Abstract
We investigated the role of gibberellins (GAs) in the effect of pat-2, a recessive mutation that induces facultative parthenocarpic fruit development in tomato (Lycopersicon esculentum Mill.) using near-isogenic lines with two different genetic backgrounds. Unpollinated wild-type Madrigal (MA/wt) and Cuarenteno (CU/wt) ovaries degenerated, but GA3 application induced parthenocarpic fruit growth. On the contrary, parthenocarpic growth of MA/pat-2 and CU/pat-2 fruits, which occurs in the absence of pollination and hormone application, was not affected by GA3. Pollinated MA/wt and parthenocarpic MA/pat-2 ovary development was negated by paclobutrazol, and this inhibitory effect was counteracted by GA3. The main GAs of the early-13-hydroxylation pathway (GA1, GA3, GA8, GA19, GA20, GA29, GA44, GA53, and, tentatively, GA81) and two GAs of the non-13-hydroxylation pathway (GA9 and GA34) were identified in MA/wt ovaries by gas chromatography-selected ion monitoring. GAs were quantified in unpollinated ovaries at flower bud, pre-anthesis, and anthesis. In unpollinated MA/pat-2 and CU/pat-2 ovaries, the GA20 content was much higher (up to 160 times higher) and the GA19 content was lower than in the corresponding non-parthenocarpic ovaries. The application of an inhibitor of 2-oxoglutarate-dependent dioxygenases suggested that GA20 is not active per se. The pat-2 mutation may increase GA 20-oxidase activity in unpollinated ovaries, leading to a higher synthesis of GA20, the precursor of an active GA.
Fruit development can be divided usually into three phases (Gillaspy et al., 1993). The earliest phase (up to around anthesis) involves the development of the ovary and the decision to abort or to continue with growth. It is currently accepted that the coordinated action of growth signals triggers fruit-set and growth, so in the absence of pollination and fertilization, the ovary will cease cell division and abscise (Gillaspy et al., 1993; García-Martínez and Hedden, 1997). Parthenocarpy is an alternative pathway to normal fruit-set and development, in which the ovary grows into fruit without fertilization and seed formation (Lukyanenko, 1991), and can be artificially induced by the application of hormones, mainly auxins and gibberellins (GAs) (Goodwin, 1978; Schwabe and Mills, 1981). In the case of natural parthenocarpy, it has been proposed to be the result of conditions that induce a threshold concentration of growth substances in the ovary sufficient to promote growth in the absence of pollination and fertilization (Nitsch, 1970).
There is some evidence supporting the hypothesis that GAs may control, at least partially, fruit development in seeded tomato (Lycopersicon esculentum Mill.). First, fruit growth can be induced by the application of GAs to unpollinated ovaries (Schwabe and Mills, 1981; Sawhney, 1984). Second, fruits containing GA-producing seeds are larger than those with GA-deficient seeds (Groot et al., 1987). Third, GAs of the early-13-hydroxylation (GA1, GA8, GA17, GA19, GA20, GA29, and GA44) and of the non-13-hydroxylation (GA9, GA15, GA24, and GA25) biosynthetic pathways have been identified in developing pollinated fruits (Bohner et al., 1988; Koshioka et al., 1994). These GAs may be synthesized in the ovary itself, because genes coding for GA biosynthesis enzymes (copalyl diphosphate synthase, GA 20-oxidase, and GA 3β-hydroxylase) are expressed in developing tomato flowers and fruit (Rebers et al., 1999). Parthenocarpy in tomato may also depend on GAs, because natural parthenocarpic fruits contain more GA-like substances than their non-parthenocarpic counterparts early after anthesis (Mapelli et al., 1978), and tomato fruits induced by the application of 4-chlorophenoxyacetic acid (an auxin) also contain high levels of GAs (Koshioka et al., 1994).
Natural parthenocarpy in tomato has been widely studied due to its potential use as a solution to poor fruit-set at unfavorable environmental conditions (George et al., 1984). Several genotypes carrying gene(s) inducing parthenocarpy have been described and selected in tomato (Philouze, 1983; George et al., 1984; Lukyanenko, 1991; Mazzucato et al., 1998). Among the different sources of natural parthenocarpy, the Russian cv Severianin obtained by Solovjeva (cited by Philouze, 1983) is of particular interest because of its strong expressivity, its facultative character, and its simple genetic control (Philouze et al., 1980). The capability of cv Severianin to set seedless fruits with complete locule fill under unfavorable environmental conditions is mainly due to the pat-2 gene (Philouze and Maisonneuve, 1978; Nuez et al., 1986; Vardy et al., 1989), which induces a different protein in the ovary after anthesis (Barg et al., 1990). The analysis by two-dimensional PAGE of in vitro translation products of RNAs from flowers and ovaries before anthesis also shows a differential expression associated to pat-2-induced parthenocarpy (Fos and Nuez, 1996; Fos and Nuez, 1997). This suggests that the molecular events observed in pat-2 ovaries before anthesis, which are associated with parthenocarpic fruit growth, may modify the hormone content of the ovary before pollination.
In the present study, the possible role of GAs in parthenocarpic tomato fruit development controlled by pat-2 has been investigated at stages before fruit-set using near-isogenic lines. The inhibition of fruit-set by paclobutrazol and its reversal by GA3 suggested that fruit growth of both seeded wild-type (wt) and parthenocarpic pat-2 tomato depends on GAs. The quantification of endogenous GA levels in developing tomato ovaries showed the accumulation of very large amounts of GA20 in pat-2 ovaries before anthesis. However, GA20 may not be active per se, because the application of an inhibitor of 2-oxoglutarate-dependent dioxygenases decreased parthenocarpic fruit development. These results suggest that the parthenocarpic capability of pat-2 ovaries may be the result of the accumulation of GA20, leading to an early higher synthesis of active GA in the absence of pollination and fertilization.
