Abstract
Although several nucleoside diphosphate (NDP) kinase genes have been cloned in plants, little is known about the functional significance of this enzyme during plant growth and development. We introduced a chimeric gene encoding an antisense RNA of NDP kinase under the control of the Arabidopsis heat shock protein HSP81-1 promoter into rice (Oryza sativa L.) plants using the Agrobacterium tumefaciens transformation system. The expression of antisense RNA down-regulated the accumulation of mRNA, resulting in reduced enzyme activity even under the standard growth temperature (25°C) in transgenic plants. Following heat shock treatment (37°C), NDP kinase activities in some transgenic rice plants were more reduced than those grown under 25°C. The comparison of the coleoptile growth under submersion showed that cell elongation process was inhibited in antisense NDP kinase transgenic plants, suggesting that an altered guanine nucleotide level may be responsible for the processes.
Nucleoside diphosphate (NDP) kinase is a ubiquitous enzyme in eukaryotes and prokaryotes. It catalyzes the transfer of γ-phosphate from ATP to NDP through autophosphorylation (Parks and Agarwal, 1973). Therefore, it is an important enzyme for maintaining stable GTP levels through nucleotide homeostasis in various metabolic pathways such as protein and DNA synthesis and GTP-mediated signal transduction pathways. The involvement of NDP kinase in cell growth and differentiation has been demonstrated in animal systems (Steeg et al., 1988a, 1988b; Rosengard et al., 1989; Biggs et al., 1990; Leone et al., 1991). NDP kinase participates in hormone-dependent signal transduction pathways by activating guanine nucleotide-binding proteins (Kimura and Shimada, 1988, 1990; Bominaar et al., 1993). With regard to their role in growth and development in eukaryotes, there is convincing evidence that NDP kinase protein forms molecular complexes with β-tubulin, which may play a crucial role during differentiation processes (Lombardi et al., 1995).
Although several NDP kinase genes have been cloned in plants such as spinach (Nomura et al., 1992; Zhang et al., 1993), pea (Finan et al., 1994), tomato (Harris et al., 1994), and oat (Sommer and Song, 1994), little is known about the functional significance of this enzyme during plant growth and development. We previously isolated a cDNA clone encoding NDP kinase from rice (Oryza sativa L.) (Yano et al., 1993) and demonstrated that the level of the enzyme changes during seed germination and the early stages of seedling growth (Yano et al., 1995). To further understand the biological significance of NDP kinase in rice development, we used reversed genetics to suppress NDP kinase gene expression. In this study, we created transgenic rice plants in which the NDP kinase mRNA level could be negatively regulated. These plants exhibited developmental abnormalities, in particular, suppression of cell elongation processes.
MATERIALS AND METHODS
Antisense Gene Construction and Plant Transformation
Rice (Oryza sativa) NDP kinase cDNA (about 700 bp) (Yano et al., 1993) was ligated in its antisense orientation to the promoter of the Arabidopsis gene for a heat shock protein, HSP81-1 (Takahashi et al., 1992), and transcription termination sequences derived from the Agrobacterium tumefaciens nopaline synthase gene (Fig. 1A). This antisense construct was inserted into a binary vector and transferred to A. tumefaciens. A. tumefaciens harboring this chimeric vector was used to infect the seed-derived calli of the rice var. Kitaake according the method of Hiei et al. (1994). Putative transformants were selected on media containing 50 μg/mL hygromycin.
