Abstract
Introduction
Oxidative stress and glutathione dysregulation have been implicated in the etiology of schizophrenia. To date, most in vivo studies have investigated alterations in cerebral glutathione levels in patients in which the disorder is already established; however, whether oxidative stress actually predates the onset of psychosis remains unknown. In the current study, we investigated cerebral glutathione levels of antipsychotic-naïve individuals at clinical high risk for psychosis. As exploratory analyses, we also investigated the associations between cerebral glutathione levels and peripheral glutathione peroxidase activity and clinical and neuropsychological measures.
Methods
Glutathione levels were measured in the medial prefrontal cortex of 30 clinical high risk (n=26 antipsychotic naïve) and 26 healthy volunteers using 3T proton magnetic resonance spectroscopy. Each participant was assessed for glutathione peroxidase activity in plasma and genotyped for the glutamate cysteine ligase catalytic subunit polymorphism.
Results
No significant differences were observed in glutathione levels between clinical high risk and healthy volunteers in the medial prefrontal cortex (F(1,54)=0.001, P =0.98). There were no significant correlations between cerebral glutathione levels and clinical and neuropsychological measures. Similarly, no significant differences were found in peripheral glutathione peroxidase activity between clinical high risk and healthy volunteers (F(1,37)=0.15, P =0.70). However, in clinical high risk, we observed a significant effect of lifetime history of cannabis use on glutathione peroxidase activity (F(1,23)=7.41, P =0.01).
Discussion
The lack of significant differences between antipsychotic naïve clinical high risk and healthy volunteers suggests that alterations in glutathione levels in medial prefrontal cortex are not present in the clinical high risk state.
Keywords: GSH, oxidative stress, magnetic resonance spectroscopy, schizophrenia
Significance Statement
Alterations in GSH levels have only been investigated in patients in whom the disorder is already established; however, whether oxidative stress predates the onset of psychosis remains unknown. This is the first in vivo study investigating GSH levels in individuals at clinical high risk for psychosis.
Introduction
Accumulating evidence suggests a role of oxidative stress in the pathophysiology of schizophrenia (SCZ) (Yao and Keshavan, 2011; Flatow et al., 2013; Barron et al., 2017). Prooxidants such as free radicals and reactive oxygen species are constantly generated as by-products of metabolic reactions and neutralized by antioxidants. Oxidative stress occurs when there is an imbalance between the production of prooxidants and their removal by antioxidant defence mechanisms. Accumulation of prooxidants in the central nervous system can result in damage to macromolecules such as proteins, nucleic acids, lipids, and cellular membranes, ultimately leading to neuronal damage (Flatow et al., 2013). Glutathione (GSH) is the most abundant antioxidant in the central nervous system and plays an important role in protecting the brain against prooxidants. It acts as a nucleophilic scavenger and converts harmful free radicals into less reactive species.
Genetic studies have shown associations between genes involved in GSH metabolism and SCZ, suggesting a role of GSH dysregulation in psychosis (Gysin et al., 2007). Supporting this, several peripheral studies have reported decreased GSH levels in blood cells (i.e., erythrocytes and platelets) and plasma of antipsychotic naïve and chronically medicated SCZ patients (Altuntas et al., 2000; Raffa et al., 2009; Micó et al., 2011; Raffa et al., 2011). Decreased GSH levels were also reported in the cerebrospinal fluid (CSF) of untreated SCZ patients (Do et al., 2000). Similarly, postmortem studies have also reported reduced levels of GSH and glutathione peroxidase (GPx) in the prefrontal cortex (Gawryluk et al., 2011) and caudate (Yao et al., 2006) of SCZ patients. GPx is an important antioxidant enzyme that reduces hydrogen peroxide to water by converting GSH to oxidized GSH and plays an important role in regulating oxidant status. Beneficial effects of N-acetyl-L-cysteine, a GSH precursor, as an adjunctive treatment for SCZ was shown in several clinical trials, further supporting the clinical relevance of GSH in SCZ (for review, see Sommer et al., 2013).Given the known limitations associated with postmortem studies, proton