Abstract
Aims
To characterize survival in relation to achieved glycated haemoglobin (HbA1c) level within alternative glucose‐lowering regimens with differing risks of hypoglycaemia.
Methods
Data were extracted from the UK Clinical Practice Research Datalink and the corresponding Hospital Episode Statistics. Patients with type 2 diabetes prescribed glucose‐lowering therapy in monotherapy or dual therapy with metformin between 2004 and 2013 were identified. Risk of all‐cause mortality within treatment cohorts was evaluated using the Cox proportional hazards model, introducing mean HbA1c as a quarterly updated, time‐dependent covariable.
Results
There were 6646 deaths in a total follow‐up period of 374 591 years. Survival for lower (<7%) vs moderate HbA1c levels (≥7%, <8.5%) differed by cohort: metformin, adjusted hazard ratio (aHR) 1.03 (95% confidence interval [CI] 0.95‐1.12); sulphonylurea, aHR 1.11 (95% CI 0.99‐1.25); insulin, aHR 1.47 (95% CI 1.25‐1.72); combined regimens with low hypoglycaemia risk, aHR 1.02 (95% CI 0.94‐1.10); and combined regimens with higher hypoglycaemia risk excluding insulin, aHR 1.24 (95% CI 1.13‐1.35) and including insulin, aHR 1.28 (95% CI 1.18‐1.37). Higher HbA1c levels were associated with increased mortality in regimens with low hypoglycaemia risk. Post hoc analysis by HbA1c deciles revealed an elevated risk of all‐cause mortality for the lowest deciles across all cohorts, but particularly in those regimens associated with hypoglycaemia. High HbA1c was associated with no difference, or a small increase in mortality risk in regimens with increased risk of hypoglycaemia.
Conclusions
The pattern of mortality risk across the range of HbA1c differed by glucose‐lowering regimen. Lower HbA1c was associated with increased mortality risk compared with moderate control, especially in those regimens associated with hypoglycaemia. High levels of HbA1c were associated with the expected elevated mortality risk in regimens with low hypoglycaemia risk.
Keywords: diabetes, glucose, hypoglycaemia, mortality, survival
1. INTRODUCTION
Treatment guidelines for diabetes generally recommend therapeutic strategies that aim for normalization or near‐normalization of glucose control.1 These guidelines are based, in large part, on convincing evidence from the Diabetes Control and Complications Trial in type 1 diabetes and the UK Prospective Diabetes Study in type 2 diabetes, which demonstrated that improved glucose control reduced the risk of microvascular complications.2, 3
Whilst reductions in macrovascular complications and mortality have been observed in long‐term follow‐up of intensively treated subjects in these studies,2, 4 data from subsequent randomized clinical trials suggested no benefit, or an increase in mortality with intensive glucose control.5, 6 Furthermore, observational studies generally report increased mortality in those with low glycated haemoglobin (HbA1c).7 Thus, the optimum target for glucose control in patients with diabetes remains uncertain. This uncertainty is further complicated because both randomized trials8, 9 and observational studies7, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 26 have been unable to demonstrate a consistent pattern of association between levels of glucose control and adverse outcome, nor to explain why this may be the case.
One potentially influential explanation for an absence of improved survival in these studies, and the inconsistent pattern of association across the glucose control range relates to the impact of hypoglycaemia on cardiovascular event risk. Both randomized trials and observational studies included therapies that cause hypoglycaemia: primarily insulin and sulphonylureas; therefore, determination of optimal glucose control may be complicated by potentially competing influences of improved hyperglycaemia vs inducement of hypoglycaemia. Severe hypoglycaemia is associated with increased cardiovascular death and all‐cause mortality,23 but, in turn, hypoglycaemia is associated with other factors, for instance, exposure to exogenous insulin and, less often, sulphonylureas. The recent introduction of glucose‐lowering drugs that are not associated with hypoglycaemia provided an opportunity to dissect out these competing mechanisms. The aim of the present study was to characterize the risk of all‐cause mortality across the range of achieved HbA1c targets, and to determine whether this pattern varied in alternative regimens with differing risk of hypoglycaemia.
2. MATERIALS AND METHODS
2.1. Data source
This was a retrospective cohort study using primary care data from the UK Clinical Practice Research Datalink (CPRD) linked, where available, to the Hospital Episode Statistics (HES). The CPRD is a proprietary data source containing clinically rich, pseudonymized data collected in a non‐interventional manner. These data include demographics, consultations, medical history, test results and prescriptions. The CPRD is broadly representative of the UK population and contains records from approximately 14 million people. No patient consent was required for this study.
2.2. Patient selection
Patients with type 2 diabetes were identified between January 2004 and December 2013, with follow‐up to January 2015. Only practices with research‐quality data were included. Patients were selected if they had been treated with glucose‐lowering therapy, prescribed as monotherapy or dual therapy in combination with metformin. A minimum wash‐in period of 1 year from the date of registration or the up‐to‐standard date for the practice was required in order to identify treatment initiation. The up‐to‐standard date is the date at which the practice is considered to have been supplying continuous high‐quality data suitable for research. The study index date was defined as the first exposure to a relevant regimen; a patient could potentially contribute more than one regimen to the study. Exclusion criteria included diagnosis of type 1 diabetes, <91 days’ follow‐up time, and absence of HbA1c observations during the follow‐up period.
2.3. Treatment exposure
Six cohorts were selected, a priori, to provide insight into the overall impact of lower HbA1c and to differentiate between treatments that have a higher or a lower risk of hypoglycaemia. Two treatment cohorts were composed of glucose‐lowering therapies with a low risk of hypoglycaemia: the metformin monotherapy group; and a composite group treated with metformin monotherapy and acarbose, dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists, sodium‐glucose co‐transporter‐2 inhibitors or thiazolidinediones, as monotherapy or in combination with metformin. Four treatment cohorts comprised patients receiving glucose‐lowering therapies associated with a higher hypoglycaemia risk: sulphonylurea monotherapy; insulin monotherapy; a composite group of regimens comprising sulphonylureas and meglitinides prescribed as monotherapy or in combination with metformin but excluding insulin; and a composite group of regimens comprising sulphonylureas, meglitinides and insulin prescribed as monotherapy or with metformin. Treatment was considered to have been discontinued if there was a gap of >100 days between prescriptions for the same class of glucose‐lowering therapy. Patients were followed until the earliest of their date of death, date of regimen change, end of recorded data or the last prescription for the glucose‐lowering therapy of interest plus 90 days.
