Abstract
Osseous reconstruction of large bone defects remains a challenge in oral and maxillofacial surgery. In addition to autogenous bone grafts, which despite potential donor-site mobility still represent the gold standard in reconstructive surgery, many studies have investigated less invasive alternatives such as in vitro cultivation techniques. This study compared different types of seeding techniques on pure β-tricalcium phosphate scaffolds in terms of bone formation and ceramic resorption in vivo. Cylindrical scaffolds loaded with autologous cancellous bone, venous blood, bone marrow aspirate concentrate or extracorporeal in vitro cultivated bone marrow stromal cells were cultured in sheep on a perforator vessel of the musculus latissimus dorsi over a 6-month period. Histological and histomorphometric analyses revealed that scaffolds loaded with cancellous bone were superior at promoting heterotopic bone formation and ceramic degradation, with autogenous bone and bone marrow aspirate concentrate inducing in vivo formation of vital bone tissue. These results confirm that autologous bone constitutes the preferred source of osteoinductive and osteogenic material that can reliably induce heterotopic bone formation in vivo.
Keywords: heterotopic bone formation, β-tricalcium phosphate, bone marrow stromal cells, bone marrow aspirate concentrate, prevascularization, ceramic degradation
Introduction
Autogenous bone grafts remain the gold standard in oro-maxillo-facial surgery for reconstruction of large segmental bone defects [1, 2]. In cases where infection or radiation has compromised vascularization at the defect site, microsurgical bone transfer is typically performed to increase the probability of undisturbed osseointegration [3–7]. However, harvesting autogenous bone grafts can lead to donor-site morbidity, with the risk increasing according to the amount of harvested bone [8–10]. To overcome these limitations, different allogenic and xenogenic bone replacement materials have been developed for and applied to clinical use [11, 12]. However, the reconstruction of large bone defects by allo- and xenografts, which would be most beneficial for the patient, is associated with a high complication rate [13]. Therefore, alternative methods with lower donor-site morbidity and complication rates are needed.
The prefabrication of bioartificial autogenous bone grafts using tissue engineering techniques constitutes a promising strategy for generating vital bone without the aforementioned risks [14–17]. There are three key elements to bioartificial bone formation: an adequate 3D matrix with good osteoconductive, and if possible, osteoinductive properties; a large number of osteogenic cells and a sufficient supply of oxygen and nutrition. Our preliminary experiments showed that β-tricalcium phosphate (β-TCP) is an ideal matrix material for adequate seeding [18, 19]. However, larger and more complex scaffolds require a sufficient blood supply to provide nutrition to osteogenic cells [20–23]. We previously demonstrated that extrinsic or intrinsic in vivo prevascularization reliably induces the formation of vital heterotopic bone, and confirmed that autogenous bone marrow (BM) comprises a reliable source of osteogenic cells and growth factors that can be readily harvested [21, 24].
However, it is unknown whether morbidity can be further reduced by using different osteogenic cells, or whether the osteoinduction capacity of β-TCP is sufficiently high to generate bone without the need for supporting cells and growth factors. To address these issues, in this study, we utilized our established experimental model in which sheep are implanted with β-TCP scaffolds loaded with different seeding materials including cancellous bone, venous blood, BM aspirate concentrate (BMAC) or extracorporeal in vitro-cultured BM stromal cells (BMSCs). We then compared the effects of different β-TCP scaffold seeding techniques on bone formation and ceramic degradation, focusing on whether the use of an in vitro tissue engineering technique such as extracorporeal cultivation of BMSCs constitutes a reliable alternative to using autogenous cancellous bone.
Materials and methods
Animal experiments were carried out as described in our previous report [24]. Sterile cylinders (length × diameter = 25 × 14 mm) with a preformed central tunnel 7 mm in diameter consisting of pure β-TCP were used as carriers (chronOS; DePuy Synthes, USA). The protocol for animal experiments received ethical approval in accordance with German federal animal welfare legislation. Specifically, ethical approval for the experimental study in sheep was provided by the Department for Veterinary Affairs, Oldenburg, Germany (AZ: 08/1621). A total of 24 cylinders were randomly implanted into 18 healthy adult female German blackheaded sheep.
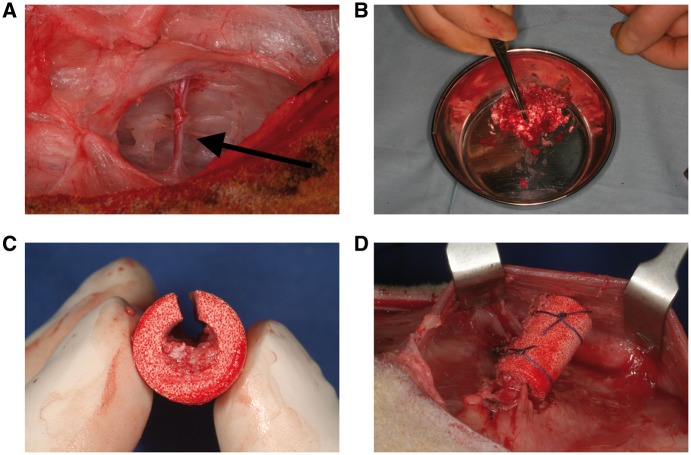
In Group A (n = 6), three bone biopsies (5 mm in diameter) were harvested from the iliac crest and morselized using an electric bone mill. The morselized bone was mixed with amorphous BMAC obtained from deep donor sites and loaded into the central cavities of predrilled blood-soaked cylinders, which were then implanted underneath the musculus latissimus dorsi on an arterio-venous vessel bundle serving as a central perfusion line, such that the whole animal acted as a natural bioreactor (Fig. 1a–d).
