Abstract
Aims
To study myocardial substrate uptake, structure and function, before and after bariatric surgery, to clarify the interaction between myocardial metabolism and cardiac remodelling in morbid obesity.
Methods
We studied 46 obese patients (age 44 ± 10 years, body mass index [BMI] 42 ± 4 kg/m2), including 18 with type 2 diabetes (T2D) before and 6 months after bariatric surgery and 25 healthy age‐matched control group subjects. Myocardial fasting free fatty acid uptake (MFAU) and insulin‐stimulated myocardial glucose uptake (MGU) were measured using positron‐emission tomography. Myocardial structure and function, and myocardial triglyceride content (MTGC) and intrathoracic fat were measured using magnetic resonance imaging and magnetic resonance spectroscopy.
Results
The morbidly obese study participants, with or without T2D, had cardiac hypertrophy, impaired myocardial function and substrate metabolism compared with the control group. Surgery led to marked weight reduction and remission of T2D in most of the participants. Postoperatively, myocardial function and structure improved and myocardial substrate metabolism normalized. Intrathoracic fat, but not MTGC, was reduced. Before surgery, BMI and MFAU correlated with left ventricular hypertrophy, and BMI, age and intrathoracic fat mass were the main variables associated with cardiac function. The improvement in whole‐body insulin sensitivity correlated positively with the increase in MGU and the decrease in MFAU.
Conclusions
In the present study, obesity and age, rather than myocardial substrate uptake, were the causes of cardiac remodelling in morbidly obese patients with or without T2D. Cardiac remodelling and impaired myocardial substrate metabolism are reversible after surgically induced weight loss and amelioration of T2D.
Keywords: cardiac function, cardiac structure, myocardial free fatty acid uptake, myocardial glucose uptake, myocardial triglyceride content, positron emission tomography, type 2 diabetes
1. INTRODUCTION
In a normal situation, free fatty acids (FFAs) are the main nutrient for the heart, and there is a balance in the uptake of FFAs for energy, via β‐oxidation, and to synthesize triglycerides. In obesity, myocardial FFA uptake (MFAU) and oxidation are elevated. The increased shift in myocardial FFA oxidation contributes to the increment in oxygen consumption, which further impairs myocardial efficiency and increases myocardial work.1, 2, 3, 4, 5 In obese rodents, the increased myocardial FFA oxidation was further shown to downshift myocardial glucose metabolism by diminishing glucose oxidation.6 In humans, myocardial insulin resistance (decreased insulin‐stimulated myocardial glucose uptake [MGU]) has been mainly found in type 2 diabetes (T2D) with or without coronary artery disease,7, 8, 9, 10, 11 but whether myocardial insulin resistance already exists in obese people is unclear. In our previous study in obese people, a very‐low‐calorie diet decreased MFAU and myocardial work but had no effect on insulin‐stimulated MGU despite a marked improvement in whole‐body and muscle insulin sensitivity.1 Thus it might be that insulin resistance in the myocardium develops in a later stage of obesity or diabetes development compared with other tissues and/or that it is associated with impaired cardiac function.
Obesity and the imbalance between the myocardial substrate uptake and oxidation result in increased myocardial adiposity, both as enlarged intrathoracic fat (epi‐ and pericardial fat) surrounding the heart and as increased myocardial triglyceride content (MTGC). Intrathoracic fat mass increases along with obesity and causes mechanical stress to the heart.12 Although intrathoracic fat is assumed to be more active in FFA metabolism than peripheral fat, direct measurements of FFA metabolism in this adipose tissue in humans in vivo are not available. While intrathoracic fat increases in proportion to obesity, accumulation of MTGC is mainly associated with insulin resistance.6, 13 Abnormal MTGC accumulation has been suggested to cause myocardial oxidative damage and interfere with myocardial contractile function leading to left ventricle (LV) hypertrophy.14, 15
In morbid obesity, bariatric surgery leads to rapid weight loss and amelioration of T2D. Bariatric surgery efficiently improves cardiac outcomes, reduces LV mass, improves diastolic function and decreases cardiac ectopic fat16, 17, 18, 19; however, it is not known whether bariatric surgery also enhances myocardial substrate metabolism, and if changes in cardiac energy metabolism are related to the improvements in cardiac structure and function.
We aimed to investigate whether morbid obesity, with or without T2D, leads to disturbed myocardial metabolism and if myocardial metabolic impairments are related to cardiac structural and functional remodelling. In addition, we aimed to investigate whether bariatric surgery‐induced rapid weight loss reversed the cardiac abnormalities found before surgery.
2. METHODS
2.1. Participants
The participant recruitment process has been described previously.20, 21 Briefly, morbidly obese participants were recruited from people undergoing bariatric surgery as part of their normal treatment at the Hospital District of Southwest Finland. The surgical inclusion criteria were age 18‐60 years and body mass index (BMI) ≥40 kg/m2 (or ≥35 kg/m2 with an additional obesity‐related comorbidity). Subjects with severe mental disorders, eating disorders or insulin‐treated T2D were excluded.
A total of 52 participants (5 men and 47 women) and 25 age‐matched healthy controls (2 men and 23 women), were recruited and included in the baseline studies. Three participants did not proceed to the surgery and another three withdrew from the study after the bariatric surgery for personal reasons. Of the 46 obese participants, 18 had T2D (T2D group) and in those without T2D (nT2D group), out of 28 participants, 11 had impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).22 Medication details are given Table S1 in File S1. Written informed consent was obtained prior to the studies. The protocols (NCT00793143/SLEEVEPASS, NCT01373892/SLEEVEPET) were approved by the local Ethics Committee.
2.2. Study design
At the screening, medical history was taken, and physical examination, anthropometric, blood and oral glucose tolerance tests were performed (Figure 1). Myocardial function, structure and MTCG, intrathoracic and intra‐abdominal fat mass were assessed with magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS). MGU was measured during euglycaemic‐hyperinsulinaemic clamp using 2‐[18F]fluoro‐2‐deoxy‐D‐glucose ([18F]FDG)‐positron‐emission tomography (PET) from 25 obese participants and 10 controls, and fasting MFAU and pericardial FFA uptake (PFAU) was measured using 14(R,S)‐[18F]fluoro‐6‐thia‐heptadecanoic acid ([18F]FTHA)‐PET from 27 obese participants and 15 controls. Thereafter, obese participants followed a very‐low‐calorie diet (800 kcal/d) for 1 month before the bariatric surgery. Of the morbidly obese participants, 22 underwent sleeve gastrectomy and 27 underwent Roux‐en‐Y gastric bypass surgery. Three participants did not proceed to the operation. The postoperational phase was conducted 6 months after surgery. Of the 49 operated participants, 3 withdrew from the postoperational studies for personal reasons.
