Abstract
Treatment of C2C12 muscle cells with metformin or the NR4A1 ligand 1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) induced NR4A1 and Glut4 messenger RNA and protein expression. Similar results were observed with buttressed (3- or 3,5-substituted) analogs of DIM-C-pPhOH, including 1,1-bis(3′-indolyl)-1-(3-chloro-4-hydroxy-5-methoxyphenyl)methane (DIM-C-pPhOH-3-Cl-5-OCH3), and the buttressed analogs were more potent than DIM-C-pPhOH NR4A1 agonists. Metformin and the bis-indole substituted analogs also induced expression of several glycolytic genes and Rab4, which has previously been linked to enhancing cell membrane accumulation of Glut4 and overall glucose uptake in C2C12 cells, and these responses were also observed after treatment with metformin and the NR4A1 ligands. The role of NR4A1 in mediating the responses induced by the bis-indoles and metformin was determined by knockdown of NR4A1, and this resulted in attenuating the gene and protein expression and enhanced glucose uptake responses induced by these compounds. Our results demonstrate that the bis-indole–derived NR4A1 ligands represent a class of drugs that enhance glucose uptake in C2C12 muscle cells, and we also show that the effects of metformin in this cell line are NR4A1-dependent.
DIM-C-pPhOH and related compounds represent a new class of NR4A1-dependent antidiabetic agents that activate glycolysis, induce Glut4, and enhance glucose uptake in C2C12 muscle cells.
The orphan nuclear receptor (NR) family has been characterized as a collection of NRs that share many structural domain similarities with other NRs; however, their endogenous ligands are unknown (1). The three NR4A receptors NR4A1 (Nur77), NR4A2 (Nurr1), and NR4A3 (Nor1) have marked structural similarities in their ligand binding domains and DNA binding domains, whereas their N-terminal (A/B) domains containing activation function 1 are highly divergent (2–5). NR4A receptors were initially defined as nerve growth factor–induced-β receptors that bind as monomers to a nerve growth factor–induced β response element (NBRE; AAAGGTCA) (5–9). NR4A receptors also bind as a homodimer or heterodimer to a Nur-responsive element (TGATATTACCTCCAAATGCCA), and both NR4A1 and NR4A2 can also bind as heterodimers with the retinoid X receptor to a DR5 motif (10, 11). The initial discovery of NR4A receptors was linked to their rapid induction by multiple stimuli in various tissues/cells and organs, and these responses play a role in coping with both exogenous and endogenous stressors (12, 13).
NR4A1, NR4A2, and NR4A3 are rapidly induced by cyclic adenosine monophosphate in mouse hepatocytes and by glucagon in mouse liver; however, knockdown/overexpression and genetic studies with NR4A1 give conflicting results with respect to the role of NR4A1 in metabolic disease (14). In db/db (diabetic) mice injected with an adenoviral-NR4A1 construct, there was an increase in blood and hepatic glucose levels, whereas a dominant negative adenoviral-NR4A1-M1 construct decreased blood glucose levels and other parameters consistent with a diabeteslike condition (14). In contrast, loss of NR4A1 in wild-type mice maintained on a high-fat diet (HFD) resulted in increased obesity and markers of insulin resistance and hepatic steatosis (15), and it was apparent that NR4A1 was particularly important for muscle glycolysis and glucose uptake in HFD-induced insulin resistance model (15, 16). Studies have identified synthetic NR4A1 ligands such as cytosporone B and a structurally related compound, [2,3,4-trimethoxy-6-(i-octanoyl)phenyl] acetate (16–18), that both enhance and decrease serum glucose levels through their activity as NR4A1 agonist and antagonist activities, respectively, based primarily on their modulation of hepatic gluconeogenesis (16, 17).
Cytosporone B and related compounds activate nuclear NR4A1 but also induce nuclear translocation of the receptor (16–18); in contrast, studies in this laboratory have identified a series of 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methane (C-DIM) compounds that act as nuclear NR4A1 ligands (19–21). 1,1-Bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) bound NR4A1 (KD ∼0.1 μM) (20) and acted as an NR4A1 antagonist in solid tumor–derived cancer cell lines and inhibited NR4A1-dependent cancer cell growth, survival, and migration (19–28). Because DIM-C-pPhOH has a short serum half-life (29), we have synthesized a series of 3- and 3,5-substituted (buttressed) analogs of DIM-C-pPhOH to inhibit metabolism (conjugation), and these compounds are significantly more potent than the parent compound as anticancer agents (30). In this study, we show that DIM-C-pPhOH and related compounds activate glycolysis, induce Glut4, and enhance glucose uptake in C2C12 muscle cells. The results indicate that C-DIM/NR4A1 ligands represent a new class of NR4A1-dependent agents that enhance glucose metabolism and also show that metformin induces similar responses in C2C12 cells that are also NR4A1-dependent.
Materials and Methods
Chemicals, antibodies, and cell culture
Antibodies against the following proteins are summarized in Table 1. The C-DIM compounds were synthesized by condensing 2 mol equivalents of indole and 1 mol equivalent of DIM-C-pPhOH, 3-chloro-4-hydroxybenzaldehyde (DIM-C-pPhOH-3-Cl), 3-chloro-4-hydroxy-5-methoxybenzaldehyde (DIM-C-pPhOH-3-Cl-5-OCH3), and 3,5-dibromo-4-hydroxybenzaldehyde (DIM-C-pPhOH-3,5-Br2) essentially as described (20), and all compounds were >98%pure. Indole and the substituted benzaldehydes metformin and 5-amino-4-carboxamide ribonucleotide (AICAR) were purchased from Sigma-Aldrich (St. Louis, MO). Mouse myoblasts C2C12 cells were obtained from American Type Culture Collection (Manassas, VA) and were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and antibiotics at 37°C in an incubator with 5% CO2 differentiation induced by switching to differentiation medium with Dulbecco’s modified Eagle medium containing 2% horse serum for 5 days.
Table 1.
Primary Antibodies
| Antigen | Source Species | Dilution | Manufacturer; Catalog No.; RRID |
|---|---|---|---|
| NR4A1/Nur77 | Rabbit | 1:1000 | Abcam; ab109180; AB_10861258 |
| Rab4 | Rabbit | 1:1000 | Cell Signaling Technology; 2167S; AB_2253579 |
| p-AMPK | Rabbit | 1:1000 | Cell Signaling Technology; 2535; AB_331250 |
| AMPK | Rabbit | 1:1000 | Cell Signaling Technology; 5831; AB_10622186 |
| GLUT4 | Mouse | 1:1000 | Cell Signaling Technology; 2213; AB_823508 |
| Monoclonal anti-flag M2 | Mouse | 1:1000 | Sigma-Aldrich; F1804; AB_262044 |
| β-Actin | Mouse | 1:10,000 | Sigma-Aldrich; A1978; AB_476692 |
Abbreviation: RRID, Research Resource Identifier.
Western blot analysis
C2C12 cells were seeded on six-well plates and treated for 24 hours with either dimethyl sulfoxide (DMSO) or different concentrations of C-DIM compounds for different time periods. Following treatment of the cells, media was aspirated and cells were washed twice in ice-cold phosphate-buffered saline (PBS) and lysed using radioimmunoprecipitation assay lysis buffer. The whole lysates were then heated for 5 minutes at 95°C, the supernatants were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and proteins were transferred to polyvinylidene difluoride membranes. The blots were incubated overnight at room temperature with primary antibodies to a 1-hour incubation with horseradish peroxidase–conjugated secondary antibodies at room temperature. The blots were then visualized via enhanced chemilumescent reagent.