Figure 1.
Surgical procedure employed in the study. (a) Exposed bottom side of the musculus latissimus dorsi showing the arterio-venous vessel bundle (arrow). (b) Morselized bone from the iliac crest. (c) Sliced cylinder from Group A partly filled with bone. (d) Cylinder implanted on the vessel bundle and fixed with sutures
In Group B (n = 6), cylinders were soaked with venous blood collected from the vena jugularis using the chronOS kit and then implanted as described (Fig. 2). In Group C (n = 6), BMAC was obtained from the left iliac crest using the MarrowStim BM aspiration and concentration system (Biomet Biologics, USA). The aspiration needle was gently inserted into the BM and flushed with anticoagulants (Liquemin; Hoffmann-La Roche AG, Germany), followed by aspiration of BM into an attached syringe. The BM was transferred to a chamber that was centrifuged at 3200 rpm for 15 min (Biomet Biologics), which separated the aspirate into three clearly visible layers; from top to bottom, these comprised the acellular plasma, nucleated cells and red blood cells. The plasma was removed and the cellular fractions were resuspended (Fig. 3a–d). The concentrated BMAC was loaded onto prepared cylinders.
Figure 2.
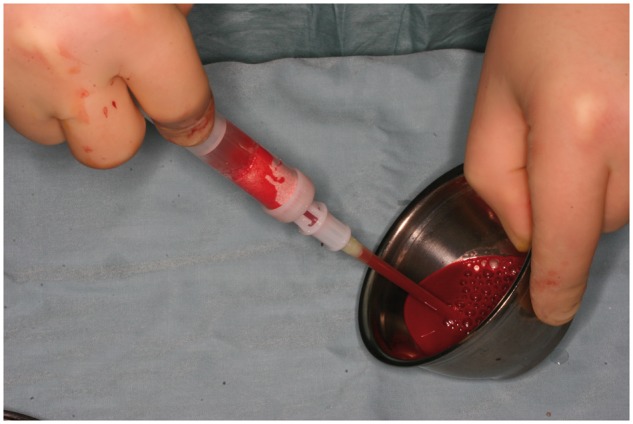
Cylinder from Group B soaked with venous blood using the chronOS kit
Figure 3.
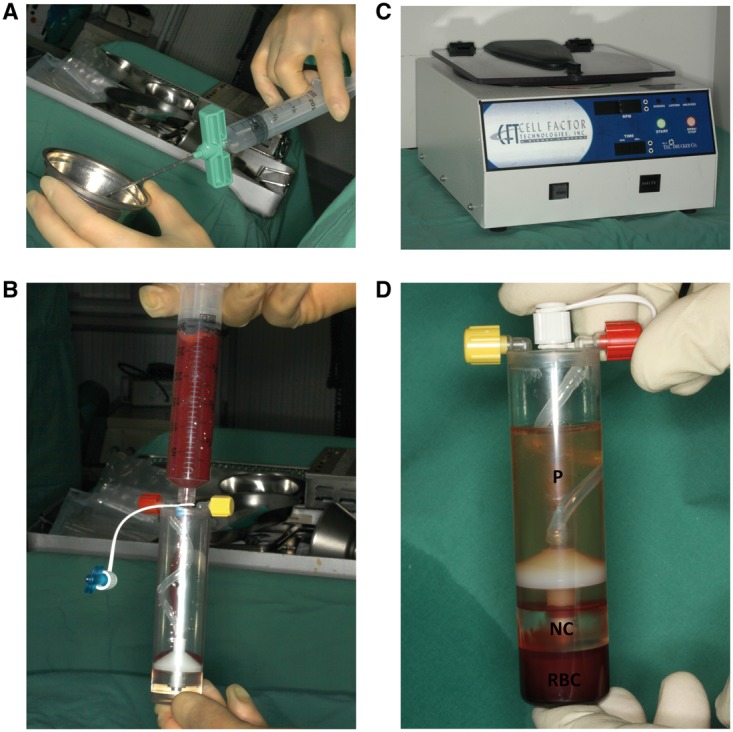
Preparation of BMAC. (a) Rinsing the aspiration needle with anticoagulant solution. (b) Separation chamber filled with BMAC. (c) Centrifuge used in the experiment. (d) Separated BMAC (NC, nucleated cell; P, plasma; RBC, red blood cell)
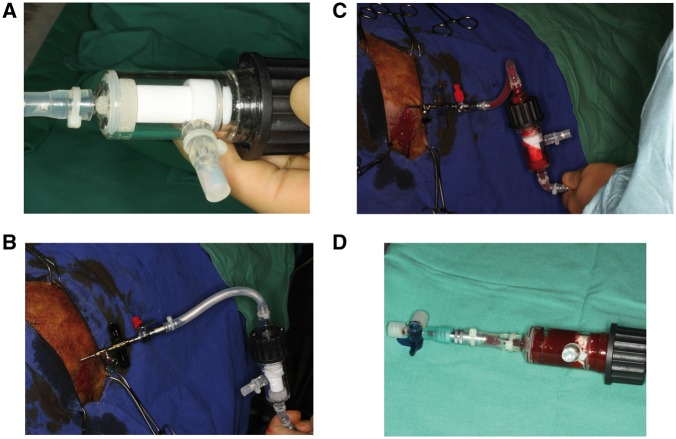
In Group D (n = 6), BMSCs from the left iliac crest were aspirated through a cylinder within a custom-made bioreactor chamber. Briefly, a stab incision was made at the most prominent aspect of the iliac crest and a Yamcidi aspiration needle was inserted into the center of the marrow cavity to a depth of ∼5 cm. The marrow was aspirated directly through a silicone tube into the porous scaffold, a technique that has been previously used for bone tissue engineering [25]. The culture chamber contained the porous β-TCP scaffold along with an inflow and two outflow valves. For cell seeding, one outflow valve was closed and another was opened to allow the aspirate to disperse through the scaffold pores. The bioreactor was filled with saline containing heparin; the same solution was used to irrigate the aspiration syringe before use (Fig. 4a–d). After cell loading, the bioreactor was filled with an angiogenic medium as described later and incubated under continuous perfusion for 3 weeks (Fig. 5).
