Abstract
Interactions of the presynaptic proteins, neuronal pentraxin 2 (NPTX2) and neurexin 2α (NRXN2α), with their respective postsynaptic functional partners, GluA4-containing glutamate (AMPA4) receptor and neuroligin 1 (NLGN1), enhance excitatory synaptic activity in some areas of the hippocampus and cerebral cortex. As early damage of such excitatory circuits in the brain tissues of participants with Alzheimer’s disease (AD) correlates with cognitive losses, plasma neuron-derived exosome (NDE) levels of these 2 pairs of specialized synaptic proteins were quantified to assess their biomarker characteristics. The NDE contents of all 4 proteins were decreased significantly in AD dementia (n = 46), and diminished levels of AMPA4 and NLGN1 correlated with the extent of cognitive loss. In a preclinical period, 6–11 yr before the onset of dementia, the NDE levels of all but NPTX2 were significantly lower than those of matched controls, and levels of all proteins declined significantly with the development of dementia. Reductions in NDE levels of these specialized excitatory synaptic proteins may therefore be indicative of the extent of cognitive loss and may reflect progression of the severity of AD.—Goetzl, E. J., Abner, E. L., Jicha, G. A., Kapogiannis, D., Schwartz, J. B. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease.
Keywords: dementia, neurodegeneration, biomarkers
Diminished synaptic function and loss of synapses are characteristic early elements of the neuropathology of Alzheimer’s disease (AD), usually attributed to the neuronal deposition of neurotoxic amyloid-β (Aβ) peptide oligomers (1, 2). The distribution and extent of brain synaptic pathology in postmortem brain tissues of patients with AD correlate generally with the severity of premortem cognitive loss (3, 4). Our initial analyses of plasma neuron-derived exosome (NDE) content of several synaptic proteins in AD demonstrated lower levels—similar to decreases in postmortem AD brain tissues—compared with those of matched controls. In cross-sectional studies, the plasma NDE levels of the presynaptic proteins, synaptotagmin and synaptophysin, and of the postsynaptic proteins, synaptopodin and neurogranin, in patients with AD were significantly lower than those for controls, whereas plasma NDE levels of the synaptic membrane protein, GAP43, were only marginally lower in patients with AD than in controls (5). The same synaptic proteins in NDEs from the plasma of participants who were initially cognitively normal but who subsequently developed definite AD dementia were at significantly lower preclinical levels than in plasma NDEs of matched controls (5). There was also a progressive decline of plasma NDE levels of synaptotagmin, synaptopodin, and GAP43, but not of synaptophysin or neurogranin, in these participants 2–10 yr later at the time of diagnosis of AD dementia.
Synaptic proteins that were investigated in our first study are widely distributed in CNS synapses and share functional properties of associating with some other synaptic proteins to form complexes that are capable of binding calcium, regulating synaptic calcium concentration, and controlling vesicle fusion, recycling, and readily releasable pool size (5). Two classes of proteins that have essential synaptic maintenance functions largely localized in excitatory circuits, rather than those of the widely distributed cluster studied by us originally, are also observed at lower levels in the postmortem brain tissues of patients with AD than in controls, and their losses appear to contribute directly to the pathogenesis of AD (6,–12).
Neuronal pentraxin 2 (NPTX2) complexes that include NPTX1 and NPTX receptors are expressed presynaptically and secreted by the excitatory synapses of pyramidal neurons of the hippocampus and cerebral cortex, where they bind specifically with the GluA4-containing glutamate (AMPA4) receptors on fast-spiking parvalbumin interneurons and thereby strengthen these excitatory synapses (9, 13). Decreased levels of NPTX2 and correspondingly diminished levels of AMPA4 in the brain tissues of patients with AD and mice with models of AD are associated with altered pyramidal neuron excitability (14). Presynaptic neurexin2α (NRXN2α) and the postsynaptic adhesion protein, neuroligin1 (NLGN1), interact trans-synaptically to ensure structural stability and functions of excitatory synapses in the hippocampus and cortex (6, 7). NLGN1 and NRXN2α both bind synaptotoxic Aβ peptide oligomers to increase their synaptic concentrations, thereby enhancing oxidative stress and promoting synaptic damage in AD (15,–17). Cognitive loss induced by the administration of Aβ peptide oligomers to mice is lessened by concurrent doses of Abs to NLGN1 and NRXN2α that diminish Aβ peptide oligomer binding by NLGN1 and NRXN2α (17).
