Abstract
To evaluate the Turkey's nationwide HPV DNA screening program on the basis of first 1 million screened women. Women over age 30 were invited for population based screening via HPV DNA and conventional cytology. Samples were collected by family physicians and the evaluations and reports had been performed in the National Central HPV laboratories. The acceptance rate for HPV based cervical cancer screening after first invitation was nearly 36.5%. Since HPV DNA tests have been implemented, cervical cancer screening rates have shown 4–5‐fold increase in primary level. Through the evaluation of all, HPV positivity was seen in 3.5%. The commonest HPV genotypes were 16, followed by 51, 31, 52 and 18. Among the 37.515 HPV positive cases, cytological abnormality rate was 19.1%. Among HPV positive cases, 16.962 cases had HPV 16 or 18 or other oncogenic HPV types with abnormal cytology (>ASC‐US). These patients were referred to colposcopy. The colposcopy referral rate was 1.6%. Among these, final clinico‐pathological data of 3.499 patients were normal in 1.985 patients, CIN1 in 708, CIN2 in 285, CIN3 in 436 and cancer in 85 patients and only pap‐smear program could miss 45.9% of ≥CIN3 cases. The results of 1 million women including the evaluation of 13 HPV genotypes with respect to prevalence, geographic distribution and abnormal cytology results shows that HPV DNA can be used in primary level settings to have a high coverage rated screening program and is very effective compared to conventional pap‐smear.
Keywords: Turkey, HPV, HPV DNA screening, cervical cancer, cytology, population based screening
Short abstract
What's new?
In Turkey, a cervical cancer screening program using Pap smear reached only a tiny proportion of the nation's women. To sidestep the logistical challenges that hindered that approach, these authors investigated a population wide HPV testing program. HPV testing is much less expensive and easier to automate than cytological testing. The screening rates have increased 5‐fold over the cytology‐based screening program, and the HPV based program made better use of the limited personnel available to interpret cytological test results. Other developing countries looking to implement cervical cancer screening programs could look to Turkey's system as a successful model.
Cervical cancer is unique among common cancers in that it can be almost totally eradicated. High risk Human Papilloma Virus (HPV) is the primary causative agent of virtually all cases, and since 2006, there are very effective HPV prophylactic vaccines.1, 2 In addition, it has been reconfirmed recently that all true cervical intraepithelial neoplasia grade 3 lesions (CIN3) can be linked to HPV while 95–98% of CIN3 will test HR‐HPV positive with a validated HPV test.3, 4 Because the development of cancer takes at least 5–10 years following HPV infection, HPV based screening offers an opportunity to prevent cervical cancer in those with persistent infections with or without precancerous morphological changes.5, 6 Further, HPV based screening has been significantly superior in detecting CIN3 lesions in randomized controlled trials and in a huge Californian pilot project and also in reducing mortality from cervical cancer in an Indian trial.7, 8, 9
Despite progress, cervical cancer remains an important worldwide public health problem with ∼530,000 new cases and 265,000 deaths each year.10 Much of this failure relates to the challenging logistic requirements for cytology based screening programs, including the need for substantial infrastructure and skilled professionals.
Turkey implemented a population based cervical screening program using the Pap smear in 2004. However, organized population screening achieved annual coverage rates of only 1–2%, much too low to be effective, although there was also a substantial amount of opportunistic screening.10, 11, 12, 13, 14, 15 The main logistical reasons for this situation were lack of coordinating infrastructure, quality assurance and insufficient laboratory cytopathology capability.11, 12, 13, 14, 15, 16, 17
Some of these limitations can be minimized by moving to HPV testing as standalone primary screening, since the test can be automated and interpretation is objective, thus not requiring subjective and tedious reading of thousands of cytology slides by cytopathologists with some percent of additional pathology verification.18, 19, 20
In 2014, Turkey redesigned the screening program including a revamped local call and recall strategy and a centralized and fully automatized monitoring of individual screening status, with HPV tests as the primary screening tool, well defined national algorithms—including extended screening intervals and referral protocols, a single nationwide centralized diagnostics laboratory, and a sustainable agreement with the diagnostics industry. The system allows for fully traceable, real time monitoring of visits and specimens by the centralized screening program.21 This article reports initial result for the first 1 million women screened.
