Abstract
Background
We report on the psychometric properties of the 16-item Concise Associated Symptom Tracking Scale self-report scale and its clinical utility.
Methods
The 5-domain (irritability, anxiety, mania, insomnia, and panic) structure of Concise Associated Symptom Tracking Scale was validated with confirmatory factor analysis in Combining Medications to Enhance Depression Outcomes trial participants at baseline (n=664). Correlations with other clinical measures were used for convergent and divergent validity. In participants with at least one postbaseline visit (n=630), worsening in each Concise Associated Symptom Tracking Scale domain was defined as ≥1.28 SD increase from baseline for each visit (weeks 1, 2, 4, and 6) only. Worsening in any domain (except mania) was defined as overall worsening. Association of domain-specific and overall worsening with remission was tested with logistic regression analyses.
Results
The 5-domain structure had adequate model fit on confirmatory factor analysis (GFI=0.93, CFI=0.89, and RMSEA=0.07). Scores on anxiety, panic, insomnia, and mania significantly correlated with Hamilton Rating Scale for Depression anxiety subscale (rs=0.27), Psychiatric Diagnostic Screening Questionnaire-panic scale (rs=0.35), sum of 3 Quick Inventory of Depressive Symptomatology Self-Report insomnia items (rs=0.55), and Altman Self-Rating Mania scale (rs=0.41), respectively. From baseline to week 6, 5.2%, 7.5%, 47.6%, 15.6%, 6.2%, and 27.6% participants (n=630) experienced irritability, anxiety, mania, insomnia, panic, and overall worsening, respectively. Participants with overall worsening were less likely to remit (31.6%) than those without any worsening (43.9%; odds ratio=0.53, 95% CI=0.36, 0.78).
Conclusion
The 16-item Concise Associated Symptom Tracking Scale self-report has acceptable psychometric properties. Clinically significant worsening of irritability, anxiety, insomnia, or panic with antidepressant treatment is associated with poorer outcomes.
Keywords: major depression, antidepressant, irritability, mania, anxiety, insomnia, panic
Significance Statement
The 16-item Concise Associated Symptom Tracking (CAST) is a valid self-report scale that measures worsening of irritability, anxiety, mania, insomnia, or panic symptoms after initiation of antidepressant medications. Depressed patients who report clinically significant increase in irritability, anxiety, insomnia, or panic at any time during the first 6 weeks of antidepressant treatment are significantly less likely to remit (31.6%) with acute-phase (12-week) antidepressant treatment compared with those who experienced no worsening. Use of this easy-to-administer self-report scale in routine clinical practice can help with early treatment optimization by identifying depressed patients who may be less likely to respond to their ongoing antidepressant treatment.
Introduction
Major depressive disorder (MDD) patients may experience emergence or worsening of irritability, anxiety, panic, insomnia, and mania/hypomania with antidepressant medications (Safer and Zito, 2006; Harada et al., 2008; Sinclair et al., 2009), but there is a dearth of valid and reliable self-report assessments of these antidepressant treatment emergent symptoms. Worsening of these symptoms may contribute to early drop-out during treatment initiation or they may suggest a need for early dose adjustments (Montgomery and Kasper, 1998; Machado and Einarson, 2010; Kostev et al., 2014).
To address these concerns, Trivedi et al. (2006) developed a 16-item self-report scale, the Concise Associated Symptom Tracking (CAST), to assess irritability, anxiety, panic, insomnia, and mania/hypomania (Trivedi et al., 2011). Validation of the easy-to-administer CAST scale in a separate sample of treatment-seeking depressed outpatients is needed prior to its use in measurement-based care protocols (Trivedi et al., 2006).
This report evaluates the psychometric properties and clinical utility of CAST in a large sample of treatment-seeking depressed outpatients who participated in the Combining Medications to Enhance Depression Outcomes (CO-MED) trial (Rush et al., 2011). To evaluate the psychometric properties, we tested the (1) validity of 5-domain (anxiety, irritability, mania, panic, and insomnia) structure with confirmatory factor analyses, (2) internal consistency with Cronbach’s α coefficient, and (3) construct validity with correlation of these CAST domains with other clinical assessments at baseline. To evaluate the clinical utility of CAST, we asked the following specific questions:
What proportion of depressed patients experience worsening in the anxiety, irritability, mania, panic, and insomnia domains of CAST over first 6 weeks of antidepressant treatment? And when?
Is symptomatic worsening on CAST domains associated with lower rates of remission even after adjusting for baseline depression severity?
Methods
Study Overview and Participants
The analytic sample for this report includes all CO-MED trial participants who completed the CAST scale at baseline (n=664). As described earlier by Rush et al. (Rush et al., 2011), participants were enrolled from 6 primary and 9 psychiatric care sites in the CO-MED trial after obtaining written informed consent. The trial was approved by the Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, and each participating regional center and clinical site and was monitored by an independent data safety and monitoring board. The inclusion and exclusion criteria have been described in detail previously by Rush et al. (Rush et al., 2011).
