Abstract
Recent observations indicate that the cross-sectional area (CSA) of vertebral bodies is on average 10% smaller in healthy newborn girls than in newborn boys, a striking difference that increases during infancy and puberty and is greatest by the time of sexual and skeletal maturity. The smaller CSA of female vertebrae is associated with greater spinal flexibility and could represent the human adaptation to fetal load in bipedal posture. Unfortunately, it also imparts a mechanical disadvantage that increases stress within the vertebrae for all physical activities. This review summarizes the potential endocrine, genetic, and environmental determinants of vertebral cross-sectional growth and current knowledge of the association between the small female vertebrae and greater risk for a broad array of spinal conditions across the lifespan.
The smaller female vertebra is associated with greater spinal flexibility and lesser vertebral strength, probably contributing to a broad array of spinal conditions across the lifespan.
Essential Points
Factors related to sex-based differences in fetal skeletal development
Newborn girls are born with smaller vertebral cross-sectional dimensions than newborn boys, which could represent the human adaptation to fetal load in bipedal posture
The greater spinal flexibility and lesser vertebral strength associated with smaller female vertebrae are likely determinants of the risk for spinal deformities in girls and vertebral fractures in older women
Among the main areas of progress in osteoporosis research is the recognition that this condition may have its antecedents in intrauterine development (1, 2). Support for the notion that osteoporosis may result from perturbations in the fetal programming of skeletal growth is based primarily on epidemiological data showing a relationship between low birth size and lower bone mass later in life (1–11). Interestingly, the association between birth weight and adult bone mass in a cohort of female twins appeared to be mediated by skeletal size (3). The importance of the fetal environment to skeletal development is further substantiated by reports that maternal smoking, nutrition, vitamin supplementation, and physical activity are linked to offspring’s bone mass (1, 12–19). However, whether birth weight predicts future fracture risk is the subject of interest and controversy (1, 8, 20–24).
Despite a broad agreement that the fetal environment shapes one’s future health, the newborn phenotypes linked to bone disease risk are unknown. As the field of imaging progresses, our understanding of fetal development is making important strides. Advances in magnetic resonance imaging (MRI) allow detailed measures of skeletal structure in healthy infants without the need for sedation, contrast, or radiation exposure (25–27). Using MRI, we recently found that newborn girls have smaller vertebrae than boys of the same length and weight (27), a striking sexual dimorphism that could represent the female adaptation to fetal load in bipedal posture and probably has major implications for women’s spinal health (27).
This review summarizes current knowledge on the differential effects of sex on human vertebral growth, spinal flexibility, and strength and their possible endocrine, genetic, and environmental determinants. It also highlights data supporting the notion that the greater prevalence of spinal deformities in girls and vertebral fractures in older women are related to small vertebrae and the downside of walking upright. Notably, although current research supports the presence of sex differences in vertebral cross-sectional area (CSA), knowledge of their clinical implications is limited, and the mechanisms for this sexual dimorphism are yet to be defined.
Bipedal Locomotion, Pregnancy, and the Evolution of the Female Spine
Erect posture and bipedal locomotion are defining features of humans associated with unique skeletal morphogenesis originating about 2 million years ago (28–36). Unlike in other primates, the bones of the lower extremities in humans evolved to be stronger and larger, with greater joint surface areas than those in the upper extremities (29). Longer legs allowed early hunter-gatherers greater stride length and increased speed and endurance for long-distance walking or running (29). The high percentage of slow-twitch muscle fibers necessary for endurance running may have derived in humans from a genetic mutation of the ACTN3 gene (37).
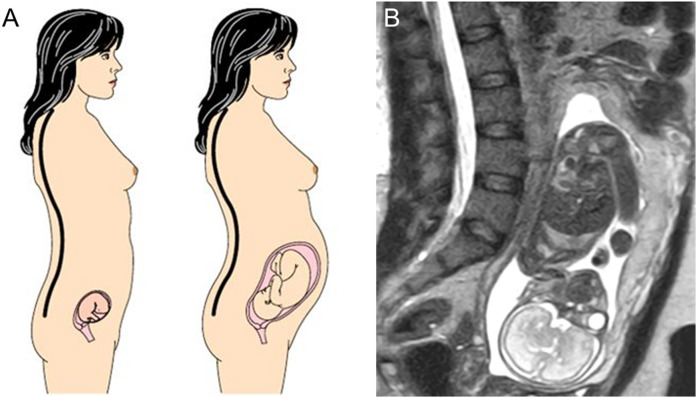
In humans, the axial skeleton also developed distinct morphological traits that allow the greater spinal mobility needed for orthograde bipedality (38–42), adaptations that are most relevant to the woman’s need to compromise between bipedal locomotion and pregnancy. During gestation, women must maintain bipedal posture and balance despite body weight increases of 15% to 25% (43–46). After 12 weeks of gestation, the uterus can no longer be contained within the pelvis and moves superiorly and anteriorly into the abdomen. To compensate for this bipedal obstetric load and counteract the shift in the center of mass, pregnant mothers habitually extend their spine and increase the degree of lumbar lordosis (LL) (Fig. 1) (42, 47, 48).
Figure 1.
(a) Schematic representation of increasing lumbar lordosis during pregnancy to compensate for fetal load and maintain a stable position of the center of mass (black and white circle with crosshairs). Adapted from (28). (b) MRI image of a 30-year-old woman at 25 weeks’ gestation showing marked lordosis. Figure 1a adapted with permission from Macmillan Publishers Ltd: Whitcome KA, Shapiro LJ, Lieberman DE. Fetal load and evolution of lumbar lordosis in bipedal hominins. Nature. 2007; 450:7172.
Relaxin, a peptide of the insulin-like growth factor (IGF) family secreted by the corpus luteum and placenta, exerts a regulatory effect on the musculoskeletal and other systems through binding to its receptor in various tissues (49–51). Serum levels of this hormone increase 10-fold during the first trimester, remaining high until late pregnancy, and then become serologically undetectable in the first few days postpartum (52). Relaxin alters the properties of cartilage and tendon by activating collagenase and facilitates the spine and pelvic flexibility necessary to accommodate the enlarging uterus. In the lumbar spine, joint laxity is most notable in the anterior and posterior longitudinal ligaments (35, 53–55). Both joint laxity and the degree of LL are less pronounced in nulliparous than multiparous women and are associated with the number of pregnancies (56, 57). The degree to which increased LL during pregnancy is the consequence of ligamentous laxity or an adaptive trait evolved by natural selection is unknown.
Although variations in lumbar vertebral morphogenesis, such as vertebral wedging and orientation of lumbar facets, have been suggested to facilitate LL (42, 45, 58), the structural basis for the greater LL in women compared with men has been poorly understood. Our limited understanding of the structural phenotypes linked to upright posture stems from a lack of animal models that can replicate human bipedalism. The mammalian vertebral column is highly variable, reflecting a wide range of adaptations to differences in lifestyles, from natural burrowers to flying bats. Even the axial skeleton of nonhuman apes lacks the lordosis and range of motion needed for the fully erect posture observed in modern humans (29, 42, 59). Although mice and rat models are useful in bone metabolism research, the growth plates of their long bones remain open throughout their lifespan, allowing continuous skeletal growth and modeling (60–62).
Sexual Dimorphism in Axial Skeletal Development
Animal investigations indicate that skeletal development begins in the embryo and is mediated by multiple signaling pathways, including Hox genes, Wnts, Hedgehogs, bone morphogenetic proteins, fibroblast growth factors, Notch/Delta, and others (63–66). Mesenchymal cells in chick and mice embryos are laid down in spatiotemporal patterns where bones of the axial and appendicular skeletons will form (67). In humans, most osteochondral progenitors become chondroblasts that initiate endochondral bone formation between the eighth and 12th week of gestation, but it is not until the third trimester that the majority of bone mineralization occurs (68, 69). Growth of the appendicular and axial skeletons results from two different processes, probably regulated by different means (70–73). In the extremities, growth in bone length occurs by endochondral bone formation at the growth plates, whereas increases in bone width occur by apposition of subperiosteal bone, a process that begins before birth and continues throughout life (73). In the vertebrae, growth occurs by endochondral ossification, which commences in the central area of the cartilage anlage and expands toward the periphery in all directions (71, 72).
The human skeleton exhibits noticeable sexual dimorphisms during intrauterine development. In the appendicular skeleton, these differences are related predominantly to the tempo and degree of skeletal maturation. Epiphyseal ossification is more advanced in girls than in boys during the third trimester of gestation, and a greater number of ossification centers are depicted in radiographs of preterm and full-term newborn girls than boys (74). Throughout childhood, the rate of skeletal maturation remains more advanced in girls, who on average achieve skeletal maturity and cessation of longitudinal growth 2 years earlier than boys (72, 75, 76).
Whereas the differences in appendicular skeletal maturation between girls and boys have been known for more than a century (77), we only recently learned that factors related to sex also program fetal vertebral development (27, 77). Previous studies examining sex differences in axial skeletal mass at birth used projection techniques that did not account for the confounding effect of vertebral size and yielded discrepant results. Although most found that sex had no effect on lumbar spine bone mass (78–81), one showed that boys had higher bone mass than girls but did not adjust for differences in body size or examine bone morphology (82). Based on three-dimensional assessments of skeletal structure, recent observations indicate that the CSAs of vertebral bodies are on average 10% smaller in healthy newborn girls than in newborn boys, a difference that was independent of gestational age, birth weight, body length, and vertebral height (Fig. 2) (27). In contrast, sex did not influence intrauterine cross-sectional growth of the appendicular skeleton, and neither the CSA nor the cortical bone area at the midshaft of the humerus differs between boys and girls (27). Because smaller vertebral cross-sectional dimensions render the female spine more flexible, a proposed explanation for the sexual dimorphism is that it improves maternal performance in posture and locomotion (27). Studies are needed to establish the generalizability of these sex differences in the cross-sectional dimensions of the axial but not appendicular skeletons in other newborn populations.
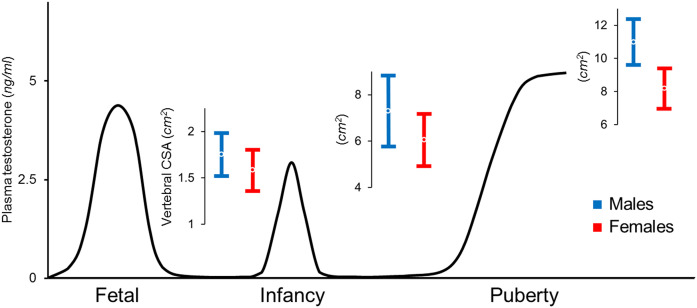
Figure 2.
Representation of plasma testosterone levels in the male fetus, infant, and adolescent (83), with overlying values for vertebral CSA (mean ± standard deviation) displaying increasing sex differences from birth to prepubertal and postpubertal stages. Female values are ~10% smaller at birth, 15% smaller during childhood, and 25% smaller at sexual maturity and young adulthood (27, 93, 134).
Although the mechanisms responsible for the smaller female vertebral body are unknown, it probably results from complex interactions involving sex steroids, growth hormone (GH), and IGF (84). Both insulin and IGF-2 are known regulators of fetal growth (85), but no investigations to date have revealed sexual dimorphism in these values. Estrogens and androgens also promote bone accretion; estrogens are particularly key for the regulation of epiphyseal function, whereas androgens promote periosteal new bone formation, which has a dramatic effect on the width of the bone (86–89). Although the absence of the SRY gene in girls may dictate the sexual dimorphism in vertebral development, the important downstream events warrant investigation. Careful evaluations of newborns with disorders of sex development, such as girls with congenital adrenal hyperplasia, could aid in deciphering the role of androgens in mediating the fetal development of the axial skeleton. Conversely, testicular feminization syndromes may demonstrate the opposite effect (90).
Sexual Dimorphism in Postnatal Axial Skeletal Development
Infancy
Infancy is a developmental stage associated with marked gains in bone and muscle. Remarkably, the sexual dimorphism in vertebral size further increases during infancy. On average, vertebral CSA in girls is 10% smaller than in boys at birth and 15% smaller at 6 months of age (27, 91), a discrepancy similar to that previously observed in prepubertal children (Fig. 2) (92, 93). This differential effect of sex on vertebral cross-sectional growth has been reported in children of European, Mexican, and African descent (92–94).
In the first few months of life, infants experience a transient activation of the hypothalamo-pituitary-gonadal axis and go through a “mini-puberty” (Fig. 2) (95–104). In male infants, testosterone concentrations can reach values that are similar to those found in men (95–97). After this postnatal surge, androgen production decreases to remain at prepubertal levels from 6 months of age until adolescence (96). In female infants, an increase in both estradiol and testosterone also occurs; however, the rise in testosterone is lower than that in boys (103, 104). The mini puberty affects the reproductive organs, causing penile and prostate growth in boys and breast enlargement in girls (90). Ultrasound measures of testicular, ovarian, and uterine volumes are also larger in the first 6 months of life than at 1 year of age (105–107). The effects of this infant gonadal activation are not confined to the reproductive organs. The greater rise in testosterone in the infant boy has been suggested to be responsible for his increased skeletal growth (108). Boys grow faster than girls during the first 6 months of life, and the greatest sex difference in growth velocity is observed concomitantly with the peak of postnatal gonadal activation (108). Old investigations in newborn male monkeys showed that blockage of the neonatal secretion of gonadotropins and testosterone results in diminished muscle mass and bone size in the axial and appendicular skeleton (109).
Infants experience remarkable skeletal development despite being largely motionless and spending most of the day asleep; this development is particularly notable in the axial skeleton (110–112). The maintenance of skeletal integrity in the presence of relative inactivity provides a strong case for an unidentified mechanism that regulates bone metabolism. Emerging data from animal investigations support a relationship between brown adipose tissue (BAT) and bone mass (113–121). Impairment in BAT function or ablation of beige fat in mice leads to development of low bone mass (118, 119). It has also been suggested that BAT influences skeletal metabolism by modulating the activity of the sympathetic nervous system (SNS) (113, 117, 118). In mice, uncoupling protein 1 (UCP1) activity exerts a protective effect on bone mass, possibly through alterations in central neuropeptide Y pathways known to regulate SNS activity (119, 122).
Additionally, a recent study found that a neutralizing antibody against follicle-stimulating hormone (FSH) increases cellular mitochondrial density, promotes brown/beige fat thermogenesis, and prevents muscle and bone loss in mice (121). Notably, human anatomical studies indicate that BAT is established in fetuses within the fifth month of gestation (123, 124), simultaneously with a decrease in fetal FSH levels (96). At the time of birth, BAT abundance peaks, as reflected by levels of UCP1, before declining concomitantly with a period of marked increases in FSH during the first 3 months of postnatal life (96). Because the greatest amounts of BAT are present at birth, followed by a period of marked bone accretion (91, 125, 126), the notion that BAT function would either directly or indirectly influence infant skeletal metabolism is a hypothesis in need of testing.
Adolescence
Variations in the accumulation and distribution of fat and muscle are important contributors to the different sexual characteristics of humans, which are already present before puberty. Compared with prepubertal boys, prepubertal girls have greater subcutaneous fat but less musculature, shorter legs, and smaller vertebral cross-sectional dimensions (93, 127). Increases in cortical thickness and CSA of the long bones in the lower extremities are driven primarily by mechanical stresses associated with increasing weight (128–132), consistent with analytic models suggesting that muscle activity related to the maintenance of upright position is a major determinant of cross-sectional bone growth. Although there are also strong correlations between weight and vertebral CSA in boys and girls, differences in weight and musculature account for a small part of the sex-related variations in vertebral CSA (93, 133). Therefore, factors other than mechanical stresses and related to sex have a role in the regulation of vertebral cross-sectional growth.
