Abstract
Background
Patients with bipolar disorder are at high risk of metabolic disturbance after mood stabilizer treatment. However, the mediators linking the two conditions remain unknown. In this study, we investigated whether fibroblast growth factor-21 (FGF21) was associated with metabolic effects and treatment response in depressed bipolar disorder patients.
Methods
We recruited 78 community-dwelling controls and 137 bipolar disorder patients; the latter were interviewed using the Chinese Version of the Modified Schedule of Affective Disorder and Schizophrenia–Life Time. Upon study entry, the bipolar disorder patients were all in a major depressive status, with 17-item Hamilton Depression Rating Scale (HDRS) scores >15. They received valproate (500–1000 mg daily) for 12 weeks, and fluoxetine 20 mg daily was permitted to treat depressive symptoms. Fasting plasma level of FGF21, lipid profiles, and body weight were collected at baseline and after 12 weeks of treatment.
Results
At baseline, the demographic characteristics, FGF21 level, and metabolic indices did not differ significantly between the controls and bipolar disorder patients. After 12 weeks of treatment, the FGF21 level (167.7±122.0 to 207.1±162.3 pg/mL, P=.001), body weight and waist circumference had increased significantly (P<.001 and P=.028, respectively). Moreover, the change in FGF21 level was significantly correlated with the changes in HDRS score (r=0.393, P=.002), total cholesterol (r=−0.344, P=.008), and low-density lipoprotein (r=−0.347, P=.007).
Conclusions
The central and peripheral mediating effects of FGF21 on bipolar disorder depression treatment might be opposite. High peripheral FGF21 levels might link regulation of metabolic effect and resistance to treatment in bipolar disorder.
Keywords: bipolar disorder, FGF21, metabolic effect, valproate
Significance Statement
Patients with bipolar disorder (BD) are at high risk of metabolic disturbance after mood stabilizer treatment, and the mediators linking the conditions remain unknown. We hypothesized that fibroblast growth factor-21 (FGF21) may not only play a role as a regulator of metabolic effects, but also possesses the therapeutic action of mood stabilizers. As the current study showed, the FGF21 level did not differ significantly between the controls and BD patients at baseline. In the BD patients after treatment, however, the FGF21 level significantly increased, and the increased amount of FGF21 was significantly correlated with the change in disease severity and metabolic indices, including the levels of total cholesterol and LDL. Further study of the mechanisms in FGF21-based therapies has the potential to uncover novel targets for drugs that could prevent metabolic disturbances, and ultimately improve overall treatment outcome of BD.
Introduction
Bipolar disorder (BD) is a mental disorder that causes impairment of functionality in daily life, resulting in costs to both patients and society (Grande et al., 2016). In addition, epidemiologic evidence has indicated increased metabolic abnormalities in BD patients, with a prevalence of metabolic syndrome of 30% to 50% (Taylor and MacQueen, 2006; Chang et al., 2009). The disorder itself, together with the medications used as mood stabilizers, could increase the metabolic risk (Yumru et al., 2008; Chang et al., 2010b; Grande et al., 2016). Metabolic abnormalities result in high medical morbidity and mortality in BD patients (Forty et al., 2014; Schoepf and Heun, 2014). Overall, comorbidities with BD and metabolic abnormalities contribute to increased long-term costs to the national health care system (Kleine-Budde et al., 2014; Jin and McCrone, 2015).
Importantly, mood stabilizers not only effectively improve mood symptoms but also induce and aggravate metabolic abnormalities in BD (Wink et al., 2017). Whether there are shared underlying neurological and physiological mechanisms that explain the therapeutic and metabolic effects remains unclear. Recent in vitro studies indicated that fibroblast growth factor 21 (FGF21) has a neuroprotective function and is markedly elevated in neurons under treatment with mood stabilizers (Leng et al., 2015). An in vivo study showed that FGF21 could regulate circadian behavior by acting on the nervous system (Bookout et al., 2013). Peripheral physiological roles of FGF21 include the maintenance of energy homeostasis under conditions of metabolic or environmental stress (Woo et al., 2013; Kim and Lee, 2014). Moreover, FGF21 can cross the blood–brain barrier and modulate phosphorylation cascades and gene expression in the whole hypothalamus (Bookout et al., 2013). FGF21 acts directly on the nervous system to induce sympathetic nerve activity that regulates metabolism (Bookout et al., 2013; Owen et al., 2014).