magnetic resonance spectroscopy (1H-MRS) allows for in vivo quantification of metabolites in the brain, including GSH. To date, 5 in vivo 1H-MRS studies have investigated GSH levels in SCZ. The first study by Do et al. (2000) reported a 52% reduction in GSH levels in the medial prefrontal cortex (mPFC) of chronic drug-free SCZ patients compared with healthy volunteers (14 SCZ, 10 healthy volunteers). However, 2 consecutive studies reported no significant differences in GSH levels in the anterior cingulate cortex (13 SCZ, 9 healthy volunteers) (Terpstra et al., 2005) or mPFC (20 SCZ, 16 healthy volunteers) (Matsuzawa et al., 2008) of SCZ patients, though the latter study reported a negative correlation between GSH levels and the severity of negative symptoms (Matsuzawa et al., 2008). Only 2 studies have investigated GSH alterations in first-episode psychosis (FEP) patients, though with conflicting results. Wood et al. (2009) reported a 22% increase in GSH levels in the medial temporal lobe of treated FEP patients compared with controls (30 FEP, 18 healthy volunteers), whereas the most recent study reported no significant differences in the mPFC of treated FEP patients (25 FEP, 33 healthy volunteers) (Xin et al., 2016); however, they reported a significant effect of a polymorphism in the gene coding for the catalytic subunit of glutamate-cysteine ligase (GCLC), independent of disease status on cerebral GSH levels. These contrasting findings may be explained by differences in GSH acquisition (i.e., methodology), long-term effect of medication, and/or phase and duration of illness. Importantly, alterations in GSH levels have only been investigated in patients in whom the disorder is already established, so, whether oxidative stress presents during the prodromal stage that precedes psychosis (i.e., individuals at clinical high risk for psychosis [CHR]) remains unknown. Furthermore, it has been suggested that preemptive treatments aiming at this redox imbalance may prevent psychosis in those at risk (Sawa and Seidman, 2014).
In the past decade, the CHR (“prodromal,” “ultra-high risk,” or “at-risk mental state”) group has become a reliably identifiable clinical constellation with clear and compelling power to predict the onset of psychosis within the near future (1–2 years) (Cannon et al., 2008). To our knowledge, this is the first in vivo 1H-MRS study investigating GSH levels in mPFC in a relatively large sample of 30 CHR (n=26 antipsychotic naive). The mPFC was chosen as the region of interest based on previous in vivo and postmortem studies that have reported alterations in GSH levels in this particular region and the known involvement of the mPFC in SCZ (Do et al., 2000; Gawryluk et al., 2011). As exploratory analyses, we also examined the associations between cerebral GSH levels and peripheral GPx activity and associations with symptom severity and neuropsychological measures. Lastly, we present results corrected for the GCLC polymorphism, as previously reported (Xin et al., 2016).
Methods
Participants
Thirty CHR and 27 healthy volunteers were initially enrolled and scanned in this study. One healthy volunteer was excluded due to motion during 1H-MRS scan. Most of the participants in the CHR group were antipsychotic-naïve (n = 26).
Individuals in the CHR group were recruited from the Focus on Youth Psychosis Prevention Clinic at the Centre for Addiction and Mental Health (CAMH), Toronto, Canada. Diagnosis and severity of psychosis-risk symptoms were assessed with the Structured Interview of Prodromal Syndromes; participants were included if they met Criteria of Prodromal Syndromes (Miller et al., 2003). Healthy volunteers were recruited from the community and did not have any history of psychoactive drug use and/or first-degree relatives with a major mental disorder. All participants were screened with the Semi-Structured Clinical Interview (First et al., 2002) for DSM-IV Axis I disorders to rule out psychiatric comorbidities and were excluded for any of the following: current DSM-IV substance abuse/dependence, pregnancy or current breastfeeding, unstable medical or neurological illness, history of severe head trauma (resulting in loss of consciousness), and the presence of metal implants precluding an MRI scan. Neurocognitive performance was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998).
This study was approved by the Research Ethics Board at CAMH. All participants provided written informed consent after procedures were explained thoroughly.