MATERIALS AND METHODS
Plant Material
Two non-parthenocarpic tomato (Lycopersicon esculentum Mill.) lines, Madrigal (MA/wt) and Cuarenteno (CU/wt), and their corresponding near-isogenic parthenocarpic lines carrying the pat-2 gene, Par54-11 (MA/pat-2) and Par14-11 (CU/pat-2), were used in the experiments. The parthenocarpic lines were generated in our laboratory from crosses of MA/wt and CU/wt with the Russian cv Severianin (source of pat-2) (Nuez et al., 1985). Line MA/pat-2 has been backcrossed to the non-parthenocarpic line MA/wt four times, and line CU/pat-2 is an F4 selection that was then backcrossed three times with the non-parthenocarpic CU/wt line.
The plants were grown during autumn-winter in an air-conditioned greenhouse set at an average temperature of 10°C in large containers (6 m × 40 cm × 50 cm depth; 18 plants per container) with sand (experiment of Fig. 1), or in 25-L pots containing a peat:soil (1:1) mixture irrigated with nutrient solution. The temperatures fluctuated according to the environment, but extremes were never higher than 21°C (day) or lower than 6°C (night).
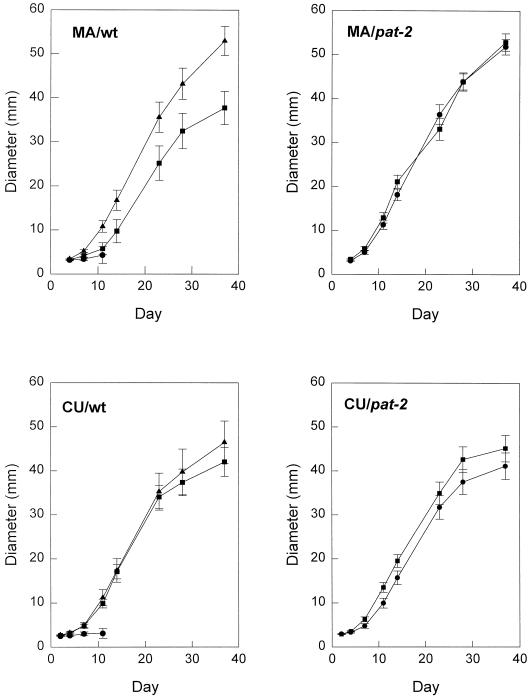
Figure 1.
Development of MA/wt, MA/pat-2, CU/wt, and CU/pat-2 fruits. The points are means ± se of eight to 12 developing fruits. ▴, Self pollinated flowers; ●, flowers emasculated and unpollinated; ▪, ovaries from emasculated flowers treated with GA3 (2 μg ovary−1).
Ovaries from flowers at the flower bud (FB), preanthesis (PR), and anthesis (AN) stage, as previously described (Fos and Nuez, 1996), were collected from the second to sixth clusters for the determination of GAs (Table I). The ovaries were frozen immediately in liquid N2 and stored at −80°C until extraction.
Table I.
Number and weight of tomato ovaries at different developmental stages collected for quantification of GAs
| Line | FB
|
PR
|
AN
|
|||
|---|---|---|---|---|---|---|
| Total no. | Ovary wt | Total no. | Ovary wt | Total no. | Ovary wt | |
| mg | mg | mg | ||||
| MA/wt | 170 | 6.1 | 70 | 12.4 | 53 | 19.7 |
| MA/pat-2 | 145 | 6.1 | 77 | 12.2 | 45 | 19.5 |
| CU/wt | 215 | 5.7 | 110 | 11.2 | 70 | 17.8 |
| CU/pat-2 | 140 | 5.9 | 70 | 11.8 | 58 | 18.1 |
Plant Hormone Application
Flowers from the second to fourth clusters were emasculated 1 d before anthesis to prevent self-pollination, and 2 to 3 d later the ovaries were treated with different amounts of GA1 (Dr. Michel Beale, Long Ashton Research Station, Long Ashton, UK), GA3 (Fluka, Sigma-Aldrich Química SA, Alcobendas, Spain), or GA20 (purchased from Dr. L. Mander, Australian National University, Canberra) at the concentrations specified in each experiment (in 20 μL of 5% [v/v] methanol containing 0.1% [v/v] Tween 80, applied directly to the ovary). Paclobutrazol (Imperial Chemical Industries, Bracknell, UK; 5 μg per ovary), an inhibitor of ent-kaurene oxidase, and 3,5-dioxo-4-butyryl-cyclohexane carboxylic acid ethyl ester (LAB 198 999 [LAB], BASF, Limburgerhof, Germany; at 1, 5, and 10 mm), an acylcyclohexadione derivative that inhibits 2-oxoglutarate-dependent dioxygenases (Rademacher et al., 1992), were applied to pollinated MA/wt and unpollinated MA/pat-2 ovaries alone or together with GAs. Control ovaries were treated with the same volume of solvent solution. Each treatment was carried out on nine to 12 ovaries (three to four plants, one cluster per plant, and three ovaries per cluster).
Extraction and Purification of GAs
Frozen material, previously ground in a mortar with liquid N2, was homogenized with a polytron homogenizer (PT-10, Kinematica, Kriens-Luzern, Switzerland) in cold 80% (v/v) methanol (1:25, w/v), stirred for 12 h at 4°C, and re-extracted twice for 30 min with one-half volume of methanol. To determine recoveries during extraction and purification and to allow GAs to be located during HPLC, the following radioactive GAs were added to the homogenized samples: [3H2]GA1 (1.39 TBq mmol−1), 16,17-dihydro[3H2]GA19 (2.45 TBq mmol−1), and [3H2]GA20 (1.41 TBq mmol−1). Methanol was removed under vacuum at 40°C, and the aqueous residue purified by solvent partitioning QAE-Sephadex A-25 (Pharmacia, Milton Keynes, UK) chromatography and C18-BondElut (500 mg; Varian, Harbor City, CA), as described by García-Martínez et al. (1991).
Reverse-Phase HPLC
Dried samples were dissolved in 10% (v/v) methanol (0.4 mL), injected onto a 4-μm C18 column (15 cm long, 3.9 mm i.d.; NovaPak, Millipore, Milford, MA), and eluted with a linear gradient of 10% to 100% (v/v) methanol containing 50 μL L−1 (v/v) acetic acid over 40 min at 1 mL min−1. Fractions were grouped according to retention times of radioactive GA markers and taken to dryness in vacuo. Retention times of standards were 17 to 18 min for GA1, 24 to 25 min for GA20, and 26 to 28 min for dihydro-GA19.