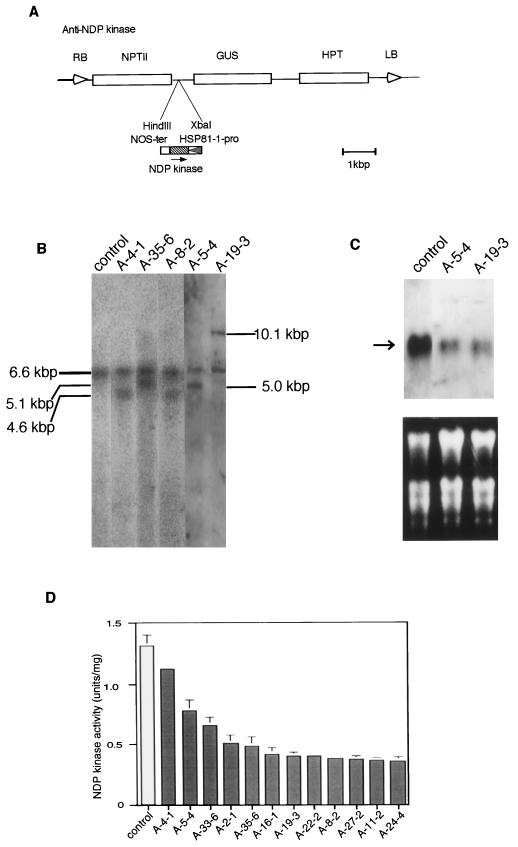
Figure 1.
A, Structure of the chimeric gene possessing an antisense DNA of NDP kinase. The heat shock promoter HSP81-1 was fused to the full-length NDP kinase cDNA and the termination sequences of the nopaline synthase gene. The cassette was then cloned into XbaI and HindIII restriction sites of the vector pHTS6.1. The original 5′ to 3′ direction of NDP kinase cDNA is indicated by the arrow. B, Southern-blot analysis of T3 transgenic rice plants. Genomic DNA was digested with XbaI and then subjected to electrophoresis followed by hybridization with the DIG-labeled NDP kinase cDNA probe. C, Northern-blot analysis of transgenic rice plants. Total RNA (10 μg) extracted from plant shoots (2-month-old) was loaded in each lane, and then hybridized with a DIG-labeled NDP kinase antisense probe. Arrow points to a position corresponding to approximately 700 nt. D, NDP kinase activity comparison of 12 different transgenic rice lines. Shoots of 12-d-old seedlings of each line (at 25°C) were assayed for NDP kinase enzyme activity. Data for individual lines represent the means ± se of three preparations.
Plant Growth Conditions
All plants (T3 generation) were grown until maturity at 25°C (12-h day length) in a growth chamber (NK System, Tokyo) (except those used for heat shock treatment).
Heat Shock Treatment
Rice seeds were germinated at 25°C under 12-h day length. For heat shock treatment, 10-d-old seedlings were kept at 37°C in a growth chamber with a 12-h day length for 2 d. For coleoptile cultivation, rice seeds were presoaked at 37°C for 2 d to induce heat shock.
DNA Analysis
Genomic DNA (10 μg) isolated from the shoots of 2-month-old plants (Shure et al., 1983) was digested with XbaI and electrophoresed in 0.7% agarose gels, followed by denaturation and blotting to a nylon membrane (Biodyne-Plus, Pall, New York). The membrane was hybridized with the digoxigenin (DIG)-labeled NDP kinase cDNA probe. Hybridization and washing were carried out according to the protocol recommended by the manufacturer (Boehringer Mannheim, Mannheim, Germany). DIG-labeled DNA was detected by chemiluminescence with disodium 3-(4-methoxyspiro[1,2-dioxetane-3,2′-{5′-chloro}tricyclo-{3.3.1.13,7}decan]4-yl) (CSPD) (Boehringer Mannheim).
Analysis of mRNA Accumulation
Total RNA was extracted from the shoots of 2-month-old plants grown at 25°C (Umeda and Uchimiya, 1994). Ten micrograms of RNA was fractionated on 5% formaldehyde/1.2% agarose gel and transferred to a nylon membrane (Biodyne-Plus, Pall) (Sambrook et al., 1989). A DIG-labeled probe encoding either the sense or antisense RNA of NDP kinase was used. Hybridization, washing, and detection were performed according to the instructions provided by the manufacturer (Boehringer Mannheim).
Protein Extraction and Enzyme Activity Analysis
Plant tissue was ground in the extraction buffer (0.15 m Tris-HCl, pH 8.0; 25% glycerol; 0.8% mercaptoethanol; 100 μm phenylmethylsulfonyl fluoride [PMSF]; 10 μg/mL leupeptin; and 10 μg/mL pepstatin A). After centrifugation at 14,000g for 10 min, the supernatant was used for enzyme assay.