Therefore, FGF21 may not only play a role as a regulator of metabolic effects but may also possess the therapeutic action of mood stabilizers. However, to date, no clinical studies have investigated the associations between FGF21, treatment outcome of mood stabilizers, and metabolic effects in BD patients. Thus, in this longitudinal study, we aimed to investigate whether FGF21 was associated with metabolic effects and the treatment response in depressed BD patients treated with valproate (VPA) and fluoxetine. In addition, bipolar II disorder (BD II) is characterized with more chronic courses and major depressive episodes than those of BD I (Vieta et al., 1997; Pallanti et al., 1999; Judd et al., 2003a). To exclude the influence of BD itself on the association between FGF21 level and treatment outcome, we only recruited the BD II patients in the current study.
Methods
Subjects
The Institutional Review Board for the Protection of Human Subjects at National Cheng Kung University Hospital approved the research protocol. All participants, recruited from outpatient settings at the National Cheng Kung University Hospital, provided written informed consent regarding their willingness to participate in the research. To assess whether BD influences the FGF21 level, we recruited healthy controls from the community after exclusion of individuals with mental illnesses by a senior psychiatrist using the Chinese version of the Mini International Neuropsychiatry Interview. All the BD patients were initially evaluated in an interview by an attending psychiatrist using the Chinese Version of the Modified Schedule of Affective Disorder and Schizophrenia–Life Time, which has a good inter-rater reliability, to determine the DSM-IV. The patients also met the following inclusion criteria: (1) diagnosis of BD II using the 2-day minimum for hypomania (Lee et al., 2013), (2) major depressive status at the time of study entry, with a 17-item Hamilton Depression Rating Scale (HDRS) score >15, and (3) drug-naïve and at first diagnosis, with no history of mood stabilizer treatment. We recruited only first-diagnosis, drug-naïve BD II patients who received their first treatment. In addition, we used 2-day minimum of hypomania in the diagnosis of BD II instead of the 4-day of criteria, because epidemiologic data suggest that 2-day duration of hypomania is more prevalent and sensitive to diagnose BD II (Judd et al., 2003b; Benazzi and Akiskal, 2006).
After enrollment in this study, the patients received VPA in the therapeutic range of 50 to 100 µg/mL for 12 weeks. Concomitant fluoxetine (20 mg/day) was permitted to treat depressive symptoms, and lorazepam (<8 mg/day) was used for nighttime sedation and to treat agitation and insomnia during the study, the dosage of which was adjusted according to the clinical manifestation and the patient’s tolerance. The mood of each patient was evaluated using the HDRS and the 11-item Young Mania Rating Scale (YMRS) at baseline and 12 weeks after the initiation of treatment.
Measurements
Fasting blood samples were collected between 8:00 am and 10:00 am. Ten milliliters of whole blood was withdrawn from the antecubital vein of each patient. Plasma, which was isolated from whole blood after centrifugation at 3000 ×g for 15 minutes at 4°C, was immediately stored at –80°C.
FGF21
The fasting plasma FGF21 level was determined using an ELISA kit (Biovender, Brno, Czech Republic) following the manufacturer’s instructions. The limit of detection was 7 pg/mL. The intra- and inter-assay coefficients of the samples were 2.4% and 3.5%, respectively. The antibodies used in this ELISA were specific for human FGF21. No cross-reactivity with human FGF19 and FGF23 had been observed. FGF21 concentration was calculated using the standard calibration curve provided with the kits. An independent assistant who was blind to the allocation of the patients examined the patients’ assessments and FGF21 levels.
Metabolic Index
Fasting total cholesterol, low-density lipoprotein (LDL), and triglyceride concentrations were measured by enzymatic methods using the automated analyzer TBA-200FR (Toshiba Lab Medical, Tokyo, Japan). In addition, the body mass index (BMI) of each patient was measured at each time point. BMI was calculated as weight (kg) divided by height squared (m2), and waist circumference was measured at the level midway between the lateral lower rib margin and the superior anterior iliac crest.
