Abstract
Endothelial dysfunction, characterized by changes in eNOS, is a common finding in chronic inflammatory vascular diseases. These states are associated with increased infectious complications. We hypothesized that alterations in eNOS would enhance the response to LPS-mediated TLR4 inflammation. Human microvascular endothelial cells were treated with sepiapterin or N-nitro-L-arginine methylester (L-NAME) to alter endogenous NO production, and small interfering RNA to knockdown eNOS. Alterations of endogenous NO by sepiapterin, and L-NAME provided no significant changes to LPS inflammation. In contrast, eNOS knockdown greatly enhanced endothelial IL-6 production and permeability in response to LPS. Knockdown of eNOS enhanced LPS-induced p38. Inhibition of p38 with SB203580 prevented IL-6 production, without altering permeability. Knockdown of p38 impaired NF-κB activation. Physical interaction between p38 and eNOS was demonstrated by immunoprecipitation, suggesting a novel, NO-independent mechanism for eNOS regulation of TLR4. In correlation, biopsy samples in patients with systemic lupus erythematous showed reduced eNOS expression with associated elevations in TLR4 and p38, suggesting an in vivo link. Thus, reduced expression of eNOS, as seen in chronic inflammatory disease, was associated with enhanced TLR4 signaling through p38. This may enhance the response to infection in patients with chronic inflammatory conditions.—Stark, R. J., Koch, S. R., Choi, H., Mace, E. H., Dikalov, S. I., Sherwood, E. R., Lamb, F. S. Endothelial nitric oxide synthase modulates Toll-like receptor 4–mediated IL-6 production and permeability via nitric oxide-independent signaling.
Keywords: inflammation, eNOS, endothelial cells, TLR-4, p38
In periods of sustained systemic inflammation, the endothelium, which serves as an interface between the blood and tissues, becomes injured (1). The chronic inflammation that induces that injury comes in many forms, from pure vascular pathologies, such as hypertension and atherosclerosis, to more systemic autoimmune diseases (1, 2). That injury disturbs the normal physiologic function of the endothelium and is termed endothelial dysfunction. Endothelial dysfunction is characterized by a change in eNOS function, which is associated with reduced production of NO and increased formation of reactive oxygen species (3, 4). Additional postulated mechanisms by which alterations in eNOS biology may lead to endothelial dysfunction include a reduction in the bioavailability of tetrahydrobiopterin (BH4), a cofactor that helps maintain dimerization of eNOS; altered phosphorylation of eNOS; and reduction in eNOS expression (5,–7). An underlying premise of endothelial dysfunction is that there is a correlation between disruption of eNOS function and endothelial injury, such as that seen in chronic inflammation. Although that correlation is well established, the contribution of eNOS and endothelial injury to acute systemic inflammation is poorly understood.
Sepsis, which is an infection-mediated, systemic, inflammatory response, carries a high burden of morbidity and mortality (8, 9). The invading pathogen sets off a cascade of normal and abnormal physiologic responses within the host’s immune system, causing the clinical features of sepsis. Integral to that innate host response is the endothelium, which can enhance coagulation and leukocyte trafficking, produce cytokines, and become more permeable, leading to barrier dysfunction (10). Although injury to the endothelium can occur via numerous mechanisms, a critical pathway is activation of the TLR (11). Those receptors recognize exogenous ligands expressed by bacteria, such as LPS, produced by gram-negative bacteria. They propagate the inflammatory response via activation of a variety of intracellular signaling pathways, including enhancement of NO production (12). It has long been postulated that the increased NO production associated with sepsis leads to worse outcomes and, potentially, adversely effects endothelial function (13, 14). However, the role of NO in sepsis remains controversial, as demonstrated in worsened outcomes in animal and clinical trials with NO inhibition (15). Consistent with that, animals overexpressing eNOS were protected in an LPS-mediated model of septic shock (16).
Specifically how eNOS, NO, and TLR modulate vascular inflammation remains unclear, but an interrelationship has been suggested (17). LPS is known to strongly activate MAPKs, and eNOS is known to possess MAPK docking sites (18, 19). Based on those studies, we sought to independently define the effect of eNOS protein expression and NO on TLR4 signaling. We hypothesized that eNOS-dependent modulation of TLR4 signaling would be mediated by changes in MAPK activation, which would lead to an altered inflammatory phenotype. By understanding those relationships, we hoped to discover new potential targets to limit the deleterious effects that alterations in eNOS biology have on infectious inflammation.
MATERIALS AND METHODS
Cells and culture
Pooled, neonatal, dermal human microvascular endothelial cells (HMVECs) were purchased from Lonza (Basel, Switzerland) and grown in Endothelial Growth Media-2 (Lonza), supplemented with 5% fetal bovine serum (FBS). HMVECs were plated at a density of approximately 30,000 cells/cm2 and grown to confluence. Experiments were conducted between the second and fifth passages. Medium was exchanged every 3 d.
Reagents
The following reagents and concentrations were used in experiments: 100 ng/ml Ultra-Pure LPS (List Biological Laboratories, Campbell, CA, USA), 100 μM L-sepiapterin (Cayman Chemicals, Ann Arbor, MI, USA), 10 μM N-nitro-L-arginine methylester (L-NAME) (MilliporeSigma, Billerica, MA, USA), 10 μM 1400W dihydrochloride (Thermo Fisher Scientific, Waltham, MA, USA), 10 μM SB203580 (Cell Signaling Technology, Danvers, MA, USA), 100 nM SCH772984 (ApexBio Technology, Houston, TX, USA).
Small interfering RNA transfection
HMVECs were treated with small interfering RNA (siRNA; scrambled siControl, sieNOS, siiNOS, or sip38), according to the manufacturer’s recommendations. In brief, siRNA were procured from Dharmacon (Lafayette, CO, USA). siRNA (25 nM) was incubated with Dharmafect (Dharmacon) in serum-free medium for 20 min. The resultant complex of siRNA–Dharmafect was added to the cells in 5% FBS medium without antibiotics for 6 h. Afterward, the transfection medium was replaced with complete medium, including antibiotics, for another 18 or 66 h before exposure to the agonist. Percentage of protein knockdown was determined by Western blot analysis.