Throughout childhood, the CSA of the vertebral bodies in prepubertal girls is ~15% smaller than that in boys matched for age, height, and weight (92), a sexual dimorphism that further increases during puberty and is greatest at sexual and skeletal maturity, when the vertebral cross-sectional dimensions are ~25% smaller in women than in men, even after differences in body size are considered (Fig. 2) (27, 92, 93, 129, 134).
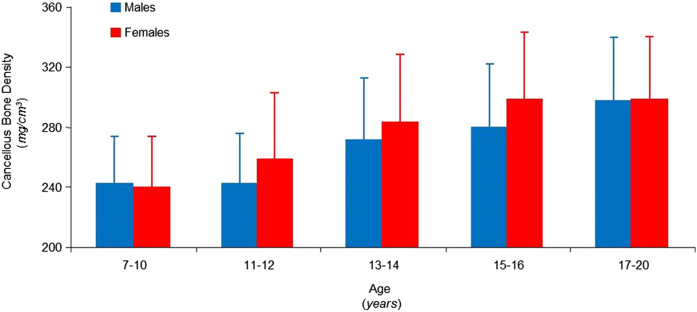
Puberty is the time of life when the greatest skeletal growth and bone accrual occur (135–141). The high activity of osteoblasts and osteoclasts during puberty is documented by the rise of bone formation and bone resorption markers in blood and urine (136, 138, 140, 142–146). Increases in both activities occur in the early stages of pubertal development in girls and reach their zenith in midpuberty. The concentration of bone metabolism markers decreases rapidly during the later stages of puberty. Conversely, the rise observed in boys is progressive and reaches its peak at later stages of pubertal development, showing a slow decrease thereafter (136, 142, 143, 146). These observations are in line with the different tempo of growth in boys and girls. Vertebral cancellous bone density does not differ in male and female subjects before the onset of puberty or by the end of the second decade of life (Fig. 3) (147). However, increases during sexual development begin and reach peak values earlier in girls than in boys (147–149). Interestingly, sex differences in bone accrual parallel differences in the rate of peak height velocities (147, 150). Peak growth velocity in boys typically is reached 2 to 3 years later, and boys continue growing for ~2 to 3 years longer than do girls (140, 150–153).
Figure 3.
Computed tomography measures of vertebral bone density in male (blue) and female (red) subjects, aged 7 to 20 years, showing no sex differences in bone density during the first decade of life and in young adulthood. Although gains in bone density during sexual development are similar for both sexes, the increase begins and reaches peak values at an earlier age in female subjects, consistent with differences in pubertal timing. Data from (147).
Several pieces of evidence suggest that differences in sex steroid actions on bone are associated with sex-specific effects of GH and its downstream effector IGF-1 on bone cells. GH directly stimulates osteoblastogenesis and bone formation (154–156). GH is secreted by the pituitary gland in a sex-dependent fashion. The female pituitary secretes GH continuously, with frequent peaks and short GH-free intervals. Conversely, male GH peaks are higher and less frequent, and the GH-free intervals last longer. These sex differences of GH secretion lead to sex dimorphism in liver gene expression that depends on STAT5b, the GH receptor downstream mediator (157–159). IGF-1 influences both linear and cross-sectional growth and exerts a direct action on growth plate chondrocytes and osteoblasts responsible for building cortical and cancellous bone (160–167). IGF-1 also promotes the synthesis of RANK-L, which is responsible for osteoclast differentiation and activation (168–170). Moreover, osteoblasts express IGF-1 under parathyroid stimulation, and several other hormones, including thyroid hormone and estrogen, induce paracrine IGF-1 secretion by osteoblasts, whereas glucocorticoids inhibit it (171–179). However, the complex interactions between sex steroids and the GH–IGF-1 system remain to be fully delineated in humans (136, 140, 180–185). In animal studies, low concentrations of estrogens stimulate the hepatic production of IGF-1, whereas large concentrations exert an inhibitory effect (180, 186). Androgens act primarily at the pituitary level but only after being converted into estrogens by the enzymatic activity of aromatase (180).
BAT was thought to disappear after infancy. However, recent data suggest that BAT is often present in children and that both the amount and the activity of BAT increase during puberty (91, 126, 140, 141, 187–191). Notably, pubertal gains in BAT during adolescence, like those for muscle, are generally greater in boys than in girls (189). Several independent groups have also reported a positive association between BAT and the amount of bone in adolescents and adults (183, 192, 193); this association may be due in part to temporal changes in endocrine secretory factors (such as IGFBP2 and Wnt10b) or confounding from the strong positive relationship of brown fat to muscle mass (183, 184, 192). BAT volume has also been associated with long bone structure (183, 184, 192). In adolescence, BAT volume is related to femoral CSA and cortical bone area (192). Additionally, young women with BAT also have thicker femoral cortices and lower Pref-1 compared with women without BAT, suggesting that BAT may be involved in the regulation of stem cell differentiation into the bone lineage at the expense of adipogenesis (183). Whether BAT is also related to vertebral cross-sectional growth is unknown.
Adulthood
The amount of bone that is gained during adolescence is the main contributor to peak bone mass (PBM) in young adulthood, which in turn is a major determinant of the risk for osteoporosis and fragility fractures in older adults (141, 185, 194–200). The time of life in which peak bone mass in the axial skeleton is attained has been the subject of controversy, with estimates based on projection techniques ranging from the third to the fifth decade of life (137). However, spinal PBM as measured by computed tomography (CT) is achieved by the time of sexual and skeletal maturity, when the amount of bone in the vertebral body and vertebral size reach peak values (Fig. 3) (147, 195, 196).
According to a recent longitudinal study, measures of vertebral volume, bone mineral density (BMD), and bone mineral content by CT scan in 50 girls at skeletal maturity remained unchanged 3 years later (137). Whether sex differences in vertebral size further increase during adulthood is controversial. Although most data indicate that little or no bone is gained from the periosteal surface of vertebral bone in adult women and the overall CSA of their vertebrae remains stable (137, 201), some studies suggest male vertebrae increase in size over life (201–205). Unlike vertebral size, cancellous bone density, the other major indicator of vertebral strength, starts to decrease in both sexes by early adulthood (193, 205–211). Previous histological examinations of vertebrae and iliac crest showed evidence that the loss of cancellous BMD may occur as early as the third decade (206–208, 210).
The basis for the lower PBM in the axial skeleton of women lies in the smaller female vertebrae because differences in vertebral bone density are less salient or nonexistent (134, 212–214). Even after differences in body size are accounted for, the CSA of vertebral bodies is 25% smaller in women than in men (134).
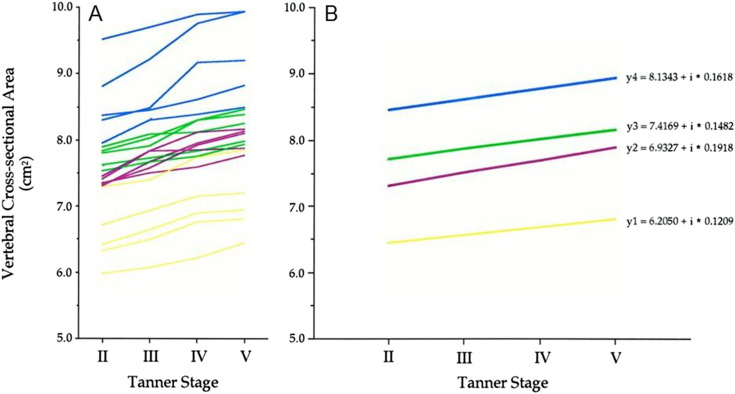
Measures of vertebral bone density and CSA track through adolescence until the time of PBM (215). Values for vertebral CSA at the beginning of puberty in both girls and boys accounted for ~90% of the variations seen at sexual maturity 3 years later (Fig. 4) (215). Tracking for this phenotype is as strong as that observed for standing height (215), knowledge that highlights our potential ability to determine which children are prone to develop low values for vertebral CSA at skeletal maturity. Dual-energy X-ray absorptiometry (DXA) measures of bone density and mineral content also track through childhood until skeletal maturity; this is true in the axial and appendicular skeleton and for both male and female subjects (216, 217).
Figure 4.
Longitudinal measurements of vertebral CSA in 20 girls from the commencement of puberty (Tanner stage 2) to its completion (Tanner stage 5). Yellow lines represent changes in values for the children in the lowest baseline quartiles; purple and green lines depict changes in values for the girls in the middle quartiles at baseline; blue lines represent changes in values for the girls in the highest baseline quartiles. Values are shown (a) for each girl and (b) for each quartile. The regression lines across Tanner stages for this phenotype differed between quartiles and did not overlap. Adapted with permission from Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85:3908–3918.
Accumulating evidence suggests that marrow mesenchymal stem cells differentiate into bone or fat through alternative activation of mutually exclusive transcriptional programs (218–222) and that bone marrow adiposity plays a critical role in affecting bone quantity (223). In healthy teenagers and young adults, the amount of bone in the axial and appendicular skeleton is negatively related to marrow adiposity measured by MRI, a reciprocal relation independent of body size and present in both sexes (224, 225). Moreover, a prospective longitudinal study in young women found that increases in femoral cortical bone thickness as measured by CT scans, were inversely related to decreases in marrow adiposity (226). To date, no study has examined the potential link between marrow adiposity and vertebral CSA.
Other Potential Determinants of Vertebral CSA
Genetic factors
Heredity is a well-known determinant of skeletal bone mass, as measured with dual photon or X-ray absorptiometry. Convergent data from sibling pairs and twin studies have estimated the heritability of spinal bone mass to be 80% of its variance (227–233). Additional support for this genetic influence comes from studies showing low lumbar bone mass in daughters of women with osteoporosis and in first-degree relatives of patients with osteoporosis (229, 234). Because measures of spinal bone mass, based on projection techniques, are greatly influenced by bone size, the reported associations in bone mass could reflect the confounding effect of vertebral geometry (235–238). Earlier candidate gene and family studies identified the vitamin D receptor, type I collagen alpha 1, and low-density lipoprotein receptor–related protein 5 as determinants of DXA measurements of BMD (239–243). In the last decade, genomewide association studies have identified >60 loci associated with BMD (244); many are also associated with bone values in childhood (245–247). However, these loci have small effect sizes and collectively explain only ~6% of the variance associated with BMD, and many more loci remain to be discovered (244). Recently, the rare variant EN1 and a common variant near SOX6 have been associated with bone mass in both childhood (246) and adulthood (245); the smaller effect sizes observed in the adult study (245) suggest that the genetic influence on bone properties is lower in adulthood than in childhood.
The obvious application of genetic studies on vertebral structure is the discovery of markers that would allow early identification of subjects at risk for spinal disease. Spondylolysis (248, 249), adolescent idiopathic scoliosis (AIS) (250, 251), and vertebral fragility fractures (244) are polygenic diseases affecting millions of people. Though widely investigated by linkage analysis and candidate gene association studies, the genetic basis of these complex diseases is still undefined. A better characterization of the phenotypes and structural basis for these deformities would greatly aid in the identification of disease predisposition genes. Greater understanding of the interactions between genetic predisposition and modifiable factors, such as physical activity and diet, in determining axial skeletal modeling would also provide new ways to prevent spinal disorders.
Behavioral and environmental factors
Bone accrual begins before birth with genetic programming and is altered by epigenetic modifications (252, 253). Dietary alterations in humans can affect that genetic program, leading to subsequent changes in skeletal mass (230, 232, 254–257). Although nutritional variables are important modifiable factors that influence bone health, the effect they have on vertebral morphogenesis is unknown. A 6-year longitudinal assessment of calcium intake on bone in a diverse cohort of 1743 children found that dietary calcium had a positive effect, after adjustment for age, height velocity, and physical activity, on bone accrual at the lumbar spine in girls (258). Because most growth-related increases in bone mass observed in DXA pediatric values are caused by increases in vertebral size, the reported gains could, in part, reflect the confounding effect of skeletal geometry on DXA measures (237).
Although the beneficial effects of nutrition on vertebral cross-sectional growth are yet to be defined, the influence of exercise on axial skeletal development is better documented. Multiple observational and interventional studies suggest that weight-bearing activities are associated with high or increased bone mass in the axial skeleton (257, 259–264). Emerging evidence suggests that exercise may increase vertebral dimensions (265–267). Using physical activity records from the Northern Finland Birth Cohort 1966, Oura et al. (266) reported that high exercise levels from 14 to 46 years of age were associated with larger MRI values for vertebral CSA at 47 years of age. Additionally, young women who participated in physical activity four or more times a week had larger vertebral CSAs in the perimenopausal years (266). Another study using anterior and lateral DXA scans found that gymnastic exposure in girls was associated with larger vertebral cross-sectional geometry (265). This benefit was observed even in girls of low gymnastic experience and years after they discontinued gymnastics. Thus, the effect of physical activity on the growing axial skeleton implicates vertebral geometric adaptation to mechanical loading that may persist well beyond activity cessation into adulthood (265, 266, 268).
Archaeological data indicate that the strength and CSA of the axial and appendicular skeleton have decreased over time alongside technological development (269, 270). Using MRI and specimens from Swedish and British bone collections, Junno et al. (270) found the CSA of the lumbar vertebra to be smaller and the vertebral height taller today than in medieval times. Higher levels of physical activity leading to bone adaptation and potential genetic selection pressures favoring more robust bone structure probably account for differences in vertebral CSA (270). A physically less demanding lifestyle could have led to selection for more fragile vertebrae.
Clinical Implications of the Female Vertebral CSA for the Growing Skeleton
The spine provides flexibility, support, and protection of the spinal cord and nerves. Phenotypic variations that have evolved to be beneficial in one set of circumstances can be detrimental in other conditions. The smaller cross-sectional dimensions of the female vertebral body, though probably facilitating the lordosis needed during pregnancy (42, 47, 48, 271), could also increase the risk for a broad array of spinal conditions across the lifespan, such as exaggerated lordosis (272), spondylolysis (273), scoliosis (274, 275), vertebral wedging (276, 277), and vertebral fractures (134, 214, 278, 279) (Table 1).
Table 1.
