Abstract
Aims
To evaluate diabetic retinopathy (DR) data from across the SUSTAIN clinical trial programme.
Materials and methods
The SUSTAIN clinical trial programme evaluated the efficacy and safety of semaglutide, a glucagon‐like peptide‐1 analogue, for the treatment of type 2 diabetes (T2D). In SUSTAIN 6, a 2‐year, pre‐approval cardiovascular outcomes trial, semaglutide was associated with a significant increase in the risk of DR complications (DRC) vs placebo. DR data from across the SUSTAIN trials were evaluated, and post hoc analyses of the SUSTAIN 6 data were conducted. These included subgroup analyses to identify at‐risk patients and a mediation analysis with initial change in glycated haemoglobin (HbA1c; percentage‐points at week 16) as a covariate, to examine the role of the magnitude of reduction in HbA1c as an intermediate factor affecting risk of DRC.
Results
There was no imbalance in DR adverse events across the SUSTAIN 1 to 5 and Japanese trials. The majority of the effect with semaglutide vs placebo in SUSTAIN 6 may be attributed to the magnitude and rapidity of HbA1c reduction during the first 16 weeks of treatment in patients who had pre‐existing DR and poor glycaemic control at baseline, and who were treated with insulin.
Conclusions
Early worsening of DR is a known phenomenon associated with the rapidity and magnitude of improvement in glycaemic control with insulin; the DRC findings in SUSTAIN 6 are consistent with this. Guidance regarding the early worsening of DR is recommended with insulin. Similar recommendations may be appropriate for semaglutide.
Keywords: antidiabetic drug, diabetic retinopathy, GLP‐1 analogue
1. INTRODUCTION
Semaglutide is a glucagon‐like peptide‐1 (GLP‐1) analogue, with an extended half‐life of ~1 week, that is in development for the treatment of type 2 diabetes (T2D).1, 2 The efficacy and safety of semaglutide has been evaluated in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical trial programme, including five global trials (SUSTAIN 1–5), two Japanese trials and one pre‐approval cardiovascular (CV) outcomes trial (SUSTAIN 6), and covers the continuum of T2D care. Semaglutide treatment led to significant and sustained improvements in glycated haemoglobin (HbA1c) and body weight vs placebo and active comparators across the SUSTAIN programme.3, 4, 5, 6, 7, 8 In SUSTAIN 6, semaglutide demonstrated a significant 26% risk reduction for the primary composite CV outcome vs placebo at 2 years.8 Microvascular complications were evaluated as a secondary endpoint; a significantly lower risk of new or worsening nephropathy was reported with semaglutide vs placebo (hazard ratio [HR] 0.64, 95% confidence interval [CI] 0.46‐0.88; P = .005); however, semaglutide was associated with a higher rate of diabetic retinopathy complications (DRC) vs placebo (HR 1.76; 95% CI 1.11‐2.78; P = .02).8
Risk factors for the development and progression of diabetic retinopathy (DR) include poor glycaemic control, long duration of diabetes and poorly controlled blood pressure.9 The beneficial effect of long‐term, stringent glycaemic control on the prevention or delay in onset or progression of DR in patients with type 1 diabetes and T2D is established10, 11, 12; however, large and rapid improvements in glycaemic control may be associated with a transient worsening of DR, which in the long term can be counterbalanced by reduction in DR with improved glycaemia.11, 13 Risk factors for DR are well recognized and should be monitored and managed in accordance with existing guidelines.14
In the present paper, we present how DR was evaluated, and the results obtained, across the full clinical development programme for semaglutide. Additionally, we present new analyses that provide further insights into the DRC findings from SUSTAIN 6.
2. MATERIALS AND METHODS
The SUSTAIN 1 to 5 and Japanese trials assessed the efficacy and safety of subcutaneous, once‐weekly semaglutide (0.5; 1.0 mg) vs placebo or active comparators in adult populations with T2D, ranging from treatment‐naïve patients to those treated with insulin (Table S1).3, 4, 5, 6, 7, 8 In the SUSTAIN 1 to 5 and Japanese trials, known proliferative retinopathy or maculopathy requiring acute treatment, according to the opinion of the investigator, were exclusion criteria, and the upper limit of HbA1c was 10% or 10.5%.
SUSTAIN 6 was a 2‐year CV outcomes trial, designed to assess the CV safety of once‐weekly semaglutide (0.5; 1.0 mg) vs volume‐matched placebo, as an add‐on to standard of care.8 The primary comparison was pooled semaglutide vs pooled placebo. Secondary microvascular outcomes included new or worsening nephropathy and DRC, both based on external event adjudication committee (EAC)‐confirmed events. The trial enrolled 3297 patients who, in contrast to the SUSTAIN 1 to 5 and Japanese trials, represented a population with advanced T2D and high CV risk.8 Importantly, there were no inclusion/exclusion criteria related to DR, and no upper limit for HbA1c.
