects were observed following systemic administration of a 5-HT1A receptor agonist, while local injection of a 5-HT1A receptor agonist into the mPFC was associated with the sustained antidepressant effects. Therefore, selective stimulation of the 5-HT1A receptor in the mPFC, and not generalized stimulation in all regions of the brain, may be involved in a sustained antidepressant action of ketamine. 5-HT1A receptor is not only localized as a presynaptic inhibitory autoreceptor on the 5-HT neuronal cell bodies in the DRN, but also as postsynaptic receptors in brain regions associated with the control of mood and cognition, such as the PFC and hippocampus (Miquel et al., 1992). Thus, the present results indicate the importance of selective stimulation of the postsynaptic 5-HT1A receptor in the mPFC for obtaining sustained antidepressant actions. This is consistent with the previous findings that the postsynaptic 5-HT1A receptor in the mPFC and limbic areas is particularly important for the antidepressant response (Blier and de Montigny, 1994; Haddjeri et al., 1998), while the loss of 5-HT1A heteroreceptor in the mPFC during adolescence is also reported to be sufficient to exert the depressive-like behavior (Garcia-Garcia et al., 2017). The importance of selective stimulation of the postsynaptic 5-HT1A receptor is also underscored by the finding reported by Assié et al. (2010) that a selective postsynaptic 5-HT1A receptor agonist, F15599, exerted a more potent and sustained antidepressant effect than 5-HT1A receptor agonists that act on both the postsynaptic and presynaptic 5-HT1A receptor.
Ketamine is rapidly eliminated from the plasma within minutes to hours (Can et al., 2016; Maxwell et al., 2006). Therefore, the sustained antidepressant effects of ketamine (effects lasting for at least 24 hours after a single administration) may be ascribed to changes in the synaptic plasticity. Indeed, ketamine has been reported to enhance synaptic protein synthesis and dendritic spine density in the mPFC (Li et al., 2010; Dong et al., 2017). Among the intracellular signaling cascades induced by postsynaptic 5-HT1A receptor stimulation, PI3K/Akt signaling plays a crucial role in synaptic plasticity (Polter et al., 2012; Islam et al., 2014; Wang et al., 2016b). In the present study, the sustained antidepressant effects of ketamine were abrogated by intra-mPFC injection of a PI3K inhibitor. In addition, ketamine increased Akt phosphorylation in the mPFC, which was attenuated by local injection of a 5-HT1A receptor antagonist or a PI3K inhibitor into the mPFC. Therefore, the sustained antidepressant actions of ketamine were exerted, presumably through 5-HT1A receptor-mediated activation of PI3K/Akt signaling in the mPFC. This notion was supported by the finding that the sustained antidepressant effect of a 5-HT1A receptor agonist was also blocked by intra-mPFC injection of a PI3K inhibitor. Ketamine has been reported to increase synaptic protein synthesis and to induce the sustained antidepressant effects through activation of mTORC1 signaling (Li et al., 2010), downstream of PI3K/Akt (Duman and Voleti, 2012; Chilmonczyk et al., 2015). Interestingly, we obtained the results in the present study that mTORC1 signaling in the mPFC is involved in not only the sustained antidepressant effects of ketamine but also the sustained antidepressant effects of a 5-HT1A receptor agonist. Therefore, 5-HT1A receptor-mediated activation of mTORC1 signaling in the mPFC can induce the sustained antidepressant effects. Collectively, the present study suggests that 5-HT1A receptor activation and its downstream PI3K/Akt/mTORC1 signaling in the mPFC may have an important role in the sustained antidepressant actions of ketamine.