Material and Methods
Women aged between 30 and 65 years (∼16 million) are invited for HPV based screening by primary level health staff (family physicians and so called KETEM screening centers) every five years. All screening processes are free of charge for the eligible individuals.
Turkey's primary health system contains 24.000 family physicians. Family physicians were trained by Ministry of Health for pap‐smear sampling and intra‐uterine device insertion before the beginning of the project. This included 5 days' theoretical training together with hands‐on practice. In addition to this, family physicians have ongoing web based training modules and on‐site trainings for all the health services they provide (including cancer screening). They have to attend to these trainings legally to be licensed by Ministry.
Population records are kept nationally by a standardized software system. This software brings the eligible target population for invitation and screening automatically to each family physician. A survey of the individuals' contact details database indicated that 83.7% of the individuals' telephone numbers and 88.7% of individuals' addresses are recorded correctly.22 Family physicians invite women via either e‐mail/telephone/face‐to‐face interviews/brochures or letter invitation according to local practice. In case of no response, a new invitation is resent annually and if no response after five years' women are recorded as “rejected screening.” The invitation process data were not recorded by centrally but are available in family physician records.
HPV DNA specimen collection kits (Qiagen HC2) are sent from one of two central laboratories in Ankara or Istanbul to the local health authorities of each province (81 in total) who distribute them to the family physicians. Two samples are taken from each woman to enable cytology testing in those found to be HPV positive without the need for a separate visit. The first sample is collected with a brush and transferred to a glass slide for conventional cytology. The second is taken with a different brush and put into 5 ml of Standard Transport Medium for HPV DNA analysis. For women who are HPV positive by Hybrid Capture2 (Qiagen), genotyping is performed with the CLART kit (Genomica). Infra‐structure and workflow of the National HPV Laboratory can be viewed at http://www.youtube.com/watch?v=IBmAflRjI10. All specimen assays were performed in two laboratories serving the entire country. Fully automated operational procedures are in place to trace specimens and deliver results on line. The laboratory capacity is an estimated 9.400 samples per day with a permanent staff of 16 people.
The Cancer Control Department of Turkish Ministry of Health is responsible for the quality assurance and monitorization of the program. There is a well‐defined standard operating procedure including some essential check‐points. These check points are both for HPV DNA analysis and for the evaluation of pap smears. They are
Sampling adequacy: Inadequate sampling taken by the family physicians is monitored by 4 pathologists in a central laboratory. Conventional cytology samples are evaluated in double blind manner at least by 2 pathologists in at least 20% of samples.
Sampling Reports: Pap‐smears of 10% of HPV positive and NILM cases are evaluated by the same 4 pathologists. The four pathologists working in the laboratories are also evaluating each other's report. For this evaluation, about 10% of slides, which were reported as normal (NILM) by one pathologist are reviewed by the other one to improve interobserver consistency and to provide quality control targeting >90% consistency in NILM reports.
HPV DNA analysis: Two control systems are used internal and external. For internal control, one negative and one positive samplings are used for each 88 patients' plate. For external quality control; re‐evaluation is done in cooperation with UK National External Quality Assessment Service (UK NEQAS) at least two times a year, using 10 external samples for validation.
HPV positive women with abnormal cytology or who are HPV 16 or 18 positive are referred for colposcopy, which is performed free of charge in a post‐screening diagnostic center, at least one of which is provided in each province of Turkey. Since 2010, Ministry of Health together with Turkish Society of Colposcopy and Cervical Pathologies is organizing basic and advanced colposcopy courses 2–3 times in a year for the gynecologists who are responsible for colposcopy referral centers.