RNA isolation and reverse transcription polymerase chain reaction
Total RNA was isolated from cultured cells according to the manufacturer’s instructions (Zymo Research, Irvine, CA). The concentration and purity of the RNA samples were determined using a nanodrop spectrophotometer. Total RNA (2 μg) was reverse transcribed using iTaq Universal SYBR Green One-Step Kit (Thermo Fisher Scientific, Grand Island, NY), and the samples were amplified using corresponding primers (NR4A1, GLUT4, PHKG1, PGAM2, PYGM, PFKM, GPD1, ENO3, and ALDO1) (Table 2). Glyceraldehyde 3-phosphate dehydrogenase was used as an endogenous control to normalize each sample.
Table 2.
List of Primers Specific for Mouse
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
| NR4A1 | ATGCCTCCCCTACCAATCTTC | CACCAGTTCCTGGAACTTGGA |
| GLUT4 | ACATACCTGACAGGGCAAGG | CGCCCTTAGTTGGTCAGAAG |
| PHKG1 | CCTTAACCGAGAAGGAAACCA | TGAGTTTGTGCAGGGTACAGA |
| PYGM | AGTGGAGGACGTGGAAAGG | GCTCAGGAATTCGGTCGTAG |
| PGAM2 | TACACCTCCATCAGCAAGGA | GCAATGGTGTCCTTGAGACTT |
| PFKM | TTGAGGAACCCTTCACCATT | TCTTCTGCACCAGATGTTCAA |
| GPD1 | AGACCTCATCACGACCTGCT | CCAGCTGCTCAATGGACTTT |
| ENO3 | TCCCGTGGTCTCCATTGAG | CCACCCCAGAGAGGAATGAG |
| ALDO1 | AATGTTCTGGCCCGTTATGC | CCGCCAGGACCTTCTCAGTA |
Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Glucose uptake
The amount of glucose uptake in C2C12 myotubes was measured using the Glucose Uptake-Glo Assay Kit (Promega, Madison, WI) according to the manufacturer’s instructions. For measuring glucose uptake in C2C12 cells based on the detection of 2-deoxyglucose-6-phosphate, after 24 hours of drug treatment, C2C12 myotubes were placed in 1 mL of glucose-free medium containing 2-deoxyglucose-6-phosphate in 96-well plates for 3 hours. The luminescence intensity in the cells was then analyzed in a luminometer.
Overexpression of NR4A1
C2C12 cells were seeded in six-well plates and allowed to differentiate for 5 days. For differentiation, C2C12 cells were washed once with PBS, and then 2% horse media was added every day. After differentiation, an NR4A1 expression plasmid was transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and after 48 hours, cells were harvested for determining protein and messenger RNA (mRNA) expression.
Immunofluorescence
C2C12 cells were seeded in Nunc chambered coverglass and allowed for differentiation, followed by various drug treatments. The cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at 37°C. Cells were then blocked and incubated overnight with primary GLUT4 antibody in buffer (5% bovine serum albumin in PBS) at 4°C, followed by incubation with Alexa Fluor conjugated secondary antibody at a dilution of 1:250 for 2 hours at room temperature. Finally, cells were observed using a Zeiss confocal fluorescence microscope.
Silencing NR4A1
C2C12 cells were seeded in six-well plates and allowed to grow to 70% confluence for 24 hours, and 2% horse serum media was changed every day during 5 days of differentiation. After differentiation, transfections were performed with transfection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Both siNR4A1 (Santa Cruz Biotechnology, Dallas, TX) and nontargeted control small interfering RNAs were used. Six hours after transfection, the medium was replaced with fresh medium and left for 48 hours. After treatment with various drugs for 24 hours, the cells were harvested for determining protein and mRNA expression after transfection.
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed using ChIP-IT Express Magnetic Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. C2C12 cells (5 × 106 cells) were seeded and allowed to differentiate for 5 days, followed by various drug treatments for 24 hours. The ChIP assay was carried out as previously described (19, 25, 27). The GLUT4 primers were 5′-TTA AGT CAA CCA GGC GCA GT-3′ (sense) and 5′-GGC TGG CCT TGA ATT TGC TG-3′ (antisense), the PYGM primers were 5′-CTG CCC GAC TTA AGG ACC TG-3′ (sense) and 5′-GCT CTG TGC CCG ACA AGC AGG-3′ (antisense), and they then respectively amplified a region of mouse promoter GLUT4 and PYGM, which contained the NR4A1 binding sequences. Polymerase chain reaction (PCR) products were resolved on a 2% agarose gel in the presence of ethidium bromide.
Animal treatment and housing
Commercially available (Jackson; catalog no. 380050), 18-week-old C57BL/6JD10 mice maintained for 12 weeks on a 60% HFD (Research Diets; catalog no. D12492) were treated with DIM-C-pPhOH-3-Cl-5-OCH3 (25 mg/kg/d) or control vehicle (corn oil) by oral gavage every second day for 8 weeks. Mice were kept in a standard housing facility in the Laboratory Animal Research and Resources Core Facility (Comparative Medicine Program, Texas A&M University). Mice were maintained under a 12-hour light cycle and at a monitored ambient temperature of 23°C. All procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Eight to 10 animals were used for each treatment group. Muscle tissue was obtained and analyzed for Glut4 mRNA by real-time PCR.
Statistical analysis
All of the experiments were repeated a minimum of three times. The data are expressed as the mean ± standard error (SE). One-way analysis of variance was used to determine statistical significance. P values <0.05 were considered statistically significant.
Results
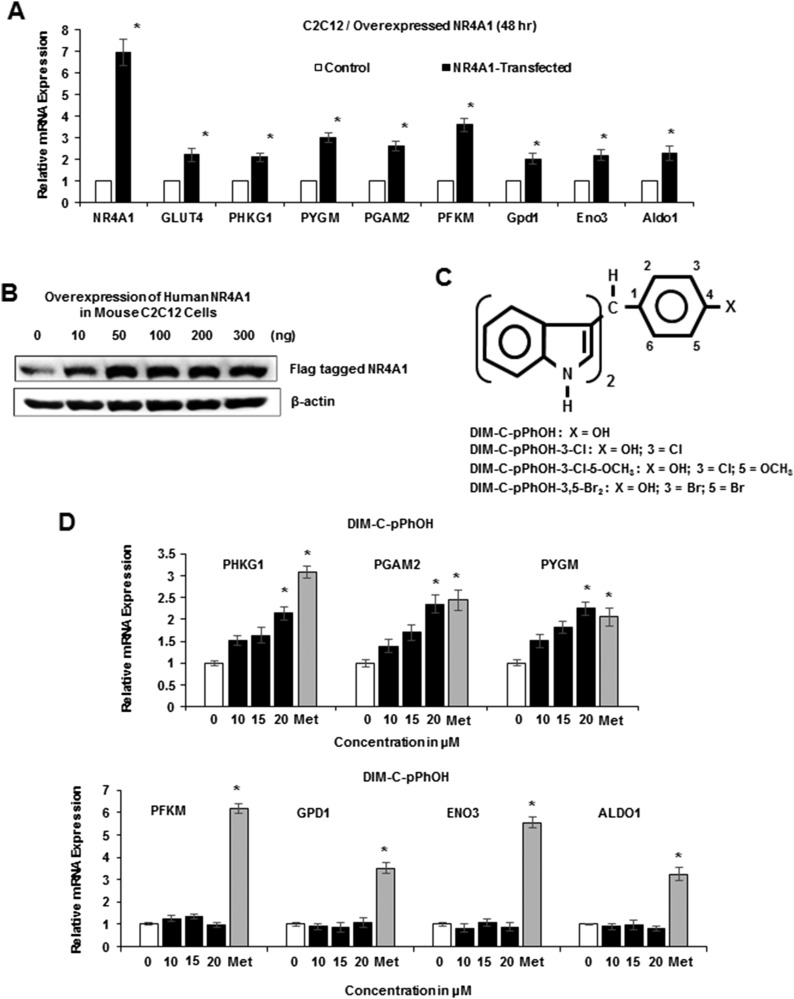
Previous studies showed that overexpression of NR4A1 protected against dietary-induced obesity in animal models, and in skeletal muscle C2C12 cells, NR4A1 overexpression was accompanied by increased levels of Glut4 mRNA and several glycolytic genes (15, 31). We also showed in C2C12 cells transfected with an NR4A1 expression plasmid increased mRNA expression of several glycolytic genes, including phosphoglycerate mutase 2 (Pgam2), phosphorylase kinase γ1 (Phkg1), muscle glycogen phosphorylase (Pygm), muscle phosphofructokinase (Pfkm), glycerol-3-phosphate dehydrogenase 1 (Gpd1), enolase-3 (Eno3), and aldolase-1 (Aldo1) (Fig. 1A). Figure 1B illustrates levels of NR4A1 protein expression (flag-tagged) in C2C12 cells transfected with the NR4A1 expression plasmid, and 100 ng was used for subsequent overexpression studies. DIM-C-pPhOH (Fig. 1C) has previously been identified as a high-affinity ligand for NR4A1 (20) (KD ∼0.1 μM), and this compound induced expression of Phkg1, Pgam2, and Pygm mRNA in differentiated C2C12 cells. In contrast, Pfkm, Gpd1, Eno3, and Aldo-1 mRNA levels were not induced by DIM-C-pPhOH, whereas metformin induced all seven glycolytic genes.