2.1. Assessment of DR across the SUSTAIN clinical trial programme
Data collection on DR (Table S2) included medical history, adverse event (AE) reporting, EAC‐confirmed DRC (SUSTAIN 6 only) and fundoscopy or fundus photography.
2.1.1. Medical history
In the SUSTAIN 1 to 5 and Japanese trials, history of DR was collected as part of the patient's medical history. In SUSTAIN 6, investigators completed a diabetes complications form at baseline, describing the patient's DR status by answering the following questions: Does the subject have DR (yes/no/unknown)? If yes, what type of DR (non‐proliferative/proliferative/unknown)? Has the patient had any of the following: macular oedema, treatment with either laser therapy/intravitreal agents, or surgery, eg, vitrectomy?
2.1.2. Fundoscopy/fundus photography
Fundoscopy/fundus photography was performed in all SUSTAIN trials at baseline, unless undertaken within 90 days prior to randomization. In SUSTAIN 6, fundoscopy/fundus photography was also performed at weeks 56 and 104 (planned end of treatment), or after premature discontinuation of treatment, and in the Japanese trials it was also performed at the planned end of treatment. Fundoscopy/fundus photography could be performed by the investigator, local ophthalmologist or optometrist according to local practice; however, no record of who performed the examinations or the type of assessment performed was kept. Furthermore, dilation of the pupil during these assessments was not mandatory, and thus not recorded, and fundus photographs were not centrally graded. Findings were categorized, as per the protocol, by the investigator as “normal,” “abnormal, not clinically significant,” or “abnormal, clinically significant.” Fundoscopic data from SUSTAIN 1 to 5 and the Japanese trials are not presented.
2.1.3. AE reporting
Diabetic retinopathy AEs were collected as part of the standard safety reporting of AEs in all SUSTAIN clinical trials.
2.1.4. EAC‐confirmed DRC (SUSTAIN 6)
Time to first event of DRC was a secondary endpoint measured in SUSTAIN 6 only. The endpoint was dichotomous (yes/no) and an event of DRC was defined as the time from randomization to first occurrence of one or more of the following: 1. need for retinal photocoagulation; 2. need for treatment with intravitreal agents; 3. vitreous haemorrhage; and 4. onset of diabetes‐related blindness, defined as a Snellen visual acuity of 20/200 [6/60] or less, or a visual field of 20° or less, in the better eye with the best correction possible at the time of the event.
Criteria were not mutually exclusive; simultaneous fulfilment of more than one of the criteria was considered a single event for the DRC endpoint.
Adjudication of DRC was performed by an external EAC, comprising two independent ophthalmologists, who were masked to treatment (Supporting Information, File S1).
Further information was requested from the sites by Novo Nordisk after the trial completion regarding the EAC‐confirmed blindness cases.
2.2. Statistical analysis
2.2.1. SUSTAIN 1 to 5 and Japanese trials
Descriptive summaries of DR AEs were completed for the SUSTAIN 1 to 5 and Japanese trials.
2.2.2. SUSTAIN 6
Post hoc subgroup analyses were conducted for time to first EAC‐confirmed DRC using unstratified Cox proportional hazards models, with an interaction between treatment (semaglutide, placebo) and subgroup variable (Supporting Information, File S2) as fixed factors.
The impact of insulin use during the trial on the risk of DRC was explored in an unstratified Cox proportional hazards model with an interaction between treatment (semaglutide, placebo) and insulin use during the trial as time‐varying covariate (Supporting Information, File S2). This analysis was carried out according to baseline DR (yes, no, unknown/missing).
A post hoc mediation analysis was conducted to evaluate the role that intermediate factors (mediators) play in describing the total effect on DRC using an unstratified Cox proportional hazards model that, in addition to treatment (semaglutide, placebo) as a fixed factor, included the following: change in HbA1c at week 16 (nadir) as a covariate, and baseline variables, HbA1c, DR (yes/no/unknown), and duration of diabetes. This analysis was also expanded to include other baseline variables (Supporting Information, File S2). A similar post hoc mediation analysis was conducted including change in systolic blood pressure (mm Hg) at week 16 as a covariate, as a substitute for HbA1c (Supporting Information, File S2).
3. RESULTS
3.1. Baseline characteristics
The baseline characteristics of patients in SUSTAIN 1 to 5 and Japanese trials vs SUSTAIN 6 are described in Table 1. The proportion of patients with DR at baseline in the SUSTAIN 1 to 5 and Japanese trials was 3.7% to 14.5%. In SUSTAIN 6, 29.4% of the trial population had known, pre‐existing DR at baseline (semaglutide, 30.9%; placebo, 27.8%), and there were similar numbers of patients with proliferative DR at baseline in both treatment groups (semaglutide, 6.3%; placebo, 6.0%; Table S3).
Table 1.