Statistical Analysis
Statistical analysis was performed using the SPSS 12.0 (SPSS Inc.,). Categorical variables were expressed as numbers and percentages, and continuous variables as means ± SDs unless otherwise specified. Categorical variables were assessed using chi-square tests, and continuous variables were assessed using t tests or paired t tests. Correlations of the FGF21 level and metabolic indices among all the subjects were assessed using linear regressions with the covariant of disease status (BD or healthy controls). Correlations of the change in FGF21 level with disease severity and metabolic effects in the BD patients were calculated using Pearson’s correlation. The change in FGF21 level was calculated as (FGF21 level at 12 weeks - FGF21 level at baseline), and the change in other indices was also calculated in a similar way. When the change in FGF21 level was a plus quantity, the FGF21 levels increased after the treatment. In addition, intent-to-treat analyses were used to assess the baseline disease severity and metabolic indices as the primary outcomes at baseline and after VPA treatment. The level of significance was set at 0.05 for 2-sided tests.
Results
We recruited 78 healthy controls and 137 BD patients. The demographic data are shown in Table 1. Age and gender did not differ significantly between the healthy controls and the BD patients. The baseline FGF21 level and metabolic indices were also not significantly different between groups, while the BD patients presented with significantly higher HDRS and YMRS scores than those of the controls. At baseline, the FGF21 level was correlated with body weight (β=0.147, P=.036), BMI (β=0.213, P=.002), waist circumference (β=0.263, P<.001), and triglyceride (β=0.329, P<.001) according to a regression model with the covariant of disease status (BD or healthy controls). There was no interaction of FGF21 level and disease status on either the disease severity score or on metabolic indices.
Table 1.
Demographic characteristics and measurements of the healthy controls and BD patients
| Controls (N=78) |
BD patients (N=137) |
BD patients after treatment |
t / χ2 | p value (95% CI)a | t / χ2 | p value (95% CI)b | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 31.0 ± 10.7 | 32.1 ± 11.6 | - | -0.690 | 0.491 (-2.0 − 4.2) | - | - |
| Gender, female (%) | 44 (56.4) | 70 (51.1) | - | 0.564 | 0.453 (-0.1 − 0.2) | - | - |
| HAMD score | 3.2 ± 1.6 | 19.4 ± 5.5 | 11.4 ± 6.9 | -25.327 | <0.001* (14.9 − 17.4) | 12.911 | <0.001* (6.8 − 9.2) |
| YMRS score | 4.1 ± 0.4 | 9.2 ± 4.0 | 6.4 ± 3.4 | -11.428 | <0.001* (4.3 − 6.0) | 8.147 | <0.001* (2.2 − 3.6) |
| FGF21 (pg/ml) | 176.0 ± 152.8 | 167.7 ± 122.0 | 207.1 ± 162.3 | 0.421 | 0.674 (-47.1 − 30.5) | -3.476 | 0.001* (-61.8 − -17.0) |
| Body weight (kg) | 64.5 ± 15.2 | 62.6 ± 14.3 | 63.5 ± 14.6 | 0.922 | 0.357 (-6.0 − 2.2) | -4.108 | <0.001* (-1.3 − -0.5) |
| BMI (kg/m2) | 23.7 ± 4.4 | 22.8 ± 4.4 | 23.2 ± 4.6 | 1.317 | 0.189 (-2.1 − 0.4) | -4.216 | <0.001* (-0.5 − -0.2) |
| Waist circumference (cm) | 79.2 ± 12.3 | 79.4 ± 12.2 | 80.3 ± 12.1 | -0.107 | 0.915 (-3.2 − 3.6) | -2.227 | 0.028* (-1.6 − -0.1) |
| Cholesterol (mg/dl) | 186.3 ± 37.2 | 183.5 ± 33.4 | 182.9 ± 34.1 | 0.573 | 0.567 (-12.6 − 6.9) | 0.302 | 0.763 (-3.2 − 4.4) |
| TG (mg/dl) | 103.2 ± 84.1 | 90.7 ± 46.9 | 94.6 ± 53.3 | 1.391 | 0.230 (-33.0 − 8.0) | -1.126 | 0.262 (-10.8 − 3.0) |
| LDL (mg/dl) | 116.4 ± 33.4 | 111.3 ± 28.5 | 111.4 ± 30.1 | 1.177 | 0.241 (-13.6 − 3.4) | -0.035 | 0.972 (-3.4 − 3.2) |
Abbreviations: YMRS, 11-item Young Mania Rating Scale; HDRS, 17-item Hamilton Depression Rating Scale; TG, triglyceride; LDL, low-density lipoprotein; BMI, body mass index.
a Compared with healthy controls; bpaired t-test in BD patients at baseline and after treatment.