Here, we report significantly lower levels of NPTX2, AMPA4, NLGN1, and NRXN2α in the plasma NDEs of patients with AD compared with those of matched controls as well as the striking progression of such diminished levels from those at a time of normal cognition in preclinical AD to those at the time of the development of AD dementia.
MATERIALS AND METHODS
Experimental design and patient evaluation
For cross-sectional studies, we retrospectively identified 28 patients with early AD—mild cognitive impairment or mild dementia—who had been evaluated extensively in the Clinical Research Unit of the National Institute on Aging (NIA; Baltimore, MD, USA) and 28 age- and gender-matched cognitively normal control participants who had donated blood at the Jewish Home of San Francisco (JHSF) during the same period of time as the patients (Table 1). For longitudinal studies, we identified 3 patients from the University of Kentucky Sanders-Brown Center on Aging, and 15 patients from JHSF with moderate AD who had provided blood at 2 times: first, when cognitively intact (AD1; Table 1), and again 6–11 yr later, after diagnosis of dementia (AD2; Table 1). Eighteen cognitively normal controls who were age and gender matched with the AD1 group were found at JHSF, and their plasmas were obtained in the same time period. One investigator (E.J.G.) supervised the identification and storage of all plasmas by the same methods and processed all plasmas together by the same procedures. Plasmas from patients in the longitudinal studies were analyzed without knowledge of the clinical data.
TABLE 1.
Characteristics of patients with AD and control participants
| Diagnosis | Total |
Age | MMSE | ADAS-cog | |
|---|---|---|---|---|---|
| n | Male/female | ||||
| Cross-sectional sets | |||||
| C | 28 | 12/16 | 73.2 ± 1.47 | 29.7 ± 0.13 | 3.32 ± 0.31 |
| AD | 28 | 12/16 | 73.1 ± 1.44 | 25.6 ± 0.83* | 13.7 ± 1.31* |
| Longitudinal sets | |||||
| C | 18 | 10/8 | 70.1 ± 1.66 | 28.3 ± 0.96 | 3.68 ± 0.45 |
| AD1 | 18 | 10/8 | 69.4 ± 1.71 | 28.7 ± 0.47 | 4.19 ± 0.57 |
| AD2 | 18 | 10/8 | 78.2 ± 1.75 | 20.2 ± 1.50* | 17.6 ± 1.64* |
AD and C are the patients and controls in the cross-sectional study of AD. AD1 and AD2 are the groups of patients with AD who were evaluated at 2 times in the longitudinal study, at a preclinical phase and after conversion to moderate dementia, respectively, and C is the control group matched to AD1 patients. ADAS-cog, AD Assessment Scale-cognitive subscale; MMSE, Mini-Mental State Examination. The significance of differences between cognitive state (MMSE and ADAS-cog) values of the groups were calculated by an unpaired Student’s t test for C vs. AD (cross-sectional sets) and for C vs. AD1 and by a paired Student’s t test for AD1 vs. AD2 (longitudinal sets). Data represent the means ± sem. *P < 0.001.
Patients with AD had mental status testing at the time of each blood sampling. Mini-Mental State Examination and the AD Assessment Scale-cognitive subscale were conducted as described previously (18). Cross-sectional patients from the NIA had amnestic mild cognitive impairment or mild dementia with a high probability of AD and a Clinical Dementia Rating global score of 0.5 or 1.0 according to NIA–Alzheimer’s Association and Informal Working Group (IWG)-2 criteria (19, 20). Each patient in the cross-sectional study had a CSF level of Aβ1–42 of <192 pg/ml and an elevated CSF level of P-T181-tau, which supported their diagnosis of AD (21). AD1/AD2 patients from JHSF and the University of Kentucky had probable AD and mild-to-moderate dementia at the AD2 stage by National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria and had a Clinical Dementia Rating global score of 1.0 at the time of the second blood collection (22).
