Abstract
Aim
Hypoglycaemia in Type 1 diabetes is associated with mortality and morbidity, especially where awareness of hypoglycaemia is impaired. Clinical pathways for access to continuous glucose monitoring (CGM) and flash glucose monitoring technologies are unclear. We assessed the impact of CGM and flash glucose monitoring in a high‐risk group of people with Type 1 diabetes.
Methods
A randomized, non‐masked parallel group study was undertaken. Adults with Type 1 diabetes using a multiple‐dose insulin‐injection regimen with a Gold score of ≥ 4 or recent severe hypoglycaemia were recruited. Following 2 weeks of blinded CGM, they were randomly assigned to CGM (Dexcom G5) or flash glucose monitoring (Abbott Freestyle Libre) for 8 weeks. The primary outcome was the difference in time spent in hypoglycaemia (below 3.3 mmol/l) from baseline to endpoint with CGM versus flash glucose monitoring.
Results
Some 40 participants were randomized to CGM (n = 20) or flash glucose monitoring (n = 20). The participants (24 men, 16 women) had a median (IQR) age of 49.6 (37.5–63.5) years, duration of diabetes of 30.0 (21.0–36.5) years and HbA1c of 56 (48–63) mmol/mol [7.3 (6.5–7.8)%]. The baseline median percentage time < 3.3 mmol/l was 4.5% in the CGM group and 6.7% in the flash glucose monitoring. At the end‐point the percentage time < 3.3 mmol/l was 2.4%, and 6.8% respectively (median between group difference −4.3%, P = 0.006). Time spent in hypoglycaemia at all thresholds, and hypoglycaemia fear, were different between groups, favouring CGM.
Conclusion
CGM more effectively reduces time spent in hypoglycaemia in people with Type 1 diabetes and impaired awareness of hypoglycaemia compared with flash glucose monitoring. (Clinical Trial Registry No: NCT03028220)
What's new?
This is the first head‐to‐head glucose monitoring study comparing continuous glucose monitoring (CGM) and flash glucose monitoring. This study addresses the highest risk group with problematic and severe hypoglycaemia.
CGM has a greater beneficial impact on hypoglycaemia outcomes than flash glucose monitoring for people at high risk of hypoglycaemia.
The data contribute to the existing CGM literature and are the first for flash glucose monitoring in a high‐risk group, expanding the evidence base.
The results are clinically relevant and support a role for CGM in the clinical pathway in people with severe hypoglycaemia or impaired awareness of hypoglycaemia.
What's new?
This is the first head‐to‐head glucose monitoring study comparing continuous glucose monitoring (CGM) and flash glucose monitoring. This study addresses the highest risk group with problematic and severe hypoglycaemia.
CGM has a greater beneficial impact on hypoglycaemia outcomes than flash glucose monitoring for people at high risk of hypoglycaemia.
The data contribute to the existing CGM literature and are the first for flash glucose monitoring in a high‐risk group, expanding the evidence base.
The results are clinically relevant and support a role for CGM in the clinical pathway in people with severe hypoglycaemia or impaired awareness of hypoglycaemia.
Introduction
Type 1 diabetes accounts for 10–15% of the worldwide diabetes prevalence and its incidence is increasing worldwide by 3–5% percent annually 1. Achieving optimal glucose control, as measured by HbA1c, reduces the risk of micro‐ and macrovascular complications, but can be challenging for people living with Type 1 diabetes due to hypoglycaemia 2, 3, 4.
Hypoglycaemia is a metabolic complication of Type 1 diabetes and is one of the major barriers to optimizing glucose self‐management. People with Type 1 diabetes on average have 1.8 self‐treated incidences of hypoglycaemia per week, and 0.2–3.2 episodes of severe hypoglycaemia, defined as hypoglycaemia requiring the assistance of a third party, annually 5, 6. Recurrent hypoglycaemia erodes hypoglycaemia awareness and impaired awareness is seen in around a quarter of people with Type 1 diabetes 7. However, this may be an underestimate, with self‐reported severe hypoglycaemia rates affected by driving regulations and other considerations 8.
Impaired awareness of hypoglycaemia increases risk of severe hypoglycaemia six‐fold. Hypoglycaemia is one of the postulated causes of the ‘dead in bed’ syndrome, which is the leading cause of death in people under 40 years of age with Type 1 diabetes 9, 10. There is a three‐ to four‐fold higher mortality in people with diabetes who self‐reported severe hypoglycaemia in the preceding 5 years 11. Hypoglycaemia is, and remains, a significant burden for people with Type 1 diabetes and carries with it mortality and morbidity.