Briefly, participants in the CO-MED trial had nonpsychotic chronic (current episode exceeded 2 years) or recurrent depression with current episode ≥2 months and a baseline 17-item Hamilton Rating Scale (HRSD17) ≥16 (Hamilton, 1960). As the primary goal of CO-MED trial was to compare outcomes of antidepressant combination vs selective serotonin reuptake inhibitor (SSRI) monotherapy, participants were assigned to one of the following treatment arms in a 1:1:1 ratio after stratification by clinical sites: (1) escitalopram plus placebo (SSRI monotherapy), (2) sustained-release (SR) bupropion plus escitalopram (bupropion-SSRI combination), and (3) extended-release (XR) venlafaxine plus mirtazapine (venlafaxine-mirtazapine combination).
Postrandomization visits were conducted at weeks 1, 2, 4, 6, 8, 10, and 12 for acute phase and weeks 16, 20, 24, and 28 for continuation phase. Study physicians used measurement-based care (Trivedi et al., 2006) to make medication dosage adjustment during the first 8 weeks based on the scores of the Quick Inventory of Depressive Symptomatology Clinician-rated version (QIDS-C) (Rush et al., 2003) scale and the Frequency, Intensity, and Burden of Side Effects Rating scale (Wisniewski et al., 2006a). Participation beyond acute phase was restricted to those who had either received an acceptable benefit (defined as QIDS-C score of ≤9 by 3 months) or had reached a score of 10 to 13 on QIDS-C and both the study physician and the participant decided to continue treatment because of substantial benefit.
Assessments
Assessments Conducted Only at Baseline
HRSD17.
The 17 items of this clinician-rated scale to assess depression severity have 3 to 5 choices that are scored from either 0 to 2 or 0 to 4 (Hamilton, 1960). The individual items are summed to measure depression severity [none (<6), mild (6–13), moderate (14–18), severe (19–3), and very severe (>24)] (Hamilton, 1960). The HRSD17 has a high inter-rater reliability (kappa=0.94) and inter-item correlations with (Cronbach’s α-0.53–0.83) (Rush et al., 1996; Trajković et al., 2011). Six items of HRSD17 (psychic anxiety, somatic anxiety, gastrointestinal somatic symptom, general somatic symptoms, hypochondriasis, and insight) was used to establish anxious features in CO-MED trial (Cleary and Guy, 1977; Fava et al., 2008). Additionally, HRSD17 score of ≥16 was used as inclusion criteria for CO-MED trial.
Psychiatric Diagnostic Screening Questionnaire (PDSQ).
This self-report questionnaire includes 139 questions with yes/no responses grouped in 13 subscales (mood disorders, eating disorder, panic disorders, generalized anxiety disorder, agoraphobia, posttraumatic stress disorder, obsessive compulsive disorder, social anxiety disorder, alcohol use disorder, drug use disorder, somatoform disorder, hypochondriasis, and psychosis), which were selected on the basis of high prevalence in epidemiologic surveys (Zimmerman and Chelminski, 2006). The mood disorder and psychosis subscales of PDSQ were not included in CO-MED, as these disorders were assessed using structured clinical interview (Rush et al., 2011). In multiple reports, PDSQ subscales have demonstrated good internal consistency and strong sensitivity in detecting these disorders compared with semistructured clinician-conducted interviews (Zimmerman and Mattia, 2001; Rush et al., 2005; Zimmerman and Chelminski, 2006).
Assessment Conducted at Baseline and Each Postrandomization Visit
Inventory of Depressive Symptomatology Clinician Rated (IDS-C).
Of the 30 items of this clinician-rated assessment of depression (each item scored from 0 to 3), 28 items are summed to generate total score (range 0–84) to indicate depression severity as follows: none (<13), mild (14–25), moderate (26–38), severe (39–48), and very severe (>49) (Rush et al., 1996). The IDS-C correlates very highly (r=0.95) with HRSD17 (Rush et al., 1996). The Cronbach’s α of IDS-C range from 0.67 to 0.94 (Rush et al., 1996; Trivedi et al., 2004). Using principal component analysis, Rush et al. (Rush et al., 1996; Trivedi et al., 2004) have previously reported a 3-factor structure for IDS-C, namely cognitive/mood, anxiety/arousal, and sleep/appetite regulation.
QIDS-C and Self-Report (QIDS-SR).
The 16 items (each scored from 0 to 3) of QIDS-C and QIDS-SR are based on the 9 criterion symptom domains of the 16 items and total score ranged from 0 to 27 (Rush et al., 2003). Both scales correlate highly with HRSD17 (0.86–0.93) and have high inter-item correlations (Cronbach’s α=0.86–0.87) (Rush et al., 2003). In the CO-MED trial, the QIDS-SR served as the measure of depressive symptoms for primary outcome, while the QIDS-C was used to monitor symptom changes and guide treatment decisions.
CAST Self-Report.
