Abstract
The prevalence of obesity, measured by body mass index, has risen to unacceptable levels in both men and women in the United States and worldwide with resultant hazardous health implications. Genetic, environmental, and behavioral factors influence the development of obesity, and both the general public and health professionals stigmatize those who suffer from the disease. Obesity is associated with and contributes to a shortened life span, type 2 diabetes mellitus, cardiovascular disease, some cancers, kidney disease, obstructive sleep apnea, gout, osteoarthritis, and hepatobiliary disease, among others. Weight loss reduces all of these diseases in a dose-related manner—the more weight lost, the better the outcome. The phenotype of “medically healthy obesity” appears to be a transient state that progresses over time to an unhealthy phenotype, especially in children and adolescents. Weight loss is best achieved by reducing energy intake and increasing energy expenditure. Programs that are effective for weight loss include peer-reviewed and approved lifestyle modification programs, diets, commercial weight-loss programs, exercise programs, medications, and surgery. Over-the-counter herbal preparations that some patients use to treat obesity have limited, if any, data documenting their efficacy or safety, and there are few regulatory requirements. Weight regain is expected in all patients, especially when treatment is discontinued. When making treatment decisions, clinicians should consider body fat distribution and individual health risks in addition to body mass index.
This Scientific Statement critically reviews the definition of obesity, providing a list of assessment methods, obesity-related diseases, and prevention measures.
Introduction
What’s past is prologue
Some would say that the obesity epidemic began in the 1980s, but history provides a broader view (1–3). Evidence of obesity in humans can be found in primitive art that dates back to the Paleolithic age (4). Two thousand five hundred years ago, Hippocrates cautioned that sudden death is more common in those who are naturally fat than lean (5).
In 1760, the English physician Malcolm Flemyng wrote that obesity can be called a disease, because it obstructs the free exercise of the animal functions and can shorten life (6). In 1810, William Wadd (Secretary of the Royal College of Surgeons in London) stated that the increase of wealth and the refinement of modern times may have banished plague and pestilence, but it has introduced nervous disorders and increased the frequency of corpulence (7).
Modern concepts of the pathophysiology of obesity date back to the end of the 18th century when Antoine Lavoisier established that life was synonymous with oxidation (8). More than 100 years later, Atwater and Rosa (9) applied the laws of thermodynamics to human beings, and during the 20th century, researchers discovered that hypothalamic tumors and tumors of the pituitary gland could cause obesity (10–12).
Obesity treatments date as far back as Hippocrates, who recommended lifestyle changes to obese patients (13, 14). Two thousand years later, William Banting (an undertaker living in London in the 19th century) wrote one of the first “popular” diet books (15).
“Drug” therapies can be traced back at least to the 10th century when Hisdai ibn Shaprut cured “Sancho the Fat” of obesity using theriac—a mixture of more than 64 ingredients (16).
The first English language texts dealing with obesity treatment were published in 1727 and 1760 and recommended chamomile soap and vinegar, as well as other remedies (6, 17, 18).
By the beginning of the 20th century, doctors were using a number of medications for treating obesity (e.g., thyroid extract, dinitrophenol, and amphetamine), often with unfortunate outcomes (19).
The discovery of leptin in 1994 (20) (a peptide produced in adipose tissue) marks the beginning of the “molecular era” for obesity. People who are deficient in this peptide become massively obese. Leptin replacement therapy completely reverses obesity for these individuals. However, leptin treatment has proven ineffective in the typical obese patient who is not leptin deficient.
Rapid advances in basic science related to maintaining an appropriate amount of body fat have provided insights into potential treatments for obesity. This newer understanding of the regulation of food intake and body weight provides the basis for promising future developments (21, 22).
Headwinds in the management of obesity
Despite progress in understanding obesity, advancements in the clinical management of the disease struggle against several headwinds.
First, obesity is a stigmatized condition. The general public and health professionals often respond negatively to overweight persons, which can negatively affect treatment (23).
Second, the desire for the cosmetic effects of weight loss often far exceeds the desire for the health benefits associated with reducing weight (24–26). This may well account for the fact that there are more women seeking help in managing obesity than men, even though the health issues related to obesity are similar between the sexes (27–29).
Although a modest 5% to 10% weight loss has proven health benefits, it often does not provide the cosmetic benefit that patients are looking for. This results in a mismatch between the patient’s goals for weight loss and what diet and exercise can realistically achieve (30). The same is true with surgical approaches to weight loss; patients often value the appearance of lost weight much more than the health benefits (25, 29, 31–33).
This stigma of obesity as a cosmetic issue vs a health issue also affects how the U.S. Food and Drug Administration (FDA) reviews drugs that manage weight loss. The FDA holds antiobesity drugs to a higher standard of review than other drugs, requiring that the risks from these medications be very low compared with drugs of other classes (34).
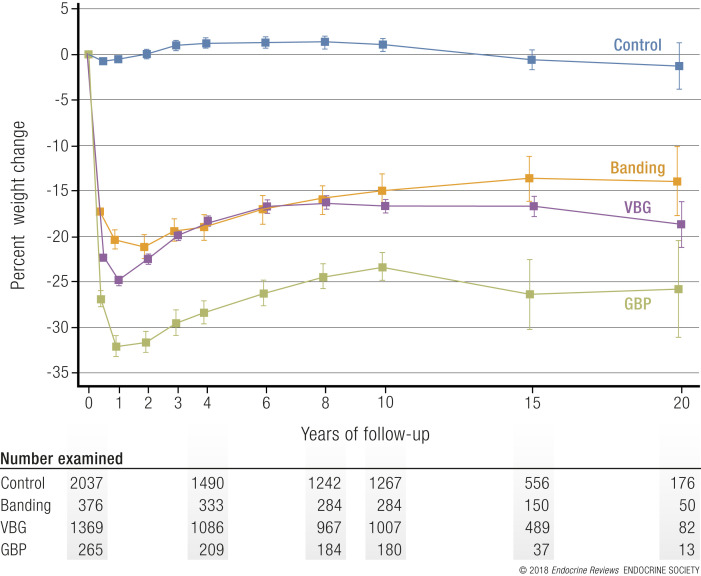
Finally, the lack of reimbursement by health insurers has resulted in poor sales of drug therapies for obesity, which only further dampens the pharmaceutical industry’s interest in developing drug therapies for obesity (35).
Defining Obesity
Introduction
Historically, the medical community defined excess weight and its associated health consequences using population-based anthropometric measurements, (i.e., sex-specific body weight and height using life insurance tables) (36, 37). However, these data only represented insured individuals based on normative standards without considering adiposity, and clinicians eventually abandoned these tables in favor of body mass index (BMI), which is a measure of body weight adjusted for height [weight (kg)/height (m2)].
The National Institutes of Health and the World Health Organization have both adopted BMI as a criterion for defining obesity (36, 38). This made interpretation simpler, eliminated the need for sex-specific height/weight tables, and provided a measurement that is better correlated with other estimates of adiposity. The measurement is based on the observation that body weight is proportional to the squared height in adults with normal body frames. In adults, classification systems (38) and obesity guidelines (39, 40) define healthy body weight as a BMI between 18.5 and 24.9 kg/m2, overweight between 25.0 and 29.9 kg/m2, and obesity ≥30 kg/m2. In children and adolescents, the U.S. Centers for Disease Control and Prevention (CDC) BMI-for-age growth charts define overweight as a BMI at or above the 90th percentile of standard weight and obesity as a BMI above the 95th percentile of standard weight.
BMI provides the most useful population-level measurement of overweight and obesity, and numerous large population studies across multiple continents have demonstrated its utility as an estimate of risk (41–43). Additionally, current assessment and management guidelines from the United States, Canada, and Europe recommend measuring BMI as a first screening step in evaluating adult and pediatric patients for obesity (39, 44–46).
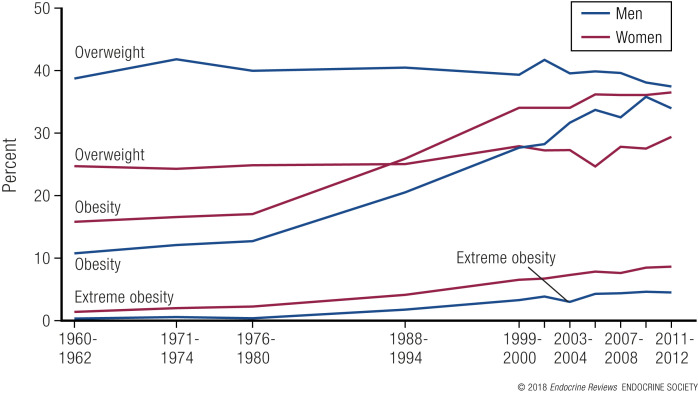
Overweight and obesity are worldwide problems (1) that affect >100 million Americans or 68.5% of the adult population. The most recent data from the 2013 to 2014 U.S. National Health and Nutrition Examination Survey indicate that obesity (defined as BMI ≥ 30 kg/m2) affects ∼35.0% of men and 40.4% of women in the United States (47). Among children and adolescents aged 2 to 19 years, the prevalence of obesity in 2011 to 2014 was 17.0% (47). Fig. 1 shows the percentage of U.S. men and women categorized as overweight, obese, or extremely obese between 1960 and 2012. The category of extreme obesity (BMI > 40 kg/m2) shows the greatest proportional change and is the most difficult group to effectively treat without surgery.
Figure 1.
Trends in the United States for adults with obesity or overweight, 1960–1962 to 2011–2012 (48).
Among adult men, the prevalence of obesity is: Hispanic, 37.9%; black, 38.0%; white, 34.7%; and Asian, 12.6%. In women, the prevalence of obesity is: black, 57.2%; Hispanic, 46.9%; white, 38.2%; and Asian, 12.4%. In children and adolescents, 17.0% of 2- to 19-year-olds are obese, with males and females equally affected (47). The prevalence of obesity among children and adolescents is: Hispanic, 21.9%; black, 19.5%; white, 14.7%; and Asian, 8.6% (47).
Limitations of the BMI
Adults
Although research had demonstrated the utility of BMI in assessing population-based mortality and disease-specific morbidity, there are two major limitations in using BMI alone to diagnose obesity in an individual.
The first is the inability of BMI to distinguish weight associated with muscle vs fat.
Population studies have demonstrated a high specificity of using BMI cutoff values to diagnose obesity but low sensitivity to identify adiposity, thus missing approximately half of people with excess fat (49). This is particularly concerning in the elderly population, where a reduced lean body mass (sarcopenia) might be misclassified as a healthy BMI (50). Dual-energy x-ray absorptiometry or air displacement plethysmography are both accurate methods to assess lean body mass and body fat, but they are expensive and thus impractical for routine clinical application.
Using bioelectric impedance to measure body water provides a relatively inexpensive measure of body fat mass vs fat-free mass (as body fat contains more water). However, this method has large interindividual variations, suggesting that this method may be insufficient for estimating individual body fat mass and fat-free mass (51).
BMI also does not distinguish body fat distribution, a known determinant of metabolic risk. Measuring fat distribution helps identify higher risk individuals, because increased visceral fat predicts the development of the metabolic syndrome, type 2 diabetes mellitus (T2DM), and total and cardiovascular mortality risk better than total body fat alone (52–55). Several anthropometric techniques are available to estimate the distribution of body fat, such as waist circumference alone, the ratio of waist circumference divided by hip circumference (waist-to-hip ratio ([WHR]), and the ratio of waist circumference divided by height (waist-to-height ratio). These measures have been associated with the risk of developing heart disease, T2DM, and other chronic problems associated with obesity (56, 57). Combining waist circumference with BMI provides a way to incorporate weight distribution into measures of obesity. Studies have demonstrated a strong link between waist circumference and BMI for both cardiovascular disease (CVD) and T2DM (58, 59). Waist circumference is most useful in individuals with a BMI of ≤35 kg/m2 (39). However, despite its promise, most clinicians only use BMI and not waist circumference as a gauge of risk from obesity. Beyond recommending annual BMI and waist circumference testing, the American Association of Clinical Endocrinologists also recommends evaluating other potential associated events (60).
Genetic factors are involved in the relationship of waist circumference to risk of CVD or T2DM. A polygenic risk score for increased WHR adjusted for BMI was significantly associated with adverse cardiometabolic traits and higher risks for both T2DM and coronary heart disease (61). A 1 standard deviation increase in WHR adjusted for BMI was associated with a 77% higher risk of T2DM (odds ratio, 1.77 [95% confidence interval (CI), 1.57 to 2.00]) and a 46% higher risk of coronary heart disease [odds ratio, 1.46 (95% CI, 1.32 to 1.62)].
Children
There has also been concern about the association between obesity and visceral or central adiposity among children and adolescents, which has led to suggestions for using waist circumference in pediatric patients as well (62). However, there are many issues with the implementation of this in routine pediatric practice, such as lack of standardized definitions of waist circumference and the inability of waist circumference to add much to the strong association between BMI and comorbidity in children (63). If clinicians are going to use waist circumference to help define obesity in children, it is likely that we will need population-based percentile values, similar to those for BMI (64–66).
Because of these limitations, BMI has also emerged as the most useful approach in children >2 years of age (46).
Are there metabolically healthy people with obesity?
Adults
In cross-sectional studies, many individuals with obesity do not manifest “associated” comorbidities, such as prediabetes, dyslipidemia, hypertension, or other comorbidities (67). These individuals often have a predominantly lower body fat distribution and normal insulin regulation of adipose tissue lipolysis (68, 69). The phenotype “metabolically healthy obesity” (MHO) meets the standard BMI cutoff point for obesity (≥30 kg/m2) but does not have other elements of the metabolic syndrome, such as insulin resistance (70, 71). They have lower levels of visceral and ectopic fat, less liver steatosis (71), and a lower degree of systemic inflammation. Among the 27 studies identified by Rey-López et al. (72), there were 30 definitions of metabolic health that relied on four criteria: blood pressure (BP), high-density lipoprotein (HDL) cholesterol, triglycerides, and plasma glucose. BMI ≥ 30 kg m2 was the main criterion for obesity. In this group of studies, the prevalence of MHO ranged between 6% and 75% (67, 72–74).
Whereas short-term cross-sectional studies suggest that MHO men and women are not at increased risk of CVD, longitudinal studies suggest that this phenotype may not be benign, and that this group is at higher risk for increased carotid artery intima-media thickness, coronary calcification, impaired vasoreactivity, and/or other cardiovascular events, as well as all-cause mortality (70, 75–79). Therefore, clinicians should view MHO as a transient or intermediary state that may progress over time to an unhealthy phenotype in many people. Cardiorespiratory fitness is one factor related to MHO. Research has shown that cardiorespiratory fitness lowers the risk of all-cause mortality for metabolically unhealthy individuals with obesity and those with and without the MHO phenotype (80–82), suggesting that the inclusion of cardiorespiratory fitness along with BMI and waist circumference may improve the assessment of risk status. Several systems are available for evaluating and staging obesity when assessing risk (80–84). Increasing physical activity might thus be a valuable recommendation for individuals with MHO. Additionally, clinicians should observe these individuals for the risk of developing cardiometabolic disease (80–86).
Children
There also has been interest in whether children and adolescents can be obese but metabolically healthy. Some pediatric patients with obesity, even some with severe obesity, have few metabolic or clinical abnormalities (83). However, the presence of obesity tends to track from childhood to adolescence and on to adulthood. Thus, there is a high likelihood that a child with obesity will become an obese adult, often with the severity of obesity increasing over time with ongoing weight gain. This makes it likely that children and adolescents with obesity, even when metabolically healthy at presentation, will develop associated diseases over time.
Age and obesity
Adults
The current guidelines for assessing obesity among adults do not consider age as an independent criterion. However, there are physiological and functional changes that occur among the aging population that may confound the interpretation of BMI and risk estimates in older people. Body composition changes associated with aging include sarcopenia, reduced bone mineral density, and the accumulation of visceral fat; BMI alone will not detect these changes (84). BMI values associated with the lowest relative mortality are slightly higher in older than in younger adults, which is often misinterpreted to suggest that obesity is not as harmful in the elderly. BMI may be a less appropriate index in the elderly because of sarcopenia (87). Centrally located fat (waist circumference) and relative loss of fat-free mass may become more important than BMI in determining the health risk associated with obesity in the elderly (88). The importance of loss of muscle mass was clearly shown in the Health ABC Study where older adults with greater thigh muscle loss had a higher risk of mortality compared with those with preserved thigh muscle, which suggests that efforts should be made to “conserve” muscle mass in old age (89).
Children
During childhood and adolescence, there are substantial changes in growth, body composition, and pubertal status. During periods of rapid growth, weight and height may be somewhat mismatched, with weight gain preceding growth in height. However, in the past three decades, children are often gaining weight at a pace much faster than what could be considered healthy or normal.
Another critical period is the time when growth in height ceases and caloric requirements decrease. If calorie intake does not adjust, weight gain is the likely result.
Furthermore, adolescence is a time of relative insulin resistance (90). Because of this insulin resistance, adolescents who are obese become more susceptible to the development of T2DM.
Prevention of Obesity
Recent trends suggest that we are making some progress in the prevention and control of the obesity epidemic using several strategies outlined below. First, the prevalence of obesity among 2- to 5-year-old children has decreased significantly since 2003 to 2004 (91). Second, it has plateaued among 6- to 11-year-olds (47). In contrast, however, obesity has continued to increase in adult women (47).
“A 2015 Cochrane review found that diet, exercise, or both reduced excessive gestational weight gain by an average of 20%.”
Strategies for preventing obesity in pregnancy
Three systematic reviews relating weight gain during pregnancy and pregnancy outcomes found that dietary interventions reduced gestational weight gain and the risks of preeclampsia, hypertension, and shoulder dystocia in infants. No differences occurred in the incidence of small-for-gestational-age infants as a result of these treatments (92–94).
A 2015 Cochrane review found that diet, exercise, or both reduced excessive gestational weight gain by an average of 20%. Dietary interventions—including low glycemic index diets, supervised or unsupervised exercise programs, and diet combined with exercise—all had comparable effects. Maternal hypertension was reduced, but preeclampsia was not. No differences were found between intervention and control groups in the risk of preterm births or macrosomia. However, a 15% reduction in macrosomia occurred among women who were overweight or had obesity. Newborn respiratory distress syndrome was also decreased in the intervention groups among mothers who were either overweight or obese (95). Maternal consumption of sugar-sweetened beverages, similar to maternal smoking, may also have long-term detrimental effects on their offspring. Gillman et al. (96) reported that at an average age of 7.7 years children of mothers who consumed two or more servings per day during the second trimester of pregnancy were both fatter and heavier. This provides an additional important piece of information to provide to the pregnant woman.
Strategies aimed at children
The two most important settings for the prevention of obesity in children and adolescents are early care and education (ECE) and schools. Children spend a lot of time in these settings, where there are great opportunities for instilling positive behaviors regarding nutrition and physical activity.
Early care and education
Although millions of young children are enrolled in ECE, there are only a few intervention studies on preventing or mitigating obesity in these settings (97). One of these studies is the Romp and Chomp Intervention conducted in Australia. This study used multiple ECE and community interventions directed at children 0 to 5 years of age. The interventions included logical and proven targets for weight control, such as reducing sugar-sweetened drinks and energy-dense foods, increasing fruit and vegetable intake and active play, and reducing television time. The study reported significant reductions in obesity prevalence in 2- and 3.5-year-old children compared with children who did not receive the interventions (98).
Because of the immense impact that policy and environmental changes in ECE could have on childhood obesity, widespread efforts are underway to develop and incorporate standards and programs to increase physical activity and improve diets in ECE settings (99). One such program is the U.S. Department of Agriculture’s Child and Adult Care Food Program, which helps child care institutions provide nutritious foods that contribute to the wellness, healthy growth, and development of young children (100).
Schools
A recent Cochrane meta-analysis of 37 studies (including 27,946 children) (101) found beneficial effects of a number of components of school-based interventions. These included: school curricula that incorporate healthy eating, physical activity, and body image; increased sessions for physical activity and the development of fundamental movement skills throughout the school week; improvements in the nutritional quality of the food that schools supply; environments and cultural practices that support children eating healthier foods and being active throughout each day; support for teachers and other staff to implement health promotion strategies and activities (e.g., professional development, capacity building activities); and parental support and home activities that encourage children to be more active, eat more nutritious foods, and spend less time in screen-based activities (101). Beneficial effects were most notable in children 6 to 12 years old.
A number of long-term studies lasting ≥12 months provide more specific information on the effects of school-based interventions. We summarized these in Table 1 (102–110).
Table 1.
Long-Term Studies or Preventive Interventions in Children and Adolescents
| Reference | Sample | Design | Results |
|---|---|---|---|
| Epstein et al., 2001 (102) | 26 children | 12-mo RCT | 1.1% decrease in overweight prevalence with increased fruits and vegetables vs 2.4% with decreased fat and sugar |
| 6–11 y old | Increased fruits and vegetables vs decreased fat and sugar | Differences not significant | |
| James et al., 2004 (103) | 644 children | 1-y intervention; classrooms randomized to reduce sugar drink consumption | No significant difference in BMI z-score |
| 7–11 y old | |||
| Ebbeling et al., 2012 (104) | 224 overweight or obese adolescents; mean age 15 y | RCT | Significantly lower rates of weight gain in intervention group |
| Sugar-free drinks and behavior modification vs untreated control | |||
| de Ruyter et al., 2012 (105) | 641 children | 18-mo RCT | Significantly lower rates of weight gain among group receiving sugar-free drinks |
| 5–12 y old | Sugar-free drinks vs drinks containing sugar at lunch | ||
| Sallis et al., 2009 (106) | 995 4th and 5th grade students | PE taught by PE instructor or teacher vs control | Some fitness measures improved in girls |
| No significant differences in changes in skinfolds | |||
| Caballero et al., 2003 (107) | 1704 Native American children 8–11 y old | 3-y study randomized by schools to control or intervention (41 schools); | No significant difference in body composition or PA |
| changes in dietary intake, increased PA, classroom curriculum changes, family involvement | |||
| Gortmaker et al., 1999 (108) | 1295 6th–7th grade students | 2-y RCT with five intervention and five control schools | Decreased prevalence of obesity in girls |
| Decreased TV, decreased fat and increased fruit and vegetable intakes, and PA | |||
| Plachta-Danielzek et al., 2011 (109) | 240 intervention and 952 nonintervention children | Nutrition intervention delivered within schools and daily running games vs controls | No significant difference in increases in overweight between intervention and control students in 8-y follow-up |
| Mean age 6 y old | Significant decreases in BMI z-scores with upper income students | ||
| Sahota et al., 2001 (110) | 636 children, 7–11 y old | Randomized by school. Teacher training, changes in school meals, and development of school actions plans to promote healthy eating and PA | No significant differences in BMI in intervention compared with control schools |
| 314 intervention | |||
| 322 control |
Abbreviations: PA, physical activity; PE, physical education; RCT, randomized controlled trial; TV, television.
Randomized controlled trials (RCTs) have shown that the reduction or elimination of sugar-sweetened drinks (often through the substitution of calorie-free beverages) has effectively reduced rates of weight gain in children and adolescents (111). These observations are consistent with the association between reductions in sugar-sweetened drinks and both the decrease in the prevalence of obesity in 2- to 5-year-old US children and the plateau in the prevalence of obesity in 2- to 19-year-old US children. The absence of a significant effect in several of these studies may indicate that a significant caloric deficit relative to the control condition was not established or sustained (112).
Compared with efforts in specific settings, clinical interventions aimed at prevention have had limited impact (113).
Strategies aimed at adults: worksites
In 2009, the Center for Disease Control’s Task Force on Community Preventive Services concluded that worksite health promotion programs that improved physical activity and/or nutrition were effective in reducing body weight and BMI (114). Studies were limited to those with at least 6 months of follow-up. A pooled effect of nine RCTs found a weight decrease of 1.3 kg, and a pooled effect of six RCTs found a decrease of 0.5 BMI units (115). Most of the studies combined informational and behavioral strategies to influence diet and physical activity; fewer studies modified the work environment (e.g., cafeteria, exercise facilities) to promote healthy choices. Recent efforts to reduce sugar-sweetened drink consumption in hospitals have effectively used labeling and choice architecture as environmental strategies to reduce sugar-sweetened drink consumption (116, 117).
Strategies for preventing obesity aimed at the entire population
Population-based messages aimed at the public concerning food and exercise require individual commitment if they are to be effective (118). If individuals follow the advice in the message, this strategy would be sufficient to overcome the epidemic of obesity. However, positive nutritional messages are often dwarfed by alternative messages urging consumption of less healthful foods, and the built environment is often a barrier to healthful exercise behaviors.
One approach might be to re-engineer the built environment to displace car use with physically active transportation options (such as walking and biking) and increase the number of accessible healthful food options (106). A systematic review by Papas et al. (119) identified 20 studies that examined the association between obesity and the numbers of outlets for physical activity and food, 18 of which were cross-sectional. Seventeen of these studies found a significant relationship between the built environment (food outlets or physical activity opportunities) and the risk of obesity. The number of recreational facilities and likelihood of overweight in adolescents were significantly related. However, few studies have examined the impact of changes in the built environment with changes in the prevalence of obesity. One exception is a study of the impact of housing changes among people living in poverty. Moving from a high-poverty neighborhood to a neighborhood of lower poverty was associated with a reduced prevalence of severe obesity (120).
Use of public transit has also been associated with increased levels of physical activity (121). For example, the implementation of a light rail system in Charlotte, North Carolina, was associated with a higher odds of meeting the daily physical activity requirement and a lower BMI (122). Neighborhood walkability appears to have much the same effect (123).
Food
Faith et al. (124) concluded that manipulating the ease of food access and/or restricting access to certain foods might influence food purchases, consumption, and possibly weight change, although this requires further research. In contrast, the food industry favors the hypothesis that obesity results from reduced levels of physical activity and strongly supports providing more places for people to exercise and more healthful food alternatives as a strategy to help overcome the obesity problem (118, 125). However, the expense of healthy food items and limited access to healthful foods in many lower income communities pose significant challenges. To address access to healthful food options, the Healthy Food Financing Initiative introduced supermarkets to underserved communities. However, this did not increase the consumption of healthful foods (126). Ideally, improved access needs to be accompanied by pricing and promotion strategies to increase consumption of more healthful products.
Some of the strategies for introducing healthful food options include introducing farmer’s markets, subsidizing the availability of fresh fruits and vegetables to school children, lowering the cost of fruits and vegetables while increasing the price of high-fat or high-sugar foods in school or worksite cafeterias, and/or changing marketing strategies. These strategies, for the most part, increase fruit and vegetable consumption (127–132). Importantly, however, note that addressing fruit and vegetable consumption alone might not be enough, as the impact of fruit and vegetable consumption on obesity prevention is uncertain. However, increased fruit and vegetable consumption does confer significant health benefits. Diets high in fruits and vegetables and low in fat and sugar lowered BP across the range of salt intake in individuals who were maintaining their body weight (127, 133).