Figure 1.
NR4A1 overexpression and effects of DIM-C-pPhOH and metformin. C2C12 cells were transfected with NR4A1 expression plasmid, and effects on (A) glycolytic gene mRNA levels and (B) NR4A1 protein expression were determined by real-time PCR and western blots of whole-cell lysates, respectively. (C) Structure of C-DIM/NR4A1 ligands used in this study. (D) C2C12 cells were treated with DMSO (control) or different concentrations of C-DIMs and 1 mM metformin, and effects on glycolytic gene expression were determined by real-time PCR. Met, metformin.
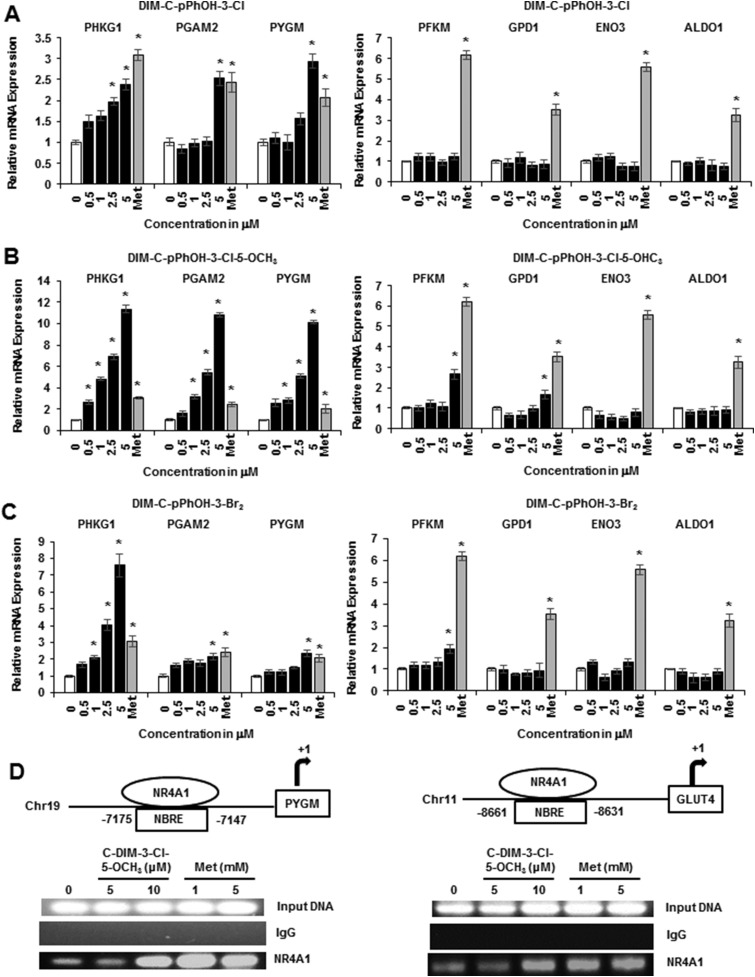
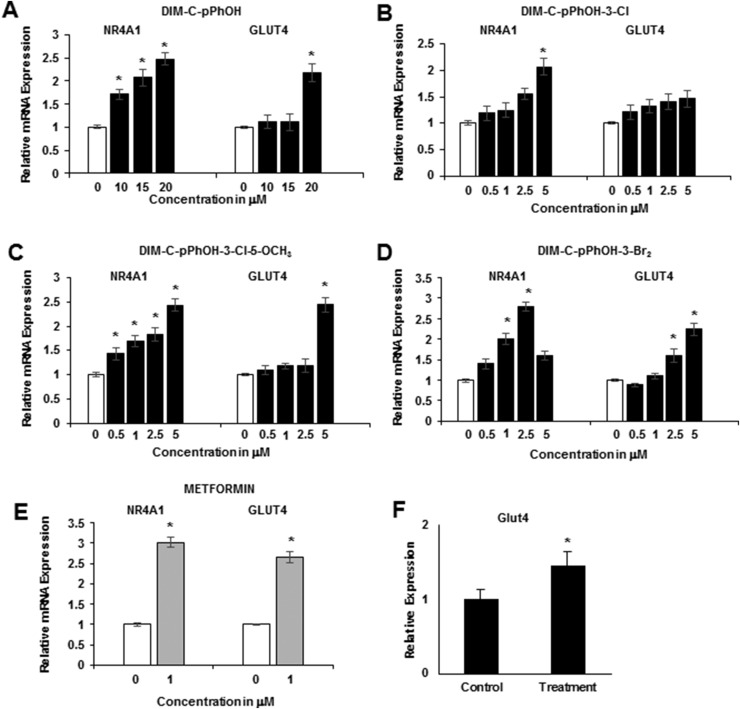
Ongoing studies in cancer cell lines show that buttressed analogs of DIM-C-pPhOH (3- and 3,5-substituted) are at least fourfold more potent than the parent compound in terms of activating NR4A1-responsive genes (30), and Fig. 2A shows that DIM-C-pPhOH-3-Cl significantly induced Phkg1, Pgam2, and Pygm at 0.5 to 5 μM concentrations but did not induce expression of Pfkm, Gpd1, Eno3, or Aldo1 mRNA. DIM-C-pPhOH-3-Cl-5-OCH3 induced expression of five glycolytic genes (Phkg1, Pgam2, Pygm, Pfkm, and Gpd1) (Fig. 2B), and DIM-C-pPhOH-3,5-Br2 induced Phkg1, Pgam2, Pygm, and Pfkm mRNA levels (Fig. 2C). Metformin induced all seven glycolytic genes; however, DIM-C-pPhOH-3-Cl-5-OCH3 was significantly more potent than metformin as an inducer of Phkg1, Pgam2, and Pygm mRNA. A previous study showed that overexpression of NR4A1 increased binding of the receptor to NBREs in the Glut4 and Pygm genes (31), and results in Fig. 2D show that treatment with metformin or DIM-C-pPhOH-3-Cl-5-OCH3 also increased NR4A1 binding to these regions as determined in a ChIP assay. The failure of the 5 μM DIM-C-pPhOH-3-Cl-5-OCH3 to recruit NR4A1 to the gene promoters may be due to the time-dependent cycling of nuclear factors on the promoter; this assay examined interactions with the promoter at only one time point (24 hours). We also observed that DIM-C-pPhOH (Fig. 3A), the buttressed analogs DIM-C-pPhOH-3-Cl (Fig. 3B), DIM-C-pPhOH-3,5-Br2 (Fig. 3C), and DIM-C-pPhOH-3-Cl-5-OCH3 (Fig. 3D), and metformin (Fig. 3E) induced NR4A1 and Glut4 mRNA levels, and for these responses, the buttressed analogs were more potent than DIM-C-pPhOH. Interestingly, we observed that metformin not only induced Glut4 (Fig. 3E) as previously described (32), but this was also accompanied by induction of NR4A1. In addition, we also observed that DIM-C-pPhOH-3-Cl-5-OCH3 induced Glut4 mRNA in muscle tissue in mice maintained on an HFD (Fig. 3F), confirming that comparable responses were observed in cell culture (C2C12 cells) and in vivo.