The 16 items of this self-report scale assess symptoms across the 5 following domains where each individual item is rated on a 5-point Likert scale with responses of “strongly disagree,” “disagree,” “neither agree nor disagree,” “agree,” and “strongly agree” corresponding to scores of 1, 2, 3, 4, and 5, respectively: anxiety (3 items, range 3–15), irritability (5 items, range 5–25), mania (4 items, range 4–20), insomnia (2 items, range 2–10), and panic (2 items, range 2–10) (Trivedi et al., 2011). The Cronbach’s α of CAST-SR was 0.78 (Trivedi et al., 2011) and high agreement between clinician rated and self-report versions of CAST with weighted Kappa ranging from 0.59 to 0.78 (Trivedi et al., 2011). The scores in each domain correlated highly with separately administered assessments. For example, anxiety and panic domains of CAST-SR correlated significantly with the scores on Beck Anxiety Inventory (r=0.51–0.59) and HRSD17 anxiety subscale (r=0.30–0.31). Similarly, the insomnia domain on CAST-SR had high correlation with the 3 insomnia items of QIDS (r=0.51). The irritability domain had significant correlations with impulsivity rating scale (r=0.39), Beck Anxiety Inventory (r=0.42), and irritability item of Clinician-Administered Rating Scale of Mania (r=0.30) (Trivedi et al., 2011).
Altman Self Rating Mania Scale (ASRM).
The ASRM is a 5item self-reported scale designed to evaluate for the presence and/or severity of manic and hypomanic symptoms over the past 7 days. Each item consists of 5 possible responses, with scores ranging from 0 to 4. Item scores are added to give a scale total score, 0 being the lowest possible score and a maximum possible of 20. The ASRM is significantly correlated to the CARS-M and the Young Mania Rating Scale (Altman et al., 1997).
Statistical Analyses
We included all CO-MED trial participants with CAST at baseline (n=664) as the analytic sample for testing the psychometric properties of CAST. For analyses of worsening of preexisting or treatment emergent symptoms (described subsequently and heretofore referred to as “worsening”) to evaluate the clinical utility, we restricted only to participants who had at least one postbaseline visit, at weeks 1, 2, 4, or 6 (n=630). As the purpose of CAST is to identify worsening, we took a conservative approach and classified missing scores as “not worsened.” We used descriptive statistics to summarize the 5 domains of CAST as well as other clinical assessments at baseline in CO-MED trial.
To validate the 5-domain structure of CAST, we used confirmatory factor analysis as implemented in SAS. We a priori defined acceptable model fit as Goodness of Fit index (GFI)≥0.90, comparative fit index (CFI) ≥0.90, and root mean square error of approximation ≤0.08 (Hooper et al., 2008). To evaluate the internal consistency, we calculated the Cronbach’s α coefficient for each CAST domain (Cronbach, 1951). We also calculated spearman’s correlation coefficient (rs) between the 5 domains of CAST and the baseline clinical assessments in CO-MED trial.
To evaluate the clinical utility, we restricted the analyses to the first 6 weeks to capture early change with treatment initiation and optimization, as CO-MED trial design included addition of a second drug (placebo, escitalopram, or mirtazapine) at week 2 with dosage adjustments recommended using MBC approach (Rush et al., 2011). We created a clinically significant change threshold of 1.28 times the SD at baseline for each domain of CAST. Next, we categorized participants who experienced increase, compared with baseline, at weeks 1, 2, 4, and 6 above this clinically significant change threshold as significantly worsening in that domain. We used 1.28 times SD threshold as it reflects large change consistent with FDA’s warning (Wisniewski et al., 2006b; Iscan et al., 2015) and our previous use of 1.28 times SD to define normal threshold (Greer et al., 2016; Jha et al., 2017). We used descriptive statistics to summarize the proportion of participants who (1) experienced worsening in each CAST domain at any time during the first 6 weeks and (2) first experienced worsening in each CAST domain at weeks 1, 2, 4, and 6. In posthoc analyses after finding that increase above the a priori defined threshold was substantially higher in mania domain compared with other domains, we also calculated the proportion of participants who experienced any worsening in domains of irritability, anxiety, insomnia, or panic (all CAST domains except mania) at any postbaseline visits during the first 6 weeks (includes visits at weeks 1, 2, 4, or 6, hereafter referred to as “overall worsening”).
We then tested the association of worsening of symptoms in each domain as well as overall worsening with remission during the acute phase of CO-MED in separate logistic regression analyses after controlling for baseline depression severity. We included baseline depression severity as a covariate, as it was associated with remission in previous reports (Friedman et al., 2012; Jha et al., 2016). Remission in CO-MED trial, the primary study outcome, was ascribed if, of the last 2 consecutive QIDS-SR scores, at least 1 was <6 while the other was <8 (Rush et al., 2011). We compared baseline clinical features between those who experienced overall worsening during the first 6 weeks vs those who did not. We repeated the above-mentioned logistic regression analysis for overall worsening after adjusting for the baseline variables that significantly differed between those with and without overall worsening during the first 6 weeks. We set the level of significance at 0.05 and used SAS 9.3 (SAS Inc) for all analyses.