Taxation provides another strategy to reduce consumption of less healthful products by increasing their price. Smed et al. (134) has shown that among Europeans, increasing the tax or reducing the subsidies on unhealthful items and reducing the tax on healthful items through the value-added tax system could shift consumption toward healthier foods (135). Because of their contribution to obesity, taxation of sugar-sweetened drinks has become a major focus in the United States. Although many municipalities have imposed sales taxes on sugar-sweetened drinks, this approach is less effective than an excise tax, which increases the price of the product on the shelf. In 2014, Berkeley, California, passed a sugar drink tax of $0.01 per ounce. A study of sugar-sweetened drinks in that city reported that consumption in low-income neighborhoods (compared with two neighboring communities) declined by 21% and water consumption increased by 63% (136). In January of 2014, Mexico imposed an excise tax of 1 peso per liter on sugar-sweetened beverages. Colchero et al. (137) reported that purchases of these taxed beverages decreased 5.5% in 2014 and 9.7% in 2015, yielding an average reduction of 7.6% during the study period. Whether this translates into improvements in health outcome is currently unknown.
Increasing physical activity
As indicated above, physical activity levels in both children and adults have declined substantially. Helping incorporate exercise into how people get from where they live to where they learn, work, shop, play, and pray has become a prominent strategy to reverse this trend. Table 2 lists 10 strategies that the CDC’s Guide for Community Preventive Services identified for increasing physical activity (138).
Table 2.
Evidence-Based Recommendations To Increase Physical Activity in Communities
| Community-wide campaigns to promote physical activity |
| Point of decision prompts for stair use |
| Individually adapted health behavior change programs |
| Enhanced school-based physical education |
| Social support in community settings |
| Creation of or enhanced access to places for physical activity combined with informational outreach activities |
| Street-scale urban design and land-use policies |
| Community-scale urban design and land-use policies |
| Active transport to school |
| Transportation and travel policies and practices |
The CDC has also released a convenient guide that focuses on how to implement these strategies (139).
China provides an interesting example of how urbanization and improved incomes reduces physical activity (140, 141). As recently as 20 years ago, the bicycle was a major mode of transport for Chinese. Since then, the automobile has relegated bicycles to museums.
Obesity and Disease
Obesity and risk of death
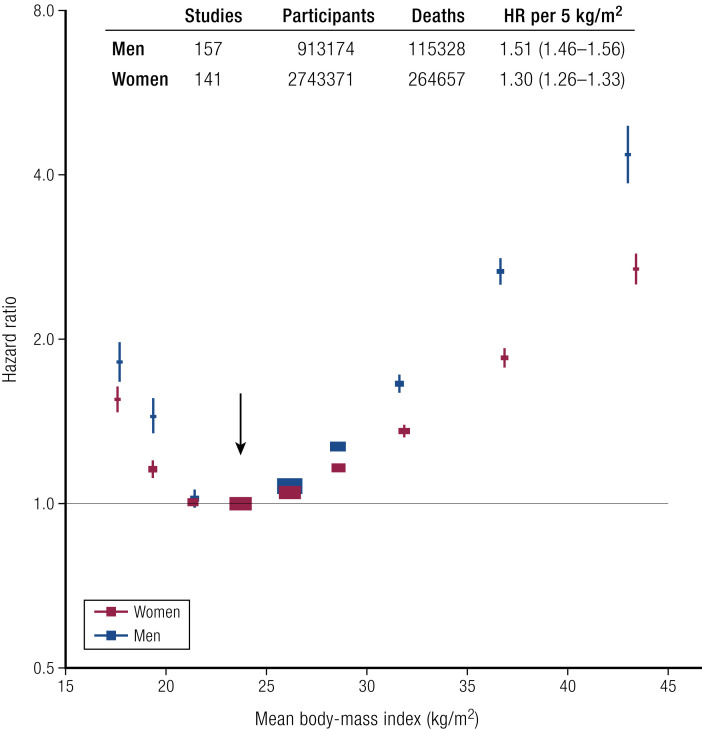
For many illnesses related to obesity, there is a curvilinear increase in risk as a function of weight (Fig. 2) (142). The Global Burden of Disease project (142) reported this relationship between BMI and all-cause mortality in 239 prospective studies that included >10 million people with a median follow-up of 13.7 years. Nearly 4 million subjects who survived 5 years were free of chronic diseases at recruitment. There was a clear J-shaped relationship between the BMI of the 385,879 who died and all-cause mortality. The lowest mortality was with a BMI of 20.0 to 25.0 kg/m2. Below this (BMI, 18.5 to 20.0 kg/ m2), mortality significantly increased by 13% [hazard ratio (HR), 1.13]. In the individuals with a BMI of 25.0 to 27.5 kg/m2, all-cause mortality increased by 7% (HR, 1.07), and with a BMI of 27.5 to 30.0 kg/m2, it increased by 20% (HR, 1.20). For grade-1 obesity (BMI 30.0 to <35.0 kg/m2), all-cause mortality increased by 45% (HR, 1.45), and for grade-2 obesity (BMI, 35.0 to <40.0 kg/m2), it increased by 94% (HR, 1.94). For those with grade-3 obesity (BMI, 40.0 to <60.0 kg/m2), all-cause mortality rose by 176% (HR, 2.76). For each 5 BMI unit increase, total mortality rose by 30%, mortality from chronic kidney disease rose by 60%, and mortality from T2DM rose by 120% (41).
Figure 2.
BMI and all-cause mortality. Vertical bars are 95% CI. The Global Mortality Collaboration, 2016 (142).
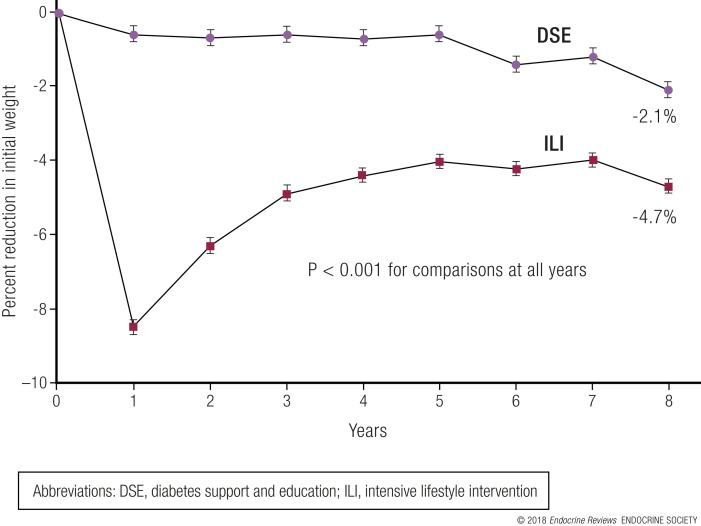
Just as weight gain can increase the risk of mortality, weight loss can reduce the risk of mortality in obese individuals. The results from the Swedish Obese Subjects Study (which compared long-term follow-up of obese patients after surgical intervention for obesity with a matched but unoperated control group) showed a 29% reduction in overall mortality after 10.9 years (143). Individuals in the Look AHEAD trial had a similar outcome after a median follow-up of 10.2 years. Those who lost at least 10% of their body weight in the first year of the study had a 21% lower risk of the primary CVD outcome [HR, 0.79 (95% CI, 0.64 to 0.98); P = 0.034] and a 24% reduced risk of the secondary outcome [HR, 0.76 (95% CI, 0.63 to 0.91); P = 0.003] compared with individuals who were weight stable or gained weight. Participants in the intensive lifestyle intervention group who lost at least 10% of their body weight had a 20% lower risk of the primary CVD outcome [HR, 0.80 (95% CI, 0.65 to 0.99); P = 0.039] and a 21% lower risk of the secondary CVD outcome [HR, 0.79 (95% CI, 0.66 to 0.95); P = 0.011] compared with the control group (144).
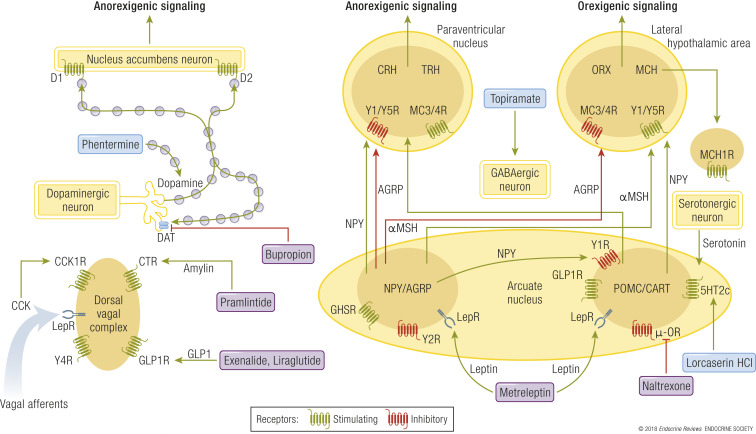
The mechanism of obesity-associated morbidity
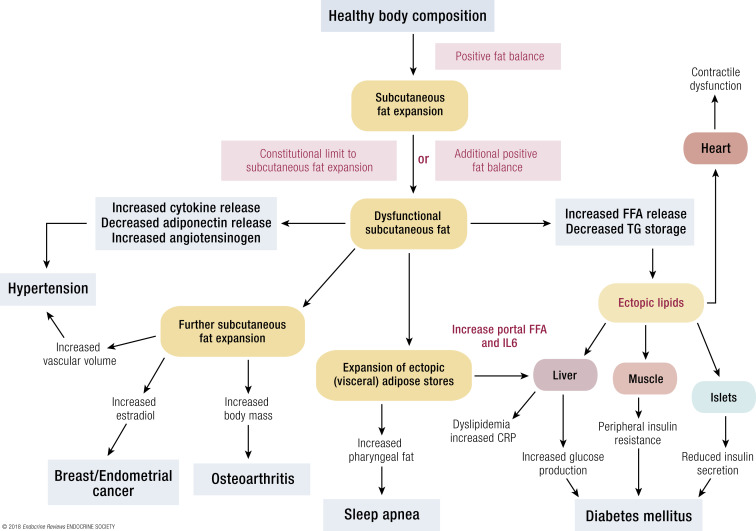
The effects of obesity on the body appear to be mediated by several major pathways. Fig. 3 shows how obesity as a disease process might lead to a variety of other diseases (145).
Figure 3.
A schematic model of the intermediary mechanisms for dyslipidemia, insulin resistance, T2DM, heart disease, hypertension, some forms of cancer, OSA, NAFLD, and osteoarthritis.
A variety of types of adipose tissue dysfunction clearly play a role in the genesis of many obesity-related diseases. These include impairments in adipocyte storage and release of fatty acids, overproduction or underproduction of “adipokines” and cytokines (146), hormonal conversion, and the adverse mechanical effects of greater tissue mass.
The pathology of obesity is closely linked with body fat distribution. Upper body/visceral or ectopic fat accumulation is a much better predictor of insulin resistance, dyslipidemia, and such than total fat. Visceral fat is considered one of the “ectopic” fat depots, along with hepatic, intramyocellular, intramuscular, and pericardial fat. Humans with the ability to respond to excess energy intake by recruiting new, healthy subcutaneous adipocytes are relatively protected from many of the metabolic consequences of obesity. Those without this ability will store excess fat in ectopic depots, including liver, visceral fat, and muscle (147). This is supported by the finding that larger subcutaneous fat cells are associated with more accumulation of visceral fat during overfeeding, because they cannot expand to store more fat (148).
One study reported that the predisposition for T2DM was associated with impaired recruitment of new adipose cells to store excess lipids in subcutaneous adipose tissue (149). Another study reported that adults who develop more leg adipocytes in response to overfeeding have a lesser increase in abdominal subcutaneous adipose size (150). Fabbrini et al. (151) showed that those with MHO are resistant to the adverse metabolic effects of moderate weight gain, whereas metabolically unhealthy people are predisposed to such adverse effects. These authors concluded that increased adipose tissue capacity for lipogenesis might help protect people with MHO from weight gain–induced metabolic dysfunction, at least with modest weight gain during shorter periods of time.
In addition to the known toxic effects of excess fatty acids, abnormalities in the hormonal function of adipose tissue may contribute to metabolic disease. Adiponectin is the most abundant peptide produced by adipose tissue (152). It improves insulin sensitivity and vascular function. Adiponectin concentrations are inversely related to adipocyte size and visceral fat mass. In contrast, most adipokines are secreted in larger quantities as fat cells increase in size.
Researchers have discovered a large number of adipokines, but their exact role in disease is often unknown. The angiotensinogen produced by adipose tissue is a precursor for angiotensin, which can contribute to the risk for hypertension. Additionally, the aromatase enzymes in adipose tissue can convert sterols (androstenedione) to estrogen, which may explain the greater risk of breast and endometrial cancer in women with obesity, particularly postmenopausal women with obesity where estrogens derived from fat are their principal source of estrogens (153).
Type 2 diabetes mellitus
There is overwhelming evidence that BMI, central adiposity, and the increase in body weight predict future T2DM (154). A meta-analysis of prospective studies provided evidence that as upper body adiposity increases, both the risk of the metabolic syndrome and of developing T2DM also increase (155). The duration of obesity in younger compared with older individuals is also associated with a greater risk for T2DM (156). Weight gain in adult life increases the risk of developing T2DM, particularly in the age range 25 to 40 years (157).
The duration of increased body weight is also a risk factor for T2DM. For a given level of excess BMI-years in the National Longitudinal Survey, younger individuals compared with older ones (and Hispanic and black compared with white individuals) had a higher risk of developing T2DM (153).
Weight loss is clearly beneficial in reducing the risk of converting to diabetes. In the Diabetes Prevention Program, a median weight loss of 5.5% during 2.8 years reduced the risk of converting from prediabetes to diabetes by 58% (158). Similarly, bariatric surgery has repeatedly reversed diabetes to normal glucose tolerance (159–161).
Cancer
Certain forms of cancer are significantly increased in individuals who are overweight (162, 163). Males face increased risk for neoplasms of the colon, rectum, and prostate. In women, cancers of the reproductive system, including breast (164), endometrium (165), and gallbladder, are more common. Women who gained 25 kg or more after age 18 were at increased risk of breast cancer (RR 1.45 P < 0.001). Women who gained 10 kg or more after menopause were also at increased risk for breast cancer compared with women whose weight remained stable. Women who lost and maintained ≥10 kg and who did not use postmenopausal hormones were at lower risk than those who maintained weight (RR, 0.43) (166).
Breast cancer is not only related to total body fat but also may have a more important relationship to central body fat (167). This relationship to body fat may also help explain why breast cancer risk is increased at age 75 in women in the highest vs the lowest quartile of BMI (168). Circulating, unconjugated estradiol may mediate the relationship between increased body fat and breast cancer (169), as well as the relationship between increased body fat and the risk of endometrial cancer (169).
Myocardial infarction
Many studies show that as BMI increases, there is an increased risk for heart disease (170, 171) and heart failure (172). Data from the Nurses’ Health Study indicate that the risk for U.S. women developing coronary artery disease is increased 3.3-fold with a BMI > 29 kg/m2 compared with women with a BMI < 21 kg/m2 (173). A BMI of 27 to <29 kg/m2 increases the relative risk to 1.8. Weight gain also strongly affects this risk at any initial BMI. That is, at all levels of initial BMI (and within BMI categories) there was a graded increase in risk of heart disease with increasing waist circumference. Similarly, within waist circumference categories there was an increased risk of heart disease with increasing BMI (171). Major risk of CVD was increased 6% for each 1.1 kg/m2 increase in BMI among 6452 British men (174).
Central adiposity, as reflected in waist circumference, is also a strong predictor of the risk for CVD (173). When increased central adiposity is added to other components of the metabolic syndrome, the prediction is even higher. Using the National Health and Examination Survey data, Janssen et al. (175) showed that BMI predicted the risk of the metabolic syndrome in men. However, when BMI is adjusted for waist circumference as a continuous variable, waist circumference accounted for essentially all of the risk for the metabolic syndrome. In a meta-analysis including 10 studies, indices of abdominal obesity (including WHR and waist circumference) were better discriminators than BMI of cardiovascular risk factors, including T2DM, hypertension, and dyslipidemia (176).
Both atrial fibrillation (177, 178) and congestive heart failure (170, 179) have a higher risk in subjects who are overweight. In the Multi-Ethnic Study of Atherosclerosis, the risk of congestive heart failure in obesity was associated with elevated levels of inflammatory markers (interleukin-6 and C-reactive protein) and albuminuria (180).
Heart failure and the obesity paradox
Obesity increases the risk of heart failure, yet some studies have found that elevated BMI may improve survival in individuals who already have congestive heart failure, a phenomenon called “the obesity paradox” (181–183). This appears to contradict the curvilinear relationship of BMI to body weight (41, 88–91, 93).
One possible explanation is “selection bias.” This occurs when studies select individuals as higher risk because they are identified after the disease develops rather than before. A simple way to eliminate this bias is to match the start of exposure to the start of follow-up. The same is true regarding the effect of obesity on the risk of mortality (43, 184–187). Alternatively, the obesity paradox may reflect some capacity of the individual with obesity to overcome cardiovascular risk. Still another explanation for this paradox may be the difference between what BMI tells us and what the underlying fat distribution is doing. In a recent study, Padwal et al. (187) found that BMI and body fat have different predictive values for cardiovascular risk. If fat is the culprit, then measuring BMI may lead to an erroneous conclusion (187).
Hypertension and stroke
Hypertension is a global public health problem. Roughly 1 billion people worldwide are estimated to have clinically significant elevations in BP (188), with ∼50 million of them in the United States (189). Hypertension is the most important of 67 risk factors for worldwide risk of coronary heart disease, stroke, renal disease, and all-cause mortality (29). Furthermore, antihypertensive therapy results in reductions of incidence of stroke, myocardial infarction, and heart failure (190).
Among hypertensive individuals who reduced their BP levels following a successful weight-loss intervention, those who maintained weight loss also maintained lower BP levels, and those who regained weight returned to their baseline BP levels (191). In a meta-analysis of 25 studies, Neter et al. (192) found that weight loss averaging 5.1 kg after diet and/or exercise programs reduced BP by 4.4/3.5 mm Hg (systolic BP/diastolic BP). The studies with weight losses >5 kg showed larger decreases in BP than those with less weight loss.
Obstructive sleep apnea
In contrast to the relatively benign effects of excess weight on most components of respiratory function, overweight predisposes to obstructive sleep apnea (OSA), which can be severe and life-threatening (193). OSA is more common in men than women. An increased snoring index and increased maximal nocturnal sound intensity are characteristic. Nocturnal oxygen saturation is significantly reduced (194). A study of obese patients with diabetes using polysomnography showed that 30.5% of the participants had moderate OSA, and 22.6% had the severe form. Waist circumference was significantly related to the presence of OSA, and severe OSA was most likely in individuals with a higher BMI (195). Independently of obesity, OSA is associated with features of the metabolic syndrome, including hypertension, T2DM, and increased cardiovascular risk, possibly mediated by stress responses and hypoxia. Excess daytime sleepiness is an important consequence and can be a risk for driving and other tasks that require alertness (195).
Hepatobiliary disease
Gallbladder disease
Obesity is associated with an increased risk of gallbladder disease. In a meta-analysis of gallbladder disease and obesity, Aune et al. (165) reported that the risk of gallbladder disease increased even within normal BMI ranges. For each 5-unit increase in BMI, the relative risk of gallbladder disease increased 63%. For a 10 cm increase in waist circumference, the increase in relative risk was 46%.
Nonalcoholic fatty liver disease
Fatty liver disease is often associated with obesity (196). Excess liver fat without inflammation/hepatocellular injury is called nonalcoholic fatty liver disease (NAFLD), which may progress to nonalcoholic steatohepatitis (NASH) and eventually cirrhosis. The diagnosis of NAFLD requires evidence of excess liver fat in the absence of secondary causes. NASH is diagnosed when there is evidence of hepatocellular injury (most often in the context of fatty liver) and is of greater concern because it poses a genuine risk of progression to fibrosis, cirrhosis, greater risk for hepatocellular carcinoma, and cirrhosis-related liver failure.
The prevalence of NAFLD ranges from 6% to 30%, depending on the diagnostic approaches and populations studied. The estimated prevalence of NASH is 3% to 5%. Both liver fat and fibrosis were increased as a function of time in nonhuman primates fed a high-fructose diet vs nonhuman primates without the added fructose (197).
NAFLD is considered by some to be the hepatic manifestation of the metabolic syndrome (198). Fatty liver is extremely common in patients undergoing bariatric surgery (prevalence 84% to 96%). The prevalence of fatty liver in the United States has been increasing steadily from 1988 to 2008 with obesity as an independent predictor (199). In a meta-analysis of 21 studies (13 of which were prospective), Li et al. (200) found that obesity produced a 3.5-fold increased risk of developing NAFLD. Moreover, there was a dose response to rising BMI, with the relative risk increasing 1.20 for each 1 unit increase in BMI. Another meta-analysis (201) found that for each 1 unit increase in waist circumference, the odds ratio of NAFLD increased 1.07, and for each 1 unit increase in BMI, the odds ratio increased 1.25. The prevalence is greater in Hispanic than white populations and less in blacks than whites. NAFLD and NASH are also more common in persons with T2DM.
Gout and osteoarthritis
Gout
Aune et al. (202) reported on the relationship of BMI to the risk of gout in 10 prospective studies that included 27,944 cases of gout among a population of 215,739 (median follow-up of 10.5 years). The summary relative risk for a 5-unit increment in BMI was 1.55 for all studies combined (95% CI, 1.44 to 1.66). The summary relative risk per 5-unit increase in BMI was 1.62 for men (95% CI, 1.33 to 1.98) and 1.49 for women (95% CI, 1.32 to 1.68). The relative risks were 1.78, 2.67, 3.62, and 4.64 for persons with a BMI of 25, 30, 35, and 40 kg/m2, respectively, compared with persons with a BMI of 20 kg/m2. The study also associated increased risk with BMI in young adulthood, WHR, and weight gain from age 21 to 25 to midlife, but the analyses included few studies.
Osteoarthritis
Osteoarthritis is likewise significantly increased in individuals who are overweight or obese. The osteoarthritis that develops in the knees and ankles may be directly related to the trauma associated with the degree of excess body weight (203). However, the increased osteoarthritis in non–weight-bearing joints suggests that some components of the excess weight may alter cartilage and bone metabolism independent of weight bearing. Increased rates of osteoarthritis account for a significant component of the cost of overweight and for the associated disability (204). Okoro et al. (204) found that class-3 obesity (BMI > 40 kg/m2) was associated with survey-reported disability among individuals >45 years of age who reported arthritis, as well as those who did not report arthritis.
Effects of obesity during pregnancy
A narrative analysis of 22 reviews on pregnancy in women with obesity (205) showed that gestational diabetes, preeclampsia, gestational hypertension, depression, instrumental and cesarean birth, and surgical-site infection are more likely to occur in pregnant women with obesity compared with women with a healthy weight. Obesity in pregnancy is also linked to greater risk of preterm birth, large-for-gestational-age babies, fetal defects, congenital anomalies, and perinatal death. Additionally, breastfeeding initiation rates are lower, and there is greater risk of early breastfeeding cessation in women with obesity compared with healthy-weight women.
Diet, Exercise, and Lifestyle in Managing Obesity
Diet in managing obesity—food is more than calories
Introduction
The idea that single food items or diets are able to promote and maintain weight loss has stimulated numerous studies to investigate different proportions of dietary fat, protein, or carbohydrates as weight-loss diets (206) (Table 3). Underlying all of these dietary approaches, however, is the fact that to lose weight, energy balance must be negative. Although calories are the essential component of energy balance, and reducing them is important for weight loss, food consists of more than calories. When choosing a diet, it is important to select foods that you enjoy and substitute lower calorie healthy foods that can improve the quality of your diet. Macronutrient composition aside, a reduction of energy intake is still an essential component of the effectiveness of any diet. In the Diabetes Prevention Program, calorie reduction was the major predictor of weight loss (207). Reduced intake in fat was the second predictor, and physical activity was only an important predictor when the calorie intake was unchanged (207).
Table 3.