Continuous glucose monitoring (CGM) devices display an estimate of blood glucose, along with the trends in glucose changes, in real time. In addition, they provide alert and alarm features for hypo‐ and hyperglycaemia, and for times of rapid glucose change. Use of CGM is associated with a reduction in HbA1c 12, and reduced exposure to, and risk of hypoglycaemia 13 in people using insulin pump and multiple‐dose injection regimens 14. The impact on glucose and hypoglycaemia outcomes has additionally been confirmed in people with Type 1 diabetes and impaired hypoglycaemia awareness 15. Flash glucose monitoring does not provide real‐time data with alerts and alarms, but allows users to retrospectively review the preceding 8 h of continuous glucose data, along with a contemporary estimated blood glucose value and trend line. The glucose data are made available when the user chooses to swipe the reader over the sensor. In one study of people with Type 1 diabetes, flash glucose monitoring was associated with a reduction in time spent in hypoglycaemia in people with Type 1 diabetes and an HbA1c close to target 16.
International guidance supports the use of CGM for people with Type 1 diabetes 17, especially those at high risk of hypoglycaemia 18. However, the role of flash glucose monitoring in the self‐management of Type 1 diabetes is less clear, especially for people with impaired awareness of hypoglycaemia, or at high risk of severe hypoglycaemia, and despite uptake of flash glucose monitoring led by people with diabetes, evidence‐based clinical pathways to optimize access to the appropriate monitoring technologies are not available.
This study aims to assess the impact of CGM and flash glucose monitoring on hypoglycaemia in people with Type 1 diabetes and impaired awareness of hypoglycaemia using a multiple‐dose insulin injection regimen.
Methods
Study design and participants
This randomized, non‐masked parallel group study was conducted at a single specialist site in the United Kingdom (UK). Ethical approval was obtained from the National Health Service (NHS) Research Ethics Committee. Participants aged ≥ 18 years with Type 1 diabetes for > 3 years were recruited. In addition, participants had experienced a severe hypoglycaemic event in the last 12 months requiring third‐party assistance or had a Gold score of ≥ 4. Those with severe hypoglycaemia and a Gold score of < 4 may not have impaired hypoglycaemia awareness; however, severe hypoglycaemia is associated with impaired awareness of hypoglycaemia and they have therefore been included in this high‐risk study population. They had been using an intensified multiple‐dose insulin injection regimen for over 6 months and a diagnosis of Type 1 diabetes was confirmed based on clinical features and a fasting c‐peptide < 200 pmol/l. All participants had received Type 1 diabetes education, including the principles of flexible insulin therapy, either as a group or in a one‐to‐one environment from a specialist educator. Participants were excluded if they had used CGM or flash glucose monitoring within the last 6 months (except short periods of diagnostic blinded use under clinic supervision), used regular paracetamol, were pregnant or planning pregnancy, breastfeeding, enrolled in other clinical trials, had active malignancy or were under investigation for malignancy, had severe visual impairment, or reduced manual dexterity. All participants gave written informed consent.
Procedures
At study enrolment, participants gave a full medical and medication history, and underwent a physical examination and electrocardiogram. Fasting venous blood tests were taken to assess HbA1c, plasma glucose, urea and electrolytes, cortisol, and serum c‐peptide. Women of childbearing age had a urine pregnancy test. The Gold Score, Hypoglycaemia Fear Score II (HFS‐II), and Problem Areas in Diabetes (PAID) questionnaires were completed. The Gold score is given by subjective rating on a scale from 1 (always) to 7 (never) in response to the question ‘Do you know when your hypos are commencing?’. Participants meeting the inclusion criteria had a brief Type 1 diabetes education refresher. Participants then commenced a two‐week run‐in phase using the Dexcom (San Diego, CA, USA) G4 sensor with a blinded receiver running the advanced ‘505’ algorithm which stores glucose data, but does not make it available to the participant. The sensor was calibrated to capillary blood glucose values a minimum of twice daily. From these blinded CGM data baseline glucose metrics were calculated.
Participants were randomly assigned to CGM (Dexcom G5) or flash glucose monitoring (Abbott Freestyle Libre) in a 1 :1 ratio using an online randomization tool (http://www.sealedenvelope.com). Randomization was stratified by HbA1c (< 58 mmol/mol and ≥ 58 mmol/mol). The treatment period was 8 weeks.