NDP kinase enzyme activity was measured using the coupled reaction method with lactate dehydrogenase (EC 1.1.1.27) and pyruvate kinase (EC 2.7.1.40) at 25°C (Yano et al., 1995). The reaction mixture (100 μL) contained 100 mm Tris-HCl, pH 7.5, 100 mm KCl, 25 mm MgCl2, 0.3 mm NADH, 3 mm phosphoenol pyruvate, 2 mm ATP, 0.4 mm thymidine-5′-diphosphate (TDP), 1 unit of pyruvate kinase (Sigma-Aldrich, St. Louis), and 5 units of lactate dehydrogenase (Sigma-Aldrich). The reaction was initiated by adding the test sample to the reaction mixture. NDP kinase activity (units/mg protein) was calculated based on the reduction of OD340 following a decrease in NADH. One unit of enzyme activity was defined as 1 μmol of ADP production per minute at 25°C. Protein concentration was determined by Bradford dye binding assays (Bradford, 1976). Enzyme activity measurements were carried out in triplicate for each independent sample.
Measurement of Length and Width of Epidermal Cells
Samples taken from basal and upper zones of coleoptiles (about 5 mm each) were first fixed with FAA solution (5% acetic acid, 45% ethanol, and 5% formaldehyde) under a vacuum and then rendered transparent by incubation for 20 min in a solution of chloral hydrate (chloral hydrate, 200 g; glycerol, 20 g; water, 50 mL) as described by Tsuge et al. (1996). Samples were then stained with 0.1% toluidine blue dissolved in 0.1 m sodium phosphate buffer (pH 7.0) for a few minutes. The length and width of epidermal cells were measured. Individual data were obtained from about 100 cells of at least three samples.
RESULTS
Molecular Analysis of Transgenes in Host Plants
A chimeric gene that expresses an antisense RNA of NDP kinase was introduced into rice genome by A. tumefaciens-mediated transformation. We measured NDP kinase activities of young T1 seedlings from 12 independent transgenic lines. Although some variability in NDP kinase activity was noted in transgenic plant lines, a reduction of enzyme activity was observed in all lines (Fig. 1D). Among the transgenic lines, we selected two for further analysis. Southern-blot analysis showed two distinctive bands in each transgenic line (Fig. 1B): a 6.6-kb fragment, representing the endogenous NDP kinase (Yano et al., 1993), and additional fragments of different lengths, representing transgenes.
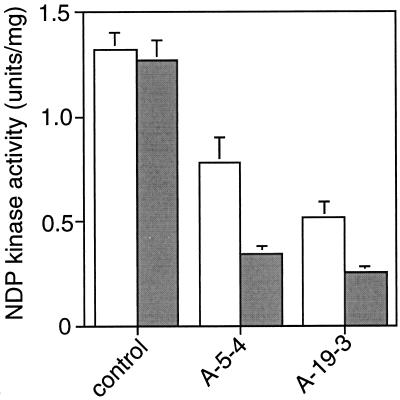
To estimate the accumulation of transcript encoding the NDP kinase, total RNA isolated from young shoots was probed with NDP kinase antisense DNA in northern blotting. In two transformants (A-5-4 and A-19-3), the mRNA level was markedly low (Fig. 1C), indicating that the expression of the antisense gene down-regulated the normal transcription level. This reduction in turn decreased NDP enzyme activity (Fig. 1D). We also confirmed the expression of antisense mRNA using sense RNA as a probe (data not shown). The Arabidopsis HSP81-1 promoter can be activated in 10-d-old rice plants even under 25°C. Heat shock treatment (37°C) for 2 d resulted in the attenuation of NDP kinase activity in transgenic plants, whereas the same treatment did not change the enzyme activity of non-transformed control plants (Fig. 2).
Figure 2.
Comparison of NDP kinase activity between normal and heat shock treatment conditions. Seeds were germinated under 25°C, and 12-d-old seedlings of each line were assayed for NDP kinase enzyme activities (white bars), or seeds were germinated for 10 d under 25°C, followed by 37°C heat shock for 2 d (shaded bars). Data for individual lines represent the means ± se of three preparations.
