Abstract
How solutes and macromolecules are removed from brain tissue is of central importance in normal brain physiology and in how toxic protein aggregates are cleared in neurodegenerative conditions, including Alzheimer’s disease (AD). Conventionally, solute transport in the narrow and tortuous extracellular space in brain parenchyma has been thought to be primarily diffusive and nondirectional. The recently proposed “glymphatic” (glial-lymphatic) hypothesis posits that solute clearance is convective and driven by active fluid transport from para-arterial to paravenous spaces though aquaporin-4 water channels in astrocyte endfeet. Glymphatic, convective solute clearance has received much attention because of its broad implications for AD and other brain pathologies and even the function of sleep. However, the theoretical plausibility of glymphatic transport has been questioned, and recent data have challenged its experimental underpinnings. A substantiated mechanism of solute clearance in the brain is of considerable importance because of its implications for pathogenic mechanisms of neurologic diseases and delivery of therapeutics.—Smith, A. J., Verkman, A. S. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation?
Keywords: aquaporin-4, water transport, brain edema, β-amyloid, sleep
The transport of small solutes and macromolecules in brain extracellular space (ECS) determines the range of action of neurotransmitters (1, 2), transport of metabolic intermediates between cells (3), and the clearance of potentially neurotoxic peptides, including β-amyloid (Aβ) and tau (4, 5). Additionally, transport in brain ECS is a major determinant of the delivery of therapeutics and the transfer of biomarkers of neurodegeneration into the periphery (6). The mechanisms and pathways for solute clearance in the brain have been studied for >150 years, and there is extensive literature on brain ECS transport and clearance mechanisms in normal physiology and in neuropathology [reviewed in Abbott (7) and Hladky and Barrand (8)].
Transport of small molecules over intermediate distances in the ECS in cortical gray matter is well described by simple diffusion (9). Solute diffusion coefficients are lower in vivo than in vitro because of the increased path length traveled by molecules (tortuosity) and mild crowding of the ECS with macromolecules (viscosity). The slowing of diffusion in vivo is generally described by the diffusional tortuosity, defined as the square root of the ratio of the in vitro and in vivo diffusion coefficients. Tortuosity is generally somewhat higher for large than for small molecules (10), in part, because of increased restrictions to the paths along which large molecules can travel. Diffusional tortuosity and the ECS volume fraction differ in different regions of the brain (11, 12). The precise structure of the ECS remains uncertain, as individual structural elements are below the resolution limit of conventional light microscopy and because of fixation artifacts, such as cellular swelling, which distort the fine structure of the ECS, as visualized by electron microscopy (13). Estimations of ECS structure, based on reconstructions from electron microscopy tomography with digital correction for fixation artifacts, predict a network of tunnels of 40–80 nm diameter connected by sheets of 10–40 nm width (2). Solute transport in ECS white matter differs from that in gray matter, as solutes can move freely in the direction of fiber tracts but are hindered in their movement perpendicular to the fibers. Early measurements of radiotracer movement in cat brain found evidence for slow convection along white-matter tracts in the direction of the ventricles but purely diffusive transport in gray matter (14).
Both gray and white matter are penetrated by blood vessels; in primate cortex, arterioles and venules are spaced ∼280 μm apart, with capillaries present at ∼3-fold higher density (15, 16), so that all areas of the interstitial space are in proximity to the vasculature. Vessels are surrounded by astrocyte endfeet that are heavily enriched in the water channel aquaporin-4 (AQP4) (17). Metabolites and ions are cleared from the interstitium by carrier-mediated transport across the vascular endothelium of the blood-brain barrier (18). For solutes that are not transported across the blood-brain barrier, the paravascular space between the endfeet and vascular endothelium is a low-resistance pathway that has long been recognized as an important route for clearance of solutes injected into the brain. Transport of solutes in the para-arteriolar spaces may be facilitated by pulsations of the vessel wall (19), although the precise mechanism by which this occurs remains controversial (20, 21). Paravenular clearance may be facilitated by cycles of venular collapse and reinflation as a result of breathing (22). The paravascular spaces communicate with the subarachnoid space, allowing clearance of solutes into the cerebrospinal fluid (CSF) and then from the brain through the arachnoid granulations or by drainage through the cribriform plate into nasal lymphatics (23, 24). The paravascular spaces also connect directly with dural lymphatics, and some solutes exit the brain along this pathway (25).