A Comparison of Various Diet Programs and Eating Plans to a Typical American Diet
| Type of Diet | Example | General Dietary Characteristics | Comments | AHA/ACC/TOS Evaluation and Others |
|---|---|---|---|---|
| Typical American diet | Carb: 50% | Low in fruits and vegetables, dairy, and whole grains | ||
| Protein: 15% | High in saturated fat and unrefined carbohydrates | |||
| Fat: 35% | ||||
| Average of 2200 kcal/d | ||||
| Balanced-nutrient, moderate-calorie approach | DASH Diet or diet based on MyPyramid food guide. Commercial diet plans such as: Diet Center, Jenny Craig, Nutrisystem, Physician’s Weight Loss, Shapedown Pediatric Program, Weight Watchers, Setpoint, Sonoma, Volumetrics | Carb: 55%–60% | Based on set pattern of selections from food lists using regular grocery store foods or prepackaged foods supplemented by fresh food items | Meta-analysis showing DASH approach better than control or healthy diets (weight mean difference 0.87–1.5 kg). |
| Protein: 15%–20% | Low in saturated fat and ample in fruits, vegetables, and fiber | |||
| Fat: 20%–30% | Recommended reasonable weight-loss goal of 0.5–2.0 pounds/wk | |||
| Usually 1200-1800 kcal/d | Prepackaged plans may limit food choices | |||
| Most recommend exercise plan | ||||
| Many encourage dietary record keeping | ||||
| Some offer weight-maintenance plans/support | ||||
| Low- and very low–fat, high-carbohydrate approach | Ornish Diet (Eat More, Weigh Less), Pritikin Diet, T-factor Diet, Choose to Lose Diet, Fit or Fat Diet | Carb: 65% | Long-term compliance with some plans may be difficult because of low level of fat | Same weight loss at 6 mo comparing 30% fat to > 40% fat; strength of evidence: moderate |
| Protein: 10%–20% | Diet can be low in calcium | |||
| Fat: ≤10%–19% | Some plans restrict healthful foods (seafood, low-fat dairy, poultry) | |||
| Limited intake of animal protein, nuts, seeds, other fats | Some encourage exercise and stress management techniques | |||
| Low energy density | Volumetrics Diet | Carb: 55% | Four food categories: | More weight loss at 6 mo with low energy-dense diet; strength of evidence: RCT |
| Protein: 10%–25% | (1) Very low density—nonstarchy fruits and vegetables, nonfat milk, broth-based soups | |||
| Fat: 20%–35% | (2) Low density—starchy fruits/vegetables, grains, breakfast cereal, low-fat meats, and mixed dishes | |||
| Focus on fruits, vegetables, and soups | (3) Medium density—meat, cheese, pizza, fries, dressings, bread, and such | |||
| (4) High density—desserts, nuts, butter, oils | ||||
| Focus on categories 1 and 2, some from 3, minimum from 4 | ||||
| Portion controlled | Use of meal replacements both liquid and solid meals | Weight loss at 1 year in Look AHEAD trial related to frequency of consuming portion-control meals | ||
| Mediterranean-style diets | Carb: 35%–40% | Eat primarily plant-based foods (fruits, vegetables, whole grains, legumes, and nuts) | ||
| Protein: 12%–20% | Healthy oils instead of saturated fats | |||
| Fat: 40%–50% | Limit red meat to a few times a month | |||
| Approximately 25%–30% of energy from monounsaturated fat | Eat fish and poultry at least twice a week | |||
| Red wine in moderation, for individuals who choose to drink alcohol | ||||
| Be active and enjoy meals with family and friends | ||||
| Low-carbohydrate, high-protein, high-fat approach | Atkins New Diet Revolution, Protein Power Diet, Stillman Diet (The Doctor’s Quick Weight Loss Diet), Carbohydrate Addict’s Diet, Scarsdale Diet | Carb: ≤20% | Promote quick weight loss (much is water loss rather than fat loss) | Same weight loss at 6 mo comparing <30 g/d vs 55% Carb–15% protein or 40% Carb and 30% protein |
| Protein: 25%–40% | Ketosis causes loss of appetite | Strength of evidence: low | ||
| Fat: ≥55%–65% | Can be too high in saturated fat | |||
| Strictly limits carbohydrates to <100–125 g/d | Low in carbohydrates, vitamins, minerals, and fiber | |||
| Not practical for long term because of rigid diet or restricted food choices | ||||
| Higher protein, moderate-carbohydrate, moderate-fat approach | The Zone Diet, Sugar Busters Diet, South Beach Diet | Carb: 40%–50% | Diet rigid and difficult to maintain | Same weight loss at 6 mo comparing 25%–30% vs 15% protein; strength of evidence: high |
| Protein: 25%–40% | Enough carbohydrates to avoid ketosis | |||
| Fat: 30%–40% | Low in carbohydrates; can be low in vitamins and minerals | |||
| Glycemic load | The Glycemic-Load Diet—Rob Thompson | Carb: 40% to >55% | Focus on low-glycemic-load foods | Same weight loss at 6 mo comparing high vs low glycemic load; strength of evidence: low |
| Protein: 15%–30% | ||||
| Fat: 30% | ||||
| Low-sugar or non–sugar-sweetened beverages | Not really a diet but just a call to reduce sugar-sweetened beverages intake as a preventive strategy | No recommendation other than to reduce/remove sugar-sweetened beverages from your overall diet plan | Meta-analyses show that consumption of sugar-sweetened beverages is related to risk of obesity, T2DM, and heart disease | Weight loss less in adolescents comparing artificial vs sugar-sweetened drinks; strength of evidence: RCT comparing artificial sweetener vs sugar-sweetened beverages |
| Novelty diets | Immune Power Diet, Rotation Diet, Cabbage Soup Diet, Beverly Hills Diet, Dr. Phil Diet | Most promote certain foods, or combinations of foods, or nutrients as having allegedly magical qualities | No scientific basis for recommendations | |
| Very low–calorie diets | Health Management Resources Program, Medifast Diet, Optifast Diet | <800 kcal/d | Requires medical supervision | |
| For clients with BMI ≥ 30 or BMI ≥ 27 with other risk factors | ||||
| May be difficult to transition to regular meals | ||||
| Weight-loss online diets | Cyberdiet, Dietwatch, eDiets, Nutrio.com | Meal plans and other tools available online | Recommend reasonable weight loss of 0.5–2.0 pounds/wk | |
| Most encourage exercise | ||||
| Some offer weight-maintenance plans/support |
Abbreviations: AHA, American Heart Association; ACC, American College of Cardiology; Carb, carbohydrate; RCT, randomized controlled trial; TOS, The Obesity Society.
A calorie deficit of 500 kcal/d produces a weekly deficit of ∼3500 kcal, which is roughly equivalent to the energy in 1 pound (0.45 kg) of fat tissue (208). Although this calculation would predict linear weight loss, weight loss is not linear; it is curvilinear. At the initial stage, weight loss tends to be more rapid, and then slows until it reaches a plateau (208–211). The initial reduction of calorie intake initiates a number of compensatory mechanisms, which tend to drive food intake up and reduce weight loss (212–214).
Several factors contribute to the different patterns of response during weight loss. The first is the initial rate of weight loss (215). In the Look AHEAD trial, a multicenter clinical trial in individuals with diabetes, those in the highest tertile of initial weight loss in the first 2 months had nearly twice as much weight loss at 4 and 8 years compared with those in the lowest tertile of weight loss in the first 2 months. This could be explained by the fact that adherence to any dietary program is critical to successful weight loss (211, 216–218).
Genetic variation can also influence weight loss, as can the biological response to different diets (219, 220). In both the Diabetes Prevention Program (219, 220) and the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) Study (220–227), individuals with the A genotype of the fat mass and obesity-associated (FTO) gene had greater weight loss when assigned the high-protein diets but not when eating the low-protein diets (222). Another analysis, which examined eight clinical trials in overweight or adults with obesity, reported that the FTO genotype did not modify the response to diet (228). Using genetic profiles may thus be of value in the future for developing personalized dietary regimens for managing obesity, but more evidence is needed for any clinical applications.
Very low–calorie diets
We define very low-calorie diets (VLCDs) as those having an energy level between 200 and 800 kcal/d. In a review comparing low-calorie diets with VLCDs, Tsai and Wadden (229) reported that VLCDs produced significantly greater short-term weight loss (16.1%) than did low-calorie diets (9.7%) but similar longer-term weight loss.
Carbohydrate subtypes, low-carbohydrate diets, and sugar-sweetened beverages
Carbohydrates, such as sugar or high-fructose corn syrup, create additional challenges to a weight-loss diet, because added sugar in beverages provides extra energy with reduced satiety, thus increasing the total energy intake (230).
“Genetic variation can also influence weight loss, as can the biological response to different diets.”
In a meta-analysis, Nordmann et al. (231) found that weight loss was greater at 6 months with low-carbohydrate diets (defined as carbohydrate intake of <60 g/d) but not at 12 months (compared with other diets). In a meta-analysis of longer trials by Tobias et al. (232), interventions with similar intensity led to a significantly greater weight loss of 1.15 kg on the low-carbohydrate diets. This is in line with a meta-analysis by Bueno et al. (233), which showed a greater weight loss of 0.91 kg with very low–carbohydrate ketogenic diets. Although both are statistically significant, the absolute difference in weight loss was quite small (∼1 kg weight reduction in a 100-kg individual). These studies over the long term are hindered by the participants’ lack of adherence to the prescribed dietary regimens.
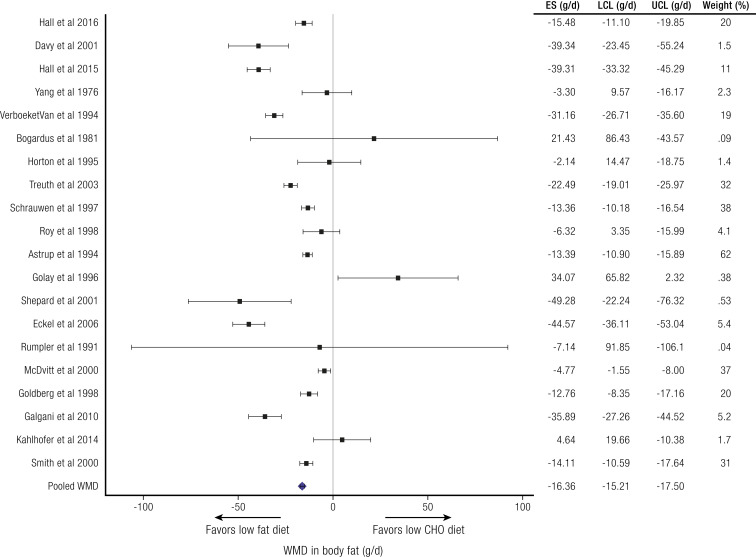
To circumvent the problem of variable effects of dietary protein in evaluating low-carbohydrate and low-fat diets (234), Hall and Guo (235) performed a meta-analysis of isocaloric low-carbohydrate/high-fat diets vs high-carbohydrate/low-fat diets where protein consumption was held constant. This analysis included 32 studies (563 subjects total), which provided all food to the subjects. Dietary carbohydrate ranged from 1% to 83% and dietary fat from 4% to 84% of total energy intake. There was a small but significant 26 kcal/d weighted mean energy expenditure difference favoring the low-fat/high-carbohydrate diets (not shown) and a small but significant 16 g/d weighted mean body fat difference favoring the low-fat/high-carbohydrate diets (Fig. 4) (235). This analysis does not support the concept of a metabolic advantage for lower carbohydrate, higher fat diets, suggesting that any benefits of such diets probably involve differences in energy intake.
Figure 4.
Weight loss comparing isocaloric low-carbohydrate/high-fat and high-carbohydrate/low-fat diets where meals were provided and protein consumption was the same. 95% horizontal CI. CHO, carbohydrate; ES, effect size; LCL, lower confidence limit; UCL, upper confidence limit; WMD, weighted mean difference. See Hall and Guo, 2017 (235).
Dietary fat, energy density, and low-fat diets
For decades, dietary recommendations for weight loss have emphasized a reduction in fat intake because of its high-energy content (9 kcal/g) compared with carbohydrates (4 kcal/g) (236). A meta-analysis of six trials reported no significant differences between low-fat diets (20 to 30 g/d or 20% of total energy) vs other weight-loss diets in terms of sustained weight loss (237).
A recent systematic review and meta-analysis compared the effects of low-fat interventions (<30% total fat) vs other dietary interventions on long-term (≥1 year) weight changes. It found that when the groups differed by >5% fat content, the higher fat interventions led to slightly greater weight loss and better adherence, although the magnitude of the differences in weight loss was small (232). The important message is that “adherence” rather than a specific diet is the important ingredient in success.
Another strategy for reducing energy density (besides reducing dietary fat intake) is to substitute foods with higher water content. One trial has compared a reduced-fat diet to a diet with extra fruits and vegetables with lower energy density. In this trial, the addition of fruits and vegetables led to greater weight loss compared with lowering fat only (238). Diets with a higher intake of fruits and vegetables evolved into the Volumetrics diet (239). The efficacy of the Volumetrics diet warrants further investigation.
Low–glycemic index diets
The glycemic index is based on the rise in blood glucose in response to test foods (240, 241). A meta-analysis by Thomas et al. (242) reported a significant but small difference in weight loss of 1.1 kg that favored low–glycemic index diets. Additionally, both total and low-density lipoprotein (LDL) cholesterol fell more with low–glycemic index diets. The long-term effects of low–glycemic index diets warrant further evaluation.
Fasting glucose may provide a clue to dietary selection. Hjorth et al. (243) have reported that individuals with higher fasting glucose who are prediabetic may respond better to a lower glycemic index diet with more fiber and whole grain.
High-protein diets
A 2-year study comparing 12% and 25% protein diets as part of a 30% fat diet (244, 245) reported that weight loss during 24 weeks was substantially greater with the higher protein diet, and that this result was maintained up to 56 weeks but not at 104 weeks.
A meta-analysis of energy-restricted, high-protein/low-fat diets compared with standard-protein/low-fat diets showed that the high-protein diet was better at reducing body weight (−0.79 kg; 95% CI, −1.50 to −0.08 kg), fat mass (−0.87 kg; 95% CI, −1.26 to −0.48 kg), and triglycerides (−0.23 mmol/L; 95% CI, −0.33 to −0.12 mmol/L) and resulted in less of a decrease in fat-free mass (0.43 kg; 95% CI, 0.09 to 0.78 kg) and resting energy expenditure (595.5 kJ/d; 95% CI, 67.0 to 1124.1 kJ/d) (246). In the intent-to-treat analysis of the POUNDS Lost Study (218), which compared 15% and 25% protein diets, there was no difference in weight loss between these diets. However, those who adhered to a higher protein diet lost more weight. When this study used urinary nitrogen loss as a measure of protein intake, those with the greater increase in protein intake lost significantly more weight (247).
Mediterranean-style diets
Mediterranean-style diets are characterized by enhanced consumption of olive oil, nuts, whole grain, fruits, and vegetables. In diabetic individuals, the Mediterranean diet produced a greater weight loss during 4 years than did a low-fat diet (248). Another meta-analysis (249) reported that Mediterranean diets reduced body weight 2.2 kg compared with low-fat diets. The Prevención con Dieta Mediterránea (PREDIMED) study from Spain showed that consumption of a high-fat Mediterranean diet (41.8% calorie from fat) resulted in a 0.43 kg weight loss (P = 0.043) and a 0.55 cm waist circumference reduction (P = 0.48) vs a comparison diet (37.4% calorie from fat) during 4.8 years of follow-up (250).
Balanced-deficit diets
Diets with a reduced content of carbohydrates, proteins, and fat (so-called “balanced-deficit diets”) have been widely used in managing obesity. In a meta-analysis, Avenell et al. (251) reported that intervention diets with an average deficit of 600 kcal/d led to a weight loss of 5.31 kg compared with controls, and the weight-loss effect lasted up to 3 years.
In a 6-month intervention, the daily use of a commercially available portion-control plate was effective in promoting weight loss among patients with obesity and T2DM when compared with a usual-care dietary group. A meta-analysis of six studies using meal replacements showed more weight loss than low-calorie diets at 3 months (252). Data from another trial showed that portion control can increase diet quality while maintaining significant weight loss during 18 months (253).
Comparison of diets with different macronutrient composition
Several RCTs have compared diets head-to-head (216, 218, 231, 254, 255). We summarize these in Table 4 (216, 218, 254–262). These studies show improvements in hemoglobin A1c (HbA1c) in patients with T2DM and improvements in triglycerides and HDL cholesterol in the groups assigned to the low-carbohydrate diet arms. One trial randomized 169 individuals with obesity to one of four popular diets, including the Atkins diet (263), The Ornish diet (264), the Weight Watchers diet (265), and the Zone diet (266). At the end of 12 months, each diet produced similar weight losses (∼5 kg). Adherence to the diets was the single most important criterion of success in these trials. In one study, a low-fat diet was compared with a low-carbohydrate diet (Atkins diet) and a Mediterranean-style diet (255). Compared with the low-fat diet, individuals assigned to the Mediterranean diet and low-carbohydrate diet had significantly greater weight loss and maintenance by 24 months (255). In a meta-analysis of numerous popular diets that included 48 unique trials, low-carbohydrate diets performed equally with low-fat diets after 12 months, with the low-carbohydrate diets resulting in 7.25 kg of weight loss (95% CI, 5.33 to 9.25 kg) compared with 7.27 kg of weight loss in the low-fat diet groups (95% CI, 5.26 to 9.34 kg) (267).
Table 4.
Weight Losses from Randomized Controlled Trials That Compared Diets With Varying Macronutrient Compositions
| Study | No., Sex, and Completers | No. of Lifestyle Sessions Provided | Dietary Intervention | Weight Change | Month | Comments/Other Results |
|---|---|---|---|---|---|---|
| Bazzano et al., 2014 (256) | 148, 88% F, 80% completed | 10 | Low Carb (<40 g/d) | −6.5 kga | 12 | Participants without CVD or diabetes; low carbohydrate diet group had greater decrease in body fat and triglycerides and greater increase in HDL cholesterol than did the low-fat group. C-reactive protein and 10-Year Framingham Risk Score improved more in low-carbohydrate group. No difference in BP response. Low-fat group had lower protein intake than in the low-carbohydrate group. |
| Low fat (<30% fat) | −2.6 kgb | |||||
| Dansinger et al., 2005 (216) | 160, 51% F, 58% completed | 4 | Atkins (Low Carb) | −2.1 kga | 12 | All patients had hypertension, dyslipidemia, and/or fasting hyperglycemia. |
| Zone (30% fat) | −3.2 kga | Weight loss was associated with level of adherence. | ||||
| Weight Watchers (Low calorie) | −3.0 kga | Each diet decreased LDL/HDL ratio. | ||||
| Ornish (10% fat) | −3.3 kga | There were no significant effects on BP or blood glucose at 12 mo. | ||||
| Das et al., 2007 (257) | 34, % F unknown, 85% completed | 52 | Low glycemic load | −7.8%a | 12 | Triglycerides and total, HDL, and LDL cholesterol decreased in both groups. |
| High glycemic load | −8.0%a | |||||
| Fabricatore et al., 2011 (258) | 79, 80% F, 63% completed | 30 | Low glycemic load | −4.5%a | 9 | All patients had T2DM. |
| Low fat | −6.4%a | There were larger reductions in HbA1c in the low–glycemic load group. | ||||
| Foster et al., 2003 (259) | 63, 68% F, 59% completed | 3 | Low carbohydrate (high protein, high fat) | −4.4%a | 12 | HDL cholesterol increased more in the low-carbohydrate group, and triglycerides were lower only in the low-carbohydrate group. |
| Conventional (high carbohydrate, low fat) | −2.5%a | Diastolic BP decreased in both groups. | ||||
| Area under the insulin curve decreased in both groups. | ||||||
| Foster et al., 2010 (260) | 307, 68% F, 63% completed | 38 | Low carbohydrate | −6.3 kga | 24 | HDL cholesterol increased more in the low-carbohydrate group. |
| Low fat | −7.4 kga | |||||
| Gardner et al., 2007 A to Z Study (254) | 311, 100% F, 80% completed | 8 | Atkins (low carb) | −4.7 kga | 12 | Increase in HDL cholesterol was larger in the Atkins than in the Ornish group. Triglyceride levels decreased more in the Atkins than in the Zone group. |
| Zone (30% fat) | −1.6 kgb | There were no differences in insulin or blood glucose between groups. | ||||
| LEARN (calorie restricted) | −2.2 kga,b | Systolic BP decreased more in Atkins than in all other groups. | ||||
| Ornish (<10% fat) | −2.6 kga,b | Diastolic BP decreased more in Atkins group than in Ornish group. | ||||
| Sacks et al., 2009 POUNDS Lost Study (218) | 811, 64% F, 80% completed | 66 | Low fat, average protein (highest carbohydrate) | −2.9 kga | 24 | LDL cholesterol decreased significantly more in lowest fat/highest carbohydrate group than in highest fat/lowest carbohydrate groups. |
| Low fat, high protein | −3.8 kga | HDL cholesterol increased more with lowest carbohydrate than with the highest carbohydrate diet. | ||||
| High fat, average protein | −3.1 kga | All diets decreased triglyceride levels similarly. | ||||
| High fat, high protein (lowest carbohydrate) | −3.5 kga | All diets, except the highest carbohydrate diet, decreased fasting insulin (greater decrease in the high-protein vs average-protein diets). | ||||
| Shai et al., 2008 DIRECT Study (255) | 322, 14% F, 85% completed | 24 | Low fat | −2.9 kga | 24 | No significant change in LDL cholesterol in any group. |
| Mediterranean | −4.4 kgb | HDL cholesterol increased in all groups, significantly more in the low-carbohydrate than low-fat group. | ||||
| Low carbohydrate | −4.7 kgb | Triglyceride levels decreased more in the low-carbohydrate than in the low-fat group. | ||||
| In diabetic patients, only the Mediterranean diet group had a decrease in fasting glucose. | ||||||
| Insulin decreased in all groups for both diabetic and nondiabetic patients. | ||||||
| All groups had a significant decrease in BP. | ||||||
| Adiponectin levels increased and leptin levels decreased in all groups. | ||||||
| Stern et al., 2004 (261) | 132, 17% F, 66% completed | 15 | Low carbohydrate | −5.1 kga | 12 | Triglyceride levels decreased more in the low-carbohydrate group than in the low-fat group. |
| Conventional (low fat) | −3.1 kga | HDL cholesterol decreased less in the low-carbohydrate group than in the low-fat group. | ||||
| Changes in total and LDL cholesterol were not significant between groups. | ||||||
| Yancy et al., 2004 (262) | 120, 76% F, 66% completed | 9 | Low-fat diet | −6.5%a | 6 | All patients were hyperlipidemic. |
| Low-carbohydrate, ketogenic diet with nutritional supplements | −12.9%b | Triglycerides decreased more and HDL cholesterol increased more in the low-carbohydrate group. |
Different letters (in superscript) indicate statistically significant differences (P ≤ 0.05) in weight loss between groups.
Abbreviations: MR, meal replacements; VLDL, very low–density lipoprotein.
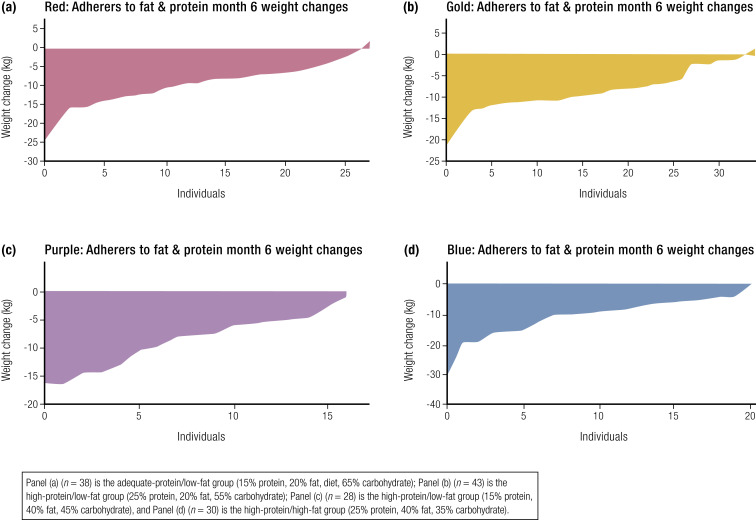
The POUNDS Lost Study (the largest trial examining macronutrient composition and weight loss) randomized participants to one of four diets, with 80% of patients providing data on body weight at the end of 2 years. The diets were: (1) 20% fat/15% protein; (2) 20% fat/25% protein; (3) 40% fat/15% protein; or (4) 40% fat/25% protein. The foods in all four diets were the same, although they differed in quantity. At the end of 6 months, 12 months, and 2 years, the weight loss was similar for all four diets (268); however, those who achieved the largest increase in protein intake lost more weight (247). The similarity of the mean weight loss in all four diet groups obscures the wide range of individual weight losses shown in Fig. 5 (243). The data from the POUNDS Lost Study are consistent with the recommendations of the American College of Cardiology/American Heart Association/Obesity Society Guideline for the Management of Overweight and Obesity in Adults, which states that “a variety of dietary approaches can produce weight loss in overweight and obese adults, and that the choice should be based on the patient’s preferences and health status” (39).
Figure 5.
Weight change from baseline to 6 months for each individual participant in the four dietary assignment groups ranked from the largest loser on the left to the most weight gain on the right. (a) (n = 38) Adequate-protein/low-fat group (15% protein, 20% fat, 65% carbohydrate); (b) (n = 43) high-protein/low-fat group (25% protein, 20% fat, 55% carbohydrate); (c) (n = 28) high-protein/low-fat group (15% protein, 40% fat, 45% carbohydrate); (d) (n = 30) high-protein/high-fat group (25% protein, 40% fat, 35% carbohydrate).
Commercial programs for weight loss
In a meta-analysis, Gudzune et al. (269) reported that the Weight Watchers diet resulted in at least a 2.6% greater weight loss than those assigned to control/education after 12 months. The Jenny Craig diet resulted in a 4.9% greater weight loss during a 12-month period vs groups receiving control/education and counseling. The Nutrisystem diet resulted in a 3.8% greater weight loss at 3 months vs control/education and counseling. VLCDs (Health Management Resources, Medifast, and Optifast) resulted in a 4.0% greater short-term weight loss than counseling, and the weight-loss effect lasted up to 6 months. The Atkins diet (not technically a commercial program, but one with affiliated diet products) resulted in 0.1% to 2.9% greater weight loss at 12 months compared with counseling (269). The differences in the amount of weight loss among various commercial diets were relatively small, and the long-term effects of these diets on weight control and chronic disease risk are still unclear.
Maintenance of long-term weight loss
As previously discussed and illustrated in the Diabetes Prevention Program (158, 270) and the Look AHEAD trial (271), maintaining weight loss is a challenge.
One study (272, 273) assigned participants to weight loss with a VLCD for 4 weeks before randomizing them to either a control diet or study diet supplemented with 48.2 g/d of protein. At the end of 3 months, the group receiving the protein supplement (to bring protein to 18% of total energy) had a 50% reduction in body-weight regain.
Data from the Women’s Health Initiative indicate that reducing dietary fat intake may be of value for long-term weight maintenance (274, 275). The study reported that body weight in the low-fat diet group and the control-diet group was similar after an average of 7.5 years of follow-up (274). However, those who maintained the lowest quintile of fat intake were 1.5 kg lighter compared with those in the top quintile of fat intake, who were 0.8 kg heavier after 7 years. A recent comprehensive meta-analysis indicated that long-term effects of low-fat diets on body weight depended on the intensity of intervention in the comparison group. When compared with other dietary interventions of similar intensity, evidence from RCTs does not support low-fat diets over other dietary interventions (232).
The National Weight Control Registry identifies additional strategies for maintaining weight loss (276), which include engaging in higher levels of physical activity (e.g., 225 to 300 min/wk), eating a low-fat, low-calorie diet (1200 to 1300 kcal/d for women), and weighing themselves frequently (once a week or more) (277, 278).
Prediction of weight gain may also be related to the ability to metabolize carbohydrates. Subjects who had a higher positive carbohydrate balance on day 15, were inactive, and ate an isocaloric high-carbohydrate diet gained less fat mass during a 4-year follow-up period (279).
Future considerations/summary
Diets with many different macronutrient compositions can result in short-term weight loss. However, weight loss reaches a plateau within the first 3 to 6 months. After that, weight is regained and often returns to baseline by 1 to 2 years.
Maintenance of long-term weight loss is strongly influenced by the ability to adhere to the dietary program. Behavioral support can significantly improve outcomes. There are variations among individuals in the response to each diet, which are larger than the difference in mean weight loss between comparison diets. Clinicians should consider genetic differences regarding dietary response to weight loss, as personalized dietary regimens might improve the efficacy of long-term weight-loss regimens.
Current data indicate that some (but not all) individuals can achieve modest long-term weight loss with any one of the diets evaluated herein. Additional research is needed to identify optimal diets for weight control and long-term health, which should extend beyond macronutrient composition and examine food quality and overall dietary patterns, as well as factors that can improve long-term compliance. The Nurses Health Study and Health Professionals Follow-up Study reported that improving diet quality was associated with less weight gain, especially in younger women or individuals who are overweight (280).
Exercise in managing obesity
Introduction
There is a significant body of evidence supporting the effect of physical activity in both short-term and long-term weight loss in adults (216, 218, 254–262).
The main components of energy expenditure (by order of magnitude) are resting energy expenditure, physical activity, and the thermic effect of food. Resting energy expenditure is the amount of energy required for a 24-hour period by the body during resting conditions. Physical activity is composed of both nonexercise activity thermogenesis and thermogenesis due to volitional activity of muscle groups. The thermic effect of food is the amount of energy (above the resting rate) used for processing and storing food.