Participants then received standardized CGM education for the CGM (Dexcom G5) or flash glucose monitoring (Abbott Freestyle Libre) devices, including the use of the absolute value, rate of change arrow and glucose trend line. Both the CGM and flash glucose monitoring systems were used non‐adjunctively (without capillary blood glucose verification before making a treatment decision), in accordance with product licences but participants were instructed to test their capillary blood glucose if symptoms of hypo‐ or hyperglycaemia occur, in case of sensor failure or if the sensor glucose is out of the device's range. Participants used the sensors with the accompanying receivers and changed the sensor according to the license (every 7 days for Dexcom G5, every 14 days for Freestyle Libre) or sooner in the event of sensor failure. There was a telephone visit 2 weeks after randomization focusing on the function of the technology and any difficulties with use. Participants attended the clinical research facility 4 weeks after randomization and data were downloaded from their CGM and flash glucose monitoring devices using the Diasend software. Eight weeks after randomization participants attended the clinical research facility for a venous blood test for HbA1c. They additionally completed the Gold Score, HFS‐II and PAID questionnaires, and data were downloaded from their CGM and flash glucose monitoring devices. Participants were provided with a contact number for technical support, but insulin titration decisions were made by the participant throughout the study. In the CGM arm of the study, low glucose alert settings were standardized at 4.4 mmol/l for all participants at randomization and could be reduced to 4 mmol/l at week 2 during the telephone visit depending on participant preference. High glucose alerts were not protocolized.
Outcomes
The primary outcome was change in time spent in hypoglycaemia (< 3.3 mmol/l) from baseline to endpoint with CGM vs. flash glucose monitoring. Secondary outcomes were percentage time spent in hypoglycaemia < 2.8, 3.5 and 3.9 mmol/l, percentage time in euglycaemia (3.9–7.8 mmol/l), percentage time spent in target (3.9–10 mmol/l), percentage time spent in hyperglycaemia > 7.8, > 10 and > 15 mmol/l, low blood glucose index (LBGI, a measure of hypoglycaemia risk derived from continuous glucose data), severe hypoglycaemia (requiring third‐party assistance to treat), hypoglycaemia risk, HbA1c, Gold Score, hypoglycaemia fear (HFS‐II) and diabetes‐related emotional distress (PAID questionnaire). Baseline continuous glucose data were taken from the first 14 days of monitoring (the run‐in phase) and endpoint outcomes calculated from the last 28 days in each treatment period.
Statistical analysis
In this pilot study we recruited n = 20 in each group (40 participants in total) which would demonstrate as significant (P < 0.05) at 80% power a 0.92 standard deviation difference in mean change from baseline, in percentage time in hypoglycaemia (< 3.3 mmol/l), between CGM and flash glucose monitoring. Data were analysed using Stata v14 (StataCorp, College Station, TX, USA). Many variables were not normally distributed and summary statistics are therefore presented as median (IQR) and median change (95% confidence interval). Outcomes at baseline and at 8 weeks were analysed for CGM and for flash glucose monitoring separately, and change from baseline to 8 weeks was compared between the two interventions. The primary outcome comparison was between CGM and flash glucose monitoring in change in percentage time in hypoglycaemia (< 3.3 mmol/l). Secondary outcome comparisons were considered as hypothesis‐generating and informative. The Wilcoxon rank sum test was used for comparing median changes between groups. Analysis was by intention to treat. No data monitoring committee was convened. The study is registered at ClincialTrials.gov, number NCT03028220.
Results
We recruited 47 participants between 22 January 2016 and 7 December 2016. Seven participants were excluded and 40 were subsequently randomized to CGM (n = 20) or flash glucose monitoring (n = 20) following the baseline run‐in period (Fig. 1, Table 1). Participants (24 men, 16 women) had a median (IQR) age of 49.5 (37.5–63.5) years, duration of diabetes of 30.0 (21.0–36.5) years, HbA1c of 56 (48–63) mmol/mol (7.3 (6.5–7.8)%), Gold Score of 5 (4–5), and episodes of self‐reported hypoglycaemia per week of 3.0 (2.0–4.5). There were no significant differences in baseline characteristics between the groups. Some 39 of the 40 participants included had a history of at least one episode of severe hypoglycaemia in the past (6 months to 7 years ago). Five participants were randomized with a history of severe hypoglycaemia in the preceding year (and a Gold score of < 4), all other participants had a Gold score of ≥ 4. Those with a Gold score of < 4 all had a Gold score of 3 at baseline. Two of these participants were randomized to the CGM group and the remaining three to the flash glucose monitoring group. All 40 randomized participants completed the intervention period. For outcomes derived from CGM data n = 19 were analysed in the CGM group due to loss of the 8‐week CGM data for one participant resulting from uploading error. A comparison of the glucose outcomes, derived from the run‐in blinded CGM data, between the CGM and flash glucose monitoring groups was performed (using a non‐parametric test) and showed no statistical difference between groups at baseline. None of the participants or their family/friends downloaded the Dexcom Share app which allows family members and friends to follow glucose trends and alarms of the individual.
Figure 1.