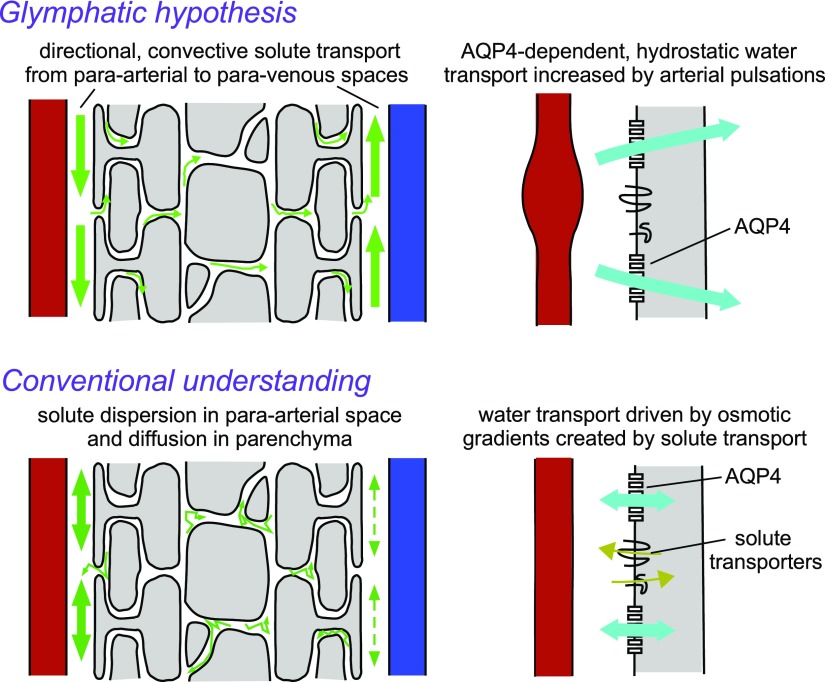
Relatively recently, Iliff et al. (26) have introduced a “glymphatic” (glial-lymphatic) hypothesis for solute clearance in brain. The glymphatic hypothesis proposes that water flow through AQP4 in astrocyte endfeet, driven by contractions of the arterial wall, generates a directional, convective flow through the brain from para-arterial to paravenous spaces (27). Expansion of the ECS during sleep is proposed to increase glymphatic convection and facilitate the clearance of toxic protein aggregates from the brain (28). Figure 1 diagrams the major differences between the glymphatic hypothesis and the conventional understanding of solute transport in the ECS. In the glymphatic hypothesis, convective solute transport through the ECS from para-arterial to paravenous spaces is driven by vascular and respiratory pulsations, requiring pressure-driven (hydrostatic) water transport through AQP4 channels in astrocyte endfeet. In the conventional understanding, solute movement in brain ECS is diffusive, passive, and nondirectional, and astrocyte AQP4 functions as a bidirectional water channel that facilitates transfer of water into and out of astrocytes in response to osmotic gradients created by solute transport. With regard to Aβ clearance, the conventional view is that the bulk of Aβ clearance occurs by receptor-mediated transcytosis across the blood-brain barrier, whereas the glymphatic hypothesis emphasizes the importance of bulk clearance into CSF along paravascular pathways.
Figure 1.
Differences between the proposed glymphatic mechanism of solute clearance (upper) and the conventional understanding of solute clearance (lower). The glymphatic mechanism predicts directional clearance of solutes, including Aβ, mainly into the paravenous space. Convective flow is generated by pulsations of the arterial wall and involves AQP4-dependent convective fluid flow through astrocyte endfeet. In contrast, the conventional understanding is that clearance of solutes is by nondirectional diffusion in the parenchyma coupled to convective or dispersive clearance in the paravascular spaces. AQP4 water channels in astrocyte endfeet facilitate rapid, bidirectional water flux between the endfoot and paravascular space, in response to osmotic gradients created by solute transport.
The glymphatic hypothesis has been received with considerable enthusiasm in academic journals (29,–31) and in the popular press (32,–34). However, it is controversial and has been the subject of skeptical reviews by us (35) and others (8, 36), and recent models of interstitial solute transport predict diffusive rather than convective transport in brain parenchyma (21, 37, 38). We recently reexamined the experimental underpinnings of the glymphatic hypothesis and reported evidence supporting the conventional view that solute transport in brain parenchyma is primarily diffusive and passive (39). Herein, we review the evidence for and against a glymphatic mechanism, with consideration of clearance of toxic protein aggregates from the brain.
IMPORTANCE OF SOLUTE CLEARANCE MECHANISMS IN THE PATHOLOGY OF ALZHEIMER’S DISEASE AND RELATED NEURODEGENERATIVE DISORDERS
Clearance of Aβ and tau from the interstitium
Alzheimer’s disease (AD) is associated with the accumulation of toxic aggregates of Aβ (plaques) and hyperphosphorylated tau (tangles) in the brain. Plaque formation involves the concentration-dependent aggregation of Aβ peptides, where concentration is determined by the balance between production and clearance mechanisms. Aβ peptides are generated from the amyloid precursor protein, a transmembrane protein expressed on neurons, by the action of various proteases (40). Monomeric Aβ can be cleared from the brain by several mechanisms, including intracellular and extracellular degradation, accumulation in plaques, transcytosis across the blood-brain barrier, and bulk clearance into the CSF (4, 41). Soluble oligomers of Aβ, which are thought to be the toxic form of the peptide, have high membrane affinity and do not move freely in the ECS (42).
As a consequence of the multiple clearance mechanisms, the kinetics of Aβ clearance in vivo is considerably more complex than that of inert membrane-impermeant tracer molecules, such as dextrans. Aβ peptides are cleared more rapidly than comparably sized dextrans (28, 43), suggesting the potentially limited importance of the paravascular clearance pathway for Aβ clearance in the healthy brain (41). However, it is possible that Aβ proteolysis and blood-brain barrier transcytosis become substantially impaired in AD, which might increase the relative importance of paravascular clearance mechanisms under certain conditions (44).
Unlike Aβ, tau is an intracellular protein that is involved in the stabilization of neuronal microtubules. Intracellular aggregates of insoluble, hyperphosphorylated tau are thought to be generated in the late stages of plaque-triggered neurodegeneration in AD (40). Recent evidence suggests that extracellular tau aggregates released from damaged or dying neurons may play an important role in disease propagation (45). Clearance of extracellular tau from the brain is much slower than that of Aβ, and specific transporters for tau exit across the blood-brain barrier have not been identified (4). Therefore, although bulk clearance may be an important route for removal of extracellular tau from the brain, it should be noted that unlike Aβ, tau does not seen to accumulate in the paravascular spaces, and instead, the spread of pathology follows the pattern of neuronal connectivity (46). Therefore, it remains unclear whether extracellular or intracellular long-range transport is the main factor in the spread of tau pathology.