Figure 1.
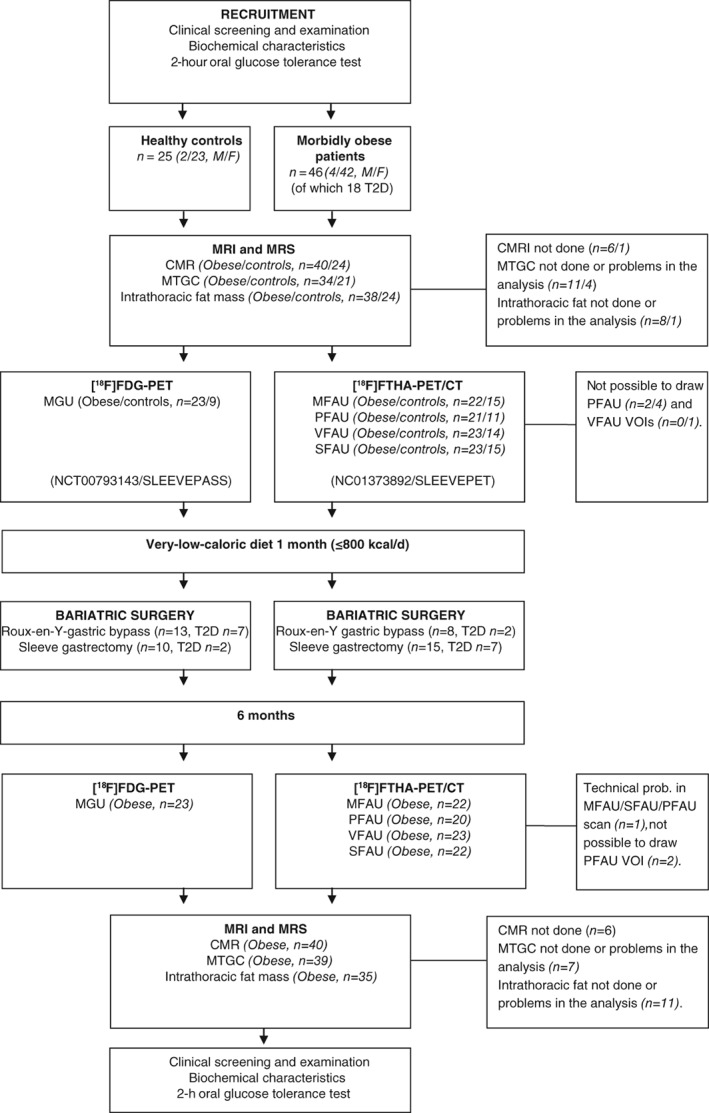
Study design. MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; CMRI, cardiac magnetic resonance imaging; MTGC, myocardial triglyceride content; PET, positron‐emission tomography; [18F]FDG, 2‐[18F]flouro‐2‐deoxy‐D‐glucose; MGU, myocardial glucose uptake; [18F]FTHA,14(R,S)‐[18F]fluoro‐6‐thia‐heptadecanoic acid; CT, computed tomography; MFAU, myocardial free fatty acid uptake; PFAU, pericardial fat free fatty acid uptake; VFAU, visceral adipose tissue free fatty acid uptake; SFAU, subcutaneous adipose tissue free fatty acid uptake; VOI, volumes of interest
2.3. PET studies
All PET studies were conducted after an overnight fast. Participants were instructed to refrain from caffeine‐containing nutrients and strenuous physical activity for 12 and 24 h and anti‐diabetic medication for 24‐72 h prior to the studies. Detailed information is provided in the Supporting Information.
2.4. Magnetic resonance imaging and magnetic resonance spectroscopy
For myocardial structure and function and intrathoracic and abdominal fat measurements, MRI was used. MTGC was determined using MRS. Details are reported in the Supporting Information.
2.5. Oral glucose tolerance test and euglycaemic‐hyperinsulinaemic clamp
An oral glucose tolerance test was performed to calculate Matsuda index23 and homeostatic model assessment of insulin resistance.24 A euglycaemic‐hyperinsulinaemic clamp was performed after a 10‐h fast.25 The insulin‐stimulated whole‐body glucose uptake (M‐value) was calculated from the glucose infusion rate and the glucose values were collected during the PET scan.
2.6. Biochemical and immunological analyses
Plasma glucose was measured in the Turku PET Centre in duplicate using the glucose oxidase method (Analox GM7 or GM9, Analox Instruments Ltd, London, UK). Other laboratory samples were sent to Turku University Hospital Central Laboratory. Glycated haemoglobin (HbA1c) was determined by HPLC (Variant II; Bio‐Rad, Hercules, California), serum insulin by time‐resolved immunofluorometric assay (AutoDELFIA, PerkinElmer Life and Analytical Sciences, Waltham, Massachusetts) and serum high‐sensitivity C‐reactive protein with the sandwich immunoassay method using an Innotrac Aio1 immunoanalyser (Innotrac Diagnostics, Turku, Finland).
2.7. Statistical analyses
Differences among the control, nT2D and T2D groups were analysed using one‐way ANOVA. Changes over time and differences between the nT2D and T2D groups and surgery methods were tested using repeated‐measures ANOVA. Tukey–Kramer's test was used to adjust the P values of pairwise comparisons. Normality of the residuals was checked and logarithmic transformation was used when necessary. Missing data were excluded from the analyses. Results are expressed using model‐based means with 95% confidence intervals. For the variables which were transformed for analyses, model‐based means were transformed back to the original scale. Correlations were calculated using Pearson's correlation (Spearman's rank for non‐normally distributed variables). Additional multiple linear regression analyses were performed to study which variables were associated with cardiac function. Analyses were performed to four response variables (LV ejection fraction, LV stroke volume, LV cardiac output and cardiac work). All of the models included BMI, age and Matsuda Index, and MFAU, MGU, MTGC and intrathoracic fat mass were added to the model separately and the models were compared using P values and adjusted R2 values. Two‐sided P values <.05 were taken to indicate statistical significance. Analyses were performed using sas System for Windows, Version 9.4 (SAS Institute Inc, Cary, North Carolina).
3. RESULTS
3.1. Marked weight reduction and remission of T2D after the bariatric surgery
Before surgery, the obese participants had impaired insulin sensitivity and increased circulating lipid levels as well as systemic inflammation compared with the healthy controls (Table 1). Among the obese participants, those in the T2D group were older, had increased systemic inflammation and tended to have higher waist‐to‐hip ratio, with no differences in whole‐body weight or abdominal fat mass compared with participants in the nT2D group. Participants in the T2D group had higher fasting glucose and HbA1c levels but no difference was found in whole‐body insulin sensitivity (M‐value or Matsuda index) between the nT2D and T2D groups. Also no differences were found in blood pressure.