After 12 weeks of treatment, improvements in HDRS and YMRS scores, representing disease severity, were observed in the BD patients (P<.001 and P<.001, respectively). The FGF21 levels (167.7 ± 122.0 and 207.1 ± 162.3 pg/mL, P=.001), body weight, and waist circumference increased significantly after the treatment (P<.001 and P=.028, respectively) (Table 1). There was no gender difference inthe increase of FGF21 levels (male: 165.6±125.1 to 203.9±159.8 pg/mL, p= 0.016 and female: 169.7±120.0 to 210.1±165.8 pg/mL, P=.011, respectively). Moreover, there was no significant correlation between the VPA concentration and FGF21 level at 12 weeks.
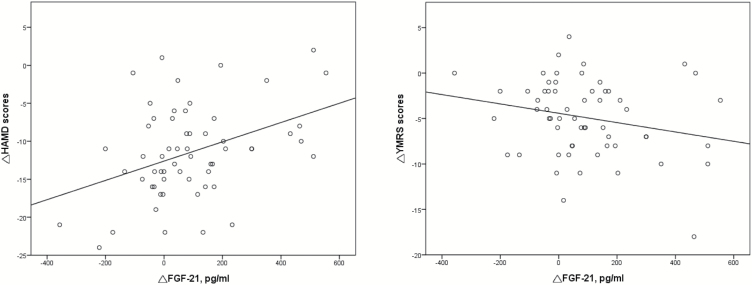
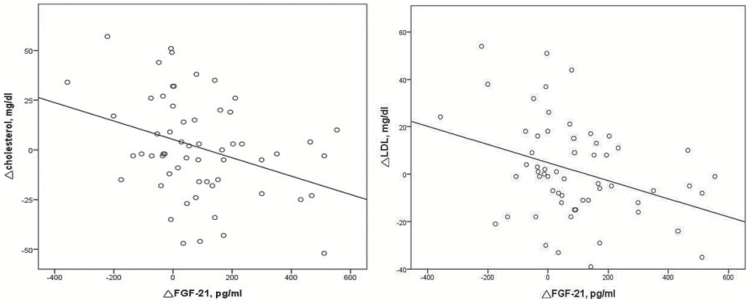
In addition, we aimed to investigate whether the change in FGF21 level was associated with treatment outcome and with metabolic effects in the BD patients (Table 2). The results showed that the change in FGF21 level was significantly correlated with the change in HAMD score (r=0.393, P=.002) but not YMRS score (r=−0.229, P=.081) (Figure 1). The BD patients who exhibited greater increases in the level of FGF21 had a poorer treatment response. The results also demonstrated that the change in FGF21 level was significantly correlated with the changes in the levels of total cholesterol (r=−0.344, P=.008) and LDL (r=−0.347, P=.007) (Figure 2).
Table 2.
Correlations of the change in FGF21 level with disease severity and metabolic effects in BD patients
| Change in FGF21 (pg/ml) | r | p value |
|---|---|---|
| △HAMD score | 0.393 | 0.002* |
| △YMRS score | −0.229 | 0.081 |
| △BMI (kg/m2) | 0.012 | 0.926 |
| △Waist circumference (cm) | −0.152 | 0.249 |
| △Cholesterol (mg/dl) | −0.344 | 0.008* |
| △TG (mg/dl) | 0.018 | 0.891 |
| △LDL (mg/dl) | −0.347 | 0.007* |
Abbreviations: YMRS, Young Mania Rating Scale; HDRS, 17-item Hamilton Depression Rating Scale; TG, triglyceride; LDL, low-density lipoprotein; BMI, body mass index.
Figure 1.
Correlation of the change in fibroblast growth factor-21 (FGF21) level with disease severity in the bipolar disorder (BD) patients. The change in FGF21 level was significantly correlated with the change in HAMD score (r=0.393, P=.002) but not the change in Young Mania Rating Scale (YMRS) score (r=−0.229, P=.081). HAMD, 17-item Hamilton Depression Rating Scale.
Figure 2.