Impaired Aβ clearance as a cause of AD
Whether impaired Aβ clearance causes AD remains unresolved, and current research seeks to determine whether treatment efforts should be targeted at clearance pathways. Genetic studies of rare, hereditable forms of AD, which are caused by mutations in the genes APP, PSEN1, and PSEN2, demonstrate that increased Aβ production or increased production of aggregation-prone isoforms, such as Aβ1–42, causes AD (40). Genetic association studies have suggested that mutations in genes that may contribute to Aβ clearance predispose to sporadic (late-onset) forms of AD (47). Variant alleles of APOE, which encode a systemically expressed protein involved in cholesterol trafficking, can increase the risk of developing AD by as much as 12-fold and have been related to alterations in Aβ clearance (48). Additional mutations in regulators of diverse physiologic processes, such as clathrin-mediated endocytosis (49) and immune cell function (50), which predispose to AD, also implicate Aβ clearance failure as an important risk factor for developing disease. However, these proteins have complex cellular functions and hence may have additional roles in AD pathogenesis.
In addition to this genetic evidence, impairment of Aβ clearance, caused by aging or brain injury, has been suggested to contribute to the pathogenesis of AD. Formation of plaques in the paravascular space, associated with development of cerebral arterial angiopathy, may inhibit clearance by the paravascular pathway (51, 52). Carare et al. (44) have suggested that a group of related neurodegenerative diseases, including cerebral arterial angiopathy, cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, and prion angiopathy, be considered as angiopathies caused by failure of protein elimination along the paravascular pathway. As described below, the glymphatic hypothesis proposes that failure of bulk clearance of Aβ, as a result of either loss of paravascular AQP4 or sleep disruption, plays an important role in the pathogenesis of AD.
EXPERIMENTAL UNDERPINNINGS OF THE GLYMPHATIC MECHANISM
The existence of a convective paravascular solute clearance pathway was well documented in early studies (19, 53, 54), and these were extended by Iliff et al. (26), who first introduced the concept of a glymphatic system based on experiments where dye transport into the mouse brain cortex was tracked following injection into CSF at the cisterna magna. Dye was initially observed in the paravascular spaces of arterioles and then seen to enter the parenchyma and then in paravenous spaces. Solute transport in brain parenchyma was found to be strongly size dependent. These results were interpreted as evidence for convective flow from para-arterial to paravenous spaces through the parenchyma. Solutes injected directly into the brain were found in both para-arterial and paravenous spaces, leading the researchers to conclude that convection occurring during the injection disrupted the endogenous directional flow. Initial evidence for glial regulation of solute transfer between the paravascular space and parenchyma was shown by reduced transfer of solutes in AQP4-deficient mice (26). This was interpreted as evidence for AQP4-dependent convection through astrocyte endfeet. Subsequent MRI of the distribution of cisternally injected, gadolinium-labeled paramagnetic probes confirmed that transfer of solutes from the CSF into the parenchyma strongly depends on solute size (55).
Subsequent work (56) suggested that vascular pulsations provide the driving force for convective fluid movement from the paravascular space through the parenchyma. A fluorescent dextran was introduced to the CSF by intracisternal injection, and its appearance in the parenchyma was followed by 2-photon imaging through a cranial window. Fluorescent dextran accumulation was slowed by ∼20% when vascular pulsation was inhibited by unilateral internal carotid artery ligation and increased by 10–40% when vascular pulsation was increased by dobutamine.
Xie et al. (28) reported substantial differences in the accumulation of cisternally applied tracers in the parenchyma of awake and anesthetized, or sleeping, mice. Cortical accumulation of cisternally injected tracers was reduced ∼10-fold in awake animals. Brain ECS volume fraction in anesthetized and awake mice was measured by the tetramethylammonium (TMA+) iontophoresis method (57). A remarkable reduction in brain ECS volume fraction was observed upon waking, from ∼23% in sleeping mice to ∼14% in awake mice, leading the researchers to conclude that the increased cell volume in awake animals results in increased hydraulic resistance of the brain ECS, which blocks convective, glymphatic solute clearance. On the basis of these results, the researchers proposed a major new hypothesis: that the function of sleep is to facilitate clearance of toxins from the brain.
The Nedergaard group (26) also reported evidence for a role of the glymphatic pathway in removing Aβ and tau from the ECS. In the original description of the glymphatic system, ∼70% of injected [125I]Aβ had cleared from the brain of wild-type mice after 1 h, whereas only ∼40% cleared from the brain of AQP4−/− mice, implying an ∼2-fold reduction in the rate of Aβ removal in the absence of AQP4. Additional studies suggested that glymphatic clearance in AD may fail as a result of mislocalization of AQP4 in astrocytes. In normal conditions, AQP4 is enriched in paravascular astrocyte endfeet (17, 58), but this polarization is lost in models of neurodegenerative diseases, including AD (59,–61). Mislocalization of AQP4 has also been described in postmortem human brain from patients with AD (62), although it is noted that the pattern of AQP4 staining in the human brain is more complex than that in rodents (63) and that the degree of AQP4 polarization observed in postmortem specimens is generally much lower than that in perfusion-fixed rodent brain (64, 65). Kress et al. (66) observed a small (10–20%) reduction of AQP4 polarization around penetrating arterioles in brains of normal, aged mice and found that AQP4 polarization was correlated with the amount of solute transferred from the paravascular space to the parenchyma. They concluded that age-related loss of AQP4 polarization could also be involved in AD pathogenesis. In additional work, knockout of AQP4, or its mislocalization following traumatic brain injury, was shown to cause accumulation of phosphorylated tau in the ECS and exacerbate the formation of neurofibrillary pathology (67).
AD is often associated with sleep disruption (68), and this led Xie and colleagues (69) to propose that sleep impairment may have a causal role in AD. A subsequent study reported ∼50% more rapid clearance of radiolabeled Aβ in rodents sleeping in the supine or lateral position than in the prone position. This finding led to the suggestion that sleep position may be an important determinant of AD susceptibility.