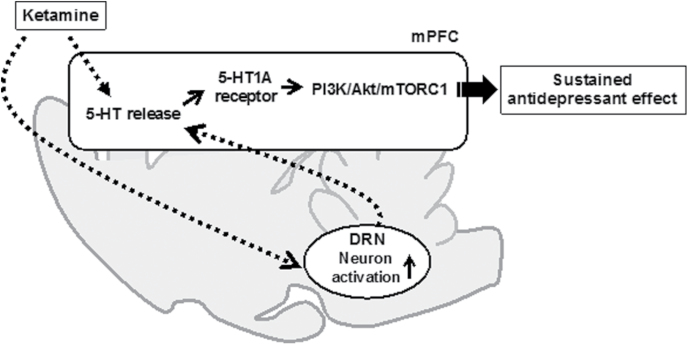
It is unknown how ketamine stimulates the postsynaptic 5-HT1A receptor in the mPFC to exert antidepressant effects. Because ketamine has negligible activity at 5-HT1A receptor (Roth et al., 2013), ketamine may indirectly activate 5-HT1A receptor and its signaling cascade. It has previously been reported that intra-mPFC injection or systemic administration of ketamine increases 5-HT release in the mPFC via activation of neurons of the DRN (Nishitani et al., 2014; Pham et al., 2017). We previously reported that ketamine activates a subpopulation of 5-HT neurons in the DRN via stimulation of AMPA receptor in the mPFC (Fukumoto et al., 2016). Therefore, it is hypothesized that ketamine increases 5-HT release in the mPFC through activation of the subpopulation of the DRN 5-HT neurons by stimulating the mPFC projections. To support this hypothesis, increase in glutamate transmission, including AMPA receptor activation, in the infralimbic cortex (a part of the mPFC) has also been shown to increase 5-HT release in the region, likely through increased activity of neurons of the DRN, resulting in an antidepressant effect (Gasull-Camós et al., 2017). However, it has also been reported that ketamine decreased, at 24 hours after administration, the firing of 5-HT neurons in the DRN (Pham et al., 2017) and that ketamine increased 5-HT release in the PFC by inhibition of 5-HT transporters (Yamamoto et al., 2013). Therefore, it is also conceivable that ketamine may increase 5-HT release in the mPFC through the local mechanisms. In either case, increased 5-HT release in the mPFC by ketamine may subsequently stimulate postsynaptic 5-HT1A receptor in the region. Taking these findings and the present results into consideration, it is suggested that increase of 5-HT release in the mPFC by ketamine stimulates the postsynaptic 5-HT1A receptor-mediated activation of the PI3K/Akt/mTORC1 cascade, leading to sustained antidepressant actions of the drugs. Nonetheless, this hypothesis remains to be further investigated. The above-mentioned hypothetical mechanisms are illustrated in Figure 6.
Figure 6.
Hypothetical mechanisms underlying the sustained antidepressant effects of ketamine. Ketamine increases serotonin (5-HT) release in the medial prefrontal cortex (mPFC) through local mechanisms such as inhibition of 5-HT transporter or through activation of the dorsal raphe nucleus (DRN) neurons by mPFC projection. Increase in 5-HT release may stimulate postsynaptic 5-HT1A receptor in the mPFC, leading to activation of phosphoinositide-3 kinase (PI3K)/Akt/mTORC1 signaling. Activation of PI3K/Akt/mTORC1 signaling has been reported to mediate the sustained antidepressant effects of ketamine (Li et al., 2010).
There are some limitations in the present study. One of the limitations is doses of 5-HT1A receptor ligands 8-OH-DPAT and WAY100635 used in this study. We used higher doses of both compounds than those used in the previous studies (Linge et al., 2016). 8-OH-DPAT has been reported to act on other receptors, particularly as an agonist for 5-HT7 receptor (Sprouse et al., 2004; Thomas et al., 1998), while WAY100635 acts as an agonist for dopamine D4 receptor (Chemel et al., 2006; Dilly and Liégeois, 2016). Therefore, roles of other receptors cannot be fully ruled out. The other limitation of the present study is lack of investigations on roles of postsynaptic 5-HT1A receptor in other brain regions, particularly in the hippocampus. Because the postsynaptic 5-HT1A receptor in the hippocampus has been implicated in depression and antidepressant actions (Santarelli et al., 2003; Samuels et al., 2015), and roles of the ventral hippocampus-mPFC pathway have been reported for antidepressant actions of ketamine (Carreno et al., 2016), it would be necessary to elucidate roles of postsynaptic 5-HT1A receptor in the hippocampus in the actions of ketamine in future studies. Moreover, in the present study, we used only the FST of naïve mice. Although the FST has been widely used to evaluate antidepressant effects, the test does not mimic some of the modifications observed in depression. Therefore, investigations by using other models, particularly models in which chronic stress is exposed are needed to confirm the role of 5-HT1A receptor and its signaling cascade in the sustained antidepressant effects of ketamine.
In conclusion, the present study demonstrated that selective stimulation of the postsynaptic 5-HT1A receptor and the subsequent activation of PI3K/Akt/mTORC1 signaling in the mPFC may be responsible for the sustained antidepressant effect of ketamine. More precise elucidation of the mechanisms of regulation of the 5-HTergic systems by ketamine may pave the way for the development of new faster, effective, and longer-acting drugs for the treatment of depression.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
We thank Dr. Shigetada Nakanishi, Professor Emeritus at Kyoto University, for the valuable discussions and advice and for his critical reading of the manuscript.
Statement of Interest
All authors are employees of Taisho Pharmaceutical Co., Ltd.