2.4. Outcome
The outcome was all‐cause mortality.
2.5. Achieved HbA1c categorization
Based on the pattern of association elucidated in a previous study7 and treatment guidelines,1 the following glucose‐control categories were selected a priori: lower HbA1c, <7%; moderate HbA1c, ≥7% < 8.5%; high HbA1c, ≥8.5% < 9.5%; and very high HbA1c, ≥9.5%.
2.6. Validation of the hypoglycaemia risk assumption
To validate that the prespecified treatment cohorts varied in hypoglycaemia risk as predicted, the hospital admission rate relating to hypoglycaemia was determined for each treatment cohort in those patients who were eligible for linkage to HES.
2.7. Statistical methods
Crude event rates were calculated for each treatment cohort and reported as events per 1000 person‐years of exposure. The relative rate was determined as the ratio of the respective crude event rates. Mid‐P confidence intervals (CIs)24 were calculated for the crude rates and the relative rate ratios. Time to and risk of death were evaluated using the Kaplan–Meier and multivariable Cox proportional hazards models.25 A significance level of .05 was chosen for this study. Unadjusted survival curves were compared using the log‐rank test. Day 0 was defined as index date plus 91 days. Based on their clinical rationale and past research,7 the following co‐variables were selected a priori and included in the multivariable models: age, gender, history of large vessel disease, Charlson morbidity index26, general practitioner contacts in the year prior to index date, smoking status, diabetes duration, systolic blood pressure, total cholesterol, and calendar year. The proportion of missing data was low (5% for total cholesterol [8464 patients], 2% for systolic blood pressure [3884 patients] and 0.2% for smoking status [350 patients]). Systolic blood pressure and total cholesterol were categorized into quartiles, plus a separate category for those with missing data, and patients with no smoking status were assumed to be non‐smokers.
Comparisons were made within each treatment cohort and not between cohorts. This is important because it eliminates glucose treatment indication bias in those on monotherapy. In the main analysis, achieved HbA1c was analysed as a mean, quarterly updated, time‐dependent co‐variable. Last observations carried forward and backwards were used to impute missing, post‐baseline values. HbA1c was also introduced into the models in the following ways in sensitivity analyses: non‐time‐dependently using mean HbA1c over follow‐up (theoretically violating the assumptions of survival models) and time‐dependently using a quarterly updated cumulative, mean value. The proportional hazards assumption was tested by examining the Pearson correlation between Schoenfeld residuals and the rank of survival time for cases that had progressed to death. Two‐sided P values are presented, and 95% CIs calculated for hazard ratios (HRs). We also report findings from an exploratory post hoc analysis, carried out to examine in more detail the structure of the association using deciles of HbA1c instead of the prespecified categories. Analyses were carried out using r‐v3.2.3 and spss‐v20.
To determine if the pattern of association between achieved HbA1c and mortality risk is the same in people with or without comorbidity, a subgroup analysis was conducted in those with a Charlson index of 1 (diabetes with no complications) and a Charlson index of ≥2 (diabetes with diabetic complications, diabetes plus non‐diabetic comorbidities or diabetes with diabetic complications plus non‐diabetic comorbidities).
2.8. Ethical approval
Studies using the CPRD are covered by ethics approval granted by the Trent Multicentre Research Ethics Committee (reference 05/MRE04/87). This study was granted CPRD Independent Scientific Advisory Committee approval on 23 February 2016, protocol number 16_007R2.
3. RESULTS
3.1. Study population
An attrition table detailing patient selection is provided in Table S1. There were 290 739 subjects with at least 1 diabetes glucose‐lowering therapy. Following exclusions, 131 315 patients were selected, with some participating in more than 1 treatment cohort and contributing more than 1 regimen to the composite treatment cohorts (number of periods of continuous therapy = 167 786). The treatment cohort with the fewest subjects was insulin monotherapy (n = 6827), and the largest was the composite cohort of regimens with a low risk of hypoglycaemia (n = 101 740; Table 1). Overall, there were 6646 deaths, with a corresponding total follow‐up of 374 591 years. The percentage of patients with a mean follow‐up HbA1c in the moderate category ranged from 42% for those prescribed metformin monotherapy (32 188 patients) to 46% for those prescribed regimens with a higher hypoglycaemia risk including insulin (30 363 patients). The percentage of patients with a mean follow‐up HbA1c in the lowest category ranged from 20% for those prescribed insulin monotherapy (1388 patients) to 47% for those prescribed metformin monotherapy (36 257 patients; Tables S2‐S7).
Table 1.