Intracellular NO assay
Glass-bottom 96-well plates were seeded with HMVECs at 30,000 cells/well 1 d before assay. Cells were exposed to LPS, with or without sepiapterin or L-NAME, for 1 or 16 h in phenol-red–free, 5% FBS medium. Afterward, supernatants were removed, cells were washed with phenol-red–free medium and exposed to 2 μM DAF-FM DA (Thermo Fisher Scientific) for 40 min in the dark at 24°C. The medium containing DAF-FM DA was then removed and replaced with fresh phenol-red–free medium, and the cells remained in the dark for an additional 10 min at 24°C. Cells were then placed in a fluorescence plate reader (FluoStar Omega; BMG Labtech, Ortenberg, Germany) and read for retained intracellular probe [excitation (ex)/emission (em) 485/520 nm], and blank wells were subtracted from the treated wells to obtain normalized well fluorescence intensity.
Cytokine and chemokine production
Culture supernatants were collected at the completion of the agonist exposure (6 or 16 h). Collected supernatants were stored at −80°C. Supernatant IL-6 (eBioscience, San Diego, CA, USA), IL-8, and granulocyte colony-stimulating factor (G-CSF; R&D Systems, Minneapolis, MN, USA) concentrations were assessed with a commercially available ELISA kit, according to the manufacturer’s specifications.
Intercellular space assay
Glass-bottom 96-well plates were used for an in vitro vascular permeability imaging assay (MilliporeSigma). Wells were treated with poly-l-lysine, glutaraldehyde, and biotinylated gelatin, per the manufacturer’s recommendations. HMVECs were seeded at a density of 30,000 cells/well and incubated for 1 d to achieve a confluent monolayer. In some experiments, the cells were then exposed to siRNA. In a later study, cells will be treated with LPS (100 ng/ml) or vehicle in the presence of sepiapterin, L-NAME, SB203580, or SCH772984, as described in Results. At 6 or 16 h after LPS exposure, supernatants were collected for ELISA, and cells were washed with 120 μl of Live Cell imaging solution (Thermo Fisher Scientific). Afterward, cells were exposed to streptavidin–fluorescein (1:2000 dilution) and stained with NucBlue (1 drop/well; Thermo Fisher Scientific) for 20 min to test for total cell count or, in some instances, ethidium homodimer (10 μM; AdipoGen, Liestal, Switzerland) for 15 min to examine cell death. Cells were then washed again with imaging solution to remove excess probe, and images were acquired on a fluorescence inverted microscope (Leica DM IRB; Leica Microsystems, Wetzlar, Germany) using filters appropriate for DAPI (ex/em 360/460 nm), ethidium (ex/em 528/617 nm), or fluorescein (ex/em 490/520 nm). Cell count was obtained via quantification of nuclear staining using ImageJ (National Institutes of Health, Bethesda, MD, USA) software, which ensured a consistent density of the endothelial layers, and monolayer integrity was assessed via quantification of the area of fluorescein staining (ImageJ), as an indicator of intercellular gap size.
Western blot and electrophoresis
Cell lysates were collected at 1 and 16 h after agonist exposure, as described in Results. For eNOS dimer and monomer determination, cells lysates were kept between 0 and 4°C and were maintained in 2-mercaptoethanol–free sample buffer. Protein extracts (50 μg/sample) were separated by SDS electrophoresis on a polyacrylamide gel (10%) and transferred to nitrocellulose membranes. Membranes were blocked with Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA) for 1 h at room-temperature. Membranes were incubated with primary antibodies overnight at 4°C on a rocker. Antibodies were as follows: IKKαβ, phospho-IKKβ, p-p38, p38, p-ERK, ERK, p-JNK, JNK, p-Akt, Akt, eNOS, p-eNOS (serine 1177), p-eNOS (threonine 495) (Cell Signaling Technology), iNOS (R&D Systems), tubulin (Vanderbilt Antibody Core; Vanderbilt Antibody and Protein Resource, Nashville, TN, USA). Afterward, membranes were incubated with fluorescent secondary antibodies and analyzed using the Odyssey Imaging System (Li-Cor Biosciences). Protein quantification was performed via densitometry and normalized as a ratio of expressed protein to tubulin, phosphorylated protein to respective total protein, or dimer to monomer ratio.
Immunoprecipitation
Cells were exposed to siRNA for 72 h, then lysed in buffer (0.02 M Tris base, 1 mM EDTA, 20 mM NaCl, 1% nonidet P40, protease inhibitor cocktail, and PMSF at pH 7.4). Lysed cells were nutated for 1 h at 4°C, then centrifuged at 20,000 g for 30 min. Supernatants were precleared with protein-G sepharose beads for 30 min at 4°C. After centrifugation, the supernatants (500 µg) were incubated with anti-eNOS antibody (2 µg, clone 49G3; Cell Signaling Technology) for 1.5 h, then incubated with protein-G sepharose for an additional 1 h. Beads were then washed with lysis buffer, resuspended in sample buffer containing SDS, and boiled. Associated proteins were then analyzed by Western blot using the following antibodies: p38 and eNOS (6H2; Cell Signaling Technology).
Gene profiles of synovial biopsies
Gene array profiles of synovial biopsies were obtained from the publically available National Centers for Biotechnology Information (NCBI; Bethesda, MD, USA) Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) database. Values were obtained from GEO DataSet (GSE36700), initially collected by Nzeusseu Toukap et al. (20). The database was queried for NOS3 (eNOS, ID: NM_000603), MAPK14 (p38, ID: L35253.1), and TLR4 (TLR4, ID: NM_003266) from samples run on an Affymetrix Human Genome U133 Plus 2.0 Array (Thermo Fisher Scientific).