1H-Magnetic Resonance Spectroscopy
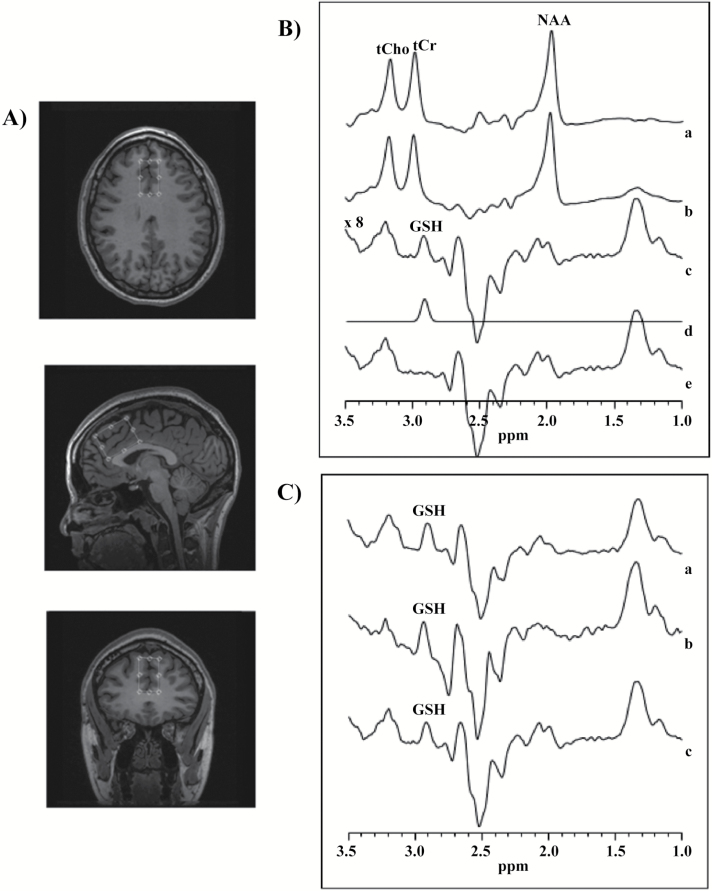
The GSH measurements were acquired on a 3T 750 MR scanner (General Electric HealthCare), equipped with an 8-channel head coil for excitation and reception. To minimize head motion, each subject was positioned at the center of the head coil with tape strapped across the forehead and restraint soft padding around the head. A 24-cc (20 x 40 x 30 mm3) single voxel 1H-MRS was carefully placed in the mPFC (Figure 1A). Magnet homogeneity was adjusted using the manufacture automated shimming routine. For a conventional single voxel PRESS data acquisition acquired at moderate echo time of 68 milliseconds, as used in this study, GSH resonance at 2.95 ppm is invisible due to the strong overlap by the up to 10-fold stronger total creatine resonance. The standard J-editing MEGA-PRESS method used in this study has been described elsewhere (Mescher et al., 1998; Wang et al., 2006; Shungu, 2012; Weiduschat et al., 2014; Lapidus et al., 2014). Briefly, a pair of frequency selective inversion pulses (Sailasuta et al., 2001) with pulse width of 14.4 milliseconds were used to invert coupled GSH resonances. The frequencies of the edited RF pulses were cycled in an interleave manner between the “on” condition at the frequency of GSH α-cysteinyl resonance (at 4.56 ppm) and the “off” condition at 7.5 ppm using TE/TR = 68/1500 milliseconds. Prior to subtraction of the on from the off condition (Figure 1B), the raw 1H-MRS datasets were combined in the time domain based on coil sensitivity (Wright and Wald, 1997), followed by frequency correction using the unsuppressed water signal. The subtracted spectrum results in J-edited GSH spectrum at 2.9 ppm (Figure 1C). Data acquisition parameters were as follows: spectral bandwidth = 5 kHz, number of excitations = 528 (512 of water suppressed, and 16 of water unsuppressed), number of data points = 4096. A 3-Hz Gaussian filter was applied to the difference data and zero-filled to 8192 points, which was then Fourier transformed.
Figure 1.
(A) Axial, sagittal, and coronal views of the voxel placement in the medial prefrontal cortex (mPFC). (B) Typical fitting results from a patient, (a) 1H-MRS subspectra acquired for the “on” condition (see text), (b) “off” condition, (c) subtracted to obtain clean glutathione (GSH) resonance at 2.9 ppm, (d) frequency domain model-fitting of edited GSH resonance only, and (e) residual of the difference between c and d. (C) Representation of the subtracted subspectra from 3 subjects.