GA Identification
GAs were identified in extracts of non-parthenocarpic MA ovaries, collected from developing flowers before pollination (from FB to AN stage). A total mixed population of 365 ovaries (3,535 mg fresh weight [FW]) were extracted and purified as described above. Dried samples from pooled HPLC fractions were dissolved in 0.5 mL of methanol and methylated with ethereal diazomethane for 15 min at room temperature. They were then dried in vacuo and trimethylsilylated with N-methyl-n-trimethylsilyltrifluoroacetamide (Pierce, Rockford, IL) for 30 min at 90°C. GA20 was also identified in extracts from a pool of MA/pat-2 and CU/pat-2 ovaries (267 and 268, respectively) collected from flowers from FB to AN.
The analyses were carried out with a gas chromatograph (model 5890, Hewlett-Packard, Palo Alto, CA) coupled to a mass-selective detector (model 5971A, Hewlett-Packard) using an HP-1 capillary column (25 m long, 0.2-mm i.d., 0.33-μm film thickness), under the conditions described by Hedden et al. (1989). The samples (1 μL) were co-injected with 0.1 μL of Parafilm extract in n-hexane to determine the Kovats Retention Index (KRI) values (Gaskin et al., 1971). The initial oven temperature was maintained at 60°C for 1 min and then increased at 20°C min−1 to 240°C and at 4°C min−1 to 300°C. The MS ion source was operated at 70 eV and 175°C to 185°C, the injector temperature at 220°C, and the interface temperature at 280°C. The samples were run using the selected ion monitoring (SIM) mode.
GA Quantification
The samples were extracted and purified as described above. The number and FW of ovaries collected for GA quantification are shown in Table I, and aliquots of approximately 300 mg FW were used for quantification. For these analyses, the appropriate amounts of the following internal standards were added to the samples at the time of extraction: [17-2H2]GA1, [17-2H2]GA3, [17-2H2]GA8, [17-2H2]GA19, [17-2H2]GA20, and [17-2H2]GA29 (purchased from Prof. L. Mander, Australian National University, Canberra).
The chromatographic and spectrometric conditions described above were also used in quantitative analysis. The concentrations of GAs in the original extracts were determined from the peak area ratios of the following pairs of ions: 506/508 for GA1, 504/506 for GA3, 594/596 for GA8, 434/436 for GA19, 418/420 for GA20, and 506/508 for GA29 by references to calibration curves. Additional ions were also monitored to confirm the identify of the GAs quantified.
Statistical Methods
Statistical treatments of the data were made by analysis of variance using the Fisher's lsd procedure to discriminate among the means (Statgraphics Plus program, version 3.1 for Windows, Statistical Graphics, Rockville, MD).
RESULTS
Growth of Non-Parthenocarpic and Parthenocarpic Ovaries
Unpollinated MA/wt and CU/wt ovaries grew very little compared with pollinated ovaries, and abscised about 10 d after anthesis (Fig. 1). In contrast, unpollinated MA/pat-2 and CU/pat-2 ovaries developed similarly to pollinated wt ovaries, leading to mature fruits essentially identical to wt seeded fruits except for the absence of seeds (Fig. 1). It is already known that the final weight of seedless and seeded pat-2 fruits in both the MA and CU backgrounds are similar (Nuez et al., 1991).
The application of GA3 (2 μg) induced set of unpollinated MA/wt and CU/wt ovaries, and the final size and weight of parthenocarpic wt fruits induced by GA3 were similar to those developed from pollinated ovaries in CU plants, but significantly smaller in MA plants (Fig. 1). The application of GA3 did not alter the growth of unpollinated MA/pat-2 and CU/pat-2 ovaries (Fig. 1).
Inhibition of Tomato Fruit Growth with Paclobutrazol
The means of the final diameters and weights of pollinated MA/wt and parthenocarpic MA/pat-2 fruits were not significantly different (Table II). However, the application of paclobutrazol (5 μg) prevented the growth of both pollinated MA/wt (all the ovaries abscised) and unpollinated MA/pat-2 (only one ovary developed, although significantly less than the control) ovaries and the application of GA3 (2 μg) fully reverted this inhibition (Table II).
Table II.
Inhibition of fruit-set and growth of seeded MA/wt and parthenocarpic MA/pat-2 fruits by paclobutrazol
| Treatment | wt (Pollinated)
|
pat-2 (Unpollinated)
|
||||
|---|---|---|---|---|---|---|
| Fruit-seta | Diameter | wt | Fruit-seta | Diameter | wt | |
| cm | g | cm | g | |||
| Control | 9 /12 | 4.9a | 56.5a | 9 /12 | 4.6a | 39.1a |
| Paclobutrazol | 0 /10 | — | — | 1 /12 | 1.7b | 2.8b |
| GA3 | 12 /12 | 5.2a | 66.0a | 12 /12 | 4.1a | 35.8a |
| Paclobutrazol + GA3 | 10 /12 | 4.5a | 43.5a | 12 /12 | 4.1a | 30.9a |
The experiments were carried out on hand-pollinated (the day equivalent to anthesis) (wt) or unpollinated (pat-2) tomato ovaries with MA genetic background. Paclobutrazol (5 μg) and GA3 (2 μg) were applied in 20 μL of solution per ovary at the day equivalent to anthesis, and developed fruits (those having a weight higher than 1 g) collected 65 to 68 d later. Control ovaries were treated with a similar volume of solvent. Fruit-set, Number of fruits developed over the total number of treated ovaries. Values of diameter and weight are means of those of developed fruits. At each column, values with different letter were significantly different (P < 0.05).
Fruits developed/treated ovaries.
Identification of GAs in Tomato Ovaries before Pollination
The identification of GAs in an extract of developing tomato ovaries (a mixed population from FB to AN) of the non-parthenocarpic line MA/wt was carried out by comparing the relative abundance of at least five characteristic ions and KRI values with those of authentic standards. The following GAs of the early-13-hydroxylation biosynthetic pathway could be identified: GA1, GA8, GA19, GA20, GA29, GA44, and GA53. GA3, as well as two members of the non-13-hydroxylation pathway, GA9 and GA34, were also identified in young ovaries. In addition, a compound with the same KRI as GA81, a 2α-hydroxy derivative of GA20, was also tentatively identified for the first time in tomato (Table III).