Analysis of NDP Kinase in Coleoptile under Submersion
During the course of the investigation, it became apparent that the growth of anti-NDP kinase plants was affected. At the adult stage (about 60 d after sowing), transgenic plants were shorter than non-transformed plants, which could be seen by a shift in the distribution peak toward dwarfism in transgenic plants.
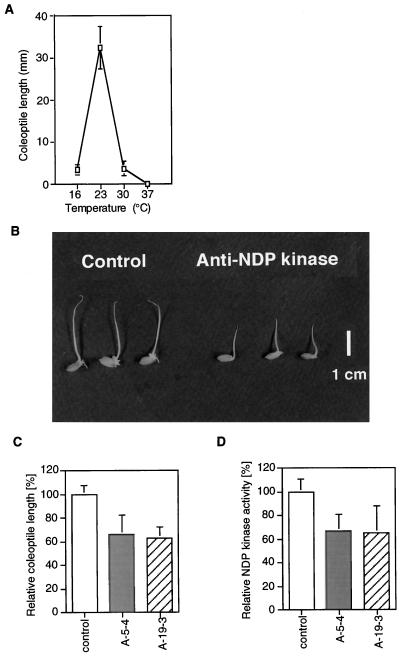
Rice seedlings that germinate under water elongate their coleoptiles. Such elongation is mostly caused by an increased cell volume. In the next series of experiments, we used this system to examine the optimal condition for maximum elongation of the coleoptile. Thus, seeds were presoaked at 37°C for 2 d for heat shock treatment, and submersed at 16°C to 37°C for another 4 d (all under darkness). The longest coleoptile was found in rice seedlings submerged at 23°C for 4 d (Fig. 3A). Seeds were presoaked at 37°C for 2 d, followed by incubation at 23°C for 4 d before sampling. Under these conditions, a marked reduction of coleoptile length was noted in anti-NDP kinase transgenic plants (Fig. 3B). The average coleoptile length of two antisense transformants (A-5-4 and A-19-3) was approximately 60% of control plants (Fig. 3C). Further analysis of the data showed that the relative level of NDP kinase activity in coleoptiles was proportional to the relative length of the coleoptile, as shown in Figure 3D.
Figure 3.
A, Trial experiment for establishing the optimal conditions for coleoptile elongation. B, Comparison of coleoptiles in non-transformants and two antisense NDP kinase transgenic rice lines. Relative coleoptile length (C) and NDP kinase activity (D) of the two transgenic rice lines are presented as a percentage of non-transformed plants.
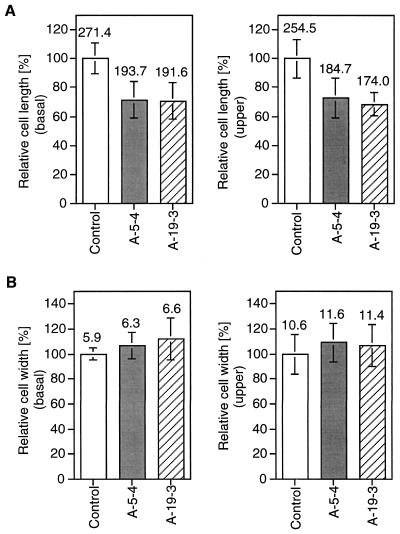
To determine whether the reduction of coleoptile growth in anti-NDP kinase plants was caused by decreased cell elongation, epidermal layers of the basal and uppermost zones of coleoptile (about 5-mm segment each) were examined. As shown in Figure 4, the length but not the width of the epidermal layers was shortened. Thus, cell elongation was inhibited in the longitudinal axis when NDP kinase enzyme activity was diminished.
Figure 4.
Comparison of epidermal cell size of coleoptiles in non-transformants and transgenic plants (A-5-4 and A-19-3). Data for cell length (A) and width (B) were obtained from epidermal cells (about 100 cells from at least three samples for each data). Samples from the basal (5 mm from the base) and upper (5 mm from the top) portions of the coleoptiles were used. Numerals on each bar are means of cell size (μm).