THEORETICAL AND EXPERIMENTAL CHALLENGES TO THE GLYMPHATIC HYPOTHESIS
Theoretical challenges
Although the glymphatic mechanism has garnered broad interest because of its novelty and implications for normal brain physiology and disease, it appears to contradict a large body of experimental evidence, its physical plausibility has been questioned from a theoretical perspective, and recent experimental evidence has challenged its major underpinnings. The glymphatic mechanism posits active, convective, directional solute transport from para-arterial to paravenous spaces through brain ECS that depends on solute size and on functional AQP4 water channels.
From a theoretical perspective, active, convective water and solute transport though brain parenchyma and its dependence on AQP4 water transport seem counterintuitive for several reasons. The ECS makes up only ∼20% of brain volume and is physically very narrow and tortuous, suggesting the need for large pressure differences between the para-arterial and paravenous spaces to drive significant convection. However, available evidence from both invasive and noninvasive methods suggests that pressure gradients in different areas of the CSF and interstitial fluid (ISF) are small (70, 71). Furthermore, how AQP4, a water-selective channel, could facilitate convective fluid transport has been questioned, because water driven across an AQP4-containing membrane by a hydrostatic pressure difference would instantaneously create an osmotic imbalance that opposes water transport and returns the pressure-driven transported water to the original side of the membrane (35). Physiologically significant pressure-driven water transport across cell membranes in mammals is quite unexpected.
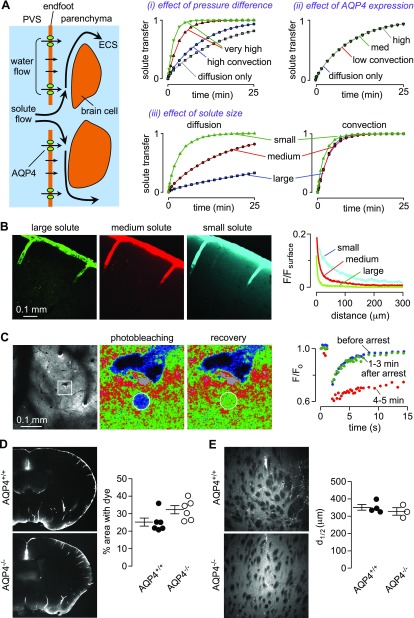
Modeling studies have attempted to predict the dynamics of fluid flow in the paravascular space (20, 72), and more recently, mathematical modeling has been done to investigate the plausibility of glymphatic transport and to determine under what conditions significant glymphatic transport is possible. In work from our laboratory (37), glymphatic solute transport between para-arterial and paravenous spaces was modeled by solving the Navier-Stokes and convection-diffusion equations using realistic ECS geometry, as illustrated schematically in Fig. 2A. The effects of para-arterial to paravenous pressure difference, ECS volume fraction, solute diffusion coefficient, and AQP4 water permeability were analyzed. The conclusions of the model included the following: the need for an unrealistic, several-mmHg sustained pressure difference between para-arterial and paravenous fluid to drive significant convection (Fig. 2Ai), with no added benefit of pulsatile pressure fluctuations; absence of significant AQP4 effect on convection (Fig. 2Aii); and diffusion (without convection) in the ECS accounting for experimental studies of solute movement in brain (Fig. 2Aiii). Details and computational results are reported in Jin et al. (37). A recently published study (38) extended this structure-based modeling approach to a 3-dimensional reconstruction of neuropil and came to the same conclusion—that the hydraulic resistance of the parenchyma is too high for significant convection to occur.
Figure 2.
Theoretical and experimental evidence against a glymphatic mechanism of solute clearance in the brain. A) Model of solute transport between the paravascular space (PVS) and parenchyma. Computational results from modeling indicate the following: very large hydrostatic pressures are required to drive significant convection through brain parenchyma (i); endfoot water permeability does not significantly affect convective flow (ii); and diffusive solute transport is size dependent, whereas convective solute transport is not (iii). See Jin et al. (37) for computational details. B) Fluorescent dextrans of different molecular sizes and colors (10-kDa Alexa 647-dextran, 70-kDa tetramethylrhodamine-dextran, 2000-kDa FITC-dextran) were coinjected into the cisterna magna, and brains were fixed after 1 h. Quantification of the drop in fluorescence intensity normalized to surface intensity (F/Fsurface) demonstrates that transport into the parenchyma is strongly size dependent, as expected for diffusive transport. C) Fluorescent dextran injected into the cortex (left panel) was used to measure local solute transport rates by 2-photon photobleaching (middle 2 panels). Photobleaching recovery rates are unchanged in the minutes immediately following cardiorespiratory arrest (right panel), suggesting that cardiac and respiratory pulsations do not play an important role in driving solute transport in the parenchyma. F/F0, ratio of fluorescence intensity to intensity at the start of the experiment. D) Similar distribution of FITC-albumin in the brain at 1 h after intracisternal injection in AQP4+/+ and AQP4−/− mice. Each point (right) represents data from a different mouse. E) Distribution of fluorescently labeled Aβ1–40 after injection into the parenchyma of AQP4+/+ or AQP4−/− mice shows nondirectional and similar diffusion of the peptide into brain parenchyma. The distance at which fluorescence decreased to half the intensity at the injection site (d1/2) was not affected by genotype. For panels B–E, see Smith et al. (39) for experimental details and analyses.
Asgari et al. (73) proposed an interesting idea about AQP4-dependent transastrocytic convective flow—that AQP4 facilitates intracellular convective flow. Their modeling study concluded that endfoot water permeability is unlikely to affect the rate of solute flow into the brain but also proposed that AQP4-dependent, intracellular convective flow through the astrocyte syncytium might facilitate a modest increase in extracellular solute movement. However, there are several concerns in postulating intracellular flow, including the osmotic effects described earlier and the very narrow astrocyte processes containing cytoskeletal elements (37). In addition, it remains unclear to what extent AQP4-dependent water transport into the astrocyte cytosol will cause local swelling, as opposed to long-range fluid flow, as the hydraulic resistance of cytosol is significant (74). Further work has addressed the mechanisms of fluid transport in the paravascular space and suggested that dispersion, where back and forth pulsation enhances diffusion, could account for rapid solute transport in the para-arterial space in the absence of bulk, directional fluid flow (21).