Baseline characteristics across the SUSTAIN clinical trial programme
| Mean (SD) | SUSTAIN 1N = 387 | SUSTAIN 2N = 1225 | SUSTAIN 3N = 809 | SUSTAIN 4N = 1082 | SUSTAIN 5N = 396 | JP SUSTAIN MONON = 308 | JP SUSTAIN OADN = 601 | SUSTAIN 6N = 3297 |
|---|---|---|---|---|---|---|---|---|
| Age, years | 53.7 (11.3) | 55.1 (10.0) | 56.6 (10.7) | 56.5 (10.4) | 58.8 (10.1) | 58.3 (10.7) | 58.5 (10.3) | 64.6 (7.4) |
| HbA1c, % | 8.1 (0.9) | 8.1 (0.9) | 8.3 (1.0) | 8.2 (0.9) | 8.4 (0.8) | 8.1 (0.9) | 8.1 (0.9) | 8.7 (1.5) |
| T2D duration, years | 4.2 (5.5) | 6.6 (5.1) | 9.2 (6.3) | 8.6 (6.3) | 13.3 (7.8) | 8.0 (6.3) | 8.8 (6.4) | 13.9 (8.1) |
| Systolic blood pressure, mm Hg | 128.8 (13.2) | 132.6 (14.9) | 133.5 (14.5) | 132.1 (15.3) | 134.8 (16.0) | 129.1 (14.8) | 129.2 (13.0) | 135.6 (17.1) |
| Medical history of DR, n (%) | 15 (3.9) | 94 (7.7) | 30 (3.7) | 50 (4.6) | 55 (13.9) | 42 (13.6) | 87 (14.5) | 969 (29.4) |
Abbreviations: DR, diabetic retinopathy; JP, Japanese; MONO, monotherapy; OAD, oral antidiabetic drug; T2D, type 2 diabetes.
3.2. Diabetic retinopathy AEs
The DR AEs reported in the SUSTAIN 1 to 5 and Japanese trials were balanced across treatments, all events were mild or moderate and there were no serious AEs (Table 2). In SUSTAIN 6, a greater proportion of AEs was reported in semaglutide‐ vs placebo‐treated patients. The majority of AEs were mild or moderate; few were reported as serious AEs (Table 2).
Table 2.
Diabetic retinopathy adverse events with their respective investigator‐assessed severity as reported across the SUSTAIN clinical trial programme
| SUSTAIN 1‐5 and Japanese SUSTAIN trials | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg N = 1373 | Semaglutide 1.0 mg N = 1777 | Comparators N = 1657 | ||||||||||
| n | (%) | E | R | n | (%) | E | R | n | (%) | E | R | |
| All events | 32 | (2.1) | 35 | 2.6 | 30 | (1.5) | 36 | 1.9 | 31 | (2.0) | 31 | 2.1 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Moderate | 3 | (0.2) | 4 | 0.3 | 3 | (0.2) | 5 | 0.3 | 6 | (0.4) | 6 | 0.4 |
| Mild | 29 | (1.9) | 31 | 2.3 | 27 | (1.3) | 31 | 1.6 | 25 | (1.6) | 25 | 1.7 |
| SAEs | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| SUSTAIN 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg N = 826 | Semaglutide 1.0 mg N = 822 | Placebo N = 1649 | ||||||||||
| n | (%) | E | R | n | (%) | E | R | n | (%) | E | R | |
| All events | 74 | (9.0) | 86 | 5.0 | 82 | (10.0) | 99 | 5.8 | 125 | (7.6) | 145 | 4.3 |
| Severe | 5 | (0.6) | 6 | 0.4 | 5 | (0.6) | 5 | 0.3 | 7 | (0.4) | 7 | 0.2 |
| Moderate | 27 | (3.3) | 33 | 1.9 | 22 | (2.7) | 25 | 1.5 | 35 | (2.1) | 37 | 1.1 |
| Mild | 44 | (5.3) | 47 | 2.8 | 57 | (6.9) | 69 | 4.1 | 86 | (5.2) | 101 | 3.0 |
| SAEs | 6 | (0.7) | 7 | 0.4 | 5 | (0.6) | 6 | 0.4 | 8 | (0.5) | 8 | 0.2 |
Abbreviations: E, events; R, events per 100 patient‐years of observation; SAE, serious adverse event.
Proportions for SUSTAIN 1 to 5 and Japanese SUSTAIN trials are adjusted proportions. Mild, no or transient symptoms, no interference with the subject's daily activities; moderate, marked symptoms, moderate interference with the subject's daily activities; severe, considerable interference with the subject's daily activities; unacceptable.
3.3. DRC in SUSTAIN 6
More patients treated with semaglutide (50 [3.0%]) vs placebo (29 [1.8%]) experienced EAC‐confirmed DRC;8 more adjudicated events were confirmed with semaglutide vs placebo for all four components of the DRC endpoint (Table S4). Although 79 patients had DRC, there were 98 events in total, as some patients had more than one event (Supporting Information, File S1 and Table S4).
3.3.1. Baseline characteristics of patients with DRC
Compared with the overall trial population, patients with EAC‐confirmed DRC were characterized by pre‐existing DR, a longer mean diabetes duration, higher mean HbA1c levels at baseline and greater proportions of patients receiving insulin treatment at trial entry (Table S3).