Table 1.
Clinical and metabolic characteristics of the control and obese groups at baseline and 6 months after the bariatric surgery
| Baseline | Baseline | P | After surgery | After surgery | P | Time | Time6Group | ||
|---|---|---|---|---|---|---|---|---|---|
| Control | Obese nT2D | Obese T2D | nT2D vs T2D | Obese nT2D | Obese T2D | nT2D vs T2D | |||
| Age, years | 45 (42‐50) | 42 (38‐45) | 49 (45‐53) | 0.02 | |||||
| Height, cm | 168 | 166 | 167 | 0.74 | |||||
| Weight, kga | 64.4 (61.5‐67.4) | 117.3 (112.6‐122.3)4 | 114.4 (108.6‐120.6)4 | 0.74 | 89.5 (85.0‐94.1)4 | 88.6 (83.2‐94.4)4 | 0.97 | <0.001 | 0.76 |
| BMI, kg/m2a | 22.9 (22.0‐23.8) | 42.6 (41.2‐44.1)4 | 41.0 (39.2‐42.9)4 | 0.36 | 32.6 (31.2‐34.1)4 | 31.7 (30.0‐33.6)4 | 0.74 | <0.001 | 0.82 |
| BSA, m‐2a | 1.7 (1.7‐1.8) | 2.2 (2.2‐2.3)4 | 2.2 (2.1‐2.3)4 | 0.97 | 2.0 (1.9‐2.0)4 | 2.0 (1.9‐2.0)4 | 0.99 | <0.001 | 0.78 |
| WHR | 0.80 (0.76‐0.83) | 0.88 (0.85‐0.91)4 | 0.94 (0.90‐0.98)4 | 0.06 | 0.87 (0.84‐0.90)5 | 0.90 (0.87‐0.94)4 | 0.40 | 0.003 | 0.053 |
| Body fat, %a | 31.8 (28.7‐34.6) | 50.6 (48.9‐52.2)4 | 48.4 (46.1‐50.5)4 | 0.25 | 43.4 (41.6‐45.2)4 | 41.7 (39.3‐44.0)4 | 0.48 | <0.001 | 0.50 |
| SAT, kga | 4.2 (3.8‐4.8) | 18.6 (16.9‐20.5)4 | 16.0 (14.1‐18.2)4 | 0.17 | 11.8 (10.4‐13.4)4 | 10.1 (8.5‐12.0)4 | 0.31 | <0.001 | 0.21 |
| VAT, kg | 0.9 (0.7‐1.2) | 3.4 (2.8‐4.1)4 | 4.0 (3.1‐5.1)4 | 0.58 | 1.8 (1.4‐2.2)5 | 2.4 (1.8‐3.3)4 | 0.20 | <0.001 | 0.46 |
| HR, bpm | 66 (62‐70) | 69 (65‐72) | 67 (63‐72) | 0.86 | 62 (58‐66) | 61 (56‐66) | 0.95 | 0.001 | 0.68 |
| RR systole, mm Hg | 129 (122‐135) | 127 (121‐134) | 138 (131‐146) | 0.06 | 126 (121‐131) | 129 (122‐135) | 0.79 | 0.016 | 0.06 |
| RR diastole, mm Hg | 81 (77‐84) | 85 (81‐88) | 87 (83‐91) | 0.64 | 80 (76‐83) | 81 (77‐86) | 0.87 | 0.002 | 0.70 |
| Laboratory values | |||||||||
| Cholesterol, mmol/L | 4.7 (4.3‐5.0) | 4.3 (4.0‐4.6) | 4.3 (3.9‐4.6) | 0.97 | 4.1 (3.8‐4.4)6 | 4.3 (4.0‐4.7) | 0.54 | 0.60 | 0.36 |
| HDL, mmol/L | 1.9 (1.7‐2.0) | 1.3 (1.2‐1.4)4 | 1.2 (1.0‐1.3)4 | 0.33 | 1.5 (1.4‐1.6)4 | 1.4 (1.2‐1.5)4 | 0.43 | <0.001 | 0.53 |
| LDL, mmol/L | 2.5 (2.2‐2.8) | 2.5 (2.2‐2.7) | 2.4 (2.1‐2.8) | 0.95 | 2.2 (2.0‐2.5) | 2.4 (2.1‐2.8) | 0.48 | 0.22 | 0.29 |
| Triglyceride, mmol/La | 0.66 (0.57‐0.76) | 1.07 (0.94‐1.224 | 1.41 (1.18‐1.68)4 | 0.65 | 0.85 (0.74‐0.98)6 | 1.08 (0.91‐1.28)4 | 0.09 | <0.001 | 0.52 |
| FFA, mmol/La | 0.45 (0.39‐0.52) | 0.62 (0.54‐0.71)5 | 0.63 (0.63‐0.75)6 | 0.99 | 0.52 (0.48‐0.66) | 0.65 (0.49‐0.72) | 0.98 | 0.47 | 0.78 |
| Glucose, mmol/La | 5.33 (5.08‐5.59) | 5.65 (5.41‐5.90) | 7.20 (6.82‐7.61)4 | <0.001 | 5.14 (4.93‐5.35) | 5.68 (5.40‐5.97) | 0.007 | <0.001 | <0.001 |
| Insulin, mU/La | 4.7 (3.6‐6.1) | 11.1 (8.8‐14.1)4 | 17.3 (12.7‐23.4)4 | 0.07 | 5.6 (4.5‐7.1) | 8.1 (6.1‐10.6)6 | 0.12 | <0.001 | 0.39 |
| HbA1c, % | 5.64 (5.46‐5.81) | 5.62 (5.46‐5.78) | 6.44 (6.24‐6.65)4 | <0.001 | 5.38 (5.30‐5.51) | 5.73 (5.57‐5.88) | 0.002 | <0.001 | <0.001 |
| HOMA‐IRa | 1.1 (0.8‐1.5) | 2.8 (2.1‐3.6)4 | 5.5 (3.9‐7.8)4 | 0.007 | 1.3 (1.0‐1.7) | 2.1 (1.5‐2.9)5 | 0.07 | <0.001 | 0.25 |
| Matsuda indexa | 14.2 (10.1‐20.0) | 5.8 (4.3‐7.9)4 | 3.8 (2.6‐5.6)4 | 0.21 | 11.6 (8.2‐16.3) | 8.5 (5.5‐13.2) | 0.52 | <0.001 | 0.73 |
| M‐value, μmol/min/kg | 40.3 (35.8‐44.8) | 14.2 (10.3‐18.0)4 | 10.1 (5.3‐14.9)4 | 0.38 | 25.3 (20.7‐29.9)4 | 20.2 (14.0‐25.9)4 | 0.35 | <0.001 | 0.76 |
| Cytokines | |||||||||