Results
Of the 664 CO-MED trial participants who completed CAST at baseline, most were females (68.1%), white (64.0%), and earned <$2000 (62.9%) per month (also see supplementary Table 1). We found that mean (SD) scores of irritability (5 items), anxiety (3 items), mania (4 items), insomnia (2 items), and panic (2 items) domains were 17.34 (3.80), 9.24 (2.95), 7.61 (2.79), 7.06 (2.33), and 4.64 (2.19), respectively. At weeks 1, 2, 4, or 6, compared with baseline, an increase ≥4.86, 3.78, 3.57, 2.98, and 2.80 was defined as worsening in irritability, anxiety, mania, insomnia, and panic domains, respectively. We have provided detailed frequency of responses to individual items of CAST-SR in Table 2 along with the mean (SD) for these items.
Table 2.
Factor Loading of Individual Items and Correlation among Individual Domains of CAST Obtained from Confirmatory Factor Analysis
| Anxiety | Irritability | Mania | Insomnia | Panic | |
|---|---|---|---|---|---|
| Standardized factor loadings | |||||
| I feel anxious all the time | 0.42 | ||||
| I have been feeling really good lately | 0.50 | ||||
| I feel as if I am going to have a heart attack | 0.68 | ||||
| I wish people would just leave me alone | 0.49 | ||||
| I have been having more trouble sleeping than usual | 0.79 | ||||
| I am feeling restless, as if I have to move constantly | 0.84 | ||||
| I suddenly feel very confident | 0.71 | ||||
| I am more talkative than normal | 0.60 | ||||
| I feel very uptight | 0.48 | ||||
| I find myself saying or doing things without thinking | 0.41 | ||||
| I can feel my heart racing | 0.86 | ||||
| Lately everything seems to be annoying me | 0.80 | ||||
| I slept very little last night | 0.75 | ||||
| I cannot sit still | 0.85 | ||||
| I find people get on my nerves easily | 0.76 | ||||
| I have been having lots of great ideas | 0.61 | ||||
| Correlation coefficient | |||||
| Anxiety | 1 | ||||
| Irritability | 0.51 | 1 | |||
| Mania | 0.15 | -0.09 | 1 | ||
| Insomnia | 0.37 | 0.37 | 0.01 | 1 | |
| Panic | 0.44 | 0.36 | 0.17 | 0.33 | 1 |
CAST, Concise Associated Symptom Tracking Scale.
Table 1.
Response to Individual Items of CAST at Baseline in CO-MED Trial Participants (n = 664)
| Domain of CAST | Strongly Disagree | Disagree | Neither Agree nor Disagree | Agree | Strongly Agree | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No.# | Individual Items | N | % | N | % | N | % | N | % | N | % | Mean | SD | |
| 1 | I feel anxious all the time | Anxiety | 55 | 8.3 | 148 | 22.3 | 121 | 18.2 | 221 | 33.3 | 119 | 17.9 | 3.30 | 1.23 |
| 2 | I have been feeling really good lately | Mania | 308 | 46.4 | 270 | 40.7 | 48 | 7.2 | 27 | 4.1 | 11 | 1.7 | 1.74 | 0.88 |
| 3 | I feel as if I am going to have a heart attack | Panic | 304 | 45.8 | 171 | 25.8 | 90 | 13.6 | 74 | 11.1 | 25 | 3.8 | 2.01 | 1.17 |
| 4 | I wish people would just leave me alone | Irritability | 54 | 8.1 | 91 | 13.7 | 153 | 23.0 | 239 | 36.0 | 127 | 19.1 | 3.44 | 1.18 |
| 5 | I have been having more trouble sleeping than usual | Insomnia | 50 | 7.5 | 108 | 16.3 | 77 | 11.6 | 226 | 34.0 | 203 | 30.6 | 3.64 | 1.27 |
| 6 | I am feeling restless, as if I have to move constantly | Anxiety | 84 | 12.6 | 150 | 22.6 | 124 | 18.7 | 233 | 35.1 | 73 | 11.0 | 3.09 | 1.23 |
| 7 | I suddenly feel very confident | Mania | 308 | 46.4 | 250 | 37.6 | 66 | 9.9 | 32 | 4.8 | 8 | 1.2 | 1.77 | 0.90 |
| 8 | I am more talkative than normal | Mania | 247 | 37.2 | 271 | 40.8 | 88 | 13.2 | 51 | 7.7 | 7 | 1.1 | 1.95 | 0.95 |
| 9 | I feel very uptight | Irritability | 56 | 8.4 | 102 | 15.4 | 110 | 16.6 | 285 | 42.9 | 111 | 16.7 | 3.44 | 1.18 |
| 10 | I find myself saying or doing things without thinking | Irritability | 86 | 13.0 | 202 | 30.4 | 120 | 18.1 | 205 | 30.9 | 51 | 7.7 | 2.90 | 1.20 |
| 12 | I can feel my heart racing | Panic | 159 | 24.0 | 193 | 29.1 | 95 | 14.3 | 170 | 25.6 | 47 | 7.1 | 2.63 | 1.29 |
| 13 | Lately everything seems to be annoying me | Irritability | 17 | 2.6 | 87 | 13.1 | 111 | 16.7 | 295 | 44.4 | 154 | 23.2 | 3.73 | 1.04 |
| 14 | I slept very little last night | Insomnia | 65 | 9.8 | 147 | 22.1 | 65 | 9.8 | 216 | 32.5 | 171 | 25.8 | 3.42 | 1.34 |
| 15 | I cannot sit still | Anxiety | 104 | 15.7 | 185 | 27.9 | 129 | 19.4 | 199 | 30.0 | 47 | 7.1 | 2.85 | 1.21 |
| 16 | I find people get on my nerves easily | Irritability | 21 | 3.2 | 65 | 9.8 | 91 | 13.7 | 317 | 47.7 | 170 | 25.6 | 3.83 | 1.02 |
| 17 | I have been having lots of great ideas | Mania | 218 | 32.8 | 249 | 37.5 | 99 | 14.9 | 69 | 10.4 | 29 | 4.4 | 2.16 | 1.12 |
CO-MED, Combining Medications to Enhance Depression Outcomes.