All the screening data are registered in a web‐based central national health information system since the beginning of the project to avoid data loss. However, postscreening data (colposcopy and final pathology results) were collected regionally (from the secondary and tertiary health care centers) by the cancer registry staff since the registries were not linked to screening until 2016. Therefore, data on histologic findings is currently incomplete and only available data (35%) is reported here. When compared with each other; these two groups (patients with final follow up data vs. patients with lost to follow up) were almost similar with respect to age. However, a great majority of lost to follow‐up patients was from Istanbul and Aegean Regions (p < 0.005) where the use of private clinic is common. The Ministry of Health, Cancer Control Department has 81 cancer registries in all 81 provinces of the country, achieving 100% coverage by year 2013 for cancer but linkage with screen detected CIN has only just begun. All registries are linked to screening units recently within 2016 and since then ministry of health started to collect data for CIN 1, 2 and 3. The ministry of health is planning to collect the remaining pathology data (65%) including that for histology performed in private clinics by 2018 and biopsy/treatments results will be reported when full data is available (Fig. 1).
Figure 1.
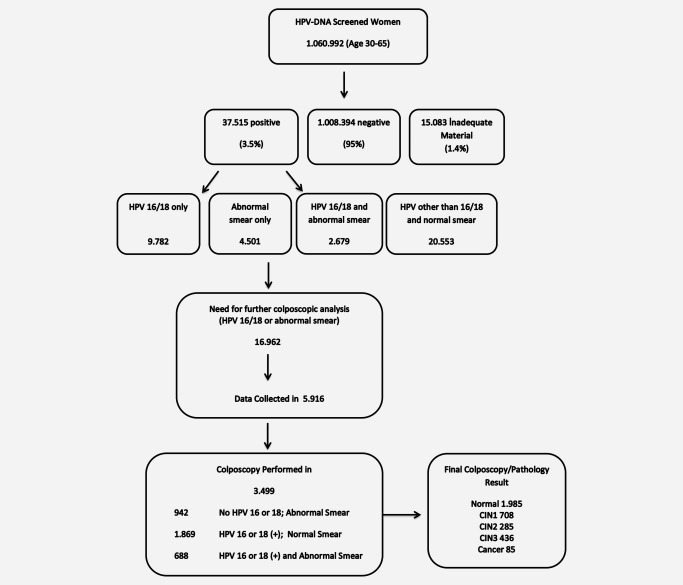
Study flow chart.
Results
Based on surveys of General Practitioners there was ∼36.5% acceptance rate for HPV based cervical cancer screening after first invitations.22 This rate was 63.5% for ages 30–45 years, 32.7% for ages 45–60 years and 13.5% for ages 60 years and older. Attendance rate among those who accepted the first invitation was 82.8%. The most common invitation method was by telephone including SMS (60% of the invitations), followed by face‐to‐face invitations (30% of invitations). These had a higher acceptance rate (about 80–90% for telephone). For the remaining 10% of women, especially in highly populated provinces which has a higher incidence of young and working population, letters, leaflets, brochures or social media were used for invitations, but the acceptance rates were lowest for these methods, being about 30–40%.22
For the cytology based program on average 25,000 women were screened per month since 2004. After implementation of HPV based testing, these numbers have increased three times in the first 14 months (averaging 63,893 per month) and four to five folds in the next years of the project (Fig. 2). For the first 1 million screened women (n = 1,060,992) from August 2103 to October 2014, the mean age was 45.6 years. Assuming 20% of 5 year‐target population (16 million) was invited, the participation rates were 28.0, 33.8, 41.6, 34.5, 32.4, 23.3 and 15.2% of annual target population for ages 30–34, 35–39, 40–44, 45–49, 50–54, 55–59 and 60–65, respectively. The highest rates were seen in the Middle‐ and North‐Eastern Anatolia regions.
Figure 2.
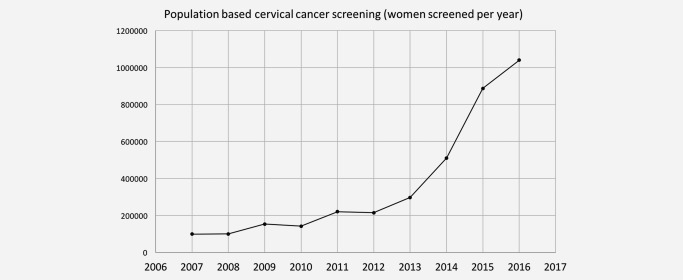
Annual screening numbers in the national screening program. HPV based screening began in 2014.