Energy expenditure from physical activity is directly related to body weight. However, it is unclear to what extent reductions in energy expenditure from physical activity relate to the epidemic of obesity that has developed during the last 30 years. Most measurements of energy expenditure are not precise or easy to use. Therefore, reliable longitudinal data are lacking.
Two recent studies have concluded that the current epidemic of obesity is more the result of an increase of energy intake than a decrease in energy expenditure (281–283), but this is not the universal opinion (280).
Genetic factors of physical activity
There is an important genetic component associated with the extent to which individuals engage in physical activity (284). In a study examining regular exercise among identical and fraternal twins that included both same and opposite sex pairs, environmental factors shared by children at age 13 accounted for 78% to 84% of sports participation, whereas genetic differences provided almost no contribution. By age 17 to 18 the genetic influences represented 36% of the variance in the level of participation in sports, and by age 18 to 20, genetic factors accounted for almost all (85%) of the differences in participation in sports (284, 285).
Resistance vs aerobic exercise
Although most research on the effects of physical activity on body weight has focused on aerobic types of physical activity, there is also evidence suggesting that resistance exercise may have some effect on weight loss. Resistance exercise may influence body weight by increasing lean body mass, which will result in an increase in resting metabolic rate. Resistance exercise also improves one’s strength, which may result in more free-living physical activity and thus increased total daily energy expenditure (286). However, the vast majority of data indicate that resistance exercise only results in minimal reductions in body weight or body fatness (286–288).
Vigorous vs moderate exercise
A study of >4500 adults from the U.S. National Health and Nutrition Examination Survey showed that greater physical activity was associated with a lower BMI (289). This relationship only existed with moderate- to vigorous-intensity physical activity and not with low-intensity physical activity. These data imply that there is an intensity threshold of physical activity that is necessary to affect body weight and prevent excessive weight gain.
Physical activity declines with age
Despite the benefit of physical activity in weight loss, physical activity appears to decline during adolescence and remains low in most adults (290, 291). In a longitudinal study of adolescent girls, the level of activity declined in both black and white girls each year during adolescence. By age 17, black girls engaged in almost no spontaneous physical activity and white girls only engaged in very modest amounts of spontaneous physical activity (292). We do not have a comparable study in adolescent males.
Sedentary behavior
There is keen interest in the influence of sedentary behavior on a variety of health-related outcomes, including overweight and obesity. Energy expenditure in occupational activities has declined by ∼140 kcal/d since 1960 in the United States, and this reduction in energy expenditure accounts for a significant portion of the increase in mean U.S. body weights for women and men since 1960 (293).
Much of the early literature in this area focused on the association between television viewing as an indicator of sedentary behavior and the risk of obesity. Television viewing is positively associated with the risk of gaining weight and the development of obesity (294, 295).
Treatment of patients who are overweight or obese using exercise with and without diet
Studies on obesity have evaluated exercise as a sole treatment, in combination with diets, and as a way to maintain weight loss. Östman et al. (296) performed a Medline search for studies related to physical exercise and overweight and identified six relevant RCTs. Five had a treatment interval of 12 months, and all had a dropout rate of <40%. Table 5 (296–304) has been adapted from this study with the addition of two newer trials, one 16 months long and one 8 months long. The effects from diet are significantly greater than those from exercise, but increasing physical activity may have important benefits on improving BP and cardiometabolic risk factors.
Table 5.
Clinical Trials of Exercise in Individuals Who Are Overweight or Obese
| Authors | Inclusion Criteria | Intervention Groups | Duration | No. Patients/No. Follow-up | Results | Comments |
|---|---|---|---|---|---|---|
| Wood et al., 1988 (297) | Men 120%–160% overweight | (1) Diet (−1 kg/wk); fat reduced by 30% | 1 year | (1) 51/42 | (1) BW −7.2 kg | TG and HDL cholesterol improved |
| (2) Individual instruction (−1 kg/wk); exercise (60%–80% of maximal physical capacity, 40–50 min, three to four times per week) | (2) 52/47 | Fat −5.9 kg | Diet and physical activity yield the same reduction in weight and fat at the same negative calorie balance | |||
| (3) Control | (3) 52/42 | (2) BW −4.0 kg | ||||
| Fat −4.2 kg | ||||||
| (3) BW +0.6 kg | ||||||
| Fat −0.3 kg | ||||||
| Wing et al., 1988 (298) | Women: 30–60 y with T2DM; >20% above ideal weight | (1) Diet (−1000 kcal/d) + walking (3 miles, three times per week) | 12 mo | (1) 12 | (1) BW −7.9 kg | HbA1C was reduced and medications were reduced in groups 1 and 2 |
| (2) Free diet + walking (3 miles, four times per week) | (2) 15 | (2) BW −7.9 kg | ||||
| (3) Diet + stretching | (3) 13 | (3) BW −3.8 kg | ||||
| Wood et al., 1991 (299) | Men and women: 25–49 y; overweight 120%–160% | (1) Diet (moderate reduction of energy, fat, cholesterol) | Bottom of form | (1) 87/71 | Men: | BP decreased in groups 1 and 2 (both men and women) |
| (2) Diet (as above) + exercise (60%–80% of maximal physical capacity, 25-45 min, three times per week) | (2) 90/81 | (1) BW −5.1 kg | Cholesterol decreased in groups 1 and 2 (women) | |||
| (3) Control | (3) 87/79 | (2) BW −8.7 kg | HDL cholesterol increased in group 2 (both men and women) | |||
| (3) BW +1.7 kg | TG decreased in group 2 (men) | |||||
| Women: | ||||||
| (1) BW −4.2 kg | ||||||
| (2) BW −5.5 kg | ||||||
| (3) BW +1.3 kg | ||||||
| Svendsen et al., 1994 (300) | Women: 49-58 y; BMI 25–42 kg/m2 | (1) Diet (4.2 MJ/d = 1000 kcal/d) | 2 wk with 6 mo follow-up | (1) 51/47 | 12 wk: | TG decreased and |
| (2) Diet (as above) + exercise (submaximal aerobics and body building) | (2) 49/47 | (1) BW −6.6 kg | HDL cholesterol increased | |||
| (3) Control | (3) 21/16 | (2) BW −10.9 kg | There was no effect from physical activity | |||
| (3) BW 0.0 kg | ||||||
| 6 mo: | ||||||
| (1) BW −8.0 kg | ||||||
| (2) BW −8.0 kg | ||||||
| (3) BW 0.0 kg | ||||||
| Pritchard et al., 1997 (301) | Men: overweight mean BMI 29 kg/m2 | (1) Diet (−500 kcal/d) + low fat | 12 mo | 66/60 | (1) BW −6.3 kg | Diet was “self-controlled” |
| (2) Exercise (65%–75% of maximal physical capacity, 45 min, three to seven times per week) | (2) BW −2.6 kg | |||||
| (3) Control | (3) BW +0.9 kg | |||||
| Irwin et al., 2003 (302) | Overweight nonsmoking postmenopausal women age 50–75 y with a BMI > 25 kg/m2 or BMI 24–25 and body fat > 33% by DXA who were sedentary at baseline (<60 min/wk of moderate to vigorous activity) and maximal oxygen uptake of <25 mL/kg/min | (1) Exercise [at least 45 min, moderate intensity, 5 d/wk, 12 mo (months 1–3 they attended three sessions per week; months 4–12 they attended one session per week)] | 12 mo | (1) 87/84 | (1) 3 mo: | Participants were advised to maintain usual diet |
| (2) Stretching (weekly sessions of 45 min for 12 mo) | (2) 86/86 | BW −0.5 | Weight loss was related to degree of exercise | |||
| 12 mo: | ||||||
| BW −1.3 kg | ||||||
| Fat −1.4 kg | ||||||
| VAT −8.5 cm2 | ||||||
| (2) 3 mo: | ||||||
| BW 0.0 kg | ||||||
| 12 mo: | ||||||
| BW 0.1 kg | ||||||
| Fat −0.1 kg | ||||||
| VAT 0.1 cm2 | ||||||
| Donnelly et al., 2003 (303) | Overweight men and women age 17–36 y with a BMI of 25.0–34.9 kg/m2 | (1) Exercise (400 kcal/d, 5 d/wk with walking on a treadmill at 55%–70% of maximal physical capacity uptake) | 16 mo of verified exercise | (1) 87/41 | (1) Men | Exercise produced weight loss in men and prevented weight gain in women |
| (2) Control | (2) 44/33 | BW −5.2 kg | ||||
| Fat −4.9 kg | ||||||
| VAT −22.4 cm2 | ||||||
| Women | ||||||
| BW +0.4 kg | ||||||
| (1) Fat −0.2 kg | ||||||
| VAT −3.2 cm2 | ||||||
| (2) Men | ||||||
| BW −0.5 kg | ||||||
| Fat −0.7 kg | ||||||
| VAT −6.3 cm2 | ||||||
| Women | ||||||
| BW +2.9 kg | ||||||
| Fat +2.0 kg | ||||||
| VAT +3.1 cm2 | ||||||
| Slentz et al., 2004 (304) | Men and women age 40–60 y and BMI of 25–35 kg/m2 and mild to moderate lipid abnormalities | (1) Exercise [high amount/vigorous intensity, calorically equivalent to ∼20 miles (32.0 km) of jogging per week at 65%–80% maximal physical capacity] | 8 mo of observed exercise | (1) 44/17 | (1) BW −3.5 kg | Subjects were counseled not to change diets and encouraged to maintain body weight. |
| (2) Exercise [low amount/vigorous intensity, equivalent to ∼12 miles (19.2 km) of jogging per week at 65%–80%] | (2) 52/24 | Fat 4.9 kg | ||||
| (3) Exercise [low amount/moderate intensity, equivalent to ∼12 miles (19.2 km) of walking per week at 40%–55%] | (3) 42/14 | Waist −3.4 cm | ||||
| (4) Control | (4) 44/7 | (2) BW −1.1 kg | ||||
| Fat −2.6 kg | ||||||
| Waist −1.4 cm | ||||||
| (3) BW −1.3 kg | ||||||
| Fat −2.0 kg | ||||||
| Waist −1.1 cm | ||||||
| (4) BW 1.1 kg | ||||||
| Fat 0.5 kg | ||||||
| Waist 0.8 cm |
Abbreviations: BW, body weight; DXA, dual x-ray absorptiometry; TG, triglyceride; VAT, visceral adipose tissue.
Adapted and updated from Östman et al., 2004 (296).
Behavioral Therapy in Managing Obesity
Behavioral modifications and/or lifestyle interventions have been an important part of weight-loss programs for more than half a century (305–307). Data from two large RCTs, the Look AHEAD trial and the Diabetes Prevention Program, support the efficacy of these approaches. These studies are the gold standard and are notable for the frequency of contact, the emphasis on individualizing therapy, and the long-term emphasis on maintaining weight loss. Fig. 6 shows data from the Look AHEAD trial. The best outcomes are with frequent, face-to-face interventions. However, incorporating this in primary care is challenging.
Figure 6.
Mean (±SE) weight losses during 8 years for participants randomly assigned to an intensive lifestyle intervention or diabetes support and education (usual-care group). Differences between groups were significant (P < 0.001) at all years. DSE, diabetes support and education; ILI, intensive lifestyle intervention.
In a meta-analysis of behavioral weight-loss programs, LeBlanc et al. (308) reported a mean weight loss of −3.01 kg (95% CI, −4.02 to −2.01 kg) favoring the behavioral strategy, but the range of mean values was quite large (−0.71 to −8.30 kg).
Lifestyle interventions may also be effective for preventing weight regain (269, 309). Patients who participated in group sessions every other week for 1 year after weight reduction maintained 13 kg of their 13.2 kg end-of-treatment weight loss (309). The most successful patients monitor their weight frequently and respond quickly to small increases in weight (276). This can be daily or several times a week, but some daily variation (−0.5 to 1.0 kg) is to be expected from fluctuations in body water.
Lifestyle methods
Self-monitoring
Self-monitoring involves recording the type and amount of foods and beverages consumed, along with their calorie content and weight gain. Self-monitoring helps patients identify their eating patterns (including times and places associated with consumption) and also helps patients select targets for reducing calorie intake (310) (Table 6).
Table 6.
Key Components of Comprehensive Behavioral Weight-Loss Interventions to Achieve a 7% to 10% Weight Loss
| Component | Weight Loss | Weight-Loss Maintenance |
|---|---|---|
| Frequency and duration of treatment contact | • Weekly contact, in person or by telephone, for 20–26 wk (Internet/e-mail contact yields smaller weight loss) | • Every-other-week contact for 52 wk (or longer) |
| • Group or individual contact | • (Monthly contact likely adequate) | |
| • Group or individual contact | ||
| Dietary prescription | • Low-calorie diet (1200-1500 kcal for those <250 pounds; 1500–1800 kcal for those ≥250 pounds | • Consumption of a hypocaloric diet to maintain reduced body weight |
| • Typical macronutrient composition: ≤30% fat (≤7% saturated fat), 15%–25% protein, remainder from carbohydrate (diet composition based on individual needs or preferences) | • Typical macronutrient composition similar to that for weight loss | |
| Physical activity prescription | • 180 min/wk of moderately vigorous aerobic activity (e.g., brisk walking), strength training also desirable | • 200-300 min/wk of moderately vigorous aerobic activity (e.g., brisk walking), strength training also desirable |
| Behavior therapy prescription | • Daily monitoring of food intake and physical activity by use of paper or electronic diaries | • Occasional to daily monitoring of food intake and physical activity by use of similar diaries |
| • Weekly monitoring of weight | • Twice weekly to daily monitoring of weight | |
| • Structured curriculum of behavior change (e.g., Diabetes Prevention Program) | • Curriculum of behavior change, including relapse prevention and individualized problem solving | |
| • Regular feedback from an interventionist | • Periodic feedback from an interventionist |
Stimulus control
Techniques of stimulus control teach patients to manage external cues, such as the sight or smell of food, as well as times, places, and events associated with eating (278, 310). By decreasing exposure to problem foods, patients are less likely to overeat.
Goal setting
Goal setting helps patients make objective, measurable changes in eating, activity, and related behaviors (278, 310). They are guided in setting specific targets for calorie intake, minutes of physical activity, and frequency of self-monitoring.
Problem solving
Problem solving teaches patients to analyze challenges they have in adhering to their diet and activity prescriptions (278, 310, 311). Patients learn to identify a number of possible solutions to the problem, pick the most promising one, and then implement it. They learn to identify cognitive distortions (e.g., “I will never be able to lose weight because I ate that dessert”) and to replace them with rational responses (e.g., “One hundred fifty calories of cake is not going to hinder my weight loss, particularly if I walk after dinner”) (312). It is important for patients to remember that the 150 calories needs to be “subtracted” in the future either with exercise or by reducing intake of some other carbohydrate/fat-containing foods.
Short-term efficacy
The structured behavioral programs, as described above, produce an average loss of 7 to 10 kg in the first 6 months but with great variability. Some lose no weight; others lose >10%. Seven to 10 kg weight loss is generally equivalent to a reduction of 7% to 10% of initial weight, because 100 kg is the average weight for patients in many studies (38, 307). Patients require a high-intensity intervention to achieve these losses; lower intensity treatment is not as effective (38). Approximately 50% to 70% of patients achieve a ≥5% reduction in initial weight, a criterion for clinically meaningful weight loss (38). Individuals with the best attendance and greatest consistency in keeping self-monitoring records achieve the largest weight losses (277).
New developments in the delivery of behavioral treatment
Telephone-delivered programs
Sherwood et al. (313) demonstrated that during a 6-month period, patients who received 20 intervention session phone calls lost an average of 4.9 kg; those who received 10 intervention calls lost 3.2 kg, and those who were self-directed lost 2.3 kg. Appel et al. (133) reported that a group that received weekly telephone coaching for 3 months, an Internet program for recording food intake and physical activity, and monthly coaching for an additional 18 months lost a mean of 6.1 kg at 6 months. The weight loss was generally well maintained at 24 months (4.6 kg) and was not significantly different than what another group achieved using an intensive in-person intervention (5.1 kg at 24 months) (133).
Perri et al. (314) demonstrated that women who were enrolled in extended-care programs that included problem-solving counseling delivered in 26 biweekly sessions via telephone or face-to-face regained only 1.2 kg in 1 year of treatment, vs 3.7 kg for those in a newsletter-only group.
“By decreasing exposure to problem foods, patients are less likely to overeat.”
Several studies that used structured dietary interventions (i.e., meal replacements and/or portion-controlled entrees) reported roughly equivalent weight losses when the same behavioral intervention was delivered in person or by telephone (315–317).
Digitally-delivered programs
Tate et al. (318) demonstrated that patients who were provided with a directory of Internet resources for weight management and also received 24 weekly lessons over 6 months via e-mail (where patients submitted their food and activity records online and received online feedback from an interventionist) lost 4.1 kg vs patients who only received the directory. In a 1-year follow-up study, Tate et al. (318) demonstrated that patients assigned to a low-intensity Internet intervention with the addition of weekly behavioral counseling lost 4.4 kg, whereas those receiving only the low-intensity Internet intervention lost 2.0 kg.
Harvey-Berino et al. (319) compared the same 24-session intervention provided either via Internet or on site. In 6 months, the on-site program resulted in 8.0 kg weight loss vs 5.5 kg for the Internet-only group.
These studies underscore the importance of patients keeping records of their food intake and physical activity and receiving feedback from a trained interventionist. Educational instruction (i.e., information) alone is not sufficient to induce clinically meaningful weight loss.
These studies also suggest that the most successful Internet programs are those in which therapists provide weekly e-mail feedback to patients. However, on-site behavioral programs still provide better results (320).
The reduced efficacy of Internet programs, however, is offset by the potentially greater accessibility and affordability of this approach, compared with traditional behavioral treatment.
Despite their popularity, little is known about the effectiveness of smart-phone applications for weight management. A recent study that compared usual primary care with or without the MyFitnessPal application revealed essentially no weight-loss difference between the two approaches during 6 months (321).
Medication in Managing Obesity
Early history
Medications for managing obesity have a long and checkered history (322). Treatment in the 18th century included soap (6, 17) and vinegar mixed with a number of purgatives (18). Some treatments also used tobacco, a strategy people still use today to prevent weight gain.
In the late 19th and early to mid-20th century, three major groups of medications came into use: thyroid hormone, dinitrophenol, and amphetamine. Clinicians prescribed both thyroid extract and dinitrophenol (a product of the aniline dye industry) until negative side effects became evident (19).
Amphetamine became popular after 1937 when Nathanson (323) noted that 10 of 40 patients treated with amphetamine for narcolepsy had marked loss of appetite and weight. However, the abuse potential of amphetamines soon became apparent (324), and clinicians stopped prescribing them as a way to manage obesity.
Aminorex, another member of the amphetamine-like group, emerged in Austria and Switzerland in 1968, but it was removed from the market in 1972 due to associated pulmonary hypertension (325). Table 7 lists several drugs for obesity management that were associated with significant detrimental side effects (326).
Table 7.
Some Medications Used in the Past for Managing Body Weight That Were Withdrawn or Are Not Approved in the United States
| Drug | Year Introduced or Withdrawn | Comments |
|---|---|---|
| Thyroid | 1892 | Mimics endogenous thyroxine/triiodothyronine |
| Associated with tachycardia and increase in metabolic rate | ||
| Dinitrophenol | 1932 | Uncouples oxidative phosphorylation |
| Associated with cataracts, neuropathy, and death | ||
| Amphetamine | 1937 | Noradrenergic-dopaminergic drug |
| Associated with recreational abuse and pulmonary hypertension | ||
| Aminorex | 1965 | Noradrenergic drug |
| Associated with pulmonary hypertension | ||
| Fenfluramine, dexfenfluramine | 1997 | Serotonergic drugs |
| Both associated with cardiac valvulopathy and primary pulmonary hypertension | ||
| Phenylpropanolamine | 1998 | Noradrenergic agonist |
| Associated with strokes and cardiovascular deaths | ||
| Ephedra alkaloids | 2003 | Noradrenergic drugs |
| Associated with heart attacks, strokes, and death | ||
| Rimonabant | 2008 | Cannabinoid receptor antagonist |
| Associated with depression and suicidality | ||
| Sibutramine | 2010 | Norepinephrine-serotonin reuptake inhibitor |
| Associated with elevated BP and death |
From the end of World War II through 1994, there was considerable research on monoaminergic drugs. Researchers discovered that injecting norepinephrine into the central nervous system of experimental animals reduced food intake and activated thermogenesis. This resulted in a search for thermogenic drugs that could work through monoaminergic receptors.
During this period, researchers also synthesized many derivatives of amphetamine for treating obesity (327), along with serotonergic drugs and multiple monoamine reuptake inhibitors.
More recent drug development: continuing difficulties
The discovery of leptin in 1994 (20) marks the beginning of modern approaches to identifying drugs for treating obesity. Leptin is a peptide made primarily in adipose tissue. Its absence is associated with massive obesity in animals and human beings. Treatment with leptin reverses the obesity caused by leptin deficiency, indicating that there is a clear-cut molecular–genetic mechanism and a highly effective treatment of at least one type of obesity. However, because leptin failed to show adequate weight loss in obese persons who are not leptin deficient, trials were stopped (328, 329). The discovery of leptin opened a flood of research to discover new treatments, some of which were withdrawn from the market due to health risks (22).
Medications approved by the FDA for treating obesity
In Table 8 (330) we list medications that are FDA approved for weight management in patients with obesity and divide them into two groups. First are the agents approved for long-term treatment of obesity. These include orlistat, lorcaserin, liraglutide, the combination of phentermine/topiramate extended release (PHEN/TPM ER), and the combination of naltrexone and bupropion sustained release (SR).
Table 8.
Drugs Approved by the FDA for Managing Patients With Obesity
| Generic Name (Year of Approval) | Trade Name(s) | Dosage | DEA Schedule |
|---|---|---|---|
| Pancreatic lipase inhibitors FDA approved for long-term use | |||
| Orlistat (1999) | Xenical | 120 mg, three times daily before meals | Not scheduled |
| Orlistat (2007) | Alli (over-the-counter) | 60 mg three times daily before meals | Not scheduled |
| Serotinin-2C receptor agonists FDA approved for long-term use | |||
| Lorcaserin (2012) | Belviq | 10 mg, two times daily or 20 mg/d | IV |
| Glucagon-like peptide-1 agonists FDA approved for long-term use | |||
| Liraglutide (2015) | Saxenda | 3 mg/d: begin at 0.6 mg/d for week 1 and increase by 0.6 mg/d each week to reach 3 mg/d at week 4 | Not scheduled |
| Combination drugs FDA approved for long-term use | |||
| PHEN/TPM ER (2012) | Qsymia | 3.75 mg/23 mg | IV |
| 7.5 mg/46 mg | |||
| 11.25 mg/69 mg | |||
| 15 mg/92 mg | |||
| Naltrexone SR/bupropion SR (2014) | Contrave | 8 mg/32 mg tablets: one in am for week 1, one in am and one in pm for week 2, two in am and one in pm for week 3, two in am and two in pm for week 4 | Not scheduled |
| Noradrenergic drugs FDA approved for short-term use | |||
| Diethylpropion (1959) | Tenuate | 25 mg, three times daily | IV |
| Tenuate dospan | 75 mg, every morning | ||
| Phentermine (1959) | Adipex and many others | 15–30 mg/d | IV |
| Benzphetamine (1960) | Didrex | 25–50 mg, three times daily | III |
| Phendimetrazine (1959) | Bontril | 17.5–70 mg, three times daily | III |
| Prelu-2 | 105 mg/d | ||
Abbreviation: DEA, Drug Enforcement Agency.
See Bray and Ryan, 2012 (330).
The second group consists of older, sympathomimetic drugs that are FDA approved for short-term use, usually considered <12 weeks. The FDA did not use modern standards to evaluate these “short-term” medications for safety and efficacy. The FDA approved them using only data from small, short-term studies, and there are no cardiovascular outcome studies for these agents.
Importantly, note that in all the clinical trials evaluating these agents, the drug-vs-placebo study also included lifestyle interventions, such as diet and/or exercise, which contribute to the overall weight loss reported.
Also important to note, these drugs are all contraindicated for pregnant women, as is weight loss per se. Because weight loss can increase fertility, all women in a weight-management program that use medications should be cautioned about the need for contraception. If pregnancy does occur while a patient is taking any of these medications, the patient should immediately stop the medication and contact a medical professional.
Listed below are brief assessments of these drugs’ action, efficacy, and safety. More detailed information is in Figs. 7 (331) and 8 and Table 8.
Figure 7.
Diagram of the sites within the central nervous system where medications can have their effects. See Apovian et al., 2015 (331).
Figure 8.
Randomized controlled trial data showing weight loss with orlistat, lorcaserin, liraglutide, phentermine/topiramate, and naltrexone/bupropion. NB, naltrexone/bupropion; Phen, phentermine; SE, standard error; SR, sustained release; tid, three times a day; Top, topiramate.
Orlistat
Orlistat is a potent and selective inhibitor of pancreatic lipase that reduces intestinal digestion of fat. One clinical trial resulted in weight loss of 9% of body weight at 1 year, compared with ∼5.5% in the placebo group (332) (Fig. 8). Another study achieved a weight loss of 11% compared with 6% in the placebo-treated group and a reduction of 37% in the development of T2DM in patients who had impaired glucose tolerance (333). In a meta-analysis of 31 studies using orlistat (Table 9), the maximal weight loss (by modeling) was −6.65 kg, and half the maximal effect occurred by 35.4 weeks (334).
Table 9.
Weight Loss Associated With Use of Orlistat, Lorcaserin, Liraglutide, Topiramate/Phentermine, and Naltrexone/Bupropion
| Drug/Placebo | No. of Trials | Maximal Weight Loss (kg) | Weeks to Half Maximal Weight Loss (wk) | Drop Rate (%) |
|---|---|---|---|---|
| Orlistat | 31 | −6.65 | 35.4 | 29.0 |
| Lorcaserin | 5 | −5.39 | 19.3 | 40.9 |
| Liraglutide | 3 | −7.68 | 12.7 | 24.3 |
| Topiramate/phentermine | 6 | −15.6 | 29.8 | 34.9 |
| Naltrexone/bupropion | 6 | −13.2 | 35.2 | 49.1 |
| Placebo | 51 | −2.71 | 12.3 |
Maximal weight loss is the modeled maximal effect and does not contain the placebo effect.
Data are from Dong et al., 2017 (334).
Orlistat is the only medication the FDA approved for weight management in adolescents with obesity (335). Adherence to orlistat use falls off rapidly after initial prescription (336). Orlistat can cause small but significant decreases in fat-soluble vitamins, and clinicians should advise patients to take vitamin supplements. Rare cases of severe liver injury have been reported with patients taking orlistat. A causal relationship has not been established, but patients who take orlistat should contact their health care provider if itching, jaundice, pale color stools, or anorexia develop (330).
Lorcaserin
Lorcaserin selectively targets the serotonin-2c receptors to reduce food intake (337), but it has low affinity for the serotonin-2b receptors on heart valves.