Statistical analysis
For endothelial cell-culture experiments, data are expressed as means ± se of multiple, individual experiments. Comparisons of treatment groups and conditions were performed via an unpaired Student’s t test for single comparisons and 1-way ANOVA, with Bonferroni correction, for multiple-group comparisons. For gene profile expression of biopsy samples, data are expressed as medians (5–95%) ± se of individual samples. Comparisons of groups were performed via the Kruskal-Wallis test, with the Dunn correction for multiple-group comparisons, and linear regression for direct expression comparison. All analysis was performed with GraphPad Prism 5.03 statistical software (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Sepiapterin enhanced eNOS dimerization and NO production in endothelial cells
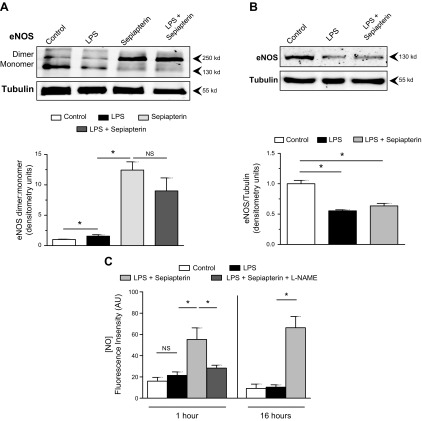
Alterations in eNOS are one of the hallmarks of endothelial dysfunction and can occur via multiple mechanisms, including dimerization, altered phosphorylation or dephosphorylation, and reduction in total protein. To examine the relationship between LPS and NO production by eNOS, we exposed HMVECs to LPS for 16 h in the presence or absence of sepiapterin, which promotes production of BH4 and associated eNOS dimerization. In that conformation, eNOS produces NO (21). In resting HMVECs, eNOS was present in both dimer and monomer forms (Fig. 1A). When exposed to LPS, the dimer to monomer ratio increased; however, that appeared to be due more to the loss of the monomer band. The addition of sepiapterin for 16 h significantly increased the amount of dimerized eNOS, independent of LPS. Exposure of endothelial cells to LPS significantly suppressed the total amount of eNOS in the cells, and that effect was not altered by the presence of sepiapterin (Fig. 1B). LPS did not induce changes in eNOS phosphorylation at serine 1177 or threonine 495 at 1 h of exposure (Supplemental Fig. 1A). To examine the effect of eNOS dimerization on NO production, cells were exposed to LPS under control conditions or in the presence of sepiapterin for either 1 or 16 h. At both times, sepiapterin significantly increased the amount of NO detected by DAF-FM DA, and that effect was suppressed by L-NAME (Fig. 1C). Those experiments demonstrated that, as expected, sepiapterin enhanced eNOS dimerization and NO production. The primary effect of LPS on eNOS appeared to be a reduction in total protein.
Figure 1.
Sepiapterin-induced eNOS dimerization and NO production. A) HMVECs were treated with LPS (100 ng/ml) or sepiapterin (100 μM), alone or in combination, for 16 h. Ratios of the density of the dimer band (260 kDa) to the monomer band (130 kDa) were determined by Western blot (n = 4/group). B) Total eNOS normalized to α-tubulin for HMVECs exposed to LPS or LPS with sepiapterin for 16 h (n = 4/group). C) Relative fluorescence intensity of DAF FM DA (2 μM) as an indication of intracellular NO production after 1 or 16 h in the presence of LPS with or without sepiapterin and L-NAME (10 μM) (n = 12/group). NS, nonsignificant. *P < 0.05 between compared groups.
Sepiapterin and L-NAME had limited effects on LPS-induced IL-6 production and intercellular space
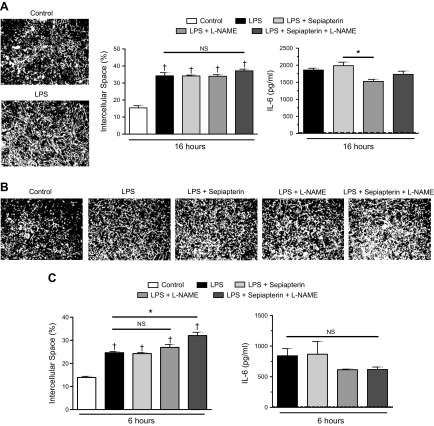
NO has been postulated as having an important role in regulating the inflammatory response of endothelial cells, including permeability and cytokine production (22, 23). To test whether eNOS dimerization and the associated generation of endogenous NO altered the endothelial response to LPS, HMVECs were exposed to sepiapterin, L-NAME, or a combination in the presence of LPS. The addition of LPS for 16 h significantly increased the amount of exposed biotin-labeled gelatin, a predicted indicator of increased permeability (Fig. 2A). Despite the increase in endothelial NO production induced by sepiapterin, there was no difference in endothelial intercellular space compared with cells treated with LPS alone. Likewise, L-NAME failed to induce any significant alteration in intercellular space, with or without the presence of sepiapterin. When examining IL-6 production 16 h after LPS exposure, once again, there was little effect of altering eNOS-derived NO. Although there was a statistically significant difference between the response to LPS and sepiapterin compared with LPS and L-NAME, that effect was small. Because changes in endothelial intercellular space in response to endotoxin can occur in the first hours after exposure, we also tested an earlier time, 6 h after LPS (24). Similar to the later time, 6 h of exposure to LPS increased intercellular space (Fig. 2B, C). At that earlier point, both sepiapterin and L-NAME alone had no significant effects on intercellular space or IL-6 production; however, in combination, there was a subtle increase in endothelial intercellular space in the presence of LPS. Despite that, these results suggested that enhanced endogenous NO production by sepiapterin-mediated eNOS dimerization and inhibition of NO by L-NAME had no prominent effects on intercellular space or cytokine production during exposure to LPS.
Figure 2.
Neither sepiapterin nor L-NAME significantly altered LPS-induced intercellular space or IL-6 production. A) HMVECs were exposed to combinations of LPS (100 ng/ml), sepiapterin (100 μM), or L-NAME (10 μM) for 16 h. Representative images of intercellular space under control conditions or with LPS (white, intercellular space; black, cells) (left). Percentage of exposed intercellular space among treatment groups (middle). Amount of IL-6 produced among treatment groups as measured by ELISA (right); dashed line indicates control group IL-6 levels (n = 4/group). B) Representative images of HMVECs exposed to LPS, sepiapterin, or L-NAME for 6 h. C) Percentage of exposed intercellular space (left) and IL-6 production (right) among groups after 6 h of treatment (n = 4/group). Dashed line indicates control condition IL-6 levels. NS, nonsignificant. *P < 0.05 between compared groups, †P < 0.05 between group compared with control conditions.