Overall 95.1% of the samples were HPV negative, 1.4% were inadequate, and the remaining 3.5% (n = 37,515) were HPV DNA positive (Table 1). Among those with inadequate sampling, 0.94% did not provide a valid HC2 result and the remainder were due to leakage during the transport process. HPV positivity rate by age groups was 4.3% (30–34), 4.0% (35–39), 3.6% (40–44), 3.2% (45–59) and 2.8% (60–65), respectively. The highest positivity rates were seen in Istanbul and Mediterranean regions. The commonest HPV genotypes were 16, followed by 51, 31, 52 and 18 (Table 1, Fig. 3).
Table 1.
HPV genotypes among 37.515 HC2 positive cases (50,064 different types) by age groups (n, %)
| Total | 30–44 | 45–54 | 55–65 | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Case | % | Case | % | Case | % | Case | % |
| HPV16 | 10,373 | 20.7 | 6,172 | 22.3 | 2,794 | 19.3 | 1,407 | 17.9 |
| HPV18 | 2,561 | 5.1 | 1,470 | 5.3 | 732 | 5.1 | 359 | 4.6 |
| HPV31 | 4,357 | 8.7 | 2,408 | 8.7 | 1,271 | 8.8 | 678 | 8.6 |
| HPV33 | 1,064 | 2.1 | 542 | 2.0 | 313 | 2.2 | 209 | 2.7 |
| HPV35 | 2,298 | 4.6 | 1,265 | 4.6 | 655 | 4.5 | 378 | 4.8 |
| HPV39 | 2,774 | 5.5 | 1,642 | 5.9 | 777 | 5.4 | 355 | 4.5 |
| HPV45 | 1,603 | 3.2 | 947 | 3.4 | 430 | 3.0 | 226 | 2.9 |
| HPV51 | 5,420 | 10.8 | 2,994 | 10.8 | 1,537 | 10.6 | 889 | 11.3 |
| HPV52 | 3,547 | 7.1 | 1,943 | 7.0 | 1,015 | 7.0 | 589 | 7.5 |
| HPV56 | 2,838 | 5.7 | 1,419 | 5.1 | 887 | 6.1 | 532 | 6.8 |
| HPV58 | 2,536 | 5.1 | 1,250 | 4.5 | 764 | 5.3 | 522 | 6.6 |
| HPV59 | 2,096 | 4.2 | 1,132 | 4.1 | 624 | 4.3 | 340 | 4.3 |
| HPV68 | 2,307 | 4.6 | 1,223 | 4.4 | 706 | 4.9 | 378 | 4.8 |
| HPV73 | 4 | 0.0 | 3 | 0.0 | 1 | 0.0 | 0 | 0.0 |
| Other | 6,286 | 12.6 | 3,326 | 12.0 | 1,965 | 13.6 | 995 | 12.7 |
| Total | 50,064 | 100 | 27,736 | 100 | 14,471 | 100 | 7,857 | 100 |
Figure 3.
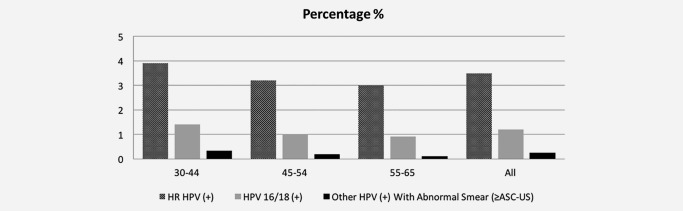
Percentage of HPV positivity among different age groups (excluding inadequate materials, all vs. 16/18 vs. others with abnormal cytology).
Among the 37,515 HPV positive women, cytology results were normal or infection only (including koilocytosis) in 66.7%; inadequate in 14.1% and abnormal in 19.1% (N = 7179). Of these the cytology findings were ASC‐US in 2290 (6.1%), LSIL in 4354 (11.6%), ASC‐H in 75 (0.2%), HSIL in 310 (0.8%), AGC in 150 (0.4%) and others (including carcinoma) in 55 (0.1%) women.