| CRP, mg/La | 0.59 (0.41‐0.85) | 3.40 (2.41‐4.81)4 | 2.69 (1.71‐4.24)4 | 0.69 | 1.03 (0.73‐1.47)(6) | 1.01 (0.65‐1.56) | 0.99 | <0.001 | 0.56 |
| TNFα, pg/mLa | 3.44 (2.68‐4.41) | 4.35 (3.47‐5.46) | 6.30 (4.74‐8.37)5 | 0.11 | 4.27 (3.40‐5.38) | 6.14 (4.62‐8.18)5 | 0.13 | 0.41 | 0.65 |
| MCP‐1, pg/mL | 235 (179‐292) | 249 (199‐299) | 382 (319‐446)5 | 0.005 | 264 (219‐309) | 339 (282‐396)6 | 0.11 | 0.44 | 0.02 |
| IL‐6, pg/mL | 1.87 (1.32‐2.66) | 2.76 (2.10‐3.63) | 2.67 (1.94‐3.68) | 0.99 | 1.64 (1.24‐2.18) | 2.26 (1.57‐3.25) | 0.36 | 0.009 | 0.17 |
| IL‐8, pg/mL | 4.42 (3.82‐5.12) | 4.70 (4.11‐5.37) | 7.70 (6.51‐9.11)4 | <0.001 | 5.38 (4.49‐6.46) | 7.10 (5.68‐8.88)5 | 0.14 | 0.58 | 0.04 |
BMI, body mass index; BSA, body surface area; CRP, C‐reactive protein; FFA, free fatty acids; HbA1c, glycated haemoglobin; HOMA‐IR, homeostatic model assessment of insulin resistance; HR, heart rate; IL, interleukin 6; MCP‐1, monocyte chemoattractant protein‐1; M‐value, insulin‐stimulated whole‐body glucose uptake; RR, blood pressure; SAT, abdominal subcutaneous adipose tissue mass; T2D, type 2 diabetes; nT2D, without T2D; TNFα, tumour necrosis factor α; WHR, waist‐to‐hip ratio; VAT, visceral adipose tissue mass.
Data are mean and 95% confidence intervals. Statistically significant values are bolded.
***P < .001. **P < .01. *P < .05.
Non‐normally distributed variable.
Surgery led to marked reductions in whole‐body weight (−23%), abdominal subcutaneous and visceral adipose tissue and insulin resistance in both obese groups, although the participants remained obese after surgery (Table 1). After surgery, diabetes remission occurred in 67% of the T2D group.
3.2. Bariatric surgery leads to decreases in intrathoracic fat mass but not in MTGC
Compared with controls, obese participants had 56% more intrathoracic fat and markedly elevated whole‐LV MTGC (Figures 2A,B). We found no differences between obese participants in the nT2D and those in the T2D group with regard to MTGC or intrathoracic fat mass (Table S1 in File S1). Postoperationally, intrathoracic fat mass decreased but did not reach the control level. No change was found in MTGC. Correlations are shown in Table S3a in File S1.
Figure 2.
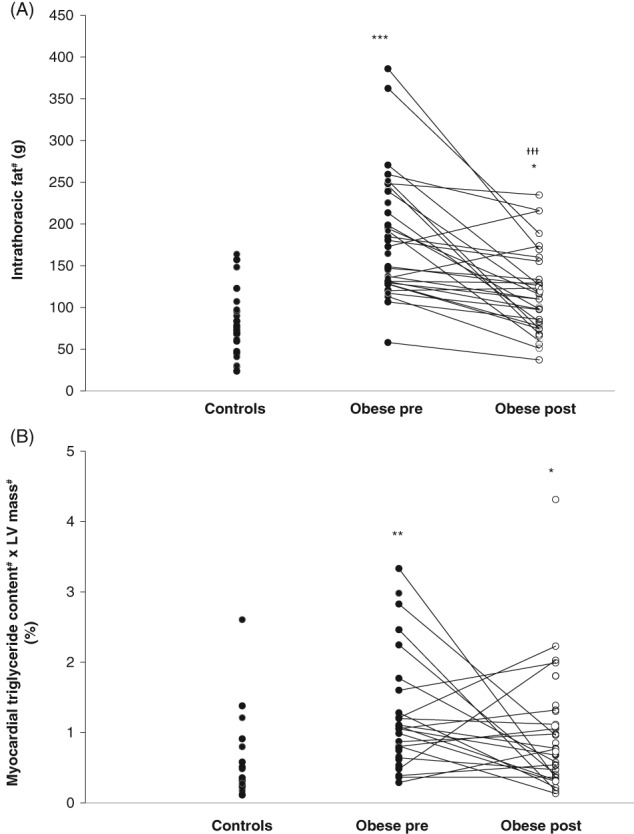
A, Intrathoracic fat mass and B, whole‐left ventricle (LV) myocardial triglyceride content (MTGC) in controls and all obese participants. *P < .05, **P < .01, ***P < .001 vs controls and †††P < .001 pre‐ vs post‐surgery. #Non‐normally distributed variable
3.3. Normalization in MFAU, PFAU and myocardial insulin resistance
Before surgery, fasting MFAU was higher in obese participants in the T2D but not in the nT2D group compared with controls, yet no difference was observed between the two obese groups (Figure 3A). Preoperatively, the nT2D group had, and the T2D group tended to have, higher fasting FFA levels measured during PET scanning compared with controls (Table S2 in File S1). Postoperatively, MFAU decreased in both obese groups without any group differences and normalized to control level. This change was not related to FFA availability, as FFA levels remained elevated postoperatively and fractional myocardial FFA uptake (MFAUKi) decreased in both obese groups without any differences between the groups (nT2D: 0.079 (0.064‐0.095) vs 0.066 (0.054‐0.078) and T2D: 0.090 (0.071‐0.109) vs 0.063 (0.049‐0.077); pre‐ vs post‐surgery P = .045). Correlations are shown in Table S3a in File S1.
Figure 3.