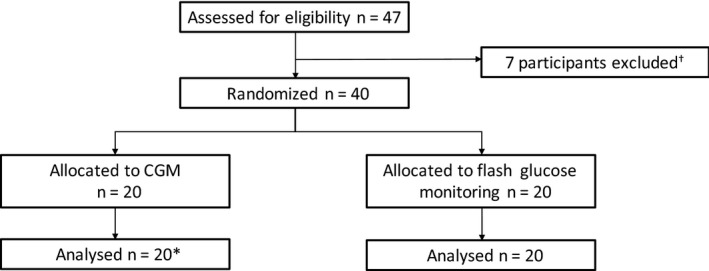
Participant recruitment. Results are expressed as median (IQR). *For outcomes derived from continuous glucose monitoring (CGM) data, n = 19 were analysed in the G5 group due to loss of the 8‐week CGM data for one participant (uploading error). †Reasons for participant exclusion were: severe hypoglycaemia in run‐in (1), failed to comply with visit schedule (3), dropped out due interference of study with exercise programme (1), dropped out as they did not feel they could commit to the study (2).
Table 1.
Baseline characteristics of study participants. Results are expressed as median (IQR)
| CGM (n = 20) | Flash glucose monitoring (n = 20) | All participants (n = 40) | |
|---|---|---|---|
| Gender (male : female) | 12 : 8 | 12 : 8 | 24 : 16 |
| Age (years) | 50.5 (45.0–64.5) | 48.5 (34.0–63.0) | 49.5 (37.5–63.5) |
| Duration of diabetes (years) | 30.0 (25.0–36.0) | 28.0 (16.5–36.5) | 30.0 (21.0–36.5) |
| Gold score | 5 (5–6) | 5 (4–5) | 5 (4–5) |
| HbA1c (mmol/mol) | 57 (49–62) | 55 (48–65) | 56 (48–63) |
| HbA1c (%) | 7.4 (6.6–7.8) | 7.2 (6.5–8.1) | 7.3 (6.5–7.8) |
| Self‐reported hypoglycaemia/week | 3.0 (2.0–4.0) | 2.5 (1.7–4.7) | 3.0 (2.0–4.5) |
CGM, continuous glucose monitoring.
Median percentage time < 3.3 mmol/l fell from 4.5% to 2.4% in the CGM group and changed from to 6.7% to 6.8% in the flash glucose monitoring group (Table 2). For the primary outcome comparison, the median changes from baseline to end‐point for participants using CGM and flash glucose monitoring were −3.0% and +1.3%< respectively (P = 0.006). Accordingly, the median net effect of CGM relative to flash glucose monitoring was a reduction of 4.3% in percentage time < 3.3 mmol/l.
Table 2.
Median percentage time (and IQR) spent within various glucose ranges, at baseline (weeks −2 to 0) and endpoint (4–8 weeks), and median change (and 95% confidence interval) in percentage time for continuous and flash glucose monitoring
| Percentage time within defined glucose range | Continuous glucose monitoring (n = 19) Median (IQR) | Flash glucose monitoring (n = 20) Median (IQR) | Median change from baseline (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline (−2 to 0 weeks) | Endpoint (4 to 8 weeks) | Baseline (−2 to 0 weeks) | Endpoint (4 to 8 weeks) | Continuous glucose monitoring | Flash glucose monitoring | P‐value | |
| < 2.8 mmol/l | 2.3 (0.6–10.7) | 0.9 (0.2–1.8) | 4.1 (2.5–5.9) | 3.8 (3.0–6.4) | −1.2 (−4.3 to −0.5) | 1.3 (−1.0 to 2.4) | 0.003 |
| < 3.3 mmol/l | 4.5 (1.9–14.1) | 2.4 (1.0–5.1) | 6.7 (4.8–9.5) | 6.8 (4.8–11.7) | −3.0 (−5.0 to −0.3) | 1.3 (−1.4 to 3.6) | 0.006 |
| < 3.5 mmol/l | 5.5 (3.1–15.7) | 3.5 (1.8–6.3) | 8.0 (5.7–10.7) | 8.2 (6.0–13.2) | −2.8 (−4.7 to −0.3) | 2.0 (−1.0 to 4.7) | 0.004 |
| < 3.9 mmol/l | 8.8 (5.7–19.5) | 6.2 (3.1–10.2) | 11.9 (8.8–13.7) | 11.0 (8.2–17.0) | −2.7 (−6.1 to −0.1) | 0.6 (−2.1 to 5.4) | 0.01 |
| > 7.8 mmol/l | 48.8 (40.8–70.0) | 49.0 (36.6–58.1) | 50.3 (43.9–58.6) | 47.1 (37.4–53.5) | −3.4 (−10.5 to 1.4) | −5.9 (−15.0 to 5.6) | 0.57 |
| > 10 mmol/l | 33.3 (25.2–49.9) | 26.7 (16.9–37.4) | 35.0 (21.9–38.7) | 28.0 (18.0–32.1) | −8.6 (−13.0 to −1.1) | −7.0 (−16.9 to 1.7) | 0.71 |
| > 15 mmol/l | 10.0 (1.6–20.4) | 4.2 (1.2–9.7) | 5.9 (2.7–9.2) | 2.6 (1.2–5.1) | −4.9 (−8.6 to −0.7) | −3.1 (−5.3 to −0.4) | 0.48 |
| 3.9–7.8 mmol/l | 31.7 (24.1–43.8) | 43.7 (34.7–52.3) | 34.8 (30.2–44.1) | 40.4 (34.7–45.3) | 10.6 (3.3 to 14.4) | 5.9 (−2.4 to 9.0) | 0.15 |
| 3.9–10 mmol/l | 50.2 (40.8–66.5) | 65.9 (53.5–74.8) | 54.1 (47.5–64.5) | 60.0 (54.5–67.8) | 12.7 (7.2 to 15.8) | 5.3 (1.1 to 11.7) | 0.05 |
Within‐group changes and significance levels for between group differences for all CGM outcomes are reported in Table2. The same directionality of change and between‐group differences were found for hypoglycaemia outcomes when overnight CGM data only (22:00 h to 07:00 h) were analysed (Table 3). No significant between group differences in change in time in target or in time spent above hyperglycaemic thresholds were observed.