The three clinical studies that provided the data for lorcaserin’s approval reported modest weight loss (see Fig. 8 for one of these trials). In a meta-analysis of five studies using lorcaserin (Table 9), the maximal weight loss (by modeling) was −5.39 kg, and half the maximal effect occurred by 19.3 weeks (334). They also showed improvements in cardiovascular risk factors (338–341).
In preclinical toxicology studies in rats, there were more brain and mammary tumors. This may reflect the fact that the drug does not reach the high concentrations in the central nervous system of human beings that is does in rats (341).
Liraglutide
Liraglutide is a GLP-1 agonist that has a 97% homology to GLP-1. The molecular change extends the circulating half-life from 1 to 2 minutes to 13 hours. Clinicians prescribe this drug in combination with a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial BMI of >30 kg/m2 or in adult patients with a BMI of >27 kg/m2 who have T2DM, hypertension, or dyslipidemia.
One study (342) that administered daily subcutaneous injections of liraglutide at 1.2, 1.8, 2.4, or 3.0 mg produced mean weight losses of 3.8, 5.4, 6.1, and 7.8 kg, respectively, after 1 year of treatment, compared with a loss of 2.0 kg in the placebo-treated group and 3.9 kg in the orlistat-treated comparator group. Another larger trial reported that after 56 weeks, liraglutide reduced body weight by 8.4 kg compared with 2.8 kg in the placebo-treated group (on average) (343) (see Fig. 8). In another trial (344), those receiving liraglutide for weight maintenance (after initially losing weight from a low-calorie diet) lost an additional 6.8 kg compared with no additional weight loss in the placebo group. Furthermore, only about half of the placebo group was able to maintain the weight they lost due to diet. In a meta-analysis of three studies using liraglutide (Table 9), the maximal weight loss (by modeling) was −7.68 kg, and half the maximal effect occurred by 12.7 weeks (334).
Liraglutide is contraindicated in people with a family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Clinicians should not prescribe liraglutide for patients with a history of pancreatitis and should discontinue liraglutide if acute pancreatitis develops. If weight loss does not exceed 4% by 16 weeks, patients should stop taking liraglutide. Two cardiovascular outcome trials studied liraglutide (1.8 mg/d) (345) and the long-acting version, semaglutide (0.5 or 1.0 mg weekly) (346). In patients with T2DM, liraglutide lowered the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (345). Semaglutide lowered the rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke (346).
PHEN/TPM ER
PHEN/TPM ER has lower doses of phentermine than clinicians usually prescribe for phentermine alone. Phentermine acts to reduce appetite through increasing norepinephrine in the hypothalamus. Topiramate may reduce appetite through its effect on GABA receptors.
Two clinical studies (347, 348) provided the efficacy and safety data for the approval of PHEN/TPM ER (341) (see Fig. 8 for one of these trials). The patients in these two studies had higher risk profiles due to excess weight. PHEN/TPM ER produced weight losses of 9.3% and 10.7% with the middle and high doses, respectively, compared with 2.2% in the placebo group. This weight loss is larger than observed in clinical trials with single drugs (349). In a meta-analysis of six studies using phentermine/topiramate (Table 9), the maximal weight loss (by modeling) was 15.6 kg, and half the maximal effect occurred by 29.8 weeks (some of which was related to the titration schedule) (334).
Improvements in BP, glycemic measures, HDL cholesterol, and triglycerides occurred with both the recommended and the top doses of the medication in these trials (347, 348). Improvements in risk factors were related to the amount of weight loss. In patients with OSA, this combination reduced the severity of symptoms (349).
Taking topiramate in the first trimester of pregnancy may increase risk of cleft lip/cleft palate in infants. Therefore, clinicians must inform women of childbearing potential of this risk and conduct a pregnancy test before prescribing PHEN/TPM ER. Glaucoma is a rare side effect of topiramate, and the drug is contraindicated in glaucoma. PHEN/TPM ER is also contraindicated in hyperthyroidism within 14 days of treatment with monoamine oxidase inhibitors and in patients with hypersensitivity to any of the ingredients in the medication. Topiramate is a carbonic anhydrase inhibitor that often produces tingling in the fingers and may change the taste for carbonated beverages. Other potential issues include risk of kidney stones (associated with topiramate) and increased heart rate in patients susceptible to phentermine.
Naltrexone/bupropion combination
Bupropion is approved as a single agent for depression and for smoking cessation. It reduces food intake by acting on adrenergic and dopaminergic receptors in the hypothalamus. It has a modest effect on weight loss. Bupropion stimulates the pro-opiomelanocortin neurons in the hypothalamus to produce pro-opiomelanocortin, which is further processed to produce both α-melanocyte stimulating hormone (which reduces food intake) and β-endorphin (which stimulates feeding). Naltrexone blocks this effect of β-endorphin, thus allowing the inhibitory effects of α-melanocyte stimulating hormone to reduce food intake by acting on the melanocortin-4 receptor system (350).
Three studies of the combination drug naltrexone/bupropion provided the basis for its approval.
In one study (351), weight loss at 56 weeks was 5.0% for a lower dose of naltrexone/bupropion (16 mg per day/360 mg per day) and 6.1% for a higher dose (32 mg per day/360 mg per day), compared with placebo. Treatment also improved waist circumference, fasting glucose, fasting insulin, homeostasis assessment model of insulin resistance (HOMA-IR), and HDL cholesterol, but there was a transient increase in BP.
In a second study that included an intensive behavioral modification program (352), weight loss at 56 weeks was about 9% for naltrexone/bupropion (32 mg per day/360 mg per day) vs about 1.8% (Fig. 8) for placebo. The study also reported significant improvements in weight, waist circumference, insulin, homeostatic model assessment of insulin resistance, HDL cholesterol, triglycerides, and quality of life.
In a third study, weight loss at week 56 was 6.4% with naltrexone/bupropion (32 mg per day/360 mg per day) compared with 1.2% with placebo (353). As in the other studies, there were improvements in cardiometabolic risk markers, weight-related quality of life, and control of eating.
Finally, naltrexone/bupropion use in patients with T2DM resulted in significantly greater weight reduction (5.0% vs 1.8% in the placebo group) and significantly greater reductions in HbA1c (−0.6 vs −0.1%; P < 0.001) (354). There was also improvement in triglycerides and HDL cholesterol compared with placebo.
Efficacy of weight loss with the naltrexone/bupropion combination at 1 year is higher than lorcaserin but not as high as PHEN/TPM ER and is associated with improvements in risk factors (350, 351, 355). In a meta-analysis of six studies using naltrexone/bupropion (Table 9), the maximal weight loss (by modeling) was −13.2 kg, and half the maximal effect occurred by 35.2 weeks (probably related to the titration schedule) (334).
Because bupropion increases pulse and both bupropion and naltrexone increase BP, an ongoing study is examining cardiovascular outcomes (345).
Comparison of medications approved for chronic weight management
There are no head-to-head comparisons of these medications. However, there is an analysis of 28 RCTs of weight-loss medications that included trials with orlistat, lorcaserin, liraglutide, naltrexone/bupropion, and PHEN/TPM ER. The inclusion criteria and background lifestyle interventions differed across studies, so we must interpret results with caution.
Attrition rates were 30% to 45% across these trials. All five agents were associated with significantly greater weight loss at 1 year than placebo. Collectively, these studies reported a weight loss of >5% in 23% of patients treated with placebo, 44% of patients treated with orlistat, 49% of patients treated with lorcaserin, 55% of patients treated with naltrexone/bupropion SR, 63% of patients treated with liraglutide, and 75% of patients treated with PHEN/TPM ER. The highest odds ratio for treatment-related discontinuation of the trial was with liraglutide and naltrexone/bupropion (356).
Drugs approved by the FDA for short-term treatment of patients with obesity
We group the sympathomimetic drugs benzphetamine, diethylpropion, phendimetrazine, and phentermine together, because they are noradrenergic drugs that the FDA tested and approved before 1973. The U.S. Drug Enforcement Agency classifies phentermine and diethylpropion as schedule IV drugs and benzphetamine and phendimetrazine as schedule III drugs. This regulatory classification indicates the government’s idea that these drugs have the potential for abuse, although this potential appears to be low (322). These drugs are approved for only a “few weeks” (usually 12 weeks).
Phentermine
Efficacy of phentermine.
The FDA approved phentermine as a single agent in 1959, and it remains the most commonly prescribed drug for weight loss in the United States (350). There are few current data to evaluate its long-term efficacy.
A 6-month study of phentermine reported that 15 mg/d resulted in 4.6% weight loss at 6 months compared with 2.1% for placebo (341). In another 6-month study of phentermine, weight loss was 5.5% for phentermine at 7.5 mg/d and 6.1% for phentermine at 15 mg/d compared with 1.7% for the placebo group (357). Finally, a study from Korea (358) reported that after 12 weeks, mean weight loss for phentermine was 8.1 ± 3.9 kg vs 1.7 ± 2.9 kg for placebo patients. Weight loss with phentermine may not be greatly enhanced by increasing doses beyond 15 mg (353).
Safety of phentermine.
Phentermine is part of a group of drugs called sympathomimetic drugs. These drugs produce central excitation, manifested as dry mouth, insomnia, or nervousness. This effect is most obvious shortly after the drug is started and wanes substantially with continued use. Sympathomimetic drugs may also increase heart rate and BP. The prescribing information usually recommends that the drugs not be given to individuals with a history of CVD (358–360).
Lacking good quantitative measures of the effects of sympathomimetic drugs on heart rate and pulse, we recommend caution in prescribing drugs in this group. According to the Endocrine Society Guidelines (331), clinicians should not prescribe sympathomimetic drugs to persons with a history of CVD and elevated BP.
Best practices for medications approved for weight management
The 2013 American Heart Association/American College of Cardiology/The Obesity Society “guideline for the management of overweight and obesity in adults” (39) and the 2015 Endocrine Society clinical practice guideline on obesity pharmacotherapy (331) both agree that clinicians may consider prescribing weight-reducing drug therapies for patients who: (1) struggle to achieve weight goals, (2) meet label indications (BMI > 30 kg/m2 or BMI > 27 kg/m2 with comorbidity), and (3) need to lose weight for health reasons (such as osteoarthritis, prediabetes, fatty liver, or other conditions). Furthermore, the American Association of Clinical Endocrinologists/American College of Endocrinology “comprehensive clinical practice guidelines for medical care of patients with obesity” from 2016 (60) indicate that clinicians may consider pharmacotherapy as a first-line treatment of weight reduction if patients present with one or more severe comorbidities and would benefit from weight loss of ≥10%. Those guidelines do not require that patients fail lifestyle therapy before clinicians prescribe medications.
Medicating the patient for other chronic conditions who is also overweight or obese
For patients who are overweight or obese, the 2015 Endocrine Society clinical practice guidelines on obesity pharmacotherapy (331) recommended that providers consider body weight when prescribing medications for other chronic health conditions, so that at-risk patients can avoid medications that promote weight gain. The guideline recommends that patients use medications that are weight neutral or associated with weight loss.
In managing patients with obesity, the guideline also advises that providers review medications at every visit and discuss weight effects with patients, so that patients at risk for weight gain can share in the decision process when choosing medications. Additionally, the guideline cautions against prescribing medications known to be associated with weight loss if they have no proven beneficial effect on the patient’s other identified health issues (331).
What is the current status of clinical adoption of medications for chronic weight management?
According to the Awareness, Care and Treatment in Obesity Management study (345), there are a number of misconceptions regarding obesity shared by providers and patients alike, specifically that obesity is not a disease, that patients have the primary responsibility for their problem and for its treatment, that prevention is more important than treatment, and that the risks of treatment should be low.
At present, the FDA has approved nine agents (five for long-term use and four for short-term use). For newer drugs, the time since approval of these medications is too short to know whether and how they will be used. However, older data (which predate the current medication landscape) indicate there are some serious concerns about how diet medications are used, such as: patients using prescription weight-loss pills who do not meet the BMI criterion for these medications; family, friends, and other nonphysicians providing medications; the use of nonprescription diet products; using pills after they were withdrawn from the market; low 1-year persistent use rates; and co-using narcotic and antidepressants (35, 336, 361, 362).
Dietary supplements, over-the-counter products, and other treatments with unproven efficacy and unknown safety
The Dietary Supplement Health Education Act of 1994 provided the framework for an expansion in the use of non–FDA-approved, over-the-counter products in the United States billed as “dietary supplements.” As a result, there has been a proliferation in the use of these products.
This legislation helped undercut the credibility of legitimate weight-management practices by allowing the promotion of agents that are often unsafe, ineffective, and have unproven health claims. As long as the claim is not for disease treatment per se, and products are generally recognized as safe, they can be promoted for health claims. These agents are regulated by the U.S. Federal Trade Commission but not by the FDA, and thus they do not undergo the rigorous testing and review exercised by the FDA when it approves pharmaceutical preparations for patients who are overweight or obese.
Blanck et al. (363) reported that 15.2% of adults (20.6% of women and 9.7% of men) have used a weight-loss supplement, and 8.7% have used one in the past year (11.3% of women and 6.0% of men). Almost 10% (10.2%) used them for ≥12 months.
Pillitteri et al. (364) reported that females, the young, the less educated, and those with lower incomes are more likely to use these products. Many respondents thought that dietary supplements are safer than prescription drugs, and many overestimated the degree of regulatory screening of these products.
Clinicians should be aware and knowledgeable about these products when they begin discussing weight management with patients, since patients have likely taken them or may currently be taking them. Table 10 (365) provides a list of herbal and complementary medications and treatments that claim to improve weight loss. Evidence to support the effectiveness for weight loss or the safety of these preparations is usually nonexistent. Moreover, variability in the composition of these products adds an additional uncertainty to their use. We thus think that the public would be better served if the dietary supplements were held to a higher standard and were overseen by the FDA.
Table 10.
Complementary and Over-the-Counter Products Used for Weight Loss
| Ingredient | Proposed Mechanism of Action | Evidence of Efficacy | Safety Concerns |
|---|---|---|---|
| Chromium | Increases lean muscle mass; promotes fat loss; and reduces food intake, hunger levels, and fat cravings | Several clinical trials of varying methodological quality | No safety concerns reported at recommended intakes (25-45 μg/d for adults) |
| Research findings: minimal effect on body weight and body fat | Reported adverse effects: headache, watery stools, constipation, weakness, vertigo, nausea, vomiting, and urticaria (hives) | ||
| β-Hydroxy β-methylbutyrate | Metabolite of leucine, produced in 0.3 g/d, but taken in doses of 30–60 g/d | Used in conditions of muscle wasting and to augment muscle in athletes | In humans, no reported adverse in young adults or older adults when β-Hydroxy β-methylbutyrate is taken in doses of 3 g/d for up to 1 y |
| β-Hydroxy β-methylbutyrate supplementation can preserve lean muscle mass in older adults (according to a 2015 meta-analysis) | |||
| Pyruvate | Increases lipolysis and energy expenditure | Few clinical trials of weak methodological quality | Few safety concerns reported |
| Research findings: possible minimal effect on body weight and body fat | Reported adverse effects: diarrhea, gas, bloating, and (possibly) decreased HDL levels | ||
| Conjugated linoleic acid | Promotes apoptosis in adipose tissue | Several clinical trials | Few safety concerns reported |
| Research findings: minimal effect on body weight and body fat | Reported adverse effects: abdominal discomfort and pain, constipation, diarrhea, loose stools, dyspepsia, and (possibly) adverse effects on blood lipid profiles | ||
| Calcium | Increases lipolysis and fat accumulation, decreases fat absorption | Several large clinical trials | No safety concerns reported at recommended intakes (1000-1200 mg/d for adults) |
| Research findings: no effect on body weight, weight loss, or prevention of weight gain based on clinical trials | Reported adverse effects: constipation, kidney stones, and interference with zinc and iron absorption at intakes >2000–2500 mg for adults | ||
| Green tea (Camellia sinensis) and green tea extract | Increases energy expenditure and fat oxidation | Several clinical trials of good methodological quality studied green tea catechins with and without caffeine | No safety concerns reported when used as a beverage |
| Reduces lipogenesis and fat absorption | Research findings: possible modest effect on body weight | Contains caffeine | |
| Some safety concerns reported for green tea extract | |||
| Reported adverse effects (for green tea extract): constipation, abdominal discomfort, nausea, increased BP, liver damage | |||
| Green coffee bean extract (Coffea aribica, Coffea canephora, Coffea robusta) | Inhibits fat accumulation | Few clinical trials, all of poor methodological quality | Few safety concerns reported but not rigorously studied |
| Modulates glucose metabolism | Research findings: possible modest effect on body weight | Contains caffeine | |
| Reported adverse effects: headache and urinary tract infections | |||
| Caffeine (as added caffeine or from guarana, kola nut, yerba mate, or other herbs) | Stimulates central nervous system | Short-term clinical trials of combination products | Safety concerns not usually reported at doses <400 mg/d for adults, significant safety concerns at higher doses |
| Increases thermogenesis and fat oxidation | |||
| Research findings: possible modest effect on body weight or decreased weight gain over time | Reported adverse effects: nervousness, jitteriness, vomiting, and tachycardia | ||
| Forskolin (Plectranthus barbatus) | Activates the enzyme adenylyl cyclase | One clinical trial | Forskolin should be used with caution or avoided altogether in women who are pregnant |
| Increases intracellular levels of cyclic adenosine monophosphate | Research findings: oral ingestion of forskolin (250 mg of 10% forskolin extract twice a day) for a 12-wk period was shown to favorably alter body composition while concurrently increasing bone mass and serum free testosterone levels in overweight and obese men | ||
| Fucoxanthin | Increases energy expenditure and fatty acid oxidation | Studied only in combination with pomegranate seed oil in one trial in humans | No safety concerns reported but not rigorously studied |
| Suppresses adipocyte differentiation and lipid accumulation | Research findings: insufficient research to draw firm conclusions | Reported adverse effects: none known | |
| Hydroxycitric acid (Garcinia cambogia) | Inhibits lipogenesis | Several short-term clinical trials of varying methodological quality | Few safety concerns reported |
| Suppresses food intake | Research findings: little to no effect on body weight | Reported adverse effects: headache, nausea, upper respiratory tract symptoms, and gastrointestinal symptoms | |
| Yohimbe (Pausinystalia yohimbe, yohimbine) | Has hyperadrenergic effects | Very little research on yohimbe for weight loss | Significant safety concerns reported |
| Research findings: no effect on body weight; insufficient research to draw firm conclusions | Reported adverse effects: headache, anxiety, agitation, hypertension, and tachycardia | ||
| Hoodia (Hoodia gordonii) | Suppresses appetite | Very little published research in humans | Some safety concerns reported, increases heart rate and BP |
| Reduces food intake | Research findings: no effect on energy intake or body weight based on results from one study | Reported adverse effects: headache, dizziness, nausea, and vomiting | |
| Raspberry ketone | Alters lipid metabolism | Studied only in combination with other ingredients | No safety concerns reported but not rigorously studied |
| Research findings: insufficient research to draw firm conclusions | Reported adverse effects: none known | ||
| Guar gum | Acts as bulking agent in gut, delays gastric emptying | Several clinical trials of good methodological quality | Few safety concerns reported with currently available formulations |
| Increases feelings of satiety | Research findings: no effect on body weight | Reported adverse effects: abdominal pain, flatulence, diarrhea, nausea, and cramps | |
| Bitter orange (synephrine) | Increases energy expenditure and lipolysis | Small clinical trials of poor methodological quality | Some safety concerns reported |
| Acts as a mild appetite suppressant | Research findings: possible effect on resting metabolic rate and energy expenditure; inconclusive effects on weight loss | Reported adverse effects: chest pain, anxiety, and increased BP and heart rate | |
| Chitosan | Binds dietary fat in the digestive tract | Small clinical trials, mostly of poor methodological quality | Few safety concerns reported, could cause allergic reactions |
| Research findings: minimal effect on body weight | Reported adverse effects: flatulence, bloating, constipation, indigestion, nausea, and heartburn | ||
| Glucomannan | Increases feelings of satiety and fullness | Several clinical trials of varying methodological quality, mostly focused on effects on lipid and blood glucose levels | Significant safety concerns reported with tablet forms, which might cause esophageal obstructions, but few safety concerns with other forms |
| Prolongs gastric emptying time | Research findings: little to no effect on body weight | Reported adverse effects: loose stools, flatulence, diarrhea, constipation, and abdominal discomfort | |
| White kidney bean (Phaseolus vulgaris) | Interferes with breakdown and absorption of carbohydrates by acting as a “starch blocker” | Several clinical trials of varying methodological quality | Few safety concerns reported |
| Research findings: possible modest effect on body weight and body fat | Reported adverse effects: headache, soft stools, flatulence, and constipation |
See National Institutes of Health, Office of Dietary Supplements, 2015 (365).
Drug targets
Effective drugs to treat obesity have been slow to arise, but efforts are still underway to develop novel, effective, and transformative medications that would have the effect on treating obesity that statins had for high cholesterol or thiazides had for hypertension (366).
Surgery in Managing Obesity
Introduction
Surgical strategies (including the use of medical devices) for the purpose of inducing and maintaining clinically significant weight loss have emerged and evolved during the last 50 years. Surgeons performed ∼196,000 bariatric procedures in 2015 in the United States.
Sleeve gastrectomy (SG) is the most common procedure (53.8%), followed by Roux-en-Y gastric bypass (RYGB), 23.1%; laparoscopic adjustable gastric banding (LAGB), 5.7%; biliopancreatic diversion with or without duodenal switch, 0.6%; and revision and others, 16.8% (367). SG and RYGB together are the most popular procedures (77%), whereas LAGB has become less popular due to poor long-term results. We list the three most common surgical procedures in Fig. 9 (368).
Figure 9.
The three most commonly performed bariatric surgical operations. (a) The laparoscopic gastric band is placed around the upper stomach to restrict the transit of ingested food. (b) Laparoscopic sleeve gastrectomy involves separation of the greater curvature from the omentum and splenic attachments. (c) RYGB involves the rearrangement of the alimentary canal, such that injected food bypasses most of the stomach, all of the duodenum, and a portion of the proximal jejunum. See Nielsen et al., 2014 (368).
Evidence now indicates that that some of these bariatric procedures (which were intended to either physically limit the ingestion of food or produce malabsorption of energy-containing nutrients) actually produce durable weight loss and health benefits by altering metabolic processes, reducing appetite, and inducing satiety early after meal ingestion.
Sleeve gastrectomy
In SG, surgeons use a linear cutting stapler to make a narrow gastric tube along the lesser curvature of the stomach and remove the remaining 75% to 80% of the gastric body and fundus (369, 370).
The lack of gastrojejunal anastomosis has theoretic benefits, such as reducing the risk of micronutrient deficiencies and peptic ulcer disease. Although some restriction of food intake may occur, gastric emptying is accelerated.
Roux-en-Y gastric bypass
RYGB refers to procedures in which a small (∼30 to 60 mL) gastric pouch is created just distal to the gastroesophageal junction with a stapling device. Most of the stomach is therefore disconnected (but not excised) from the alimentary stream.
The small gastric pouch is the restrictive component of this procedure. RYGB permits ingested food to pass directly from the esophagus through the small stomach pouch and proceed directly into the jejunum, with little or no gastric or duodenal phase of digestion, because food never enters the body of the stomach or the duodenum.
RYGB became a predominant weight-loss procedure in the 1990s and is used worldwide today. The development and demonstration of the safety and efficacy of minimally invasive (laparoscopic) techniques, the recognition of severe obesity as a disease, and the health benefits of bariatric surgery have led to a progressive increase in the number of gastric bypass procedures performed (143, 160, 371, 372).
Laparoscopic adjustable gastric banding
LAGB constricts the upper stomach by placing a mechanical device encircling the stomach just beyond the gastroesophageal junction, thus creating a small (30 to 60 mL) pouch. The tightness of the band is adjusted by inflating a linear balloon fixed within the wall of the band. The balloon is connected to a subcutaneous port, so clinicians can tighten the band via a relatively simple percutaneous injection procedure. The band is intended to reduce the amount of food consumed (373).
Biliopancreatic diversion with (or without) duodenal switch
Biliopancreatic diversion with or without duodenal switch is a complex procedure in which ∼80% of the body of the stomach is resected, creating a tubular stomach (SG) based on the lesser curvature of the stomach. An anastomosis between the proximal duodenum and bypassed intestine creates a degree of malabsorption of nutrients. This procedure is infrequently performed because of a relatively high incidence of short-term and long-term complications, including micronutrient deficiencies (369).
Vagal blockade
In this procedure, leads are placed about the vagal trunks at the diaphragm to produce intermittent vagal blockade. Weight loss occurs by reducing appetite and inducing early satiety. The intermittent blockade is designed to avoid the neural adaptation that occurred with truncal vagatomy for peptic ulcer disease. Weight loss, although modest, is superior to sham-treated controls yet less successful than conventional surgical procedures, such as SG and gastric bypass (374). Despite a better safety profile than adjustable banding, intermittent vagal blockade has limited efficacy. This coupled with adverse events make it a less desirable intervention for resolving obesity and associated comorbidities (375).
Gastrointestinal endoscopic interventions or devices
Several devices, placed either by gastrointestinal endoscopy or suturing procedures, have become available. The FDA approved two gastric balloons in 2015 and another in 2016. Clinicians can fill the Orbera intragastric balloon system with 400 to 700 mL of saline. The ReShape integrated dual balloon system contains two connected, saline-filled balloons. In 2016 the FDA approved the Obalon balloon system, which expands with air after insertion. Technical improvements to these devices have resulted in a favorable safety profile (376). The present protocol requires removal of the intragastric balloon 3 to 6 months after placement, which is a limitation to the long-term efficacy of this intervention. The balloon can be replaced for those who regain weight (377). In August 2017, the FDA sent a letter to health care providers noting seven deaths associated with liquid-filled intragastric balloon systems used to treat obesity. Four of the reports involved the Orbera intragastric balloon system and one with the ReShape integrated dual balloon system. Two earlier deaths were also noted.
Researchers have also developed a specially designed percutaneous gastrostomy tube and apparatus, called the AspireAssist device, that allows patients to directly remove ingested food from the stomach (378). After 1 year with this device, patients lost 12.1% compared with 3.6% in the control group. This aspiration technique requires available facilities to discard the aspirated food and is not for everyone.
Additionally, endoscopic placement of a duodenal–jejunal luminal sleeve is under evaluation (379). In a study that examined endoscopic ablation of duodenal mucosa to enhance glycemic control of T2DM (380), reduction of HbA1c persisted 6 months after ablation.