Clinical Studies Assessing Vertebral CSA in Relation to LL, Spondylolysis, Scoliosis, and Vertebral Wedging
| Study (Ref.) | n | Age (y) | Imaging Modality | Outcomes | Findings |
|---|---|---|---|---|---|
| Wren et al., 2017 (272) | 40 girls and 40 boys | 9–13 | MRI | Vertebral CSA | Significant negative correlation between vertebral CSA and LL in girls and boys. |
| LL | Girls have significantly smaller vertebral CSA and greater degree of LL. | ||||
| Wren et al., 2017 (273) | Spondylolysis: 16 girls and 19 boys | 9–14 | MRI | Vertebral CSA | Girls and boys with spondylolysis have smaller vertebral CSA and greater LL. |
| Controls: 36 girls and 50 boys | LL | Significant negative correlation between vertebral CSA and LL. | |||
| Spondylolysis | |||||
| Ponrartana et al., 2016 (274) | AIS: 35 girls and 11 boys | 10–15 | MRI | Vertebral CSA | Girls and boys with AIS have significantly smaller vertebral CSA and taller IVD. |
| Controls: 35 girls and 11 boys | CT | IVD height | Girls have significantly taller IVD | ||
| AIS | |||||
| Wren et al., 2017 (276) | AIS: 25 girls | 9–15 | MRI | Vertebral CSA | Significant negative correlation between vertebral CSA and lateral thoracic vertebral wedging. |
| Lateral thoracic wedging | |||||
| 50 girls and 50 boys | 9–15 | MRI | Vertebral CSA | Significant negative correlation between vertebral CSA and anterior lumbar vertebral wedging. | |
| Sagittal lumbar wedging | |||||
| Poorghasamians et al., 2017 (277) | 27 girls | 9–13 | MRI | Changes in vertebral CSA and sagittal lumbar wedging | Progression of lumbar vertebral wedging is associated with lesser vertebral cross-sectional growth, whereas regression is the consequence of greater cross-sectional growth. |
The height of the intervertebral disc (IVD), the compliance of its fibrous cartilage, and the dimensions of adjacent vertebrae are major determinants of spinal mobility (133, 275, 280). A greater range of motion occurs when the IVD is tall or the vertebral CSA is small. For comparable disc thickness and stiffness, the smaller female vertebral CSA results in greater flexion and extension and lateral flexion (133), a notion consistent with knowledge that the lumbar spine of young girls has greater flexibility than that of boys (281–283). Previous radiographic studies suggest that children with slender vertebrae have greater range of motion of the spine than those with larger vertebral bodies (284, 285). More recently, bending flexibility was found to be associated with smaller vertebral CSA and taller IVDs in healthy girls (275).
Spinal curvatures
LL is the anterior curvature of the lumbar spine, expressed in humans as a response to upright posture, which develops during childhood, increases throughout adolescence (286), and is more prominent in women than in men (133, 287–290). Support for the notion that smaller female vertebrae represent the human adaptation to bipedal obstetric load comes from a recent study showing that when compared with adolescent boys, adolescent girls had significantly smaller vertebral CSA and a greater degree of lumbar curvature (272). Notably, differences in LL between boys and girls were closely related to sex differences in vertebral CSA (272).
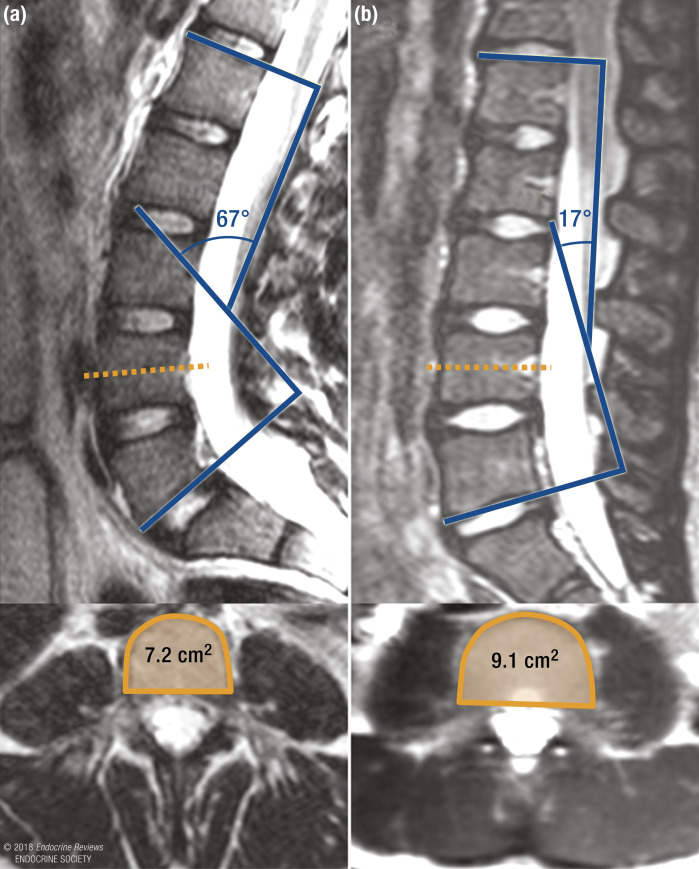
Spondylolysis is a stress fracture of the pars interarticularis of the vertebra, which occurs most commonly in the lower lumbar spine of adolescents with prominent LL (291–293). This condition affects ~6% of children and is thought to be the result of mechanical stresses from hyperextension of the spine (291, 294–297). Recent data show that in addition to a greater degree of LL, boys and girls with spondylolysis have significantly smaller vertebral CSA when compared with controls (Fig. 5), a difference that persisted even after the data were adjusted for height and weight (273). Whether the small vertebral body size found in patients with spondylolysis also translates to a small pars interarticularis and neural arch is unknown. Facet height, width, and orientation have all been proposed in the pathogenesis of spondylolysis (298, 299). Studies are needed to determine the degree to which exaggerated LL (mediated by small vertebral CSA) and small pars interarticularis predict spondylolysis.
Figure 5.
MRI images of the lumbar spine showing a greater LL angle (67° vs 17°) and smaller fourth lumbar vertebral CSA (7.2 cm2 vs 9.1 cm2) in (a) a 12-year-old girl with spondylolysis and (b) a 12-year-old girl without spondylolysis.
Scoliosis affects ~7 million people in the United States, mostly adolescents, and 85% of cases are considered idiopathic (300–303). Beyond knowledge that female sex and a family history are major risk factors for its development (304), the specific genes and phenotypes that confer susceptibility or resistance to the deformity are unknown. Recent evidence suggests that girls with AIS have taller IVDs relative to vertebral width and greater spinal flexibility when compared with peers without spinal deformity (275). These findings are in agreement with data showing that girls who participate in sports, such as gymnastics and dancing, have both greater joint flexibility and a higher risk of developing AIS (305–310). Additional support for a role of vertebral morphology in the development of spinal deformity comes from two previous radiographic studies showing that girls with slender vertebrae have a greater tendency to develop progressive scoliosis than those with larger vertebral body widths (284, 285). Results from a preliminary retrospective study also show that MRI measures of lumbar vertebral CSA in children with AIS were significantly smaller and the IVDs taller than those obtained in children without spinal deformities, as measured with CT scans (274). Prospective studies using the same imaging modality in patients and controls are needed to validate these findings.
Multiple reports based on dual photon or X-ray absorptiometry showed lower spinal mass in children with AIS (311–317). Measurements of spinal bone mass at the time of diagnosis have even been suggested as a prognostic factor of curve progression (318). Because patients with AIS have substantially thicker IVDs (274), which have lower radiographic attenuation than bone, low spinal bone measures could, in part, reflect the confounding effect of a greater proportion of intervertebral fibrocartilage in the spine (235, 237, 238).
Vertebral wedging
Just as the term plasticity in engineering refers to the ability of solid material to undergo deformation in response to load, skeletal plasticity refers to the ability of growing bone to alter modeling as a consequence of mechanical stresses (319). Wedging of the vertebral body, a key structural characteristic of spinal curvatures (45, 276, 320, 321), is the result of a simultaneous increase in longitudinal growth on the convex side of a curve and inhibition on the concave side (322–324). In LL, greater axial loading in the posterior portions of the vertebral body leads to anterior-posterior wedging of the lumbar vertebrae (320). In scoliosis, compressive loading on the concave side of the curve inhibits longitudinal growth, and tensile loading on the convex side accelerates it, resulting in lateral wedging of the thoracic vertebrae (322–327). Previous studies have shown that the degrees of anterior wedging in LL and lateral wedging in scoliosis are both inversely correlated to vertebral CSA (276, 277).
Unlike the permanent deformations that adult vertebrae sustain under load, asymmetrical vertebral growth in children has the capacity to change shape in response to mechanical stresses (328, 329). In the immature skeleton, vertebral cross-sectional growth is an important determinant of the plasticity of the vertebral body; regression of vertebral wedging is associated with greater vertebral cross-sectional growth, whereas progression is the consequence of lesser cross-sectional growth (277). Makino et al. (327) recently showed the degree of wedging in thoracic vertebrae to diminish a year after posterior corrective surgery for AIS. A similar phenomenon occurs in pediatric patients with vertebral fractures secondary to leukemia or hypercortisolism (330–336). In contrast to the mature skeleton, these pathological fractures often regain normal dimensions after treatment (330, 337–339). Interestingly, older teenagers, like adults, are less likely to regain vertebral body height (339, 340), supporting the notion that vertebral body plasticity is a property of the immature axial skeleton.
Clinical Implications of the Female Vertebral CSA for the Aging Skeleton
Vertebral fragility fractures
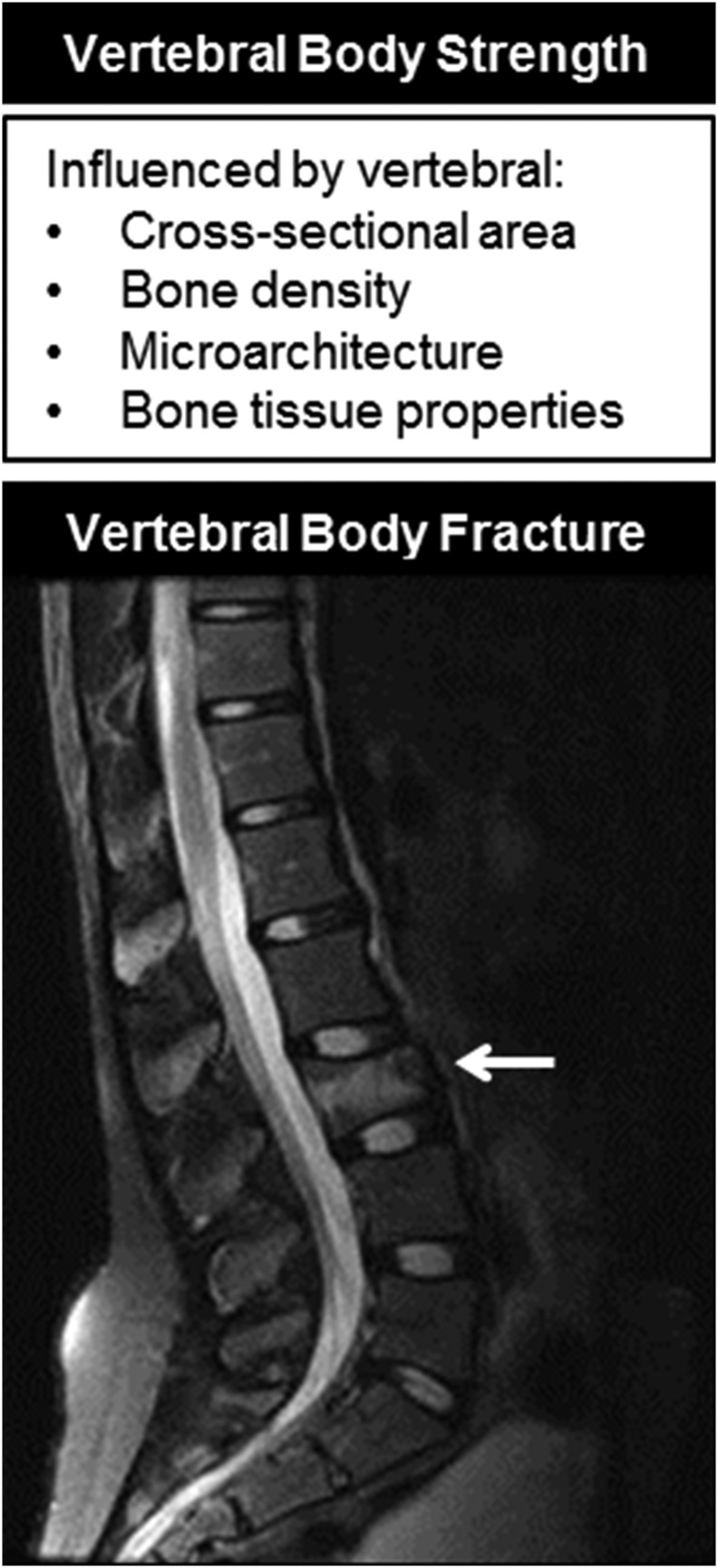
The smaller female vertebra confers a biomechanical disadvantage that increases the stress within vertebrae for all physical activities and limits the loading capacity of the spine for life (134, 203, 214, 341). During compression along the longitudinal axis of a vertebral body, its loading capacity is directly proportional to its CSA and to the material, or compressive, strength of the bone (Fig. 6). Conversely, mechanical stresses are inversely proportional to CSA (stress = load/CSA); therefore, vertebrae with a smaller CSA have higher stresses than vertebrae with a larger CSA for a similar load. It has been estimated that sex differences in vertebral size result in 30% to 40% greater mechanical stress within vertebral bodies in young women than in men for equivalent loads (134). Not surprisingly, vertebral fractures, the most common clinical manifestation of osteoporosis, have a higher prevalence in women than men (342–345).
Figure 6.
The strength of the vertebral body is determined by vertebral CSA, bone density, microarchitecture, and bone tissue properties. Fractures (arrow) occur when the loads applied to a vertebral body exceed its strength.
Approximately 25% of all postmenopausal women in the United States are diagnosed with vertebral fractures, caused by the inability of the vertebral body to withstand the loads associated with normal daily activities (340–342, 346–348). However, most vertebral fractures are clinically silent (349, 350), highlighting the difficulties in assessing their true prevalence and justifying the varying results between studies of sex differences in vertebral fractures. In the Framingham cohort, a greater percentage of men had vertebral fractures than women in the younger age groups, but more women had vertebral fractures in the oldest age group, consistent with the notion that young men have more frequent exposure to high-load activities and injuries than young women (345). Available data indicate that men at all ages have greater vertebral strength, largely because of their larger CSA (214, 351).
Cancellous bone density alone is not a sufficient explanation for vertebral fractures. Many women with low bone density values, regardless of technique, do not experience fractures, and there is substantial overlap in bone density between women with and without radiographic evidence of vertebral compression (278, 352, 353). In an attempt to understand why some patients with low vertebral bone density do not have fractures, other properties of bone that contribute to its strength, such as the vertebral dimensions and material properties and microarchitecture of cancellous bone, have been considered in the pathogenesis of osteoporotic fractures (202–204, 278, 279, 352, 354–361) (Fig. 6).
Several clinical studies have also examined the role of vertebral cross-sectional dimensions as a possible determinant of vertebral fractures (Table 2) (201, 202, 214, 278, 279, 361). Overall, when compared with men and women without fragility fractures, patients with fractures had, on average, 7.7% smaller vertebral CSA (279). Notably, in a case-control study of matched pairs of older women with reduced bone density and spinal osteoporosis, whose main difference was the absence or presence of vertebral fractures, the CSAs of the unaffected vertebrae in the fracture group were ~8% smaller than in the nonfracture group (278).
Table 2.