Baseline characteristics by glucose‐lowering regimen
| Characteristic | Insulin | Metformin | Sulphonylureas | Regimens with a low risk of hypoglycaemia | Regimens with higher hypoglycaemia risk excluding insulin | Regimens with higher hypoglycaemia risk including insulin |
|---|---|---|---|---|---|---|
| N | 6827 | 76 821 | 14 834 | 101 740 | 50 094 | 66 046 |
| Males, n (%) | 3819(56) | 43 985 (57) | 7675 (52) | 58 299 (57) | 29 213 (58) | 38 372 (58) |
| Age at index, mean (SD), years | 66.3 (12.9) | 62.5 (12.4) | 69.7 (12.3) | 62.1 (12.2) | 65.3 (12.5) | 65 (12.5) |
| Duration of diabetes, median (IQR), years | 7.5 (3‐12.9) | 0.5 (0‐3.1) | 2.6 (0.4 to 6.1) | 1.4 (0.1‐4.5) | 3.9 (1.4‐7.2) | 4.7 (1.7‐8.6) |
| Ever smoked, n (%) | 4063 (60) | 44 157 (57) | 8377 (56) | 58 559 (58) | 28 844 (58) | 38 343 (58) |
| Serum creatinine, median (IQR), μmol/L | 99 (77‐143) | 82 (71‐95) | 91 (76‐119) | 82 (70‐95) | 84 (72‐100) | 85 (72‐102) |
| BMI, mean (SD), kg/m2 | 29.8 (6.6) | 32.4 (6.4) | 29.3 (6) | 32.6 (6.4) | 30.6 (6.1) | 30.7 (6.2) |
| Systolic blood pressure, mean (SD), mmHg | 134.1 (18.5) | 137 (16.1) | 135.4 (17.4) | 136.5 (15.9) | 135.3 (16) | 135.2 (16.3) |
| Total cholesterol, mean (SD), mmol/L | 4.5 (1.4) | 4.9 (1.3) | 4.7 (1.3) | 4.8 (1.2) | 4.5 (1.2) | 4.5 (1.2) |
| Charlson comorbidity index, median (IQR) | 3 (2‐5) | 2 (1‐3) | 3 (1‐4) | 2 (1‐3) | 2 (1‐3) | 2 (1‐3) |
| Prior cancer, n (%) | 870 (13) | 5863 (8) | 1918 (13) | 7594 (7) | 4759 (10) | 6335 (10) |
| Prior large vessel disease, n (%) | 2501 (37) | 15 173 (20) | 4537 (31) | 19 878 (20%) | 12 148 (24) | 17 216 (26) |
| Prior vision problems, n (%) | 2982 (44) | 15 382 (20) | 4941 (33) | 23 102 (23) | 15 799 (32) | 22 633 (34) |
| Prior microalbuminuria, n (%) | 720 (11) | 1738 (2) | 860 (6%) | 3352 (3) | 2960 (14) | 4598 (7) |
| Prior renal, n (%) | 2482 (36) | 7888 (10) | 4066 (27) | 11 444 (11) | 9102 (18) | 13 193 (20) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
3.2. Baseline characteristics
The baseline characteristics varied by cohort (Table 1). For example, the youngest mean age at treatment initiation was in the composite cohort of regimens with a low risk of hypoglycaemia, with a mean age of 62.1 years. The oldest mean age was in those in the sulphonylurea monotherapy cohort, at 69.7 years. A summary of the baseline characteristics by treatment cohort is provided in Table 1; more detailed descriptions by HbA1c category are provided in Tables S2 to S7. There were differences between those with lower and moderate mean HbA1c values within the 6 cohorts. Age at treatment initiation was generally younger in those with moderate mean HbA1c, except for insulin monotherapy where those with moderate control vs lower control were older (67.3 vs 65.8 years; P = .001). Modifiable risk factors such as smoking status, blood pressure and total cholesterol concentration did not vary markedly between moderate and lower HbA1c categories. Patients in the lower HbA1c category had a shorter duration of diabetes.
3.3. Crude admission rates for hypoglycaemia
The hospital admission rate for hypoglycaemia varied by treatment cohort. For example, the value for the insulin monotherapy cohort was 42.2 (95% CI 37.9‐46.5) admissions per 1000 person‐years of exposure. For the composite cohort of regimens with lower hypoglycaemia risk, this was 0.8 (95% CI 0.6‐0.9) admissions per 1000 person‐years of exposure (Figure S1). When compared with moderate control, the rate of admissions for hypoglycaemia was higher for those in the lower mean follow‐up HbA1c category treated with regimens defined a priori to have a high risk of hypoglycaemia. The rate of hypoglycaemia was also higher in those with very high mean follow‐up HbA1c treated with insulin monotherapy and regimens with a higher risk of hypoglycaemia including insulin.
3.4. Crude mortality rates
The overall crude mortality rate was 17.7 deaths per 1000 person‐years of exposure. This differed between glucose‐lowering cohorts; the highest rate was in those treated with insulin monotherapy, with a crude rate of 53.5 deaths per 1000 person‐years, and the lowest rate was in the composite group of regimens with low risk of hypoglycaemia, with a crude rate of 13.5 deaths per 1000 person‐years (Table 2). The Kaplan–Meier survival curves by mean HbA1c categories, and by treatment cohort are shown in Figure 1. These showed that the rate of progression to death was more rapid for those with lower mean HbA1c vs moderate.
Table 2.