Figure 4.
Seeding of BMSCs (a) cylinder from Group D inside the bioreactor. (b) Insertion of the Yamcidi aspiration needle into the iliac crest. (c) Aspiration of BM through the bioreactor. (d) Bioreactor containing the soaked cylinder
Figure 5.
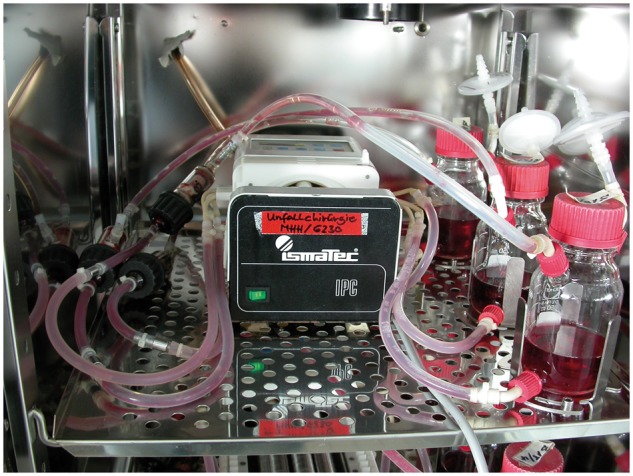
Bioreactor with the continuous flow perfusion system inside the incubator
During incubation, the bioreactor was rotated at 5° min−1 for 24 h. The scaffold was perfused with 500 ml of angiogenic medium composed of Dulbecco’s modified Eagle’s medium/Ham’s F-12 (Biochrom, Germany), 10% fetal calf serum (Gibco, Germany), 100 U penicillin, 0.2 mg/ml streptomycin (Biochrom), 0.5 µg/ml amphotericin B (Biochrom), 5 µg/ml ascorbic acid (Sigma-Aldrich Chemie GmbH, Germany), 0.02 nM/ml dexamethasone (Merck, Germany) and 30 ng/ml of the Toll-like receptor-2/6 agonist macrophage-activating lipopeptide (MALP)-2 (Axxora GmbH, Germany) to induce angiogenesis. The bioreactor was incubated at 37 °C and culture was performed with a medium flow of 2 ml min−1 at 5% CO2. Every third day, 50 ml of culture medium was replaced with fresh medium. After the culture period ended, the bioreactor was disconnected and transferred to the operating room at 5 °C, and the cylinders were then implanted in the described manner.
At 6 months after the operation, animals were euthanized after deep sedation (1 mg/kg midazolam by intramuscular injection and 5 mg/kg propofol with 80 mg/kg pentobarbital by intravenous injection). Cylinders were removed from the latissimus dorsi muscle and fixed in 3.5% neutral buffered formalin, embedded in methylmethacrylate and sectioned perpendicular to the axis of the cylinder using a modified inner-hole diamond saw. Non-decalcified sections with a thickness of 30 mm were surface-stained with Alizarin-methylene blue for standard light microscopy and histomorphometric analyses. Digital images of each section were acquired using a Zeiss Axio Imager microscope fitted with an AxioCam MRc digital camera and AxioVision v.4.5 software (Carl Zeiss, Germany). The AxioVision module MosaiX was used to generate images of whole cylinder cross sections. Total bone and residual ceramic areas were quantified using analy-SIS v.3.0 image analysis software (Olympus Soft Imaging Solutions, Germany). A total of six sections distributed evenly throughout each cylinder were used for histomorphometric evaluations.
Results
All animals survived surgery without any complications and were capable of ingestion and rumination immediately afterward. The site of implantation was examined daily and showed no signs of infection or rejection until animals were sacrificed. There was no postoperative swelling of the thoracic wall and no indication of compromised mobility.
Explanted constructs in Group A had a hard consistency with no flexibility. In all cylinders, the central vessel bundle was easily detectable after separation of the two halves (Fig. 6). There was bleeding throughout the surface, providing evidence for tissue regeneration. Bone formation was observed inside the cylinders by microscopy. A substantial portion of the β-TCP scaffolds was degraded and replaced with osseous tissue. Osteogenic activity was evidenced by the presence of osteoblasts and osteoids. Bony infiltration of β-TCP with conversion to bone was observed throughout the implant. Well-vascularized soft fibrous tissue had invaded the remaining parts of the matrix material (Figs 7 and 8).
Figure 6.