Table 3.
Median percentage time (and IQR) spent within various glucose ranges overnight (22.00–07.00), at baseline (weeks −2 to 0) and endpoint (4–8 weeks), and the median change (and 95% confidence interval) in percentage time for continuous and flash glucose monitoring
| Percentage time within defined glucose range | Continuous glucose monitoring (n = 19) Median (IQR) | Flash glucose monitoring (n = 20) Median (IQR) | Median change from baseline(95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline (−2 to 0 weeks) | Endpoint (4–8 weeks) | Baseline (−2 to 0 weeks) | Endpoint (4–8 weeks) | Continuous glucose monitoring | Flash glucose monitoring | P‐value | |
| < 2.8 mmol/l | 4.1 (0.5–13.1) | 0.5 (0.0–2.3) | 5.1 (1.7–8.2) | 6.1 (3.6–10.8) | −2.7 (−6.1 to −0.5) | 1.2 (−1.5 to 4.8) | 0.001 |
| < 3.3 mmol/l | 6.1 (2.9–17.3) | 1.4 (0.4–5.7) | 8.3 (2.7–12.2) | 8.8 (6.0–16.7) | −4.4 (−6.9 to 0.0) | 1.6 (−1.6 to 6.0) | < 0.001 |
| < 3.5 mmol/l | 7.0 (3.9–18.7) | 2.7 (0.6–7.1) | 9.5 (4.0–13.6) | 10.2 (7.7–18.9) | −5.2 (−6.4 to 0.0) | 2.2 (−0.9 to 6.6) | 0.001 |
| < 3.9 mmol/l | 9.6 (5.2–20.7) | 5.5 (1.5–10.5) | 13.0 (6.7–17.1) | 12.6 (10.1–22.0) | −4.8 (−9.5 to −0.7) | 3.1 (−2.7 to 6.8) | 0.004 |
| > 7.8 mmol/l | 51.9 (36.9–68.9) | 52.4 (35.5–63.3) | 49.4 (34.4–64.6) | 43.4 (30.5–60.7) | −1.9 (−11.1 to 9.4) | −7.0 (−12.7 to 3.4) | 0.41 |
| > 10 mmol/l | 33.8 (13.5–53.1) | 26.7 (11.2–44.6) | 30.0 (16.6–44.9) | 24.0 (13.5–32.5) | −4.4 (−15.4 to 9.5) | −9.9 (−15.7 to −4.3) | 0.36 |
| > 15 mmol/l | 8.5 (1.0–13.8) | 5.1 (0.5–8.3) | 5.4 (2.1–9.8) | 1.1 (0.7–4.6) | −4.1 (−6.1 to 0.0) | −2.9 (−6.1 to −1.4) | 0.70 |
| 3.9–7.8 mmol/l | 31.8 (21.8–46.6) | 42.8 (29.2–49.5) | 37.6 (25.2–46.7) | 41.4 (30.5–46.6) | 13.0 (−4.1 to 19.6) | 4.1 (−1.0 to 11.0) | 0.16 |
| 3.9–10 mmol/l | 47.8 (39.2–65.9) | 62.6 (51.7–72.7) | 53.9 (42.3–67.5) | 59.5 (52.1–64.2) | 14.1 (−1.5 to 23.7) | 5.2 (0.7 to 11.6) | 0.20 |
No episodes of severe hypoglycaemia were reported during the 8‐week intervention phase in either group.