A greater proportion of patients with DRC had pre‐existing DR at baseline vs the overall trial population (66 [83.5%] vs 969 [29.4%], respectively; 23 [29.1%] vs 202 [6.1%] with proliferative DR, and 14 [17.7%] vs 112 [3.4%] of these had received laser therapy or treatment with intravitreal agents prior to enrolment, respectively). The proportions of patients were similar in each treatment arm (42 [84.0%] and 24 [82.8%] for semaglutide and placebo, respectively; Table S3 and Figure 1A). For semaglutide‐treated patients with no known pre‐existing DR at baseline, the risk of DRC was low and there was no statistically significant difference vs placebo (Table S3 and Figure 1B); the number (proportion) of patients with no pre‐existing DR at baseline who developed an event was 5 (10.0%) for semaglutide and 4 (13.8%) for placebo. Overall, a higher risk for DRC with semaglutide vs placebo was observed in patients with proliferative and non‐proliferative DR at baseline (Figure S1). There was no evidence of a dose‐dependent effect on DRC with semaglutide (Figure S2): 25 patients in each dose group had an event.
Figure 1.
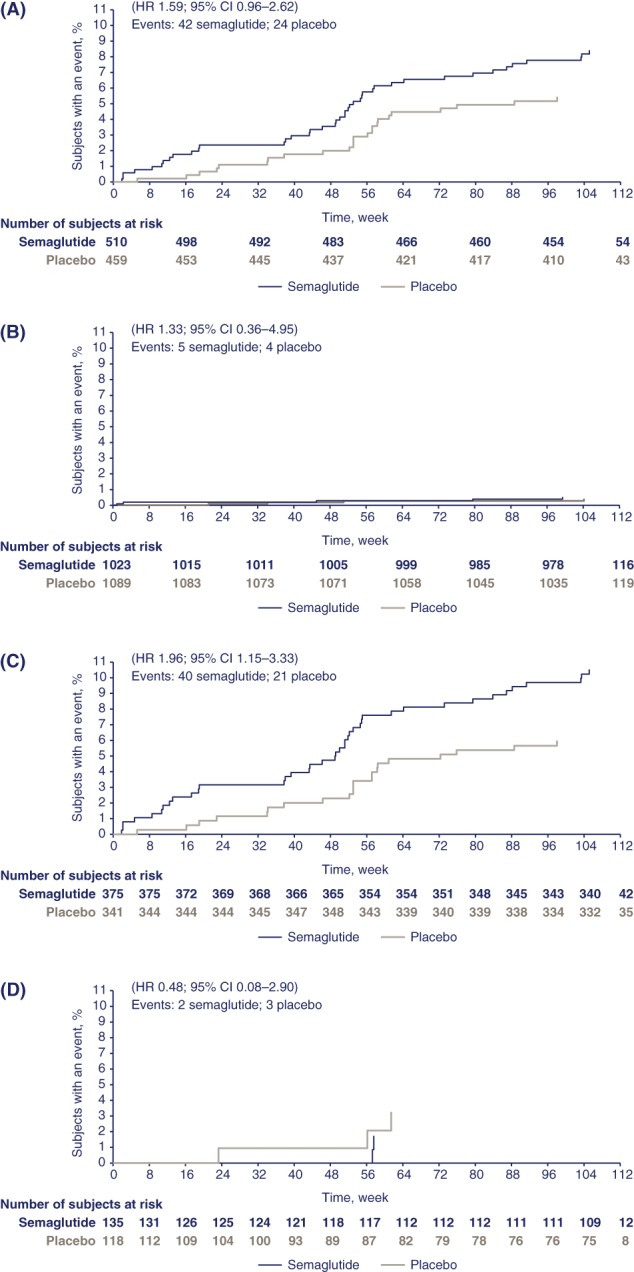
Kaplan–Meier plots showing time to first event adjudication committee (EAC)‐confirmed diabetic retinopathy complications (DRC) in SUSTAIN 6 by: baseline diabetic retinopathy (DR) status, yes (A), no (B); in patients with DR at baseline with insulin use prior to event, yes (C), no (D). The proportion of patients with events with “unknown” status of DR at baseline was 3 for semaglutide and 1 for placebo (hazard ratio [HR] 2.73; 95% confidence interval [CI] 0.28−26.26); the proportion of patients with events with “unknown” status of DR at baseline with insulin use “yes” was 1 for semaglutide and 1 for placebo (HR 1.29; 95% CI 0.07−22.15). The incidence rate per 100 patient‐years for DRC with semaglutide vs placebo by baseline DR status was 4.16 and 2.63 (with DR); 0.24 and 0.18 (without DR); and 1.33 and 0.49 (unknown/missing), respectively
In patients with pre‐existing DR at baseline, the risk of DRC was further increased in patients treated with insulin prior to the event (Figure 1C,D). No increase in risk was associated with prior insulin use in patients without known pre‐existing DR at baseline.