IDL-based software (XSOS) was used to quantify GSH within the volume of interest. The area of GSH resonance was obtained by modeling the GSH peak area as a linear combination of pseudo-Voigt lineshape functions and then fitted in the frequency domain using a highly optimized public-domain Levenberg–Marquardt nonlinear least-squares minimization routine, MPFIT (Markwardt, 2009). The pseudo-Voigt lineshape function enabled more precise analysis of lineshapes that consist of mixtures of Lorentzian and Gaussian functions (Marshall et al., 2000), as is often the case for in vivo spectra. The GSH/H2O peak area ratio was reported. Typical unsuppressed water resonance frequency width at half maximum intensity (FWHM) is between 8 and 10 Hz. Examination was rejected if FWHM was >11 Hz or head motion resulting in incomplete subtraction.
The data acquisition approach employed in this study has been successfully used in numerous brain pathologies (Lapidus et al., 2014; Weiduschat et al., 2014). In addition, the strength of using MPFIT to estimate the GSH/H2O ratio has recently been reported (Shungu et al., 2016). Given that GSH levels in the human brain are very low, we acquired the 1H-MRS signal from a larger voxel size, 24 cc (3 times larger than a typical single 1H-MRS voxel of 8 cc), and a rather long acquisition time of 15 minutes (compared with 3–5 minutes from a typical single voxel 1H-MRS scan). Moreover, we routinely obtained a signal to noise ratio of GSH to unsuppressed water signal between 0.001 and 0.002. For our specific MR scanner, we performed test-retest reliability scans on the J-edited data acquisition and data processing. The coefficient of variation for intra- and inter- subject variability was 20% and 17%, respectively. Duplicate examinations were delayed by 1 to 2 hours after the first scan, representing test-retest scans.
In addition, tissue composition was measured to determine the fractions of gray matter, white matter, and CSF within the volume of interest. Briefly, from the 1H-MRS raw data file and 3D T1-weighted images, mPFC voxel mask, and volume images were created using an in-house Matlab-based code. Segment function available from Statistical Parametric Mapping version 8 (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used to determine fractional tissue composition within the voxel. GSH was corrected for CSF fraction as previously described elsewhere (Harris et al., 2015).
Peripheral GPx Enzymatic Activity in Plasma
Peripheral GPx activity (nmol/min/mL) was obtained from 39 participants (25 CHR and 14 healthy volunteers) for whom blood samples were available. Blood samples were taken on average within 15.03 days of the 1H-MRS GSH scan. GPx enzymatic activity was assessed in plasma using the GPx assay kit from Cayman Chemical according to the manufacturer instructions (CaymenChem, www.caymanchem.com/pdfs/703102.pdf). We chose to measure the levels of GPx instead of GSH in plasma, as samples had not been deproteinized prior to freezing, hence compromising blood GSH quantification (Tipple and Rogers, 2012). In our sample, the intra-assay CV was calculated to be 11.79% and the inter-assay CV was calculated to be 2.02%. These values are considered to be within the acceptable range for this assay (www.salimetrics.com/assets/ documents/Spit_Tips_-_Inter__Intra_ Assay_ Coefficients_ of_ Variability.pdf).
Genotyping of GCLC Polymorphism
A GAG trinucleotide repeat polymorphism in the GCLC gene encoding for the rate-limiting enzyme for GSH synthesis has been shown to influence GSH in the periphery and to be associated with SCZ (Gysin et al., 2007). GCLC high-risk genotypes were 30% more frequent in patients and associated with lower GSH levels in fibroblasts when challenged with oxidative stress (Gysin et al., 2007), and lower cerebral GSH levels, compared with GCLC low-risk genotypes (Xin et al., 2016). In this study, 40 participants (27 CHR and 13 healthy volunteers) for whom blood samples were available were genotyped for GCLC trinucleotide polymorphism and were categorized based on the polymorphism as high risk (7/8, 8/8, 8/9, 9/9) or low risk (7/7, 7/9), as described elsewhere (Gysin et al., 2007). Details of genotyping procedures are provided in the supplementary material.