Figure 2.
Effects of C-DIM/NR4A1 ligands and metformin on glycolytic gene expression. C2C12 cells were treated with DMSO (control), 1 mM metformin, or different concentrations of the buttressed C-DIM compounds (A) DIM-C-pPhOH-3-Cl, (B) DIM-C-pPhOH-3-Cl-5-OCH3, and (C) DIM-C-pPhOH-3,5-Br2 for 24 hours, and mRNA levels were determined by real-time PCR. Experiments were determined in triplicate for each data point and results are presented as means ± SE. Significant (P < 0.05) induction is indicated (*). (D) C2C12 cells were treated with metformin or DIM-C-pPhOH-3-Cl-5-OCH3,, and recruitment of NR4A1 to the NBRE regions of the PYGM and GLUT4 promoter was determined in a ChiP assay. IgG, immunoglobulin G; Met, metformin.
Figure 3.
Metformin and C-DIM/NR4A1 ligands induce NR4A1 and Glut4 gene expression. C2C12 cells were treated with (A) DIM-C-pPhOH, (B) DIM-C-pPhOH-3-Cl, (C) DIM-C-pPhOH-3,5-Br2, (D) DIM-C-pPhOH-3-Cl-5-OCH3, and (E) metformin, and induction of NR4A1 and Glut4 mRNA was determined by real-time PCR. (F) Glut4 mRNA expression in muscle from mice maintained on an HFD was determined by real-time PCR as outlined in “Materials and Methods.” Results are expressed as means ± SE for at least three replicated determinations, and significant (P < 0.05) induction is indicated (*).
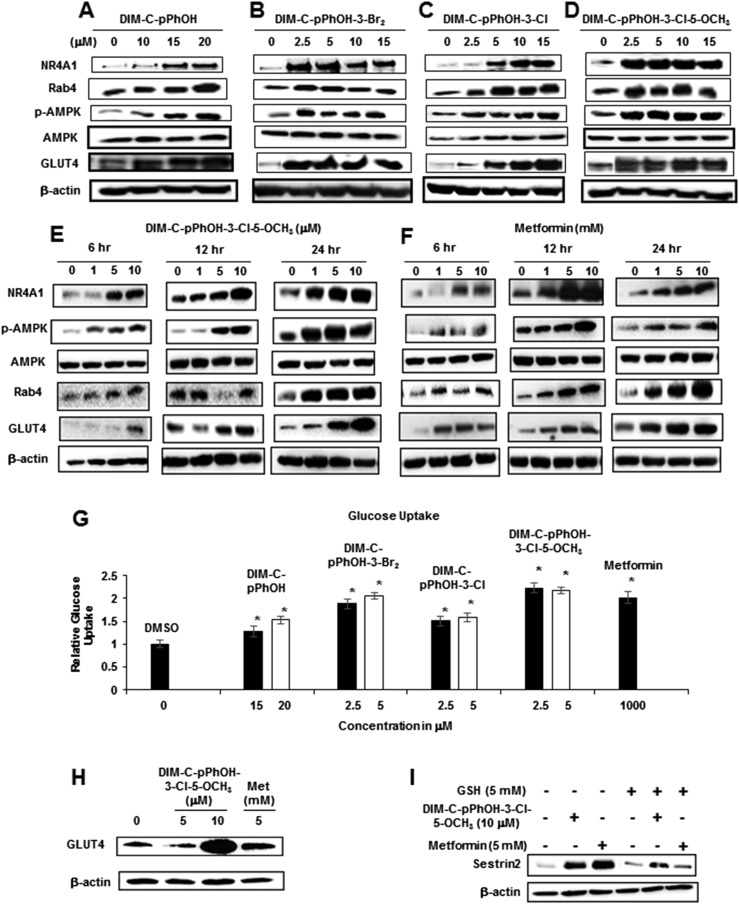
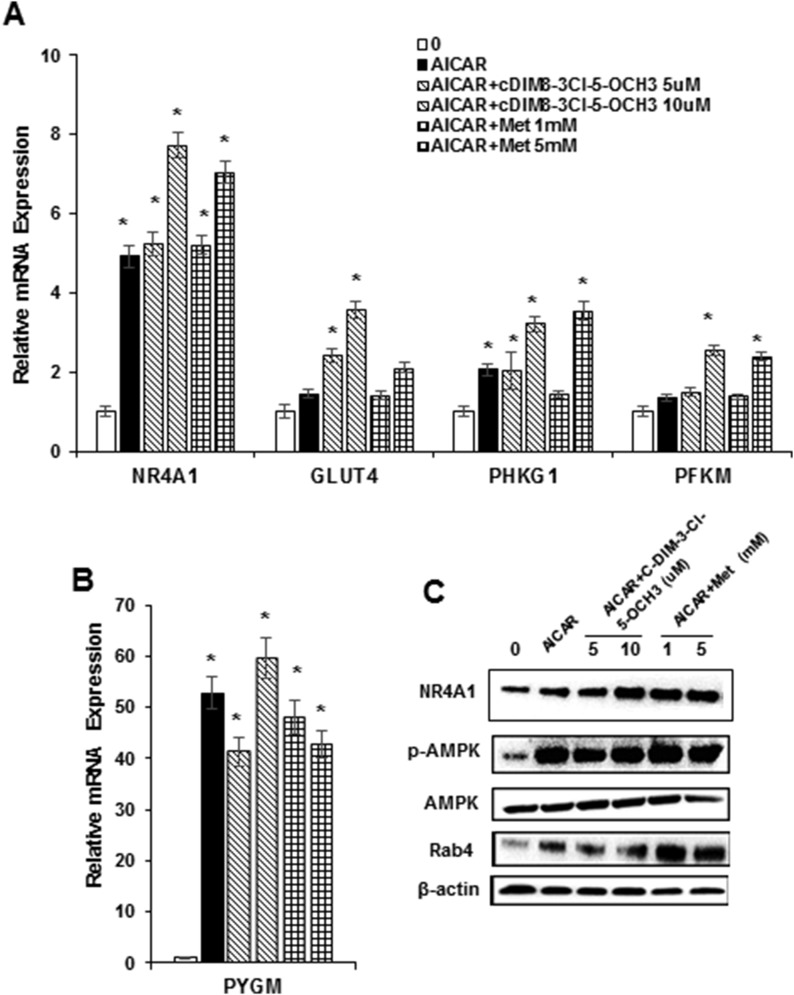
Figure 4A–4D summarize the concentration-dependent effects of DIM-C-pPhOH, DIM-C-pPhOH-3,5-Br2, DIM-C-pPhOH-3-Cl, and DIM-C-pPhOH-3-Cl-5-OCH3 on expression of several gene products associated with antidiabetic activity in C2C12 cells. All NR4A1 ligands induced expression of NR4A1 and Glut4 proteins, and this correlated with their effects on expression of the corresponding genes (Fig. 3). A previous publication indicated that metformin activated 5' adenosine monophosphate–activated protein kinase (AMPK)–dependent induction of Rab4, which subsequently plays a role in membrane uptake of Glut4 (32), and in this study, the NR4A1 ligands also activated AMPK and induced Rab4 protein expression. A direct comparison of the concentration- and time-dependent effects of DIM-C-pPhOH-3-Cl-5-OCH3 and metformin on this same set of responses was also determined (Fig. 4E and 4F), and we observed activation of AMPK by concentrations as low as 1 μM DIM-C-pPhOH-3-Cl-5-OCH3 and 1 mM metformin within 6 hours after treatment. Induction of Rab4 and NR4A1 was observed only after 12 to 24 hours of treatment. Previous studies show that activation of AMPK and induction of Rab4 by metformin are associated with Glut4 membrane localization and increased glucose uptake in C2C12 cells (32), and results in Fig. 4G show that metformin and the C-DIM/NR4A1 ligands induced glucose uptake into C2C12 cells. These results are consistent with the western blot data in Fig. 4A–4F showing induction of Glut4 protein. We also observed that DIM-C-pPhOH-3-Cl-5-OCH3 and metformin induced Glut1 expression in C2C12 cells (Fig. 4H), and these same compounds also induced sestrin 2 (Fig. 4I), which is an upstream activator of AMPK (33, 34). Induction of sestrin 2 by C-DIM/NR4A1 ligands in cancer cells was reactive oxygen species–dependent and reversed after cotreatment with glutathione (22–24), and similar results were observed in C2C12 cells (Fig. 4I). The C-DIM/NR4A1 compounds had