3.3.2. Glycaemic control
Semaglutide‐treated patients experienced significant and sustained reductions in HbA1c from baseline vs placebo in the overall trial population (Figure 2A,B).8 The reduction in HbA1c with semaglutide vs placebo in patients with DRC was 1.9% and 2.5% at week 16 with semaglutide 0.5 and 1.0 mg, respectively, vs 0.9% and 1.3% with placebo 0.5 and 1.0 mg (Figure 2C) and reductions in HbA1c were greater vs the overall trial population until the end of the trial (week 104, Figure 2D). A similar pattern for the incidence rate of DRC with semaglutide or placebo was seen in patients with pre‐existing DR, when stratified according to change in HbA1c by week 16. The incidence rate of confirmed events was highest in patients with HbA1c reductions >1.5% with semaglutide or placebo (Figure 3). In patients without pre‐existing DR, the incidence rate of DRC was low and similar between treatment groups, regardless of the magnitude of HbA1c reduction (Figure 3A).
Figure 2.
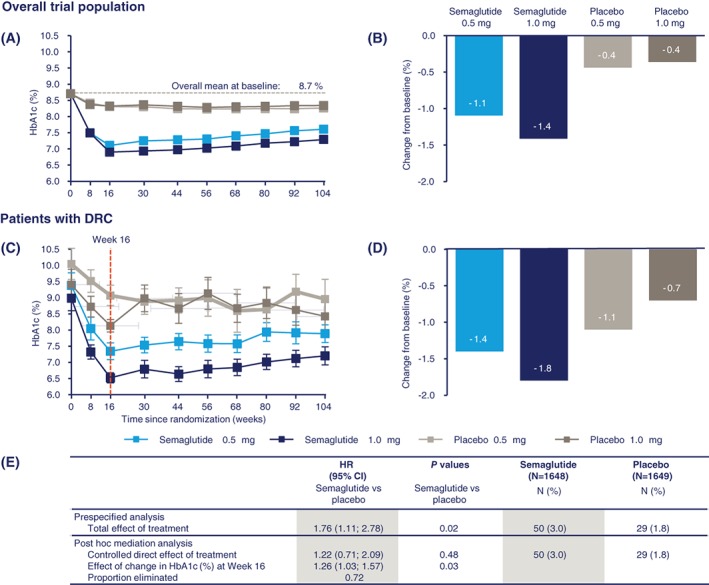
Glycated haemoglobin (HbA1c) in SUSTAIN 6: Change in HbA1c over time and from baseline to week 104 in the overall population (A and B); change in HbA1c over time and from baseline to week 104 in subjects with diabetic retinopathy complications (DRC; C and D); Post hoc mediation analysis of the effect of change in HbA1c (%) at week 16 for time to first (external) event adjudication committee (EAC)‐confirmed DRC (E). Overall trial population, n = 3297; patients with DRC, n = 79. Values are estimated means (± SE) from a mixed model for repeated measurements analysis using “in‐trial” data from patients in the full analysis set. The table summarizes the results of a post hoc mediation analysis for time to first EAC‐confirmed DRC, together with the results of the prespecified analysis. The mediation analysis assesses the effect of change in HbA1c at week 16 on time to first DRC. This is analysed by an unstratified Cox proportional hazards model, which in addition to treatment (semaglutide, placebo) as a fixed factor also includes “change in HbA1c (% points) at week 16” as a covariate as well as confounding variables “HbA1c at baseline,” “retinopathy at baseline” (“yes,” “no,” “unknown/missing”) and “baseline duration of diabetes.” Variables were deemed to be confounding if they were significantly associated with both a change in HbA1c at week 16 and time to first retinopathy complications. This was analysed by use of separate univariate ANCOVAs and Cox proportional hazards models. Other considered confounders were “gender” and “body weight” at baseline. Missing values of HbA1c were imputed as predicted values from a mixed model for repeated measurements. “Proportion eliminated” is calculated as: (total effect of treatment − controlled direct effect of treatment)/(total effect of treatment −1); ie, the absolute risk reduction from the mediation analysis divided by the total excess risk. Panel A is from Marso SP, Bain SC, Consoli A. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 375(19):1834–1844. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. CI, confidence interval; HR, hazard ratio
Figure 3.
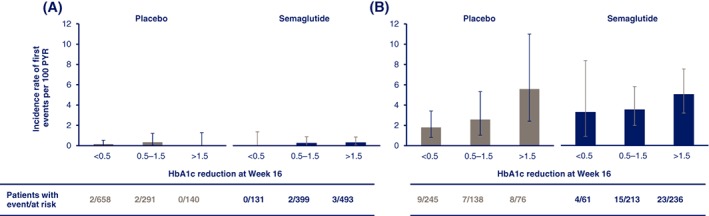
Incidence of diabetic retinopathy complications (DRC) in SUSTAIN 6 by baseline diabetic retinopathy (DR) and glycated haemoglobin (HbA1c) reduction: patients without baseline DR (A); patients with known pre‐existing DR (B). Values are observed incidence rates per 100 patient‐years of risk (PYR) with error bars representing 95% confidence intervals (CIs). A patient's risk time is defined as the time from randomization to first event or censoring
A post hoc mediation analysis showed that when controlling for HbA1c reduction at week 16, for semaglutide vs placebo, the HR for DRC is reduced to 1.22 (95% CI 0.71‐2.09; P = .48; Figure 2E); therefore, the majority of the treatment effect could be attributed to the reduction in HbA1c at week 16. Expansion of the mediation analysis to include other baseline variables did not change the mediator analysis results (Supporting Information, File S3).