Comparisons of Vertebral CSA, Width, and Volume in Subjects With and Without Vertebral Body Fracture
| Study (Ref.) | n | Age (y) | Imaging Modality | Outcomes | Findings |
|---|---|---|---|---|---|
| Gilsanz et al., 1995 (278) | Vertebral fracture: 32 women | 60–89 | CT | Vertebral CSA | Women with vertebral fractures have significantly smaller vertebral CSA. |
| Controls: 32 women | Vertebral fractures | ||||
| Duan et al., 2001 (343) | Vertebral fracture: 76 (50 women) | 18–92 | DXA | Vertebral CSAa | Women and men with vertebral fractures have significantly smaller CSA. |
| Controls: 1013 (686 women) | Vertebral fractures | ||||
| Nielsen et al., 1993 (355) | Vertebral fracture: 62 women | 66.8 ± 8.1b | DXA | Vertebral width | Women with vertebral fractures have smaller vertebral width, but the difference did not reach statistical significance. |
| Controls: 415 women | 53.0 ± 6.5 | Vertebral fractures | |||
| Vega et al., 1998 (358) | Vertebral fracture: 30 men | 47–83 | DXA | Vertebral width | Men with vertebral fractures have significantly smaller vertebral width. |
| Controls: 26 men | Vertebral fractures | ||||
| Seeman et al., 2001 (204) | Vertebral fracture: 95 men | 17–91 | DXA | Vertebral width | Men with vertebral fractures have significantly smaller vertebral width. |
| Controls: 395 men | Vertebral fractures | ||||
| Ito et al., 1997 (211) | Vertebral fracture: 58 women | 55–79 | CT | Vertebral volumec | Women with vertebral fracture have smaller vertebral volume, but the difference did not reach statistical significance. |
| Controls: 65 women | Vertebral fractures | ||||
| Duan et al., 1999 (359) | Vertebral fracture: 163 women | 18–87 | DXA | Vertebral volumed | Women with vertebral fractures have significantly smaller vertebral volume. |
| Controls: 479 women | Vertebral fractures |
Vertebral CSA calculated as π × width/2 × depth/2.
Age range not provided.
Vertebral volume calculated as vertebral body area × mean vertebral height (vertebral CSA values were not provided).
Vertebral volume calculated as (projected area)3/2.
Vertebral fracture cascade
The main risk factor for sustaining a vertebral fracture is having suffered a previous one (362–367), a phenomenon often called the vertebral fracture cascade (364, 366). It has been estimated that the risk of another vertebral fracture is more than four times that of the first fracture and significantly greater than the recurrence rate associated with other fragility fractures (367). Why one vertebral fracture is an imminent risk for a subsequent fracture is unclear (366, 368). It should be noted that when compared with men, women are more likely to sustain first and subsequent fractures (367). Data also show that this elevated risk for vertebral fracture recurrence in women persists even after adjustment for age and BMD (362). Clearly, studies are needed to establish the potential role of vertebral CSA in the vertebral fracture cascade and whether patients who refracture are those with the smallest vertebral cross-sectional dimensions.
Conclusions and Future Directions
We now know that factors related to sex program fetal vertebral growth and give rise to the outmost phenotypic difference between the male and female skeletons. During evolution, the female axial skeleton probably reacted to the unique requirements of pregnancy by modeling the CSA of the vertebral body to facilitate spinal flexibility. Unfortunately, this programming not only renders the female spine more flexible but also lessens its strength. Even though this concept has only recently been formalized, a strong argument can be made that smaller vertebral CSA is a key risk factor for spinal deformities in adolescence and vertebral fractures in older adults.
Well-designed studies across multiple pediatric populations are needed to definitively establish the role of vertebral CSA as a risk for exaggerated lordosis, spondylolysis, and scoliosis. Studies are also needed to better examine vertebral cross-sectional growth as a determinant of vertebral plasticity in the immature spine and to investigate the potential for wedge deformities to reshape or resolve over time. A better understanding of the differences in vertebral growth and the reshaping potential of the growing vertebral body between sexes could help explain the 10 times greater prevalence of severe scoliosis in girls than boys. It could also aid in the design of preventive and corrective treatments for wedge deformities in children.
Additionally, sex discrepancies in vertebral CSA probably contribute to the higher incidence of fragility fractures in the axial skeleton of older women than men. Studies are needed to determine the strength of this phenotype in predicting vertebral fracture and to provide reference standards for vertebral CSA measures in adults of varying ages and body sizes; only then would we be in a position to establish a threshold value for vertebral fragility fractures. Future work should also compare the strength of vertebral size measures with bone density values in predicting fracture risk, specifically in women with a history of previous vertebral fracture because they are at substantial risk for additional fractures. This research could play a crucial role in the development of an integrated predictive index for fracture risk based on vertebral size and bone density, alongside sex, age, weight, and clinical history.
Although current data suggest that deficient vertebral cross-sectional growth in early life could be associated with an array of adverse spinal conditions, they do not preclude the possibility that vertebral growth can be optimized through mechanical or dietary interventions. Physical activity and mechanical loading in children are known to influence bone density, shape, and size, especially when performed frequently and regularly. Although much more work is needed, it is tempting to think that the clinical relevance of vertebral cross-sectional growth will soon be better delineated. Such knowledge will provide a more rational way to diagnose, prevent, and treat spinal pathologies in the young and in older adults.
Acknowledgments
The authors thank Dr. Erika Rubesova and Ramon Gilsanz for their insightful contributions and Ms. Patricia C. Aggabao and Mr. Ervin Poorghasamians for their technical assistance on this manuscript.
Financial Support: This research was supported by the Department of the Army (DAMD17-01-1-0817) and the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR41853, R01AR052744), National Institute of Child Health and Human Development (N01HD13333), National Institute of Diabetes and Digestive and Kidney Diseases (R21DK090778), and National Institute of General Medical Sciences (U54GM115516).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- AIS
adolescent idiopathic scoliosis
- BAT
brown adipose tissue
- BMD
bone mineral density
- CSA
cross-sectional area
- CT
computed tomography
- DXA
dual-energy X-ray absorptiometry
- FSH
follicle-stimulating hormone
- GH
growth hormone
- IGF
insulin-like growth factor
- IVD
intervertebral disc
- LL
lumbar lordosis
- MRI
magnetic resonance imaging
- PBM
peak bone mass
- SNS
sympathetic nervous system
- UCP1
uncoupling protein 1
References
- 1. Dennison EM, Harvey NC, Cooper C. Programming of osteoporosis and impact on osteoporosis risk. Clin Obstet Gynecol. 2013;56(3):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wood CL, Stenson C, Embleton N. The developmental origins of osteoporosis. Curr Genomics. 2015;16(6):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford). 2003;42(6):791–796. [DOI] [PubMed] [Google Scholar]
- 4. Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57(4):582–586. [DOI] [PubMed] [Google Scholar]
- 5. Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17(3):337–347. [DOI] [PubMed] [Google Scholar]
- 6. Harvey NC, Mahon PA, Robinson SM, Nisbet CE, Javaid MK, Crozier SR, Inskip HM, Godfrey KM, Arden NK, Dennison EM, Cooper C; SWS Study Group . Different indices of fetal growth predict bone size and volumetric density at 4 years of age. J Bone Miner Res. 2010;25(4):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harvey NC, Mahon PA, Kim M, Cole ZA, Robinson SM, Javaid K, Inskip HM, Godfrey KM, Dennison EM, Cooper C; SWS Study Group . Intrauterine growth and postnatal skeletal development: findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2012;26(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexander BT, Henry Dasinger J, Intapad S. Effect of low birth weight on women’s health. Clin Ther. 2014;36(12):1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuh D, Wills AK, Shah I, Prentice A, Hardy R, Adams JE, Ward K, Cooper C; National Survey for Health and Development (NSHD) Scientific and Data Collection Team . Growth from birth to adulthood and bone phenotype in early old age: a British birth cohort study. J Bone Miner Res. 2014;29(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christoffersen T, Ahmed LA, Daltveit AK, Dennison EM, Evensen EK, Furberg AS, Gracia-Marco L, Grimnes G, Nilsen OA, Schei B, Tell GS, Vlachopoulos D, Winther A, Emaus N. The influence of birth weight and length on bone mineral density and content in adolescence: The Tromsø Study, Fit Futures [published correction appears in Arch Osteoporos. 2017;12:62] Arch Osteoporos. 2017;12(1):54. [DOI] [PubMed] [Google Scholar]
- 11. Balasuriya CND, Evensen KAI, Mosti MP, Brubakk AM, Jacobsen GW, Indredavik MS, Schei B, Stunes AK, Syversen U. Peak bone mass and bone microarchitecture in adults born with low birth weight preterm or at term: a cohort study. J Clin Endocrinol Metab. 2017;102(7):2491–2500. [DOI] [PubMed] [Google Scholar]
- 12. Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16(9):1694–1703. [DOI] [PubMed] [Google Scholar]
- 13. Cole ZA, Gale CR, Javaid MK, Robinson SM, Law C, Boucher BJ, Crozier SR, Godfrey KM, Dennison EM, Cooper C. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J Bone Miner Res. 2009;24(4):663–668. [DOI] [PubMed] [Google Scholar]
- 14. Harvey NC, Javaid MK, Arden NK, Poole JR, Crozier SR, Robinson SM, Inskip HM, Godfrey KM, Dennison EM, Cooper C; SWS Study Team . Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. J Dev Orig Health Dis. 2010;1(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodfellow LR, Earl S, Cooper C, Harvey NC. Maternal diet, behaviour and offspring skeletal health. Int J Environ Res Public Health. 2010;7(4):1760–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, Noble JA, Cooper C, Papageorghiou AT. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. 2012;97(11):E2070–E2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen SB, Rasmussen MA, Olsen SF, Vestergaard P, Mølgaard C, Halldorsson TI, Strøm M. Maternal dietary patterns during pregnancy in relation to offspring forearm fractures: prospective study from the Danish National Birth Cohort. Nutrients. 2015;7(4):2382–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D’Angelo S, Crozier SR, Moon RJ, Arden NK, Dennison EM, Godfrey KM, Inskip HM, Prentice A, Mughal MZ, Eastell R, Reid DM, Javaid MK; MAVIDOS Study Group . Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(5):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bisson M, Tremblay F, St-Onge O, Robitaille J, Pronovost E, Simonyan D, Marc I. Influence of maternal physical activity on infant’s body composition. Pediatr Obes. 2017;12(suppl 1):38–46. [DOI] [PubMed] [Google Scholar]
- 20. Jones IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: evidence from a birth cohort study. Am J Epidemiol. 2004;159(4):343–350. [DOI] [PubMed] [Google Scholar]
- 21. Hallal PC, Siqueira FV, Menezes AM, Araújo CL, Norris SA, Victora CG. The role of early life variables on the risk of fractures from birth to early adolescence: a prospective birth cohort study. Osteoporos Int. 2009;20(11):1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byberg L, Michaëlsson K, Goodman A, Zethelius B, Koupil I. Birth weight is not associated with risk of fracture: results from two Swedish cohort studies. J Bone Miner Res. 2014;29(10):2152–2160. [DOI] [PubMed] [Google Scholar]
- 23. Mikkola TM, von Bonsdorff MB, Osmond C, Salonen MK, Kajantie E, Eriksson JG. Association of body size at birth and childhood growth with hip fractures in older age: an exploratory follow-up of the Helsinki Birth Cohort Study. J Bone Miner Res. 2017;32(6):1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maruyama H, Amari S, Fujinaga H, Fujino S, Nagasawa J, Wada Y, Tsukamoto K, Ito Y. Bone fracture in severe small-for-gestational-age, extremely low birth weight infants: a single-center analysis. Early Hum Dev. 2017;106-107:75–78. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert C, Babyn P. MR imaging of the neonatal musculoskeletal system. Magn Reson Imaging Clin N Am. 2011;19(4):841–858, ix. [DOI] [PubMed] [Google Scholar]
- 26. Jaimes C, Chauvin NA, Delgado J, Jaramillo D. MR imaging of normal epiphyseal development and common epiphyseal disorders. Radiographics. 2014;34(2):449–471. [DOI] [PubMed] [Google Scholar]
- 27. Ponrartana S, Aggabao PC, Dharmavaram NL, Fisher CL, Friedlich P, Devaskar SU, Gilsanz V. Sexual dimorphism in newborn vertebrae and its potential implications. J Pediatr. 2015;167(2):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitt D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J Exp Biol. 2003;206(Pt 9):1437–1448. [DOI] [PubMed] [Google Scholar]
- 29. Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432(7015):345–352. [DOI] [PubMed] [Google Scholar]
- 30. Young NM, Wagner GP, Hallgrímsson B. Development and the evolvability of human limbs. Proc Natl Acad Sci USA. 2010;107(8):3400–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niemitz C. The evolution of the upright posture and gait—a review and a new synthesis. Naturwissenschaften. 2010;97(3):241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carrier DR. The advantage of standing up to fight and the evolution of habitual bipedalism in hominins. PLoS One. 2011;6(5):e19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wagner H, Liebetrau A, Schinowski D, Wulf T, de Lussanet MH. Spinal lordosis optimizes the requirements for a stable erect posture. Theor Biol Med Model. 2012;9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pons JL, Moreno JC, Torricelli D, Taylor JS. Principles of human locomotion: a review. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:6941–6944. [DOI] [PubMed] [Google Scholar]
- 35. Gruss LT, Schmitt D. The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams SA, Middleton ER, Villamil CI, Shattuck MR. Vertebral numbers and human evolution. Am J Phys Anthropol. 2016;159(suppl 61):S19–S36. [DOI] [PubMed] [Google Scholar]
- 37. Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. 2003;73(3):627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crompton RH, Vereecke EE, Thorpe SK. Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. J Anat. 2008;212(4):501–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Been E, Barash A, Marom A, Aizenberg I, Kramer PA. A new model for calculating the lumbar lordosis angle in early hominids and in the spine of the Neanderthal from Kebara. Anat Rec (Hoboken). 2010;293(7):1140–1145. [DOI] [PubMed] [Google Scholar]
- 40. Sparrey CJ, Bailey JF, Safaee M, Clark AJ, Lafage V, Schwab F, Smith JS, Ames CP. Etiology of lumbar lordosis and its pathophysiology: a review of the evolution of lumbar lordosis, and the mechanics and biology of lumbar degeneration. Neurosurg Focus. 2014;36(5):E1. [DOI] [PubMed] [Google Scholar]
- 41. Been E, Gómez-Olivencia A, Shefi S, Soudack M, Bastir M, Barash A. Evolution of spinopelvic alignment in hominins. Anat Rec (Hoboken). 2017;300(5):900–911. [DOI] [PubMed] [Google Scholar]
- 42. Whitcome KK, Shapiro LJ, Lieberman DE. Fetal load and the evolution of lumbar lordosis in bipedal hominins [published correction appears in Nature. 2012;487(7405):128] Nature. 2007;450(7172):1075–1078. [DOI] [PubMed] [Google Scholar]