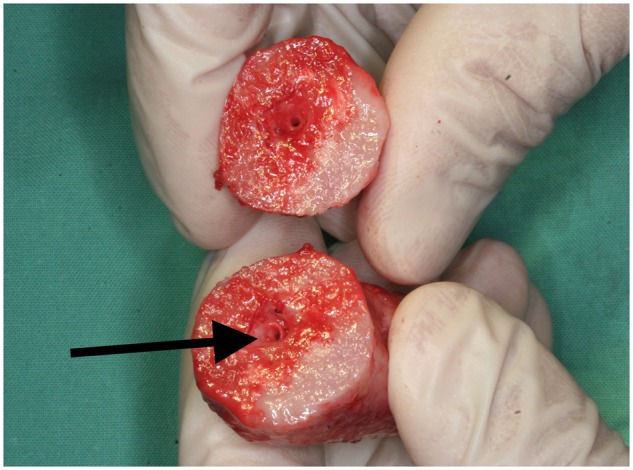
Sliced cylinder from Group A after explantation showing the intact blood vessel bundle (arrow) and vital bone
Figure 7.
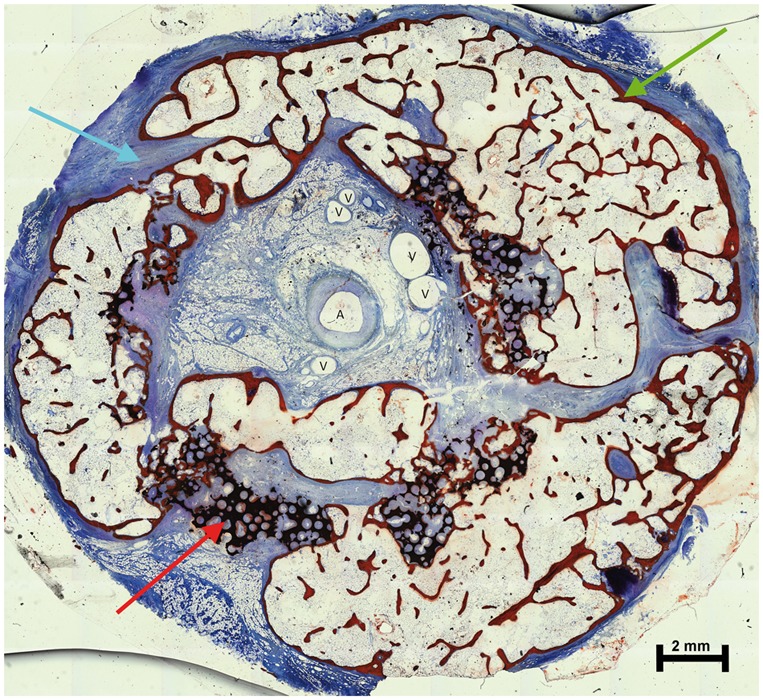
Cross section of a cylinder from Group A, surface-stained with Alizarin-methylene blue (red arrow, residual ceramic material; blue arrow, fibrous soft tissue; green arrow, bone; A, artery and V, vein)
Figure 8.
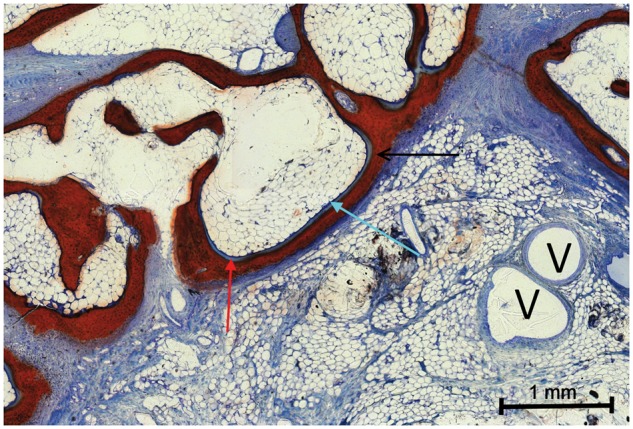
Magnification of Fig. 7 (red arrow, osteoid; blue arrow, osteoblast; black arrow, osteocytes with mature bone and V, vein)
Only five cylinders were recovered from Group D. Examination by palpation revealed that the cylinders from Groups B–D were not rigid but instead exhibited 3D flexibility. After separation, the implanted vascular bundle was detectable in the soft fibrous tissue. A significant amount of residual ceramic material was macroscopically observed, suggesting that the cylinders had been crushed rather than degraded. Only a small amount of bone was observed in Group C by microscopy, whereas Groups B and D showed no signs of newly formed bone, consistent with the lack of stability of the implant (Fig. 9).
Figure 9.