minimal effects on insulin-induced glucose uptake in C2C12 cells (data not shown). We also observed induction of Glut1 protein by both metformin and DIM-C-pPhOH-3-Cl-5-OCH3 (data not shown). AICAR activates AMPK, and treatment of C2C12 cells with this compound induced expression of NR4A1, Glut4, PHKG1, and PFKM mRNA (Fig. 5A) and PYGM mRNA (Fig. 5B), demonstrating the importance of AMPK activation in mediating expression of these genes. Combinations of AICAR plus metformin or DIM-C-pPhOH-3-Cl-5-OCH3 further enhanced expression of these genes (Fig. 5A and 5B). AICAR and the same binary drug combinations also increased NR4A1, p-AMPK, and Rab4 protein expression (Fig. 5C), further confirming the role of activated MAPK in mediating the C-DIM/metformin-mediated responses.
Figure 4.
C-DIM/NR4A1 ligands and metformin induce glucose uptake and gene products associated with this response. C2C12 cells were treated with DMSO and (A) DIM-C-pPhOH, (B) DIM-C-pPhOH-3,5-Br2, (C) DIM-C-pPhOH-3-Cl, and (D) DIM-C-pPhOH-3-Cl-5-OCH3 for 24 hours, and whole-cell lysates were analyzed by western blots. C2C12 cells were treated with (E) DIM-C-pPhOH-3-Cl-5-OCH3 and (F) metformin for 6, 12, and 24 hours, and whole cell lysates were analyzed by western blots. (G) C2C12 cells were treated with C-DIM compounds and metformin for 24 hours, and glucose uptake in the cells was determined using the Glucose Uptake-Glo Assay Kit (Promega). Results are expressed as means ± SE for at least three replicated determinations, significant (P < 0.05) glucose uptake is indicated (*), and glucose uptake in control cells was 1.0. (H) DIM-C-pPhOH-3-Cl-5-OCH3 and metformin treatment also induced GLUT1 expression as determined by western blot. (I) C2C12 cells were treated with DIM-C-pPhOH-3-Cl-5-OCH3 and metformin alone or in combination with 5 mM glutathione (GSH) for 12 hours, and whole-cell lysates were analyzed by western blots for sestrin 2. Met, metformin.
Figure 5.
Role of AMPK in activation of GLUT4 and glycolytic genes. C2C12 cells were treated for 12 hours with AICAR alone (500 μM) or in combination with DIM-C-pPhOH-3-Cl-5-OCH3 or metformin, and effects on (A) NR4A1, GLUT4, PHKG1, and PFKM and (B) PYGM mRNA levels were determined by real-time PCR. Results are means ± SE (three replicates), and significant (P < 0.05) induction is indicated (*). (C) Cells were treated with AICAR, DIM-C-pPhOH-3-Cl-5-OCH3, and metformin (as described previously), and after 24 hours, whole cell lysates were analyzed in a western blot. Met, metformin.
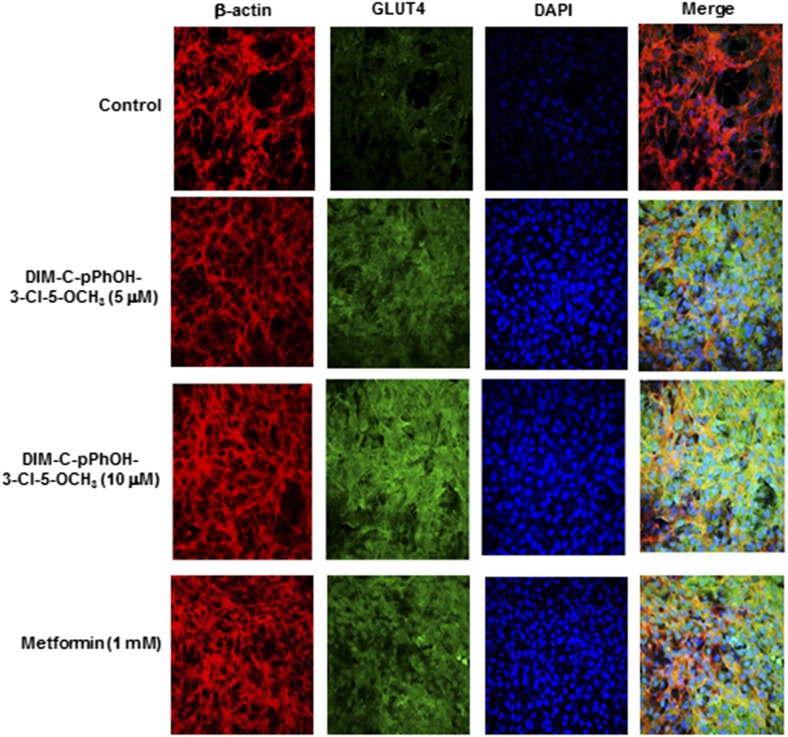
Results in Fig. 6 summarize confocal microscopic analysis of Glut4 expression in C2C12 cells treated with DIM-C-pPhOH-3-Cl-5-OCH3 (5 and 10 μM) and 1 mM metformin. Cells were fixed and immunostained with β-actin, Glut4, and 4′,6-diamidino-2-phenylindole (DAPI) and analyzed by confocal microscopy. The individual merged images show that both DIM-C-pPhOH-3-Cl-5-OCH3 and metformin induce Glut4 staining (green), and the merged images show that Glut4 is primarily extranuclear.
Figure 6.
Confocal microscopic analysis of Glut4 induction. C2C12 cells were treated with DMSO (control), 5 and 10 μM DIM-C-pPhOH-3-Cl-5-OCH3, or 1 mM metformin for 24 hours. Cells were then fixed, immunostained with β-actin, Glut4 antibodies, and DAPI, merged, and analyzed by confocal microscopy.