Statistical Analysis
The statistical analyses for the primary outcome measure, cerebral GSH levels, were performed using univariate ANOVA, with GSH levels (corrected and uncorrected for CSF fraction) as the dependent variable and group (CHR vs healthy volunteers) as the independent variable. Similarly, to test for differences in GPx activity between groups, a univariate ANOVA was performed with GPx activity as the dependent variable and group as the independent variable. To study the potential effects of GCLC polymorphism on cerebral GSH levels and GPx activity, the analysis was repeated adding GCLC genotype (high-risk vs low-risk) as a fixed factor. In addition, we explored the potential effects of confounding factors such as age, other drugs of abuse, antipsychotic use, or tobacco use on cerebral GSH levels and GPx activity. Demographic measures were compared using chi-square tests for categorical variables and ANOVA for continuous variables. Bivariate correlations were performed to examine the associations between cerebral GSH levels and peripheral GPx activity or clinical and neuropsychological measures. We also explored the associations between GPx activity and clinical and neuropsychological measures. These analyses were repeated using partial correlations to control for the effects of the GCLC polymorphism. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk), with P<0.05 considered to be significant.
Results
Demographic and clinical information is shown in Table 1. The groups did not differ according to gender. Although the CHR group was significantly younger than the healthy volunteers (F(1,54)=8.98, P=0.004), the difference in mean age between groups was <3 years. Most of the participants in the CHR group had no lifetime history exposure to antipsychotics (n=26 were antipsychotic naïve out of n=30). Four CHR individuals were currently on low-dose antipsychotic treatment with risperidone (0.5 mg and 1 mg), quetiapine (75 mg), or aripiprazole (5 mg). All participants had a negative urine drug screen, except for one CHR who had a positive urine drug screen for cannabis (but was not a regular user). Based on the GCLC genotyping, 27 participants had low-risk (7/7 and 7/9) and 13 participants had high-risk (7/8, 8/8, 8/9, and 9/9) genotypes (supplementary Table 1). One healthy volunteer and one CHR had less common GCLC genotype variants (healthy volunteer: 9/10, CHR: 7/10) and were excluded from the analyses involving genotype. Unsuppressed water linewidth for GSH data acquisition, FWHM, was 7.04 ± 0.92 Hz for healthy volunteers and 7.53 ± 1.14 Hz for CHR. No significant differences were observed in FWHM (P>0.05) between groups (supplementary Table 2).
Table 1.
Demographic and clinical measures of the participants
| Demographics | Healthy volunteers (n = 26) | Clinical high risk (n = 30) | |||
|---|---|---|---|---|---|
| Age (years), SD | 22.77 + 4.05 | 20.33 + 1.73 | F= 8.98 | P = 0.004 | |
| Gender | Male | 10 | 15 | χ2=0.75 | P = 0.39 |
| Female | 16 | 15 | |||
| Drug use (current)1 | Nicotine | 0 | 6 | χ2= 14.67 | P < 0.001 |
| Cannabis | 0 | 1 | χ2= 0.88 | P = 0.35 | |
| Lifetime recreational history of drug use (>10 times lifetime) | Cannabis | 0 | 13 | ||
| MDMA | 0 | 1 | |||
| Cocaine | 0 | 1 | |||
| LSD | 0 | 1 | |||
| Barbiturate | 0 | 1 | |||
| Anti-psychotic use2 | 0 | 4 | |||
| SOPS | Total | 35.20 +10.99 | |||
| Positive | 11.00 + 3.36 | ||||
| Negative | 11.07 + 5.64 | ||||
| Disorganization | 3.33 + 2.18 | ||||
| General | 8.87 + 4.17 | ||||
| RBANS | Total | 90.60 + 13.99 | |||
| Immediate memory | 94.30 + 13.90 | ||||
| Visuospatial memory | 88.23 + 13.70 | ||||
| Language | 85.87 + 21.59 | ||||
| Attention | 101.63 + 17.21 | ||||
| Delayed memory | 94.70 + 9.09 |
*SOPS, Scale of Prodromal Symptoms; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status
1All participants had a negative urine drug screen for cannabis, ethanol, methadone, and cocaine at baseline except one CHR who had a positive urine drug screen for cannabis.
2CHR were currently on antipsychotic treatment with 0.5 mg and another with 1.0 mg of risperidone, one with 75 mg of quetiapine and the last one with 5 mg aripiprazole.