Liposuction
Liposuction (also known as lipoplasty or suction-assisted lipectomy) is the most common esthetic procedure performed in the United States, with >400,000 cases performed annually (381). Although not generally considered to be a bariatric procedure, clinicians remove and contour subcutaneous fat by aspiration after injecting physiologic saline. As techniques have improved, it is now possible to remove significant amounts of subcutaneous adipose tissue without affecting the amount of visceral fat. In a study to examine the effects of this procedure, Klein et al. (381) studied seven diabetic women who were overweight and eight women with normal glucose tolerance that were overweight before and after liposuction. One week after assessing insulin sensitivity, the subjects underwent large volume tumescent liposuction, which consists of removing >4 L of aspirate injected into the fat beneath the skin. There was a significant loss of subcutaneous fat, but no change in the visceral fat. Subjects were reassessed 10 to 12 weeks after the surgery. The nondiabetic women lost 6.3 kg of body weight and 9.1 kg of body fat, which reduced body fat by 6.3%. The diabetic women had a similar response with a weight loss of 7.9 kg, a reduction in body fat of 10.5 kg, and a reduction in percentage fat of 6.7%. Waist circumference was also significantly reduced. Despite these significant reductions in body fat, there were no changes in BP, lipids, or cytokines (tumor necrosis factor-α, interleukin-6), or C-reactive protein. There was also no improvement in insulin sensitivity, suggesting that removal of subcutaneous adipose tissue without reducing ectopic fat depots has little influence on the risk factors related to being overweight.
Indications for bariatric surgery
Criteria for bariatric surgery
The National Institutes of Health Consensus Panel in 1991 established the initial criteria for surgical interventions for obesity (382). The panel concluded that individuals with BMI ≥ 35 kg/m2 with a related comorbidity or BMI ≥ 40 kg/m2 were appropriate candidates for bariatric surgery. An additional criterion was failure of medical treatment to accomplish sustained weight loss. These criteria have been variably interpreted for many years but have remained essentially unchanged until the present.
In evaluating the outcome for any procedure, we need criteria for “successful” treatment. Weight loss is highly variable with all interventions. For example, intense lifestyle interventions in the Look AHEAD trial produced an average of 8.6% weight loss at 1 year and ∼5% at 4 years. However, this average covers considerable variability. In this study, the bottom 25% of participants lost <3% of their body weight in contrast to the top 25% who lost 12 kg (Fig. 10b) (383).
Figure 10.
(a) Percentage weight trajectories. See Courcoulas et al., 2013 (383). (b) Percentage of participants in the intensive lifestyle intervention and diabetes support and education groups who achieved different categorical weight losses at year 8. See Look AHEAD Research Group, 2014 (271).
During active weight loss after surgery, BP decreases to a point where antihypertensive drugs may be discontinued. In addition, the requirement for hypoglycemic medications in patients with T2DM may also be diminished or discontinued. However, after weight stabilization, the results are less clear, as hypertension commonly reoccurs. Additionally, if weight is regained, comorbidities that were present at baseline may reappear. As a result, the question of what constitutes “successful medical therapy” is open to interpretation. Therefore, additional criteria for surgical interventions should include an understanding of operative risk and the ability to manage obesity and comorbid disease after surgery.
A recent joint statement by international diabetes organizations has indicated that bariatric or metabolic surgery procedures are a consideration for patients with poorly controlled T2DM and a BMI of 30 to 35 kg/m2 (380). The Endocrine Society has also released pediatric guidelines for bariatric surgery (62).
Preoperative assessment
Preoperative assessment of potential bariatric surgical candidates includes confirming the patient’s understanding of the basic procedure(s) proposed and what he or she needs to do to help make the treatment successful. It also includes determining the patient’s dedication and motivation to make the behavioral changes necessary for a satisfactory outcome. Fig. 11 (384) contains a flowchart for managing bariatric patients with obesity.
Figure 11.
Obesity management flow. Summarized from the 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults (39). * refers to comorbid conditions. Reproduced with permission from Beamish et al., 2016 (384).
The patient must also understand the risks associated with the procedure, and clinicians need to assess all related comorbid conditions and manage these conditions preoperatively. At a minimum, clinicians should meet standard guidelines for cancer screening, given the increased risk for common cancers in those with obesity (including breast and colon cancers). Clinicians should also identify and correct micronutrient deficiencies.
Many centers require preoperative weight loss, which may decrease the risk of perioperative medical complications of anesthesia and abdominal surgery. However, the role of preoperative weight loss in determining longer term outcomes (such as weight loss beyond 1 year) has not been demonstrated.
Outcomes of bariatric surgery
Safety
There is little or no disagreement about the benefits of weight loss among individuals with severe obesity, particularly those with comorbid conditions. These benefits, however, must be considered in the context of potential surgical complications. A population-based study in 2004 reported 2% mortality after RYGB (385). In response, the bariatric surgical community enacted several changes to improve safety. This included identifying the importance of surgeon experience and the experience of the particular surgical center; the establishment of pathways, care protocols, and quality initiatives; and the incorporation of all these aspects of care into the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program administered by the American Society for Metabolic and Bariatric Surgery and the American College of Surgeons.
The addition of laparoscopic procedures also contributed to improved safety. Recently, the Longitudinal Assessment of Bariatric Surgery (LABORATORIES) (a multicenter bariatric surgery research consortium funded by the National Institutes of Health) reported a 30-day overall bariatric surgery mortality rate of 0.3%. For laparoscopic RYGB, it reported a 30-day mortality rate of 0.2% (386).
A serious complication occurred in 4.1% of all patients. Factors that predicted a major complication include high BMI, extreme OSA, inability to walk 200 feet, and a history of deep vein thrombosis. Other studies have reported different risk profiles. Studies consistently report that the experience of both the surgeon and the surgical center are predictors of safety (386).
Mid-term and longer term complications have been well described, although determining their incidence is limited by a progressively greater number of patients lost to follow-up (387). These include, but are not limited to, intestinal obstruction, marginal ulcer, ventral hernia, and gallstones. Metabolic complications reported include nephrolithiasis, osteoporosis, and hypoglycemia. Mineral and vitamin deficiencies and weight regain are reported in variable numbers of patients. Micronutrient deficiencies following gastric bypass include: iron, 33% to 55%; calcium/vitamin D, 24% to 60%; vitamin B12, 24% to 70%; copper, 10% to 15%; and thiamine, <5% (388). Established guidelines recommend routine nutrient supplementation to include multivitamins, vitamin B12, iron, minerals, calcium, and vitamin D (389, 390).
Perioperative complications specific to LAGB are less frequent, with near zero mortality. Longer term complications, however, continue to occur at a rate of ∼2% per year. These longer term complications include erosion of the gastric wall by the band and slippage or herniation of the body of the stomach, thereby creating obstructions within the band. Inadequate weight loss is the most common cause of LAGB failure. Complications following other device placement procedures occur but are infrequent and generally less severe. However, there is a tradeoff between reduced complication rates and the severity of complications vs efficacy of weight loss.
In summary, both perioperative and longer term complications occur after all bariatric surgical procedures. Multiple steps have been taken in recent years to reduce perioperative mortality and serious complications. Prospective data collection, analysis, and reporting to individual centers through the accreditation program will continue to identify complications and stimulate appropriate quality improvement initiatives.
Weight loss
The high degree of variability of weight loss following all interventions (including intense lifestyle intervention, medications, and virtually all bariatric surgical procedures) speaks to the complexity of severe obesity (Fig. 10a and 10b). We can define obesity in terms of excess weight represented by BMI. However, the genetic, behavioral, and environmental factors that underlie excessive weight gain in life are exceedingly complex and variable.
The same factors influence variability observed in the weight-loss phenotypes following surgery. After RYGB, for example, the LABORATORIES Consortium reported patterns of similar and rapid weight loss among patients 6 months after surgery by stratifying weight loss into five separate trajectories, ranging from 12% to 45% total body weight loss 3 years after surgery (391) (Fig. 10a). These weight-loss trajectories persist through 7 years (391).
Weight loss following LAGB is similarly variable, but only one-half of the total body weight loss seen with RYGB is seen after LAGB (on average). Thus, the commonly reported mean weight loss among populations undergoing bariatric surgery is of limited use for predicting results for individuals who are contemplating surgical treatment.
There have been many efforts to identify preoperative clinical predictors of postoperative weight loss. However, although research has established some statistical correlations, the extent of the variability explained by a number of clinical covariates has been disappointing (391).
The single best predictors of sustained postoperative weight loss (identified by the LABORATORIES Consortium) are postoperative eating and lifestyle behaviors. Specifically, subjects who self-monitor (e.g., frequent weighing), avoid eating when full, and who avoid snacking between meals appear to experience the greatest weight loss (392). The weight loss following RYGB, compared with interventions other than surgery (Fig. 10a and 10b), demonstrates that even the poorest weight loss following gastric bypass is comparable to the best reported weight loss for nonsurgical interventions (393). A third study found changes from baseline after 5 years in the surgical groups were superior to the changes seen with medical therapy. Body weight decreased 23% with gastric bypass, 19% with SG, and 5% with drug therapies (161). We must interpret these outcomes with the caveat that the requirement for surgical intervention is the failure of patients to accomplish sustained weight loss via other means, thereby creating a selected population.
Related outcomes/remission of T2DM
The remarkable remission of T2DM following RYGB has generated much interest given the prevalence of T2DM and the severity of this disease (394, 395) (Table 11) (396, 397).
Table 11.
Weight Loss and Reversal of Diabetes Mellitus after Metabolic/Bariatric Surgery
| Procedure | Excess Weight Loss (%) | Resolution of T2DM (%) |
|---|---|---|
| Gastric banding | 46.2 | 56.7 |
| Gastroplasty | 55.5 | 79.7 |
| RYGB | 59.7 | 80.3 |
| Biliopancreatic diversion | 63.6 | 95.1 |
Analysis of the data from the LABORATORIES Consortium has demonstrated that both weight loss and the neuroendocrine effects specific to gastric bypass contribute to the remission of T2DM (159). The durability of the remission in many participants was sustained through year 7 (391).
The Swedish Obese Subjects study reported that ∼50% of gastric bypass patients who were in T2DM remission at year 2 had recurrence by year 10 (160). Weight loss in these groups is shown in Fig. 12. Gastric banding was the predominant procedure in the Swedish Obese Subjects trial. This suggests that maintaining weight loss, as well as the incretin stimulation associated with RYGB, contributes to the durability of the remission. Reports of T2DM remission among patients with considerably less severe obesity (BMI 27 to 40 kg/m2) have led to several RCTs in this patient population (398).
Figure 12.
Mean weight change percentages from baseline for controls and the three surgery groups during 20 years in the Swedish obese subjects study. Data shown for controls obtaining usual care and for surgery patients obtaining banding, vertically banded gastroplasty, or gastric bypass at baseline. Percentage weight changes from the baseline examination and onward are based on data available on 1 July 2011. Error bars represent 95% CIs. Vertical error bars represent SEM. GBP, gastric bypass; VBG, vertically banded gastroplasty. See Sjöström et al., 2012 (393).
Most recently, SG has taken a dominant place in the spectrum of procedures used for weight loss worldwide (399, 400). Although the weight loss and T2DM remission following SG appear to be slightly less than that following gastric bypass, lower perioperative complication rates, shorter lengths of stay, and lower costs have made SG an attractive bariatric surgical procedure (395, 401). The mechanism and durability of this improved glycemic control, including the role of diet-induced weight loss, have not been determined (60).
Overall, there is considerable evidence favoring RYGB, LAGB, and now SG as superior methods for controlling or inducing remission of T2DM, vs intense medical treatment (380). As a result, the term “metabolic” surgery has become popular. The concept that clinicians should consider surgical intervention for patients with poorly controlled T2DM and patient with less severe obesity (class I) with T2DM (rather than having BMI be the primary indication for surgery) has gained widespread international support (380). Remission of dyslipidemia is also seen in most patients following effective surgical weight loss, whereas remission of hypertension is less frequent.
Bariatric/metabolic surgery in adolescents
Owing to the lack of effectiveness of nonsurgical options for treating severe obesity in young patients and the demonstrated safety and efficacy of bariatric surgery in adults, clinicians increasingly use surgical procedures to induce weight loss in selected adolescents with severe obesity. The rationale for and expectation of bariatric treatment in adolescents are to provide significant and durable weight reduction, correct existing health problems, and prevent expected comorbidities in those at risk.
Lifestyle modification and even pharmacotherapy in adolescents with severe obesity are associated with unsatisfactory outcomes, and any weight reduction seen may not be sustained. Conversely, growing evidence indicates that surgery results in 25% to 35% weight reduction in severely obese adolescents. In the Teen-LABORATORIES study, SG and RYGB were the most commonly performed procedures in adolescents, and 3-year outcomes demonstrated a similar weight loss of nearly 30% for these procedures (402).
The lower complexity of SG and the lower theoretical risk of at least some micronutrient deficiencies associated with RYGB make SG an attractive option for most adolescents, despite fewer published studies of SG in adolescent age groups.
In 2017, investigators in the United States (403) and Sweden (404) simultaneously reported long-term outcomes for weight loss and comorbidities in adolescents who underwent RYGB. Eight-year (United States) and 5-year (Sweden) post-RYGB surgery follow-up assessments indicated 30% and 28% BMI reductions, respectively. Both research groups documented important improvements in health.
In the U.S. study, remission of T2DM occurred in 88% (n = 7). The study did not report any incident T2DM during the 8 years. The study also reported dyslipidemia remission in 64% (n = 29) and incident dyslipidemia in four of eight subjects who did not have dyslipidemia at baseline. The study reported hypertension remission in 76% (n = 19) and incident hypertension in only 10% (3 of 29) participants without hypertension at baseline.
The Swedish study reported similar health improvements, with remission of comorbid conditions in 74% to 100% of participants. The study reported remission of T2DM in 3 of 3 participants, disturbed glucose homeostasis in 18 of 21, dyslipidemia in 43 of 52, elevated BP in 11 of 12, inflammation (high-sensitivity C-reactive protein ≥ 2 mg/L) in 45 of 61, and elevated liver enzymes in 19 of 19 participants.
Both studies also reported long-term nutritional effects. The U.S. study reported mild anemia in 46% (n = 25), hyperparathyroidism in 45% (n = 22), and low vitamin B12 levels in 16% (n = 8) at long-term follow-up. At 5 years in the Swedish study, 63% (46 of 73) had vitamin D (25-hydroxy vitamin D) insufficiency (<50 nmol/L) and 66% (51 of 77) had low ferritin and/or iron levels. The prevalence of anemia rose from 10% (8 of 78) to 32% (25 of 77), and 22% had low vitamin B12 levels.
In summary, the two long-term, prospective studies demonstrate excellent durability of weight loss and response of comorbidities for adolescents who have RYGB surgery. These studies also reported the typical nutritional consequences of RYGB that we see in studies in adults, and this must be taken into consideration when counseling patients about long-term risks of RYGB.
Current expert opinion recommends that clinicians should use criteria similar to those used for adults when selecting adolescents for weight-loss surgery (399, 405). Surgery is generally recommended for adolescents with a BMI ≥ 40 kg/m2 and a weight-related comorbid condition or impairment in quality of life. It is also recommended for those with a BMI of ≥35 kg/m2 with significant current comorbidities, such as T2DM, dyslipidemia, OSA, hypertension, NASH, or pseudotumor cerebri (395, 400).
Where Do We Go From Here?
In this Endocrine Society Scientific statement titled “The Science of Obesity Management: An Endocrine Society Scientific Statement,” we have documented the rising prevalence of obesity in both men and women in the United States and worldwide with resultant hazardous health implications. The prevalence of obesity is correlated with income disparity both between developed countries and between the states of the United States (406).
Obesity results in part from environmental and behavioral factors, and both the public and health care professionals alike stigmatize the condition. The opportunity to move from a neighborhood with a high level of poverty to one with a lower level of poverty was associated with modest but potentially important reductions in the prevalence of extreme obesity and diabetes (120), supporting the relationship between income inequality and obesity. Because the prevalence of obesity has strong social and environmental components, this may provide a basis for future approaches. The study by Christakis and Fowler (407) showed that “friends” of an individual with obesity were more likely to also be obese.
Obesity is lower when there are more opportunities for physical activity as part of everyday life, as shown by the slower rise in obesity among more active individuals during 10 years (123). Sleep time is a modifiable behavior, and the observation that preschool-aged children with early weekday bedtimes were one-half as likely as children with late bedtimes to be obese as adolescents offers further opportunities for intervention in the environment (408). These observations may provide the potential for more effective preventive strategies utilizing social engineering.
Genetic factors also play a role. Recently, insufficiency in the gene TRIM28 was shown to produce polyphenic obesity in both mice and humans. In this setting, both lean and obese phenotypes can arise from identical genotypes through dysregulation of an imprinted gene network (409). This finding and other genetic research into the mechanisms behind obesity may provide new genetic strategies for helping this segment of the population.
The hazards of obesity are many, including a shortened life span, T2DM, CVD, some cancers, kidney disease, OSA, gout, osteoarthritis, and hepatobiliary disease, among others. As might be expected, weight loss reduces all of these diseases in a dose-related manner.
The phenotype of MHO appears to be a transient state that progresses over time to an unhealthy phenotype, especially in children and adolescents. Understanding in more detail how complications of obesity develop will provide new opportunities for prevention of these negative outcomes.
Of particular interest are reports that two diabetes medications (liraglutide and empagliflozin) (345, 410) also produce weight loss and are cardioprotective. Particularly striking is the fact that these two drugs reduce cardiovascular death to a greater extent than statins. This opens a whole new paradigm for managing patients with obesity and diabetes in relationship to their complications.
One of the unexplained issues in all treatment strategies is the marked variability in response of any form of treatment of obesity. Efforts to understand the biological basis of this variability may provide new insights into its treatment. The POUNDS Lost Study population of 811 individuals randomized to one of four diets (20% vs 40% fat and 15% vs 25% protein) has provided many genetic clues to help us better understand factors that modulate dietary response (206, 411). The ability to combine several measures to predict responses to environmental factors may expand the option for personalized medicine. An algorithm that integrates blood parameters, dietary habits, anthropometrics, physical activity, and gut microbiota measured in a sample cohort (412) showed that these factors accurately predict personalized postprandial glycemic response to real-life meals. Similar strategies might well be developed for obesity. Many genes affect the response to diets, opening the possibility of “personalized medicine” for managing obesity.
The public commonly uses over-the-counter herbal preparations to manage obesity, but evidence documenting their efficacy or safety is usually absent. We think that the public would be well served by more regulatory requirements regarding sale and use of these products.
We can expect to see weight regain in all patients when they discontinue obesity treatments. When making treatment decisions, clinicians should consider body fat distribution and individual health risks in addition to BMI. Because all treatments have considerable variability in their outcome, it is important to know when to stop treatment as well as when to begin. Surgical strategies have demonstrated greater weight loss that outlasts other treatment options.
As the knowledge base underpinning obesity continues to expand, the options for treating patients with obesity should also expand, offering hope for future conquest of this problem. One fascinating new strategy is the combination of peptides acting on receptors in the gastrointestinal track into a single molecule acting on two or more receptors, called coagonists and triagonists. Using glucagon-like peptide-1, glucagon, and glucose-insulin peptide as the background for these molecules, peptides have been shown to enhance weight loss and the decline in glucose, opening a fascinating new horizon (413, 414).
Finally, improved techniques for modulating food transit through the gastrointestinal track and its absorption also offer new strategies for dealing with the devastating epidemic posed by obesity. It is clear that food is more than calories and that dietary choices and diet quality play a role in long-term weight change (415), and this provides other opportunities for public health programs. The so-called “obesogens” in the food supply offer another opportunity for making the food supply less likely to contribute to obesity (416). Control of obesity is the most important public health strategy for the prevention of diabetes and its devastating consequences. With all of these opportunities on the horizon, we are optimistic about the future of treatment and prevention of obesity.
Acknowledgments
We acknowledge science writer Eric A. Vohr for his contribution to this scientific statement.
Financial Support: Work on this paper was supported in part by Bill and Melinda Gates Foundation Grant OPP1070441 to A.A., National Institutes of Health Grants DK40484 and DK45343 to M.D.J., and by National Institutes of Health Grant DK046200 to F.B.H.
Disclaimer Statement: The Endocrine Society develops scientific statements to be of assistance to clinicians by providing guidance and recommendations for particular areas of practice. One should not consider this scientific statement inclusive of all proper approaches or methods or exclusive of others. It cannot guarantee any specific outcome, nor does it establish a standard of care. It is not intended to dictate the treatment of a particular patient. Health care providers must make treatment decisions based on their best judgment and each patient’s individual circumstances, needs, and preferences. The Endocrine Society makes no warranty, express or implied, regarding this scientific statement and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. The Endocrine Society shall not be liable for direct, indirect, special, incidental, or consequential damages related to the use of the information contained herein.
Disclosure Summary: G.B. is on the Herbalife Nutrition Advisory Council, is a consultant to Medifast, and is a consultant to Novo Nordisk. M.D.J. serves as a consultant to Novo Nordisk, Merck, and Jannsen, serves on the advisory board of Weight Watchers International, and serves on the board of directors of the Partnership for a Healthier America. R.F.K. serves on the advisory board to Novo Nordisk, Weight Watchers, Retrofit, and Zafgen. S.R.D. serves as a consultant for Sanofi and is on the Data Monitoring Committee for Novo Nordisk. T.A.W. serves on advisory boards for Novo Nordisk, Nutrisystem, and Weight Watchers, and has received grant support on behalf of the University of Pennsylvania from Eisai Pharmaceutical, Novo Nordisk, and Weight Watchers. F.B.H. receives research support from Metagenics and the California Walnut Commission. J.M.J. reports personal fees from Weight Watchers International for serving on the Scientific Advisory Board. D.H.R. serves as consultant to Novo Nordisk, Orexigen, Janssen, Merck, Astra Xenica, and KVK Tech, serves as speaker for Novo Nordisk, Orexigen, Eisai, and Kwang Dong, and has an equity position in Scientific Intake and Gila Therapeutics. The remaining authors have nothing to disclose.
Abbreviations:
- BMI
body mass index
- BP
blood pressure
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CVD
cardiovascular disease
- ECE
early care and education
- ER
extended release
- FDA
Food and Drug Administration
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HR
hazard ratio
- LAGB
laparoscopic adjustable gastric banding
- LDL
low-density lipoprotein
- MHO
metabolically healthy obesity
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OSA
obstructive sleep apnea
- PHEN/TPM ER
phentermine/topiramate extended release
- RCT
randomized controlled trial
- RYGB
Roux-en-Y gastric bypass
- SG
sleeve gastrectomy
- SR
sustained release
- T2DM
type 2 diabetes mellitus
- VLCD
very low–calorie diet
- WHR
waist-to-hip ratio.
References
- 1. Bray GA. Obesity: historical development of scientific and cultural ideas. Int J Obes. 1990;14(11):909–926. [PubMed] [Google Scholar]
- 2. Bray G. Battle of the Bulge: A History of Obesity Research. Philadelphia, PA: Dorrance Publishing; 2007. [Google Scholar]
- 3. Gilman S. Obesity: The Biography. Oxford, U.K.: Oxford University Press; 2010. [Google Scholar]
- 4. Angeli W. Die Venus von willendorf. Vienna, Austria: Edition Wien; 1989. [Google Scholar]
- 5. Hippocrates Oeuvres Complètes: Traduction Nouvelle Avec le Texte Grec en Regard, Collationné sur les Manuscrits et Toutes les Éditions; Accompagnée d’une ... Suivie d’une Table Générale. Paris, France: J.B. Bailliere; 1839. [Google Scholar]
- 6. Flemyng M. A Discourse on the Nature, Causes, and Cure of Corpulency. Illustrated by a Remarkable Case. Read Before the Royal Society November 1757. London, England: L. Davis and C. Reymers; 1760. [Google Scholar]
- 7. Wadd W. Cursory Remarks on Corpulence; or Obesity Considered as a Disease: With a Critical Examination of Ancient and Modern Opinion, Relative to Its Causes and cure. London: J. Callow; 1816.
- 8. Lavoisier AL. Traité Élémentaire de Chimie: Présenté dans un Ordre Nouveau et d’Après les Découvertes Modernes: Avec Figures. Paris, France: Cuchet; 1789. [Google Scholar]
- 9. Atwater WO, Rosa EB. A new respiration calorimeter and experiments on the conservation of energy in the human body, II. Phys Rev Ser I. 1899;9:214–251. [Google Scholar]
- 10. Cushing H. The pituitary body and its disorders. Clinical states produced by disorders of the hypophysis cerebri. Am J Med Sci. 1912;144(6):891. [Google Scholar]
- 11. Babinski M. Tumeur du corps pituitaire sans acromégalie et avec de développement des organes génitaux. Rev Neurol. 1900;8:531–533. [Google Scholar]
- 12. Fröhlich A. Ein Fall von Tumor der Hypophysis cerebri ohne Akromegalie. Wiener Klin Rdsch; 1901:883–886. [Google Scholar]
- 13. Precope J. Hippocrates on Diet and Hygiene. London, England: Zeno; 1952.
- 14. Foxcroft L. Calories and Corsets: A History of Dieting Over 2000 Years. London, U.K.: Profile Books; 2011. [Google Scholar]
- 15. Banting W. Letter on corpulence, addressed to the public. 1869. Obes Res. 1993;1(2):153–163. [DOI] [PubMed] [Google Scholar]
- 16. Rössner S. Sancho the Fat: King of León, Spain. Obes Rev. 2011;12(11):995. [DOI] [PubMed] [Google Scholar]
- 17. Short T. Discourse Concerning the Causes and Effects of Corpulency: Together With the Method for Its Prevention and Cure. 2nd ed.London, England: J. Roberts; 1727. [Google Scholar]
- 18. Bonetus T. Sepulchretum, Sive Anatomia Practica, ex Cadaveribus Morbo Denatis, Proponens Historias Omnium Humani Corporis Affectum. Geneva, Switzerland: Sumptibus Cramer & Perachon; 1700. [Google Scholar]
- 19. Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol. 2007;48(2):115–117. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz L, ed. Preface. The Cambridge Companion to Paradise Lost. New York, NY: Cambridge University Press; 2014:xi–xiv. [Google Scholar]
- 22. Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puhl RM, Latner JD. Stigma, obesity, and the health of the nation’s children. Psychol Bull. 2007;133(4):557–580. [DOI] [PubMed] [Google Scholar]
- 24. Linné Y, Hemmingsson E, Adolfsson B, Ramsten J, Rössner S. Patient expectations of obesity treatment-the experience from a day-care unit. Int J Obes Relat Metab Disord. 2002;26(5):739–741. [DOI] [PubMed] [Google Scholar]
- 25. Kaly P, Orellana S, Torrella T, Takagishi C, Saff-Koche L, Murr MM. Unrealistic weight loss expectations in candidates for bariatric surgery. Surg Obes Relat Dis. 2008;4(1):6–10. [DOI] [PubMed] [Google Scholar]
- 26. Dutton GR, Perri MG, Stine CC, Goble M, Van Vessem N. Comparison of physician weight loss goals for obese male and female patients. Prev Med. 2010;50(4):186–188. [DOI] [PubMed] [Google Scholar]
- 27. Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26(2):262–273. [DOI] [PubMed] [Google Scholar]
- 28. Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG. Reduced hypophagic effects of d-fenfluramine and the 5-HT2C receptor agonist mCPP in 5-HT1B receptor knockout mice. Psychopharmacology (Berl). 2004;176(1):39–49. [DOI] [PubMed] [Google Scholar]
- 29. Heal DJ, Gosden J, Smith SL. A review of late-stage CNS drug candidates for the treatment of obesity. Int J Obes. 2013;37(1):107–117. [DOI] [PubMed] [Google Scholar]
- 30. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65(1):79–85. [DOI] [PubMed] [Google Scholar]
- 31. Smiertka JK, MacPherson BH. Bariatric surgery postoperative behavioral change: The importance of ongoing assessment and teaching. Obes Surg. 1995;5(3):337–340. [DOI] [PubMed] [Google Scholar]
- 32. Rabner JG, Greenstein RJ. Obesity surgery: expectation and reality. Int J Obes. 1991;15(12):841–845. [PubMed] [Google Scholar]
- 33. Wee CC, Jones DB, Davis RB, Bourland AC, Hamel MB. Understanding patients’ value of weight loss and expectations for bariatric surgery. Obes Surg. 2006;16(4):496–500. [DOI] [PubMed] [Google Scholar]
- 34. Weintraub M, Bray GA. Drug treatment of obesity. Med Clin North Am. 1989;73(1):237–249. [DOI] [PubMed] [Google Scholar]
- 35. Bolen SD, Clark JM, Richards TM, Shore AD, Goodwin SM, Weiner JP. Trends in and patterns of obesity reduction medication use in an insured cohort. Obesity (Silver Spring). 2010;18(1):206–209. [DOI] [PubMed] [Google Scholar]
- 36. Harrison GG. Height-weight tables. Ann Intern Med. 1985;103(6, Pt 2):989–994. [DOI] [PubMed] [Google Scholar]
- 37. Bray GA, Greenway FL, Molitch ME, Dahms WT, Atkinson RL, Hamilton K. Use of anthropometric measures to assess weight loss. Am J Clin Nutr. 1978;31(5):769–773. [DOI] [PubMed] [Google Scholar]
- 38. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 39. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25, Suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. [DOI] [PubMed] [Google Scholar]
- 41. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R; Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H; Obesity Management Task Force of the European Association for the Study of Obesity . European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lau DCW, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E; Obesity Canada Clinical Practice Guidelines Expert Panel . 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176(8):S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barlow SE; Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 47. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fryar C. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2009–2010. Available at: https://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.pdf. Accessed 4 April 2017.