No. refers to the item numbers originally reported in the 17-item CAST as reported in Figure 1 of Trivedi et al. primary CAST paper (Trivedi et al., 2011). Note that 16-item CAST SR excluded item 11 as it loaded on 2 different factors.
Psychometric Properties of the CAST Scale
Validity of 5-Domain Structure of CAST
We found GFI=0.93, CFI=0.89, and RMSEA=0.07 for the 5-domain structure of CAST using confirmatory factor analysis. As 2 of the 3 a priori-defined criteria were met, we found the model fit to be acceptable. The standardized factor loadings for the anxiety, irritability, mania, insomnia, and panic ranged from 0.42 to 0.85, 0.41 to 0.80, 0.50 to 0.71, 0.7 to 0.79, and 0.68 to 0.86, respectively (Table 2). The domains of irritability, anxiety, insomnia, and panic were significantly correlated with each other (correlation coefficient=0.33–0.51) (Table 2). Mania domain had poor correlation with other domains of CAST (correlation coefficient= -0.09–0.15) (Table 2).
Internal Consistency
The Cronbach’s α of irritability, anxiety, mania, insomnia, and panic domains were 0.83, 0.87, 0.84, 0.92, and 0.92, respectively.
Construct Validity
Irritability, anxiety, insomnia, and panic domains were positively correlated with measures of overall depression severity included at baseline, namely QIDS-SR (rs=0.31–0.45), HRSD17 (rs=0.26–0.40), and IDS-C (rs=0.29–0.41) (Table 3). Conversely, mania domain was negatively correlated with QIDS-SR (rs= -0.30), HRSD17 (rs= -0.15), and IDS-C (rs= -0.22). Anxiety domain was positive correlated with anxious features on HRSD17(rs=0.28), anxiety/arousal factor of IDS-C (rs=0.37), and PDSQ generalized anxiety disorder subscale (rs=0.29). While there were no measures specifically of irritability in CO-MED trial, scores on irritability were significantly correlated with the mood/cognitive subscale of IDS-C (rs=0.38) and poorly correlated with scales of mania (rs=0.08) and nonanxiety subscales of PDSQ (rs=0.0.07–0.11) (Table 3). Mania, insomnia, and panic domains were positive correlated with ASRM (rs=0.41), sum of insomnia items of QIDS-SR (rs=0.55), and PDSQ panic subscales (rs=0.35), respectively.
Table 3.
Spearman Correlation Coefficients of CAST Domains with Baseline Clinical Characteristics in CO-MED Trial
| Anxiety | Irritability | Mania | Insomnia | Panic | |
|---|---|---|---|---|---|
| QIDS-SR | 0.35 | 0.45 | -0.30 | 0.31 | 0.31 |
| HRSD17 | 0.26 | 0.31 | -0.15 | 0.40 | 0.29 |
| IDS-C | 0.29 | 0.41 | -0.22 | 0.37 | 0.33 |
| IDS-C factor 1 | 0.18 | 0.38 | -0.28 | 0.24 | 0.22 |
| IDS-C factor 2 | 0.38 | 0.32 | -0.05 | 0.49 | 0.39 |
| IDS-C factor 3 | 0.09 | 0.22 | -0.14 | 0.37 | 0.18 |
| HRSD anxiety | 0.27 | 0.26 | -0.05 | 0.19 | 0.33 |
| ASRM | 0.12 | 0.08 | 0.41 | 0.04 | 0.10 |
| QIDS-SR insomnia total | 0.11 | 0.20 | -0.07 | 0.55 | 0.14 |
| PDSQ generalized anxiety disorder | 0.29 | 0.27 | -0.02 | 0.24 | 0.28 |
| PDSQ panic | 0.22 | 0.17 | -0.03 | 0.21 | 0.35 |
| PDSQ agoraphobia | 0.22 | 0.22 | 0.02 | 0.19 | 0.20 |
| PDSQ alcohol use disorder | 0.07 | 0.07 | 0.09 | 0.04 | 0.05 |
| PDSQ bulimia | 0.06 | 0.09 | -0.01 | 0.02 | 0.04 |
| PDSQ hypochondriasis | 0.13 | 0.08 | 0.02 | 0.11 | 0.16 |
| PDSQ drug use disorder | 0.07 | 0.08 | 0.08 | 0.01 | 0.05 |
| PDSQ obsessive compulsive disorder | 0.20 | 0.20 | 0.12 | 0.12 | 0.18 |
| PDSQ posttraumatic stress disorder | 0.26 | 0.20 | 0.04 | 0.14 | 0.23 |
| PDSQ social anxiety | 0.21 | 0.29 | 0.03 | 0.17 | 0.25 |
| PDSQ somatic | 0.01 | 0.11 | -0.01 | 0.05 | 0.07 |
Clinical Utility of CAST Scale
What Proportion of Depressed Patients Experience Worsening in the Anxiety, Irritability, Mania, Panic, and Insomnia Domains of CAST during First 6 Weeks of Antidepressant Treatment? And When?