DISCUSSION
NDP kinase is a ubiquitous housekeeping enzyme (Parks and Agarwal, 1973). For example, it provides nucleoside triphosphates for nucleic acid synthesis, CTP for lipid synthesis, UTP for polysaccharide synthesis, and GTP for protein elongation. Apart from these basic functions, other specific roles have been identified. In mammals, reduced expression of nm23 (non-metastatic 23rd clone), a homologous gene of NDP kinase, correlates with increased metastatic potential (Steeg et al., 1988a, 1988b; Bevilaqua et al., 1989). This suggests that nm23 may act as a metastasis suppressor gene in mammalian cells (Steeg et al., 1988a; Bevilaqua et al., 1989). In Drosophila the NDP kinase encoded by the awd gene is essential for development (Biggs et al., 1990).
The association of NDP kinase with microtubule protein has been observed in mammalian cell system (Nickerson and Wells, 1984; Lombardi et al., 1995). Nm23-M1 protein (NDP kinase) formed complexes with β-tubulin and that the number of complexes increased during the differentiation process of murine cells. Therefore, NDP kinase may play a role in the cell growth pathway.
Harris et al. (1994) reported up-regulation of NDP kinase gene by wounding in tomatoes. Moisyadi et al. (1994) provided biochemical evidence indicating that sugarcane NDP kinase may be modulated by heat shock. However, little is known about their biological functions, in particular, their roles in growth and development. To explore this, the full-length cDNA (Yano et al., 1993) was fused to its antisense orientation to a heat shock promoter of the Arabidopsis HSP81-1 gene (Takahashi et al., 1992). This promoter was selected because NDP kinase seems to play a crucial role in various developmental processes in other organisms, so the expression of such a gene needs to be strictly regulated. If the antisense NDP kinase gene is constitutively expressed at high levels in plants, as driven by the 35S promoter, it will influence the growth of rice plants. On the other hand, the gene driven by the heat shock promoter is conditionally expressed. The foreign protein expression under the regulation of Arabidopsis HSP81-1 promoter was demonstrated to be a useful experimental system (Ueda et al., 1996).
As described in “Results,” characterization of the T3 generation of transgenic plants was performed at the DNA, RNA, and enzyme activity levels. Southern hybridization confirmed the integration of the chimeric gene in transgenic plants. Northern and enzyme activity analyses confirmed the expression of antisense RNA, which led to the effective suppression of NDP kinase mRNA, thus lowering enzyme activity. The suppression levels varied among transgenic plant lines, possibly due to the random integration site of the antisense construct (Stockhaus et al., 1990). Considering the level of inhibition, other types of NDP kinase must be working.
It has been reported that the abnormal wing disc (awd)/NDP kinase protein co-localizes with microtubules and that the lack of awd may cause a defect of spindle microtubule polymerization (Biggs et al., 1990). In murine cells, a molecular interaction between Nm23-M1/NDP kinase protein and β-tubulin has been described; the formation of this complex might be involved in the differentiation process (Lombardi et al., 1995). In anti-NDP kinase transgenic rice plants, the growth defect was manifested by dwarfism of the whole plant. Therefore, we analyzed whether reduced levels of NDP kinase activity were related to cell growth processes. In higher plants, cortical microtubules are thought to regulate cell morphogenesis by defining the direction of cellulose microfibril deposition (Green, 1980; Giddings and Staehelin, 1991). In this scenario, if NDP kinase protein is also related to microtubules in plant cells, we should expect a modification of cell shape or size in antisense transgenic plants. Furthermore, the reduction of NDP kinase activities may trigger the decreases in GTP and CTP, which in turn may lower the steady-state levels of UTP essential for the production of cell wall mass. We used the rice coleoptile system to confirm this hypothesis. By comparing coleoptile lengths, NDP kinase enzyme activities, and cell size analysis data in non-transformants and anti-NDP kinase plants, we found that the cell elongation process was predominantly inhibited in epidermal cells of coleoptiles in antisense plants.