Correlation of the change in fibroblast growth factor-21 (FGF21) level with metabolic effects in bipolar disorder (BD) patients. The change in FGF21 level was significantly correlated with the changes in the levels of cholesterol (r=−0.344, P=.008) and LDL (r=−0.347, P=.007).LDL, low-density lipoprotein.
Discussion
To our knowledge, this was the first clinical study to identify associations between FGF21 level, mood stabilizer treatment response, and metabolic effects in BD patients. In the current study, we found that at baseline, the FGF21 level did not differ significantly between the healthy controls and BD patients. However, in the BD patients, the FGF21 level significantly increased after VPA treatment, and the increased amount of FGF21 was significantly correlated with the treatment outcome and metabolic effects. The results suggested that FGF21 could be a common mediator of the treatment outcome with mood symptoms and metabolic effects in VPA-treated BD patients.
FGF21 is a novel endogenous regulator of glucose, lipid metabolism, and energy balance (Badman et al., 2007; Potthoff et al., 2009; Woo et al., 2013; Patel et al., 2015). Human studies have shown that the plasma concentration of FGF21 is positively associated with BMI, fat mass, and waist circumference (Tan et al., 2011; Heianza et al., 2016). In this study, we found that the plasma levels of FGF21 were similar to that reported in a previous study (Tan et al., 2011). And the plasma FGF21 level was correlated with BMI and waist circumference, regardless of disease status. Studies have demonstrated that liver-derived FGF21 can act as an insulin sensitizer to overcome prolonged fasting-induced insulin resistance by maximizing glucose uptake and adipose tissue lipolysis (Badman et al., 2007; Inagaki et al., 2007). Interestingly, our previous studies showed that the glucose level was significantly lower in BD patients under chronic VPA treatment, though the blood lipid levels were significantly higher (Chang et al., 2009, 2010b). In this study, we observed an increase in the level of FGF21 after VPA treatment. Taken together, these results demonstrate that FGF21 could be a mediator of VPA-induced metabolic effects.
Our study also demonstrated that the plasma FGF21 level increased after VPA treatment in BD patients; however, the underlying mechanisms remain unknown (Szalowska et al., 2014). In physiological conditions, the expression of FGF21 can be induced in response to diverse stressors in multiple organs (Kim and Lee, 2014). Currently, in vitro studies using neuron/glial cultures demonstrate the induction of FGF21 expression by VPA throught its histone deacetylase inhibition effects (Leng et al., 2016). Further studies are need to clarify the underlying mechanisms for the FGF21 induction after in systemic VPA administration.
In addition to epilepsy treatment, VPA has also been used to treat BD depression (Grande et al., 2016). Although there is no direct evidence of the mediatng effect of FGF21 in BD treatment, in vitro studies have shown FGF21 mRNA upregulation in neuron and glia after lithium/VPA co-treatment, and the production of FGF21 has been found to be associated with a critical role in neuroprotective effect against glutamate neurotoxicity through an Akt dependent pathway (Leng et al., 2015, 2016). Recent in vivo animal study has demonstrated that FGF21 regulates circadian behavior and metabolism through its central effects (Bookout et al., 2013; Leng et al., 2015; Patel et al., 2015). In addition, the central effects of FGF21 are important in terms of stress adaptation (Bookout et al., 2013; Patel et al., 2015). FGF21 can modulate cognitive function by restoring synaptic plasticity, dendritic spine density, and brain mitochondrial function (Sa-nguanmoo et al., 2016). However, the peripheral effect of FGF21 on BD remains unclear. Our results showed the possible central and peripheral mediating effects of FGF21 on BD depression treatment might be opposite. Those with increased peripheral FGF21 levels were associated with poor treatment response (Figure 1) and less significant increase in blood lipid levels (Figure 2). As the FGF21 level was correlated with body weight, BMI, and, waist circumference at baseline for these patients, we proposed the changed in FGF21 level after treatment might represent a tendency to regulate metabolic effects (Chang et al., 2009, 2010a, 2010b; Fisher et al., 2010). Furthermore, although evidences have linked metabolic dysfunction to resistance to treatment in BD (Calkin et al., 2015; Mansur et al., 2016a, 2016b), further study is needed to clarify the understanding mechanism of FGF21 and mood stabilizer treatment in peripheral and in central effects.