Table III.
GAs present in unpollinated tomato ovaries
| HPLC Fraction | GA | Origin | KRI | Characteristic Ions m/z |
|---|---|---|---|---|
| mL | % relative intensity of base peak | |||
| 7–12 | GA8 | Ovaries | 2,832 | 594(M+,100), 535(16), 448(77), 379(95), 375(98) |
| Stand | 2,832 | 594(M+,100), 535(6), 448(60), 379(52), 375(54) | ||
| GA29 | Ovaries | 2,701 | 506(M+,100), 491(14), 447(11), 389(14), 375(45), 235(48) | |
| Stand | 2,698 | 506(M+,100), 491(13), 447(12), 389(117), 375(33), 235(31) | ||
| GA81 | Ovaries | 2,698 | 506(M+,100), 491(24), 459(12), 447(38), 389(31), 375(56), 303(125) | |
| Stand | 2,694 | 506(M+,100), 491(10), 459(21), 447(12), 389(10), 375(85), 303(167) | ||
| 17–18 | GA1 | Ovaries | 2,694 | 506(M+,100), 491(13), 448(32), 376(59), 375(30), 313(20), 207(112) |
| Stand | 2,694 | 506(M+,100), 491(12), 448(28), 376(410), 375(27), 313(21), 207(106) | ||
| GA3 | Ovaries | 2,714 | 504(M+,100), 489(9), 475(4), 370(13), 347(14) | |
| Stand | 2,714 | 504(M+,100), 489(9), 475(9), 370(9), 347(16) | ||
| 23–25 | GA20 | Ovaries | 2,520 | 418(M+,100), 403(19), 375(78), 359(18), 301(17), 235(17), 207(145) |
| Stand | 2,523 | 418(M+,100), 403(17), 375(67), 359(15), 301(20), 235(13), 207(59) | ||
| 26–28 | GA19 | Ovaries | 2,629 | 462(M+,9), 434(100), 402(73), 375(59), 374(98), 238(100 |
| Stand | 2,631 | 462(M+,8), 434(100), 402(46), 375(59), 374(86), 238(42) | ||
| GA44 | Ovaries | 2,823 | 432(M+,3), 417(3), 404(2), 373(7), 238(7), 208(25), 207(100) | |
| Stand | 2,823 | 432(M+,3), 417(3), 404(2), 373(2), 238(4), 208(23), 207(100) | ||
| GA9 | Ovaries | 2,367 | 330(M+,19), 298(100), 286(8), 270(78), 243(59), 227(21) | |
| Stand | 2,365 | 330(M+,10), 298(100), 286(8), 270(78), 243(53), 227(29) | ||
| GA34 | Ovaries | 2,692 | 506(M+,100), 459(9), 416(11), 387(16), 372(16), 356(8), 288(45) | |
| Stand | 2,690 | 506(M+,100), 459(11), 416(13), 387(13), 372(17), 356(16), 288(34) | ||
| 29–33 | GA53 | Ovaries | 2,532 | 448(M+,100), 433(24), 419(16), 416(48), 389(55) |
| Stand | 2,531 | 448(M+,100), 433(21), 419(19), 416(52), 389(58) |
Ovaries were collected from MA/wt flowers between the FB and the AN stage. Compounds were identified as methyl esters trimethylsilyl ethers by comparison of selected ions and KRIs with those of standards (Stand).
Quantification of GAs from Tomato Ovaries
The content (nanograms per gram FW) of GA1, GA8, GA19, GA20, and GA29 in ovaries from flowers at three developmental stages (FB, PR, and AN) of the non-parthenocarpic tomato lines MA/wt and CU/wt and their respective near-isogenic parthenocarpic (pat-2) lines were determined by GC-SIM (Fig. 2). GA3 was also quantitated but it was always at very low level or undetected (results not presented).
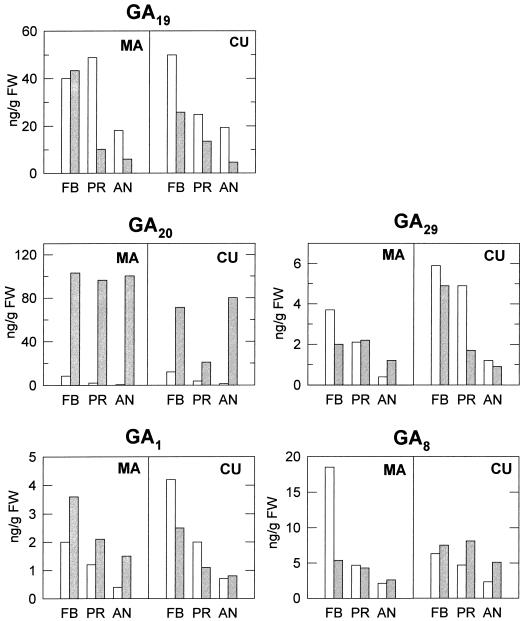
Figure 2.
Concentration of GA1, GA8, GA19, GA20, and GA29 in unpollinated ovaries of non-parthenocarpic (MA/wt and CU/wt) and parthenocarpic (MA/pat-2 and CU/pat-2) near-isogenic lines of tomato at three developmental stages. FB, Flower bud; PR, preanthesis; AN, anthesis. White bars, wt; shaded bars, pat-2.