- 43. Jensen RK, Doucet S, Treitz T. Changes in segment mass and mass distribution during pregnancy. J Biomech. 1996;29(2):251–256. [DOI] [PubMed] [Google Scholar]
- 44. Kramer PA. The costs of human locomotion: maternal investment in child transport. Am J Phys Anthropol. 1998;107(1):71–85. [DOI] [PubMed] [Google Scholar]
- 45. Masharawi Y, Dar G, Peleg S, Steinberg N, Medlej B, May H, Abbas J, Hershkovitz I. A morphological adaptation of the thoracic and lumbar vertebrae to lumbar hyperlordosis in young and adult females. Eur Spine J. 2010;19(5):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hay O, Dar G, Abbas J, Stein D, May H, Masharawi Y, Peled N, Hershkovitz I. The lumbar lordosis in males and females, revisited. PLoS One. 2015;10(8):e0133685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pawłowski B, Grabarczyk M. Center of body mass and the evolution of female body shape. Am J Hum Biol. 2003;15(2):144–150. [DOI] [PubMed] [Google Scholar]
- 48. Opala-Berdzik A, Błaszczyk JW, Bacik B, Cieślińska-Świder J, Świder D, Sobota G, Markiewicz A. Static postural stability in women during and after pregnancy: a prospective longitudinal study. PLoS One. 2015;10(6):e0124207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–480. [DOI] [PubMed] [Google Scholar]
- 50. Dehghan F, Haerian BS, Muniandy S, Yusof A, Dragoo JL, Salleh N. The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports. 2014;24(4):e220–e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patil NA, Rosengren KJ, Separovic F, Wade JD, Bathgate RAD, Hossain MA. Relaxin family peptides: structure-activity relationship studies. Br J Pharmacol. 2017;174(10):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bell RJ, Eddie LW, Lester AR, Wood EC, Johnston PD, Niall HD. Relaxin in human pregnancy serum measured with an homologous radioimmunoassay. Obstet Gynecol. 1987;69(4):585–589. [PubMed] [Google Scholar]
- 53. Kristiansson P, Svärdsudd K, von Schoultz B. Serum relaxin, symphyseal pain, and back pain during pregnancy. Am J Obstet Gynecol. 1996;175(5):1342–1347. [DOI] [PubMed] [Google Scholar]
- 54. Wu WH, Meijer OG, Bruijn SM, Hu H, van Dieën JH, Lamoth CJ, van Royen BJ, Beek PJ. Gait in pregnancy-related pelvic girdle pain: amplitudes, timing, and coordination of horizontal trunk rotations. Eur Spine J. 2008;17(9):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aldabe D, Ribeiro DC, Milosavljevic S, Dawn Bussey M. Pregnancy-related pelvic girdle pain and its relationship with relaxin levels during pregnancy: a systematic review. Eur Spine J. 2012;21(9):1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lindgren A, Kristiansson P. Finger joint laxity, number of previous pregnancies and pregnancy induced back pain in a cohort study. BMC Pregnancy Childbirth. 2014;14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoo H, Shin D, Song C. Changes in the spinal curvature, degree of pain, balance ability, and gait ability according to pregnancy period in pregnant and nonpregnant women. J Phys Ther Sci. 2015;27(1):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Masharawi Y, Rothschild B, Dar G, Peleg S, Robinson D, Been E, Hershkovitz I. Facet orientation in the thoracolumbar spine: three-dimensional anatomic and biomechanical analysis. Spine. 2004;29(16):1755–1763. [DOI] [PubMed] [Google Scholar]
- 59. Asher RJ, Lin KH, Kardjilov N, Hautier L. Variability and constraint in the mammalian vertebral column. J Evol Biol. 2011;24(5):1080–1090. [DOI] [PubMed] [Google Scholar]
- 60. Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001;1(3):193–207. [PubMed] [Google Scholar]
- 61. Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–430. [PMC free article] [PubMed] [Google Scholar]
- 62. Bagi CM, Berryman E, Moalli MR. Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med. 2011;61(1):76–85. [PMC free article] [PubMed] [Google Scholar]
- 63. Yang Y. Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr. 2009;19(3):197–218. [DOI] [PubMed] [Google Scholar]
- 64. Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today. 2013;99(3):170–191. [DOI] [PubMed] [Google Scholar]
- 65. Kobayashi T, Kronenberg HM. Overview of skeletal development. Methods Mol Biol. 2014;1130:3–12. [DOI] [PubMed] [Google Scholar]
- 66. Naruse C, Shibata S, Tamura M, Kawaguchi T, Abe K, Sugihara K, Kato T, Nishiuchi T, Wakana S, Ikawa M, Asano M. New insights into the role of Jmjd3 and Utx in axial skeletal formation in mice. FASEB J. 2017;31(6):2252–2266. [DOI] [PubMed] [Google Scholar]
- 67. Fernández-Terán MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev Dyn. 2006;235(9):2521–2537. [DOI] [PubMed] [Google Scholar]
- 68. Trotter M, Hixon BB. Sequential changes in weight, density, and percentage ash weight of human skeletons from an early fetal period through old age. Anat Rec. 1974;179(1):1–18. [DOI] [PubMed] [Google Scholar]
- 69. Kovacs CS. Bone metabolism in the fetus and neonate. Pediatr Nephrol. 2014;29(5):793–803. [DOI] [PubMed] [Google Scholar]
- 70. Carter DR, Van Der Meulen MC, Beaupré GS. Mechanical factors in bone growth and development. Bone. 1996;18(1suppl)5S–10S. [DOI] [PubMed] [Google Scholar]
- 71. Zhang G. An evo-devo view on the origin of the backbone: evolutionary development of the vertebrae. Integr Comp Biol. 2009;49(2):178–186. [DOI] [PubMed] [Google Scholar]
- 72. Albert MA, Maier CA. Epiphyseal union of the cervical vertebral centra: its relationship to skeletal age and maturation of thoracic vertebral centra. J Forensic Sci. 2013;58(6):1568–1574. [DOI] [PubMed] [Google Scholar]
- 73. Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reynolds EL, Asakawa T. Skeletal development in infancy; standards for clinical use. Am J Roentgenol Radium Ther. 1951;65(3):403–410. [PubMed] [Google Scholar]
- 75. Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed.Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 76. Gilsanz V, Ratib O. Bone development In: Gilsanz V, Ratib O, eds. Hand Bone Age. New York, NY: Springer-Verlag Berlin Heidelberg; 2005:2–8. [Google Scholar]
- 77. Todd TW. Atlas of Skeletal Maturation of the Hand. St. Louis, MO: The C.V. Mosby Company; 1937. [Google Scholar]
- 78. Salle BL, Braillon P, Glorieux FH, Brunet J, Cavero E, Meunier PJ. Lumbar bone mineral content measured by dual energy X-ray absorptiometry in newborns and infants. Acta Paediatr. 1992;81(12):953–958. [DOI] [PubMed] [Google Scholar]
- 79. Namgung R, Tsang RC. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc. 2000;59(1):55–63. [DOI] [PubMed] [Google Scholar]
- 80. Koo WW, Hockman EM. Physiologic predictors of lumbar spine bone mass in neonates. Pediatr Res. 2000;48(4):485–489. [DOI] [PubMed] [Google Scholar]
- 81. Ahmad I, Nemet D, Eliakim A, Koeppel R, Grochow D, Coussens M, Gallitto S, Rich J, Pontello A, Leu SY, Cooper DM, Waffarn F. Body composition and its components in preterm and term newborns: a cross-sectional, multimodal investigation. Am J Hum Biol. 2010;22(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Holroyd CR, Harvey NC, Crozier SR, Winder NR, Mahon PA, Ntami G, Godfrey KM, Inskip HM, Cooper C; SWS Study Group . Placental size at 19 weeks predicts offspring bone mass at birth: findings from the Southampton Women’s Survey. Placenta. 2012;33(8):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Griffin JE, Wilson JD. Disorders of the testes and the male reproductive tract In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. 10th ed.Philadelphia, PA: Elsevier; 2003:709–770. [Google Scholar]
- 84. Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 2010;207(2):127–134. [DOI] [PubMed] [Google Scholar]
- 85. Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009;215(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tanner JM, Whitehouse RH, Hughes PC, Carter BS. Relative importance of growth hormone and sex steroids for the growth at puberty of trunk length, limb length, and muscle width in growth hormone-deficient children. J Pediatr. 1976;89(6):1000–1008. [DOI] [PubMed] [Google Scholar]
- 87. Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3(5):414–421. [DOI] [PubMed] [Google Scholar]
- 88. Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25(3):617–626. [DOI] [PubMed] [Google Scholar]
- 89. Callewaert F, Boonen S, Vanderschueren D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab. 2010;21(2):89–95. [DOI] [PubMed] [Google Scholar]
- 90. Lin-Su K, New MI. Ambiguous genitalia in the newborn In: Gleason CA, Devaskar SU, eds. Avery’s Diseases of the Newborn. 9th ed.Philadelphia, PA: Elsevier Saunders; 2012:1286–1306. [Google Scholar]
- 91. Ponrartana S, Aggabao PC, Chavez TA, Dharmavaram NL, Gilsanz V. Changes in brown adipose tissue and muscle development during infancy. J Pediatr. 2016;173:116–121. [DOI] [PubMed] [Google Scholar]
- 92. Gilsanz V, Boechat MI, Roe TF, Loro ML, Sayre JW, Goodman WG. Gender differences in vertebral body sizes in children and adolescents. Radiology. 1994;190(3):673–677. [DOI] [PubMed] [Google Scholar]
- 93. Arfai K, Pitukcheewanont PD, Goran MI, Tavare CJ, Heller L, Gilsanz V. Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology. 2002;224(2):338–344. [DOI] [PubMed] [Google Scholar]
- 94. Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83(5):1420–1427. [DOI] [PubMed] [Google Scholar]
- 95. Forest MG, Sizonenko PC, Cathiard AM, Bertrand J. Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy. J Clin Invest. 1974;53(3):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Winter JS, Hughes IA, Reyes FI, Faiman C. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J Clin Endocrinol Metab. 1976;42(4):679–686. [DOI] [PubMed] [Google Scholar]
- 97. Bolton NJ, Tapanainen J, Koivisto M, Vihko R. Circulating sex hormone-binding globulin and testosterone in newborns and infants. Clin Endocrinol (Oxf). 1989;31(2):201–207. [DOI] [PubMed] [Google Scholar]
- 98. Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90(5):3122–3127. [DOI] [PubMed] [Google Scholar]
- 99. Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Dunkel L, Sankilampi U. Transient postnatal secretion of androgen hormones is associated with acne and sebaceous gland hypertrophy in early infancy. J Clin Endocrinol Metab. 2013;98(1):199–206. [DOI] [PubMed] [Google Scholar]
- 100. Grinspon RP, Loreti N, Braslavsky D, Valeri C, Schteingart H, Ballerini MG, Bedecarrás P, Ambao V, Gottlieb S, Ropelato MG, Bergadá I, Campo SM, Rey RA. Spreading the clinical window for diagnosing fetal-onset hypogonadism in boys. Front Endocrinol (Lausanne). 2014;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kurtoğlu S, Baştuğ O. Mini puberty and its interpretation. Turk Pediatri Ars. 2014;49(3):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dwyer AA, Phan-Hug F, Hauschild M, Elowe-Gruau E, Pitteloud N. Transition in endocrinology: hypogonadism in adolescence. Eur J Endocrinol. 2015;173(1):R15–R24. [DOI] [PubMed] [Google Scholar]
- 103. Copeland KC, Chernausek S. Mini-puberty and growth. Pediatrics. 2016;138(1):e20161301. [DOI] [PubMed] [Google Scholar]
- 104. Contreras M, Raisingani M, Chandler DW, Curtin WD, Barillas J, Brar PC, Prasad K, Shah B, David R. Salivary testosterone during the minipuberty of infancy. Horm Res Paediatr. 2017;87(2):111–115. [DOI] [PubMed] [Google Scholar]
- 105. Cohen HL, Shapiro MA, Mandel FS, Shapiro ML. Normal ovaries in neonates and infants: a sonographic study of 77 patients 1 day to 24 months old. AJR Am J Roentgenol. 1993;160(3):583–586. [DOI] [PubMed] [Google Scholar]
- 106. Kuijper EA, van Kooten J, Verbeke JI, van Rooijen M, Lambalk CB. Ultrasonographically measured testicular volumes in 0- to 6-year-old boys. Hum Reprod. 2008;23(4):792–796. [DOI] [PubMed] [Google Scholar]
- 107. Nguyen RH, Umbach DM, Parad RB, Stroehla B, Rogan WJ, Estroff JA. US assessment of estrogen-responsive organ growth among healthy term infants: piloting methods for assessing estrogenic activity. Pediatr Radiol. 2011;41(5):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kiviranta P, Kuiri-Hänninen T, Saari A, Lamidi ML, Dunkel L, Sankilampi U. Transient postnatal gonadal activation and growth velocity in infancy. Pediatrics. 2016;138(1):e20153561. [DOI] [PubMed] [Google Scholar]
- 109. Mann DR, Akinbami MA, Gould KG, Tanner JM, Wallen K. Neonatal treatment of male monkeys with a gonadotropin-releasing hormone agonist alters differentiation of central nervous system centers that regulate sexual and skeletal development. J Clin Endocrinol Metab. 1993;76(5):1319–1324. [DOI] [PubMed] [Google Scholar]
- 110. Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. [DOI] [PubMed] [Google Scholar]
- 111. Feigelman S. The first year In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, eds. Nelson Textbook of Pediatrics. 18th ed.Philadelphia, PA: Saunders Elsevier; 2007:43–48. [Google Scholar]
- 112. Blair PS, Humphreys JS, Gringras P, Taheri S, Scott N, Emond A, Henderson J, Fleming PJ. Childhood sleep duration and associated demographic characteristics in an English cohort. Sleep (Basel). 2012;35(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sviderskaya EV, Novak EK, Swank RT, Bennett DC. The murine misty mutation: phenotypic effects on melanocytes, platelets and brown fat. Genetics. 1998;148(1):381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170(2):620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure [published correction appears in Nature. 2009;459:122] Nature. 2008;454(7207):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466(7310):1110–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Motyl KJ, Rosen CJ. Temperatures rising: brown fat and bone. Discov Med. 2011;11(58):179–185. [PMC free article] [PubMed] [Google Scholar]
- 118. Motyl KJ, Bishop KA, DeMambro VE, Bornstein SA, Le P, Kawai M, Lotinun S, Horowitz MC, Baron R, Bouxsein ML, Rosen CJ. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res. 2013;28(9):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nguyen AD, Lee NJ, Wee NK, Zhang L, Enriquez RF, Khor EC, Nie T, Wu D, Sainsbury A, Baldock PA, Herzog H.et al. Uncoupling protein-1 is protective of bone mass under mild cold stress conditions. Bone. 2018;106:167–178. [DOI] [PubMed] [Google Scholar]
- 120. Lidell ME, Enerbäck S. Brown adipose tissue and bone. Int J Obes Suppl. 2015; 5(S1, suppl 1)S23–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P, Zhou Y, Zhu LL, Oberlin DJ, Davies TF, Reagan MR, Brown A, Kumar TR, Epstein S, Iqbal J, Avadhani NG, New MI, Molina H, van Klinken JB, Guo EX, Buettner C, Haider S, Bian Z, Sun L, Rosen CJ, Zaidi M. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lecka-Czernik B, Rosen CJ. Skeletal integration of energy homeostasis: translational implications. Bone. 2016;82:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Merklin RJ. Growth and distribution of human fetal brown fat. Anat Rec. 1974;178(3):637–645. [DOI] [PubMed] [Google Scholar]
- 124. Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48(2):243–256. [DOI] [PubMed] [Google Scholar]
- 125. Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond). 1986;71(3):291–297. [DOI] [PubMed] [Google Scholar]
- 126. Symonds ME. Brown adipose tissue growth and development. Scientifica (Cairo). 2013;2013:305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fredriks AM, van Buuren S, van Heel WJ, Dijkman-Neerincx RH, Verloove-Vanhorick SP, Wit JM. Nationwide age references for sitting height, leg length, and sitting height/height ratio, and their diagnostic value for disproportionate growth disorders. Arch Dis Child. 2005;90(8):807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. van der Meulen MC, Beaupré GS, Carter DR. Mechanobiologic influences in long bone cross-sectional growth. Bone. 1993;14(4):635–642. [DOI] [PubMed] [Google Scholar]
- 129. Gilsanz V, Kovanlikaya A, Costin G, Roe TF, Sayre J, Kaufman F. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J Clin Endocrinol Metab. 1997;82(5):1603–1607. [DOI] [PubMed] [Google Scholar]
- 130. Carpenter RD, Carter DR. The mechanobiological effects of periosteal surface loads. Biomech Model Mechanobiol. 2008;7(3):227–242. [DOI] [PubMed] [Google Scholar]