3.3.3. Blood pressure control
Additional post hoc analyses were performed to assess the role of blood pressure control in the DRC finding; however, there was no indication of an effect (Supporting Information, File S3).
3.4. Events evaluated as diabetes‐related blindness in SUSTAIN 6
In total, the EAC evaluated that 6 patients met the criteria for events of diabetes‐related blindness: 5 with semaglutide; 1 with placebo (Table S5). All 5 semaglutide‐treated patients had pre‐existing proliferative DR and other eye diseases (eg, cataract), and all had received treatment with laser therapy and/or intravitreal agents prior to entering the trial. The placebo‐treated patient with diabetes‐related blindness had no known pre‐existing DR.
Further information was available for 3 of the 5 semaglutide‐treated patients post event, none of whom continued to fulfill the criteria for diabetes‐related blindness (2 patients 18 months post event, and 1 patient, 21 days post event).
3.5. Fundoscopy/fundus photographs in SUSTAIN 6
Table S6 shows fundoscopy/fundus photograph assessments at baseline and end of treatment. After 2 years, the proportion of patients in each category was similar in the two treatment arms.
4. DISCUSSION
The SUSTAIN clinical trial programme was designed to evaluate the efficacy and safety of semaglutide across the continuum in patients with T2D; the trials were not specifically designed to investigate DR. Although there was no increase in DR AEs in the SUSTAIN 1 to 5 or Japanese trials with semaglutide vs comparators, there was a significant 76% increase in the risk of DRC with semaglutide vs placebo in SUSTAIN 6.8
The increased risk of DRC in SUSTAIN 6 was identified using a dichotomous secondary endpoint, thus only allowing the identification of predetermined events, and the trial design does not provide a sensitive assessment of progression of retinal changes over time. Furthermore, clinical interpretation of the DRC results is challenging for several reasons: the endpoint is a mixture of treatments and diagnoses, it does not take into account pre‐existing eye disease and there was no routine assessment of visual acuity. Lastly, as the severity of DR was not formally assessed at baseline nor graded during the trial, any potential changes in DR severity cannot be determined from the data collected in SUSTAIN 6.
Semaglutide treatment in SUSTAIN 6 led to more pronounced reductions in HbA1c vs placebo;8 the difference occurred despite investigators being masked to treatment assignment and encouraged throughout the trial to actively treat hyperglycaemia by adding antihyperglycaemic agents as per local guidelines. A post hoc mediation analysis suggests that the increase in DRC seen with semaglutide vs placebo may be associated with the large and rapid decline in HbA1c during the first 16 weeks of treatment. Furthermore, the majority of patients with DRC events were treated with insulin prior to, or at the time of, the event. While no data are available to support an interaction with insulin, use of insulin therapy appears to identify those patients at highest risk of DRC, and includes those with known risk factors for DR, including longer duration of diabetes and high baseline HbA1c. The combination of pre‐existing DR and insulin use may be important, therefore, for identifying the patients at the highest risk of developing DRC. Importantly, there was no increase in risk of DRC with semaglutide vs placebo in patients without pre‐existing DR, regardless of whether they were receiving insulin treatment.
Rapid and marked reductions in HbA1c, as a result of improved glycaemic control initiated during pregnancy, bariatric surgery or intensified insulin treatment, have previously been associated with transitory worsening of DR11, 12, 15, 16, 17, 18, 19; this has also been reported in patients with type 1 diabetes in the Diabetes Control and Complications Trial (DCCT),12 and in newly diagnosed patients with T2D in the UK Prospective Diabetes Study (UKPDS 33).11 It is important to note that the DCCT excluded patients with proliferative retinopathy, and used a more sensitive assessment method with graded retinal imaging to chart the progression of DR.12 Although the characteristics of patients in the DCCT are different from those in SUSTAIN 6, the increased risk of DRC may be a manifestation of an “early worsening” phenomenon attributable to the large and rapid reduction in HbA1c. Consequently, patient profiling and risk assessment before intensification of treatment may help identify patients whose eyes require close monitoring.
Other agents causing abrupt glycaemic improvement (eg, insulin) have warnings in their prescribing information about the potential association with temporary worsening of DR.20 In the insulin glargine clinical development programme, more frequent DR progression was reported with insulin glargine vs NPH in patients with T2D;21 however, a subsequent 5‐year DR trial, employing 7‐field Early Treatment Diabetic Retinopathy Study fundus photographic assessment, showed no detrimental effect with insulin glargine vs NPH on the long‐term progression of DR.22
As a CV outcomes trial, SUSTAIN 6 was, by design, different from other SUSTAIN trials, and indeed from other phase III development programmes. These differences relate to the inclusion of patients with pre‐existing advanced DR requiring acute treatment in SUSTAIN 6. Additionally, patients were older, had a higher baseline HbA1c and a longer duration of diabetes, and a higher proportion had pre‐existing DR vs those included in the other SUSTAIN trials. All of these factors increased the overall risk of DR in the SUSTAIN 6 population.