In non-parthenocarpic ovaries, the highest GA concentrations were found at the FB stage: about 40 to 50 ng g−1 FW for GA19, 10 to 15 ng g − FW for GA20, 4 to 6 ng g−1 FW for GA29, 2 to 4 ng g−1 FW for GA1, and 7 to 17 ng g−1 FW for GA8. The concentrations of all GAs decreased progressively during ovary development in the two non-parthenocarpic lines. In the case of GA19, its content was higher in non-parthenocarpic than in pat-2 ovaries (three to five times higher with the MA background, and two to four times higher with the CU background), except at the FB stage with the MA background, which were very similar (Fig. 2). In contrast, the level of GA20, the immediate product of GA19 metabolism was clearly higher in pat-2 than in wt ovaries at the three stages of flower development analyzed: between 10 and 160 times with the MA background, and between five and 50 times higher with the CU background (Fig. 2). The concentration of GA20 was particularly high and constant (about 100 ng g−1 FW) in pat-2 ovaries with MA background. The content of GA1 was higher in pat-2 ovaries with MA (about 2- to 4-fold), but not with CU background (where similar or higher GA1 levels were found in non-parthenocarpic ovaries). The content of GA8 was lower in pat-2 than in non-parthenocarpic ovaries in MA background at the FB stage (about 3-fold), and similar at later developmental stages (Fig. 2), whereas with the CU background it was higher (about 2-fold at PR and AN stages) in pat-2 ovaries. The differences in the contents of GA29 between non-parthenocarpic and pat-2 ovaries were either not consistent (MA background) or not significant (CU background, except at PR stage, where the pat-2 ovaries contained about three times less GA29).
To check that the m/z ion 418, used to quantitate GA20, was not contaminated in pat-2 ovaries with a similar ion from another compound with the same retention time as GA20, extracts from pools of MA/pat-2 and CU/pat-2 ovaries (from FB to AN flowers) were analyzed by GC-SIM measuring the abundance of the same ions used to identify GA20 in MA/wt ovaries (Table II). The results showed clearly the presence of uncontaminated GA20 in both kinds of pat-2 ovaries (Table IV).
Table IV.
Identification of GA20 in unpollinated MA/pat-2 and CU/pat-2 tomato ovaries
| Material | KRI | Characteristic Ions m/z |
|---|---|---|
| % relative intensity of base peak | ||
| MA/pat-2 | 2,525 | 418(M+,100), 403(17), 375(68), 359(21), 301(29), 235(18), 207(57) |
| CU/pat-2 | 2,527 | 418(M+,100), 403(17), 375(67), 359(19), 301(25), 235(19), 207(63) |
| GA20 | 2,523 | 418(M+,100), 403(17), 375(67), 359(15), 301(20), 235(13), 207(59) |
Ovaries were collected from MA/pat-2 and CU/pat-2 flowers between the FB and the AN stage. Methyl esters trimethylsilyl ethers derivative compounds in the appropriate HPLC fractions (23–25 mL) were analyzed by GC-SIM, and the abundance of characteristic m/z GA20 ions in the extracts were compared with those of authentic GA20.
Effect of GA1, GA20, and LAB on the Growth of Unpollinated Tomato Ovaries
The application of GA1 (0.02, 0.2, and 2 μg) induced parthenocarpic growth of unpollinated MA/wt ovaries in a dose-responsive manner, and 2 μg of GA20 had an effect similar to the same amount of GA1 (Table V). Lower amounts of GA20 (0.2 and 0.02 μg) were not active. The effect of GA20 on parthenocarpic growth of MA/wt ovaries was not affected by the simultaneous application of 1 mm LAB, but it was significantly reduced by 5 mm LAB (Table V), suggesting that GA20 needs to be metabolized to be active. A higher concentration of the inhibitor (10 mm) did not elicit further reduction of fruit-set and growth.
Table V.
Effect of application of GA1, GA20, and LAB on parthenocarpic fruit growth of MA/wt unpollinated tomato ovaries
| Treatment | Fruit-Seta | Diameter | wt |
|---|---|---|---|
| cm | g | ||
| Control | 0 /12 | — | |
| GA1 (μg) | |||
| 0.02 | 3 /12 | 2.7a | 9.8a |
| 0.2 | 5 /12 | 2.2a | 5.2a |
| 2 | 11 /12 | 4.1b | 26.4b |
| GA20 (μg) | |||
| 0.02 | 0 /12 | — | |
| 0.2 | 0 /12 | — | |
| 2 | 10 /12 | 3.8b | 24.8b |
| 2 + LAB (1 mm) | 9 /12 | 4.1b | 35.8b |
| 2 + LAB (5 mm) | 4 /12 | 1.7a | 2.5a |
| 2 + LAB (10 mm) | 4 /12 | 1.9a | 2.7a |
Ovaries from emasculated flowers were treated with solutions (20 μL per ovary) containing different amounts of GA1 or GA20, or with mixtures of GA20 (2 μg) and LAB (1, 5, and 10 mm), and developed fruits (those having a weight higher than 1 g) collected 65 to 68 d later. Control ovaries were treated with an equal volume of solvent solution. Values of diameter and weight are means of those developed fruits. At each column, values with a different letter were significantly different (P < 0.05).
Fruits developed/treated ovaries.
In MA/pat-2 ovaries, the application of GA1 or GA20 (2 μg) did not affect the growth of parthenocarpic fruits developed in the absence of pollination (Table VI, experiment I), as was observed previously with GA3 (Fig. 1). The smaller final size of the fruits in this experiment may be due to the use of 25-L pots with a peat/soil mixture rather than large containers with sand. LAB (1 mm) did not have any effect when applied together with 2 μg GA20, as occurred in MA/wt, whereas 5 mm LAB also significantly reduced fruit-set and growth (Table VI, experiment I). In a separate experiment, the application of 5 mm LAB alone also inhibited significantly fruit-set and growth, and this inhibition was fully reverted by the simultaneous application of 2 μg of GA1 (Table VI, experiment II).
Table VI.
Effect of application of GA1, GA20, and LAB on parthenocarpic fruit growth of MA/pat-2 unpollinated tomato ovaries
| Treatment | Fruit-Seta | Diameter | wt |
|---|---|---|---|
| cm | g | ||
| Experiment I | |||
| Control | 9 /12 | 4.6a | 39.1a |
| GA1 | 10 /12 | 4.4a | 32.5a |
| GA20 | 10 /12 | 4.4a | 33.9a |
| GA20 + 1 mm LAB | 7 /9 | 4.5a | 38.0a |
| GA20 + 5 mm LAB | 7 /12 | 2.1b | 7.6b |
| Experiment II | |||
| Control | 12 /15 | 4.4a | 40.9a |
| LAB (5 mm) | 9 /15 | 2.6b | 15.4b |
| LAB (5 mm) + GA1 | 12 /12 | 4.1a | 36.1a |
Ovaries from emasculated flowers were treated with solutions (20 μL per ovary) containing GA1 (2 μg), GA20 (2 μg), or mixtures of GA1 and GA20 and LAB (1 and 5 mm), and developed fruits (those having a weight higher than 1 g) were collected 65 to 68 d later. Control ovaries were treated with an equal volume of solvent solution. Values of diameter and weight are means of those of developed fruits. At each column, for each experiment, values with a different letter were significantly different (P < 0.05).