There were some limitations of the present study. The first was the relatively small sample size from a single site. The second was the lack of control of confounding factors such as diet, exercise, socioeconomic status, and general health status. The third limitation was the length of the study period, although we were able to detect changes in metabolic indices and treatment outcome.
In conclusion, this was the first longitudinal study to report correlations between FGF21 level, treatment response and metabolic effects in BD patients treated with VPA. Our novel results suggested that FGF21 could be a common mediator of a shared mechanism of mood response and metabolic effects in BD patients treated with VPA and fluoxetine. FGF21-based therapies could potentially represent novel targets to prevent and treat a variety of mood and metabolic disorders (Gimeno and Moller, 2014; So and Leung, 2016).
Acknowledgments
The authors thank Chien Ting Lin for administrative support.
Funding
This study was financially supported by the the Ministry of Science and Technology of Taiwan (MOST 104-2320-B-006 -024, MOST 105-2320-B-006-014, MOST 106-2320-B-006-040, and MOST 105-2321-B-006-020) and National Cheng Kung University Hospital (NCKUH-10301003 and NCKUH-10509004). This research also received funding (D105-35A06) from the Headquarters of University Advancement at the National Cheng Kung University, which is sponsored by the Ministry of Education, Taiwan.
Statement of Interest
None.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E(2007)Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437. [DOI] [PubMed] [Google Scholar]
- Benazzi F, Akiskal H(2006)The duration of hypomania in bipolar-II disorder in private practice: methodology and validation. J Affect Disord 96:189–196. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MHM, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA(2013)FGF21 regulates circadian behavior and metabolism by acting on the nervous system. Nat Med 19:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin CV, Ruzickova M, Uher R, Hajek T, Slaney CM, Garnham JS, O’Donovan MC, Alda M(2015)Insulin resistance and outcome in bipolar disorder. Br J Psychiatry 206:52–57. [DOI] [PubMed] [Google Scholar]
- Chang HH, Chou CH, Chen PS, Gean PW, Huang HC, Lin CY, Yang YK, Lu RB(2009)High prevalence of metabolic disturbances in patients with bipolar disorder in Taiwan. J Affect Disord 117:124–129. [DOI] [PubMed] [Google Scholar]
- Chang HH, Gean PW, Chou CH, Yang YK, Tsai HC, Lu RB, Chen PS (2010a) C825T polymorphism of the GNB3 gene on valproate-related metabolic abnormalities in bipolar disorder patients. J Clin Psychopharmacol 30:512–517. [DOI] [PubMed] [Google Scholar]
- Chang HH, Yang YK, Gean PW, Huang HC, Chen PS, Lu RB (2010b) The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord 124:319–323. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E(2010)Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59:2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forty L, Ulanova A, Jones L, Jones I, Gordon-Smith K, Fraser C, Farmer A, McGuffin P, Lewis CM, Hosang GM, Rivera M, Craddock N(2014)Comorbid medical illness in bipolar disorder. Br J Psychiatry 205:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE, Moller DE(2014)FGF21-based pharmacotherapy – potential utility for metabolic disorders. Trend Endocrinol Metab 25:303–311. [DOI] [PubMed] [Google Scholar]
- Grande I, Berk M, Birmaher B, Vieta E(2016)Bipolar disorder. The Lancet 387:1561–1572. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Ma W, Huang T, Wang T, Zheng Y, Smith SR, Bray GA, Sacks FM, Qi L(2016)Macronutrient intake–associated FGF21 genotype modifies effects of weight-loss diets on 2-year changes of central adiposity and body composition: the POUNDS lost trial. Diabetes Care 39:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA(2007)Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425. [DOI] [PubMed] [Google Scholar]