Limited data are available regarding DR with GLP‐1 receptor agonists. In the LEADER trial, the CV outcomes trial of liraglutide, the HR for the DRC endpoint disfavoured liraglutide, although the difference was not significant (HR 1.15; 95% CI 0.87‐1.52; P = .33).23 With exenatide, a retrospective analysis of patients receiving treatment twice daily for longer than 6 months, showed that DR had progressed in 30% of patients and progression of DR was associated with greater reductions in HbA1c; there was no comparator.24 In the same group of patients, DR had improved or remained stable after follow‐up, suggesting that early worsening did not progress.25 With albiglutide, a higher incidence of on‐therapy DR AEs was reported vs placebo (3.6% vs 1.7%), but not with comparators.26 Importantly, only one of these studies applied robust methods for assessing DR prospectively25; whereas the majority of the DR findings relating to GLP‐1 receptor agonists were based on standard AE reporting and not retinal imaging. The patient populations and the inclusion/exclusion criteria differ across the clinical development programmes, making clinical interpretation difficult.
Although the DRC data in SUSTAIN 6 are consistent with an early worsening phenomenon secondary to glycaemic improvement in patients with pre‐existing DR, it is important to consider alternative aetiologies. Animal toxicology studies with semaglutide and liraglutide in non‐diabetic animals have shown no evidence for a direct GLP‐1 receptor agonist effect on the retina, and semaglutide data have shown no treatment‐related changes (including DR) in the eyes of any of the species studied.27
The increased risk of DRC with semaglutide vs placebo in patients with poor glycaemic control and pre‐existing DR in SUSTAIN 6 must be viewed in the wider context of the overall benefits with semaglutide treatment, including reduction in HbA1c, body weight and CV risk reduction.8
The beneficial effect of intensive glycaemic control on microvascular outcomes in the long term is well described, and optimization of glycaemic control remains the cornerstone of diabetes management and the prevention, or prevention of progression, of microvascular diseases such as DR and nephropathy. Improved glycaemic control had a beneficial effect on DR in both the DCCT and the UKPDS in the long term, and the magnitude of benefit on the risk of DR continued to increase over time, and was more pronounced in patients with DR at baseline.11, 12
Although fundoscopic assessments were collected in SUSTAIN 6, there are significant limitations to the granularity of these data, and it is necessary to interpret them with caution. However, they do provide reassurance that the retinal changes observed with semaglutide are similar to those seen with placebo at the end of treatment. Furthermore, events that the EAC evaluated as “onset of diabetes‐related blindness” were not annulled if blindness resolved spontaneously or as a result of treatment, as occurred in all 3 patients for whom further information was available, out of the 5 semaglutide‐treated patients. In this context, it is important to note that there are further limitations associated with the collection of the DRC data: the definition of onset of diabetes‐related blindness did not exclude other sight‐threatening conditions and visual acuity was only recorded once at the time of the event. Regarding vitreous haemorrhage, no data are available on duration or severity.
In conclusion, an increase in DRC was observed in SUSTAIN 6 in high‐risk patients, and the data are consistent with the phenomenon of early worsening of pre‐existing DR, secondary to an initial, rapid improvement in glycaemic control. This conclusion is supported by a post hoc mediation analysis, which suggests that the DRC finding in SUSTAIN 6 is seen in patients with pre‐existing DR and is primarily attributable to the magnitude and rapidity of reduction in HbA1c during the first 16 weeks of the trial. Additionally, there was no evidence for an increase in DR AE reporting in the SUSTAIN 1 to 5 or Japanese trials with semaglutide vs comparators. While further clinical evidence is required to understand fully the impact of semaglutide in general and on the progression of DR, it should be noted that physicians are alerted to the risk of worsening DR associated with intensified treatment in the prescribing information for insulins; similar recommendations may be appropriate when initiating other efficacious treatments, such as semaglutide.
Supporting information
File S1.
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial‐site staff who were involved in the conduct of the trial.
We also thank Eirik Quamme Bergan, MD, Mikkel Agersnap, MD, PhD and Oluf Hansen, MSc (all from Novo Nordisk) for their review and input into the manuscript. Madeleine Nowak, MSci (AXON Communications), provided medical writing and editorial assistance and received compensation from Novo Nordisk.