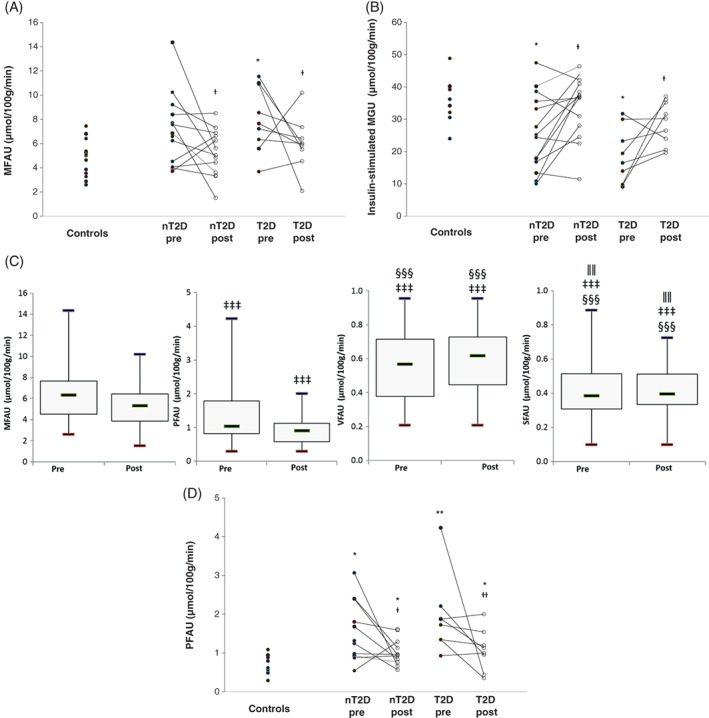
Myocardial (MFAU) (A) and pericardial fat free fatty acid (FFA) uptake (PFAU) (D) at fast and myocardial insulin‐stimulated glucose uptake (MGU) (B) in control and obese participants with type 2 diabetes (T2D) or without T2D (nT2D) before and after bariatric surgery. (C) Fasting free fatty acid (FFA) uptake (FAU) in myocardium, pericardial fat and in abdominal subcutaneous (SFAU) and visceral adipose tissue (VFAU) in all obese participants before and after bariatric surgery. Figures 3A, B and D: *P < .01, **P < .01, ***P < .001 vs controls and †pre‐ vs post‐surgery. Figure 3C, ‡vs MFAU, §vs PFAU and ǁvs VFAU at the corresponding time point. #Non‐normally distributed variable
Both obese groups had impaired insulin‐stimulated MGU before surgery compared with controls (Figure 3B). After surgery, MGU improved similarly in both obese groups and normalized to the control level. Preoperatively, FFA concentrations during the MGU scan were higher in the T2D group (P = .038) compared with the controls and tended to be higher in the T2D compared with the nT2D group (P = .072; Table S2 in File S1). The FFA concentration correlated inversely with MGU in the whole study group (rP = −0.54, P < .001). Post‐surgery, FFA levels during the clamp normalized to control values. Correlations are shown in Table S3a in File S1.
The rate of PFAU was lower than MFAU, but higher than visceral (VFAU) or abdominal subcutaneous adipose tissue FFA uptake (SFAU) (all P < .001; Figure 3C). At baseline, PFAU was higher in obese participants compared with controls, with no differences within the two obese groups (Figure 3D). The difference persisted also after accounting for myocardial fractional uptake of [18F]FTHA as a covariate (P = .014; data not shown). PFAU decreased after surgery, but remained higher in participants with T2D and tended to be higher in the nT2D group compared with the control group. We found no change in VFAU or SFAU (Table S2 in File S1). No differences were observed between surgical procedures in any of the PET, MRI or MRS measurements.
3.4. Reduction in LV mass and improvements in myocardial function
In absolute terms, LV mass was greater in obese compared with control participants at baseline and reduced after the operation, and was still higher in obese participants compared with controls after surgery (Table 2). We found no differences in LV mass between the obese nT2D and T2D groups at baseline or after surgery. Pre‐surgery, LV and right ventricle (RV) volumes were greater in the nT2D group in end‐diastole and end‐systole and in the T2D group in end‐diastole compared with controls. We detected no changes in LV and RV volumes after surgery in the obese groups. No differences were found between obese participants in the nT2D and T2D groups in any of the cardiac structure measures. Correlations are shown in Table S3b in File S1.
Table 2.
Cardiac structure and function in the control group and obese groups at baseline and 6 months after the bariatric surgery
| Baseline | Baseline | P | After surgery | After surgery | P | Time | Time15Group | ||
|---|---|---|---|---|---|---|---|---|---|
| Control | Obese nT2D | Obese T2D | nT2D vs T2D | Obese nT2D | Obese T2D | nT2D vs T2D | |||
| Function | |||||||||
| EF, % | |||||||||
| LV | 66.8 (65.1‐68.6) | 68.1 (66.5‐69.6) | 70.2 (68.2‐72.3) | 0.22 | 66.7 (65.0‐68.4) | 67.3 (65.1‐69.5) | 0.92 | 0.013 | 0.39 |
| RV | 57.2 (55.0‐59.3) | 57.8 (55.8‐59.8) | 58.4 (56.5‐60.2) | 0.63 | 58.7 (56.5‐60.2) | 58.7 (56.3‐61.1) | 0.97 | 0.73 | 0.65 |
| SV, mL | |||||||||
| LV | 89.5 (82.6‐96.5) | 117.8 (111.4‐124.3)13 | 114.4 (105.9‐122.9)13 | 0.80 | 111.9 (104.5‐119.3)13 | 105.1 (95.5‐114.7)15 | 0.50 | 0.028 | 0.48 |
| RV | 80.3 (72.7‐87.9) | 107.3 (100.9‐113.6)13 | 96.4 (79.7‐113.1)13 | 0.70 | 109.3 (101.9‐116.7)13 | 100.3 (90.7‐19.9)14 | 0.31 | 0.06 | 0.37 |
| CO, mL/min | |||||||||
| LV | 5.2 (4.7‐5.7) | 6.9 (6.4‐7.4)13 | 6.6 (6.0‐7.2)13 | 0.50 | 6.1 (5.7‐6.6)15 | 5.9 (5.0‐6.2) | 0.34 | <0.001 | 0.18 |
| RV | 4.7 (4.3‐5.1) | 5.4 (5.1‐5.8)14 | 4.6 (3.7‐5.5)15 | 0.81 | 5.6 (5.2‐6.0)15 | 4.9 (4.4‐5.4) | 0.13 | <0.001 | 0.13 |
| LVwork, mm Hg /L/min | |||||||||
| 509 (446‐571) | 675 (614‐737) 14 | 696 (620‐771)13 | 0.91 | 575 (520‐629) | 555 (486‐625) | 0.90 | <0.001 | 0.15 | |
| LVwork per gram of tissue (mm Hg/ L/min/g) | |||||||||
| 6.46 (5.83‐7.09) | 6.21 (5.59‐6.83) | 6.48 (5.72‐7.24) | 0.85 | 5.91 (5.34‐6.48) | 5.49 (4.77‐6.21) | 0.63 | 0.002 | 0.038 | |
| Structure | |||||||||
| ED volume, mL | |||||||||
| LV | 134.0 (123.6‐144.3) | 173.3 (163.7‐182.9)13 | 163.8 (151.1‐176.5)14 | 0.46 | 167.4 (157.1‐177.8)13 | 156.1 (142.7‐169.5)15 | 0.38 | 0.13 | 0.55 |
| RV | 140.4 (129.9‐150.9) | 175.4 (165.6‐185.1)13 | 165.8 (152.9‐178.7)14 | 0.47 | 174.3 (163.9‐184.7)13 | 158.3 (144.9‐171.7) | 0.15 | 0.23 | 0.17 |
| ES volume, mL | |||||||||
| LVa | 43.5 (40.0‐47.4) | 52.9 (49.3‐56.7)14 | 51.7 (42.9‐62.3) | 0.15 | 54.6 (50.3‐59.3)13 | 49.7 (44.7‐55.2) | 0.33 | 0.39 | 0.93 |
| RV | 60.1 (54.3‐65.8) | 74.1 (68.8‐79.5)14 | 67.6 (60.5‐74.7) | 0.32 | 72.8 (67.6‐78.0)14 | 65.1 (58.4‐71.8) | 0.18 | 0.23 | 0.42 |