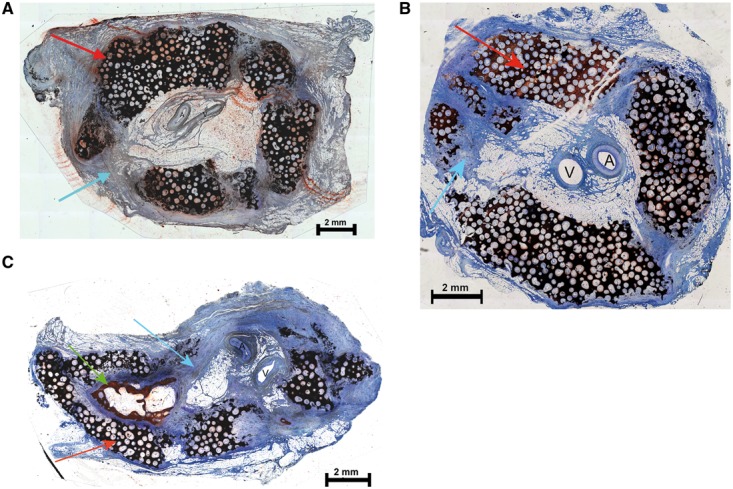
Histological analysis of cylindrical scaffolds. Cross section of a representative cylinder from Group B (a), Group C (b) and Group D (c). All sections were surface-stained with Alizarin-methylene blue (red arrow, residual ceramic material; blue arrow, fibrous soft tissue; green arrow, bone; A, artery and V, vein)
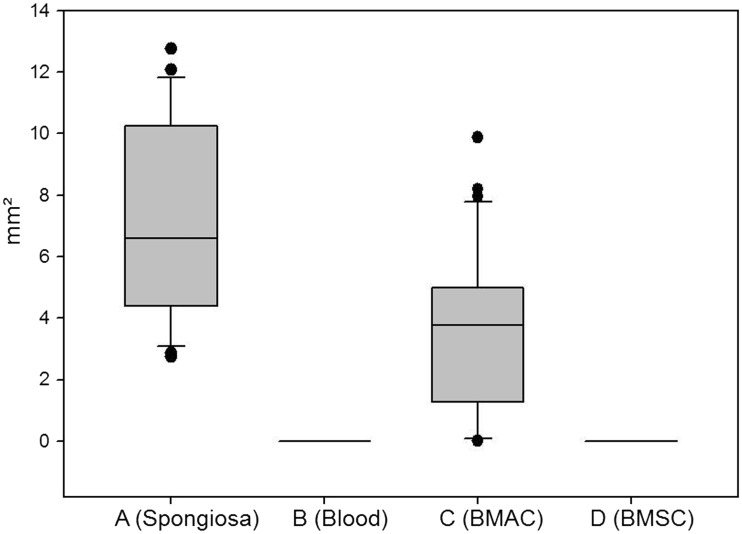
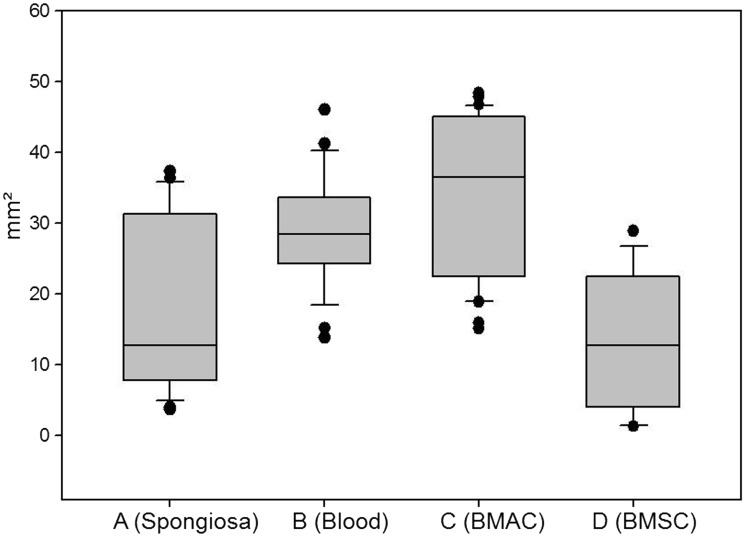
More bone formation was observed in Group A than in the other groups (Figs 10 and 11; Table 1). It was difficult to distinguish the effects of degradation and crushing; however, the cylinders from Group A appeared more degraded than those from the other groups (Fig. 12 and Table 2).
Figure 10.
Bone area (mm2) in Groups A–D. For A–C, n = 6; for D, n = 5
Table 1.
Area of bonea
| Group | Mean | SD | P |
|---|---|---|---|
| mm² | mm² | ||
| A (Spongiosa) | 7.03 | 3.22 | |
| B (Blood) | 0 | 0 | <0.001 |
| C (BMAC) | 3.57 | 2.50 | <0.001 |
| D (BMSC) | 0 | 0 | <0.001 |
aT-test of Groups B–D versus Group A. P < 0.001 indicates significantly more bone formation in Group A compared with the indicated group.
Figure 12.
Area of residual ceramic (mm2) in Groups A–D. For A–C, n = 6; for D, n = 5
Table 2.
Area of residual ceramica
| Group | Median | 25% | 75% | P |
|---|---|---|---|---|
| mm² | mm² | mm² | ||
| A (Spongiosa) | 12.77 | 7.83 | 30.21 | |
| B (Blood) | 28.47 | 24.51 | 33.31 | 0.003 |
| C (BMAC) | 36.51 | 22.9 | 45.06 | <0.001 |
| D (BMSC) | 12.77 | 4.13 | 21.72 | 0.16 |
aP, Rank sum test of Groups B–D versus Group A.
Figure 11.