- Jin H, McCrone P(2015)Cost-of-illness studies for bipolar disorder: systematic review of international studies. Pharmacoeconomics 33:341–353. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB (2003a) A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 60:261–269. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Maser J, Rice JA, Solomon DA, Keller MB (2003b) The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders?J Affect Disord 73:19–32. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lee MS(2014)FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J 38:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Budde K, Touil E, Moock J, Bramesfeld A, Kawohl W, Rossler W(2014)Cost of illness for bipolar disorder: a systematic review of the economic burden. Bipolar Disord 16:337–353. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Chen S-L, Chang Y-H, Chen PS, Huang S-Y, Tzeng N-S, Wang Y-S, Wang L-J, Lee IH, Wang T-Y, Yeh TL, Yang YK, Hong J-S, Lu R-B(2013)Inflammation’s association with metabolic profiles before and after a twelve-week clinical trial in drug-naïve patients with bipolar II disorder. PLoS ONE 8:e66847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wang Z, Tsai LK, Leeds P, Fessler EB, Wang J, Chuang DM(2015)FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol Psychiatry 20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wang J, Wang Z, Liao H-M, Wei M, Leeds P, Chuang D-M(2016)Valproic acid and other HDAC inhibitors upregulate FGF21 gene expression and promote process elongation in glia by inhibiting HDAC2 and 3. Int J Neuropsychopharmacol 19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur RB, Rizzo LB, Santos CM, Asevedo E, Cunha GR, Noto MN, Pedrini M, Zeni M, Cordeiro Q, McIntyre RS, Brietzke E (2016a) Impaired glucose metabolism moderates the course of illness in bipolar disorder. J Affect Disord 195:57–62. [DOI] [PubMed] [Google Scholar]
- Mansur RB, Rizzo LB, Santos CM, Asevedo E, Cunha GR, Noto MN, Pedrini M, Zeni M, Cordeiro Q, McIntyre RS, Brietzke E (2016b) Adipokines, metabolic dysfunction and illness course in bipolar disorder. J Psychiatr Res 74:63–69. [DOI] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ(2014)FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S, Quercioli L, Pazzagli A, Rossi A, Dell’Osso L, Pini S, Cassano GB(1999)Awareness of illness and subjective experience of cognitive complaints in patients with bipolar I and bipolar II disorder. Am J Psychiatry 156:1094–1096. [DOI] [PubMed] [Google Scholar]
- Patel R, Bookout AL, Magomedova L, Owen BM, Consiglio GP, Shimizu M, Zhang Y, Mangelsdorf DJ, Kliewer SA, Cummins CL(2015)Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol Endocrinol 29:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC(2009)FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 106:10853–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-nguanmoo P, Tanajak P, Kerdphoo S, Satjaritanun P, Wang X, Liang G, Li X, Jiang C, Pratchayasakul W, Chattipakorn N, Chattipakorn SC(2016)FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav 85:86–95. [DOI] [PubMed] [Google Scholar]
- Schoepf D, Heun R(2014)Bipolar disorder and comorbidity: increased prevalence and increased relevance of comorbidity for hospital-based mortality during a 12.5-year observation period in general hospital admissions. J Affect Disord 169:170–178. [DOI] [PubMed] [Google Scholar]
- So WY, Leung PS(2016)Fibroblast growth factor 21 as an emerging therapeutic target for type 2 diabetes mellitus. Med Res Rev 36:672–704. [DOI] [PubMed] [Google Scholar]
- Szalowska E, van der Burg B, Man H-Y, Hendriksen PJM, Peijnenburg AACM(2014)Model steatogenic compounds (amiodarone, valproic acid, and tetracycline) alter lipid metabolism by different mechanisms in mouse liver slices. PLoS ONE 9:e86795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS(2011)Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes 60:2758–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, MacQueen G(2006)Associations between bipolar disorder and metabolic syndrome: a review. J Clin Psychiatry 67:1034–1041. [DOI] [PubMed] [Google Scholar]
- Vieta E, Gasto C, Otero A, Nieto E, Vallejo J(1997)Differential features between bipolar I and bipolar II disorder. Compr Psychiatry 38:98–101. [DOI] [PubMed] [Google Scholar]
- Wink LK, Minshawi NF, Shaffer RC, Plawecki MH, Posey DJ, Horn PS, Adams R, Pedapati EV, Schaefer TL, McDougle CJ, Swiezy NB, Erickson CA(2017)d-Cycloserine enhances durability of social skills training in autism spectrum disorder. Mol Autism 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y, Xu A, Wang Y, Lam K(2013)Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol (Oxf) 78:489–496. [DOI] [PubMed] [Google Scholar]
- Yumru M, Savas E, Gergerlioglu HS, Baflarali K, Kalenderoglu A, Savas HA, Buyukbas S(2008)The relationship of metabolic syndrome, serum leptin levels and treatment in bipolar disorder. Bulletin of Clinical Psychopharmacology 18:79–83. [Google Scholar]