References
- Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ(2012)Ketamine for depression: where do we go from here?Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A(2010)F15599, a highly selective post-synaptic 5-HT(1A) receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol 13:1285–1298. [DOI] [PubMed] [Google Scholar]
- Barreto RA, Walker FR, Dunkley PR, Day TA, Smith DW(2012)Fluoxetine prevents development of an early stress-related molecular signature in the rat infralimbic medial prefrontal cortex. Implications for depression?BMC Neurosci 13:125. doi: 10.1186/1471-2202-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH(2000)Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C(1994)Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15:220–226. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD(2016)Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther 359:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ(2016)Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE(2006)WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 188:244–251. [DOI] [PubMed] [Google Scholar]
- Chilmonczyk Z, Bojarski AJ, Pilc A, Sylte I(2015)Functional selectivity and antidepressant activity of serotonin 1A receptor ligands. Int J Mol Sci 16:18474–18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilly S, Liégeois JF(2016)Structural insights into 5-HT1A/D4 selectivity of WAY-100635 analogues: molecular modeling, synthesis, and in vitro binding. J Chem Inf Model 56:1324–1331. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, Chaki S, Hashimoto K(2017)Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol 20:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Müller HK, Elfving B, Sanchez C, Wegener G(2016)Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl) 233:2813–2825. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R(2004)Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29:1321–1330. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH(2016)Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Voleti B(2012)Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, Aghajanian GK, Duman RS(2015)Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A 112:8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2014)Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231:2291–2298. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2016)The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Meng Q, Canetta S, Gardier AM, Guiard BP, Kellendonk C, Dranovsky A, Leonardo ED(2017)Serotonin signaling through prefrontal Cortex 5-HT1A receptors during adolescence can determine baseline mood-related behaviors. Cell Rep 18:1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull-Camós J, Tarrés-Gatius M, Artigas F, Castañé A(2017)Glial GLT-1 blockade in infralimbic cortex as a new strategy to evoke rapid antidepressant-like effects in rats. Transl Psychiatry 7:e1038. doi: 10.1038/tp.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard DM, Wohleb ES, Duman RS(2016)Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A(2013)Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C(1998)Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci 18:10150–10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E(2010)mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, Nyer M, Cassano P, Mischoulon D, Alpert JE, Brown EN, Nock MK, Fava M, Cusin C(2016)Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry 77:e719–725. [DOI] [PubMed] [Google Scholar]
- Islam MR, Moriguchi S, Tagashira H, Fukunaga K(2014)Rivastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience 272:116–130. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication (2003)National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S(2011)Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61:1419–1423. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS(2013)Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linge R, Jiménez-Sánchez L, Campa L, Pilar-Cuéllar F, Vidal R, Pazos A, Adell A, Díaz Á(2016)Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 103:16–26. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Somboonthum P, Suzuki M, Asano S, Baba A(1995)Antidepressant-like effect by postsynaptic 5-HT1A receptor activation in mice. Eur J Pharmacol 280:235–238. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ(2006)Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 316:315–324. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Doucet E, Riad M, Adrien J, Vergé D, Hamon M(1992)Effect of the selective lesion of serotoninergic neurons on the regional distribution of 5-HT1A receptor mRNA in the rat brain. Brain Res Mol Brain Res 14:357–362. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S(2014)Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17:1321–1326. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM(2017)Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Polter AM, Yang S, Jope RS, Li X(2012)Functional significance of glycogen synthase kinase-3 regulation by serotonin. Cell Signal 24:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M(1977)Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Price RB, Mathew SJ(2015)Does ketamine have anti-suicidal properties? Current status and future directions. CNS Drugs 29:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, Iversen L(2013)The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS One 8:e59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M(2006)Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madroñal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R(2015)5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci 18:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R(2003)Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A(2004)8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 46:52–62. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC(2009)Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol 12:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Gittins SA, Collin LL, Middlemiss DN, Riley G, Hagan J, Gloger I, Ellis CE, Forbes IT, Brown AM(1998)Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br J Pharmacol 124:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhou C, Zhu D, Wang X, Fang L, Zhong J, Mao Q, Sun L, Gong X, Xia J, Lian B, Xie P (2016a) Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry 16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Ren QG, Gong WG, Wu D, Tang X, Li XL, Wu FF, Bai F, Xu L, Zhang ZJ (2016b) Escitalopram attenuates β-amyloid-induced tau hyperphosphorylation in primary hippocampal neurons through the 5-HT1A receptor mediated Akt/GSK-3β pathway. Oncotarget 7:13328–13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, Domino EF(2013)Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology 38:2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.