Frequency of deaths, total exposure and crude event rates by mean follow‐up HbA1c category
| Parameter | Lower (<7%) | Moderate (≥7% and <8.5%) | High (≥8.5% and <9.5%) | Very high (≥9.5%) | Overall |
|---|---|---|---|---|---|
| Metformin | |||||
| Number of deaths | 1708 | 928 | 49 | 34 | 2719 |
| Person‐years of exposure | 104 268 | 72 022 | 6666 | 3095 | 186 051 |
| Mean follow‐up, years | 2.88 | 2.24 | 1.25 | 1.02 | 2.42 |
| Crude event rate per 1000 person‐years (95% CI) | 16.4 (15.6‐17.2) | 12.9 (12.1‐13.7) | 7.4 (5.3‐9.4) | 11.0 (7.3‐14.7) | 14.6 (14.1‐15.2) |
| Crude rate ratio | 1.27 (1.17‐1.38) | 1 | 0.57 (0.42‐0.75) | 0.85 (0.6‐1.19) | |
| Sulphonylureas | |||||
| Number of deaths | 768 | 505 | 73 | 29 | 1375 |
| Person‐years of exposure | 12 564 | 11 899 | 2214 | 1147 | 27 823 |
| Mean follow‐up, years | 2.20 | 1.90 | 1.28 | 1.03 | 1.88 |
| Crude event rate per 1000 person‐years (95% CI) | 61.1 (56.8‐65.5) | 42.4 (38.7‐46.1) | 33.0 (25.4‐40.5) | 25.3 (16.1‐34.5) | 49.4 (46.8‐52) |
| Crude rate ratio | 1.44 (1.29‐1.61) | 1 | 0.78 (0.6‐0.99) | 0.60 (0.4‐0.85) | |
| Insulin | |||||
| Number of deaths | 182 | 389 | 170 | 108 | 849 |
| Person‐years of exposure | 2818 | 7847 | 3307 | 1898 | 15 870 |
| Mean follow‐up, years | 2.03 | 2.60 | 2.33 | 1.90 | 2.32 |
| Crude event rate per 1000 person‐years (95% CI) | 64.6 (55.2‐74) | 49.6 (44.6‐54.5) | 51.4 (43.7‐59.1) | 56.9 (46.2‐67.6) | 53.5 (49.9‐57.1) |
| Crude rate ratio | 1.30 (1.09‐1.55) | 1 | 1.04 (0.86‐1.24) | 1.15 (0.92‐1.42) | |
| Regimens with a low hypoglycaemia risk | |||||
| Number of deaths | 1928 | 1128 | 72 | 41 | 3169 |
| Person‐years of exposure | 125 054 | 93 951 | 10 006 | 4952 | 233 962 |
| Mean follow‐up, years | 2.81 | 2.14 | 1.21 | 0.97 | 2.30 |
| Crude event rate per 1000 person‐years (95% CI) | 15.4 (14.7‐16.1) | 12.0 (11.3‐12.7) | 7.2 (5.5‐8.9) | 8.3 (5.7‐10.8) | 13.5 (13.1‐14) |
| Crude rate ratio | 1.28 (1.19‐1.38) | 1 | 0.60 (0.47‐0.76) | 0.69 (0.5‐0.93) | |
| Regimens with a higher hypoglycaemia risk excluding insulin | |||||
| Number of deaths | 1280 | 959 | 138 | 64 | 2441 |
| Person‐years of exposure | 38 958 | 49 927 | 9691 | 4760 | 103 336 |
| Mean follow‐up, years | 2.23 | 2.17 | 1.60 | 1.32 | 2.06 |
| Crude event rate per 1000 person‐years (95% CI) | 32.9 (31.1‐34.7) | 19.2 (18‐20.4) | 14.2 (11.9‐16.6) | 13.4 (10.2‐16.7) | 23.6 (22.7‐24.6) |
| Crude rate ratio | 1.71 (1.57‐1.86) | 1 | 0.74 (0.62‐0.88) | 0.70 (0.54‐0.9) | |
| Regimens with a higher hypoglycaemia risk including insulin | |||||
| Number of deaths | 1504 | 1444 | 334 | 195 | 3477 |
| Person‐years of exposure | 44 597 | 68 873 | 17 828 | 9331 | 140 628 |
| Mean follow‐up, years | 2.21 | 2.27 | 1.87 | 1.57 | 2.13 |
| Crude event rate per 1000 person‐years (95% CI) | 33.7 (32‐35.4) | 21.0 (19.9‐22) | 18.7 (16.7‐20.7) | 20.9 (18‐23.8) | 24.7 (23.9‐25.5) |
| Crude rate ratio | 1.61 (1.5‐1.73) | 1 | 0.89 (0.79‐1.01) | 1.00 (0.86‐1.16) | |
Abbreviation: CI, confidence interval.
Figure 1.
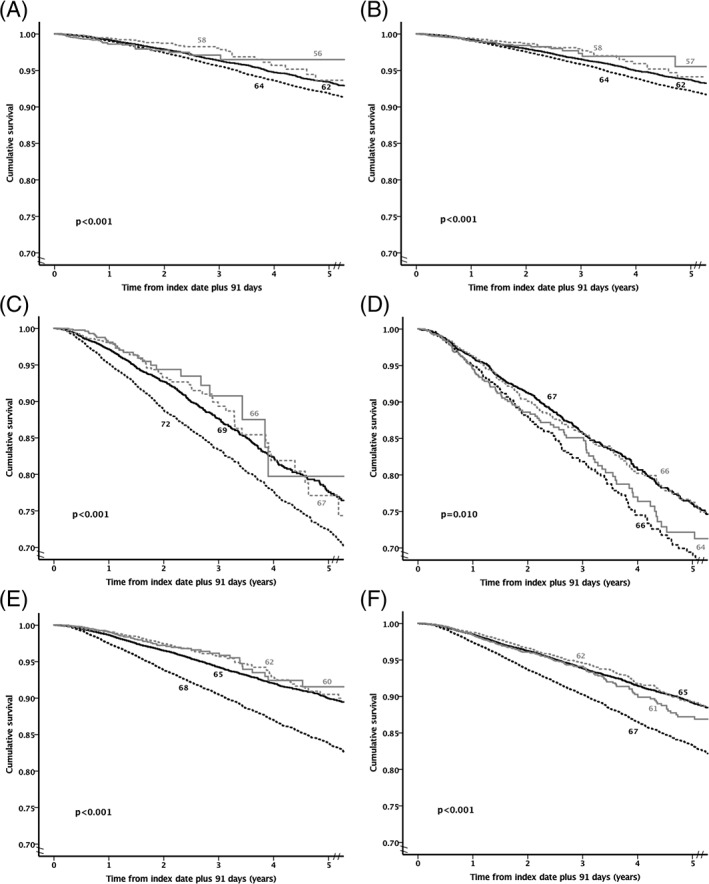
Kaplan–Meier survival curves by glucose‐lowering regimen. A, Metformin monotherapy. B, Regimens with a low hypoglycaemia risk. C, Sulphonylurea monotherapy. D, Insulin monotherapy. E, Regimens with a higher hypoglycaemia risk excluding insulin. F, Regimens with a higher hypoglycaemia risk including insulin. Black dashed line = lower glycated haemoglobin (HbA1c; <7%), black solid line = moderate HbA1c (≥7% and <8.5%), grey dashed line = high HbA1c (≥8.5% and <9.5%) and grey solid line = very high HbA1c (≥9.5%). The number next to each line is the mean age for each cohort
The crude death rate was consistently higher in patients in the lower mean HbA1c category than in those in the moderate mean HbA1c category across all 6 cohorts (Table 2). Patients in the composite cohort of regimens with a higher risk of hypoglycaemia excluding insulin had the highest relative increase in crude death rate between the lower and moderate mean HbA1c categories: relative rate ratio 1.71 (95% CI 1.57‐1.86). The lowest relative death rate between the lower and moderate mean HbA1c categories was in the metformin monotherapy cohort: 1.27 (95% CI 1.17‐1.38). The crude mortality rates are shown in Figures S2 and S3.