Among HPV positive cases, 16,962 cases had HPV 16 or 18 or other oncogenic HPV types with abnormal cytology (≥ASC‐US; Fig. 2). According to the national guidelines, these patients were referred to colposcopy centers for further evaluation by colposcopy [colposcopy referral rate of 1.6%, (16.962/1.060.992)]. However, clinico‐pathological data of 5.916 (35%) patients could be collected from cancer registry centers and colposcopy was performed to 3.499 of these cases (59.1%) and remaining 2.417 patients were not managed by national algorithms (repeat cytology, repeat HPV Testing, simple follow up, directly surgery etc.; Fig. 1). Among patients who were evaluated by an initial colposcopy in accordance with national guidelines (n = 3.499), 3.190 (91.2%) had undergone a diagnostic procedure under colposcopy (punch, local electrosurgical excision procedure‐LEEP, endocervical curettege‐ECC, conization, etc.) while 309 (8.8%) had only a colposcopy. Final colposcopic/pathological results of these 3.499 patients were normal in 1.985 patients, CIN1 in 708, CIN2 in 285, CIN3 in 436 and cancer in 85 patients. Of these cancers, 31 were microinvasive cancers while 54 was invasive cancer (8 adenocarcinoma, 45 squamous cancers and 1 adeno‐squamous cancer). For patients directed for colposcopy, risk of having a CIN3+ lesion was 14.9%; while normal results were seen in 57.0%.
Among 521 ≥ CIN3 lesions, cytological results were NILM in 24 (4.6%), insufficient in 58 (11.1%), ASC‐US in 74 (14.2%), LSIL in 116 (22.3%), HSIL in 41 (7.9%), ASC‐H in 5 (1%), AGC in 14 (2.7%), adenocarcinoma in situ in 1 (0.2%) and infection without malignancy in 188 (36.2%). Therefore, excluding the insufficient samples, only pap‐smear program could miss 45.9% of ≥CIN3 cases. Similarly, evaluating the HPV genotypes among those 521 ≥CIN3 cases revealed that a screening without a reflex cytology (based only on HPV 16 or 18 positivity for colposcopy referral) could miss 12.7% of ≥CIN3 cases.
Discussion
HPV DNA testing is currently recommended for primary screening by many professional societies and official agencies such as European Union, IARC and WHO.18, 23 Many countries now convert their system into HPV DNA screening such as Netherlands, Australia and Norway. This is the first report in the world using HPV DNA test for screening over millions of ladies in a middle income country. It has both scientific and organizational advantages, and in Turkey the screening rates have increased 5–6‐fold compared with the previous cytology based program, largely due to better use of limited man‐power by using automated central testing and avoiding short term re‐sampling and frequent hospital revisits which occur with cytology. Turkey has a mostly Muslim population living in a range of environments including both the metropolis of Istanbul and sparsely populated provincial areas. The majority of these patients accepted HPV screening after either face‐to‐face or telephone invitations. Centralized testing facilitated cost control and provides a good model for similar countries. Only four pathologists were needed to read all cytology slides of HPV Positive women and the results were available on the internet within 10 days. The low HPV DNA positivity rate (3.5%) facilitated the use of HPV as the primary test and this is relevant for other countries with low HPV prevalence rates. HPV positivity was highest among ages 30–34 and decreasing with advancing ages. Positivity rate by age groups was 4.3% (30–34), 4.0% (35–39), 3.6% (40–44), 3.2% (45–59) and 2.8% (60–65), respectively.
The role of liquid based cytology (LBC) vs. conventional cytology has been thoroughly debated. In the beginning, we used using conventional cytology. According to legal requirements there have to be at least four companies whose kits meet the mentioned criteria prepared to tender. At the beginning, there were just two companies who had an LBC product (Hologic and BD). Therefore, our advisory board has decided to allow either conventional or LBC. A 5% cost was allowed for companies who offered LBC.
However, including LBC tubes to the system may have disadvantages such as; increasing the cost of instrumentation and increasing the possible cost of disposal. If we assume that 95% of the LBC samples will be disposed arised by having Negative HPV‐DNA results; this may cause unnecessary consumption and cost simultaneously. Due to this, Ministry of Health plans to make a cost efficiency analysis before the next tender to give a final decision.