- 49. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34(5):791–799. [DOI] [PubMed] [Google Scholar]
- 50. Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32(6):959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berstad P, Randby A, Seim Ekeland G, Ulveland H, Omland T, Almendingen K. Body fat and fat-free mass measured by bioelectric impedance spectroscopy and dual-energy X-ray absorptiometry in obese and non-obese adults. Br J Nutr. 2012;107(8):1192–1200. [DOI] [PubMed] [Google Scholar]
- 52. Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. [DOI] [PubMed] [Google Scholar]
- 53. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629–639. [DOI] [PubMed] [Google Scholar]
- 54. Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163(11):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. 1956. Obes Res. 1996;4(2):204–212. [DOI] [PubMed] [Google Scholar]
- 56. Pouliot M-C, Després J-P, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73(7):460–468. [DOI] [PubMed] [Google Scholar]
- 57. Lean MEJ, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311(6998):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KAA, Smith SC Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116(17):1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami H-O, Ebbert JO, English DR, Gapstur SM, Giles GG, Horn-Ross PL, Park Y, Patel AV, Robien K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hartge P, Bernstein L, Berrington de Gonzalez A. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Executive summary. Complete guidelines available at https://www.aace.com/publications/guidelines. Endocr Pract. 2016;22(7):842–884. [DOI] [PubMed] [Google Scholar]
- 61. Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, Kathiresan S. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317(6):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA. Pediatric obesity-assessment, treatment, and prevention: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sardinha LB, Santos DA, Silva AM, Grøntved A, Andersen LB, Ekelund U. A comparison between BMI, waist circumference, and waist-to-height ratio for identifying cardio-metabolic risk in children and adolescents. PLoS One. 2016;11(2):e0149351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for disease control and prevention 2000 growth charts for the united states: improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109(1):45–60. [DOI] [PubMed] [Google Scholar]
- 65. Kahn HS, Imperatore G, Cheng YJ. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr. 2005;146(4):482–488. [DOI] [PubMed] [Google Scholar]
- 66. Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, Grant SFA, McCormack SE, Zemel BS. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137(5):e20153492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168(15):1617–1624. [DOI] [PubMed] [Google Scholar]
- 68. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11, Suppl 1):S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152–162. [DOI] [PubMed] [Google Scholar]
- 70. Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, Tran T, Blaha MJ, Santos RD, Sposito A, Al-Mallah MH, Blankstein R, Budoff MJ, Nasir K. Beyond BMI: the “metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—a systematic review. BMC Public Health. 2014;14(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Camhi SM, Katzmarzyk PT. Differences in body composition between metabolically healthy obese and metabolically abnormal obese adults. Int J Obes. 2014;38(8):1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15(10):781–790. [DOI] [PubMed] [Google Scholar]
- 73. van Vliet-Ostaptchouk JV, Nuotio M-L, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T, Joensuu A, Newby C, Pang C, Partinen E, Reischl E, Schwienbacher C, Tammesoo M-L, Swertz MA, Burton P, Ferretti V, Fortier I, Giepmans L, Harris JR, Hillege HL, Holmen J, Jula A, Kootstra-Ros JE, Kvaløy K, Holmen TL, Männistö S, Metspalu A, Midthjell K, Murtagh MJ, Peters A, Pramstaller PP, Saaristo T, Salomaa V, Stolk RP, Uusitupa M, van der Harst P, van der Klauw MM, Waldenberger M, Perola M, Wolffenbuttel BHR. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring H-U. Identification and characterization of metabolically benign obesity in humans. Obstet Gynecol Surv. 2009;64(1):30–31. [DOI] [PubMed] [Google Scholar]
- 75. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–769. [DOI] [PubMed] [Google Scholar]
- 76. Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65(1):101–102. [DOI] [PubMed] [Google Scholar]
- 77. Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(9):956–966. [DOI] [PubMed] [Google Scholar]
- 78. Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Boyko EJ. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes. 2015;39(9):1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jae SY, Franklin B, Choi Y-H, Fernhall B. Metabolically healthy obesity and carotid intima-media thickness: effects of cardiorespiratory fitness. Mayo Clin Proc. 2015;90(9):1217–1224. [DOI] [PubMed] [Google Scholar]
- 80. Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34(5):389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ricketts TA, Sui X, Lavie CJ, Blair SN, Ross R. Addition of cardiorespiratory fitness within an obesity risk classification model identifies men at increased risk of all-cause mortality. Am J Med. 2016;129(5):536.e13–536.e20. [DOI] [PubMed] [Google Scholar]
- 82. McAuley PA, Blaha MJ, Keteyian SJ, Brawner CA, Al Rifai M, Dardari ZA, Ehrman JK, Al-Mallah MH. Fitness, fatness, and mortality: the FIT (Henry Ford exercise testing) project. Am J Med. 2016;129(9):960–965.e1. [DOI] [PubMed] [Google Scholar]
- 83. Daniels SR. The consequences of childhood overweight and obesity. Future Child. 2006;16(1):47–67. [DOI] [PubMed] [Google Scholar]
- 84. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society . Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13(11):1849–1863. [DOI] [PubMed] [Google Scholar]
- 85. National Clinical Guideline Centre (U.K.) Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. Partial Update of cg43. London, U.K: National Institute for Health and Care Excellence (U.K.); 2014. [PubMed] [Google Scholar]
- 86. Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes. 2009;33(3):289–295. [DOI] [PubMed] [Google Scholar]
- 87. Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull. 2011;97(1):169–196. [DOI] [PubMed] [Google Scholar]
- 88. Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes. 2005;29(9):1011–1029. [DOI] [PubMed] [Google Scholar]
- 89. Santanasto AJ, Goodpaster BH, Kritchevsky SB, Miljkovic I, Satterfield S, Schwartz AV, Cummings SR, Boudreau RM, Harris TB, Newman AB. Body composition remodeling and mortality: the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2017;72(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dietz WH, Economos CD. Progress in the control of childhood obesity. Pediatrics. 2015;135(3):e559–e561. [DOI] [PubMed] [Google Scholar]
- 92. Thangaratinam S, Rogozińska E, Jolly K, Glinkowski S, Duda W, Borowiack E, Roseboom T, Tomlinson J, Walczak J, Kunz R, Mol BW, Coomarasamy A, Khan KS. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess. 2012;16(31):iii–iv, 1–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, Kunz R, Mol BW, Coomarasamy A, Khan KS. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tanentsapf I, Heitmann BL, Adegboye ARA. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or Exercise, or Both, for Preventing Excessive Weight Gain in Pregnancy. Cochrane Database of Systematic Reviews. Wiley-Blackwell; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during pregnancy and childhood adiposity. Pediatrics. 2017;140(2):e20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Larson N, Ward DS, Neelon SB, Story M. What role can child-care settings play in obesity prevention? A review of the evidence and call for research efforts. J Am Diet Assoc. 2011;111(9):1343–1362. [DOI] [PubMed] [Google Scholar]
- 98. de Silva-Sanigorski AM, Bell AC, Kremer P, Nichols M, Crellin M, Smith M, Sharp S, de Groot F, Carpenter L, Boak R, Robertson N, Swinburn BA. Reducing obesity in early childhood: results from Romp & Chomp, an Australian community-wide intervention program. Am J Clin Nutr. 2010;91(4):831–840. [DOI] [PubMed] [Google Scholar]
- 99. Evaluating obesity prevention efforts: what have we learned? Available at: https://publichealth.gwu.edu/sites/default/files/downloads/Redstone-Center/Evaluating Obesity Prevention Efforts What Have We Learned.pdf. Accessed 31 July 2016.
- 100. United States Department of Agriculture Food and Nutrition Services. Child and adult care food program. Available at: https://www.fns.usda.gov/cacfp/child-and-adult-care-food-program. Accessed 12 March 2017.
- 101. Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011; (12):CD001871. [DOI] [PubMed]
- 102. Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obes Res. 2001;9(3):171–178. [DOI] [PubMed] [Google Scholar]
- 103. James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ. 2004;328(7450):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367(15):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–1406. [DOI] [PubMed] [Google Scholar]
- 106. Sallis JF, Glanz K. Physical activity and food environments: solutions to the obesity epidemic. Milbank Q. 2009;87(1):123–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Caballero B, Clay T, Davis SM, Ethelbah B, Rock BH, Lohman T, Norman J, Story M, Stone EJ, Stephenson L, Stevens J; Pathways Study Research Group . Pathways: a school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am J Clin Nutr. 2003;78(5):1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gortmaker SL, Peterson K, Wiecha J, Sobol AM, Dixit S, Fox MK, Laird N. Reducing obesity via a school-based interdisciplinary intervention among youth: Planet Health. Arch Pediatr Adolesc Med. 1999;153(4):409–418. [DOI] [PubMed] [Google Scholar]
- 109. Plachta-Danielzik S, Landsberg B, Lange D, Seiberl J, Müller MJ. Eight-year follow-up of school-based intervention on childhood overweight—the Kiel Obesity Prevention Study. Obes Facts. 2011;4(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sahota P, Rudolf MC, Dixey R, Hill AJ, Barth JH, Cade J. Randomised controlled trial of primary school based intervention to reduce risk factors for obesity. BMJ. 2001;323(7320):1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dietz WH, Gortmaker SL. New strategies to prioritize nutrition, physical activity, and obesity interventions. Am J Prev Med. 2016;51(5):e145–e150. [DOI] [PubMed] [Google Scholar]
- 113. Kamath CC, Vickers KS, Ehrlich A, McGovern L, Johnson J, Singhal V, Paulo R, Hettinger A, Erwin PJ, Montori VM. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4606–4615. [DOI] [PubMed] [Google Scholar]
- 114. Task Force on Community Preventive Services A recommendation to improve employee weight status through worksite health promotion programs targeting nutrition, physical activity, or both. Am J Prev Med. 2009;37(4):358–359. [DOI] [PubMed] [Google Scholar]
- 115. Anderson LM, Quinn TA, Glanz K, Ramirez G, Kahwati LC, Johnson DB, Buchanan LR, Archer WR, Chattopadhyay S, Kalra GP, Katz DL; Task Force on Community Preventive Services . The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. Am J Prev Med. 2009;37(4):340–357. [DOI] [PubMed] [Google Scholar]
- 116. Thorndike AN, Sonnenberg L, Riis J, Barraclough S, Levy DE. A 2-phase labeling and choice architecture intervention to improve healthy food and beverage choices. Am J Public Health. 2012;102(3):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Boston Public Health Commission Healthy beverages. Available at: http://www.bphc.org/whatwedo/healthy-eating-active-living/healthy-beverages/Pages/Healthy-Beverages.aspx. Accessed 7 February 2014.
- 118. Jeffery RW, French SA. Preventing weight gain in adults: the pound of prevention study. Am J Public Health. 1999;89(5):747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29(1):129–143. [DOI] [PubMed] [Google Scholar]
- 120. Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011;365(16):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Besser LM, Dannenberg AL. Walking to public transit: steps to help meet physical activity recommendations. Am J Prev Med. 2005;29(4):273–280. [DOI] [PubMed] [Google Scholar]
- 122. MacDonald JM, Stokes RJ, Cohen DA, Kofner A, Ridgeway GK. The effect of light rail transit on body mass index and physical activity. Am J Prev Med. 2010;39(2):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, Matheson FI, Kaufman-Shriqui V, Rosella LC, Manuel DG, Booth GL. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA. 2016;315(20):2211–2220. [DOI] [PubMed] [Google Scholar]
- 124. Faith MS, Fontaine KR, Baskin ML, Allison DB. Toward the reduction of population obesity: macrolevel environmental approaches to the problems of food, eating, and obesity. Psychol Bull. 2007;133(2):205–226. [DOI] [PubMed] [Google Scholar]
- 125. James W, Gill T. Prevention of obesity. In: Bray GA, Bouchard C, eds. Handbook of Obesity: Clinical Applications. 3rd ed.Boca Raton, FL: CRC Press; 2008:157–175. [Google Scholar]
- 126. Dubowitz T, Ghosh-Dastidar M, Cohen DA, Beckman R, Steiner ED, Hunter GP, Flórez KR, Huang C, Vaughan CA, Sloan JC, Zenk SN, Cummins S, Collins RL. Diet and perceptions change with supermarket introduction in a food desert, but not because of supermarket use. Health Aff (Millwood). 2015;34(11):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG, Karanja N, Lin P-H, Aickin M, Most-Windhauser MM, Moore TJ, Proschan MA, Cutler JA; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 128. Kennedy BM, Paeratakul S, Ryan DH, Bray GA. Socioeconomic status and health disparity in the United States. J Hum Behav Soc Environ. 2007;15(2–3):13–23. [Google Scholar]
- 129. Glanz K, Yaroch AL. Strategies for increasing fruit and vegetable intake in grocery stores and communities: policy, pricing, and environmental change. Prev Med. 2004;39(Suppl 2):S75–S80. [DOI] [PubMed] [Google Scholar]
- 130. Glanz K, Hoelscher D. Increasing fruit and vegetable intake by changing environments, policy and pricing: restaurant-based research, strategies, and recommendations. Prev Med. 2004;39(Suppl 2):S88–S93. [DOI] [PubMed] [Google Scholar]
- 131. Buzby M. Evaluation of the USDA fruit and vegetable program: report to congress. Available at: https://www.ers.usda.gov/webdocs/publications/efan03006/30826_efan03006_002.pdf. Accessed 8 January 2018.
- 132. French SA, Wechsler H. School-based research and initiatives: fruit and vegetable environment, policy, and pricing workshop. Prev Med. 2004;39(Suppl 2):S101–S107. [DOI] [PubMed] [Google Scholar]
- 133. Appel LJ, Clark JM, Yeh H-C, Wang N-Y, Coughlin JW, Daumit G, Miller ER III, Dalcin A, Jerome GJ, Geller S, Noronha G, Pozefsky T, Charleston J, Reynolds JB, Durkin N, Rubin RR, Louis TA, Brancati FL. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Smed S, Jensen JD, Denver S. Socio-economic characteristics and the effect of taxation as a health policy instrument. Food Policy. 2007;32(5–6):624–639. [Google Scholar]
- 135. Brownell KD, Farley T, Willett WC, Popkin BM, Chaloupka FJ, Thompson JW, Ludwig DS. The public health and economic benefits of taxing sugar-sweetened beverages. N Engl J Med. 2009;361(16):1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Falbe J, Thompson HR, Becker CM, Rojas N, McCulloch CE, Madsen KA. Impact of the Berkeley excise tax on sugar-sweetened beverage consumption. Am J Public Health. 2016;106(10):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Colchero MA, Rivera-Dommarco J, Popkin BM, Ng SW. In Mexico, evidence of sustained consumer response two years after implementing a sugar-sweetened beverage tax. Health Aff (Millwood). 2017;36(3):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Centers for Disease Control and Prevention Environmental and policy approaches to increase physical activity: creation of or enhanced access to places for physical activity combined with informational outreach activities. Available at: http://www.thecommunityguide.org/pa/environmental-policy/improvingaccess.html. Accessed 5 April 2015.
- 139. Centers for Disease Control and Prevention Strategies to Prevent Obesity and Other Chronic Diseases: The CDC Guide to Strategies to Increase Physical Activity in the Community. Atlanta, GA: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 140. Zhai F, Wang H, Du S, He Y, Wang Z, Ge K, Popkin BM. Prospective study on nutrition transition in China. Nutr Rev. 2009;67(Suppl 1):S56–S61. [DOI] [PubMed] [Google Scholar]
- 141. Monda KL, Adair LS, Zhai F, Popkin BM. Longitudinal relationships between occupational and domestic physical activity patterns and body weight in China. Eur J Clin Nutr. 2008;62(11):1318–1325. [DOI] [PubMed] [Google Scholar]
- 142. Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O’Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Zh, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB; The Global Mortality Collaboration . Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos A-K, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Ågren G, Carlsson LMS; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 144. Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. [DOI] [PubMed] [Google Scholar]
- 146. Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37(3):753–768, x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26(1):13. [DOI] [PubMed] [Google Scholar]
- 148. Bray GA, Redman LM, de Jonge L, Rood J, Smith SR. Effect of three levels of dietary protein on metabolic phenotype of healthy individuals with 8 weeks of overfeeding. J Clin Endocrinol Metab. 2016;101(7):2836–2843. [DOI] [PubMed] [Google Scholar]
- 149. Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS One. 2011;6(4):e18284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107(42):18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, Fraterrigo G, Okunade AL, Patterson BW, Klein S. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125(2):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hvidtfeldt UA, Gunter MJ, Lange T, Chlebowski RT, Lane D, Farhat GN, Freiberg MS, Keiding N, Lee JS, Prentice R, Tjønneland A, Vitolins MZ, Wassertheil-Smoller S, Strickler HD, Rod NH. Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 155. Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–819. [DOI] [PubMed] [Google Scholar]
- 156. Sasai H, Sairenchi T, Iso H, Irie F, Otaka E, Tanaka K, Ota H, Muto T. Relationship between obesity and incident diabetes in middle-aged and older Japanese adults: the Ibaraki Prefectural Health Study. Mayo Clin Proc. 2010;85(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–563. [DOI] [PubMed] [Google Scholar]
- 158. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Purnell JQ, Selzer F, Wahed AS, Pender J, Pories W, Pomp A, Dakin G, Mitchell J, Garcia L, Staten MA, McCloskey C, Cummings DE, Flum DR, Courcoulas A, Wolfe BM. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the longitudinal assessment of bariatric surgery study. Diabetes Care. 2016;39(7):1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. [DOI] [PubMed] [Google Scholar]
- 161. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Brawer R, Brisbon N, Plumb J. Obesity and cancer. Prim Care. 2009;36(3):509–531. [DOI] [PubMed] [Google Scholar]
- 163. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 164. Rosner B, Eliassen AH, Toriola AT, Hankinson SE, Willett WC, Natarajan L, Colditz GA. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat. 2015;150(3):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Aune D, Navarro Rosenblatt DA, Chan DSM, Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV, Norat T. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol. 2015;26(8):1635–1648. [DOI] [PubMed] [Google Scholar]
- 166. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. [DOI] [PubMed] [Google Scholar]
- 167. van Kruijsdijk RCM, van der Wall E, Visseren FLJ. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. [DOI] [PubMed] [Google Scholar]
- 168. Sweeney C, Blair CK, Anderson KE, Lazovich D, Folsom AR. Risk factors for breast cancer in elderly women. Am J Epidemiol. 2004;160(9):868–875. [DOI] [PubMed] [Google Scholar]
- 169. Schairer C, Fuhrman BJ, Boyd-Morin J, Genkinger JM, Gail MH, Hoover RN, Ziegler RG. Quantifying the role of circulating unconjugated estradiol in mediating the body mass index-breast cancer association. Cancer Epidemiol Biomarkers Prev. 2016;25(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. [DOI] [PubMed] [Google Scholar]
- 171. Canoy D, Cairns BJ, Balkwill A, Wright FL, Green J, Reeves G, Beral V; Million Women Study Collaborators . Body mass index and incident coronary heart disease in women: a population-based prospective study. BMC Med. 2013;11(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Flint AJ, Hu FB, Glynn RJ, Caspard H, Manson JE, Willett WC, Rimm EB. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring). 2010;18(2):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Emberson JR, Whincup PH, Morris RW, Wannamethee SG, Shaper AG. Lifestyle and cardiovascular disease in middle-aged British men: the effect of adjusting for within-person variation. Eur Heart J. 2005;26(17):1774–1782. [DOI] [PubMed] [Google Scholar]
- 175. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. [DOI] [PubMed] [Google Scholar]
- 176. Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–653. [DOI] [PubMed] [Google Scholar]
- 177. Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. [DOI] [PubMed] [Google Scholar]
- 178. Tsang TSM, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29(18):2227–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639–649. [DOI] [PubMed] [Google Scholar]
- 180. Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JAC. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–1783. [DOI] [PubMed] [Google Scholar]
- 181. Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122(12):1106–1114. [DOI] [PubMed] [Google Scholar]
- 182. Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV, Ventura HO. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58(4):393–400. [DOI] [PubMed] [Google Scholar]
- 183. Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol. 2018;15(1):45–56. [DOI] [PubMed] [Google Scholar]
- 184. Lajous M, Banack HR, Kaufman JS, Hernán MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128(4):334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. [DOI] [PubMed] [Google Scholar]
- 186. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014;127(6):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship among body fat percentage, body mass index, and all-cause mortality. Ann Intern Med. 2016;164(8):532–541. [DOI] [PubMed] [Google Scholar]
- 188. World Health Organization World health statistics 2015. Available at: http://www.who.int/gho/publications/world_health_statistics/2015/en/. Accessed March 2017.
- 189. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the united states: National health and nutrition examination survey, 2011–2012. NCHS Data Brief. 2013;2013(133):1–8. [PubMed] [Google Scholar]
- 190. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. [DOI] [PubMed] [Google Scholar]
- 191. Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J; Trials for the Hypertension Prevention Research Group . Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134(1):1–11. [DOI] [PubMed] [Google Scholar]
- 192. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884. [DOI] [PubMed] [Google Scholar]
- 193. Coccagna G, Pollini A, Provini F. Cardiovascular disorders and obstructive sleep apnea syndrome. Clin Exp Hypertens. 2006;28(3–4):217–224. [DOI] [PubMed] [Google Scholar]
- 194. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985). 2005;99(4):1592–1599. [DOI] [PubMed] [Google Scholar]
- 195. Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST; Sleep AHEAD Research Group . Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–S10. [DOI] [PubMed] [Google Scholar]
- 197. Cydylo MA, Davis AT, Kavanagh K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity (Silver Spring). 2017;25(2):290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239(1):192–202. [DOI] [PubMed] [Google Scholar]
- 199. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–530.e1. [DOI] [PubMed] [Google Scholar]
- 200. Li L, Gan Y, Li W, Wu C, Lu Z. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity (Silver Spring). 2016;24(8):1786–1802. [DOI] [PubMed] [Google Scholar]
- 201. Pang Q, Zhang JY, Song SD, Qu K, Xu XS, Liu SS, Liu C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015;21(5):1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Aune D, Norat T, Vatten LJ. Body mass index and the risk of gout: a systematic review and dose-response meta-analysis of prospective studies. Eur J Nutr. 2014;53(8):1591–1601. [DOI] [PubMed] [Google Scholar]
- 203. Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. [DOI] [PubMed] [Google Scholar]
- 204. Okoro CA, Hootman JM, Strine TW, Balluz LS, Mokdad AH. Disability, arthritis, and body weight among adults 45 years and older. Obes Res. 2004;12(5):854–861. [DOI] [PubMed] [Google Scholar]
- 205. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–638. [DOI] [PubMed] [Google Scholar]
- 206. Bray GA, Siri-Tarino PW. The role of macronutrient content in the diet for weight management. Endocrinol Metab Clin North Am. 2016;45(3):581–604. [DOI] [PubMed] [Google Scholar]
- 207. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Antonetti VW. The equations governing weight change in human beings. Am J Clin Nutr. 1973;26(1):64–71. [DOI] [PubMed] [Google Scholar]
- 209. Thomas DM, Gonzalez MC, Pereira AZ, Redman LM, Heymsfield SB. Time to correctly predict the amount of weight loss with dieting. J Acad Nutr Diet. 2014;114(6):857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Thomas DM, Weedermann M, Fuemmeler BF, Martin CK, Dhurandhar NV, Bredlau C, Heymsfield SB, Ravussin E, Bouchard C. Dynamic model predicting overweight, obesity, and extreme obesity prevalence trends. Obesity (Silver Spring). 2014;22(2):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. [DOI] [PubMed] [Google Scholar]
- 213. Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. Hepatic, adipocyte, enteric and pancreatic hormones: response to dietary macronutrient composition and relationship with metabolism. Nutr Metab (Lond). 2017;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gavrieli A, Mantzoros CS. Novel molecules regulating energy homeostasis: physiology and regulation by macronutrient intake and weight loss. Endocrinol Metab (Seoul). 2016;31(3):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Espeland MA, Bray GA, Neiberg R, Rejeski WJ, Knowler WC, Lang W, Cheskin LJ, Williamson D, Lewis CB, Wing R; Look Ahead Study Group . Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) research group. Ann Epidemiol. 2009;19(10):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 217. Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. Int J Obes. 2008;32(6):985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Delahanty LM, Pan Q, Jablonski KA, Watson KE, McCaffery JM, Shuldiner A, Kahn SE, Knowler WC, Florez JC, Franks PW; Diabetes Prevention Program Research Group . Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care. 2012;35(2):363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Papandonatos GD, Pan Q, Pajewski NM, Delahanty LM, Peter I, Erar B, Ahmad S, Harden M, Chen L, Fontanillas P, Wagenknecht LE, Kahn SE, Wing RR, Jablonski KA, Huggins GS, Knowler WC, Florez JC, McCaffery JM, Franks PW; Diabetes Prevention Program and the Look AHEAD Research Groups . Genetic predisposition to weight loss and regain with lifestyle intervention: analyses from the diabetes prevention program and the look ahead randomized controlled trials. Diabetes. 2015;64(12):4312–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. Weight-loss diets modify glucose-dependent insulinotropic polypeptide receptor rs2287019 genotype effects on changes in body weight, fasting glucose, and insulin resistance: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr. 2012;95(2):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, Bray GA, Qi L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012;61(11):3005–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhang X, Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. APOA5 genotype modulates 2-y changes in lipid profile in response to weight-loss diet intervention: the Pounds Lost Trial. Am J Clin Nutr. 2012;96(4):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Qi Q, Xu M, Wu H, Liang L, Champagne CM, Bray GA, Sacks FM, Qi L. IRS1 genotype modulates metabolic syndrome reversion in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Diabetes Care. 2013;36(11):3442–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Xu M, Qi Q, Liang J, Bray GA, Hu FB, Sacks FM, Qi L. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2013;127(12):1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mirzaei K, Xu M, Qi Q, de Jonge L, Bray GA, Sacks F, Qi L. Variants in glucose- and circadian rhythm-related genes affect the response of energy expenditure to weight-loss diets: the POUNDS LOST Trial. Am J Clin Nutr. 2014;99(2):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zheng Y, Huang T, Zhang X, Rood J, Bray GA, Sacks FM, Qi L. Dietary fat modifies the effects of fto genotype on changes in insulin sensitivity. J Nutr. 2015;145(5):977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Livingstone KM, Celis-Morales C, Papandonatos GD, Erar B, Florez JC, Jablonski KA, Razquin C, Marti A, Heianza Y, Huang T, Sacks FM, Svendstrup M, Sui X, Church TS, Jääskeläinen T, Lindström J, Tuomilehto J, Uusitupa M, Rankinen T, Saris WH, Hansen T, Pedersen O, Astrup A, Sørensen TI, Qi L, Bray GA, Martinez-Gonzalez MA, Martinez JA, Franks PW, McCaffery JM, Lara J, Mathers JC. FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ. 2016;354:i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring). 2006;14(8):1283–1293. [DOI] [PubMed] [Google Scholar]
- 230. Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care. 2014;37(4):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–293. [DOI] [PubMed] [Google Scholar]
- 232. Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Bueno NB, de Melo ISV, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1178–1187. [DOI] [PubMed] [Google Scholar]
- 234. Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–1727.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Astrup A, Grunwald GK, Melanson EL, Saris WHM, Hill JO. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord. 2000;24(12):1545–1552. [DOI] [PubMed] [Google Scholar]
- 237. Pirozzo S, Summerbell C, Cameron C, Glasziou P. Should we recommend low-fat diets for obesity? Obes Rev. 2003;4(2):83–90. [DOI] [PubMed] [Google Scholar]
- 238. Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85(6):1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rolls BJ, Barnett RA. Volumetrics. A Systematic Lifetime Approach to Eating. Proven Methods for Satisfying Hunger. Increase Food Volume Without Gaining Weight. Sound Recipes and Menus for Weight Loss. New York, NY: Harper-Collins, Inc;2000.