- 131. Doube M, Wiktorowicz-Conroy A, Christiansen P, Hutchinson JR, Shefelbine S. Three-dimensional geometric analysis of felid limb bone allometry [published correction appears in PLoS One. 2009;4(4)] PLoS One. 2009;4(3):e4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Biver E, Perréard Lopreno G, Hars M, van Rietbergen B, Vallée JP, Ferrari S, Besse M, Rizzoli R. Occupation-dependent loading increases bone strength in men. Osteoporos Int. 2016;27(3):1169–1179. [DOI] [PubMed] [Google Scholar]
- 133. Middleditch A, Oliver J. Structure of the vertebral column & normal movement. In: Middleditch A, Oliver J, eds. Functional Anatomy of the Spine. Oxford, England: Butterworth Heinemann; 2005:1–62, 173–208. [Google Scholar]
- 134. Gilsanz V, Boechat MI, Gilsanz R, Loro ML, Roe TF, Goodman WG. Gender differences in vertebral sizes in adults: biomechanical implications. Radiology. 1994;190(3):678–682. [DOI] [PubMed] [Google Scholar]
- 135. Saggese G, Baroncelli GI, Bertelloni S. Puberty and bone development. Best Pract Res Clin Endocrinol Metab. 2002;16(1):53–64. [DOI] [PubMed] [Google Scholar]
- 136. Fares JE, Choucair M, Nabulsi M, Salamoun M, Shahine CH, Fuleihan G-H. Effect of gender, puberty, and vitamin D status on biochemical markers of bone remodeling. Bone. 2003;33(2):242–247. [DOI] [PubMed] [Google Scholar]
- 137. Wren TA, Kim PS, Janicka A, Sanchez M, Gilsanz V. Timing of peak bone mass: discrepancies between CT and DXA. J Clin Endocrinol Metab. 2007;92(3):938–941. [DOI] [PubMed] [Google Scholar]
- 138. Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46(2):294–305. [DOI] [PubMed] [Google Scholar]
- 139. Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporos Int. 2011;22(10):2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev. 2014;35(5):820–847. [DOI] [PubMed] [Google Scholar]
- 141. Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK. The determinants of peak bone mass. J Pediatr. 2017;180:261–269. [DOI] [PubMed] [Google Scholar]
- 142. Mora S, Prinster C, Proverbio MC, Bellini A, de Poli SC, Weber G, Abbiati G, Chiumello G. Urinary markers of bone turnover in healthy children and adolescents: age-related changes and effect of puberty. Calcif Tissue Int. 1998;63(5):369–374. [DOI] [PubMed] [Google Scholar]
- 143. Mora S, Pitukcheewanont P, Kaufman FR, Nelson JC, Gilsanz V. Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J Bone Miner Res. 1999;14(10):1664–1671. [DOI] [PubMed] [Google Scholar]
- 144. van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol (Oxf). 2002;57(1):107–116. [DOI] [PubMed] [Google Scholar]
- 145. Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A, Finkenstedt G, Högler W. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92(2):443–449. [DOI] [PubMed] [Google Scholar]
- 146. Mora S, Cafarelli L, Erba P, Puzzovio M, Zamproni I, Giacomet V, Viganò A. Differential effect of age, gender and puberty on bone formation rate assessed by measurement of bone-specific alkaline phosphatase in healthy Italian children and adolescents. J Bone Miner Metab. 2009;27(6):721–726. [DOI] [PubMed] [Google Scholar]
- 147. Gilsanz V, Perez FJ, Campbell PP, Dorey FJ, Lee DC, Wren TA. Quantitative CT reference values for vertebral trabecular bone density in children and young adults. Radiology. 2009;250(1):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gilsanz V, Gibbens DT, Roe TF, Carlson M, Senac MO, Boechat MI, Huang HK, Schulz EE, Libanati CR, Cann CC. Vertebral bone density in children: effect of puberty. Radiology. 1988;166(3):847–850. [DOI] [PubMed] [Google Scholar]
- 149. Arabi A, Nabulsi M, Maalouf J, Choucair M, Khalifé H, Vieth R, El-Hajj Fuleihan G. Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone. 2004;35(5):1169–1179. [DOI] [PubMed] [Google Scholar]
- 150. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–329. [DOI] [PubMed] [Google Scholar]
- 151. Rogol AD, Clark PA, Roemmich JN. Growth and pubertal development in children and adolescents: effects of diet and physical activity. Am J Clin Nutr. 2000;72(2, suppl)521S–528S. [DOI] [PubMed] [Google Scholar]
- 152. Marcell AV. Adolescence In: Kliegman RM, Jenson HB, Behrman RE, Stanton BF, eds. Nelson Textbook of Pediatrics. 18th ed.Philadelphia, PA: Saunders Elsevier; 2007:60–65. [Google Scholar]
- 153. Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, Zemel BS. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kassem M, Blum W, Ristelli J, Mosekilde L, Eriksen EF. Growth hormone stimulates proliferation and differentiation of normal human osteoblast-like cells in vitro. Calcif Tissue Int. 1993;52(3):222–226. [DOI] [PubMed] [Google Scholar]
- 155. Joung YH, Lim EJ, Darvin P, Chung SC, Jang JW, Do Park K, Lee HK, Kim HS, Park T, Yang YM. MSM enhances GH signaling via the Jak2/STAT5b pathway in osteoblast-like cells and osteoblast differentiation through the activation of STAT5b in MSCs. PLoS One. 2012;7(10):e47477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporosis. Int J Endocrinol. 2014;2014:235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142(9):3836–3841. [DOI] [PubMed] [Google Scholar]
- 158. Wiwi CA, Waxman DJ. Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450. Growth Factors. 2004;22(2):79–88. [DOI] [PubMed] [Google Scholar]
- 159. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333–1351. [DOI] [PubMed] [Google Scholar]
- 160. Yakar S, Rosen CJ. From mouse to man: redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp Biol Med (Maywood). 2003;228(3):245–252. [DOI] [PubMed] [Google Scholar]
- 161. Yakar S, Courtland HW, Clemmons D. IGF-1 and bone: new discoveries from mouse models. J Bone Miner Res. 2010;25(12):2543–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41(2):323–333, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wang Y, Bikle DD, Chang W. Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res. 2013;1(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, Bikle DD. IGF-I Signaling in osterix-expressing cells regulates secondary ossification center formation, growth plate maturation, and metaphyseal formation during postnatal bone development. J Bone Miner Res. 2015;30(12):2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Maridas DE, DeMambro VE, Le PT, Nagano K, Baron R, Mohan S, Rosen CJ. IGFBP-4 regulates adult skeletal growth in a sex-specific manner. J Endocrinol. 2017;233(1):131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Youssef A, Aboalola D, Han VK. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017;2017:9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, Tanaka K, Kumegawa M. Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology. 1992;131(3):1075–1080. [DOI] [PubMed] [Google Scholar]
- 169. Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21(9):1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sheng MH, Lau KH, Baylink DJ. Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J Bone Metab. 2014;21(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ernst M, Rodan GA. Estradiol regulation of insulin-like growth factor-I expression in osteoblastic cells: evidence for transcriptional control. Mol Endocrinol. 1991;5(8):1081–1089. [DOI] [PubMed] [Google Scholar]
- 172. Lakatos P, Caplice MD, Khanna V, Stern PH. Thyroid hormones increase insulin-like growth factor I content in the medium of rat bone tissue. J Bone Miner Res. 1993;8(12):1475–1481. [DOI] [PubMed] [Google Scholar]
- 173. Richards RG, DiAugustine RP, Petrusz P, Clark GC, Sebastian J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci USA. 1996;93(21):12002–12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15(10):1781–1789. [DOI] [PubMed] [Google Scholar]
- 175. Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29(5):535–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fritton JC, Emerton KB, Sun H, Kawashima Y, Mejia W, Wu Y, Rosen CJ, Panus D, Bouxsein M, Majeska RJ, Schaffler MB, Yakar S. Growth hormone protects against ovariectomy-induced bone loss in states of low circulating insulin-like growth factor (IGF-1). J Bone Miner Res. 2010;25(2):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lee JH, Hwang KJ, Kim MY, Lim YJ, Seol IJ, Jin HJ, Jang YK, Choi SJ, Oh W, Cho YH, Lee YH. Human parathyroid hormone increases the mRNA expression of the IGF system and hematopoietic growth factors in osteoblasts, but does not influence expression in mesenchymal stem cells. J Pediatr Hematol Oncol. 2012;34(7):491–496. [DOI] [PubMed] [Google Scholar]
- 178. Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37(2):135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lindsey RC, Mohan S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol Cell Endocrinol. 2016;432:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Vanderschueren D, Vandenput L, Boonen S. Reversing sex steroid deficiency and optimizing skeletal development in the adolescent with gonadal failure. Endocr Dev. 2005;8:150–165. [DOI] [PubMed] [Google Scholar]
- 181. Rogol AD. Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev. 2010;17:77–85. [DOI] [PubMed] [Google Scholar]
- 182. Wit JM, Camacho-Hübner C. Endocrine regulation of longitudinal bone growth. Endocr Dev. 2011;21:30–41. [DOI] [PubMed] [Google Scholar]
- 183. Bredella MA, Fazeli PK, Lecka-Czernik B, Rosen CJ, Klibanski A. IGFBP-2 is a negative predictor of cold-induced brown fat and bone mineral density in young non-obese women. Bone. 2013;53(2):336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bredella MA, Gill CM, Rosen CJ, Klibanski A, Torriani M. Positive effects of brown adipose tissue on femoral bone structure. Bone. 2014;58:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gilsanz V, Gibbens DT, Carlson M, Boechat MI, Cann CE, Schulz EE. Peak trabecular vertebral density: a comparison of adolescent and adult females. Calcif Tissue Int. 1988;43(4):260–262. [DOI] [PubMed] [Google Scholar]
- 186. Venken K, Schuit F, Van Lommel L, Tsukamoto K, Kopchick JJ, Coschigano K, Ohlsson C, Movérare S, Boonen S, Bouillon R, Vanderschueren D. Growth without growth hormone receptor: estradiol is a major growth hormone-independent regulator of hepatic IGF-I synthesis. J Bone Miner Res. 2005;20(12):2138–2149. [DOI] [PubMed] [Google Scholar]
- 187. Gelfand MJ, O’hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35(10):984–990. [DOI] [PubMed] [Google Scholar]
- 188. Drubach LA, Palmer EL III, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr. 2011;159(6):939–944. [DOI] [PubMed] [Google Scholar]
- 189. Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160(4):604–609.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55(6):1597–1606. [DOI] [PubMed] [Google Scholar]
- 191. Rogers NH. Brown adipose tissue during puberty and with aging. Ann Med. 2015;47(2):142–149. [DOI] [PubMed] [Google Scholar]
- 192. Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97(8):2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lee P, Brychta RJ, Collins MT, Linderman J, Smith S, Herscovitch P, Millo C, Chen KY, Celi FS. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos Int. 2013;24(4):1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Block JE, Smith R, Glueer CC, Steiger P, Ettinger B, Genant HK. Models of spinal trabecular bone loss as determined by quantitative computed tomography. J Bone Miner Res. 1989;4(2):249–257. [DOI] [PubMed] [Google Scholar]
- 195. Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporos Int. 1994;4(suppl 1):7–13. [DOI] [PubMed] [Google Scholar]
- 197. Sabatier JP, Guaydier-Souquières G, Laroche D, Benmalek A, Fournier L, Guillon-Metz F, Delavenne J, Denis AY. Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int. 1996;6(2):141–148. [DOI] [PubMed] [Google Scholar]
- 198. Löfman O, Larsson L, Toss G. Bone mineral density in diagnosis of osteoporosis: reference population, definition of peak bone mass, and measured site determine prevalence. J Clin Densitom. 2000;3(2):177–186. [DOI] [PubMed] [Google Scholar]
- 199. Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32(1):39–63. [DOI] [PubMed] [Google Scholar]
- 200. Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15(4):263–273. [DOI] [PubMed] [Google Scholar]
- 201. Mosekilde L, Mosekilde L. Sex differences in age-related changes in vertebral body size, density and biomechanical competence in normal individuals. Bone. 1990;11(2):67–73. [DOI] [PubMed] [Google Scholar]
- 202. Mosekilde L, Mosekilde L. Normal vertebral body size and compressive strength: relations to age and to vertebral and iliac trabecular bone compressive strength. Bone. 1986;7(3):207–212. [DOI] [PubMed] [Google Scholar]
- 203. Mosekilde L. Age-related changes in bone mass, structure, and strength: effects of loading. Z Rheumatol. 2000;59(suppl 1):1–9. [DOI] [PubMed] [Google Scholar]
- 204. Seeman E, Duan Y, Fong C, Edmonds J. Fracture site-specific deficits in bone size and volumetric density in men with spine or hip fractures. J Bone Miner Res. 2001;16(1):120–127. [DOI] [PubMed] [Google Scholar]
- 205. Riggs BL, Melton Iii LJ III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–1954. [DOI] [PubMed] [Google Scholar]
- 206. Villanueva AR, Sedlin ED, Frost HM. Variations in osteoblastic activity with age by the osteoid seam index. Anat Rec. 1963;146(3):209–213. [DOI] [PubMed] [Google Scholar]
- 207. Weaver JK, Chalmers J. Cancellous bone: its strength and changes with aging and an evaluation of some methods for measuring its mineral content. J Bone Joint Surg Am. 1966;48(2):289–298. [PubMed] [Google Scholar]
- 208. Merz WA, Schenk RK. A quantitative histological study on bone formation in human cancellous bone. Acta Anat (Basel). 1970;76(1):1–15. [DOI] [PubMed] [Google Scholar]
- 209. Arnold JS. Amount and quality of trabecular bone in osteoporotic vertebral fractures. Clin Endocrinol Metab. 1973;2(2):221–238. [DOI] [PubMed] [Google Scholar]
- 210. Marcus R, Kosek J, Pfefferbaum A, Horning S. Age-related loss of trabecular bone in premenopausal women: a biopsy study. Calcif Tissue Int. 1983;35(4–5):406–409. [DOI] [PubMed] [Google Scholar]
- 211. Ito M, Lang TF, Jergas M, Ohki M, Takada M, Nakamura T, Hayashi K, Genant HK. Spinal trabecular bone loss and fracture in American and Japanese women. Calcif Tissue Int. 1997;61(2):123–128. [DOI] [PubMed] [Google Scholar]
- 212. Kalender WA, Felsenberg D, Louis O, Lopez P, Klotz E, Osteaux M, Fraga J. Reference values for trabecular and cortical vertebral bone density in single and dual-energy quantitative computed tomography. Eur J Radiol. 1989;9(2):75–80. [PubMed] [Google Scholar]
- 213. Fournier PE, Rizzoli R, Slosman DO, Buchs B, Bonjour JP. Relative contribution of vertebral body and posterior arch in female and male lumbar spine peak bone mass. Osteoporos Int. 1994;4(5):264–272. [DOI] [PubMed] [Google Scholar]
- 214. Bouxsein ML, Melton LJ III, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, Khosla S. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J Bone Miner Res. 2006;21(9):1475–1482. [DOI] [PubMed] [Google Scholar]
- 215. Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab. 2000;85(10):3908–3918. [DOI] [PubMed] [Google Scholar]
- 216. Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, Huang X, Frederick MM, Winer KK, Zemel BS. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95(4):1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, Winer KK, Gilsanz V; Bone Mineral Density in Childhood Study Group . Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164(6):1280–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. [DOI] [PubMed] [Google Scholar]
- 219. Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79(4):557–565. [DOI] [PubMed] [Google Scholar]
- 220. Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19(8):458–466. [DOI] [PubMed] [Google Scholar]
- 221. Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309(5737):1074–1078. [DOI] [PubMed] [Google Scholar]
- 222. Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Veldhuis-Vlug AG, Rosen CJ. Mechanisms of marrow adiposity and its implications for skeletal health. Metabolism. 2017;67:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96(3):782–786. [DOI] [PubMed] [Google Scholar]