At baseline 90% (18/20) of participants in the CGM group and 85% (17/20) had a Gold score ≥ 4; at the 8‐week end‐point this was reduced to 60% (12/20) in both groups, indicating restored self‐reported hypoglycaemia awareness in a proportion of individuals. However, no significant difference was observed in overall Gold score from baseline to end‐point between the two groups (Table 4). No between‐group differences in HbA1c change were noted at 8 weeks.
Table 4.
Median (and IQR) LBGI, Gold score, HbA1c, HFS‐total scores, HFS‐Behaviour subscores, HFS‐Worry subscores and PAID scores at baseline and endpoint (at 8 weeks), and the median change (and 95% confidence interval) for continuous and flash glucose monitoring
| Continuous glucose monitoring (n = 19) Median (IQR) | Flash glucose monitoring (n = 20) Median (IQR) | Median change from baseline (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Endpoint (at 8 weeks) | Baseline | Endpoint (at 8 weeks) | Continuous glucose monitoring | Flash glucose monitoring | P‐value | |
| LBGI | 7.0 (5.4–12.3) | 5.3 (3.2–6.3) | 8.5 (5.9–9.8) | 9.1 (7.2–10.7) | −3.5 (−4.9 to −0.9) | 0.9 (−0.2 to 3.0) | < 0.001 |
| Gold score | 5 (5–6) | 4.5 (3.0 ‐ 5.0) | 5 (4–5) | 5.0 (3.5–6.0) | 0.0 (−1.0 to 0.0) | 0.0 (−0.8 to 0.0) | 0.23 |
| HbA1c (mmol/mol) | 57 (49–62) | 54 (45–61) | 55 (48–65) | 51 (48–59) | −1.5 (−8.6 to −1.0) | −4.5 (−5.8 to 0.0) | 0.91 |
| HbA1c (%) | 7.4 (6.6–7.8) | 7.1 (6.3–7.7) | 7.2 (6.5–8.1) | 6.8 (6.5–7.5) | −0.15 (−0.8 to −0.05) | −0.35 (−0.6 to 0.0) | |
| HFS‐total score | 59.5 (37.0–78.0) | 49.5 (28.0–74.0) | 42.5 (32.0–56.5) | 42.0 (28.5–65.5) | −6.5 (−10.8 to −2.2) | −2.0 (−3.8 to 2.8) | 0.02 |
| HFS‐Behaviour subscore | 21.0 (13.5–31.0) | 20.0 (10.5–26.0) | 17.5 (12.5–24.5) | 15.0 (11.5–25.5) | −2.0 (−3.8 to −0.1) | −0.5 (−3.0 to 1.8) | 0.36 |
| HFS‐Worry subscore | 40.5 (24.0–52.5) | 30.0 (17.5–44.0) | 27.5 (18.0–34.5) | 31.0 (15.5–46.0) | −4.5 (−7.8 to −0.1) | 0.5 (−3.0 to 2.8) | 0.02 |
| PAID score | 31.0 (13.5–45.5) | 28.5 (17.5–43.0) | 19.0 (14.0–46.0) | 22.0 (11.5–40.0) | −1.0 (−5.7 to 4.8) | −1 (−5.0 to 2.0) | 0.82 |
The change in hypoglycaemia between group difference was significant (P = 0.02; Table 4). This difference was accounted for by changes in the worry sub‐score of the HFS‐II (P = 0.02 for the between group difference). No within or between group differences were noted in HFS‐II behaviour sub‐score, and PAID scores.
Discussion
The results from this randomized parallel group pilot study suggest that an 8‐week intervention with CGM has a greater benefit in reducing time in hypoglycaemia compared with flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Both CGM and flash glucose monitoring improved HbA1c and percentage time spent in glucose target (3.9–7.8 and 3.9–10 mmol/l) over 8 weeks. Finally, within‐ and between‐group improvements in overall hypoglycaemia fear and the worry sub‐scale of the hypoglycaemia fear survey were seen with CGM. Awareness of hypoglycaemia remained unchanged with both glucose monitoring devices.
This is the first direct comparator study of continuous glucose recording technologies assessing glucose outcomes and aimed to provide supporting evidence for clinical pathways implementing CGM and flash glucose monitoring technologies. Around 25% of people with Type 1 diabetes have impaired awareness of hypoglycaemia, and the associations with severe hypoglycaemia confer a burden of mortality and morbidity. The data from this study suggest that alerts and alarms are important for this high‐risk group, and that evidence‐based clinical pathways must include a measure of hypoglycaemia awareness prior to implementing monitoring technologies where flash glucose monitoring may not be first choice. A measure of hypoglycaemia awareness is already included in the National Institute for Health and Care Excellence (NICE) guideline for Type 1 diabetes in adults 18. In the IMPACT study 16 flash glucose monitoring reduced time in hypoglycaemia, a finding we have not replicated, but IMPACT excluded people with impaired awareness of hypoglycaemia and recruited participants with a lower mean HbA1c. These differences in the population recruited may further indicate the importance of selecting the appropriate technology for individuals with Type 1 diabetes.