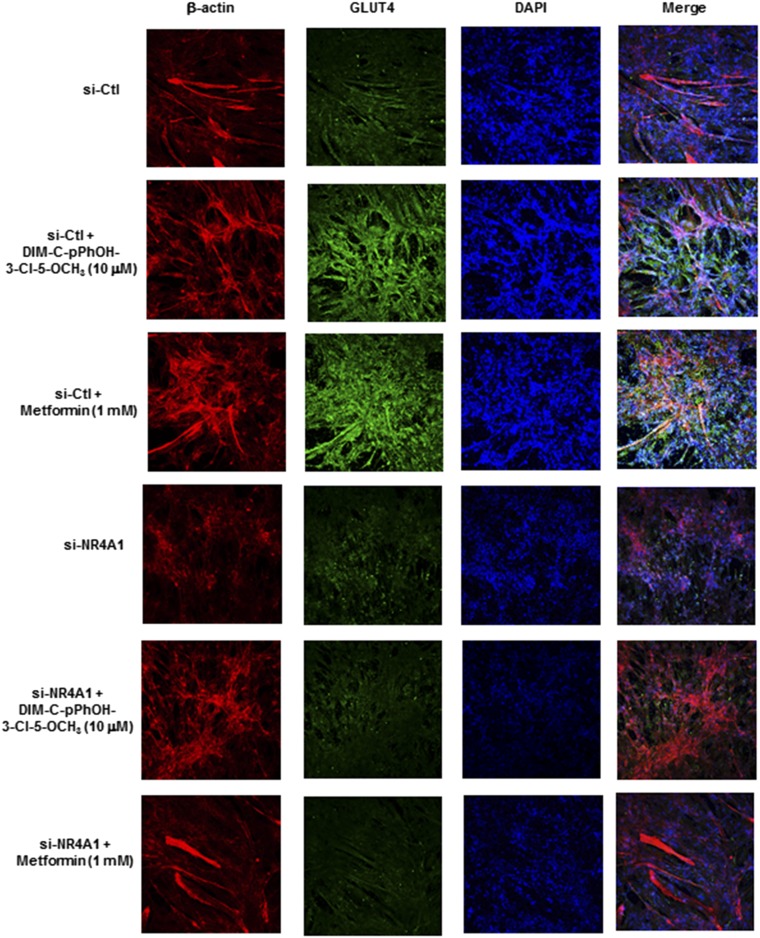
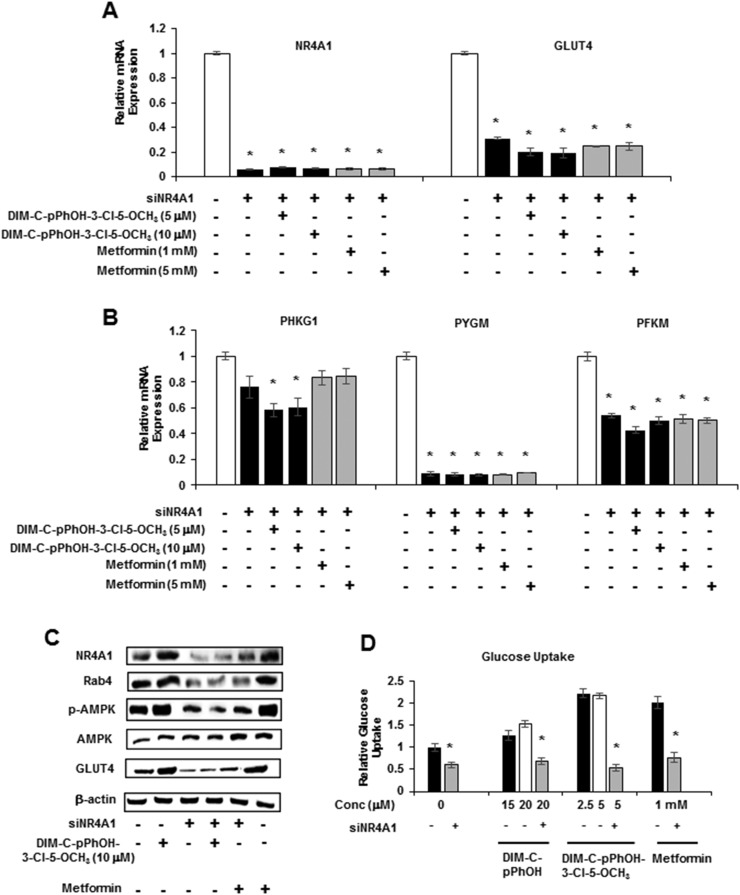
Results summarized in Figs. 1–6 demonstrate that, with the exception of some glycolytic genes, the C-DIM/NR4A1 ligands and metformin induced a similar spectrum of genes/responses, suggesting a possible common mechanism of action that involves NR4A1, and this was further investigated by RNA interference and knockdown of NR4A1 (siNR4A1). Immunostaining and confocal microscopy were also used to investigate the role of NR4A1 in mediating the induction of Glut4 by 10 μM DIM-C-pPhOH-3-Cl-5-OCH3 and 1 mM metformin (Fig. 7). Both compounds induced Glut4 expression, as evidenced by enhanced immunostaining (green), and there was colocalization with β-actin (extranuclear). Moreover, in cells transfected with siNR4A1 (knockdown) and treated with the compounds, there was a marked reduction in Glut4 immunostaining, suggesting that Glut4 induction by DIM-C-pPhOH-3-Cl-5-OCH3 and metformin was NR4A1-dependent. We also observed that, in C2C12 cells treated with 5 and 10 μM DIM-C-pPhOH-3-Cl-5-OCH3 or 1 and 5 mM metformin, the induction of Glut4 and NR4A1 mRNA and Phkg1, Pygm, and Pfkm (Figs. 3 and 4) was significantly decreased after knockdown of NR4A1 (Fig. 8A and 8B). Moreover, DIM-C-pPhOH-3-Cl-5-OCH3– and metformin-induced Glut4 and Rab4 proteins and activation of AMPK were also significantly decreased by NR4A1 knockdown (Fig. 8C), and we also observed that siNR4A1 decreased basal and DIM-C-pPhOH-3-Cl-5-OCH3–induced glucose uptake in C2C12 cells (Fig. 8D). These results demonstrate that the effects of C-DIM/NR4A1 ligands and metformin on glucose metabolism and uptake in C2C12 cells are NR4A1-dependent.
Figure 7.
Confocal microscopic analysis of Glut4 induction and effects of NR4A1 knockdown. C2C12 cells were transfected with si-Ctl (nonspecific oligonucleotide) or siNR4A1. After 72 hours, cells were treated with DMSO (control), 10 μM DIM-C-pPhOH-3-Cl-5-OCH3, or 1 mM metformin. Cells were then fixed, immunostained with β-actin, Glut4 antibodies, and DAPI, merged, and analyzed by confocal microscopy.
Figure 8.
NR4A1 knockdown attenuates the effects of DIM-C-pPhOH-3-Cl-5-OCH3 and metformin. C2C12 cells were transfected with si-Ctl (nonspecific oligonucleotide; white bar) or siNR4A1. After 72 hours, cells were treated with DIM-C-pPhOH-3-Cl-5-OCH3 or metformin, and effects on (A) NR4A1/Glut4 and (B) glycolytic gene expression were determined. (C) C2C12 cells were treated as described in (A), and whole cell lysates were analyzed by western blots. (D) Cells were treated as described in (A–C), and effects of siNR4A1 on compound-induced glucose uptake were determined (glucose uptake in control cells was set at 1.0). (A, B, D) Results are expressed as means ± SE for three replicate determinations. Significantly (P < 0.05) decreased activity due to NR4A1 knockdown is indicated (*). Conc, concentration.