- 225. Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, Albu J, Engelson E, Kotler D, Pi-Sunyer X, Gilsanz V. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur J Clin Nutr. 2012;66(9):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95(6):2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80(3):706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Christian JC, Yu PL, Slemenda CW, Johnston CC Jr. Heritability of bone mass: a longitudinal study in aging male twins. Am J Hum Genet. 1989;44(3):429–433. [PMC free article] [PubMed] [Google Scholar]
- 229. Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jerums G. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320(9):554–558. [DOI] [PubMed] [Google Scholar]
- 230. Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8(1):1–9. [DOI] [PubMed] [Google Scholar]
- 231. Kelly PJ, Nguyen T, Hopper J, Pocock N, Sambrook P, Eisman J. Changes in axial bone density with age: a twin study. J Bone Miner Res. 1993;8(1):11–17. [DOI] [PubMed] [Google Scholar]
- 232. Brown LB, Streeten EA, Shapiro JR, McBride D, Shuldiner AR, Peyser PA, Mitchell BD. Genetic and environmental influences on bone mineral density in pre- and post-menopausal women. Osteoporos Int. 2005;16(12):1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations [published correction appears in Osteoporos Int. 2016;27(4)1387] Osteoporos Int. 2016;274(4):1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Evans RA, Marel GM, Lancaster EK, Kos S, Evans M, Wong SY. Bone mass is low in relatives of osteoporotic patients. Ann Intern Med. 1988;109(11):870–873. [DOI] [PubMed] [Google Scholar]
- 235. Kröger H, Kotaniemi A, Vainio P, Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner. 1992;17(1):75–85. [DOI] [PubMed] [Google Scholar]
- 236. Fewtrell MS; British Paediatric & Adolescent Bone Group . Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child. 2003;88(9):795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone acquisition in healthy children and adolescents: comparisons of dual-energy x-ray absorptiometry and computed tomography measures. J Clin Endocrinol Metab. 2005;90(4):1925–1928. [DOI] [PubMed] [Google Scholar]
- 238. Engelke K, Glüer CC. Quality and performance measures in bone densitometry: part 1: errors and diagnosis. Osteoporos Int. 2006;17(9):1283–1292. [DOI] [PubMed] [Google Scholar]
- 239. Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet. 1996;14(2):203–205. [DOI] [PubMed] [Google Scholar]
- 240. Sainz J, Van Tornout JM, Sayre J, Kaufman F, Gilsanz V. Association of collagen type 1 alpha1 gene polymorphism with bone density in early childhood. J Clin Endocrinol Metab. 1999;84(3):853–855. [DOI] [PubMed] [Google Scholar]
- 241. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. [DOI] [PubMed] [Google Scholar]
- 242. Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Boroń D, Kamiński A, Kotrych D, Bogacz A, Uzar I, Mrozikiewicz PM, Czerny B. Polymorphism of vitamin D3 receptor and its relation to mineral bone density in perimenopausal women. Osteoporos Int. 2015;26(3):1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Liu YJ, Zhang L, Papasian CJ, Deng HW. Genome-wide association studies for osteoporosis: a 2013 update. J Bone Metab. 2014;21(2):99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gómez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellström D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren Ö, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussière J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Åkesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB; AOGC Consortium; UK10K Consortium . Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Mitchell JA, Chesi A, McCormack SE, Roy SM, Cousminer DL, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Kelly A, Zemel BS, Grant SF. Rare EN1 variants and pediatric bone mass. J Bone Miner Res. 2016;31(8):1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Chesi A, Mitchell JA, Kalkwarf HJ, Bradfield JP, Lappe JM, Cousminer DL, Roy SM, McCormack SE, Gilsanz V, Oberfield SE, Hakonarson H, Shepherd JA, Kelly A, Zemel BS, Grant SF. A genomewide association study identifies two sex-specific loci, at SPTB and IZUMO3, influencing pediatric bone mineral density at multiple skeletal sites. J Bone Miner Res. 2017;32(6):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Roh HL, Lee JS, Suh KT, Kim JI, Lee HS, Goh TS, Park SH. Association between estrogen receptor gene polymorphism and back pain intensity in female patients with degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2013;26(2):E53–E57. [DOI] [PubMed] [Google Scholar]
- 249. Cai T, Yang L, Cai W, Guo S, Yu P, Li J, Hu X, Yan M, Shao Q, Jin Y, Sun ZS, Luo ZJ. Dysplastic spondylolysis is caused by mutations in the diastrophic dysplasia sulfate transporter gene. Proc Natl Acad Sci USA. 2015;112(26):8064–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Gao X, Gotway G, Rathjen K, Johnston C, Sparagana S, Wise CA. Genomic analyses of patients with unexplained early onset scoliosis. Spine Deform. 2014;2(5):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Haller G, Alvarado D, Mccall K, Yang P, Cruchaga C, Harms M, Goate A, Willing M, Morcuende JA, Baschal E, Miller NH, Wise C, Dobbs MB, Gurnett CA. A polygenic burden of rare variants across extracellular matrix genes among individuals with adolescent idiopathic scoliosis. Hum Mol Genet. 2016;25(1):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Park-Min KH. Epigenetic regulation of bone cells. Connect Tissue Res. 2017;58(1):76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Husain A, Jeffries MA. Epigenetics and bone remodeling. Curr Osteoporos Rep. 2017;15(5):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Abrams SA, Stuff JE. Calcium metabolism in girls: current dietary intakes lead to low rates of calcium absorption and retention during puberty. Am J Clin Nutr. 1994;60(5):739–743. [DOI] [PubMed] [Google Scholar]
- 255. Kitchin B, Morgan SL. Not just calcium and vitamin D: other nutritional considerations in osteoporosis. Curr Rheumatol Rep. 2007;9(1):85–92. [DOI] [PubMed] [Google Scholar]
- 256. Cvijetic S, Baric IC, Satalic Z, Keser I, Bobic J. Influence of nutrition and lifestyle on bone mineral density in children from adoptive and biological families. J Epidemiol. 2014;24(3):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Chevalley T, Bonjour JP, van Rietbergen B, Ferrari S, Rizzoli R. Tracking of environmental determinants of bone structure and strength development in healthy boys: an eight-year follow up study on the positive interaction between physical activity and protein intake from prepuberty to mid-late adolescence. J Bone Miner Res. 2014;29(10):2182–2192. [DOI] [PubMed] [Google Scholar]
- 258. Lappe JM, Watson P, Gilsanz V, Hangartner T, Kalkwarf HJ, Oberfield S, Shepherd J, Winer KK, Zemel B. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J Bone Miner Res. 2015;30(1):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kirchner EM, Lewis RD, O’Connor PJ. Effect of past gymnastics participation on adult bone mass. J Appl Physiol (1985). 1996;80(1):226–232. [DOI] [PubMed] [Google Scholar]
- 260. Zanker CL, Gannon L, Cooke CB, Gee KL, Oldroyd B, Truscott JG. Differences in bone density, body composition, physical activity, and diet between child gymnasts and untrained children 7-8 years of age. J Bone Miner Res. 2003;18(6):1043–1050. [DOI] [PubMed] [Google Scholar]
- 261. Specker BL. Influence of rapid growth on skeletal adaptation to exercise. J Musculoskelet Neuronal Interact. 2006;6(2):147–153. [PubMed] [Google Scholar]
- 262. Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuller A, Durski S, Snow C. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. 2008;42(4):710–718. [DOI] [PubMed] [Google Scholar]
- 263. Janz KF, Letuchy EM, Francis SL, Metcalf KM, Burns TL, Levy SM. Objectively measured physical activity predicts hip and spine bone mineral content in children and adolescents ages 5–15 years: Iowa Bone Development Study. Front Endocrinol (Lausanne). 2014;5:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Specker B, Thiex NW, Sudhagoni RG. Does exercise influence pediatric bone? A systematic review. Clin Orthop Relat Res. 2015;473(11):3658–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Dowthwaite JN, Rosenbaum PF, Scerpella TA. Mechanical loading during growth is associated with plane-specific differences in vertebral geometry: a cross-sectional analysis comparing artistic gymnasts vs. non-gymnasts. Bone. 2011;49(5):1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Oura P, Paananen M, Niinimäki J, Tammelin T, Herrala S, Auvinen J, Korpelainen R, Junno JA, Karppinen J. Effects of leisure-time physical activity on vertebral dimensions in the Northern Finland Birth Cohort 1966. Sci Rep. 2016;6(1):27844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Ren J, Brann LS, Bruening KS, Scerpella TA, Dowthwaite JN. Relationships among diet, physical activity, and dual plane dual-energy X-ray absorptiometry bone outcomes in pre-pubertal girls. Arch Osteoporos. 2017;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Ruff CB. Gracilization of the modern human skeleton: the latent strength in our slender bones teaches lessons about human lives, current and past. Am Sci. 2006;94(6):508–514. [Google Scholar]
- 270. Junno JA, Niskanen M, Nieminen MT, Maijanen H, Niinimäki J, Bloigu R, Tuukkanen J. Temporal trends in vertebral size and shape from medieval to modern-day. PLoS One. 2009;4(3):e4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wren TA, Ponrartana S, Gilsanz V. Vertebral cross-sectional area: an orphan phenotype with potential implications for female spinal health. Osteoporos Int. 2017;28(4):1179–1189. [DOI] [PubMed] [Google Scholar]
- 272. Wren TAL, Aggabao PC, Poorghasamians E, Chavez TA, Ponrartana S, Gilsanz V. Association between vertebral cross-sectional area and lumbar lordosis angle in adolescents. PLoS One. 2017;12(2):e0172844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Wren TAL, Ponrartana S, Aggabao PC, Poorghasamians E, Skaggs DL, Gilsanz V. Increased lumbar lordosis and smaller vertebral cross-sectional area are associated with spondylolysis [published online ahead of print October 31, 2017]. Spine. [DOI] [PubMed]
- 274. Ponrartana S, Fisher CL, Aggabao PC, Chavez TA, Broom AM, Wren TA, Skaggs DL, Gilsanz V. Small vertebral cross-sectional area and tall intervertebral disc in adolescent idiopathic scoliosis. Pediatr Radiol. 2016;46(10):1424–1429. [DOI] [PubMed] [Google Scholar]
- 275. Wren TAL, Ponrartana S, Poorghasamians E, Moreau S, Aggabao PC, Zaslow TL, Edison BR, Gilsanz V. Biomechanical modeling of spine flexibility and its relationship to spinal range of motion and idiopathic scoliosis. Spine Deform. 2017;5(4):225–230. [DOI] [PubMed] [Google Scholar]
- 276. Wren TAL, Ponrartana S, Aggabao PC, Poorghasamians E, Gilsanz V. Association between vertebral cross-sectional area and vertebral wedging in children and adolescents: a cross-sectional analysis. J Bone Miner Res. 2017;32(11):2257–2262. [DOI] [PubMed] [Google Scholar]
- 277. Poorghasamians E, Aggabao PC, Wren TAL, Ponrartana S, Gilsanz V. Vertebral cross-sectional growth: a predictor of vertebral wedging in the immature skeleton. PLoS One. 2017;12(12):e0190225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Gilsanz V, Loro ML, Roe TF, Sayre J, Gilsanz R, Schulz EE. Vertebral size in elderly women with osteoporosis. Mechanical implications and relationship to fractures. J Clin Invest. 1995;95(5):2332–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Ruyssen-Witrand A, Gossec L, Kolta S, Dougados M, Roux C. Vertebral dimensions as risk factor of vertebral fracture in osteoporotic patients: a systematic literature review. Osteoporos Int. 2007;18(9):1271–1278. [DOI] [PubMed] [Google Scholar]
- 280. Kim HJ, Yeom JS, Lee DB, Kang KT, Chang BS, Lee CK. Association of benign joint hypermobility with spinal segmental motion and its clinical implication in active young males. Spine. 2013;38(16):E1013–E1019. [DOI] [PubMed] [Google Scholar]
- 281. Haley SM, Tada WL, Carmichael EM. Spinal mobility in young children. A normative study. Phys Ther. 1986;66(11):1697–1703. [DOI] [PubMed] [Google Scholar]
- 282. Twomey LT, Taylor JR. Lumbar posture, movement and mechanics In: Twomey LT, Taylor JR, eds. Physical Therapy of the Low Back. Edinburgh, Scotland: Churchill Livingstone; 1987:51–84. [Google Scholar]
- 283. Penha PJ, Casarotto RA, Sacco ICN, Marques AP, João SMA. Qualitative postural analysis among boys and girls of seven to ten years of age. Rev Bras Fisioter. 2008;12(5):386–391. [Google Scholar]
- 284. Taylor JR, Twomey LT. Sexual dimorphism in human vertebral body shape. J Anat. 1984;138(Pt 2):281–286. [PMC free article] [PubMed] [Google Scholar]
- 285. Hodson GC. Vertebral body dimensions: an aid to diagnosis of severely progressive adolescent idiopathic scoliosis? Aust J Physiother. 1984;30(2):39–41. [DOI] [PubMed] [Google Scholar]
- 286. Shefi S, Soudack M, Konen E, Been E. Development of the lumbar lordotic curvature in children from age 2 to 20 years. Spine. 2013;38(10):E602–E608. [DOI] [PubMed] [Google Scholar]
- 287. Fernand R, Fox DE. Evaluation of lumbar lordosis. A prospective and retrospective study. Spine. 1985;10(9):799–803. [DOI] [PubMed] [Google Scholar]
- 288. Nissinen M. Spinal posture during pubertal growth. Acta Paediatr. 1995;84(3):308–312. [DOI] [PubMed] [Google Scholar]
- 289. Smith AJ, O'Sullivan PB, Beales DJ, de Klerk N, Straker LM. Trajectories of childhood body mass index are associated with adolescent sagittal standing posture. Int J Pediatr Obes. 2011;6(2–2):e97–106. [DOI] [PubMed] [Google Scholar]
- 290. Dolphens M, Cagnie B, Vleeming A, Vanderstraeten G, Danneels L. Gender differences in sagittal standing alignment before pubertal peak growth: the importance of subclassification and implications for spinopelvic loading. J Anat. 2013;223(6):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine. 2007;32(1):120–125. [DOI] [PubMed] [Google Scholar]
- 292. Been E, Li L, Hunter DJ, Kalichman L. Geometry of the vertebral bodies and the intervertebral discs in lumbar segments adjacent to spondylolysis and spondylolisthesis: pilot study. Eur Spine J. 2011;20(7):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Chung SB, Lee S, Kim H, Lee SH, Kim ES, Eoh W. Significance of interfacet distance, facet joint orientation, and lumbar lordosis in spondylolysis. Clin Anat. 2012;25(3):391–397. [DOI] [PubMed] [Google Scholar]
- 294. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007;6(1):62–66. [DOI] [PubMed] [Google Scholar]
- 295. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656–671. [PubMed] [Google Scholar]
- 296. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683–700. [DOI] [PubMed] [Google Scholar]
- 297. Lawrence KJ, Elser T, Stromberg R. Lumbar spondylolysis in the adolescent athlete. Phys Ther Sport. 2016;20:56–60. [DOI] [PubMed] [Google Scholar]
- 298. Masharawi Y, Dar G, Peleg S, Steinberg N, Alperovitch-Najenson D, Salame K, Hershkovitz I. Lumbar facet anatomy changes in spondylolysis: a comparative skeletal study. Eur Spine J. 2007;16(7):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Don AS, Robertson PA. Facet joint orientation in spondylolysis and isthmic spondylolisthesis. J Spinal Disord Tech. 2008;21(2):112–115. [DOI] [PubMed] [Google Scholar]
- 300. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527–1537. [DOI] [PubMed] [Google Scholar]
- 301. Burwell RG, Dangerfield PH, Moulton A, Grivas TB, Cheng JC. Whither the etiopathogenesis (and scoliogeny) of adolescent idiopathic scoliosis? Incorporating presentations on scoliogeny at the 2012 IRSSD and SRS meetings. Scoliosis. 2013;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Dayer R, Haumont T, Belaieff W, Lascombes P. Idiopathic scoliosis: etiological concepts and hypotheses. J Child Orthop. 2013;7(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. National Scoliosis Foundation Information and support. 2016. http://www.scoliosis.org/info.php. Accessed 4 May 2017.