Fruits developed/treated ovaries.
DISCUSSION
The application of GA3 induced fruit-set and development of unpollinated ovaries in the non-parthenocarpic tomato MA/wt and CU/wt lines (Fig. 1), which is in agreement with previous results in different tomato cultivars (Bünger-Kibler and Bangerth, 1982; Sjut and Bangerth, 1982; Alabadí et al., 1996). GA1 and GA20 were also active in inducing parthenocarpic fruit development, at least in MA/wt (Table V). The application of paclobutrazol, an inhibitor of GA biosynthesis, negated the development of seeded MA/wt fruits, and this effect was fully counteracted by GA3 (Table II). These results suggest that GAs may be involved in tomato fruit development. The final size of GA3-induced fruits of the MA/wt tomato line were smaller than seeded fruits, but those of the CU/wt tomato line were not. This indicates that, depending on the genetic background, more than a single application of GA3 may be necessary to mimic the effect of pollination and fertilization. Alternatively, other growth factors (e.g. auxins; Gillaspy et al., 1993) in addition to GAs may also be requested for the normal fruit growth of tomato. Unpollinated ovaries of the MA/pat-2 and CU/pat-2 lines developed similarly to pollinated ovaries of their near-isogenic wt lines, and their growth was not altered by GA3 treatment (Fig. 1). The application of paclobutrazol prevented the growth of seedless parthenocarpic MA/pat-2 ovaries, and this effect was negated by the application of GA3 (Table II). This indicates that parthenocarpic fruit-set and development in pat-2 also depend on GAs, and suggests that the capacity of pat-2 ovaries to set and develop may be due to their higher content of active GAs in the absence of pollination and/or fertilization, as found for the level of GA-like substances in pat (another parthenocarpic gene) tomato fruits at early stages of development (Mapelli et al., 1978).
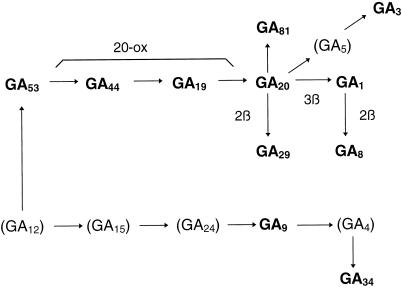
The following members of the early-13-hydroxylation pathway of GA biosynthesis: GA53, GA44, GA19, GA20, GA29, GA1, and GA8, were identified in developing non-parthenocarpic (MA/wt) tomato ovaries before anthesis (Table III). These GAs, with the exception of GA53, plus GA17 were previously identified in the pericarp of 20-d-old fruits (Bohner et al., 1988) and in entire 10-d-old pollinated fruits (Koshioka et al., 1994). GA3, previously identified in vegetative tissues of tomato (Grünzweig et al., 1997), has now been found in unpollinated ovaries and, as in maize, may come from GA20 via GA5 (Fujioka et al., 1990). The identification of GA9 and GA34 (Table II), two members of the non-13-hydroxylation pathway, supports that both biosynthetic pathways are present simultaneously in the ovaries before pollination (see Fig. 3). This is in contrast to previous results showing that GA15, GA24, and GA25 (from the non-13-hydroxylation pathway) were only present in seeds of 20-d-old pollinated fruits (Bohner et al., 1988).
Figure 3.
Possible pathways of GA biosynthesis in developing unpollinated tomato ovaries. The GAs in parentheses have not been identified in unpollinated ovaries.
It has also been reported that GAs could not be detected in pollinated tomato ovaries until several days after anthesis (Koshioka et al., 1994). The presence of GAs in unpollinated ovaries, however, as shown in this work, suggests that the developing ovaries are capable of GA biosynthesis. This idea is supported by the recent observation that genes coding for copalyl diphosphate synthase, GA 20-oxidase, and GA 3β-hydroxylase are expressed during flower and early fruit development in tomato, and that a GA 20-oxidase gene (Le20ox-2) is localized in the placenta tissue of young, unpollinated tomato ovaries (Rebers et al., 1999). Our results also suggest that the GA biosynthetic capability of the ovary may be reduced or disappear once it has developed completely, around the time of anthesis, and be triggered again as a consequence of pollination, because the concentration of the quantitated GAs decreased progressively during ovary development in wt tomato plants (Fig. 2). This agrees with previous results in pea, in which GA8, GA19, and GA20, present in emasculated, unpollinated ovaries, rapidly decreased in the absence of pollination (García-Martínez et al., 1991).
The quantitation of GAs showed that the concentration of GA20 in pat-2 ovaries before pollination was much higher than in non-parthenocarpic ovaries (Fig. 2). This difference was found at the three stages of ovary development studied (FB, PR, and AN) and with two different genetic backgrounds (MA and CU). The accumulation of GA20 in pat-2 ovaries was confirmed by identifying this GA by GC-SIM in both MA/pat-2 and CU/pat-2 ovaries (Table IV). Although the levels of GA29 and GA8 catabolite are not known, the higher content of GA20 may not be due to a blockage of GA20 metabolism. First, there was not, as would be expected if the metabolism of GA20 to GA29 were decreased, a large reduction in the amount of GA29 in pat-2 ovaries (Fig. 2). Second, the higher amount of GA1 (the product of 3β-hydroxylation) in MA/pat-2 ovaries and a similar or higher amount of GA1 plus GA8 (the metabolic product of GA1) in CU/pat-2 ovaries do not support a blockage of GA20 to GA1 metabolism. The accumulation of GA20 in pat-2 ovaries may rather be due to a higher GA 20-oxidase activity, since the level of GA19 (the immediate biosynthetic precursor of GA20) in pat-2 ovaries was generally much lower than in non-parthenocarpic ovaries (Fig. 2). The recent isolation of three GA 20-oxidase cDNA clones of tomato, whose transcripts were detected in flower buds and during early fruit development (Rebers et al., 1999), should allow testing whether the expression of these genes is enhanced in pat-2 ovaries.