Although seemingly robust, conclusions from modeling are, of course, dependent on model assumptions, which can be difficult to justify for a system as complex as the brain. Limitations of existing models include failure to account for distortion of brain structure in response to pressure pulsation (compliance) and effects of activity-dependent solute transport between the endfoot and paravascular space. We also note that existing models do not include the extracellular matrix, which will reduce the cross-sectional area available for convective fluid flow and increase hydraulic resistance (41) so that model results provide an upper limit on convective rates, making it even less likely that convection can occur.
With regards to the role of sleep in solute clearance from the brain and the hypothesis that sleep deprivation impairs Aβ clearance, sleep impairment has been associated with development of AD and other neurodegenerative conditions and often precedes the development of other symptoms (68). In addition, sleep deprivation increases amyloid plaque deposition in mice (75). A circadian pattern in the CSF concentration of Aβ has been identified in which Aβ concentration is increased during wakefulness (76), although the magnitude of circadian variation is small and has not been consistently seen (77). The large transfer of fluid from the intracellular to extracellular compartment on wake-to-sleep transition, as observed by Xie et al. (28), requires a large transfer of solutes (mainly NaCl) into the ECS, and it has been questioned how this can occur (8).
Experimental challenges
Motivated by our interest in understanding the role of AQP4 in astrocyte endfeet and questions raised by mathematical modeling of brain solute transport, we undertook a reexamination of the experimental underpinnings of the glymphatic hypothesis (39). Major predictions of the glymphatic hypothesis that were tested experimentally include the following: 1) approximately solute size-independent transport in brain parenchyma, 2) active, directional solute transport in brain parenchyma, and 3) reduced transport of solutes from CSF into the parenchyma in AQP4-deficient animals.
Experimental investigation of the size dependence of solute movement in brain parenchyma was done by injection of dextrans of 10, 70, and 2000 kDa molecular size into the CSF by intracisternal injection or directly into the ISF by intraparenchymal injection. Experimental results shown in Fig. 2B and in Fig. 2 of Smith et al. (39) demonstrate strongly size-dependent dextran transport into the parenchyma from the CSF and within the parenchyma. This is consistent with modeling results for diffusive transport where it is assumed that the effects of convective sieving are small, as solute size is less than half of the size of the gaps between cells (78). In addition, low MW dextrans distributed throughout the parenchyma without evidence for directional transport toward paravenous spaces. Further studies investigated the role of active, directional solute transport in the cortex of live mice using 2-photon microscopy to visualize fluorescent dextran injected into the cortex (Fig. 2C, left). Photobleaching in a circular region adjacent to a vessel revealed that recovery occurred isotropically without directional solute movement (Fig. 2C, middle 2 panels). A particularly compelling experimental finding was that transport of a fluorescent dextran was unimpaired for several minutes following cardiorespiratory arrest, with marked slowing at later times following anoxic depolarization and brain-cell swelling (Fig. 2C, right). The contribution of cardiac and respiratory pulsations to parenchymal solute transport thus appears to be insignificant. Experimental details of the data shown in Fig. 2B–E are provided in Smith et al. (39).
Experiments were also done to replicate the original study of Iliff et al. (26), where it was concluded that AQP4 is required for parenchymal accumulation of tracers introduced to the CSF by intracisternal injection. With the use of the original and independent analysis methods, we failed to demonstrate reduced movement of fluorescent albumin from the CSF space into brain parenchyma of AQP4−/− mice or AQP4−/− rats (compared with matched wild-type animals; Fig. 2D). Lastly, the distribution of fluorescently labeled Aβ1–40 peptide at 60 min after injection showed similar and nondirectional diffusion away from the injection site in wild-type and AQP4−/− mice (Fig. 2E).
The reasons for the discrepancies in experimental data in this field remain unclear. As discussed earlier, the in vivo imaging data of Iliff et al. (26) that led to the original assertion of directional fluid transport in brain parenchyma are open to multiple interpretations and might equally represent diffusion down a concentration gradient. The marked size-dependent differences in solute accumulation in the parenchyma, as a result of glymphatic transport, also seem largely consistent with diffusive transport, and the photobleaching approaches that we have introduced, which allow solute transport in parenchyma and paravascular space to be assessed independently in live animals, demonstrate that pulsation-driven solute flow is restricted to the paravascular space. The prior finding of slowed solute transport following unilateral carotid artery occlusion (56) might be the consequence of ischemic tissue swelling. The reasons for differences in tracer accumulation following AQP4 deletion between our work and the experiments of Iliff et al. (26) are not known but might be related to differences in experimental methodology, such as choice of anesthetic or the use of constant flow brain injection, where intracranial pressure can rise considerably. Other confounding factors may be the use of threshold-based image analysis methods to determine solute uptake, which is inherently nonquantitative, or the low statistical power in the original studies. It remains possible that AQP4 may play some role in regulating structure or solute flow in the paravascular space with consequent effect on Aβ clearance by this pathway or that AQP4 deletion alters a different aspect of Aβ metabolism. Additional independent testing of key predictions of the glymphatic hypothesis is warranted.