In conclusion, the phenotype associated with the suppression of NDP kinase gene expression in transgenic rice plants suggests that this gene is essential for the cell elongation processes. To our knowledge, this is the first evidence to show that the gene engaged in nucleotide homeostasis is responsible for cell elongation processes in higher plants. Cell elongation mediated by phytohormones (Shibaoka, 1991; Azpiroz et al., 1998) and/or phytochrome (Zandomeni and Schopfer, 1993) has been extensively studied. Involvement of NDP kinase in such pathways, possibly downstream, cannot be excluded completely because the precise biochemical events are not fully understood, particularly with regard to the mechanisms of cell elongation. In Dictyostelium, Bominaar et al. (1993) suggested that receptor-stimulated NDP kinase contributed to the mediation of hormone action by producing GTP for the activation of GTP-binding proteins. The evidence presented here may provide new insight into the significance of the guanine nucleotide pathway in the regulation of plant cell elongation.
ACKNOWLEDGMENTS
We thank K. Yoshida and N. Katagiri for their help in the experiments.
Footnotes
This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture and Science, Japan, by a grant from the Rockefeller Foundation, and by Research for the Future from the Japan Society for the Promotion of Science (grant no. JSPS–RFTF96L00604).
LITERATURE CITED
- Azpiroz R, Wu Y, LoCascio JC, Feldmann KA. An Arabidopsis brassinosteroid-dependent mutants is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua G, Sobel ME, Liotta LA, Steeg PA. Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res. 1989;49:5185–5190. [PubMed] [Google Scholar]
- Biggs J, Hersperger E, Steeg PS, Liotta LA, Shearn A. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell. 1990;63:933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- Bominaar AA, Molijn AC, Pestel M, Veron M, Van Haastert PJM. Activation of G-proteins by receptor-stimulated nucleoside diphosphate kinase in Dictyostelium. EMBO J. 1993;12:2275–2279. doi: 10.1002/j.1460-2075.1993.tb05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Finan PM, White IR, Redpath SH, Findlay JBC, Millner PA. Molecular cloning, sequence determination and heterologous expression of nucleoside diphosphate kinase from Pisum sativum. Plant Mol Biol. 1994;25:59–67. doi: 10.1007/BF00024198. [DOI] [PubMed] [Google Scholar]
- Giddings TH, Staehelin A. Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 85–99. [Google Scholar]
- Green PB. Organogenesis: a biophysical view. Annu Rev Plant Physiol. 1980;31:51–82. [Google Scholar]
- Harris N, Taylor JE, Roberts JA. Isolation of a mRNA encoding a nucleoside diphosphate kinase from tomato that is up-regulated by wounding. Plant Mol Biol. 1994;25:739–742. doi: 10.1007/BF00029611. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Kimura N, Shimada N. Membrane-associated nucleoside diphosphate kinase from rat liver: purification, characterization, and comparison with cytosolic enzyme. J Biol Chem. 1988;263:4647–4653. [PubMed] [Google Scholar]
- Kimura N, Shimada N. Evidence for complex formation between GTP binding protein (Gs) and membrane-associated nucleoside diphosphate kinase. Biochem Biophys Res Commun. 1990;168:99–106. doi: 10.1016/0006-291x(90)91680-q. [DOI] [PubMed] [Google Scholar]
- Leone A, Flatow U, King CR, Sandeen MA, Margulies IMK, Liotta LA, Steeg PS. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991;65:25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- Lombardi D, Sacchi A, D'Agostino G, Tibursi G. The association of the Nm23–M1 protein and β-tubulin correlates with cell differentiation. Exp Cell Res. 1995;217:267–271. doi: 10.1006/excr.1995.1086. [DOI] [PubMed] [Google Scholar]
- Moisyadi S, Dharmasiri S, Harrington HM, Lukas TJ. Characterization of a low molecular mass autophosphorylating protein in cultured sugarcane cells and its identification as a nucleoside diphosphate kinase. Plant Physiol. 1994;104:1401–1409. doi: 10.1104/pp.104.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson JA, Wells WW. The microtubule-associated nucleoside diphosphate kinase. J Biol Chem. 1984;259:11297–11304. [PubMed] [Google Scholar]