The performance and procedures of all studies were approved by the respective institutional review committees of participating medical centers.
Blood and CSF sampling of patients and control participants
Ten milliliters of venous blood was drawn into 0.5 ml of saline with EDTA or a 100-U/ml final concentration of heparin and centrifuged for 15 min at 2500 g. Plasmas were stored in 0.25-ml aliquots at −80°C. CSF levels of P-T181-tau and Aβ1–42 were quantified by xMap Technology (Luminex, Austin, TX, USA) using Inno-Bia AlzBio3 Kits (Innogenetics, Ghent, Belgium).
Enrichment of plasma NDEs for extraction and ELISA quantification of proteins
Aliquots (0.25 ml) of plasma were incubated with 0.1 ml thromboplastin D (Thermo Fisher Scientific, Waltham, MA, USA), followed by the addition of calcium- and magnesium-free Dulbecco’s balanced salt solution with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Thermo Fisher Scientific) (5). After centrifugation at 3000 g for 30 min at 4°C, NDEs were harvested from the resultant supernatants by sequential ExoQuick (System Biosciences, Mountain View, CA, USA) precipitation and immunochemical enrichment with mouse anti-human CD171 (L1CAM neural adhesion protein) biotinylated Ab (clone 5G3; eBiosciences, San Diego, CA, USA) as previously described (5, 23, 24). M-PER mammalian protein extraction reagent (Thermo Fisher Scientific Life Sciences) lysates of NDEs that contained protease and phosphatase inhibitors were stored at −80°C. Astrocyte-derived exosomes (ADEs) were isolated as described previously (25) from plasmas of the same participants who provided the NDEs.
NDE and ADE proteins were quantified by ELISA kits for human tetraspanning exosome marker CD81 (American Research Products–Cusabio, Waltham, MA, USA); NPTX2 and AMPA4 (American Research Products–Cloud-Clone Corp.); and NRXN2α and NLGN1 (American Research Products—Qayee-Bio).
Mean values for all determinations of CD81 in each assay group was set at 1.00 and relative values of CD81 for each sample were used to normalize their recovery. The selective representation of the present set of synaptic proteins in NDEs, in contrast to ADEs, was demonstrated by concurrent analyses of NLGN1 and NRXN2α in both types of exosomes from plasmas of a subset of 20 control participants for the cross-sectional study. The respective levels of NLGN1 and NRXN2α were (means ± sem) 7548 ± 923 and 3921 ± 349 pg/ml for ADEs, and 174,226 ± 18,073 and 78,519 ± 7736 pg/ml for NDEs.
Statistical analyses
The Shapiro-Wilks test demonstrated that data in all sets were distributed normally. The statistical significance of differences between means for cross-sectional groups AD and C, and between longitudinal groups AD1 and C were determined by using unpaired Student’s t test, including a Bonferroni correction, and the significance of differences between means for longitudinal groups AD1 and AD2 were determined by using paired Student’s t test (Prism 6; GraphPad Software, La Jolla, CA, USA). Relationships between NDE content of a cargo synaptic protein and the corresponding cognitive score of a patient with AD were evaluated by Pearson correlation coefficients.
RESULTS
Patients with AD in the cross-sectional study had cognitive scores consistent with mild cognitive impairment or mild dementia that were significantly different from the normal range of scores for controls (Table 1). The longitudinal study participants who were evaluated initially at their AD1 preclinical phase had normal cognitive scores that were not different than those of their controls (Table 1). At the time of donation of the second blood sample, the longitudinal group was termed AD2 and had mild-to-moderate dementia and significantly worse cognitive scores than at the AD1 phase.