Knockdown of eNOS-enhanced, LPS-induced IL-6 production, and intercellular space
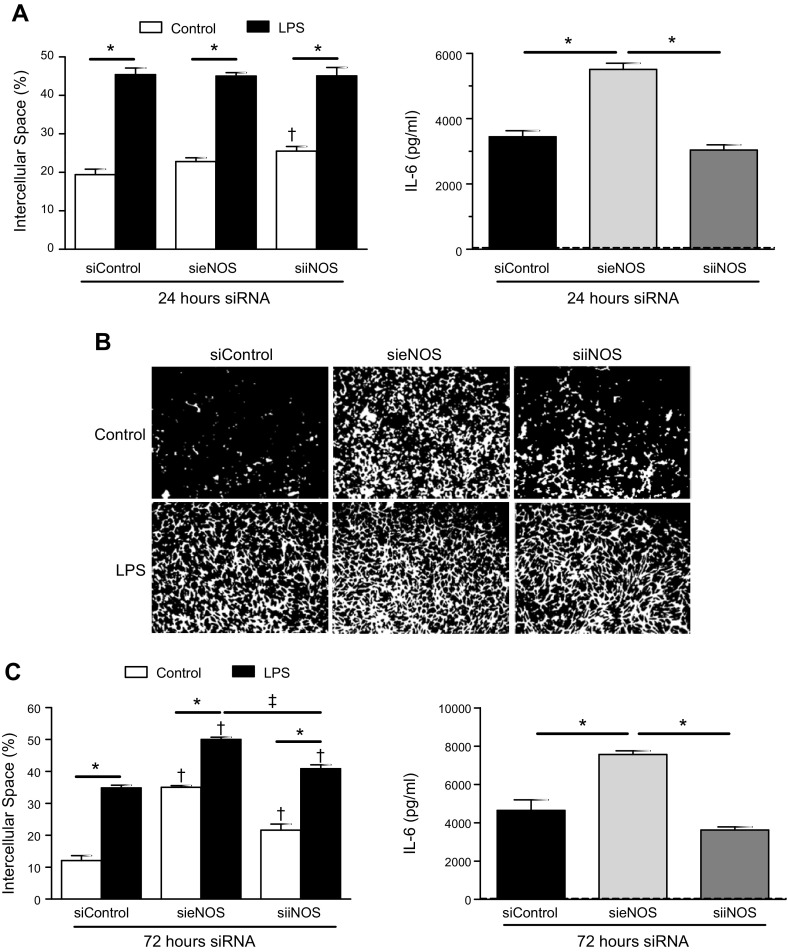
Given that eNOS-derived NO did not significantly alter the global endothelial response to LPS, we next examined the effect of siRNA knockdown on total eNOS protein, as would be seen in chronic inflammatory conditions (25). After 24 h of initial exposure to siRNA against eNOS (sieNOS), there were no notable changes to intercellular space in the knockdown groups after LPS exposure (Fig. 3A). However, the amount of IL-6 produced in the sieNOS group at 6 h after LPS was significantly increased compared with those cells treated with scrambled siRNA (siControl). Documentation of effective eNOS knockdown is provided in Supplemental Fig. 1B. To examine whether knockdown of iNOS would affect LPS-induced inflammation, cells were exposed to siRNA targeting iNOS (siiNOS) and then to LPS. Although knockdown of iNOS did not alter LPS-induced intercellular space, it did increase baseline intercellular space, albeit minimally, despite difficulty in detecting iNOS in the endothelial cell lysates (Supplemental Fig. 1C). Exposure to siiNOS did not significantly affect the IL-6 produced after LPS. The absence of an important role for iNOS was confirmed using a selective iNOS inhibitor, 1400W (Supplemental Fig. 1D), which showed no alteration in IL-6 production. Next, we extended the exposure time to siRNA to 72 h (Fig. 3B, C). Again, reduction of iNOS increased baseline intercellular space to a small, but statistically significant, degree but did not alter IL-6 production. In contrast, reduction of eNOS-enhanced, LPS-induced IL-6, and that effect was not altered by the addition of sepiapterin or L-NAME (Supplemental Fig. 2C, D). In addition, exposure to sieNOS significantly increased baseline intercellular space, which was further enhanced by LPS. Together, these result show that reduced eNOS enhanced inflammatory cytokine production and intercellular space by LPS.
Figure 3.
eNOS knockdown enhanced LPS-induced intercellular space and IL-6 production. A) HMVECs were exposed to siRNA for 24 h (siControl, sieNOS, siiNOS) and then treated with LPS (100 ng/ml) for 6 h. Percentage of exposed intercellular space (left) and IL-6 production (right) among groups (n = 4/group). B) Representative images of intercellular space under control conditions or with LPS after exposure to siControl, sieNOS, or siiNOS for 72 h (white, intercellular space; black, cells). C) Percentage of exposed intercellular space (left) and IL-6 production (right) among siRNA groups exposed for 72 h (n = 4/group). Dashed line indicates IL-6 levels for control conditions. *P < 0.05 between compared groups, †P < 0.05 between group compared with siControl, ‡P < 0.05 between sieNOS group compared with siiNOS.
Reduction of eNOS protein abundance enhanced p38 phosphorylation
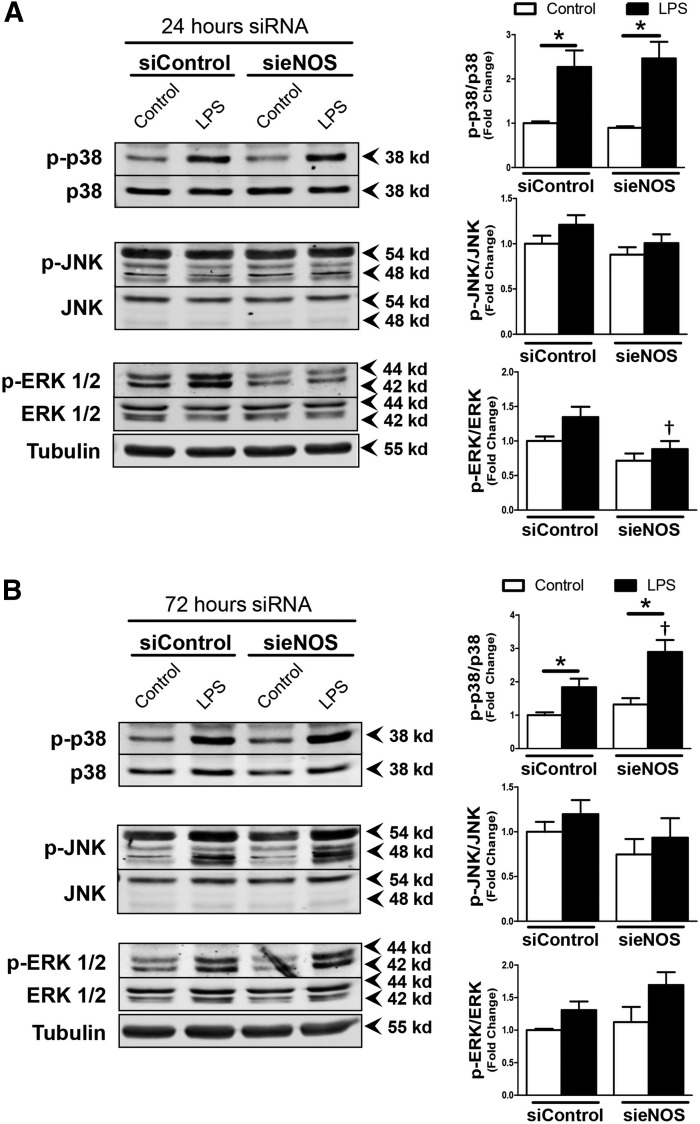
To further examine how eNOS temporally altered cytokine production and intercellular space, we focused on MAPK signaling because of the presence of postulated MAPK docking sites on eNOS (19). Despite a significant reduction in eNOS protein 24 h after exposure to sieNOS, 1 h of LPS exposure produced no significant changes in MAPK phosphorylation among the siRNA groups, with the exception of ERK1/2, which was reduced after exposure to eNOS siRNA (Fig. 4A). When siRNA exposure was extended to 72 h, followed by 1 h of LPS, an increase in p38 phosphorylation was seen in the sieNOS group compared with the siControl group (Fig. 4B). Again, JNK phosphorylation was unaffected, and ERK 1/2 phosphorylation in the sieNOS group was no longer inhibited. There was a trend toward an increase in phosphorylation of IKK, an upstream component of NF-κB activation, which was not statistically significant. There were no detectable changes in Akt phosphorylation (Supplemental Fig. 2A, B). Thus, these data suggested that reduced eNOS allowed for enhanced p38 activity, which could contribute to enhanced inflammation.