Discussion
Diabetes is a highly progressive disease, and the current trend of increasing obesity coupled with a sedentary lifestyle has resulted in a parallel increase in the diagnosis of type II diabetes in many developed countries (35, 36). Several antidiabetic drugs have been developed for treating type II diabetes, and the biguanide metformin has been one of the most successful glucose-lowering agents for treating this disease. Even patients with type I diabetes benefit from taking this drug (37). Interestingly, individuals prescribed metformin exhibit decreased incidence of several cancers, and metformin exhibits both cancer chemopreventive and chemotherapeutic activities and is currently been evaluated for treating cancer patients after diagnosis (38–40). Metformin-mediated antidiabetic and anticarcinogenic activities are complex and involve multiple pathways including inhibition of mammalian target of rapamycin pathways via activation of AMPK, inhibition glucagon action, and targeting mitochondrial genes/pathways (37, 38).
The orphan NR NR4A1 and synthetic NR4A1 ligands also modulate serum glucose levels and key gluconeogenesis and glycolytic genes in the liver and muscle, respectively, in rodent models of obesity (14–16, 31). Previous studies in this laboratory have identified C-DIMs as synthetic NR4A1 ligands for the NR (20), and in solid tumor–derived cell lines, the C-DIM/NR4A1 ligands antagonize or inactivate NR4A1-dependent pro-oncogenic activities. This study has focused on the potential effects of C-DIMs on glucose metabolism and uptake using C2C12 muscle cells as an in vitro model. This cell line is optimal for these studies because overexpression of NR4A1 in C2C12 cells induces Glut4 and glycolytic gene expression, and this is accompanied by increased glucose uptake (31). Moreover, other genes/pathways including NR4A1-dependent lipolysis, oxidative metabolism, and mitochondrial activity have also been characterized in C2C12 cells (15, 31, 41). Metformin also exhibits antidiabetic activity in C2C12 cells, including induction of Glut4 and enhanced Glut4 membrane localization and glucose uptake (35), and we therefore compared the effects of metformin to those observed for the NR4A1 ligand DIM-C-pPhOH (20) and three more potent buttressed analogs (30).
Initial studies confirmed that NR4A1 overexpression induced Glut4 and several glycolytic genes, whereas DIM-C-pPhOH primarily induced only Phkg1, Pgam2, and Pygm in C2C12 cells (Fig. 1). The three buttressed DIM-C-pPhOH (4-hydroxyphenyl) analogs DIM-C-pPhOH-3-Cl, DIM-C-pPhOH-3-Cl-5-OCH3, and DIM-C-pPhOH-3,5-Br2 contain one (3-), two, and two (3,5-) substituents, respectively, adjacent to the 4-hydroxyphenyl moiety and were more potent inducers of Glut4 and glycolytic genes than was DIM-C-pPhOH. These results are consistent with their potency in cancer cells in terms of functional activity (inhibition of tumor growth) and effects on expression of NR4A1-regulated genes (30). Interestingly, there was considerable overlap between the C-DIM/NR4A1 ligands and metformin with respect to their induction of gene expression. For example, DIM-C-pPhOH-3-Cl-5-OCH3 and metformin induced NR4A1, Glut4, Phgk1, Pgam2, Pygm, Pfkm, and Gpd1, and only two genes (Eno3 and Aldo1) induced by metformin were not activated by the C-DIM compounds. DIM-C-pPhOH and the 3-chloro- and 3,5-dibromo analogs did not induce all metformin-activated genes (e.g., Aldo1, Eno3, and Gpd1), but, nevertheless, there was considerable overlap in the induction of glycolytic gene expression by metformin and the C-DIM/NR4A1 ligands. DIM-C-pPhOH binds NR4A1 and acts as an NR4A1 antagonist/inverse agonist in cancer cell lines, thereby inhibiting the pro-oncogenic function of NR4A1 in solid tumors. In contrast, NR4A1 plays a role in regulating expression of glucose-metabolizing genes and glucose uptake in C2C12 cells, and DIM-C-pPhOH and related buttressed analogs act as agonists to enhance NR4A1-dependent responses. The tissue- and response-specific agonist or antagonist activity of DIM-C-pPhOH has previously been observed for ligands of other NRs, and this is typified by tamoxifen, an estrogen receptor ligand. Like DIM-C-pPhOH, tamoxifen is both an estrogen receptor antagonist (breast tumor) and agonist (uterus) (42), and the selective receptor modulator activity of DIM-C-pPhOH and tamoxifen is due to several factors, including tissue-specific differences in expression of cofactors and epigenetic-related differences in chromatin structure (43, 44). We also observed induction of NR4A1 and Glut4 mRNA expression by both metformin and the C-DIM/NR4A1 ligands in C2C12 cells, and in an in vivo pilot study, we confirmed that DIM-C-pPhOH-3-Cl-5-OCH3 (25 mg/kg/d) induced Glut4 mRNA in muscle from mice maintained on an HFD (Fig. 3F).
Previous studies showed that metformin induced expression of Glut4 protein, activated AMPK and Rab4, which was important for Glut4 membrane translocation, and increased glucose uptake in C2C12 muscle cells (32). Results in Figs. 3, 4, and 6 confirm that metformin induces NR4A1, Glut4, and Rab4, and this is associated with increased Glut4 in the cell membrane and increased glucose uptake into the cells. Similar results were observed for the C-DIM/NR4A1 ligands, suggesting elements of a common mechanism of action for metformin and C-DIM/NR4A1 ligands.
Because overexpression of NR4A1 induced glycolytic genes and Glut4 and glucose uptake in C2C12 cells (31), it was not unexpected that knockdown of NR4A1 (siNR4A1) inhibited these responses that were also induced by the C-DIM/NR4A1 ligands. However, knockdown of NR4A1 also inhibited these same metformin-induced responses, and induction of Rab4 and AMPK activation by metformin and C-DIMs were also inhibited after NR4A1 knockdown. Not surprisingly, the C-DIM/NR4A1 ligands also induced Glut4 and Glut4-dependent glucose uptake in C2C12 cells (Fig. 4), and these responses were also inhibited after NR4A1 knockdown (Figs. 7 and 8). These results indicate that NR4A1 mediated the activity not only of C-DIMs, but also metformin in enhancing glucose metabolism and uptake in C2C12 cells. It has been reported that activation of AMPKα was critical for the antidiabetic activity of metformin (32), and because both C-DIMs and metformin activate AMPK (Fig. 4), we hypothesized that AMPKα activation was a critical upstream regulator of induced NR4A1 expression. This was supported by a time course study showing that metformin and DIM-C-pPhOH-3-Cl-5-OCH3 induced activation of AMPK prior to induction of NR4A1 and other factors required for enhanced glucose uptake in C2C12 cells (Fig. 4). These results were also consistent with the effects of AICAR, an AMPK activator (Fig. 5). The mechanism of C-DIMs and metformin as activators of AMPK is unclear because we observed inconsistent results on their effects on mitochondrial respiration. Our results show that C-DIM/NR4A1 ligands activate AMPK, glucose metabolism genes, and glucose uptake in C2C12 cells, and these responses are NR4A1-dependent as evidenced by NR4A1 knockdown (Figs. 7 and 8). Previous studies show that C-DIM/NR4A1 ligands induce reactive oxygen species–dependent induction of sestrin 2, which activates AMPK (22–24). We have also observed similar results in C2C12 cells where DIM-C-pPhOH-3-Cl-5-OCH3 and metformin induce sestrin 2, and this response is blocked by glutathione (Fig. 4I). These results are consistent with a ligand-induced NR4A1 → sestrin 2 → AMPK pathway for induction of glucose metabolism genes and glucose uptake in C2C12 cells. However, because these same responses are also induced by direct activation of AMPK by AICAR, it is possible that NR4A1-AMPK interactions may be more complex and involve feedback pathways, and these are currently being investigated. Thus, we demonstrate that C-DIM/NR4A1 ligands exhibit antidiabetic activity in C2C12 cells and also show that NR4A1 plays a critical role in the activity of C-DIMs and metformin in this cell line. The mechanisms associated with the antidiabetic activity of C-DIMs and metformin and the role of NR4A1 in mediating these responses in various cells/tissue associated with metabolic disease are currently being further investigated in both in vitro and in vivo models of metabolic disease.