The application of GA1 and GA20 to unpollinated MA/wt tomato ovaries induced parthenocarpic fruit growth (Table V), but did not affect the parthenocarpic growth of unpollinated pat-2 ovaries (Table VI). This indicates that the endogenous content of active GA may be saturating in pat-2 ovaries. However, GA20 does not seem to be active by itself because the simultaneous application of LAB, an inhibitor of GA20 metabolism, blocked the induction by GA20 of parthenocarpy in wt ovaries (Table V). Although the application of 5 mm LAB also inhibited the parthenocarpy of unpollinated pat-2 ovaries, simultaneous treatment with GA1 reversed this effect but a GA20 treatment was ineffective (Table VI). This strongly suggests that the effect of 5 mm LAB was due to its blockage of GA20 metabolism. Furthermore, the inhibition of MA/pat-2 ovary growth by LAB (Table VI) also suggests that the accumulated GA20 needs to be converted to an active GA for the expression of parthenocarpy of pat-2 ovaries.
It has been shown that GA1 is the active GA in the control of stem elongation in many species (Graebe, 1987), and there is considerable evidence suggesting that it also regulates fruit-set and development in pea (García-Martínez and Hedden, 1997). GA20 is the immediate precursor of GA1 in many species (Graebe, 1987). The metabolic biosynthetic pathway of GA1 in reproductive tomato tissues, however, is still unknown, and GA4 as an alternative precursor, as suggested in pea fruit (Rodrigo et al., 1997), cannot be fully discarded. However, the low GA levels of members of the GA4 biosynthetic pathway in young tomato fruit (Bohner et al., 1988; Koshioka et al., 1994) suggest that the early-13-hydroxylation pathway is the main operative pathway in the ovary and fruit (Fig. 3). The MA/pat-2 unpollinated ovaries had a higher GA1 content than MA/wt ovaries (Fig. 2). In CU/pat-2 ovaries, however, the content of GA1 was not higher than in the corresponding non-parthenocarpic isoline, but they contained more GA8, the metabolic product of GA1, and the amount of GA1 plus GA8 was higher at the PR and AN stages (Fig. 2).
A correlation between the level of [3H]GA8 produced from applied [3H]GA20 and pea internode elongation has also been found (Ingram et al., 1986), indicating that the content of GA8 may be a measure of the previous GA1 content in the tissues. This suggests that pat-2 unpollinated ovaries with the CU background may also synthesize more GA1 than wt ovaries and that the flux of GA1 may be important to induce parthenocarpic growth. Therefore, pat-2, whose expression can be detected before pollination (Fos and Nuez, 1996), may induce parthenocarpy by increasing the content or production of active GA in unpollinated ovaries, probably as a result of the high accumulation of GA20. A similar situation occurs in the pea sln mutant, in which the blockage of conversion of GA20 to GA29 and GA29 catabolite produces the accumulation of GA20 in the mature seeds, leading to a high GA1 content in young seedlings and to a slender phenotype (Ross et al., 1995).
In conclusion, the results presented show that the pat-2 mutation produces the accumulation of GA20 in unpollinated tomato ovaries. Natural parthenocarpy induced by pat-2 is probably due to a higher synthesis of active GA (e.g. GA1) in the developing unpollinated ovaries as a result of the accumulation of GA20.
ACKNOWLEDGMENTS
We thank Dr. Peter Hedden for the generous gift of [3H]GA1, [3H]GA19, and [3H]GA20 for quantitative analyses and GA34, GA53, and GA81 to produce GC-MS spectra; Dr. Manuel Talón for the gift of GA9; Dr. Michel Beale for the gift of GA1; Dr. Wilhelm Rademacher for the gift of LAB 198 999; Karina Proaño for her help with the application experiments; and Dr. Isabel López-Díaz for critical reading of the manuscript.
Footnotes
This work was supported by Consellería de Cultura, Educación y Ciencia, Generalitat Valenciana (grant no. GV–D–AG–01–130–96 to F.N.) and Plan Nacional de Investigación y Ciencia, Biotecnología (grant no. BIO97–0578–C03–01 to J.L.G.M.).