3.5. Adjusted risk of death by HbA1c category
In the main analysis, the overall adjusted HRs (aHRs), compared with moderate HbA1c, for the lower, high and very high HbA1c categories were 1.16 (95% CI 1.06‐1.18), 1.18 (95% CI 1.07‐1.32) and 1.16 (95% CI 1.03‐1.32), respectively.
For metformin monotherapy and the composite cohort of regimens with a low risk of hypoglycaemia, there was no statistically significant difference in the risk of mortality between the lower and moderate HbA1c categories when HbA1c was modelled as a mean, quarterly updated, time‐dependent co‐variable (aHR 1.03 [95% CI 0.95‐1.12] and 1.02 [95% CI 0.94‐1.10], respectively); however, an increase in the risk of all‐cause mortality in the lower vs moderate HbA1c categories was observed in patients in the insulin monotherapy cohort (aHR 1.47 [95% CI 1.25‐1.72]) and in both composite cohorts of regimens with a higher hypoglycaemia risk, excluding and including insulin (aHR 1.24 [95% CI 1.13‐1.35] and 1.28 [95% CI 1.18‐1.37], respectively; Figure 2). The high and very high HbA1c categories were associated with a significantly increased risk of all‐cause mortality when compared with the moderate categories for patients in the metformin monotherapy cohort and in the composite group of regimens with a low risk of hypoglycaemia (results for the high and very high categories were aHR 1.37 [95% CI 1.09‐1.72] and 1.70 [95% CI 1.28‐2.26] for metformin, and aHR 1.27 [95% CI 1.04‐1.56] and 1.45 [95% CI 1.12‐1.89] for regimens with a lower‐risk of hypoglycaemia). The risk of all‐cause mortality was not significantly elevated in the high and very high categories for sulphonylurea monotherapy, insulin monotherapy and regimens with a higher risk of hypoglycaemia with the exception of the high HbA1c category for regimens with a higher risk of hypoglycaemia including insulin where the risk of all‐cause mortality was significantly elevated (aHR 1.13, 95% CI 1.00‐1.28, p=0.047). Sensitivity analyses were largely consistent with the primary analysis (Figure S2).
Figure 2.
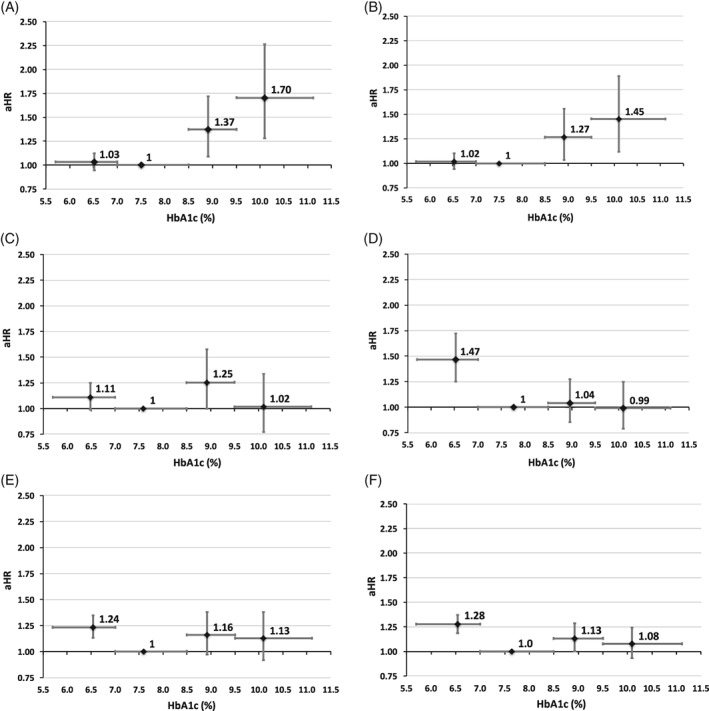
Relative risk of death by glycated haemoglobin (HbA1c) category when HbA1c is modelled as a mean, quarterly updated, time‐dependent covariate. Data from alternative models are detailed in Figure S2. A, Metformin monotherapy. B, Regimens with a low hypoglycaemia risk. C, Sulphonylurea monotherapy. D, Insulin monotherapy. E, Regimens with a higher hypoglycaemia risk excluding insulin. F, Regimens with a higher hypoglycaemia risk including insulin. Vertical error bars represent the 95% confidence interval (CI) for adjusted hazard ratio (aHR) and the horizontal error bars represent the HbA1c range
3.6. Adjusted risk of death stratified by HbA1c decile
When analysed according to HbA1c deciles there was further structure in these data (Figures 3 and S3). There was a consistent pattern of a more specific, optimal HbA1c (7.03%‐7.27%). In all 6 cohorts there was an increased association with all‐cause mortality in the lowest decile compared with the nadir. At the highest decile of HbA1c, all treatment cohorts had elevated risk, and this achieved significance in those prescribed metformin monotherapy (aHR 2.01 [95% CI 1.53‐2.65]), regimens with a lower hypoglycaemia risk (aHR 1.77 [95% CI 1.38‐2.27]) and regimens with a higher hypoglycaemia risk, excluding (aHR 1.27 [95% CI 1.02‐1.57]) and including (aHR 1.22 [95% CI 1.03‐1.44]) insulin. This was not the case, however, for all high deciles of HbA1c. Sensitivity analyses were largely consistent with the primary analysis (Figure S3). A similar pattern of association between HbA1c and all‐cause mortality was observed in those patients with a baseline Charlson score of 1 and ≥2 prescribed regimens with a low hypoglycaemia risk or regimens with a higher hypoglycaemia risk including insulin (Figure S4).