Figure 4.
p38 phosphorylation in response to LPS was enhanced by eNOS knockdown. A) Representative Western blot images of HMVECs exposed to siRNA (siControl or sieNOS) for 24 h and then treated with LPS (100 ng/ml) for 1 h (left). Images show band intensity for p-p38, p38, p-JNK, JNK, p-ERK1/2, ERK1/2, or α-tubulin with ratios of phosphorylated protein to respective total protein (right) B) Representative Western blot images of HMVECs exposed to siRNA (siControl or sieNOS) for 72 h (left) with 1 h of LPS and respective ratios of phosphorylated protein to associated total protein (right) (n = 6/group). *P < 0.05 between compared groups, †P < 0.05 between group compared to siControl.
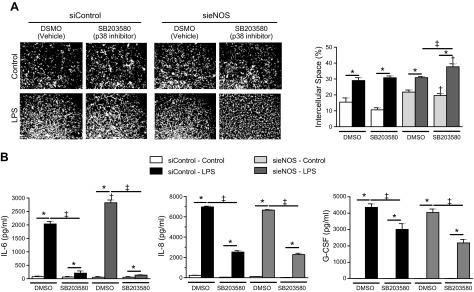
To explore that, we tested intercellular space, analyzed cytokine and chemokine production in HMVECs treated with sieNOS, and examined the effect of the selective p38 inhibitor SB203580 on the response to LPS. SB203580 did not alter the effect of LPS on intercellular space in the siControl group (Fig. 5A). Likewise, SB203580 did not prevent the enhancement of intercellular space caused by sieNOS and, instead, increased intercellular space in both vehicle and LPS treated conditions. However, p38 inhibition was associated with a significant reduction in IL-6 production in the LPS-treated siControl group (Fig. 5B). That reduction in LPS-mediated IL-6 production was also observed in the more reactive sieNOS group, where inhibition of p38 completely reduced the response to LPS to those of siControl-treated cells. Contrary to that, eNOS reduction did not affect LPS-mediated release of the chemokines IL-8 or G-CSF. Chemokine release was less dependent on p38 activity compared with IL-6 release. We also tested the effect of the selective ERK1/2 inhibitor SCH772984 because ERK1/2 has been shown previously to modulate endothelial intercellular space (26). However, inhibition of ERK1/2 did not alter IL-6 production or intercellular space (Supplemental Fig. 3). Together, these experiments suggest that eNOS protein reduction enhanced p38 MAPK activation by LPS, contributing to the greater IL-6 production in eNOS knockdown cells. However, those changes in p38 activity did not account for the increase in intercellular space in sieNOS-treated cells and had no relation to chemokine production, suggesting independent mechanisms of regulation.
Figure 5.
Inhibition of p38 prevented eNOS knockdown from enhancing IL-6 production but not intercellular space. A) Representative images of intercellular space under control conditions or 6 h of LPS (100 ng/ml), with or without the p38 inhibitor, SB203580 (10 μM), after 72 h exposure to siControl or sieNOS (white, intercellular space; black, cells) (left) and graphical representation of the percentage of exposed intercellular space (right). B) IL-6, IL-8, and G-CSF production among siRNA groups exposed with LPS and SB203580, as measured by ELISA (n = 4/group). *P < 0.05 between control or LPS-treated conditions, †P < 0.05 between siControl and respective sieNOS group, ‡P < 0.05 between vehicle control and SB203580-treated group as measured by ANOVA with the Bonferroni correction.
p38 had direct interactions with eNOS and knockdown impaired IKK activation by LPS
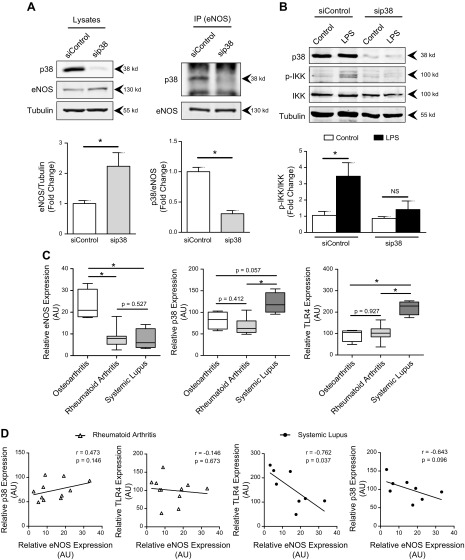
Given the apparent p38-dependence of eNOS-mediated IL-6 production, we next examined whether there was a reciprocal relationship between p38 and eNOS that affected proinflammatory activation by LPS. Cells exposed to siRNA against p38 had a significant reduction in p38 protein that correlated with an increase in total eNOS (Fig. 6A). In addition, using immunoprecipitation, eNOS protein pulled down with anti-eNOS antibody, stained for p38, and that staining was dramatically reduced in the presence of siRNA to p38, suggesting a physical interaction between the 2 proteins. Furthermore, cells in the sip38 group demonstrated a significant decrease in IKK phosphorylation after LPS compared with the siControl group (Fig. 6B).