OUTSTANDING QUESTIONS
The understanding of how Aβ is cleared from the brain remains an issue of central importance in AD. Whereas there is some evidence to suggest that impairment of paravascular transport may play a role in AD, the theoretical and experimental evidence, as reviewed here, does not support the glymphatic hypothesis, as originally conceived. The existence of pulsation-driven, AQP4-dependent convective flow through brain parenchyma has been challenged on theoretical grounds and failed independent experimental attempts at replication. Nonetheless, studies in knockout mice demonstrate that AQP4 is a determinant of ECS structure and solute transport (79, 80), and bulk clearance of fluid injected into the brain is impaired in AQP4−/− mice (81). Therefore, questions remain about the role of astrocytes and AQP4 in sculpting the ECS, with potential implications for the understanding of how monomers and small aggregates of Aβ and tau are transported. Additional questions include the following: 1) Do short-term, neuronal, activity-dependent changes in the structure of the ECS, caused by changes in cell volume, alter the transport of protein aggregates in the parenchyma and paravascular spaces? 2) Do structural and functional changes in astrocytes that occur following brain injury (reactive gliosis) affect ECS structure and transport of solutes in the parenchyma and paravascular spaces? 3) How does ECS transport differ between gray matter and white matter? 4) How do Aβ-targeted antibody therapeutics alter Aβ transport through and out of the ECS?
Advances in imaging methodologies may help in answering these questions. High-pressure freezing for fixation, which preserves tissue ultrastructure in near-native state, will help to define ECS structure. Recently, this technique has been applied to study the ultrastructure of astrocyte endfeet with some surprising results (82). Cortical surface photobleaching has provided useful information about the mechanisms of solute transport at the brain surface (81), and we have recently demonstrated that 2-photon photobleaching deep within the cortex can be applied to study solute transport in paravascular space and parenchyma separately (39). Super-resolution optical imaging allows mapping of the nanoscale distribution of fluorescent molecules in fixed tissue, including pathologic specimens (64), and may be useful in the study of brain ultrastructure (83). Finally, single particle tracking, which has shown promise for mapping solute movement in the ECS of living neural tissue (84), may permit direct visualization of solute transport patterns in brain parenchyma.
CONCLUSIONS
The popularization of the glymphatic hypothesis has brought welcomed attention to a neglected and important area of neurophysiology, and the term “glymphatic system” has become widely applied to describe the paravascular system in the brain. However, a number of researchers have expressed concern that the use of terms such as “glymphatic” and “brain lymphatics” invites confusion, as brain ISF and lymph are substantially different (36, 85). We would add to these concerns that the major mechanistic aspects of the glymphatic hypothesis are highly speculative and perhaps incorrect; therefore, the use of the term “paravascular system” may be more appropriate.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH; Bethesda, MD, USA) National Eye Institute Grant EY13574, NIH National Institute of Biomedical Imaging and Bioengineering Grant EB00415, NIH National Institute of Diabetes and Digestive and Kidney Diseases Grants DK35124, and DK72517, and a grant from the Guthy-Jackson Charitable Foundation. The authors declare no conflicts of interest.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- AQP4
aquaporin-4
- CSF
cerebrospinal fluid
- ECS
extracellular space
- ISF
interstitial fluid
AUTHOR CONTRIBUTIONS
A. J. Smith and A. S. Verkman prepared figures and wrote the manuscript.
REFERENCES
- 1.Kullmann D. M., Min M. Y., Asztely F., Rusakov D. A. (1999) Extracellular glutamate diffusion determines the occupancy of glutamate receptors at CA1 synapses in the hippocampus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinney J. P., Spacek J., Bartol T. M., Bajaj C. L., Harris K. M., Sejnowski T. J. (2013) Extracellular sheets and tunnels modulate glutamate diffusion in hippocampal neuropil. J. Comp. Neurol. 521, 448–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball K. K., Cruz N. F., Mrak R. E., Dienel G. A. (2010) Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J. Cereb. Blood Flow Metab. 30, 162–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarasoff-Conway J. M., Carare R. O., Osorio R. S., Glodzik L., Butler T., Fieremans E., Axel L., Rusinek H., Nicholson C., Zlokovic B. V., Frangione B., Blennow K., Ménard J., Zetterberg H., Wisniewski T., de Leon M. J. (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker E. N., Bacskai B. J., Arbel-Ornath M., Aldea R., Bedussi B., Morris A. W., Weller R. O., Carare R. O. (2016) Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 36, 181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolak D. J., Thorne R. G. (2013) Diffusion of macromolecules in the brain: implications for drug delivery. Mol. Pharm. 10, 1492–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott N. J. (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 45, 545–552 [DOI] [PubMed] [Google Scholar]
- 8.Hladky S. B., Barrand M. A. (2014) Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syková E., Nicholson C. (2008) Diffusion in brain extracellular space. Physiol. Rev. 88, 1277–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson C., Tao L. (1993) Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys. J. 65, 2277–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zador Z., Magzoub M., Jin S., Manley G. T., Papadopoulos M. C., Verkman A. S. (2008) Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain. FASEB J. 22, 870–879 [DOI] [PubMed] [Google Scholar]
- 12.McBain C. J., Traynelis S. F., Dingledine R. (1990) Regional variation of extracellular space in the hippocampus. Science 249, 674–677 [DOI] [PubMed] [Google Scholar]
- 13.Vanharreveld A., Crowell J., Malhotra S. K. (1965) A study of extracellular space in central nervous tissue by freeze-substitution. J. Cell Biol. 25, 117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg G. A., Kyner W. T., Estrada E. (1980) Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am. J. Physiol. 238, F42–F49 [DOI] [PubMed] [Google Scholar]