- Nomura T, Yatsunami K, Honda A, Sugimoto Y, Fukui T, Zhang J, Yamamoto J, Ichikawa A. The amino acid sequence of nucleoside diphosphate kinase I from spinach leaves, as deduced from the cDNA sequence. Arch Biochem Biophys. 1992;297:42–45. doi: 10.1016/0003-9861(92)90638-d. [DOI] [PubMed] [Google Scholar]
- Parks RE, Jr, Agarwal RP. Nucleoside diphosphokinase. In: Boyer PD, editor. The Enzymes. New York: Academic Press; 1973. pp. 307–344. [Google Scholar]
- Rosengard AM, Krutzsch HC, Shearn A, Biggs J, Barker E, Margulis IMK, King CR, Liotta LA, Steeg PS. Reduced nm23/awd protein in tumor metastasis and aberrant Drosophila development. Nature. 1989;342:177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 7.39–7.52. [Google Scholar]
- Shibaoka H. Microtubules and the regulation of cell morphogenesis by plant hormones. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. New York: Academic Press; 1991. pp. 159–168. [Google Scholar]
- Shure M, Wessler S, Fedoroff N. Molecular identification and isolation of the waxy locus in maize. Cell. 1983;35:225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Sommer D, Song PS. A plant nucleoside diphosphate kinase homologous to the human Nm23 gene product: purification and characterization. Biochim Biophys Acta. 1994;1222:464–470. doi: 10.1016/0167-4889(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Steeg P, Bevilaqua G, Pozzatti R, Liotta L, Sobel M. Altered expression of nm23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastases. Cancer Res. 1988a;48:6550–6554. [PubMed] [Google Scholar]
- Steeg PS, Bevilaqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988b;80:200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Stockhaus J, Höfer M, Renger G, Westhoff P, Wydrzynski T, Willmitzer L. Antisense RNA efficiently inhibits formation of the 10 kd polypeptide of photosystem II in transgenic potato plants: analysis of the role of the 10 kd protein. EMBO J. 1990;9:3013–3021. doi: 10.1002/j.1460-2075.1990.tb07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Naito S, Komeda Y. Isolation and analysis of the expression of two genes for the 81-kilodalton heat-shock proteins from Arabidopsis. Plant Physiol. 1992;99:383–390. doi: 10.1104/pp.99.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge T, Tsukaya H, Uchimiya H. Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development. 1996;122:1589–1600. doi: 10.1242/dev.122.5.1589. [DOI] [PubMed] [Google Scholar]
- Ueda T, Anai T, Tsukaya H, Hirata A, Uchimiya H. Characterization and subcellular localization of a small GTP-binding protein (Ara-4) from Arabidopsis: conditional expression under control of the promoter of the gene for heat-shock protein HSP81-1. Mol Gen Genet. 1996;250:533–539. doi: 10.1007/BF02174441. [DOI] [PubMed] [Google Scholar]
- Umeda M, Uchimiya H. Differential transcript levels of genes associated with glycolysis and alcohol fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiol. 1994;106:1015–1022. doi: 10.1104/pp.106.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano A, Shimazaki T, Kato A, Umeda M, Uchimiya H. Molecular cloning and nucleotide sequence cDNA encoding nucleoside diphosphate kinase of rice (Oryza sativa L.) Plant Mol Biol. 1993;23:1087–1090. doi: 10.1007/BF00021824. [DOI] [PubMed] [Google Scholar]
- Yano A, Umeda M, Uchimiya H. Expression of functional proteins of cDNA encoding rice nucleoside diphosphate kinase (NDK) in Escherichia coli and organ-related alteration of NDK activities during rice seed germination (Oryza sativa L.) Plant Mol Biol. 1995;27:1053–1058. doi: 10.1007/BF00037032. [DOI] [PubMed] [Google Scholar]
- Zandomeni K, Schopfer P. Reorientation of microtubules at the outer epidermal wall of maize coleoptiles by phytochrome, blue-light photoreceptor and auxin. Protoplasma. 1993;173:103–112. [Google Scholar]
- Zhang J, Nomura T, Yatsunami K, Honda A, Sugimoto Y, Moriwaki T, Yamamoto J, Ohta M, Fukui T, Ichikawa A. Nucleotide sequence of the cDNA encoding nucleoside diphosphate kinase II from spinach leaves. Biochim Biophys Acta. 1993;1171:304–306. doi: 10.1016/0167-4781(93)90070-t. [DOI] [PubMed] [Google Scholar]