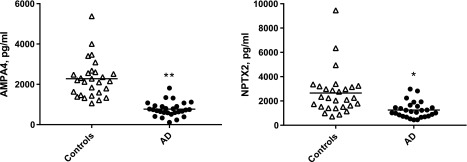
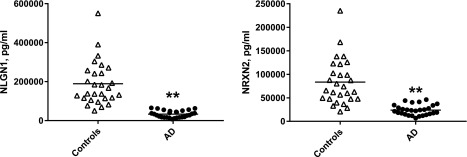
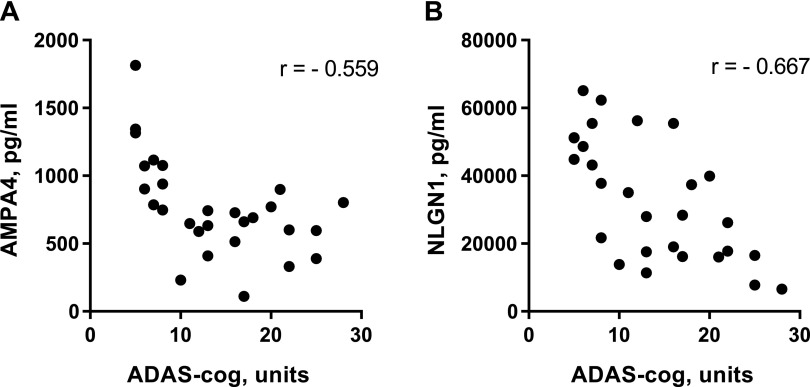
NDE levels of both synaptic proteins of the 2 sets were significantly lower than those of matched controls (Figs. 1 and 2). Values for the NLGN1–NRXN2α pair were higher than those of the AMPA4–NPTX2 pair and displayed much less overlap with control values. For NLGN1 and NRXN2α, only 4 and 5 control participant values, respectively, were in the range of those for patients with AD (Fig. 2). There were significant inverse correlations between elevated AD Assessment Scale-cognitive subscale scores and decreased NDE levels of AMPA4 and NLGN1, but not of NPTX2 or NRXN2α (Fig. 3). Similarly, there were significant positive correlations between depressed Mini-Mental State Examination scores and decreased NDE levels of AMPA4 (r = 0.621; P = 0.0004) and NLGN1 (r = 0.525; P = 0.0053), but not of NPTX2 or NRXN2α. No correlations were observed between reduced levels of CSF Aβ1–42 and decreased levels of any of the synaptic proteins. One half of patients with AD in the cross-sectional study were being treated with 5 or 10 mg/d donepezil, but there were no significant differences between levels of NDE synaptic proteins for treated and untreated subsets.
Figure 1.
NDE levels of AMPA4 and NPTX2 in cross-sectional control and AD groups. Each point represents the value for a control participant or patient with AD, and the horizontal line in point clusters is the mean level for that group. Control and AD patient values are 2276 ± 180 and 766 ± 68.0 pg/ml (means ± sem), respectively, for AMPA4 and 2656 ± 343 and 1250 ± 123 pg/ml, respectively, for NPTX2. The significance of differences between values for controls and patients with AD was calculated by using an unpaired Student’s t test. *P < 0.01, **P < 0.0001.
Figure 2.
NDE levels of NLGN1 and NRXN2α in cross-sectional control and AD groups. Each point represents the value for a control participant or patient with AD, and the horizontal line in point clusters is the mean level for that group. Control and AD patient values are 189,498 ± 21,106 and 33,155 ± 3305 pg/ml (means ± sem), respectively, for NLGN1, and 83,374 ± 9132 and 23,930 ± 2057 pg/ml, respectively, for NRXN2α. The significance of differences between values for controls and patients with AD was calculated by using an unpaired Student’s t test. **P < 0.0001.
Figure 3.
Correlations between NDE contents of specialized synaptic proteins and cognitive function of patients with AD in the cross-sectional set. Each point depicts the levels for 1 patient with AD. P = 0.0020 (A), P = 0.0001 (B).