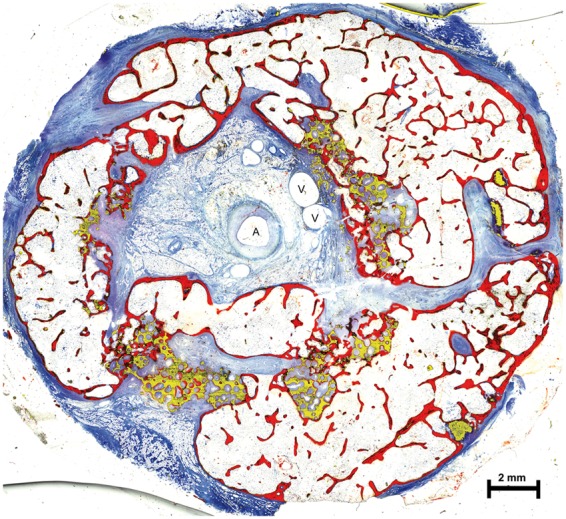
Exemplary cross section of the histomorphometric analysis of a cylinder from Group A (bone colored in red, residual ceramic material colored in yellow; A, artery and V, vein)
Discussion
BMAC is widely used in modern regenerative medicine, such as for spinal fusion in elderly patients in combination with allogeneic bone chips and demineralized bone matrix, which achieved a very good solid fusion rate of 83.9% [26, 27]. BMAC has also been used in combination with β-TCP; e.g. tibia defects in rabbits were reconstructed with β-TCP loaded with platelet-rich plasma (PRP) or BMAC, with both methods showing bony consolidation of the defect, although this was greater in the PRP group [28, 29]. A combination of BMAC, PRP and calcium phosphate granules was used to reconstruct critical-size metaphyseal defects in the proximal tibia of miniature pigs and was found to be a reasonable alternative to autologous bone grafting [30]. In another study, bone defects were treated with a combination of BMAC and hydroxyapatite or collagen, which promoted full healing after 6 months [31]. Moreover, patients with lower jaw continuous defects after benign tumor resection in whom the missing bone was replaced by a combination of allogeneic bone, bone morphogenetic protein and BMAC showed bone regeneration of a sufficiently high quality to accommodate dental implants after 12 months [32].
In this study, we found that BMAC comprises an effective osteogenic and osteoinductive material that promotes heterotopic bone formation in combination with β-TCP. However, autologous cancellous bone from the iliac crest was still superior, as significantly more new bone was formed that exhibited bone-like behavior than had been observed with BMAC [33]. In particular, cancellous bone or BM constitutes a well-known donor of osteoinductive cells as BMSCs or mesenchymal stem cells exhibit a considerable ability to regenerate bone tissue [34, 35]. Cancellous bone or marrow also contains other osteoprogenitor cells and valuable bone matrices that induce ectopic bone formation [36]. Cancellous or autologous bone is therefore widely used in bone tissue engineering procedures to vitalize different matrix materials. BMSCs have also been used to vitalize scaffolds for bone tissue engineering and induced the formation of new bone when used in conjunction with various matrix materials and growth factors [37–44]. For example, a calcium phosphate matrix with BMSCs induced subcutaneous bone formation in mice, with similar heterotopic bone formation being demonstrated in a subcutaneous pocket in sheep using hydroxyapatite as the matrix material [45, 46]. Furthermore, continuous flow perfusion has been reported to have positive effects on osteoblasts, such as stimulation of osteoblast maturation and deposition of the mineralized matrix [47].
MALP-2 promotes early implant vascularization, which plays a critical role in bone growth and repair [44, 48–50]. In this study, we attempted to induce early vascularization in vivo to enhance the survival of BMSCs loaded onto the β-TCP scaffold; however, seeded BMSCs did not stimulate new bone formation. This may be because the number of seeded cells was too low or because our measurement method was not sufficiently sensitive to detect small numbers of osteoblasts and osteocytes present within or on the surface of the β-TCP cylinders. Ultimately, the constructs were not stable after 6 months and were therefore deemed ineffective. Calcium phosphate-based ceramics are known for their osteoinductive properties, although the type of ceramic, its micro and macro structures, and the animal model that is used influence the extent of heterotopic bone formation [51–55]. In this study, we used chronOS as a matrix material. This consists of highly purified β-TCP with interconnected macro and micro pores, which would presumably induce heterotopic bone formation without the need for additional osteoinductive materials. Conversely, in our experimental model, there was no heterotopic bone formation induced by blood-soaked cylinders. This phenomenon has mostly been described in dogs, rabbits and mice; in contrast, bone formation has not been successfully induced in rats. It is therefore possible that sheep are also not a suitable model for heterotopic bone formation in muscle tissue [56].
In conclusion, cancellous bone was a reliable inducer of osteoinduction in our experimental model; however, BMAC was less effective, whereas β-TCP alone or in combination with BMSCs showed no capacity to induce heterotopic bone formation in the musculus latissimus dorsi of sheep. Future studies will investigate whether the addition of growth factors such as bone morphogenetic proteins, potentially administered via a sustained release system, might improve heterotopic bone formation in our experimental model [39, 57].
Acknowledgements
The authors gratefully thank DePuy Synthes for providing the ChronOs cylinders for this study.
Funding
This study was supported by the AO Foundation (grant no. F-07-58K).
Conflict of interest statement. None declared.