GSH Levels in mPFC
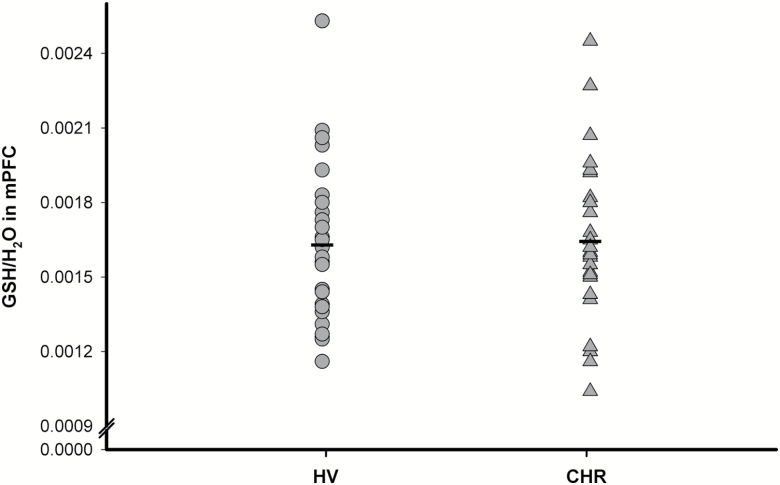
No significant differences were found in GSH levels in mPFC between groups (F(1,54)=0.001, P=0.98, 0.12% lower in CHR compared with healthy volunteers) (Figure 2). Similar results were also obtained after controlling for age, history of recreational drug use, or tobacco use, and after excluding the CHR individuals currently on antipsychotic medication (n=4). There were no significant differences in gray matter, white matter, and CSF fractions within the mPFC voxel between CHR and healthy volunteers (P>0.05; supplementary Table 2). In addition, there were no significant differences between groups after correcting GSH for CSF (supplementary Table 2). In CHR, there were no correlations between GSH levels in the mPFC and severity of psychosis-risk symptoms as measured by the SOPS and cognitive function as measured by RBANS (supplementary Table 3).
Figure 2.
Glutathione (GSH)/H2O in medial prefrontal cortex (mPFC) of clinical high risk (CHR) (n=30) and healthy volunteers (HV) (n=26).
Peripheral GPx Activity
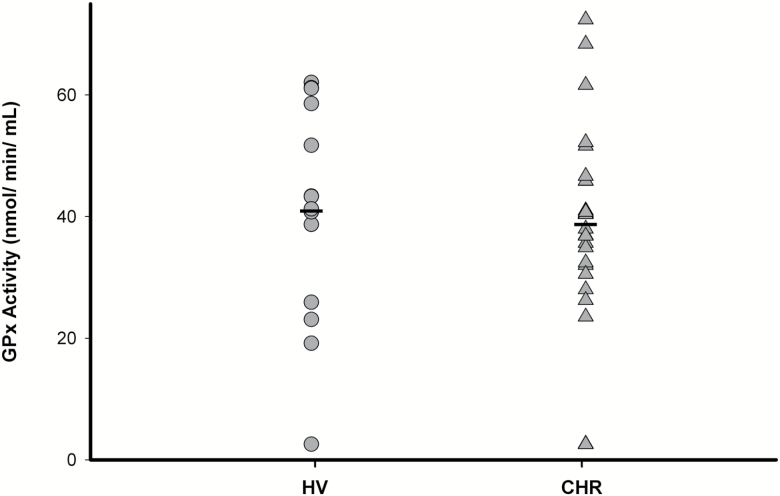
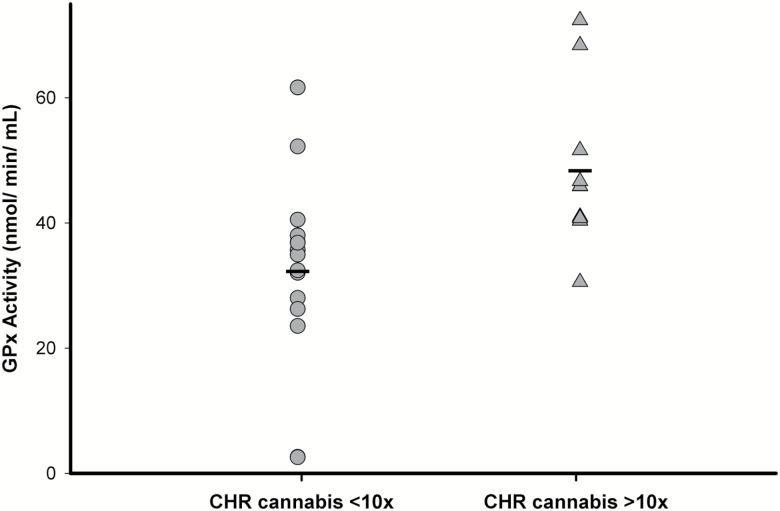
No significant differences in peripheral GPx activity were found between CHR and healthy volunteers (F(1,37)=0.15, P=0.70, 5.39% lower in CHR compared with healthy volunteers) (Figure 3). Similar results were also obtained after controlling for age or tobacco use and after excluding the CHR individuals currently on antipsychotic medication (n=4). However, in CHR, we found a significant effect of lifetime history of cannabis use on GPx activity (F(1,23)=7.41, P=0.01; Figure 4), such that CHR individuals with lifetime cannabis use >10 times (n=10) had significantly higher GPx activity (49.81%) compared with those with <10 times lifetime use (n=15). In addition, total cognitive score (r= 0.47, P=0.02), attention (r= 0.55, P=0.004), and language scores (r= 0.49, P=0.01) as measured by RBANS were significantly correlated with plasma GPx activity in CHR (supplementary Figure 1), such that higher GPx activity is associated with higher cognitive functioning across those specified domains. Only the correlation between RBANS attention score and GPx activity survived Bonferroni correction for number of RBANS subscales. In addition, there was also a trend level correlation between GPx activity and severity of prodromal negative symptoms in CHR (r= -0.35, P=0.09) (supplementary Figure 2; supplementary Table 4).
Figure 3.
Peripheral glutathione peroxidase (GPx) activity in clinical high risk (CHR) (n=25) and healthy volunteers (HV) (n=14).
Figure 4.
Peripheral glutathione peroxidase (GPx) activity in clinical high risk (CHR) with lifetime history cannabis use >10 times (n=10) compared to CHR with <10 times use (n=15).