- 15.Adams D. L., Piserchia V., Economides J. R., Horton J. C. (2015) Vascular supply of the cerebral cortex is specialized for cell layers but not columns. Cereb. Cortex 25, 3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch S., Reichold J., Schneider M., Székely G., Weber B. (2012) Topology and hemodynamics of the cortical cerebrovascular system. J. Cereb. Blood Flow Metab. 32, 952–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen S., Nagelhus E. A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O. P. (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 17, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hladky S. B., Barrand M. A. (2016) Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 13, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennels M. L., Blaumanis O. R., Grady P. A. (1990) Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol. 52, 431–439 [PubMed] [Google Scholar]
- 20.Schley D., Carare-Nnadi R., Please C. P., Perry V. H., Weller R. O. (2006) Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 238, 962–974 [DOI] [PubMed] [Google Scholar]
- 21.Asgari M., de Zélicourt D., Kurtcuoglu V. (2016) Glymphatic solute transport does not require bulk flow. Sci. Rep. 6, 38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreha-Kulaczewski S., Joseph A. A., Merboldt K. D., Ludwig H. C., Gärtner J., Frahm J. (2017) Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J. Neurosci. 37, 2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradbury M. W., Westrop R. J. (1983) Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol. 339, 519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szentistványi I., Patlak C. S., Ellis R. A., Cserr H. F. (1984) Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246, F835–F844 [DOI] [PubMed] [Google Scholar]
- 25.Weller R. O., Djuanda E., Yow H. Y., Carare R. O. (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14 [DOI] [PubMed] [Google Scholar]
- 26.Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., Benveniste H., Vates G. E., Deane R., Goldman S. A., Nagelhus E. A., Nedergaard M. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jessen N. A., Munk A. S., Lundgaard I., Nedergaard M. (2015) The glymphatic system: a beginner’s guide. Neurochem. Res. 40, 2583–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie L., Kang H., Xu Q., Chen M. J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D. J., Nicholson C., Iliff J. J., Takano T., Deane R., Nedergaard M. (2013) Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui F. K. (2015) Clearing your mind: a glymphatic system? World Neurosurg. 83, 715–717 [DOI] [PubMed] [Google Scholar]
- 30.Mendelsohn A. R., Larrick J. W. (2013) Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 16, 518–523 [DOI] [PubMed] [Google Scholar]
- 31.Nedergaard M. (2013) Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sample I. (2013) Why do we sleep? To clean our brains, say US scientists. The Guardian. Accessed August 1, 2017, at: https://www.theguardian.com/science/2013/oct/17/sleep-cleans-our-brains-say-scientists
- 33.Konnikova M. (2014) Goodnight. Sleep clean. The New York Times. Accessed August 1, 2017, at: https://www.nytimes.com/2014/01/12/opinion/sunday/goodnight-sleep-clean.html
- 34.Kohn D. (2017) When scientists saw the mouse heads glowing, they knew the discovery was big. The Washington Post. Accessed August 1, 2017, at: https://www.washingtonpost.com/national/health-science/when-scientists-saw-the-mouse-heads-glowing-they-knew-the-discovery-was-big/2017/05/19/f33cc574-246a-11e7-a1b3-faff0034e2de_story.html?utm_term=.358dbd0e201f
- 35.Smith A. J., Jin B. J., Verkman A. S. (2015) Muddying the water in brain edema? Trends Neurosci. 38, 331–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spector R., Robert Snodgrass S., Johanson C. E. (2015) A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp. Neurol. 273, 57–68 [DOI] [PubMed] [Google Scholar]
- 37.Jin B. J., Smith A. J., Verkman A. S. (2016) Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 148, 489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holter K. E., Kehlet B., Devor A., Sejnowski T. J., Dale A. M., Omholt S. W., Ottersen O. P., Nagelhus E. A., Mardal K. A., Pettersen K. H. (2017) Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl. Acad. Sci. USA 114, 9894–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith A. J., Yao X., Dix J. A., Jin B. J., Verkman A. S. (2017) Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6, e27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selkoe D. J., Hardy J. (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hladky S. B., Barrand M. A. (2017) Metabolite clearance during wakefulness and sleep. [E-pub ahead of print] Handb. Exp. Pharmacol. doi: 10.1007/164_2017_37 [DOI] [PubMed] [Google Scholar]
- 42.Hong S., Ostaszewski B. L., Yang T., O’Malley T. T., Jin M., Yanagisawa K., Li S., Bartels T., Selkoe D. J. (2014) Soluble Aβ oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron 82, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., Holtzman D. M., Miller C. A., Strickland D. K., Ghiso J., Zlokovic B. V. (2000) Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carare R. O., Hawkes C. A., Jeffrey M., Kalaria R. N., Weller R. O. (2013) Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 39, 593–611 [DOI] [PubMed] [Google Scholar]
- 45.Holmes B. B., Diamond M. I. (2014) Prion-like properties of tau protein: the importance of extracellular tau as a therapeutic target. J. Biol. Chem. 289, 19855–19861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D. H., Kopeikina K. J., Pitstick R., Sahara N., Ashe K. H., Carlson G. A., Spires-Jones T. L., Hyman B. T. (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohm C., Chen F., Sevalle J., Qamar S., Dodd R., Li Y., Schmitt-Ulms G., Fraser P. E., St George-Hyslop P. H. (2015) Current and future implications of basic and translational research on amyloid-β peptide production and removal pathways. Mol. Cell. Neurosci. 66 (Pt A), 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holtzman D. M., Herz J., Bu G. (2012) Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z., Sagare A. P., Ma Q., Halliday M. R., Kong P., Kisler K., Winkler E. A., Ramanathan A., Kanekiyo T., Bu G., Owens N. C., Rege S. V., Si G., Ahuja A., Zhu D., Miller C. A., Schneider J. A., Maeda M., Maeda T., Sugawara T., Ichida J. K., Zlokovic B. V. (2015) Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 18, 978–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeh F. L., Wang Y., Tom I., Gonzalez L. C., Sheng M. (2016) TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91, 328–340 [DOI] [PubMed] [Google Scholar]