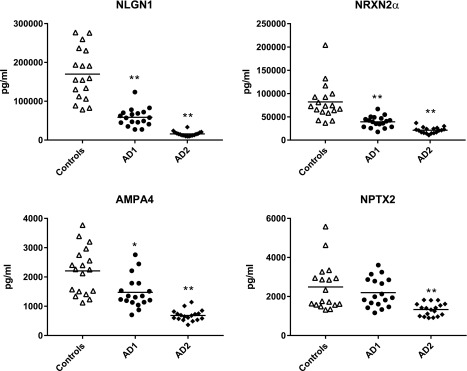
For the longitudinal series of patients with AD, NDE levels of AMPA4, NLGN1, and NRXN2α, but not NPTX2, were significantly lower than those of matched controls in the AD1 preclinical phase (Fig. 4). At the AD2 stage of mild-to-moderate dementia 6–11 yr later, NDE levels of all 4 synaptic proteins had decreased significantly for the group and in every patient compared with their levels at the AD1 phase.
Figure 4.
Courses of decline in NDE levels of specialized synaptic protein cargoes with worsening AD. Each point represents the value for a control participant or patient with AD, and the horizontal line in point clusters is the mean level for that group. Control, AD1 patient, and AD2 patient values are 169,870 ± 15,535, 58,721 ± 5451, and 15,875 ± 1405 pg/ml (means ± sem), respectively, for NLGN1; 81,968 ± 9306, 39,242 ± 2832, and 21,150 ± 1535 pg/ml, respectively, for NRXN2α; 2210 ± 188, 1476 ± 127, and 686 ± 44.1 pg/ml, respectively, for AMPA4; and 2488 ± 281, 2195 ± 175, and 1330 ± 76.2 pg/ml, respectively, for NPTX2. The significance of differences between values for controls and AD1 patients was calculated by using an unpaired Student’s t test, and for differences between values for AD1 and AD2 patients by using a paired Student’s t test. *P < 0.01, **P < 0.0001.
DISCUSSION
The methods described here permit the quantification of meaningful levels of both members of the 2 sets of excitatory synaptic proteins as well as the demonstration of significant differences between levels in patients with AD and controls and between preclinical and clinically apparent stages of AD (Figs. 1, 2, and 4). There are 4 major differences between the present results and the findings for the group of broadly distributed synaptic proteins previously reported (5).
The first is distinctive functions in specific excitatory synapses of the hippocampus and areas of the cerebral cortex. Presynaptic complexes that include NPTX2 are secreted into the excitatory synapses of pyramidal neurons of the hippocampus and cerebral cortex, bind specifically with AMPA4, and thereby mediate enhanced synaptic transmission in these circuits. Presynaptic NRXN2α and the postsynaptic adhesion protein, NLGN1, interact trans-synaptically in these excitatory synapses of the hippocampus and cortex to also ensure structural stability and enhanced synaptic function. The second distinguishing feature of these 2 protein pairs is their localization in areas that are affected very early in AD, where the NRXN2α–NLGN1 pair also may directly contribute to pathogenesis via binding and the selective concentration of neurotoxic oligomers of Aβ peptides, such as Aβ1–42 (17).
The third difference between 2 of these functionally specialized synaptic proteins and numerous other NDE cargo proteins that have been implicated in AD is the correlation between cognitive scores and the levels of AMPA4 and NLGN1 (Fig. 3). This type of correlation, that suggests value for NDE levels of these proteins as indicators of AD clinical severity, is shared only by the more broadly distributed synaptic proteins synaptopodin, synaptotagmin, and synaptophysin, but not by a wide range of other NDE cargo proteins (5). Finally, the fourth distinguishing feature of these 2 synaptic protein pairs is a striking progressive decrease in all NDE levels as patient clinical status declines from normal cognition in the preclinical stage to dementia with overt AD (Fig. 4). This progressive reduction in NDE level with declining clinical status was observed only for a synaptotagmin, GAP43, and to a much lesser extent for synaptopodin, but not for synaptophysin or neurogranin, of the more broadly distributed set of synaptic proteins (5).