| LVmass, ga | 78.3 (72.4‐84.7) | 106.7 (99.2‐114.8)13 | 106.4 (96.6‐117.2)13 | 1.00 | 95.9 (88.5‐103.9)14 | 99.6 (89.8‐110.4)14 | 0.83 | <0.001 | 0.48 |
| LVmass/height, g/m | 51.0 (33.4‐68.7) | 74.6 (65.6‐83.5)14 | 79.8 (56.1‐103.4)15 | 1.00 | 73.6 (63.1‐84.2) | 77.9 (64.2‐91.5)15 | 0.88 | <0.001 | 0.77 |
| LVmass/BSA, g/m2a | 45.6 (42.6‐48.7) | 48.5 (45.6‐51.6) | 48.6 (44.8‐52.8) | 1.00 | 46.0 (42.7‐49.6) | 47.6 (43.2‐52.4) | 0.76 | 0.07 | 0.60 |
BSA, body surface area; CO, cardiac output; ED, end‐diastolic; EF, ejection fraction; ES, end‐systolic; LV, left ventricle; RV, right ventricle; SV, stroke volume; T2D, type 2 diabetes; nT2D, without T2D.
LVwork, stroke volume × heart rate × (diastolic blood pressure + 1/3 × [systolic − diastolic blood pressure]).
LVwork per gram of tissue = LVwork/LVmass.
Data are mean and 95% confidence intervals. Statistically significant values are bolded.
***P < .001. **P < .01. *P < .05 compared with controls.
Non‐normally distributed variable.
Obese participants had higher LV and RV stroke volume, cardiac output and higher absolute LV work compared with controls (Table 2). Surgery decreased ejection fraction and stroke volume in the LV and cardiac output in the LV and RV, and LV work. When LV work was normalized to LV mass, the change differed between the two obese groups and the LV work decreased only in the T2D group (P = .001). No differences were found between surgical procedures in any of the cardiac structure or function measures. Based on multiple linear regression analyses BMI, age and intrathoracic fat mass were the main variables linked to cardiac function (Table S4 in File S1). Correlations are shown in Table S3c in File S1.
4. DISCUSSION
In the present study, we showed that morbid obesity, with or without T2D, leads to impaired myocardial metabolism, increased MFAU and MTGC accumulation, as well as myocardial insulin resistance. The novel finding is that myocardial insulin resistance exists in morbidly obese people already without T2D. Before surgery, morbidly obese participants had LV and RV hypertrophy, abnormal LV and RV function and higher absolute LV work compared with healthy controls. Bariatric surgery led to marked weight loss and amelioration of T2D in most of the participants. MFAU and insulin resistance were normalized and cardiac structure and function started to return towards control values 6 months post‐surgery. We also show, for the first time in humans, that FFA uptake in pericardial fat is higher compared with abdominal subcutaneous and visceral fat tissues and the uptake rate decreases after bariatric surgery.
4.1. Myocardial substrate metabolism
We found decreased MGU in both obese groups, whereas MFAU was increased in obese participants in the T2D group, but not in the nT2D group before surgery compared with the control group. Increased MFAU has been shown to relate to the increased FFA supply, but findings are contradictory.1, 2, 26 In the present study, participants in the nT2D group had, and participants in the T2D group tended to have, increased ambient FFA levels compared with controls, suggesting that FFA levels do not entirely explain the elevation in MFAU in people with T2D.
In addition to increased FFA supply, impairments in FFA transport and mitochondrial function have been shown to deteriorate myocardial lipid metabolism.27 In the context of our data this could mean that, because of impaired mitochondrial oxidative capacity in cardiomyocytes, the myocardium in people with T2D needs to take up more FFAs to generate the same amount of energy as the myocardium in people without T2D. The increase in MFAU in the present study may also be related to the permanent relocation of CD36, the main FFA transporter in the heart, on the sarcolemma,6, 28, 29 as well as increased levels of peroxisome proliferator‐activated receptor‐α and/or its coactivator PGC1 activation or increased carnitine palmitoyltransferase enzyme levels augmenting fatty acid oxidation.30, 31
Postoperatively, MFAU decreased to the control level in both obese groups, without differences in the decrease between the groups. We did not find any decrease in fasting FFA concentrations in the obese groups after surgery, which might have explained the decrease in MFAU, probably because the myocardium was still losing weight and in a catabolic state 6 months after surgery. Although there was no change in peripheral FFA supply, it is possible that there was a reduction in FFA supply from the adjacent epicardial fat. Epicardial fat shares the same blood supply as the myocardium, is highly lipolytic and sustains fatty acid homeostasis in coronary microcirculation.32 As intrathoracic fat mass decreased after surgery, it may be that reduced epicardial fat FFA supply plays a role in the found decrease in MFAU. Our results further suggest that the detected decrease in MFAU is dependent on an intrinsic cardiac mechanism leading to reduced extraction of fatty acids, as confirmed by the consensual reduction observed in MFAUKi (fractional extraction of FFA), probably reflecting changes in the aforementioned cell level mechanisms.