Figure 6.
p38 and eNOS had a direct reciprocal relationship in vitro and in vivo. A) Representative images of p38, eNOS, and α-tubulin Western blots from whole-cell lysates (top, left), and of p38 and eNOS after immunoprecipitation with anti-eNOS (top, right). HMVECs were exposed to p38 siRNA for 72 h. Ratios of eNOS or p38 to α-tubulin are provided below. B) Representative Western blot images of HMVEC exposed to 72 h of p38 siRNA then LPS (100 ng/ml) or control medium for 1 h with antibodies against phosphorylated IKK, total IKK, p38, and α-tubulin (top). Ratios of phosphorylated IKK to total IKK are displayed below (n = 4/group). C) Relative gene expression for eNOS, p38, and TLR4 from synovial biopsies obtained from patients with arthritis, seronegative arthritis (control), rheumatoid arthritis, or systemic lupus erythematous. D) Linear regression analysis of eNOS expression compared with TLR4 or p38 expression within respective sample populations of seronegative arthritis compared with rheumatoid arthritis or systemic lupus erythematous (n = 4–7 patients/group). *P < 0.05 between designated groups.
Because the current data were derived from cultured endothelial cells, we were interested in seeing whether there was evidence of eNOS–TLR4–p38 interactions in samples derived from patients with systemic, vascular autoimmune disease. To examine that relationship in vivo, we queried the publicly available NCBI GEO database for chronic inflammatory conditions. We used DataSet GSE36700, obtained by Nzeusseu Toukap et al. (20), from synovial biopsy samples collected from patients with rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). We focused on SLE, as well as RA, because of a postulated correlation between increased disease severity and reduced eNOS expression, similar to our in vitro model (25). Samples from patients with seropositive disease had significantly reduced eNOS expression compared with those with osteoarthritis (Fig. 6C). Concurrently, the reduction of eNOS expression in systemic lupus correlated with a trend toward increased p38 and a significant increase in TLR4 expression compared with patients in the other groups. Together, these data suggest a reciprocal relationship between eNOS and TLR4/p38 signaling in chronic, systemic inflammatory diseases.
DISCUSSION
The role of NO and its associated synthases during severe infections have been a source of much interest and debate since its discovery (13, 27). This confusion has been enhanced by animal studies showing detrimental effects of exaggerated NO-production by iNOS and potentially protective effects of eNOS, with subsequent clinical studies showing increased mortality in septic patients given nonselective NOS inhibitors (15, 16). To more specifically examine the role of eNOS in TLR4-mediated inflammation, we used several different techniques involving functional activation and inhibition of the eNOS enzyme as well as protein knockdown. Sepiapterin enhanced eNOS dimerization and thus NO production, whereas L-NAME reduced NO production. However, neither treatment conferred any significant protective or detrimental effects with regard to LPS-mediated inflammation. In contrast, knockdown of eNOS greatly enhanced both LPS-mediated IL-6 production and intercellular space, a surrogate of permeability. Knockdown of eNOS was associated with increased p38 activation by LPS. Inhibition of p38 with a pharmacologic inhibitor attenuated the increased IL-6 production induced by eNOS knockdown but had no effect on intercellular space, suggesting a divergence in the regulatory mechanisms. In congruence, p38 associated with eNOS in a manner that could be reduced with siRNA to p38 and, in turn, reduced LPS-mediated IKK phosphorylation. A reciprocal relationship between eNOS and TLR4/p38 signaling was further supported in synovial biopsies from patients with chronic, inflammatory arthritides that are associated with systemic vascular disease and endothelial dysfunction. Together, these data suggest an important role for eNOS in regulating TLR4-mediated endothelial inflammation.
NO has been suggested to have opposing roles during sepsis (13, 27). The role of eNOS-mediated NO production in the endothelial dysfunction associated with cardiovascular disease has been extensively investigated (5). Mechanisms by which eNOS-dependent NO production can be altered include BH4-mediated subunit dimerization, phosphorylation, and changes in eNOS expression (5,–7). In the current study, sepiapterin greatly enhanced eNOS dimerization and NO production in response to LPS, and as expected, L-NAME effectively inhibited NO (Fig. 1). The increase in NO production after sepiapterin was likely related to an increase in de novo BH4 synthesis; however, that appears to be offset by a reduction of eNOS protein abundance after LPS (7, 21). Furthermore, despite increased eNOS dimerization and NO production, alterations in endogenous NO failed to either enhance or reduce LPS IL-6 production or permeability (Fig. 2). These findings are contrary to the postulated concept that NO can induce permeability and suppress cytokines (22, 23, 28). The reason for this discrepancy is likely multifactorial, including the use of a different agonist (LPS vs. VEGF or platelet-activating factor) and the testing of endogenous NO vs. an exogenous NO donor. Our findings are more consistent with the observation that inhibition of endogenous NO with L-NAME enhanced baseline endothelial permeability and could be restored through the NO donor, sodium nitroprusside (29). Additionally, acetylcholine, which is known to induce eNOS phosphorylation and NO production, was shown not to induce permeability (30). Thus, it appears that the role of endogenous NO in regulating endothelial permeability depends on the mechanism of the stimulus, and regarding TLR4, NO does not appear to have a significant role.
Because endogenous, eNOS-derived NO did not appear to have a significant role in TLR4-mediated inflammation, we transitioned to investigating an alternative mechanism of endothelial dysfunction seen in chronic inflammatory conditions: reduced eNOS expression (25, 31). Using siRNA, we were able to show that reduced eNOS expression enhanced IL-6 production and altered baseline permeability after LPS in a time-dependent fashion (Figs. 3 and 4). Furthermore, that effect was specific to eNOS because siRNA to iNOS had no prominent effects, likely related the low abundance and poor induction of iNOS in endothelial cells (32). In contrast, the effect of eNOS knockdown on vascular inflammation was striking. Our data concurs with observations from eNOS knockout mice, which had higher TNF-α expression, suggestive of a link between reduced eNOS and acute-phase cytokine production (33). Similarly, transgenic mice made to overexpress eNOS were protected in a model of endotoxemia, with an associated reduction in pulmonary capillary leak, suggesting a protective role of eNOS abundance (16). Despite a potential protective role for eNOS in endothelial permeability, there are other studies demonstrating that, for VEGF-mediated permeability, the loss of eNOS, protected against permeability (34). Given these discrepant data, it is likely that eNOS regulation of permeability and cytokine production is ligand and stimulus specific. For infectious stimuli, it appears that the role of eNOS in TLR4-mediated inflammation is independent of eNOS-mediated NO production and is instead related to another functional role of eNOS protein.