Acknowledgments
We thank Fan Yang-Yi and the laboratory of Robert Chapkin for assistance.
Financial Support: This work was supported by the National Institute of Environmental Health Sciences (P30-ES023512; to S.S.), Texas AgriLife Research (to S.S.), a Sid Kyle Chair endowment (to S.S.), and a sponsored research agreement with Eli Lilly.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AICAR
5-amino-4-carboxamide ribonucleotide
- Aldo1
aldolase-1
- AMPK
5' adenosine monophosphate–activated protein kinase
- C-DIM
1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methane
- ChIP
chromatin immunoprecipitation
- DAPI
4′,6-diamidino-2-phenylindole
- DIM-C-pPhOH
1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl)methane
- DMSO
dimethyl sulfoxide
- Eno3
enolase-3
- Gpd1
glycerol-3-phosphate dehydrogenase 1
- HFD
high-fat diet
- mRNA
messenger RNA
- NBRE
nerve growth factor–induced β response element
- NR
nuclear receptor
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- Pfkm
muscle phosphofructokinase
- Pgam2
phosphoglycerate mutase 2
- Phkg1
phosphorylase kinase γ1
- Pygm
muscle glycogen phosphorylase
- SE
standard error
References
- 1. Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol. 2005;19(6):1429–1438. [DOI] [PubMed] [Google Scholar]
- 2. Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277(36):33001–33011. [DOI] [PubMed] [Google Scholar]
- 3. Giguère V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20(5):689–725. [DOI] [PubMed] [Google Scholar]
- 4. Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278(27):24776–24790. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423(6939):555–560. [DOI] [PubMed] [Google Scholar]
- 6. Wilson TE, Day ML, Pexton T, Padgett KA, Johnston M, Milbrandt J. In vivo mutational analysis of the NGFI-A zinc fingers. J Biol Chem. 1992;267(6):3718–3724. [PubMed] [Google Scholar]
- 7. Wilson TE, Padgett KA, Johnston M, Milbrandt J. A genetic method for defining DNA-binding domains: application to the nuclear receptor NGFI-B. Proc Natl Acad Sci USA. 1993;90(19):9186–9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252(5010):1296–1300. [DOI] [PubMed] [Google Scholar]
- 9. Paulsen RF, Granas K, Johnsen H, Rolseth V, Sterri S. Three related brain nuclear receptors, NGFI-B, Nurr1, and NOR-1, as transcriptional activators. J Mol Neurosci. 1995;6(4):249–255. [DOI] [PubMed] [Google Scholar]
- 10. Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9(7):769–782. [DOI] [PubMed] [Google Scholar]
- 11. Zetterström RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10(12):1656–1666. [DOI] [PubMed] [Google Scholar]
- 12. Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearen MA, Muscat GE. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol. 2010;24(10):1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12(9):1048–1055. [DOI] [PubMed] [Google Scholar]
- 15. Chao LC, Wroblewski K, Zhang Z, Pei L, Vergnes L, Ilkayeva OR, Ding SY, Reue K, Watt MJ, Newgard CB, Pilch PF, Hevener AL, Tontonoz P. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58(12):2788–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhan YY, Chen Y, Zhang Q, Zhuang JJ, Tian M, Chen HZ, Zhang LR, Zhang HK, He JP, Wang WJ, Wu R, Wang Y, Shi C, Yang K, Li AZ, Xin YZ, Li TY, Yang JY, Zheng ZH, Yu CD, Lin SC, Chang C, Huang PQ, Lin T, Wu Q. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol. 2012;8(11):897–904. [DOI] [PubMed] [Google Scholar]
- 17. Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, Li G, Xu Q, Zhang M, Weimer BC, Chen D, Cheng Z, Zhang L, Li Q, Li S, Zheng Z, Song S, Huang Y, Ye Z, Su W, Lin SC, Shen Y, Wu Q. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. 2008;4(9):548–556. [DOI] [PubMed] [Google Scholar]
- 18. Liu JJ, Zeng HN, Zhang LR, Zhan YY, Chen Y, Wang Y, Wang J, Xiang SH, Liu WJ, Wang WJ, Chen HZ, Shen YM, Su WJ, Huang PQ, Zhang HK, Wu Q. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res. 2010;70(9):3628–3637. [DOI] [PubMed] [Google Scholar]
- 19. Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, Wang H, Safe S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70(17):6824–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, Li L, Zhang Y, Wu Q, Safe S. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol Endocrinol. 2014;28(10):1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr Relat Cancer. 2015;22(5):831–840. [DOI] [PubMed] [Google Scholar]
- 23. Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, Abudayyeh A. Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma. PLoS One. 2015;10(6):e0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget. 2016;7(21):31257–31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A expression in rhabdomyosarcoma is driven by the targetable nuclear receptor NR4A1. Cancer Res. 2017;77(3):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hedrick E, Li X, Safe S. Penfluridol represses integrin expression in breast cancer through induction of reactive oxygen species and downregulation of Sp transcription factors. Mol Cancer Ther. 2017;16(1):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hedrick E, Safe S. Transforming growth factor β/NR4A1-inducible breast cancer cell migration and epithelial-to-mesenchymal transition is p38α (mitogen-activated protein kinase 14) dependent. Mol Cell Biol. 2017;37(18):e00306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Mol Cell Biol. 2016;36(9):1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Miranda BR, Miller JA, Hansen RJ, Lunghofer PJ, Safe S, Gustafson DL, Colagiovanni D, Tjalkens RB. Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;345(1):125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lacey A. NR4A1 Antagonists Target the Oncogenic PAX3-FOXO1A Gene and Induce Interleukin-24 in Rhabdomyosarcoma Cells [dissertation]. College Station, TX: Texas A&M University; 2017.
- 31. Chao LC, Zhang Z, Pei L, Saito T, Tontonoz P, Pilch PF. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol. 2007;21(9):2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JO, Lee SK, Jung JH, Kim JH, You GY, Kim SJ, Park SH, Uhm KO, Kim HS. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. J Cell Physiol. 2011;226(4):974–981. [DOI] [PubMed] [Google Scholar]
- 33. Parmigiani A, Budanov AV. Sensing the environment through sestrins: implications for cellular metabolism. Int Rev Cell Mol Biol. 2016;327:1–42. [DOI] [PubMed] [Google Scholar]
- 34. Wang M, Xu Y, Liu J, Ye J, Yuan W, Jiang H, Wang Z, Jiang H, Wan J. Recent insights into the biological functions of sestrins in health and disease. Cell Physiol Biochem. 2017;43(5):1731–1741. [DOI] [PubMed] [Google Scholar]
- 35. Ingelfinger JR, Jarcho JA. Increase in the incidence of diabetes and its implications. N Engl J Med. 2017;376(15):1473–1474. [DOI] [PubMed] [Google Scholar]
- 36. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhaw-Luximon A, Jhurry D. Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification. J Cancer Res Clin Oncol. 2016;142(10):2159–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–847. [DOI] [PubMed] [Google Scholar]
- 41. Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem. 2005;280(13):12573–12584. [DOI] [PubMed] [Google Scholar]
- 42. Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. 2007;99(5):350–356. [DOI] [PubMed] [Google Scholar]
- 43. Wittmann BM, Sherk A, McDonnell DP. Definition of functionally important mechanistic differences among selective estrogen receptor down-regulators. Cancer Res. 2007;67(19):9549–9560. [DOI] [PubMed] [Google Scholar]
- 44. Johnson AB, O’Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]