- 304. Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013;7(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Warren MP, Brooks-Gunn J, Hamilton LH, Warren LF, Hamilton WG. Scoliosis and fractures in young ballet dancers. Relation to delayed menarche and secondary amenorrhea. N Engl J Med. 1986;314(21):1348–1353. [DOI] [PubMed] [Google Scholar]
- 306. Hellström M, Jacobsson B, Swärd L, Peterson L. Radiologic abnormalities of the thoraco-lumbar spine in athletes. Acta Radiol. 1990;31(2):127–132. [PubMed] [Google Scholar]
- 307. Tanchev PI, Dzherov AD, Parushev AD, Dikov DM, Todorov MB. Scoliosis in rhythmic gymnasts. Spine. 2000;25(11):1367–1372. [DOI] [PubMed] [Google Scholar]
- 308. Meyer C, Cammarata E, Haumont T, Deviterne D, Gauchard GC, Leheup B, Lascombes P, Perrin PP. Why do idiopathic scoliosis patients participate more in gymnastics? Scand J Med Sci Sports. 2006;16(4):231–236. [DOI] [PubMed] [Google Scholar]
- 309. Schiller JR, Eberson CP. Spinal deformity and athletics. Sports Med Arthrosc Rev. 2008;16(1):26–31. [DOI] [PubMed] [Google Scholar]
- 310. Czaprowski D. Generalised joint hypermobility in caucasian girls with idiopathic scoliosis: relation with age, curve size, and curve pattern. ScientificWorldJournal. 2014;2014:370134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, Ratner ES. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7(2):168–174. [DOI] [PubMed] [Google Scholar]
- 312. Thomas KA, Cook SD, Skalley TC, Renshaw SV, Makuch RS, Gross M, Whitecloud TS III, Bennett JT. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow-up study. J Pediatr Orthop. 1992;12(2):235–240. [DOI] [PubMed] [Google Scholar]
- 313. Cheng JC, Guo X, Sher AH. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine. 1999;24(12):1218–1222. [DOI] [PubMed] [Google Scholar]
- 314. Sadat-Ali M, Al-Othman A, Bubshait D, Al-Dakheel D. Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J. 2008;17(7):944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Ishida K, Aota Y, Mitsugi N, Kono M, Higashi T, Kawai T, Yamada K, Niimura T, Kaneko K, Tanabe H, Ito Y, Katsuhata T, Saito T. Relationship between bone density and bone metabolism in adolescent idiopathic scoliosis [published correction appears in Scoliosis. 2015;10:34] Scoliosis. 2015;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 318. Hung VW, Qin L, Cheung CS, Lam TP, Ng BK, Tse YK, Guo X, Lee KM, Cheng JC. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87(12):2709–2716. [DOI] [PubMed] [Google Scholar]
- 319. Kachanov LM. Fundamentals of the Theory of Plasticity. Mineola, NY: Dover Publications, Inc; 2004:1–4. [Google Scholar]
- 320. Been E, Barash A, Marom A, Kramer PA. Vertebral bodies or discs: which contributes more to human-like lumbar lordosis? Clin Orthop Relat Res. 2010;468(7):1822–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Bailey JF, Sparrey CJ, Been E, Kramer PA. Morphological and postural sexual dimorphism of the lumbar spine facilitates greater lordosis in females. J Anat. 2016;229(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Stokes IA. Analysis and simulation of progressive adolescent scoliosis by biomechanical growth modulation. Eur Spine J. 2007;16(10):1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. de Sèze M, Cugy E. Pathogenesis of idiopathic scoliosis: a review. Ann Phys Rehabil Med. 2012;55(2):128–138. [DOI] [PubMed] [Google Scholar]
- 324. Scherrer SA, Begon M, Leardini A, Coillard C, Rivard CH, Allard P. Three-dimensional vertebral wedging in mild and moderate adolescent idiopathic scoliosis. PLoS One. 2013;8(8):e71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Modi HN, Suh SW, Song HR, Yang JH, Kim HJ, Modi CH. Differential wedging of vertebral body and intervertebral disc in thoracic and lumbar spine in adolescent idiopathic scoliosis: a cross sectional study in 150 patients. Scoliosis. 2008;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Hasler CC, Hefti F, Büchler P. Coronal plane segmental flexibility in thoracic adolescent idiopathic scoliosis assessed by fulcrum-bending radiographs. Eur Spine J. 2010;19(5):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Makino T, Kaito T, Sakai Y, Takenaka S, Sugamoto K, Yoshikawa H. Plasticity of vertebral wedge deformities in skeletally immature patients with adolescent idiopathic scoliosis after posterior corrective surgery. BMC Musculoskelet Disord. 2016;17(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Stokes IA. Mechanical effects on skeletal growth. J Musculoskelet Neuronal Interact. 2002;2(3):277–280. [PubMed] [Google Scholar]
- 329. Sarwark J, Aubin CE. Growth considerations of the immature spine. J Bone Joint Surg Am. 2007;89(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 330. Goswami R, Shah P, Ammini AC, Berry M. Healing of osteoporotic vertebral compression fractures following cure of Cushing’s syndrome. Australas Radiol. 1995;39(2):195–197. [DOI] [PubMed] [Google Scholar]
- 331. Tauchmanovà L, Pivonello R, Di Somma C, Rossi R, De Martino MC, Camera L, Klain M, Salvatore M, Lombardi G, Colao A. Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab. 2006;91(5):1779–1784. [DOI] [PubMed] [Google Scholar]
- 332. Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, Shenouda N, Lentle B, Abish S, Atkinson S, Cairney E, Dix D, Israels S, Stephure D, Wilson B, Hay J, Moher D, Rauch F, Siminoski K, Ward LM; Canadian STOPP Consortium . Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24(7):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Alos N, Grant RM, Ramsay T, Halton J, Cummings EA, Miettunen PM, Abish S, Atkinson S, Barr R, Cabral DA, Cairney E, Couch R, Dix DB, Fernandez CV, Hay J, Israels S, Laverdière C, Lentle B, Lewis V, Matzinger M, Rodd C, Shenouda N, Stein R, Stephure D, Taback S, Wilson B, Williams K, Rauch F, Siminoski K, Ward LM. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol. 2012;30(22):2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Trementino L, Appolloni G, Ceccoli L, Marcelli G, Concettoni C, Boscaro M, Arnaldi G. Bone complications in patients with Cushing’s syndrome: looking for clinical, biochemical, and genetic determinants. Osteoporos Int. 2014;25(3):913–921. [DOI] [PubMed] [Google Scholar]
- 335. Mostoufi-Moab S, Halton J. Bone morbidity in childhood leukemia: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. 2014;12(3):300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Cummings EA, Ma J, Fernandez CV, Halton J, Alos N, Miettunen PM, Jaremko JL, Ho J, Shenouda N, Matzinger MA, Lentle B, Stephure D, Stein R, Sbrocchi AM, Rodd C, Lang B, Israels S, Grant RM, Couch R, Barr R, Hay J, Rauch F, Siminoski K, Ward LM; Canadian STOPP Consortium (National Pediatric Bone Health Working Group) . Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab. 2015;100(9):3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Pandya NA, Meller ST, MacVicar D, Atra AA, Pinkerton CR. Vertebral compression fractures in acute lymphoblastic leukaemia and remodelling after treatment. Arch Dis Child. 2001;85(6):492–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Tsai PY, Tzeng WS. Images in clinical medicine. Vertebra plana with spontaneous healing. N Engl J Med. 2012;366(19):e30. [DOI] [PubMed] [Google Scholar]
- 339. Dal Osto LC, Konji VN, Halton J, Matzinger MA, Bassal M, Rauch F, Ward LM. The spectrum of recovery from fracture-induced vertebral deformity in pediatric leukemia. Pediatr Blood Cancer. 2016;63(6):1107–1110. [DOI] [PubMed] [Google Scholar]
- 340. Ippolito E, Farsetti P, Tudisco C. Vertebra plana. Long-term follow-up in five patients. J Bone Joint Surg Am. 1984;66(9):1364–1368. [PubMed] [Google Scholar]
- 341. Bruno AG, Anderson DE, D’Agostino J, Bouxsein ML. The effect of thoracic kyphosis and sagittal plane alignment on vertebral compressive loading. J Bone Miner Res. 2012;27(10):2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Melton LJ III, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129(5):1000–1011. [DOI] [PubMed] [Google Scholar]
- 343. Duan Y, Turner CH, Kim BT, Seeman E. Sexual dimorphism in vertebral fragility is more the result of gender differences in age-related bone gain than bone loss. J Bone Miner Res. 2001;16(12):2267–2275. [DOI] [PubMed] [Google Scholar]
- 344. Chen P, Krege JH, Adachi JD, Prior JC, Tenenhouse A, Brown JP, Papadimitropoulos E, Kreiger N, Olszynski WP, Josse RG, Goltzman D; CaMOS Research Group . Vertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk. J Bone Miner Res. 2009;24(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Bruno AG, Broe KE, Zhang X, Samelson EJ, Meng CA, Manoharan R, D’Agostino J, Cupples LA, Kiel DP, Bouxsein ML. Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD. J Bone Miner Res. 2014;29(3):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Old JL, Calvert M. Vertebral compression fractures in the elderly. Am Fam Physician. 2004;69(1):111–116. [PubMed] [Google Scholar]
- 347. Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68(10):1236–1242. [DOI] [PubMed] [Google Scholar]
- 348. Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017;28(5):1531–1542. [DOI] [PubMed] [Google Scholar]
- 349. Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11(7):577–582. [DOI] [PubMed] [Google Scholar]
- 350. Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE; Fracture Intervention Trial Research Group . What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20(7):1216–1222. [DOI] [PubMed] [Google Scholar]
- 351. Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res. 2011;26(5):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Wren TA, Gilsanz V. Evolving role of imaging in the evaluation of bone structure. J Bone Miner Res. 2009;24(12):1943–1945. [DOI] [PubMed] [Google Scholar]
- 353. Blake G, Adams JE, Bishop N. DXA in adults and children In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed.Hoboken, NJ: John Wiley & Sons, Inc.; 2013:249–263. [Google Scholar]
- 354. Cooper C. The epidemiology of fragility fractures: is there a role for bone quality? Calcif Tissue Int. 1993;53(suppl 1):S23–S26. [DOI] [PubMed] [Google Scholar]
- 355. Nielsen SP, Hermansen F, Bärenholdt O. Interpretation of lumbar spine densitometry in women with fractures. Osteoporos Int. 1993;3(5):276–282. [DOI] [PubMed] [Google Scholar]
- 356. Ross PD, Huang C, Davis JW, Wasnich RD. Vertebral dimension measurements improve prediction of vertebral fracture incidence. Bone. 1995;16(4suppl):257S–262S. [DOI] [PubMed] [Google Scholar]
- 357. Ito M, Ohki M, Hayashi K, Yamada M, Uetani M, Nakamura T. Relationship of spinal fracture to bone density, textural, and anthropometric parameters. Calcif Tissue Int. 1997;60(3):240–244. [DOI] [PubMed] [Google Scholar]
- 358. Vega E, Ghiringhelli G, Mautalen C, Rey Valzacchi G, Scaglia H, Zylberstein C. Bone mineral density and bone size in men with primary osteoporosis and vertebral fractures. Calcif Tissue Int. 1998;62(5):465–469. [DOI] [PubMed] [Google Scholar]
- 359. Duan Y, Parfitt A, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796–1802. [DOI] [PubMed] [Google Scholar]
- 360. Curtis EM, Harvey NC, D’Angelo S, Cooper CS, Ward KA, Taylor P, Pearson G, Cooper C. Bone mineral content and areal density, but not bone area, predict an incident fracture risk: a comparative study in a UK prospective cohort. Arch Osteoporos. 2016;11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Brinckmann P, Biggemann M, Hilweg D. Prediction of the compressive strength of human lumbar vertebrae. Spine. 1989;14(6):606–610. [PubMed] [Google Scholar]
- 362. Black DM, Arden NK, Palermo L, Pearson J, Cummings SR; Study of Osteoporotic Fractures Research Group . Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res. 1999;14(5):821–828. [DOI] [PubMed] [Google Scholar]
- 363. van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68(1):99–102. [DOI] [PubMed] [Google Scholar]
- 364. Christiansen BA, Bouxsein ML. Biomechanics of vertebral fractures and the vertebral fracture cascade. Curr Osteoporos Rep. 2010;8(4):198–204. [DOI] [PubMed] [Google Scholar]
- 365. Kendler DL, Bauer DC, Davison KS, Dian L, Hanley DA, Harris ST, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129(2):221.e1–10. [DOI] [PubMed] [Google Scholar]
- 366. Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28(6):1765–1769. [DOI] [PubMed] [Google Scholar]
- 367. Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28(3):775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Briggs AM, Wrigley TV, van Dieën JH, Phillips B, Lo SK, Greig AM, Bennell KL. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006;15(12):1785–1795. [DOI] [PubMed] [Google Scholar]