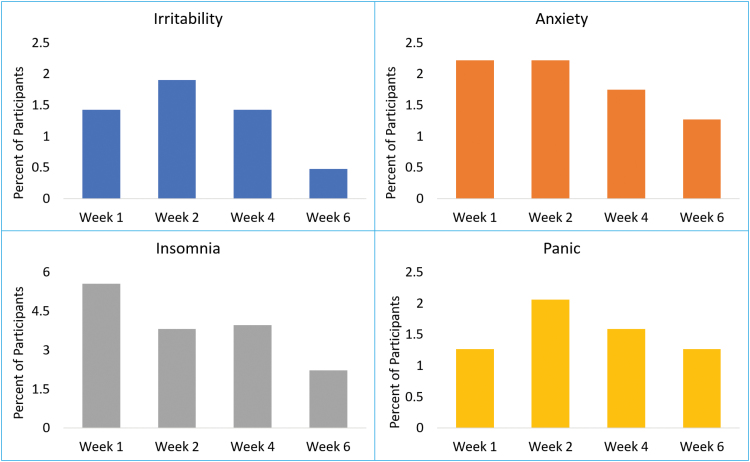
Of the 630 participants, 33 (5.2%), 47 (7.5%), 300 (47.6%), 98 (15.6%), and 39 (6.2%) met our predefined threshold for worsening in irritability, anxiety, mania, insomnia, and panic, respectively, at week 1, 2, 4, or 6. The rate of overall worsening was 27.6% (174/630). Most participants experienced worsening early in the course of treatment by week 4, as shown in Figure 1. Of the 630 participants, those receiving bupropion-SSRI experienced significantly higher rates (21/208, 10.1%) of worsening at weeks 1, 2, 4, or 6 in panic domain (χ2=8.33, df=2, P=.015) than those on SSRI monotherapy (8/212, 3.8%) or on venlafaxine-mirtazapine combination (10/210, 4.8%). We found no significant difference in rates of worsening based on treatment arm either in overall worsening (χ2=3.13, df=2, P=.21) or in irritability (χ2=0.002, df=2, P=.999), anxiety (χ2=1.52, df=2, P=.47), mania (χ2=1.41, df=2, P =.49), and insomnia (χ2=1.21, df=2, P=.55) domains (see supplementary Table 2 for proportion of participants experiencing worsening by each treatment arm).
Figure 1.
Proportion of participants who first experiences worsening in Concise Associated Symptom Tracking (CAST) domains during the first 6 weeks of the Combining Medications to Enhance Depression Outcomes (CO-MED) trial. Worsening is defined as an increase of ≥1.28 baseline SD at week 1, 2, 4, or 6 in the CAST domains of irritability, anxiety, insomnia, and panic.
Is Symptomatic Worsening on CAST Domains Associated with Lower Rates of Remission Even After Adjusting for Baseline Depression Severity?