Conflict of interest
T.V. declares personal fees from Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, Merck Sharp & Dohme, Sanofi, Novo Nordisk and Bristol‐Myers Squibb, and grants (to her institution) from Eli Lilly and Novo Nordisk. S.B. has received grants (to his institution) and personal fees from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Sanofi, Merck Sharp & Dohme, Janssen and Cellnovo. L.A.L. has received grants (to his institution) and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi, personal fees from Servier and grants (to his institution) from GlaxoSmithKline. I.L. has received grants (to her institution) and/or other fees from Novartis, GI dynamics, Pfizer, Merck and Novo Nordisk; personal fees from AstraZeneca, Sanofi and Lilly, and non‐financial (editorial) support from AstraZeneca, Sanofi, Lilly, Novo Nordisk and Boehringer Ingelheim. D.M. has received grants (to his institution) from Novo Nordisk and Takeda and reports support from Janssen, Servier and Novartis; R.S. reports grants (to his institution) and personal fees from Novo Nordisk and OM Pharma, personal fees from Bayer, and non‐financial support from Sanofi. I.C. and N.W. are full‐time employees of Novo Nordisk. M.L. reports personal fees from Novo Nordisk.
Author contributions
Author contributions to the paper were as follows. T.V.: data collection and review, writing the manuscript; S.B.: data collection and review, writing the manuscript; L.A.L.: data collection and review, writing the manuscript; IL: data collection and review, writing the manuscript; D.M.: data review, writing the manuscript; R.S.: data review, writing the manuscript; I.C.: data collection and review, writing the manuscript; N.W.: data collection and review, writing the manuscript. M.L.: data review, writing the manuscript.
Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889–897. https://doi.org/10.1111/dom.13172
Funding information Novo Nordisk A/S
REFERENCES
- 1. Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once‐weekly human GLP‐1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marbury TC, Flint A, Jacobsen JB, Derving Karsbol J, Lasseter K. Pharmacokinetics and tolerability of a single dose of semaglutide, a human glucagon‐like peptide‐1 analog, in subjects with and without renal impairment. Clin Pharmacokinet. 2017;56(11):1381‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 4. Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 5. Ahmann A, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide vs. exenatide ER in subjects with type 2 diabetes (SUSTAIN 3). American Diabetes Association, 76th Annual Scientific Sessions; June 10‐14, 2016: New Orleans, LA.
- 6. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 7. Rodbard HW, Lingvay I, Reed J, et al. Efficacy and safety of semaglutide once‐weekly vs placebo as add‐on to basal insulin alone or in combination with metformin in subjects with type 2 diabetes (SUSTAIN 5). European Association for the Study of Diabetes, 52nd Annual Meeting; September 12‐16, 2016: Munich, Germany.
- 8. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 9. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ACCORDION Eye Study Group, ACCORDION Study Group . Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the action to control cardiovascular risk in diabetes (ACCORD) follow‐on study. Diabetes Care. 2016;39(7):1089‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 12. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 13. Diabetes Control and Complications Trial Research Group . Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116(7):874‐886. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical care in diabetes‐2016 abridged for primary care providers. Clin Diabetes. 2016;34(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Management of diabetes in pregnancy. Diabetes Care. 2015;38(suppl):S77‐S79. [DOI] [PubMed] [Google Scholar]
- 16. Gorman DM, le Roux CW, Docherty NG. The effect of bariatric surgery on diabetic retinopathy: good, bad, or both? Diabetes Metab J. 2016;40(5):354‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroc Collaborative Study Group . Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. N Engl J Med. 1984;311(6):365‐372. [DOI] [PubMed] [Google Scholar]
- 18. Lauritzen T, Frost‐Larsen K, Larsen HW, Deckert T. Two‐year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes. 1985;34(suppl 3):74‐79. [DOI] [PubMed] [Google Scholar]
- 19. Dahl‐Jørgensen K. Near‐normoglycemia and late diabetic complications. The Oslo Study. Acta Endocrinol Suppl. 1987;284:1‐38. [PubMed] [Google Scholar]
- 20. Sanofi‐Aventis . Lantus® (insulin glargine), EU summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000284/WC500036082.pdf. Accessed May 31, 2017.
- 21. Davis MD, Beck RW, Home PD, Sandow J, Ferris FL. Early retinopathy progression in four randomized trials comparing insulin glargine and NPH [corrected] insulin. Exp Clin Endocrinol Diabetes. 2007;115(4):240‐243. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Fonseca V, McGill JB, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long‐term, randomised, open‐label study. Diabetologia. 2009;52(9):1778‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varadhan L, Humphreys T, Hariman C, Walker AB, Varughese GI. GLP‐1 agonist treatment: implications for diabetic retinopathy screening. Diabetes Res Clin Pract. 2011;94(3):e68‐e71. [DOI] [PubMed] [Google Scholar]
- 25. Varadhan L, Humphreys T, Walker AB, Varughese GI. The impact of improved glycaemic control with GLP‐1 receptor agonist therapy on diabetic retinopathy. Diabetes Res Clin Pract. 2014;103(3):e37‐e39. [DOI] [PubMed] [Google Scholar]
- 26. US Food & Drug Administration Medical Reviews . Albiglutide. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125431Orig1s000MedR.pdf. Accessed May 31, 2017.
- 27. Novo Nordisk . Data on file. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.