Figure 3.
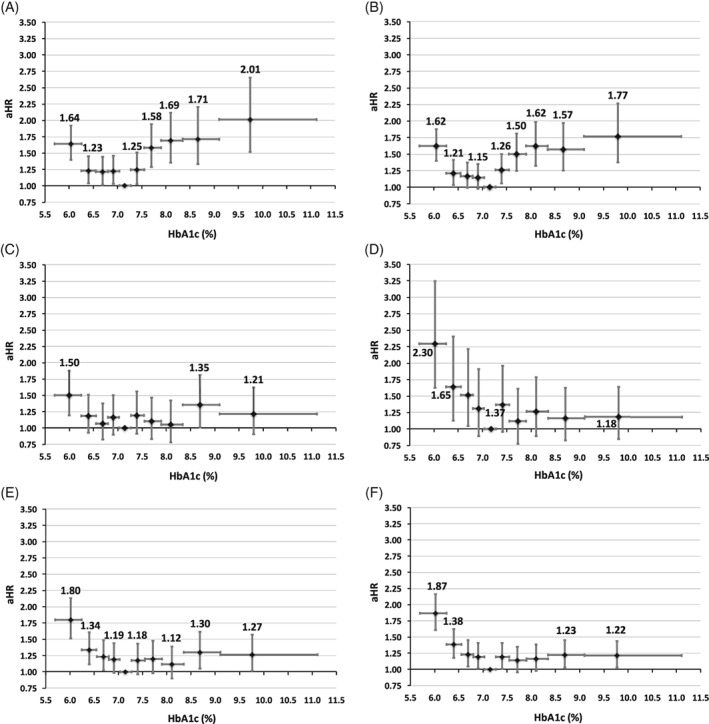
Relative risk of death by HbA1c decile when HbA1c is modelled as a mean, quarterly updated, time‐dependent covariate. Data from alternative models are detailed in Figure S3. A, Metformin monotherapy. B, Regimens with a low hypoglycaemia risk. C, Sulphonylurea monotherapy. D, Insulin monotherapy. E, Regimens with a higher hypoglycaemia risk excluding insulin. F, Regimens with a higher hypoglycaemia risk including insulin. Vertical error bars represent the 95% confidence interval (CI) for adjusted hazard ratio (aHR) and the horizontal error bars represent the glycated haemoglobin (HbA1c) range
4. DISCUSSION
Previous studies in patients with type 2 diabetes have reported that the relationship between HbA1c, all‐cause mortality and other outcomes was not a direct association but better characterized by a J‐shaped, V‐shaped or U‐shaped association.7, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 22 In this study, we not only found that the pattern of association between differing levels of HbA1c and all‐cause mortality differed within specific glucose‐lowering regimens, but also that this pattern of association differed between differing glucose‐lowering regimens. When categorized by prespecified HbA1c ranges, lower HbA1c was associated with significantly increased mortality when compared with moderate levels of control, but only in those regimens that were associated with hypoglycaemia. In regimens associated with hypoglycaemia, there was either no elevation of mortality risk at higher levels of HbA1c at the conventional level of statistical significance, or a small increase in mortality risk was observed. In contrast, in those regimens not associated with hypoglycaemia there was no increase in mortality at the lower HbA1c range, but, as might be expected, there was an increase in mortality at higher levels. In itself this does not prove that hypoglycaemia is solely or even partially responsible for these observations; however, these findings do show persuasively that there were marked differences that need somehow to be explained.
Within the prespecified categories of HbA1c, patient numbers varied, with more people in the moderate control category. Thus, we performed a post hoc analysis by HbA1c deciles that revealed further structure in these data. The findings by HbA1c category masked more detailed differences in risk patterns between regimens. All regimens were associated with elevated mortality risk at the lowest decile of HbA1c. Sulphonylurea monotherapy and insulin monotherapy resulted in considerably elevated mortality risk at low HbA1c levels, but an increase was also seen with therapies that are not typically associated with hypoglycaemia. Insofar as low glucose levels may possibly be a consequence of disease‐related morbidity, these observations may suggest that the overall increase in mortality in patients with type 2 diabetes that has been previously described may be a function of the interplay between 2 recognized factors: hypoglycaemia induced by certain glucose‐lowering drugs, against a background of a generalized association of morbidity with reduced plasma glucose concentrations.
In contrast, at higher levels of HbA1c there was no clear elevation in mortality in people treated with regimens associated with increased hypoglycaemia. While the reasons for this observation remain unknown, one possibility is that the influence of hypoglycaemia‐related mortality extends throughout the entire HbA1c range, and confounds the ability to tease out the contribution of hyperglycaemia to mortality. This hypothesis is supported by the observations in patients treated with regimens with a low hypoglycaemia risk, where the expected increase in mortality was observed in higher HbA1c categories.
In terms of the association with all‐cause mortality, the optimal level of HbA1c in these analyses was consistently slightly above 7% in all regimens, although high levels of HbA1c were not always associated with increased risk of all‐cause mortality. Variability in these patterns between alternative regimens—whilst consistent with previous observational studies—helps explain why no single, consistent pattern has yet emerged. It is likely that the pattern of HbA1c association with adverse outcome varies by regimen, and if confirmed, this would be an important consideration.