References
- 1. Bak M, Jacobson AS, Buchbinder D.. Contemporary reconstruction of the mandible. Oral Oncol 2010;46:71–6. [DOI] [PubMed] [Google Scholar]
- 2. Gellrich NC, Held U, Schoen R. et al. Alveolar zygomatic buttress: a new donor site for limited preimplant augmentation procedures. J Oral Maxillofac Surg 2007;65:275–80. [DOI] [PubMed] [Google Scholar]
- 3. Barth HD, Zimmermann EA, Schaible E. et al. Characterization of the effects of x-ray irradiation on the hierarchical structure and mechanical properties of human cortical bone. Biomaterials 2011;32:8892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goh BT, Lee S, Tideman H. et al. Mandibular reconstruction in adults: a review. Int J Oral Maxillofac Surg 2008;37:597–605. [DOI] [PubMed] [Google Scholar]
- 5. Miles BA, Goldstein DP, Gilbert RW. et al. Mandible reconstruction. Curr Opin Otolaryngol Head Neck Surg 2010;18:317–22. [DOI] [PubMed] [Google Scholar]
- 6. Foster RD, Anthony JP, Sharma A. et al. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck 1999;21:66–71. [DOI] [PubMed] [Google Scholar]
- 7. Hirsch DL, Bell RB, Dierks EJ. et al. Analysis of microvascular free flaps for reconstruction of advanced mandibular osteoradionecrosis: a retrospective cohort study. J Oral Maxillofac Surg 2008;66:2545–56. [DOI] [PubMed] [Google Scholar]
- 8. Dimitriou R, Mataliotakis GI, Angoules AG. et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 2011;42:S3–15. [DOI] [PubMed] [Google Scholar]
- 9. Farhadi J, Valderrabano V, Kunz C. et al. Free fibula donor-site morbidity: clinical and biomechanical analysis. Ann Plast Surg 2007;58:405–10. [DOI] [PubMed] [Google Scholar]
- 10. Pohlenz P, Blessmann M, Heiland M. et al. Postoperative complications in 202 cases of microvascular head and neck reconstruction. J Craniomaxillofac Surg 2007;35:311–5. [DOI] [PubMed] [Google Scholar]
- 11. Hirota M, Matsui Y, Mizuki N. et al. Combination with allogenic bone reduces early absorption of beta-tricalcium phosphate (beta-TCP) and enhances the role as a bone regeneration scaffold. Experimental animal study in rat mandibular bone defects. Dent Mater J 2009;28:153–61. [DOI] [PubMed] [Google Scholar]
- 12. Scarano A, Carinci F, Assenza B. et al. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: case series. Clin Oral Implants Res 2011;22:1125–30. [DOI] [PubMed] [Google Scholar]
- 13. Aho AJ, Ekfors T, Dean PB. et al. Incorporation and clinical results of large allografts of the extremities and pelvis. Clin Orthop Relat Res 1994;307:200–13. [PubMed] [Google Scholar]
- 14. Atala A. Engineering tissues, organs and cells. J Tissue Eng Regen Med 2007;1:83–96. [DOI] [PubMed] [Google Scholar]
- 15. Bruder SP, Fox BS.. Tissue engineering of bone. Cell based strategies. Clin Orthop Relat Res 1999;367:S68–83. [DOI] [PubMed] [Google Scholar]
- 16. Godbey WT, Atala A.. In vitro systems for tissue engineering. Ann N Y Acad Sci 2002;961:10–26. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein SA. Tissue engineering: functional assessment and clinical outcome. Ann N Y Acad Sci 2002;961:183–92. [DOI] [PubMed] [Google Scholar]
- 18. Bignon A, Chouteau J, Chevalier J. et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med 2003;14:1089–97. [DOI] [PubMed] [Google Scholar]
- 19. Eppley BL, Pietrzak WS, Blanton MW.. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 2005;16:981–9. [DOI] [PubMed] [Google Scholar]
- 20. Kneser U, Schaefer DJ, Polykandriotis E. et al. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med 2006;10:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kokemueller H, Spalthoff S, Nolff M. et al. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int J Oral Maxillofac Surg 2010;39:379–87. [DOI] [PubMed] [Google Scholar]
- 22. Polykandriotis E, Horch RE, Arkudas A. et al. Intrinsic versus extrinsic vascularization in tissue engineering. Adv Exp Med Biol 2006;585:311–26. [DOI] [PubMed] [Google Scholar]
- 23. Zimmerer R, Jehn P, Spalthoff S. et al. [Prefabrication of vascularized facial bones]. Chirurg 2015;86:254–8. [DOI] [PubMed] [Google Scholar]
- 24. Spalthoff S, Jehn P, Zimmerer R. et al. Heterotopic bone formation in the musculus latissimus dorsi of sheep using beta-tricalcium phosphate scaffolds: evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int J Oral Maxillofac Surg 2015;44:791–7. [DOI] [PubMed] [Google Scholar]
- 25. Grote K, Petri M, Liu C. et al. Toll-like receptor 2/6-dependent stimulation of mesenchymal stem cells promotes angiogenesis by paracrine factors. Eur Cell Mater 2013;26:66–79. [DOI] [PubMed] [Google Scholar]
- 26. Ajiboye RM, Hamamoto JT, Eckardt MA. et al. Clinical and radiographic outcomes of concentrated bone marrow aspirate with allograft and demineralized bone matrix for posterolateral and interbody lumbar fusion in elderly patients. Eur Spine J 2015;24:2567–72. [DOI] [PubMed] [Google Scholar]
- 27. Ajiboye RM, Eckardt MA, Hamamoto JT. et al. Outcomes of demineralized bone matrix enriched with concentrated bone marrow aspirate in lumbar fusion. Int J Spine Surg 2016;10:35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhong W, Sumita Y, Ohba S. et al. In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS One 2012;7:e40833.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batista MA, Leivas TP, Rodrigues CJ. et al. Comparison between the effects of platelet-rich plasma and bone marrow concentrate on defect consolidation in the rabbit tibia. Clinics 2011;66:1787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hakimi M, Grassmann JP, Betsch M. et al. The composite of bone marrow concentrate and PRP as an alternative to autologous bone grafting. PLoS One 2014;9:e100143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jager M, Herten M, Fochtmann U. et al. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res 2011;29:173–80. [DOI] [PubMed] [Google Scholar]