- 51.Weller R. O., Massey A., Newman T. A., Hutchings M., Kuo Y. M., Roher A. E. (1998) Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol. 153, 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weller R. O., Subash M., Preston S. D., Mazanti I., Carare R. O. (2008) Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 18, 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cserr H. F., Cooper D. N., Suri P. K., Patlak C. S. (1981) Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 240, F319–F328 [DOI] [PubMed] [Google Scholar]
- 54.Ichimura T., Fraser P. A., Cserr H. F. (1991) Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 545, 103–113 [DOI] [PubMed] [Google Scholar]
- 55.Iliff J. J., Lee H., Yu M., Feng T., Logan J., Nedergaard M., Benveniste H. (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iliff J. J., Wang M., Zeppenfeld D. M., Venkataraman A., Plog B. A., Liao Y., Deane R., Nedergaard M. (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicholson C. (1993) Ion-selective microelectrodes and diffusion measurements as tools to explore the brain cell microenvironment. J. Neurosci. Methods 48, 199–213 [DOI] [PubMed] [Google Scholar]
- 58.Smith A. J., Jin B. J., Ratelade J., Verkman A. S. (2014) Aggregation state determines the localization and function of M1- and M23-aquaporin-4 in astrocytes. J. Cell Biol. 204, 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner E., Enzmann G. U., Lin S., Ghavampour S., Hannocks M. J., Zuber B., Rüegg M. A., Sorokin L., Engelhardt B. (2012) Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia 60, 1646–1659 [DOI] [PubMed] [Google Scholar]
- 60.Wilcock D. M., Vitek M. P., Colton C. A. (2009) Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 159, 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren Z., Iliff J. J., Yang L., Yang J., Chen X., Chen M. J., Giese R. N., Wang B., Shi X., Nedergaard M. (2013) ‘Hit & run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 33, 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeppenfeld D. M., Simon M., Haswell J. D., D’Abreo D., Murchison C., Quinn J. F., Grafe M. R., Woltjer R. L., Kaye J., Iliff J. J. (2017) Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol. 74, 91–99 [DOI] [PubMed] [Google Scholar]
- 63.Sosunov A. A., Wu X., Tsankova N. M., Guilfoyle E., McKhann G. M., II, Goldman J. E. (2014) Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J. Neurosci. 34, 2285–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith A. J., Verkman A. S. (2015) Superresolution imaging of aquaporin-4 cluster size in antibody-stained paraffin brain sections. Biophys. J. 109, 2511–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eidsvaag V. A., Enger R., Hansson H. A., Eide P. K., Nagelhus E. A. (2017) Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia 65, 964–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kress B. T., Iliff J. J., Xia M., Wang M., Wei H. S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J. A., Plog B. A., Ding F., Deane R., Nedergaard M. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliff J. J., Chen M. J., Plog B. A., Zeppenfeld D. M., Soltero M., Yang L., Singh I., Deane R., Nedergaard M. (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musiek E. S., Holtzman D. M. (2016) Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee H., Xie L., Yu M., Kang H., Feng T., Deane R., Logan J., Nedergaard M., Benveniste H. (2015) The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penn R. D., Lee M. C., Linninger A. A., Miesel K., Lu S. N., Stylos L. (2005) Pressure gradients in the brain in an experimental model of hydrocephalus. J. Neurosurg. 102, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 71.Alperin N. J., Lee S. H., Loth F., Raksin P. B., Lichtor T. (2000) MR-intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology 217, 877–885 [DOI] [PubMed] [Google Scholar]
- 72.Bilston L. E., Fletcher D. F., Brodbelt A. R., Stoodley M. A. (2003) Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: a computational model. Comput. Methods Biomech. Biomed. Engin. 6, 235–241 [DOI] [PubMed] [Google Scholar]
- 73.Asgari M., de Zélicourt D., Kurtcuoglu V. (2015) How astrocyte networks may contribute to cerebral metabolite clearance. Sci. Rep. 5, 15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charras G. T., Yarrow J. C., Horton M. A., Mahadevan L., Mitchison T. J. (2005) Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang J. E., Lim M. M., Bateman R. J., Lee J. J., Smyth L. P., Cirrito J. R., Fujiki N., Nishino S., Holtzman D. M. (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roh J. H., Huang Y., Bero A. W., Kasten T., Stewart F. R., Bateman R. J., Holtzman D. M. (2012) Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci. Transl. Med. 4, 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cicognola C., Chiasserini D., Eusebi P., Andreasson U., Vanderstichele H., Zetterberg H., Parnetti L., Blennow K. (2016) No diurnal variation of classical and candidate biomarkers of alzheimer’s disease in CSF. Mol. Neurodegener. 11, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dechadilok P., Deen W. M. (2009) Electrostatic and electrokinetic effects on hindered convection in pores. J. Colloid Interface Sci. 338, 135–144 [DOI] [PubMed] [Google Scholar]
- 79.Yao X., Hrabetová S., Nicholson C., Manley G. T. (2008) Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J. Neurosci. 28, 5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Binder D. K., Papadopoulos M. C., Haggie P. M., Verkman A. S. (2004) In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 24, 8049–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Papadopoulos M. C., Manley G. T., Krishna S., Verkman A. S. (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 18, 1291–1293 [DOI] [PubMed] [Google Scholar]
- 82.Korogod N., Petersen C. C., Knott G. W. (2015) Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 4, e05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heller J. P., Michaluk P., Sugao K., Rusakov D. A. (2017) Probing nano-organization of astroglia with multi-color super-resolution microscopy. J. Neurosci. Res. 95, 2159–2171 [DOI] [PubMed] [Google Scholar]
- 84.Godin A. G., Varela J. A., Gao Z., Danné N., Dupuis J. P., Lounis B., Groc L., Cognet L. (2017) Single-nanotube tracking reveals the nanoscale organization of the extracellular space in the live brain. Nat. Nanotechnol. 12, 238–243 [DOI] [PubMed] [Google Scholar]
- 85.Pollak T. A., Drndarski S., Stone J. M., David A. S., McGuire P., Abbott N. J. (2017) The blood-brain barrier in psychosis. [E-pub ahead of print] Lancet Psychiatry doi: 10.1016/S2215-0366(17)30293-6 [DOI] [PubMed] [Google Scholar]