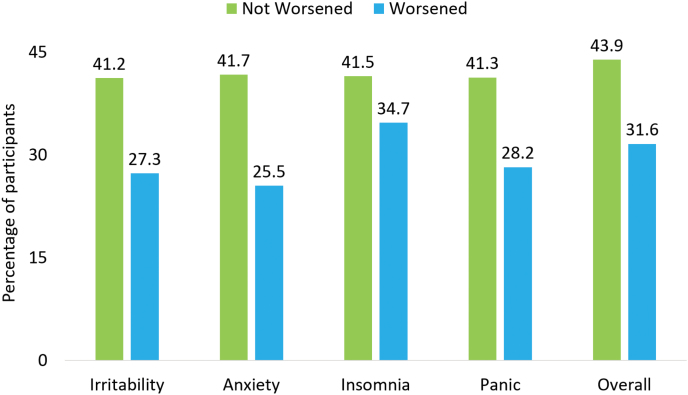
Yes. As shown in figure 2, participants with worsening of irritability, anxiety, insomnia, and panic during the first 6 weeks of CO-MED trial had numerically lower rates of remission compared with those who reported no worsening in these domains. Using separate logistic regression analyses that controlled for baseline severity for each CAST domain and overall worsening, we found lower likelihood of remission with worsening in anxiety (OR= 0.43, 95% CI= 0.21, 0.86), insomnia (OR=0.62, 95% CI=0.39, 0.99), and panic (OR=0.47, 95% CI=0.22, 0.99) domains. Conversely, we found that participants with a 1.28 SD increase in the mania domain were more likely to remit (49.3%) compared with those without this increase (32.4, number needed to treat [NNT]=5.9); this difference was statistically significant even after controlling for baseline severity (OR=2.22, 95% CI=1.59, 3.11). The lower likelihood of remission with worsening in irritability domain was not statistically significant (OR=0.47, 95% CI=0.21, 1.05). Participants with overall worsening were less likely to remit (31.6%) than those without any worsening (43.9%, NNT= 8.1) even after controlling for baseline depression severity (OR=0.53, 95% CI= 0.36, 0.78).
Figure 2.
Observed rates of remission during the Combining Medications to Enhance Depression Outcomes (CO-MED) trial based on overall and individual Concise Associated Symptom Tracking (CAST) domain-specific worsening. Remission was ascribed if, of the last 2 consecutive quick inventory of depressive symptomatology self-report scores during 12-week-long acute-phase, one was <6 and the other was <8. Worsening is defined as an increase of ≥1.28 baseline SD at week 1, 2, 4, or 6 in the CAST domains of irritability, anxiety, insomnia, and panic.
Discussion
We found confirmatory evidence for the 5-domain structure and psychometric properties of the 16-item CAST scale in a large sample of depressed outpatients. One in 4 (27.6%) participants reported worsening in at least one of the CAST domains (except mania) during the first 6 weeks. Participants with any worsening were significantly less likely to attain remission (NNT=8.1) compared with those with no overall worsening. A clinically significant increase in mania domain was associated with higher rate of remission (NNT=5.9) compared with those with no increase. The association of overall worsening with lower likelihood of remission continued to be significant even after controlling for baseline depression severity.
An unexpected finding of our report is the high rate of change in mania domain. This may be related to the wording of our questions where improved depression with treatment was reflected in clinically significant increase in mania symptoms. As valid self-report measures for manic/hypomanic symptoms such as ASRM are already used frequently, a shortened 12-item version of CAST, which excludes mania items, may be clinically useful. Alternatively, a different threshold of clinically significant change, other than the 1.28 times baseline SD, may be able to detect worsening manic/hypomanic symptoms in mania domain.
Among all CAST domains excluding mania, rates of worsening were highest for insomnia (15.6%) and lowest for irritability (5.2%). The rate of insomnia worsening with SSRI monotherapy (14.2%) was comparable with previously reported rates of treatment emergent insomnia (14%) with escitalopram 20 mg/d (Iscan et al., 2015). The rate of worsening insomnia (17.8%) and anxiety (8.2%) with bupropion-SSRI is similar to the treatment-emergent insomnia (16%) and anxiety (6%) reported previously with bupropion 400-mg/d dose (Wisniewski et al., 2006b). Interestingly, we found that worsening on CAST domains did not differ among treatment arms, except the bupropion-SSRI arm had significantly higher rates of worsened panic compared with SSRI monotherapy and venlafaxine-mirtazapine.
There are several limitations of our report. Validation of psychometric properties of CAST was not a primary goal of CO-MED trial. Hence, study design did not include any measures of irritability, anxiety, or panic beyond the baseline visit. Additionally, our use of threshold of 1.28 times baseline SD may have captured only severe worsening of symptoms and missed our participants with mild-moderate worsening. As the primary purpose of this report was to evaluate worsening, we did not test for improvement in these symptom domains with treatment and the potential clinical implications.
In conclusion, we find that 16-item CAST has good psychometric properties and can detect clinically significant worsening across domains of irritability, anxiety, insomnia, and panic with significant treatment implications. Use of CAST in clinical practice can help identify depressed patients who may be less likely to respond to their ongoing antidepressant treatment.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
The authors thank the clinical staff at each clinical site for their assistance with this project and all the study participants, and Jeremy Kee, MA, for administrative support.