- 32. Melville JC, Nassari NN, Hanna IA. et al. Immediate transoral allogeneic bone grafting for large mandibular defects. Less morbidity, more bone. A paradigm in benign tumor mandibular reconstruction? J Oral Maxillofac Surg 2017;75:828–38. [DOI] [PubMed] [Google Scholar]
- 33. Zimmerer RM, Jehn P, Kokemuller H. et al. In vivo tissue engineered bone versus autologous bone: stability and structure. Int J Oral Maxillofac Surg 2017;46:385–93. [DOI] [PubMed] [Google Scholar]
- 34. Mravic M, Péault B, James AW.. Current trends in bone tissue engineering. BioMed Res Int 2014;2014:865270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jayakumar P, Di Silvio L.. Osteoblasts in bone tissue engineering. Proc Inst Mech Eng H 2010;224:1415–40. [DOI] [PubMed] [Google Scholar]
- 36. Kinoshita Y, Maeda H.. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci World J 2013;2013:863157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hempel U, Muller K, Preissler C. et al. Human bone marrow stromal cells: a reliable, challenging tool for in vitro osteogenesis and bone tissue engineering approaches. Stem Cells Int 2016;2016:7842191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gomez-Barrena E, Rosset P, Muller I. et al. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med 2011;15:1266–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arpornmaeklong P, Pripatnanont P, Kittidumkerng W. et al. Effects of autogenous growth factors on heterotopic bone formation of osteogenic cells in small animal model. J Craniomaxillofac Surg 2012;40:332–40. [DOI] [PubMed] [Google Scholar]
- 40. Gallego L, Perez-Basterrechea M, Garcia-Consuegra L. et al. Repair of segmental mandibular bone defects in sheep using bone marrow stromal cells and autologous serum scaffold: a pilot study. J Clin Periodontol 2015;42:1143–51. [DOI] [PubMed] [Google Scholar]
- 41. Idaszek J, Bruinink A, Święszkowski W.. Ternary composite scaffolds with tailorable degradation rate and highly improved colonization by human bone marrow stromal cells. J Biomed Mater Res A 2015;103:2394–404. [DOI] [PubMed] [Google Scholar]
- 42. Mankani MH, Kuznetsov SA, Avila NA. et al. Bone formation in transplants of human bone marrow stromal cells and hydroxyapatite-tricalcium phosphate: prediction with quantitative CT in mice. Radiology 2004;230:369–76. [DOI] [PubMed] [Google Scholar]
- 43. Masaoka T, Yoshii T, Yuasa M. et al. Bone defect regeneration by a combination of a beta-tricalcium phosphate scaffold and bone marrow stromal cells in a non-human primate model. Open Biomed Eng J 2016;10:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pelissier P, Villars F, Mathoulin-Pelissier S. et al. Influences of vascularization and osteogenic cells on heterotopic bone formation within a madreporic ceramic in rats. Plast Reconstr Surg 2003;111:1932–41. [DOI] [PubMed] [Google Scholar]
- 45. Brennan MA, Renaud A, Amiaud J. et al. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res Ther 2014;5:114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weigand A, Beier JP, Schmid R. et al. Bone tissue engineering under xenogeneic-free conditions in a large animal model as a basis for early clinical applicability. Tissue Eng Part A 2017;23:208–22. [DOI] [PubMed] [Google Scholar]
- 47. Bancroft GN, Sikavitsas VI, van den Dolder J. et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 2002;99:12600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grasser C, Scheuer C, Parakenings J. et al. Effects of macrophage-activating lipopeptide-2 (MALP-2) on the vascularisation of implanted polyurethane scaffolds seeded with microvascular fragments. Eur Cell Mater 2016;32:74–86. [DOI] [PubMed] [Google Scholar]
- 49. Grote K, Schuett H, Salguero G. et al. Toll-like receptor 2/6 stimulation promotes angiogenesis via GM-CSF as a potential strategy for immune defense and tissue regeneration. Blood 2010;115:2543–52. [DOI] [PubMed] [Google Scholar]
- 50. Kanczler JM, Oreffo RO.. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 2008;15:100–14. [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Zhang B, Bao C. et al. Ectopic osteoid and bone formation by three calcium-phosphate ceramics in rats, rabbits and dogs. PLoS One 2014;9:e107044.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng L, Wang T, Zhu J. et al. Osteoinduction of calcium phosphate ceramics in four kinds of animals for 1 year: dog, rabbit, rat, and mouse. Transplant Proc 2016;48:1309–14. [DOI] [PubMed] [Google Scholar]
- 53. Kondo N, Ogose A, Tokunaga K. et al. Osteoinduction with highly purified beta-tricalcium phosphate in dog dorsal muscles and the proliferation of osteoclasts before heterotopic bone formation. Biomaterials 2006;27:4419–27. [DOI] [PubMed] [Google Scholar]
- 54. Denry I, Kuhn LT.. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater 2016;32:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chan O, Coathup MJ, Nesbitt A. et al. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater 2012;8:2788–94. [DOI] [PubMed] [Google Scholar]
- 56. Le Nihouannen D, Saffarzadeh A, Aguado E. et al. Osteogenic properties of calcium phosphate ceramics and fibrin glue based composites. J Mater Sci Mater Med 2007;18:225–35. [DOI] [PubMed] [Google Scholar]
- 57. Seo BB, Koh JT, Song SC.. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017;122:91–104. [DOI] [PubMed] [Google Scholar]