We did not see an improvement in self‐reported awareness of hypoglycaemia measured by Gold score with either CGM or flash glucose monitoring. The lack of improvement in Gold score with CGM is consistent with findings seen in the IN CONTROL study 15 and in a retrospective audit 19. The HypoCOMPaSS study showed that restoration of hypoglycaemia awareness can be achieved, but that self‐monitoring capillary blood glucose and CGM have an equivalent effect on impaired awareness of hypoglycaemia 20. However, the study designs and technologies implemented differ, and further research is warranted to explore the impacts of technology as an adjunct to education in people with Type 1 diabetes and impaired awareness of hypoglycaemia. A limitation to evaluating the Gold score after the use of CGM or flash glucose monitoring is that it is a subjective score and therefore does not distinguish whether those who restored their hypoglycaemia awareness had true recurrence of hypoglycaemia awareness from symptoms or whether the glucose monitoring was providing ‘electronic awareness’ by seeing the glucose trace falling or hearing the alarms with CGM.
Our study is limited by small numbers and a short follow‐up period, but the population and study design are comparable with previous reports in highly selected high‐risk groups. The baseline estimate of glucose data was derived from blinded CGM in both groups, but the final glucose data was derived from either CGM or flash glucose monitoring. Therefore, a further limitation is the comparison between CGM and FGM data, where accuracy may not be equivalent, so glucose outcomes may not be directly comparable. This applies when evaluating the difference from baseline to endpoint within the flash glucose monitoring group and when comparing the two groups. However, the devices were used in line with license and the relative published accuracies, expressed as a mean absolute difference, are between 11% and 13% for real‐world use 21, 22, 23, 24. Another limitation of our study is that stratification at randomization was based on HbA1c alone and does not consider other factors such as age, gender and diabetes duration. It is also important to note that the reported times within range reported are not independent (for example the percentage time spent < 3.3mmol outcome includes percentage time spent < 2.8 mmol/l). We recognize that the inclusion of participants with severe hypoglycaemia and a Gold score of < 4 makes the study population heterogeneous as those five participants with a Gold score of < 4 may not have impaired awareness of hypoglycaemia. This is a limitation, but these participants belong to a high‐risk population and were randomized in an equal distribution (two in the CGM group and five in the flash glucose monitoring group). The strength of the study lies in its novelty and the clearly defined homogeneous group of those at highest risk of challenging hypoglycaemia.
A new consensus for reporting hypoglycaemia in studies as < 3.0 mmol/l was recently recommended by The International Hypoglycaemia Study Group 25, but this was not the case at the time of study design. The percentage time spent at glucose < 3.0 mmol/l was therefore not a predetermined study outcome in this study, but when analysed post hoc the baseline vs. endpoint values were (3.1 vs. 1.5) and (4.7 vs. 5.0) in the CGM group and flash glucose monitoring group respectively and there was a significant difference in median change from baseline between groups (P = 0.004), suggesting benefit with CGM.
The uptake of flash glucose monitoring has been striking but, as yet, the technology has not been widely incorporated into clinical guidelines where its role has been unclear. The IMPACT study selected a specific group of people with HbA1c values close to target and showed no change in HbA1c but a reduction in time spent in hypoglycaemia compared with self‐monitoring of capillary blood glucose 16. This study adds to the IMPACT and DIAMOND studies and suggests that CGM is preferable to flash glucose monitoring for people with Type 1 diabetes using a multiple‐dose injection regimen with HbA1c values above target, and for those with challenging hypoglycaemia.
One possible mechanism for the findings in our study is the impact of alerts and alarms on behaviour and it is striking to note that, alongside a reduction in exposure to hypoglycaemia, we have demonstrated a reduction in hypoglycaemia fear and worry. The changes to hypoglycaemia fear should be confirmed in a larger study with a more heterogeneous population.
Conclusion
In summary, our pilot data suggest that CGM has a greater beneficial impact on hypoglycaemia outcomes than flash glucose monitoring for people with impaired hypoglycaemia awareness. Additionally, CGM has a beneficial impact on hypoglycaemia fear, one of the major barriers to optimal glucose control. The data suggest that careful assessment of hypoglycaemia awareness is critical to selecting the appropriate glucose monitoring technology and that evidence‐based clinical pathways for monitoring should be different for people with impaired awareness.