Another debate may be the role of extensive HPV genotyping, instead of just HPV 16 and 18 genotyping although HPV 16–18 genotyping already seems to be sufficient for triage of the patients to colposcopy together with reflex cytology results. The main reason for performing such extended genotyping results from not clinical necessity, it totally depends on the need of epidemiologic mapping, which comes out of Turkey's geographical and population‐based features such as having heterogeneous distribution of the population and bridging eastern countries to the west. The detailed data per different ages and regions is planned to be used for shaping our future policies on HPV prevention, vaccination and screening either nationally or regionally.
Another interesting finding was the difference in most commonly seen HPV types. Even if the HPV DNA is lower in prevalence, genotype distributions showed characteristics of a bridge between Asia, Africa and Europe having number one HPV type of each continent in top five HPV genotypes of Turkey.24 The most common cytological abnormality was LSIL as expected followed by ASC‐US, HSIL and ASC‐H. Among patients with cytological abnormalities, HPV 16 was the most commonly identified genotype, followed by HPV 51, 31, 52 and 18.
Unfortunately, 65% of the final pathology results of the screened females is lacking and the ministry of health is planning to collect these missing data including that for histology performed in private clinics by 2018. Furthermore, a great majority of the positive cases were evaluated without colposcopy, which is actually out of the scope of national algorithms. In addition to this, excisional procedures had been performed more than expected (91.2%) among the patients who were managed by colposcopy which may reflect poor quality in colposcopic evaluations. This liberal biopsy decision may be attributable to some reasons such as; inadequate experience of the gynecologists during the first year of the project, fear of possible malpractice and lower fertility effect concerns due to screening age interval (>30 women). When 65% lost to follow up rates in colposcopy, referrals‐missing data and inadequate quality of performed colposcopies are taken into consideration we noticed that as Turkey we should now focus on follow‐up organization of the screened ladies.
Apart from these facts, our evaluation shows that the existing data is sufficient to make an extrapolation and evaluate the efficacy of the HPV DNA screening for Turkey. Current algorithm seems to be fair in keeping the balance between unnecessary colposcopies (false positives) and detection of preinvasive lesions. Colposcopy referral was only 1.6%. Furthermore, evaluation of 521 ≥CIN3 cases revealed a detection rate of 48.2% for Pap‐Smear, 87.3% for HPV Plus Genotyping for 16 and 18. It is important to mention that; the given CIN3 case rate is minimal when lost to follow up cases and further CIN3+ cases, which arise out of persistent high risk HPV are taken into account.
In conclusion, this program has demonstrated the feasibility of an HPV based screening program in a developing country with a large population in varied geographic conditions in a more effective way compared to conventional cytology. This may open new perspectives for similar countries with low screening rates due to organizational problems.
Acknowledgement
The authors express their gratitude for Turkish Society of Gynecological Oncology (Dr. Ali Ayhan and Dr. Macit Arvas), Turkish Society of Cervical Pathologies and Colposcopy (Dr. Kunter Yuce and Dr. Nejat Ozgul), Society for the Clinical Microbiologists of Turkey (Dr. Selim Badur, Dr. Ahmet Pinar and Dr. Gulendam Bozdayi), Turkish Society of Cytopathology (Dr. Pinar Firat and Dr. Binnur Onal), Federation of Turkish Pathology Societies (Dr. Alp Usubutun), Federation of Family Physicians Societies (AHEF), Turkish Academicians (Dr. Faruk Kose, Dr. Polat Dursun, and Dr. M. Coskun Salman) as national advisory board, WHO‐IARC (Rengaswamy Sankaranarayanan and Dr. Parta Basu), Dr. Xavier Bosch, Dr. Marc Arbyn, Dr. Jack Cuzick and Dr. Vesna Kesic as international advisory board for all the efforts used in preparation, implementation and monetarization of this project.