The decrease in MFAU post‐surgery could also be attributable to the increased utilization of intramyocardial triglycerides. Baseline MTGC in the whole LV mass were higher in obese participants than in controls. After surgery, although there was a marked reduction in intrathoracic and abdominal fat masses, we found no change in absolute MTGC per unit mass or per whole LV mass, and no correlation between MTGC and MFAU; therefore, our data do not support the hypothesis that the use of intramyocardial triglycerides could explain the change in MFAU. Even though our negative result is in agreement with the post‐bariatric surgery results of Gaborit et al.,18 the absence of MTGC decline after surgery remains unexpected because MTGC has been shown to decrease after diet and exercise with smaller weight reduction.1, 33, 34 Potential explanations are the lack of change in FFA levels, or the fact that patients undergoing bariatric surgery in Finland need to lose 5% of weight to show motivation before surgery, which might affect baseline MTGC levels.
Before surgery, insulin‐stimulated MGU was decreased in both obese groups compared with the controls. To our knowledge, this is the first evidence that morbid obesity, in the absence of T2D, is characterized by myocardial insulin resistance. In the whole group, ambient FFA levels during the [18F]FDG‐PET scan correlated inversely with MGU, consistent with the existing knowledge of substrate competition. Although MGU did not differ between the nT2D and T2D groups before surgery, it was positively correlated with whole‐body insulin sensitivity and was more severe in proportion to glucose intolerance progression (Figure 3B). The T2D group also had higher ambient FFA levels during MGU measurements compared with the control (P < .04) and nT2D (P = .07) groups, indicating worse suppression of lipolysis during hyperinsulinaemia (Table S2 in File S1). After operation, insulin‐suppressed FFA levels were improved and no longer different from controls, and MGU was enhanced and normalized to control levels in both obese groups.
Both impaired MGU and increased MFAU were associated with increased BMI and especially MGU with abdominal obesity, but interestingly, only MFAU correlated with the accumulation of intrathoracic fat and MGU inversely with MTGC before surgery. Furthermore, we found an association between the improvement in whole‐body insulin sensitivity and the improvement in MGU and decrease in MFAU; thus, our results suggest that in morbidly obese people the increased MTGC with whole‐body adiposity and decreased whole‐body insulin sensitivity predict myocardial insulin resistance. We have previously reported no change in MGU, regardless of a marked improvement in whole‐body and muscle insulin sensitivity, after a short‐term very‐low‐calorie diet; in that study weight loss was less pronounced and MTGC reduction not significant. It may be that more marked and/or longer‐term changes are required to sustain a measurable MGU elevation.
In the present study, we chose to group obese participants into an nT2D (with and without IFG/IGT) and a T2D group using the American Diabetes Association criteria22 to be consistent and allow comparability with our other publications with this same study population21, 35; however, we also re‐analysed the main data in obese participants with normal glucose tolerance (nT2D) vs obese participants with either IFG, IGT or T2D (Supporting Information). Based on this grouping, before the surgery, MFAU was increased and MGU decreased in the T2D but not in the nT2D group compared with controls, suggesting that in “healthy obesity” myocardial substrate metabolism is normal. The grouping had no significant effect on myocardial structure and function results.
4.2. Cardiac structure and function
Before surgery, the obese groups had enlarged cardiac volumes and LV mass. When LV mass was corrected for body surface area, the differences in LV mass between the obese and control groups disappeared. Both obese groups had also increased stroke volume, cardiac output and myocardial work compared with the controls, but no difference was measured in ejection fraction, as found previously.36 Despite the differences, all cardiac parameters were within the normal range. Bariatric surgery reduced LV mass but did not alter chamber dimensions, in agreement with previous results.17 Also stroke volume, cardiac output, LV ejection fraction and myocardial work decreased after surgery.
We did not find differences between the obese nT2D and T2D groups in cardiac structure or function before surgery, possibly because of the short (<3 years) history of T2D. In both obese groups, cardiac work was increased and cardiac volumes enlarged as normally observed in obesity. After surgery the LV work index improved in both groups.
We found a positive correlation between MFAU and LV mass before surgery, suggesting that the elevation in FFA utilization may progressively strain the heart, as suggested previously.1, 3 We found no correlation between MGU and cardiac structure or function. Using multiple linear regression analyses, we found that BMI, age and intrathoracic fat mass were the main variables associated with cardiac function in our study population.
4.3. Pericardial fat FFA uptake
To our knowledge, this is the first study in humans in which FFA uptake was quantitated non‐invasively in pericardial fat surrounding the heart. It is known that pericardial and epicardial fat tissues are different anatomically and biochemically37; however, it is unclear whether pericardial fat is distinct from visceral fat and whether pericardial fat has an independent pathogenic role. Our novel data show that PFAU is markedly lower compared with MFAU but higher than VFAU or SFAU. We also found that PFAU was higher in obese participants compared with controls before surgery; however, it was not different between participants in the nT2D and T2D groups. After surgery PFAU, but not VFAU or SFAU, decreased in both obese groups and was still higher in T2D compared with controls. Pericardial fat is located in the close vicinity of the myocardium and thus it is possible that PFAU may have been affected by a spill‐over effect (cross‐contamination of activity from the nearby myocardium), leading to an overestimation. To discount this potential confounder, we repeated the analyses of PFAU also after accounting for MFAUKi and we obtained similar results. [18F]FDG‐PET scans were performed with the PET scanner without computed tomography, thus it was not possible to localize pericardial fat and pericardial fat glucose uptake. Based on our results, pericardial fat differs from abdominal subcutaneous and visceral fat with more active lipid metabolism and susceptibility to weight‐loss‐induced metabolic changes.
4.4. Study limitations
The present study has some limitations. The [18F]FDG and [18F]FTHA‐PET scans were performed in two different subject populations. This was done to avoid too high radiation exposure for an individual. In addition, the post‐surgery studies were performed when participants were still losing weight and in a catabolic state, and the number of study participants was too small to reliably compare the two surgical techniques.
4.5. Conclusions
In this study, morbidly obese participants had impaired myocardial energy metabolism and cardiac remodelling without differences in the measured cardiac parameters between those with T2D and those without. Bariatric surgery‐induced weight loss and amelioration of T2D led to the normalization of myocardial insulin resistance and MFAU and improved myocardial function and work. Our results suggest that, in the present study, BMI, age and intrathoracic fat mass were more closely related to cardiac remodelling than myocardial substrate uptake in morbidly obese people. Importantly, our results show that cardiac remodelling and impaired myocardial substrate metabolism are reversible after marked weight loss. Our data also provide the first evidence that pericardial fat is more avid in FFA uptake and more responsive to weight loss than abdominal subcutaneous and visceral fat tissues, suggesting greater metabolic flexibility.
Supporting information
File S1. Supplementary Material.