In our prior study of NDE levels of a more broadly distributed set of synaptic proteins, decreases for 16 patients with frontotemporal dementia (FTD) compared with those of matched controls approached the magnitude of decreases that were observed for patients with AD only for one synaptotagmin and synaptopodin (5). Limited supplies of plasmas from this same group of patients with FTD and controls permitted the generation now of only fragmentary information for 6 of them. For patients with FTD and matched controls, NDE levels were 116,244 ± 13,085 and 153,286 ± 17,902 pg/ml (means ± sem), respectively, for NLGN1 and 58,114 ± 6370 and 75,192 ± 8934 pg/ml, respectively, for NRXN2α. Although the decreases of these 2 synaptic proteins in patients with FTD seem to be less than for patients with AD, the number of participants is too low for meaningful statistical analyses and data from more patients are required.
Several variables may affect the laboratory results reported here. Diminished NDE levels of cargo proteins reflect lower neuronal concentrations of these proteins, but may also be influenced by less efficient loading of some proteins into NDEs as the disease progresses. There also is the potential involvement of altered postloading proteolysis. Interpretation of the results is also subject to several limitations. One such issue is sample size, such that findings may not be generalizable to larger and different populations. Nonetheless, the findings reported here advance our understanding of which synaptic proteins may be affected in early AD. The possibility of establishing this new set of specialized excitatory synaptic proteins as useful biomarkers of the stage and severity of AD will depend on additional analyses of NDE trafficking and protein handling, as well as clinical investigations of larger groups of patients over longer periods of time.
ACKNOWLEDGMENTS
The authors thank Judith H. Goetzl (Jewish Home of San Francisco) for expert preparation of the illustrations. This work was supported by a grant from the Biomarkers Across Neurodegenerative Diseases 2 (BAND2) program of the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research United Kingdom, and the Weston Brain Institute (to E.J.G.); and by the U.S. National Institutes of Health (NIH) National Institute on Aging (NIA) (Grant P30028383; to G.A.J.). D.K. was supported by the Intramural Research Program of the NIH NIA. E.J.G. has filed an application with the U.S. Patent Office for the platform and methodologies described in this report. The remaining authors declare no conflicts of interest.
Glossary
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- AD1
cognitively intact
- AD2
after diagnosis of Alzheimer’s disease dementia
- ADE
astrocyte-derived exosome
- AMPA4
GluA4-containing glutamate
- FTD
frontotemporal dementia
- JHSF
Jewish Home of San Francisco
- NDE
neuron-derived exosome
- NIA
National Institute on Aging
- NLGN1
neuroligin 1
- NPTX2
neuronal pentraxin 2
- NRXN2α
neurexin 2α
AUTHOR CONTRIBUTIONS
E. J. Goetzl developed the initial concept and approach; E. J. Goetzl and J. B. Schwartz designed the study; E. J. Goetzl performed the exosome isolations and ELISAs; E. L. Abner, G. A. Jicha, D. Kapogiannis, and J. B. Schwartz selected and evaluated the patients and control participants; and E. J. Goetzl, E. L. Abner, D. Kapogiannis, and J. B. Schwartz prepared and edited the manuscript.