Conflict of Interest: Nothing to report
References
- 1. Walboomers JM, Jacobs MV, Manos M, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- 2. Harper DM, DeMars LR. HPV vaccines ‐ a review of the first decade. Gynecol Oncol 2017;146:196–204. [DOI] [PubMed] [Google Scholar]
- 3. Petry KU, Cox JT, Johnson K, et al. Evaluating HPV‐negative CIN2+ in the ATHENA trial. Int J Cancer 2016;138:2932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30:F88–99. [DOI] [PubMed] [Google Scholar]
- 5. Broutet N, Eckert LO, Ullrich A, et al., eds. Comprehensive Cervical Cancer Control: A Guide to Essential Practice, 2nd edn Geneva: World Health Organization Publications, 2014. 408 p. [PubMed] [Google Scholar]
- 6. Catarino R, Petignat P, Dongui G, et al. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J Clin Oncol 2015;6:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet 2014;383:524–32. [DOI] [PubMed] [Google Scholar]
- 8. Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360:1385–94. [DOI] [PubMed] [Google Scholar]
- 10. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 11. Açikgöz A, Ergör G. Cervical cancer risk levels in Turkey and compliance to the national cervical cancer screening standard. Asian Pac J Cancer Prev. 2011;12:923–7. [PubMed] [Google Scholar]
- 12. Sevil S, Kevser O, Aleattin U, et al. The frequency of having pap‐smear tests among women between 15–64 years old and the evaluation of the level of their knowledge. J Pak Med Assoc 2013;63:873–7. [PubMed] [Google Scholar]
- 13. Daloglu FT, Karakaya YA, Balta H, et al. Cervical cytological screening results of 8,495 cases in Turkey–common inflammation but infrequent epithelial cell abnormalities? Asian Pac J Cancer Prev 2014;15:5127–31. [DOI] [PubMed] [Google Scholar]
- 14. Mehmetoglu HC, Sadikoglu G, Ozcakir A, et al. Pap smear screening in the primary health care setting: a study from Turkey. N Am J Med Sci 2010;2:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demirhindi H, Nazlican E, Akbaba M. Cervical cancer screening in Turkey: a community‐based experience after 60 years of pap smear usage. Asian Pac J Cancer Prev 2012;13:6497–500. [DOI] [PubMed] [Google Scholar]
- 16. Tuncer M, eds. National Cancer Control Program 2009–2015, 1st edn Ankara: Turkish Ministry of Health Publication; No 760, 2009. 108 p. [Google Scholar]
- 17. Keskinkılıc B, Gultekin M, Akarca AS, et al., eds. Turkey Cancer Control Program. Ankara: Turkish Ministry of Health Publication, 2016. 90 p. [Google Scholar]
- 18. Anttila A, Arbyn M, Vuyst de H, et al., eds. European Guidelines for Quality Assurance in Cervical Cancer Screening, 2nd edn, Supplements. Luxembourg: European Union Publications, 2015. 194 p. [Google Scholar]
- 19. Wright TC, Jr , Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid‐based cytology: insights from the ATHENA trial. Int J Cancer 2014;134:1835–43. [DOI] [PubMed] [Google Scholar]
- 20. Mayrand MH, Duarte‐Franco E, Coutlée F, et al. Randomized controlled trial of human papillomavirus testing versus Pap cytology in the primary screening for cervical cancer precursors: design, methods and preliminary accrual results of the Canadian cervical cancer screening trial (CCCaST). Int J Cancer 2006;119:615–23. [DOI] [PubMed] [Google Scholar]
- 21.Turkish Public Health. Available at: http://kanser.gov.tr/Dosya/tarama/serviks.pdf. Accessed on December 15, 2017.
- 22.AHEF. Available at: http://www.ahef.org.tr/Haber/1917/KANSER-TARAMALARINDA-UYGULAMA-ANKETI.aspx. Accessed on December 15, 2017.
- 23. Huh WK, Ault KA, Chelmow D, et al. Use of primary high‐risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol 2015;136:178–82. [DOI] [PubMed] [Google Scholar]
- 24. Bruni L, Diaz M, Castellsagué X, et al. Cervical human papillomavirus prevalence in 5 continents: meta‐analysis of 1 million women with normal cytological findings. J Infect Dis 2010;202:1789–99. [DOI] [PubMed] [Google Scholar]