LITERATURE CITED
- Alabadí D, Agüero MS, Pérez-Amador MA, Carbonell J. Arginase, arginine decarboxylase, ornithine decarboxylase, and polyamines in tomato ovaries: changes in unpollinated ovaries and parthenocarpic fruits induced by auxin and gibberellin. Plant Physiol. 1996;112:1237–1244. doi: 10.1104/pp.112.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg R, Meir E, Lapushner D, Frankel R, Salts Y. Differential regulation of a fruit-specific 62 kDa protein in developing parthenocarpic (pat-2/pat-2) and seeded tomato fruits. Physiol Plant. 1990;80:417–424. [Google Scholar]
- Bohner J, Hedden P, Bora-Haber E, Bangerth F. Identification and quantitation of gibberellins in fruits of Lycopersicon esculentum, and their relationship to fruit size in L. esculentum and L. pimpinellifolium. Physiol Plant. 1988;73:348–353. [Google Scholar]
- Bünger-Kibler S, Bangerth F. Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul. 1982;1:143–154. [Google Scholar]
- Fos M, Nuez F. Molecular expression of genes involved in parthenocarpic fruit set in tomato. Physiol Plant. 1996;98:165–171. [Google Scholar]
- Fos M, Nuez F. Expression of genes associated to natural parthenocarpy in tomato ovaries. J Plant Physiol. 1997;151:235–238. [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Phynney BO, Gaskin P, MacMillan J, Takahashi N. Gibberellin A3 is biosynthesized from gibberellin A20 via gibberellin A5 is shoots of Zea mays L. Plant Physiol. 1990;94:127–131. doi: 10.1104/pp.94.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Hedden P. Gibberellins and fruit development. In: Tomás-Barberán FA, Robins RJ, editors. Phytochemistry of Fruit and Vegetables. Oxford: Clarendon Press; 1997. pp. 263–286. [Google Scholar]
- García-Martínez JL, Santes C, Croker S, Hedden P. Identification, quantitation and distribution of gibberellins in fruits of Pisum sativum L. cv. Alaska during pod development. Planta. 1991;184:53–60. doi: 10.1007/BF00208236. [DOI] [PubMed] [Google Scholar]
- Gaskin P, MacMillan J, Firn RD, Pryce RJ. “Parafilm,” a convenient source of n-alkane standards for determination of GA chromatographic retention indexes. Phytochemistry. 1971;10:1155–1157. [Google Scholar]
- George WL, Scott JW, Splittstoesser WE. Parthenocarpy in tomato. Hortic Rev. 1984;6:65–84. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PB. Phytohormones and fruit growth. In: Letham DS, Goodwin PB, Higgins TJV, editors. Phytohormones and Related Compounds. A Comprehensive Treatise. II. Amsterdam: Elsevier/North-Holland; 1978. pp. 175–214. [Google Scholar]
- Graebe JE. Gibberellin biosynthesis and control. Annu Rev Plant Physiol. 1987;38:419–465. [Google Scholar]
- Groot SPC, Bruinsma J, Karssen CM. The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiol Plant. 1987;71:184–190. [Google Scholar]
- Grünzweig JM, Rabinowitch HD, Katan J, Wodner M, Ben-Tal Y. Endogenous gibberellins in foliage of tomato (Lycopersicon esculentum) Phytochemistry. 1997;46:811–815. [Google Scholar]
- Hedden P, Croker SJ, Rademacher W, Jung J. Effects of the triazole plant growth retardant Bas111.W on gibberellins levels in oilseed rape, Brassica napus. Physiol Plant. 1989;75:445–451. [Google Scholar]
- Ingram TJ, Reid JB, MacMillan J. The quantitative relationship between gibberellin A1 and internode growth in Pisum sativum L. Planta. 1986;168:414–420. doi: 10.1007/BF00392370. [DOI] [PubMed] [Google Scholar]
- Koshioka M, Nishijima T, Yamazaki H, Nonaka M, Mander LN. Analysis of gibberellins in growing fruits of Lycopersicon esculentum after pollination or treatment with 4-chlorophenoxyacetic acid. J Hortic Sci. 1994;69:171–179. [Google Scholar]
- Lukyanenko AN. Parthenocarpy in tomato. In: Kalloo G, editor. Genetic Improvement of Tomato, Monographs on Theoretical and Applied Genetics 14. Berlin: Springer-Verlag; 1991. pp. 167–178. [Google Scholar]
- Mapelli S, Frova C, Torti G, Soressi GP. Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978;19:1281–1288. [Google Scholar]
- Mazzucato A, Taddei AR, Soressi GP. The parthenocarpic fruit (pat) mutant of tomato (Lycopersicon esculentum Mill.) sets seedless fruits and has aberrant anther and ovule development. Development. 1998;125:107–114. doi: 10.1242/dev.125.1.107. [DOI] [PubMed] [Google Scholar]
- Nitsch J. Hormonal factors in growth and development. In: Hulme AC, editor. The Biochemistry of Fruits and Their Products. II. London: Academic Press; 1970. pp. 427–472. [Google Scholar]
- Nuez F, Báguena M, Díez MJ, Cuartero J. Condiciones de producción de semilla en materiales partenocárpicos de tomate. Acta Hortic. 1991;8:83–91. [Google Scholar]
- Nuez F, Costa J, Cuartero J. Genetics of the parthenocarpy for tomato varieties “Sub-Artic Plenty,” “75/59” and “Severianín.”. Z Pflanzenzucht. 1986;96:200–206. [Google Scholar]
- Nuez F, Díez MJ, Cuartero J, Costa J. Líneas partenocárpicas experimentales de tomate. An Inst Nac Invest Agrar Ser Agri. 1985;28:47–59. [Google Scholar]
- Philouze J. Parthenocarpie naturelle chez la tomate. I. Revue bibliographique. Agronomie. 1983;3:311–320. [Google Scholar]
- Philouze J, Laterrot H, Maisonneuve B. Rapport d'activité 1979/80 Station d'Amélioration des Plantes Maraichères d'Avignon-Montfavet. Institut National de la Recherche Agronomique, Montfavet. 1980. I. Etude de l'aptitude à la parthénocarpie naturelle; pp. 91–99. [Google Scholar]
- Philouze J, Maisonneuve B. Heredity of the natural ability to set parthenocarpic fruits in the soviet variety Severianin. Tomato Genet Coop. 1978;28:12–13. [Google Scholar]
- Rademacher W, Temple-Smith KE, Griggs DL, Hedden P. The mode of action of acylcyclohexanediones: a new type of growth retardant. In: Karssen CM, Van Loon LC, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 571–577. [Google Scholar]
- Rebers M, Kaneta T, Kawaide H, Yamaguchi S, Yang YY, Imai R, Sekimoto H, Kamiya Y. Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J. 1999;17:241–250. doi: 10.1046/j.1365-313x.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, García-Martínez JL, Santes CM, Gaskin P, Hedden P. The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L. and the identification of gibberellins A4 and A7 in young seeds. Planta. 1997;201:446–455. [Google Scholar]
- Ross JJ, Reid JB, Swain SM, Hasan O, Poole AT, Hedden P, Willis CL. Genetic regulation of gibberellin deactivation in Pisum. Plant J. 1995;7:513–523. [Google Scholar]
- Sawhney VK. Gibberellins and fruit formation in tomato: a review. Sci Hortic. 1984;22:1–8. [Google Scholar]
- Schwabe WW, Mills JJ. Hormones and parthenocarpic fruit set: a literature survey. Hortic Abstr. 1981;51:661–698. [Google Scholar]
- Sjut V, Bangerth F. Induced parthenocarpy: a way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.): 1. Extractable hormones. Plant Growth Regul. 1982;1:243–251. [Google Scholar]
- Vardy E, Lapushner D, Genizi A, Hewitt J. Genetics of parthenocarpy in tomato under a low temperature regime: II. Cultivar Severianin Euphytica. 1989;41:9–15. [Google Scholar]