Statement of Interest
Drs. Jha, Minhajuddin, and South do not report any personal, financial, or professional relationships or conflicting interests. Dr. Trivedi has in the past 24 months consulted or served on the advisory boards of Alkermes Inc., Akili Interactive Inc., Allergan, Arcadia Pharmaceuticals Inc., AstraZeneca, Brintellix, BMS, Cerecor, Global Medical Education Inc., Health Research Associates, Lundbeck, Medscape, MSI Methylation Sciences Inc., Merck, Naurex Inc., Navitor, Nestle Health Science – Pamlab Inc., One Carbon Therapeutics, Otsuka America Pharmaceuticals Inc., PamLab, Pfizer Inc., Roche, SHIRE Development, and Takeda Pharmaceuticals Inc; has conducted research activities with NIMH, NIDA, J&J, Janssen Research, and Development LLC; and has claimed royalties from Janssen Research and Development LLC. Dr. Rush has received consulting fees from Akili Inc., the American Psychiatric Association, Brain Resource Ltd., Compass Inc., Curbstone Consultant LLC., Eli Lilly, Emmes Corp., Holmusk, Liva-Nova, Lundbeck A/S, National Institute of Drug Abuse, Santium Inc., Sunovion, Taj Medical, and Takeda USA; speaking fees from Live Nova; and royalties from Guilford Publications and the University of Texas Southwestern Medical Center.
References
- Altman EG, Hedeker D, Peterson JL, Davis JM(1997)The Altman self-rating mania scale. Biol Psychiatry 42:948–955. [DOI] [PubMed] [Google Scholar]
- Cleary P, Guy W(1977)Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res 1:115–120. [Google Scholar]
- Cronbach LJ.(1951)Coefficient alpha and the internal structure of tests. Psychometrika 16:297–334. [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH(2008)Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 165:342–351. [DOI] [PubMed] [Google Scholar]
- Friedman ES, Davis LL, Zisook S, Wisniewski SR, Trivedi MH, Fava M, Rush AJ(2012)Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur neuropsychopharmacol 22:183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer TL, Trombello JM, Rethorst CD, Carmody TJ, Jha MK, Liao A, Grannemann BD, Chambliss HO, Church TS, Trivedi MH(2016)Improvements in psychosocial functioning and health-related quality of life following exercise augmentation in patients with treatment response but nonremitted major depressive disorder: results from the tread study. Depress Anxiety 33:870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.(1960)A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Sakamoto K, Ishigooka J(2008)Incidence and predictors of activation syndrome induced by antidepressants. Depress Anxiety 25:1014–1019. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M(2008)Structural equation modelling: guidelines for determining model fit. EJBRM 6:53–60. [Google Scholar]
- Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, Fava M, McGrath PJ, Weissman M, Kurian BT, Adams P, Weyandt S, Toups M, Carmody T, McInnis M, Cusin C, Cooper C, Oquendo MA, Parsey RV, DeLorenzo C(2015)Test-retest reliability of freesurfer measurements within and between sites: effects of visual approval process. Hum Brain Mapp 36:3472–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Greer TL, Carmody T, Rush AJ, Trivedi MH(2016)Early improvement in work productivity predicts future clinical course in depressed outpatients: findings from the CO-MED trial. Am J Psychiatry 173:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Teer RB, Minhajuddin A, Greer TL, Rush AJ, Trivedi MH(2017)Daily activity level improvement with antidepressant medications predicts long-term clinical outcomes in outpatients with major depressive disorder. Neuropsychiatr Dis Treat 13:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostev K, Rex J, Eith T, Heilmaier C(2014)Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger Med Sci 12:Doc15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M, Einarson TR(2010)Comparison of SSRIs and SNRIs in major depressive disorder: a meta-analysis of head-to-head randomized clinical trials. J Clin Pharm Ther 35:177–188. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Kasper S(1998)Side effects, dropouts from treatment and cost consequences. Int Clinic Psychopharmacol 13:S1–S6. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH(1996)The inventory of depressive symptomatology (IDS): psychometric properties. J Psychol Med 26:477–486. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB(2003)The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Biggs MM, Shores-Wilson K, Shelton RC, Luther JF, Thomas B, Trivedi MH(2005)Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord 87:43–55. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR(2011)Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry 168:689–701. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM(2006)Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J child adolesc psychopharmacol 16:159–169. [DOI] [PubMed] [Google Scholar]
- Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A, Srivastava S, Nutt DJ, Davies SJC(2009)Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry 194:483–490. [DOI] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J(2011)Reliability of the Hamilton rating scale for depression: a meta-analysis over a period of 49years. J Psychiatr Res 189:1–9. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Wisniewski SR, Morris DW, Fava M, Kurian BT, Gollan JK, Nierenberg AA, Warden D, Gaynes BN, Luther JF, Rush AJ(2011)Concise Associated Symptoms Tracking scale: a brief self-report and clinician rating of symptoms associated with suicidality. J Psychiatr Res 72:765–774. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM(2004)The inventory of depressive symptomatology, clinician rating (IDS-C) and self-report (IDS-SR), and the quick inventory of depressive symptomatology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. J Psychol Med 34:73–82. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M(2006)Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA (2006a) Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract 12:71–79. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA, Investigators S (2006b) Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract 12:71–79. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI(2001)A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry 58:787–794. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I(2006)A scale to screen for DSM-IV Axis I disorders in psychiatric out-patients: performance of the psychiatric diagnostic screening questionnaire. J Psychol Med 36:1601–1611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.