REFERENCES
- 1.Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D. J. (2002) Alzheimer’s disease is a synaptic failure. Science 298, 789–791 [DOI] [PubMed] [Google Scholar]
- 3.DeKosky S. T., Scheff S. W. (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 [DOI] [PubMed] [Google Scholar]
- 4.Clare R., King V. G., Wirenfeldt M., Vinters H. V. (2010) Synapse loss in dementias. J. Neurosci. Res. 88, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetzl E. J., Kapogiannis D., Schwartz J. B., Lobach I. V., Goetzl L., Abner E. L., Jicha G. A., Karydas A. M., Boxer A., Miller B. L. (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 30, 4141–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Südhof T. C. (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler-Llavina G. J., Fuccillo M. V., Ko J., Südhof T. C., Malenka R. C. (2011) The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc. Natl. Acad. Sci. USA 108, 16502–16509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bie B., Wu J., Yang H., Xu J. J., Brown D. L., Naguib M. (2014) Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat. Neurosci. 17, 223–231 [DOI] [PubMed] [Google Scholar]
- 9.Chang M. C., Park J. M., Pelkey K. A., Grabenstatter H. L., Xu D., Linden D. J., Sutula T. P., McBain C. J., Worley P. F. (2010) Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 13, 1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner M. W., Veitch D. P., Aisen P. S., Beckett L. A., Cairns N. J., Cedarbaum J., Green R. C., Harvey D., Jack C. R., Jagust W., Luthman J., Morris J. C., Petersen R. C., Saykin A. J., Shaw L., Shen L., Schwarz A., Toga A. W., Trojanowski J. Q.; Alzheimer’s Disease Neuroimaging Initiative (2015) 2014 update of the Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 11, e1–e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelkey K. A., Barksdale E., Craig M. T., Yuan X., Sukumaran M., Vargish G. A., Mitchell R. M., Wyeth M. S., Petralia R. S., Chittajallu R., Karlsson R. M., Cameron H. A., Murata Y., Colonnese M. T., Worley P. F., McBain C. J. (2016) Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 90, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S. J., Wei M., Zhang C., Maxeiner S., Pak C., Calado Botelho S., Trotter J., Sterky F. H., Südhof T. C. (2017) Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J. Neurosci. 37, 1062–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu D., Hopf C., Reddy R., Cho R. W., Guo L., Lanahan A., Petralia R. S., Wenthold R. J., O’Brien R. J., Worley P. (2003) Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39, 513–528 [DOI] [PubMed] [Google Scholar]
- 14.Xiao M. F., Xu D., Craig M. T., Pelkey K. A., Chien C. C., Shi Y., Zhang J., Resnick S., Pletnikova O., Salmon D., Brewer J., Edland S., Wegiel J., Tycko B., Savonenko A., Reeves R. H., Troncoso J. C., McBain C. J., Galasko D., Worley P. F. (2017) NPTX2 and cognitive dysfunction in Alzheimer’s disease. eLife 6, e23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinamarca M. C., Di Luca M., Godoy J. A., Inestrosa N. C. (2015) The soluble extracellular fragment of neuroligin-1 targets Aβ oligomers to the postsynaptic region of excitatory synapses. Biochem. Biophys. Res. Commun. 466, 66–71 [DOI] [PubMed] [Google Scholar]
- 16.Tristán-Clavijo E., Camacho-Garcia R. J., Robles-Lanuza E., Ruiz A., van der Zee J., Van Broeckhoven C., Hernandez I., Martinez-Mir A., Scholl F. G. (2015) A truncating mutation in Alzheimer’s disease inactivates neuroligin-1 synaptic function. Neurobiol. Aging 36, 3171–3175 [DOI] [PubMed] [Google Scholar]
- 17.Brito-Moreira J., Lourenco M. V., Oliveira M. M., Ribeiro F. C., Ledo J. H., Diniz L. P., Vital J. F. S., Magdesian M. H., Melo H. M., Barros-Aragão F., de Souza J. M., Alves-Leon S. V., Gomes F. C. A., Clarke J. R., Figueiredo C. P., De Felice F. G., Ferreira S. T. (2017) Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J. Biol. Chem. 292, 7327–7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano S. J., Posner H. B., Moline M. L., Hurt S. W., Swartz J., Hsu T., Hobart J. C. (2010) The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 81, 1363–1368 [DOI] [PubMed] [Google Scholar]
- 19.Petersen R. C. (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 [DOI] [PubMed] [Google Scholar]
- 20.Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R. Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw L. M., Vanderstichele H., Knapik-Czajka M., Clark C. M., Aisen P. S., Petersen R. C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V. M., Trojanowski J. Q.; Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 [DOI] [PubMed] [Google Scholar]
- 23.Kapogiannis D., Boxer A., Schwartz J. B., Abner E. L., Biragyn A., Masharani U., Frassetto L., Petersen R. C., Miller B. L., Goetzl E. J. (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 29, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustapic M., Eitan E., Werner J. K. Jr., Berkowitz S. T., Lazaropoulos M. P., Tran J., Goetzl E. J., Kapogiannis D. (2017) Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front. Neurosci. 11, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetzl E. J., Mustapic M., Kapogiannis D., Eitan E., Lobach I. V., Goetzl L., Schwartz J. B., Miller B. L. (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]