To explore that possibility, we quantified MAPK phosphorylation in cells with reduced eNOS expression and observed enhanced activation of p38 after LPS (Fig. 4). This relationship with p38 was responsible for the increased cytokine production because a p38 inhibitor blocked nearly all IL-6 production in response to LPS and completely abrogated the increment in IL-6 production observed in sieNOS cells. However, sieNOS had no effect on chemokine production, as eNOS knockdown did not impair LPS-mediated IL-8 or G-CSF production. Furthermore, IL-8 and G-CSF were less affected by p38 inhibition, suggesting eNOS and p38 have both synergistic and independent roles in mediating endothelial inflammation. This was further seen in examining the intercellular space in which p38 inhibition had no effect on LPS-mediated intercellular space, despite eNOS knockdown (Fig. 5). Although it has been suggested previously that p38 is involved in the regulation of LPS-induced permeability, the effect was shown to be small and thus, p38 was not likely to be a conserved mediator of both cytokine production and permeability (35). In further experiments, we found that eNOS had a direct association with p38 that could be altered by p38 knockdown. Furthermore, p38 knockdown was associated with reduced NF-κB activation by LPS (Fig. 6). Knockdown of p38 reduced NF-κB activation to a greater degree than eNOS knockdown had enhanced signaling NF-κB and was an unexpected finding. Although a p38–NF-κB pathway has been established, little is known about how eNOS reduction, or NO, directly affects NF-κB (36, 37). Given that NF-κB is further downstream and subject to multiple regulatory pathways, it is likely that, although p38 regulates IL-6 production through NF-κB, eNOS has differential or counter-regulatory effects on NF-κB, which explains how it regulates cytokine production and permeability independently. In the case of mechanisms regulating LPS-mediated endothelial inflammation, both IL-6 production and intercellular space were enhanced by eNOS knockdown, but the eNOS–p38 interaction only appeared to regulate cytokine production, whereas eNOS enhanced permeability either synergistically or additively with TLR4 via an unknown mechanism that is independent of p38. That interaction between p38 and eNOS driving IL-6 response is consistent with a previous study demonstrating that eNOS contains a MAPK docking site and direct binding between recombinant p38 and eNOS proteins (19). To our knowledge, the current work represents the first demonstration of that interaction in living cells. The observation that eNOS deficiency adversely affects TLR4-mediated inflammation in animal models, coupled with the current observation that eNOS reduction enhances LPS responses, points to an ability of eNOS to modulate TLR4 signaling (17, 18).
The clinical relevance of that relationship was demonstrated in biopsy samples from patients with SLE and RA. Both diseases were associated with dramatically suppressed eNOS mRNA levels. Further, patients with SLE had an associated reciprocal relationship between eNOS and TLR4 and p38 expression (Fig. 6C). Interestingly, the relationships of reduced eNOS and increased TLR4 individually have been postulated to correlate with disease severity in SLE (25, 38). Although a disease-specific reciprocal interaction has not been demonstrated, it has been noted that endothelial dysfunction is more prevalent in patients with SLE compared with those with RA (39). Further, it has been demonstrated that patients with SLE have higher circulating levels of LPS and that those higher levels of LPS regulate inflammatory gene expression in a p38-dependent manner (39, 40). What role eNOS expression and its associated alterations in TLR4-p38 signaling have in these clinical outcomes is unknown, but our data support the concept of a direct relationship among eNOS, TLR4, and p38, which may regulate the endothelial response to infectious challenge in chronic vascular inflammation.
In summary, the current study demonstrates that LPS-mediated endothelial injury was enhanced by the reduction of eNOS protein abundance in a NO-independent manner. The potentiated response of eNOS knockdown cells was p38 dependent regarding IL-6 production but not endothelial permeability, suggesting independent mechanisms of LPS-induced and eNOS-regulated IL-6 production and permeability. The relationship between eNOS and p38 abundance was reciprocal, and the ability of eNOS to modulate p38 activity appeared to involve a direct, binding relationship. That reciprocal relationship among eNOS, p38, and TLR4 was observed in biopsy samples from patients with SLE. Although the direct implications of that relationship have not been fully explored, this work provides a foundation for elucidating the mechanisms of endothelial dysfunction in chronic inflammation and its relationship to sepsis outcomes in these patients.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants K08-GM117367 (to R.J.S.) and R01-GM104306 (to E.R.S.) and NIH National Heart, Lung, and Blood Institute Grant R01-HL128386 (to F.S.L.), and by American Heart Association Grant 16SDG30610002 (to H.C.). The authors declare no conflicts of interest.
Glossary
- BH4
tetrahydrobiopterin
- DAF-FM DA
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- FBS
fetal bovine serum
- G-CSF
granulocyte colony-stimulating factor
- HMVEC
human dermal microvascular endothelial cell
- L-NAME
N-nitro-L-arginine methylester
- RA
rheumatoid arthritis
- siRNA
small interfering RNA
- SLE
systemic lupus erythematosus
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. J. Stark and F. S. Lamb designed the research; R. J. Stark, S. R. Koch, H. Choi, E. H. Mace, S. I. Dikalov, E. R. Sherwood, and F. S. Lamb analyzed the data; and R. J. Stark, S. R. Koch, H. Choi, E. H. Mace, S. I. Dikalov, E. R. Sherwood, and F. S. Lamb wrote the paper.