Funding sources
Dexcom funded the investigator‐initiated study and provided materials. The study was sponsored by Imperial College London.
Competing interests
NO has received honoraria for speaking and advisory board participation from Abbott Diabetes, Dexcom, Medtronic Diabetes and Roche Diabetes.
Acknowledgements
This paper presents independent research funded by Dexcom and supported by the NIHR CRF and BRC at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of Dexcom, the NHS, the NIHR or the Department of Health. The authors would like to thank all of the study participants for their valuable time.
Author contributions
MR, ES, NJ and SA ran the clinical study. MR performed the statistical analysis. NO and MR wrote the first draft of the report. NO designed the study, and AEL, MR and NO wrote the protocol. NO is the guarantor of the study.
Diabet. Med. 35, 483–490 (2018)
References
- 1. International Diabetes Federation (IDF) . IDF Diabetes Atlas ‐ Seventh Edition. Brussels: International Diabetes Federation, 2015. https://doi.org/10.1289/image.ehp.v119.i03. [Google Scholar]
- 2. The Diabetes Control and Complications Trial Study Research . Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 1991; 90: 450–459. [PubMed] [Google Scholar]
- 3. DCCT Study Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with Type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Östenson CG, Geelhoed‐Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen‐Bjergaard U. Self‐reported non‐severe hypoglycaemic events in Europe. Diabet Med 2014; 31: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014; 10: 711–722. [DOI] [PubMed] [Google Scholar]
- 7. Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen‐Bjergaard U, Færch L, Allingbjerg ML, Agesen R, Thorsteinsson B. The influence of new European Union driver's license legislation on reporting of severe hypoglycemia by patients with Type 1 diabetes. Diabetes Care 2015; 38: 29–33. [DOI] [PubMed] [Google Scholar]
- 9. Dahlquist G, Källén B. Mortality in childhood‐onset Type 1 diabetes: a population‐based study. Diabetes Care 2005; 28: 2384–2387. [DOI] [PubMed] [Google Scholar]
- 10. Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long‐term mortality in a nationwide cohort of childhood‐onset Type 1 diabetic patients in Norway. Diabetologia 2006; 49: 298–305. [DOI] [PubMed] [Google Scholar]
- 11. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012; 35: 1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in Type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta‐analysis of randomised controlled trials using individual patient data. BMJ 2011; 343: d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El‐Laboudi AH, Godsland IF, Johnston DG, Oliver NS. Measures of glycemic variability in Type 1 diabetes and the effect of real‐time continuous glucose monitoring. Diabetes Technol Ther 2016; 18: 806–812. [DOI] [PubMed] [Google Scholar]
- 14. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S et al Effect of continuous glucose monitoring on glycemic control in adults with Type 1 diabetes using insulin injections. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 15. van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed‐Duijvestijn PH, Kramer MH et al Continuous glucose monitoring for patients with Type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol 2016; 4: 893–902. [DOI] [PubMed] [Google Scholar]
- 16. Bolinder J, Antuna R, Geelhoed‐duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in Type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care 2017; 40 (Suppl 1). [Google Scholar]
- 18. National Institute for Health and Care Excellence (NICE) . Type 1 Diabetes in Adults: Diagnosis and Management. NICE guideline 17. Available at https://www.nice.org.uk/guidance/ng17 Last accessed 1 November 2017.
- 19. Choudhary P, Ramasamy S, Green L, Gallen G, Pender S, Brackenridge A et al Real‐time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia‐unaware patients with Type 1 diabetes. Diabetes Care 2013; 36: 4160–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina‐Solomon A et al Recovery of hypoglycemia awareness in long‐standing Type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self‐monitoring (HypoCOMPaSS). Diabetes Care 2014; 37: 2114–2122. [DOI] [PubMed] [Google Scholar]
- 21. Andelin M, Kropff J, Matuleviciene V, Joseph JI, Attvall S, Theodorsson E et al Assessing the accuracy of continuous glucose monitoring (CGM) calibrated with capillary values using capillary or venous glucose levels as a reference. J Diabetes Sci Technol 2016; 10: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taleb N, Emami A, Suppere C, Messier V, Legault L, Chiasson JL et al Comparison of two continuous glucose monitoring systems, Dexcom G4 Platinum and Medtronic Paradigm Veo Enlite System, at rest and during exercise. Diabetes Technol Ther 2016; 18: 561–567. https://doi.org/10.1089/dia.2015.0394. [DOI] [PubMed] [Google Scholar]
- 23. Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A multicenter evaluation of the performance and usability of a novel glucose monitoring system in chinese adults with diabetes. J Diabetes Sci Technol 2017; 11: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015; 9: 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017; 40: 155–157. [DOI] [PubMed] [Google Scholar]