These data may also help explain the lack of demonstrable mortality benefits in previous studies of intensive control such as the ACCORD5 and the VADT studies.5, 6 To the extent that an increase in mortality is associated with therapies that increase insulin availability in a non‐glucose‐dependent manner (eg, sulphonylureas and insulin), studies of intensive glucose‐lowering may be confounded by the competing influences of reversal of hyperglycaemia and risk of hypoglycaemia. The ORIGIN trial27 examined the outcome of low‐dose, basal insulin compared with standard care, and the insulin arm did not reduce either all‐cause mortality or cardiovascular mortality; however, patients in the insulin group received concomitant metformin—shown to markedly attenuate mortality risk in people treated with insulin, in particular at low doses28—and insulin was quite frequently used in the standard care arm. In a recent observational study characterizing the outcome of care of those exposed to metformin, where the outcome was major adverse cardiovascular events or all‐cause mortality, there was no evidence of increased adverse outcomes at low HbA1c levels.29 A further observational study in 2010 reported that there was no difference in all‐cause mortality across the HbA1c range, but this study was subject to immortal time bias and other limitations30.
The risk‐to‐benefit calculation in choosing between alternative glucose‐lowering drugs involves a complex balancing exercise between competing risks due to pleiotropic effects such as hypoglycaemia, hyperglycaemia, weight gain, renal disease, cancer‐related properties, haemodynamic changes, hyperlipidaemia and direct cardiovascular injury. Although there are no competing risks when analysing all‐cause mortality, the potential harms or benefits from these drugs may differentially influence microvascular and macrovascular outcomes and may impact differing patient phenotypes in differing ways. Intensive glucose control undoubtedly results in improved microvascular disease outcome, but, in the present study, we confirm that there is a serious downside risk that must be taken into account when HbA1c is lowered, particularly with therapies that are associated with an increased risk of hypoglycaemia.
These data and the study design had a number of strengths and limitations. The study included routine observations from a very large number of people, from a national, real‐world setting. There was no standardization of the timing of observations such as HbA1c. We believe that this will have introduced noise but not bias because there is inherent variability in the measurement and timing of glucose control in routine care. One general criticism of retrospective, observational studies is indication bias, that is, that patients are often treated differentially with differing drugs at differing doses in a non‐random way. Unusually, this was less of a concern here because the comparisons of the pattern of association between differing levels of HbA1c were only made in a statistical way within the same regimens and compared only differing levels of glucose control. However, it is also possible to examine the general patterns of association and determine that they differed markedly between the 6 regimens that were characterized. Nevertheless, multivariable models were used to account for recorded differences between HbA1c categories. HbA1c varies over time for many reasons. The time‐dependent statistical models helped account for this, but they remain statistical models. To increase confidence in our findings, we set out, a priori, to use various analytical approaches and to determine if our findings remained consistent. In the main, there was consistency between alternative approaches. There were differences in phenotype between groups, so the possibility of residual confounding is a consideration that needs to be borne in mind. There could be several reasons why treatment intensification was delayed in those with poor glucose control including clinical inertia, non‐compliance, age, comorbidities, or restricted access to care and there may therefore be underlying differences between patients across the HbA1c range that could not be fully adjusted for in our statistical model.
In summary, lower HbA1c was associated with increased mortality risk compared with moderate levels, especially with regimens that are associated with hypoglycaemia. High levels of HbA1c were consistently associated with the expected elevated mortality risk in those regimens that have a lower risk of hypoglycaemia. These data suggest that, in the individualization of glycaemic targets for patients, consideration needs to be given to the classes of glucose‐lowering therapy that are being used, with less aggressive targets in those patients who are being treated with therapies associated with hypoglycaemia. Lower HbA1c was associated with increased mortality risk compared with moderate control, especially in those regimens associated with hypoglycaemia.
Supporting information
Table S1. Attrition table.
Table S2. Baseline characteristics for metformin monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S3. Baseline characteristics for sulfonylurea monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S4. Baseline characteristics for insulin monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S5. Baseline characteristics for regimens with a low hypoglycaemia risk by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S6. Baseline characteristics for regimens with a higher hypoglycaemia risk excluding insulin by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S7. Baseline characteristics for regimens with a higher hypoglycaemia risk including insulin by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Figure S1. Hospital admission rate for hypoglycaemia (a) by treatment cohort and (b) by treatment cohort and mean follow‐up HbA1c.
Figure S2. Relative risk of death by HbA1c category using 5 alternative methods of risk estimation for (a) metformin monotherapy, (b) regimens with low hypoglycaemia risk, (c) sulfonylurea monotherapy, (d) insulin monotherapy, (e) regimens with higher hypoglycaemia risk (excluding insulin) and (f) regimens with higher hypoglycaemia risk (including insulin).
Figure S3. Relative risk of death by HbA1c decile using 5 alternative methods of risk estimation for (a) metformin monotherapy, (b) regimens with low hypoglycaemia risk, (c) sulfonylurea monotherapy, (d) insulin monotherapy, (e) regimens with higher hypoglycaemia risk (excluding insulin) and (f) regimens with higher hypoglycaemia risk (including insulin).
Figure S4 Relative risk of death in patients with low baseline morbidity (Charlson index of 1 due–diabetes) and a higher baseline morbidity (Charlson index >1) by HbA1c decile when HbA1c was modelled as a mean, quarterly updated, time‐dependent covariate (aHR, adjusted hazard ratio) for (a) regimens with a low risk of hypoglycaemia and (b) regimens with a higher risk of hypoglycaemia including insulin.
ACKNOWLEDGMENTS
This study was supported by Merck Sharp and Dohme & Co. Employees of the sponsor contributed to this study as co‐authors, as detailed.
Conflict of interest
All authors have completed the unified competing interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) C.J.C., S.E.H., S.J.J., C.Ll.M. are employed by, and C.J.C. is a director of Pharmatelligence, a research consultancy receiving funding from Merck for the submitted work; (2) B.V., S.N.R., B.A., and S.S.E. are employees of Merck (i.e. MSD outside of the US&Canada), the sponsor of the study. J.R.P. has no competing interests to declare.
Author contributions
All co‐authors contributed to the study design. S.J.J. extracted these data. S.H. carried out the data analysis. All co‐authors jointly interpreted these data. C.J.C. wrote the first draft of the manuscript, and all co‐authors edited the manuscript.