REFERENCES
- 1.Castellon X., Bogdanova V. (2016) Chronic inflammatory diseases and endothelial dysfunction. Aging Dis. 7, 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandes R. P. (2014) Endothelial dysfunction and hypertension. Hypertension 64, 924–928 [DOI] [PubMed] [Google Scholar]
- 3.Deanfield J. E., Halcox J. P., Rabelink T. J. (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115, 1285–1295 [DOI] [PubMed] [Google Scholar]
- 4.Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B. S., Karoui H., Tordo P., Pritchard K. A., Jr (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. USA 95, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Förstermann U., Münzel T. (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113, 1708–1714 [DOI] [PubMed] [Google Scholar]
- 6.Kolluru G. K., Siamwala J. H., Chatterjee S. (2010) eNOS phosphorylation in health and disease. Biochimie 92, 1186–1198 [DOI] [PubMed] [Google Scholar]
- 7.Cardaropoli S., Silvagno F., Morra E., Pescarmona G. P., Todros T. (2003) Infectious and inflammatory stimuli decrease endothelial nitric oxide synthase activity in vitro. J. Hypertens. 21, 2103–2110 [DOI] [PubMed] [Google Scholar]
- 8.Balk R. A. (2014) Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence 5, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr F. B., Yende S., Angus D. C. (2014) Epidemiology of severe sepsis. Virulence 5, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aird W. C. (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101, 3765–3777 [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto H., Ono S., Efron P. A., Scumpia P. O., Moldawer L. L., Mochizuki H. (2008) Role of Toll-like receptors in the development of sepsis. Shock 29, 315–321 [DOI] [PubMed] [Google Scholar]
- 12.Liu Q., Ding J. L. (2016) The molecular mechanisms of TLR-signaling cooperation in cytokine regulation. Immunol. Cell Biol. 94, 538–542 [DOI] [PubMed] [Google Scholar]
- 13.Kirkebøen K. A., Strand O. A. (1999) The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol. Scand. 43, 275–288 [DOI] [PubMed] [Google Scholar]
- 14.Woods M. E., Wen G., Olano J. P. (2005) Nitric oxide as a mediator of increased microvascular permeability during acute rickettsioses. Ann. N. Y. Acad. Sci. 1063, 239–245 [DOI] [PubMed] [Google Scholar]
- 15.Hauser B., Bracht H., Matejovic M., Radermacher P., Venkatesh B. (2005) Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth. Analg. 101, 488–498 [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T., Kawashima S., Ohashi Y., Ozaki M., Ueyama T., Ishida T., Inoue N., Hirata K., Akita H., Yokoyama M. (2000) Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation 101, 931–937 [DOI] [PubMed] [Google Scholar]
- 17.Yazji I., Sodhi C. P., Lee E. K., Good M., Egan C. E., Afrazi A., Neal M. D., Jia H., Lin J., Ma C., Branca M. F., Prindle T., Richardson W. M., Ozolek J., Billiar T. R., Binion D. G., Gladwin M. T., Hackam D. J. (2013) Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc. Natl. Acad. Sci. USA 110, 9451–9456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark R., Choi H., Koch S., Lamb F., Sherwood E. (2015) Monophosphoryl lipid A inhibits the cytokine response of endothelial cells challenged with LPS. Innate Immun. 21, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrestensen C. A., McMurry J. L., Salerno J. C. (2012) MAP kinases bind endothelial nitric oxide synthase. FEBS Open Bio 2, 51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nzeusseu Toukap A., Galant C., Theate I., Maudoux A. L., Lories R. J., Houssiau F. A., Lauwerys B. R. (2007) Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 56, 1579–1588 [DOI] [PubMed] [Google Scholar]
- 21.Gross S. S., Levi R. (1992) Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J. Biol. Chem. 267, 25722–25729 [PubMed] [Google Scholar]
- 22.De Caterina R., Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A. Jr., Shin W. S., Liao J. K. (1995) Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 96, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durán W. N., Breslin J. W., Sánchez F. A. (2010) The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 87, 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vries H. E., Blom-Roosemalen M. C., de Boer A. G., van Berkel T. J., Breimer D. D., Kuiper J. (1996) Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J. Pharmacol. Exp. Ther. 277, 1418–1423 [PubMed] [Google Scholar]
- 25.AlFadhli S. (2013) Influence of endothelial nitric oxide synthase gene intron-4 27bp repeat polymorphism on its expression in autoimmune diseases. Dis. Markers 34, 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varma S., Breslin J. W., Lal B. K., Pappas P. J., Hobson R. W., II, Durán W. N. (2002) p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc. Res. 63, 172–178 [DOI] [PubMed] [Google Scholar]
- 27.De Cruz S. J., Kenyon N. J., Sandrock C. E. (2009) Bench-to-bedside review: the role of nitric oxide in sepsis. Expert Rev. Respir. Med. 3, 511–521 [DOI] [PubMed] [Google Scholar]
- 28.Yang B., Cai B., Deng P., Wu X., Guan Y., Zhang B., Cai W., Schaper J., Schaper W. (2015) Nitric oxide increases arterial endotheial permeability through mediating VE-cadherin expression during arteriogenesis. PLoS One 10, e0127931. 10.1371/journal.pone.0127931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubes P., Granger D. N. (1992) Nitric oxide modulates microvascular permeability. Am. J. Physiol. 262, H611–H615 [DOI] [PubMed] [Google Scholar]
- 30.Ramírez M. M., Quardt S. M., Kim D., Oshiro H., Minnicozzi M., Durán W. N. (1995) Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc. Res. 50, 223–234 [DOI] [PubMed] [Google Scholar]
- 31.Steyers C. M., III, Miller F. J., Jr (2014) Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 15, 11324–11349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreger H., Ludwig A., Weller A., Baumann G., Stangl V., Stangl K. (2016) Epigenetic suppression of iNOS expression in human endothelial cells: a potential role of Ezh2-mediated H3K27me3. Genomics 107, 145–149 [DOI] [PubMed] [Google Scholar]
- 33.Flaherty M. P., Brown M., Grupp I. L., Schultz J. E., Murphree S. S., Jones W. K. (2007) eNOS deficient mice develop progressive cardiac hypertrophy with altered cytokine and calcium handling protein expression. Cardiovasc. Toxicol. 7, 165–177 [DOI] [PubMed] [Google Scholar]
- 34.Di Lorenzo A., Lin M. I., Murata T., Landskroner-Eiger S., Schleicher M., Kothiya M., Iwakiri Y., Yu J., Huang P. L., Sessa W. C. (2013) eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 126, 5541–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Hu J., He T., Zhang Q., Yang X., Lan X., Zhang D., Mei H., Chen B., Huang Y. (2015) P38/MAPK contributes to endothelial barrier dysfunction via MAP4 phosphorylation-dependent microtubule disassembly in inflammation-induced acute lung injury. Sci. Rep. 5, 8895. 10.1038/srep08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig R., Larkin A., Mingo A. M., Thuerauf D. J., Andrews C., McDonough P. M., Glembotski C. C. (2000) p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release: evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 275, 23814–23824 [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi Y. (2010) The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 88, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 38.Liu B., Yang Y., Dai J., Medzhitov R., Freudenberg M. A., Zhang P. L., Li Z. (2006) TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J. Immunol. 177, 6880–6888 [DOI] [PubMed] [Google Scholar]
- 39.Santos M. J., Carmona-Fernandes D., Canhão H., Canas da Silva J., Fonseca J. E., Gil V. (2012) Early vascular alterations in SLE and RA patients—a step towards understanding the associated cardiovascular risk. PLoS One 7, e44668. 10.1371/journal.pone.0044668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi L., Zhang Z., Yu A. M., Wang W., Wei Z., Akhter E., Maurer K., Costa Reis P., Song L., Petri M., Sullivan K. E. (2014) The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One 9, e93846. 10.1371/journal.pone.0093846 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.