- 240. Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157(8):773–779. [DOI] [PubMed] [Google Scholar]
- 241. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–2102. [DOI] [PubMed] [Google Scholar]
- 242. Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007; (3):CD005105. [DOI] [PMC free article] [PubMed]
- 243. Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, Poulsen SK, Larsen TM, Sørensen TI, Zohar Y, Astrup A. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am J Clin Nutr. 2017;106(2):499–505. [DOI] [PubMed] [Google Scholar]
- 244. Skov AR, Toubro S, Rønn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23(5):528–536. [DOI] [PubMed] [Google Scholar]
- 245. Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord. 2004;28(10):1283–1290. [DOI] [PubMed] [Google Scholar]
- 246. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–1298. [DOI] [PubMed] [Google Scholar]
- 247. Bray GA, Ryan DH, Johnson W, Champagne CM, Johnson CM, Rood J, Williamson DA, Sacks FM. Markers of dietary protein intake are associated with successful weight loss in the POUNDS Lost trial. Clin Obes. 2017;7(3):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Esposito K, Ida Maiorino M, Ciotola M, Di Palo C, Scognamiglio P, Gicchino M, Petrizzo M, Saccomanno F, Beneduce F, Ceriello A, Giugliano D. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Obstet Gynecol Surv. 2010;65(6):379–380. [DOI] [PubMed] [Google Scholar]
- 249. Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011;124(9):841–851.e2. [DOI] [PubMed] [Google Scholar]
- 250. Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Chiva-Blanch G, Fiol M, Gómez-Gracia E, Arós F, Lapetra J, Serra-Majem L, Pintó X, Buil-Cosiales P, Sorlí JV, Muñoz MA, Basora-Gallisá J, Lamuela-Raventós RM, Serra-Mir M, Ros E; PREDIMED Study Investigators . Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(8):666–676. [DOI] [PubMed] [Google Scholar]
- 251. Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8(21):iii–iv, 1–182. [DOI] [PubMed] [Google Scholar]
- 252. Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27(5):537–549. [DOI] [PubMed] [Google Scholar]
- 253. Ptomey LT, Willis EA, Goetz JR, Lee J, Szabo-Reed AN, Sullivan DK, Donnelly JE. Portion-controlled meals provide increases in diet quality during weight loss and maintenance. J Hum Nutr Diet. 2016;29(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–977. [DOI] [PubMed] [Google Scholar]
- 255. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group . Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. [DOI] [PubMed] [Google Scholar]
- 256. Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, Chen CS, Klag MJ, Whelton PK, He J. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161(5):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85(4):1023–1030. [DOI] [PubMed] [Google Scholar]
- 258. Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, Ludwig DS. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. [DOI] [PubMed] [Google Scholar]
- 260. Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ, Zemel B, Wadden TA, Tenhave T, Newcomb CW, Klein S. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–785. [DOI] [PubMed] [Google Scholar]
- 262. Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140(10):769–777. [DOI] [PubMed] [Google Scholar]
- 263. Atkins R. Dr. Atkins’ Diet Revolution: The High Calorie Way to Stay Thin Forever. New York, NY: David McKay; 1972. [Google Scholar]
- 264. Ornish D. What if Americans ate less fat? JAMA. 1992;267(3):362, author reply 363–364. [DOI] [PubMed] [Google Scholar]
- 265. Nidetch J. The Story of Weight Watchers. New York, NY: New American Library; 1972. [Google Scholar]
- 266. Sears B. The Zone: A Revolutionary Life Plan to Put Your Body in Total Balance for Permanent Weight Loss. New York, NY: HarperCollins Publishers; 1995. [Google Scholar]
- 267. Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, Ball GD, Busse JW, Thorlund K, Guyatt G, Jansen JP, Mills EJ. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–933. [DOI] [PubMed] [Google Scholar]
- 268. National Institutes of Health Pounds lost study. Available at: https://www.nhlbi.nih.gov/health/resources/heart/other-pounds-lost. Accessed 31 January 2017.
- 269. Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, Lee CJ, Bleich SN, Clark JM. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162(7):501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Venditti EM, Bray GA, Carrion-Petersen ML, Delahanty LM, Edelstein SL, Hamman RF, Hoskin MA, Knowler WC, Ma Y; Diabetes Prevention Program Research Group . First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes. 2008;32(10):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Look AHEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Westerterp-Plantenga MS, Lejeune MPGM, Nijs I, van Ooijen M, Kovacs EMR. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28(1):57–64. [DOI] [PubMed] [Google Scholar]
- 273. Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AFH, Martinez JA, Handjieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WHM, Astrup A; Diet, Obesity, and Genes (Diogenes) Project . Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, Rodabough RJ, Snetselaar L, Thomson C, Tinker L, Vitolins M, Prentice R. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295(1):39–49. [DOI] [PubMed] [Google Scholar]
- 275. Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 2012;345:e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21(1):323–341. [DOI] [PubMed] [Google Scholar]
- 277. Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. [DOI] [PubMed] [Google Scholar]
- 278. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity. 2013;21(9):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK, Sharp TA, Francis CC, Hill JO. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr. 2006;83(4):803–808. [DOI] [PubMed] [Google Scholar]
- 280. Fung TT, Pan A, Hou T, Chiuve SE, Tobias DK, Mozaffarian D, Willett WC, Hu FB. Long-term change in diet quality is associated with body weight change in men and women. J Nutr. 2015;145(8):1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Bray GA. From farm to fat cell: why aren’t we all fat? Metabolism. 2015;64(3):349–353. [DOI] [PubMed] [Google Scholar]
- 282. Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes. 2008;32(8):1256–1263. [DOI] [PubMed] [Google Scholar]
- 283. Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–1456. [DOI] [PubMed] [Google Scholar]
- 284. Stubbe JH, Boomsma DI, De Geus EJ. Sports participation during adolescence: a shift from environmental to genetic factors. Med Sci Sports Exerc. 2005;37(4):563–570. [DOI] [PubMed] [Google Scholar]
- 285. Samaras K, Kelly PJ, Chiano MN, Spector TD, Campbell LV. Genetic and environmental influences on total-body and central abdominal fat: the effect of physical activity in female twins. Ann Intern Med. 1999;130(11):873–882. [DOI] [PubMed] [Google Scholar]
- 286. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine . American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. [DOI] [PubMed] [Google Scholar]
- 287. Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. US Department of Health and Human Services Physical activity guidelines advisory committee report 2008. Available at: https://health.gov/paguidelines/guidelines/. Accessed 10 March 2017. [DOI] [PubMed]
- 289. Fan JX, Brown BB, Hanson H, Kowaleski-Jones L, Smith KR, Zick CD. Moderate to vigorous physical activity and weight outcomes: does every minute count? Am J Health Promot. 2013;28(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Kimm SY, Glynn NW, Kriska AM, Fitzgerald SL, Aaron DJ, Similo SL, McMahon RP, Barton BA. Longitudinal changes in physical activity in a biracial cohort during adolescence. Med Sci Sports Exerc. 2000;32(8):1445–1454. [DOI] [PubMed] [Google Scholar]
- 291. DeLany JP, Bray GA, Harsha DW, Volaufova J. Energy expenditure in African American and white boys and girls in a 2-y follow-up of the Baton Rouge Children’s Study. Am J Clin Nutr. 2004;79(2):268–273. [DOI] [PubMed] [Google Scholar]
- 292. Kimm SY, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, Crawford PB, Sabry ZI, Liu K. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347(10):709–715. [DOI] [PubMed] [Google Scholar]
- 293. Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ball K, Brown W, Crawford D. Who does not gain weight? Prevalence and predictors of weight maintenance in young women. Int J Obes Relat Metab Disord. 2002;26(12):1570–1578. [DOI] [PubMed] [Google Scholar]
- 295. Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. [DOI] [PubMed] [Google Scholar]
- 296. Östman M, Britton E, Jonsson E. Physical exercise In: Östman M, Britton E, Jonsson E, eds. Treating and Preventing Obesity. Weinheim, Germany: Wiley-VCH Verlag; 2004:142–143. [Google Scholar]
- 297. Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, Superko HR, Fortmann SP, Albers JJ, Vranizan KM, Ellsworth NM, Terry RB, Haskell WL. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319(18):1173–1179. [DOI] [PubMed] [Google Scholar]
- 298. Wing RR, Epstein LH, Paternostro-Bayles M, Kriska A, Nowalk MP, Gooding W. Exercise in a behavioural weight control programme for obese patients with type 2 (non-insulin-dependent) diabetes. Diabetologia. 1988;31(12):902–909. [DOI] [PubMed] [Google Scholar]
- 299. Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325(7):461–466. [DOI] [PubMed] [Google Scholar]
- 300. Svendsen OL, Hassager C, Christiansen C. Six months’ follow-up on exercise added to a short-term diet in overweight postmenopausal women—effects on body composition, resting metabolic rate, cardiovascular risk factors and bone. Int J Obes Relat Metab Disord. 1994;18(10):692–698. [PubMed] [Google Scholar]
- 301. Pritchard JE, Nowson CA, Wark JD. A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc. 1997;97(1):37–42. [DOI] [PubMed] [Google Scholar]
- 302. Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan A. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. [DOI] [PubMed] [Google Scholar]
- 303. Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Hellan K, Hise M, Fennessey PV, Sonko B, Sharp T, Jakicic JM, Blair SN, Tran ZV, Mayo M, Gibson C, Washburn RA. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163(11):1343–1350. [DOI] [PubMed] [Google Scholar]
- 304. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med. 2004;164(1):31–39. [DOI] [PubMed] [Google Scholar]
- 305. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947–1956. [DOI] [PubMed] [Google Scholar]
- 306. Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, Hu FB, Hubbard VS, Jakicic JM, Kushner RF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 . Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22(Suppl 2):S5–S39. [DOI] [PubMed] [Google Scholar]
- 307. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. LeBlanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434–447. [DOI] [PubMed] [Google Scholar]
- 309. Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity: an evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol. 1984;52(3):404–413. [DOI] [PubMed] [Google Scholar]
- 310. Alamuddin N, Wadden TA. Behavioral treatment of the patient with obesity. Endocrinol Metab Clin North Am. 2016;45(3):565–580. [DOI] [PubMed] [Google Scholar]
- 311. Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–1330. [DOI] [PubMed] [Google Scholar]
- 312. Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, Wilk J, Weinstock R, Buckenmeyer P, Berkowitz RI, Steen SN. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65(2):269–277. [DOI] [PubMed] [Google Scholar]
- 313. Sherwood NE, Jeffery RW, Welsh EM, Vanwormer J, Hotop AM. The drop it at last study: six-month results of a phone-based weight loss trial. Am J Health Promot. 2010;24(6):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, Dale MS, Daniels MJ, Radcliff TA, Martin AD. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, Gibson C, Sullivan DK. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes. 2007;31(8):1270–1276. [DOI] [PubMed] [Google Scholar]
- 316. Donnelly JE, Goetz J, Gibson C, Sullivan DK, Lee R, Smith BK, Lambourne K, Mayo MS, Hunt S, Lee JH, Honas JJ, Washburn RA. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity (Silver Spring). 2013;21(10):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304(16):1803–1810. [DOI] [PubMed] [Google Scholar]
- 318. Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285(9):1172–1177. [DOI] [PubMed] [Google Scholar]
- 319. Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, Skelly J. Internet delivered behavioral obesity treatment. Prev Med. 2010;51(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE; Diabetes Prevention Program Research Group . Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54(4):1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Svetkey LP, Batch BC, Lin P-H, Intille SS, Corsino L, Tyson CC, Bosworth HB, Grambow SC, Voils C, Loria C, Gallis JA, Schwager J, Bennett GG. Cell phone intervention for you (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23(11):2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59(2):151–184. [DOI] [PubMed] [Google Scholar]
- 323. Nathanson MH. The central action of betaaminopropylbenzene (benzedrine): clinical observations. J Am Med Assoc. 1937;108(7):528–531. [Google Scholar]
- 324. Kasant O. The Amphetamines: Toxicity and Addiction. Ontario, Canada: Alcoholism and Drug Addiction Research Foundation of Ontario; 1966. [Google Scholar]
- 325. Fishman AP. Aminorex to fen/phen: an epidemic foretold. Circulation. 1999;99(1):156–161. [DOI] [PubMed] [Google Scholar]
- 326. Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, Perry BH. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722–733. [DOI] [PubMed] [Google Scholar]
- 327. Heil GC, Ross ST. Agents affecting appetite. Annu Rep Med Chem. 1973;(8):42–51.
- 328. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–1575. [DOI] [PubMed] [Google Scholar]
- 329. Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009;17(9):1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Bray GA, Ryan DH. Medical therapy for the patient with obesity. Circulation. 2012;125(13):1695–1703. [DOI] [PubMed] [Google Scholar]
- 331. Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD; Endocrine Society . Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. [DOI] [PubMed] [Google Scholar]
- 332. Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M; European Multicentre Orlistat Study Group . Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167–172. [DOI] [PubMed] [Google Scholar]
- 333. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. [DOI] [PubMed] [Google Scholar]
- 334. Dong Z, Xu L, Lv Y, Zheng Q, Li L. Comparative efficacy of five long-term weight loss drugs: quantitative information for medication guidelines. Obes Rev. 2017;18(12):1377–1383. [DOI] [PubMed] [Google Scholar]
- 335. Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes. 2007;31(10):1567–1570. [DOI] [PubMed] [Google Scholar]
- 337. Halford JCG, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. [DOI] [PubMed] [Google Scholar]
- 338. Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group . Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256. [DOI] [PubMed] [Google Scholar]
- 339. Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM; BLOSSOM Clinical Trial Group . A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–3077. [DOI] [PubMed] [Google Scholar]
- 340. O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20(7):1426–1436. [DOI] [PubMed] [Google Scholar]
- 341. Food and Drug Administration FDA briefing information: meeting of the endocrinologic and metabolic drugs advisory committee. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM303198.pdf. Accessed 8 January 2018.
- 342. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner S, Savolainen MJ, Van Gaal L; NN8022-1807 Investigators . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JPH; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 344. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L; NN8022-1923 Investigators . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37(11):1443–1451. [DOI] [PubMed] [Google Scholar]
- 345. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 347. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. [DOI] [PubMed] [Google Scholar]
- 348. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring). 2009;17(9):1730–1735. [DOI] [PubMed] [Google Scholar]
- 351. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E; COR-I Study Group . Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. [DOI] [PubMed] [Google Scholar]
- 352. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E; COR-II Study Group . A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, Klassen P, Fujioka K; COR-Diabetes Study Group . Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Ali KF, Shukla AP, Aronne LJ. Bupropion-SR plus naltrexone-SR for the treatment of mild-to-moderate obesity. Expert Rev Clin Pharmacol. 2016;9(1):27–34. [DOI] [PubMed] [Google Scholar]
- 356. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.U.S. Food and Drug Administration. New drug applications 22580: Vi-0521 qnexa (phentermine/topiramate). Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292315.pdf. Accessed 15 June 2017.
- 358. Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12(10):876–882. [DOI] [PubMed] [Google Scholar]
- 359. Addy C, Jumes P, Rosko K, Li S, Li H, Maes A, Johnson-Levonas AO, Chodakewitz J, Stoch SA, Wagner JA. Pharmacokinetics, safety, and tolerability of phentermine in healthy participants receiving taranabant, a novel cannabinoid-1 receptor (CB1R) inverse agonist. J Clin Pharmacol. 2009;49(10):1228–1238. [DOI] [PubMed] [Google Scholar]
- 360. Kim KK, Cho H-J, Kang H-C, Youn B-B, Lee K-R. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med J. 2006;47(5):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Blanck HM, Khan LK, Serdula MK. Prescription weight loss pill use among Americans: patterns of pill use and lessons learned from the fen-phen market withdrawal. Prev Med. 2004;39(6):1243–1248. [DOI] [PubMed] [Google Scholar]
- 362. Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy. 2013;33(12):1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Blanck HM, Serdula MK, Gillespie C, Galuska DA, Sharpe PA, Conway JM, Khan LK, Ainsworth BE. Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc. 2007;107(3):441–447. [DOI] [PubMed] [Google Scholar]
- 364. Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring). 2008;16(4):790–796. [DOI] [PubMed] [Google Scholar]
- 365.National Institutes of Health Office of Dietary Supplements. Dietary supplements for weight loss. https://ods.od.nih.gov/factsheets/WeightLoss-HealthProfessional/. Accessed 31 January 2016.
- 366. George M, Rajaram M, Shanmugam E. New and emerging drug molecules against obesity. J Cardiovasc Pharmacol Ther. 2014;19(1):65–76. [DOI] [PubMed] [Google Scholar]
- 367. Ponce J, DeMaria EJ, Nguyen NT, Hutter M, Sudan R, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis. 2016;12(9):1637–1639. [DOI] [PubMed] [Google Scholar]
- 368. Nielsen S, Svane MS, Bojsen-Møller KN, Madsbad S. Effects of bariatric surgery on weight loss and quality of life. Anaplastology. 2014;3:136. [Google Scholar]
- 369. Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg. 2003;13(4):649–654. [DOI] [PubMed] [Google Scholar]
- 370. Zundel N. Laparoscopic sleeve gastrectomy: Technique and outcomes In: Nguyen NT, Blackstone RP, Morton JM, Ponce J, Rosenthal R, eds. American Society for Metabolic and Bariatric Surgery: Textbook of Bariatric Surgery. Vol. 1 New York, NY: Springer; 2015:205–210. [Google Scholar]
- 371. Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, Wolfe BM. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234(3):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated bariatric surgery centers of excellence using the bariatric outcomes longitudinal database. Surg Obes Relat Dis. 2010;6(4):347–355. [DOI] [PubMed] [Google Scholar]
- 373. O’Brien PE, Dixon JB. Lap-band: outcomes and results. J Laparoendosc Adv Surg Tech A. 2003;13(4):265–270. [DOI] [PubMed] [Google Scholar]
- 374. Shikora SA, Wolfe BM, Apovian CM, Anvari M, Sarwer DB, Gibbons RD, Ikramuddin S, Miller CJ, Knudson MB, Tweden KS, Sarr MG, Billington CJ. Sustained weight loss with vagal nerve blockade but not with sham: 18-month results of the recharge trial. J Obes. 2015;2015:365604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Shikora SA, Bergenstal R, Bessler M, Brody F, Foster G, Frank A, Gold M, Klein S, Kushner R, Sarwer DB. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009;5(1):31–37. [DOI] [PubMed] [Google Scholar]
- 376. Saber AA, Shoar S, Almadani MW, Zundel N, Alkuwari MJ, Bashah MM, Rosenthal RJ. Efficacy of first-time intragastric balloon in weight loss: a systematic review and meta-analysis of randomized controlled trials. Obes Surg. 2017;27(2):277–287. [DOI] [PubMed] [Google Scholar]
- 377. Mion F, Ibrahim M, Marjoux S, Ponchon T, Dugardeyn S, Roman S, Deviere J. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg. 2013;23(5):730–733. [DOI] [PubMed] [Google Scholar]
- 378. Klein S, Kinney J, Jeejeebhoy K, Alpers D, Hellerstein M, Murray M, Twomey P. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Clin Nutr. 1997;16(4):193–218. [DOI] [PubMed] [Google Scholar]
- 379. Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, Deal SE, Kuwada TS, Heniford BT. Duodenal- jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov. 2007;14(4):275–278. [DOI] [PubMed] [Google Scholar]
- 380. Rajagopalan H, Cherrington AD, Thompson CC, Kaplan LM, Rubino F, Mingrone G, Becerra P, Rodriguez P, Vignolo P, Caplan J, Rodriguez L, Galvao Neto MP. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care. 2016;39(12):2254–2261. [DOI] [PubMed] [Google Scholar]
- 381. Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350(25):2549–2557. [DOI] [PubMed] [Google Scholar]
- 382. Gastrointestinal surgery for severe obesity consensus statement. Nutrition Today 1991;26:32–35. [PubMed] [Google Scholar]
- 383. Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Beamish AJ, Olbers T, Kelly AS, Inge TH. Cardiovascular effects of bariatric surgery. Nature Rev Cardiol 2016;13(12):730–743. [DOI] [PubMed] [Google Scholar]
- 385. Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294(15):1903–1908. [DOI] [PubMed] [Google Scholar]
- 386. Smith MD, Patterson E, Wahed AS, Belle SH, Bessler M, Courcoulas AP, Flum D, Halpin V, Mitchell JE, Pomp A, Pories WJ, Wolfe B. Relationship between surgeon volume and adverse outcomes after RYGB in Longitudinal Assessment of Bariatric Surgery (LABS) study. Surg Obes Relat Dis. 2010;6(2):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Puzziferri N, Roshek TB III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118(11):1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Gletsu-Miller N, Wright BN. Mineral malnutrition following bariatric surgery. Adv Nutr. 2013;4(5):506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery. Obesity (Silver Spring). 2013;21(Suppl 1):S1–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, Melissa Kalarchian PD, Mitchell JE, Patterson E, Pomp A, Pories WJ, Spaniolas K, Steffen K, Wolfe BM, Belle SH. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015;11(5):1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Mitchell JE, Christian NJ, Flum DR, Pomp A, Pories WJ, Wolfe BM, Courcoulas AP, Belle SH. Postoperative behavioral variables and weight change 3 years after bariatric surgery. JAMA Surg. 2016;151(8):752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 394. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–973. [DOI] [PubMed] [Google Scholar]
- 396. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. [DOI] [PubMed] [Google Scholar]
- 397. Bray GA. A Guide to Obesity and the Metabolic Syndrome: Origins and Treatment. New York, NY: CRC Press; 2011:265. [Google Scholar]
- 398. Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Michalsky M, Reichard K, Inge T, Pratt J, Lenders C; American Society for Metabolic and Bariatric Surgery . ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8(1):1–7. [DOI] [PubMed] [Google Scholar]
- 400. Sieber P, Gass M, Kern B, Peters T, Slawik M, Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(2):243–249. [DOI] [PubMed] [Google Scholar]
- 401. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ESH, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, Helmrath MA. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5(3):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Olbers T, Beamish AJ, Gronowitz E, Flodmark C-E, Dahlgren J, Bruze G, Ekbom K, Friberg P, Göthberg G, Järvholm K, Karlsson J, Mårild S, Neovius M, Peltonen M, Marcus C. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5(3):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Pratt JSA, Lenders CM, Dionne EA, Hoppin AG, Hsu GLK, Inge TH, Lawlor DF, Marino MF, Meyers AF, Rosenblum JL, Sanchez VM. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring). 2009;17(5):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Pickett K, Wilkinson RP. The Spirit Level: Why Greater Equality Makes Societies Stronger. New York, NY: Bloomsbury Press; 2011. [Google Scholar]
- 407. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379. [DOI] [PubMed] [Google Scholar]
- 408. Anderson SE, Andridge R, Whitaker RC. Bedtime in preschool-aged children and risk for adolescent obesity. J Pediatr. 2016;176:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Dalgaard K, Landgraf K, Heyne S, Lempradl A, Longinotto J, Gossens K, Ruf M, Orthofer M, Strogantsev R, Selvaraj M, Lu TT, Casas E, Teperino R, Surani MA, Zvetkova I, Rimmington D, Tung YCL, Lam B, Larder R, Yeo GS, O’Rahilly S, Vavouri T, Whitelaw E, Penninger JM, Jenuwein T, Cheung C-L, Ferguson-Smith AC, Coll AP, Körner A, Pospisilik JA. Trim28 haploinsufficiency triggers bi-stable epigenetic obesity. Cell. 2016;164(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 411. Huang T, Zheng Y, Hruby A, Williamson DA, Bray GA, Shen Y, Sacks FM, Qi L. Dietary protein modifies the effect of the MC4R genotype on 2-year changes in appetite and food craving: the POUNDS Lost Trial. J Nutr. 2017;147(3):439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. [DOI] [PubMed] [Google Scholar]
- 413. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–766. [DOI] [PubMed] [Google Scholar]
- 414. Tschöp MH, Finan B, Clemmensen C, Gelfanov V, Perez-Tilve D, Müller TD, DiMarchi RD. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24(1):51–62. [DOI] [PubMed] [Google Scholar]
- 415. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214(5):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]