Currie CJ, Holden SE, Jenkins‐Jones S, et al. Impact of differing glucose‐lowering regimens on the pattern of association between glucose control and survival. Diabetes Obes Metab. 2018;20:821–830. https://doi.org/10.1111/dom.13155
Funding information Merck Sharp and Dohme
REFERENCES
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. [DOI] [PubMed] [Google Scholar]
- 3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 4. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, ME M, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129‐139. [DOI] [PubMed] [Google Scholar]
- 7. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481‐489. [DOI] [PubMed] [Google Scholar]
- 8. Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373:1765‐1772. [DOI] [PubMed] [Google Scholar]
- 9. Boussageon R, Bejan‐Angoulvant T, Saadatian‐Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d4169 https://doi.org/10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Östgren CJ, Sundström J, Svennblad B, Lohm L, Nilsson PM, Johansson G. Associations of HbA1c and educational level with risk of cardiovascular events in 32,871 drug‐treated patients with type 2 diabetes: a cohort study in primary care. Diabet Med. 2013;30:e170‐e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paprott R, Schaffrath Rosario A, Busch MA, et al. Association between hemoglobin A1c and all‐cause mortality: results of the mortality follow‐up of the German National Health Interview and examination survey 1998. Diabetes Care. 2015;38:249‐256. [DOI] [PubMed] [Google Scholar]
- 12. Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1C and all‐cause mortality during a median 3.4‐year follow‐up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nichols GA, Joshua‐Gotlib S, Parasuraman S. Glycemic control and risk of cardiovascular disease hospitalization and all‐cause mortality. J Am Coll Cardiol. 2013;62:121‐127. [DOI] [PubMed] [Google Scholar]
- 15. Kontopantelis E, Springate DA, Reeves D, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia. 2015;58:505‐518. [DOI] [PubMed] [Google Scholar]
- 16. Park C, Guallar E, Linton JA, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monami M, Vitale V, Lamanna C, et al. HbA1c levels and all‐cause mortality in type 2 diabetic patients: epidemiological evidence of the need for personalised therapeutic targets. Nutr Metab Cardiovasc Dis. 2013;23:300‐306. [DOI] [PubMed] [Google Scholar]
- 18. Fagher K, Löndahl M. The impact of metabolic control and QTc prolongation on all‐cause mortality in patients with type 2 diabetes and foot ulcers. Diabetologia. 2013;56:1140‐1147. [DOI] [PubMed] [Google Scholar]
- 19. Nicholas J, Charlton J, Dregan A, Gulliford MC. Recent HbA1c values and mortality risk in type 2 diabetes. Population‐based case‐control study. PLoS ONE. 2013;8:e68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grembowski D, Ralston JD, Anderson ML. Hemoglobin A1c, comorbid conditions and all‐cause mortality in older patients with diabetes: a retrospective 9‐year cohort study. Diabetes Res Clin Pract. 2014;106:373‐382. [DOI] [PubMed] [Google Scholar]
- 21. Chiang HH, Tseng FY, Wang CY, et al. All‐cause mortality in patients with type 2 diabetes in association with achieved hemoglobin A1c, systolic blood pressure, and low‐density lipoprotein cholesterol levels. PLoS ONE. 2014;9:e109501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. HbA1c and all‐cause mortality risk among patients with type 2 diabetes. Int J Cardiol. 2016;202:490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410‐1418. [DOI] [PubMed] [Google Scholar]
- 24. Rothmann KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 25. Kleinbaum DG, Klein M. Survival Analysis: A Self‐Learning Text. 2nd ed. New York: Springer; 2005. [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1989;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 27. The ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319‐328. [DOI] [PubMed] [Google Scholar]
- 28. Holden SE, Jenkins‐Jones S, Currie CJ. Association between insulin monotherapy versus insulin plus metformin and the risk of all‐cause mortality and other serious outcomes: a retrospective cohort study. PLoS ONE. 2016;11:e0153594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Svensson E, Baggesen LM, Johnsen SP, et al. Early glycemic control and magnitude of HbA1c reduction predict cardiovascular events and mortality: population‐based cohort study of 24,752 metformin initiators. Diabetes Care. 2017;40:800‐807. [DOI] [PubMed] [Google Scholar]
- 30. Eeg‐Olofsson K, Cederholm J, Nilsson PM, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med. 2010;268:471‐482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Attrition table.
Table S2. Baseline characteristics for metformin monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S3. Baseline characteristics for sulfonylurea monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S4. Baseline characteristics for insulin monotherapy by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S5. Baseline characteristics for regimens with a low hypoglycaemia risk by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S6. Baseline characteristics for regimens with a higher hypoglycaemia risk excluding insulin by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Table S7. Baseline characteristics for regimens with a higher hypoglycaemia risk including insulin by (a) mean follow‐up HbA1c category and (b) mean follow‐up HbA1c decile.
Figure S1. Hospital admission rate for hypoglycaemia (a) by treatment cohort and (b) by treatment cohort and mean follow‐up HbA1c.
Figure S2. Relative risk of death by HbA1c category using 5 alternative methods of risk estimation for (a) metformin monotherapy, (b) regimens with low hypoglycaemia risk, (c) sulfonylurea monotherapy, (d) insulin monotherapy, (e) regimens with higher hypoglycaemia risk (excluding insulin) and (f) regimens with higher hypoglycaemia risk (including insulin).
Figure S3. Relative risk of death by HbA1c decile using 5 alternative methods of risk estimation for (a) metformin monotherapy, (b) regimens with low hypoglycaemia risk, (c) sulfonylurea monotherapy, (d) insulin monotherapy, (e) regimens with higher hypoglycaemia risk (excluding insulin) and (f) regimens with higher hypoglycaemia risk (including insulin).
Figure S4 Relative risk of death in patients with low baseline morbidity (Charlson index of 1 due–diabetes) and a higher baseline morbidity (Charlson index >1) by HbA1c decile when HbA1c was modelled as a mean, quarterly updated, time‐dependent covariate (aHR, adjusted hazard ratio) for (a) regimens with a low risk of hypoglycaemia and (b) regimens with a higher risk of hypoglycaemia including insulin.