ACKNOWLEDGMENTS
This study was conducted within the Centre of Excellence in Cardiovascular and Metabolic Diseases, supported by the Academy of Finland, the University of Turku, the Turku University Hospital, and Åbo Academy University. Additional financing was received from the Sigrid‐Juselius Foundation, Turku University Hospital, Finnish Diabetes Association, Finnish Cultural Foundation and Turku University Foundation. The authors thank the study nurse Mia Koutu and Behnoush Foroutan for their valuable work and the staff of Turku PET Centre for skillful assistance.
Conflict of interest
None declared.
Author contributions
J.C.H. was involved in the study subject recruitment, data acquisition, analysed the PET and MRI data and wrote the manuscript. J.P., M.S. and V.S. performed MRI data acquisition and analysis and edited the manuscript. S.H. was the responsible statistician and edited the manuscript. M.S. and K.A.V. were involved in subject recruitment, data acquisition and edited the manuscript. P.D. analysed the abdominal fat distribution and edited the manuscript. P.S. was involved in the study design, patient recruitment and surgical procedures and edited the manuscript. T.G. and S.F. were involved in the [18F]FDG and [18F]FTHA tracer production and analysis and edited the manuscript. R.L. and P.I. contributed to and edited the manuscript. P.N. is the principal investigator of this study and was involved in the project planning, analysis, and writing of the manuscript. P.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Parts of this study have been presented in an abstract form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 2011.
Hannukainen JC, Lautamäki R, Pärkkä J, et al. Reversibility of myocardial metabolism and remodelling in morbidly obese patients 6 months after bariatric surgery. Diabetes Obes Metab. 2018;20:963–973. https://doi.org/10.1111/dom.13183
Funding information Turun Yliopisto; Suomen Akatemia; Suomen Kulttuurirahasto; Diabetesliitto; Turku University Hospital; Sigrid Juséliuksen Säätiö
REFERENCES
- 1. Viljanen AP, Karmi A, Borra R, et al. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol. 2009;103:1721‐1726. [DOI] [PubMed] [Google Scholar]
- 2. Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191‐2196. [DOI] [PubMed] [Google Scholar]
- 3. Mather KJ, Hutchins GD, Perry K, et al. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16‐[18F]fluoro‐4‐thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab. 2016;310:E452‐E460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labbe SM, Noll C, Grenier‐Larouche T, et al. Improved cardiac function and dietary fatty acid metabolism after modest weight loss in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2014;306:E1388‐E1396. [DOI] [PubMed] [Google Scholar]
- 5. Lin CH, Kurup S, Herrero P, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring). 2011;19:1804‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341‐5349. [DOI] [PubMed] [Google Scholar]
- 7. Voipio‐Pulkki LM, Nuutila P, Knuuti MJ, et al. Heart and skeletal muscle glucose disposal in type 2 diabetic patients as determined by positron emission tomography. J Nucl Med. 1993;34:2064‐2067. [PubMed] [Google Scholar]
- 8. Yokoyama I, Yonekura K, Ohtake T, et al. Role of insulin resistance in heart and skeletal muscle F‐18 fluorodeoxyglucose uptake in patients with non‐insulin‐dependent diabetes mellitus. J Nucl Cardiol. 2000;7:242‐248. [DOI] [PubMed] [Google Scholar]
- 9. Ohtake T, Yokoyama I, Watanabe T, et al. Myocardial glucose metabolism in noninsulin‐dependent diabetes mellitus patients evaluated by FDG‐PET. J Nucl Med. 1995;36:456‐463. [PubMed] [Google Scholar]
- 10. Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282‐E290. [DOI] [PubMed] [Google Scholar]
- 11. Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020‐3024. [DOI] [PubMed] [Google Scholar]
- 12. Iacobellis G, Leonetti F, Singh N, Sharma M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272‐273. [DOI] [PubMed] [Google Scholar]
- 13. Iozzo P, Lautamäki R, Borra R, et al. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94(11):4472‐4482. [DOI] [PubMed] [Google Scholar]
- 14. Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417‐423. [DOI] [PubMed] [Google Scholar]
- 15. Levelt E, Mahmod M, Piechnik SK, et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. 2016;65:44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ippisch HM, Inge TH, Daniels SR, et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51:1342‐1348. [DOI] [PubMed] [Google Scholar]
- 17. Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763‐1777. [DOI] [PubMed] [Google Scholar]
- 18. Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381‐1389. [DOI] [PubMed] [Google Scholar]
- 19. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718‐726. [DOI] [PubMed] [Google Scholar]
- 20. Honka H, Koffert J, Hannukainen JC, et al. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab. 2015;100:2015‐2023. [DOI] [PubMed] [Google Scholar]
- 21. Dadson P, Landini L, Helmio M, et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292‐299. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care. 2010;33(Suppl 1):S62‐S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing ‐ comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 24. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 25. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214‐E223. [DOI] [PubMed] [Google Scholar]
- 26. Rijzewijk LJ, van der Meer RW, Lamb HJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524‐1532. [DOI] [PubMed] [Google Scholar]
- 27. Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554‐564. [DOI] [PubMed] [Google Scholar]
- 28. Aguer C, Mercier J, Man CY, et al. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 2010;53:1151‐1163. [DOI] [PubMed] [Google Scholar]
- 29. Coort SL, Hasselbaink DM, Koonen DP, et al. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes. 2004;53:1655‐1663. [DOI] [PubMed] [Google Scholar]
- 30. Coort SL, Bonen A, van der Vusse GJ, Glatz JF, Luiken JJ. Cardiac substrate uptake and metabolism in obesity and type‐2 diabetes: role of sarcolemmal substrate transporters. Mol Cell Biochem. 2007;299:5‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692‐1700. [DOI] [PubMed] [Google Scholar]
- 32. Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253‐261. [DOI] [PubMed] [Google Scholar]
- 33. Schrauwen‐Hinderling VB, Hesselink MK, Meex R, et al. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J Clin Endocrinol Metab. 2010;95:1932‐1938. [DOI] [PubMed] [Google Scholar]
- 34. Utz W, Engeli S, Haufe S, et al. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int J Cardiol. 2013;167:905‐909. [DOI] [PubMed] [Google Scholar]
- 35. Immonen H, Hannukainen JC, Iozzo P, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non‐diabetic patients. J Hepatol. 2014;60(2):377‐383. [DOI] [PubMed] [Google Scholar]
- 36. Di Bello V, Santini F, Di Cori A, et al. Effects of bariatric surgery on early myocardial alterations in adult severely obese subjects. Cardiology. 2008;109:241‐248. [DOI] [PubMed] [Google Scholar]
- 37. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907‐917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supplementary Material.