Correlation between Cerebral GSH Levels and Peripheral GPx Activity
No significant correlations were found between cerebral GSH levels and peripheral GPx activity in CHR or healthy volunteers and in the sample as a whole (P>0.05).
GCLC Polymorphism
We explored the effect of GCLC polymorphism on GSH in the mPFC and GPx activity. The lack of group effect on GSH in the mPFC (F(1,36)=0.00, P=0.99; group*genotype: F(1,36)=0.43, P=0.52; supplementary Figure 3) and peripheral GPx activity (F(1,33)=0.1, P=0.91; group*genotype: F(1,33)= 2.16, P=0.15) was not altered after controlling for GCLC genotype. Furthermore, there were no differences in GSH levels in the mPFC between high-risk and low-risk GCLC genotypes, independent of disease status (F(1,36)=0.04, P=0.85, 1.29% higher in high risk compared with low risk; supplementary Figure 4). Similarly, no significant differences in peripheral GPx activity were observed between GCLC high-risk and low-risk genotypes (F(1,35)=0.03, P=0.87, 2.45% higher in high risk compared with low risk; supplementary Figure 5).
Discussion
This is the first in vivo study investigating cerebral GSH levels in individuals at CHR for psychosis. In this study, we observed no significant differences in GSH levels in the mPFC between CHR and healthy volunteers. Furthermore, we did not observe any correlations between cerebral GSH levels and symptom severity and neuropsychological measures. We also did not observe any significant group effect on GPx activity. However, in CHR, we found a significant effect of lifetime history of cannabis use on GPx activity. We also found a significant positive association between GPx activity and cognition in CHR.
Our results in CHR are in line with 3 in vivo 1H-MRS studies investigating alterations in GSH levels in psychosis, including the most recent study that reported no significant differences between treated FEP and healthy volunteers (Xin et al., 2016). Two other studies, using MEGA-PRESS, also reported no significant differences between treated chronic SCZ patients and controls (Terpstra et al., 2005; Matsuzawa et al., 2008). In addition, consistent with our findings, a genome-wide association study failed to reveal any significant associations between glutathione-related genes and SCZ (Consortium, 2014). Our findings suggest that GSH levels in mPFC are not significantly altered in CHR, consistent with previous studies in treated FEP and SCZ patients.
The findings of this study contrast with an earlier 1H-MRS study in SCZ reporting lower GSH levels in brain and CSF of drug-free SCZ patients compared with healthy controls at 1.5T (Do et al., 2000). The reasons underlying this discrepancy may be explained by differences in the populations (CHR vs established schizophrenia) and methodology used for GSH quantification (double quantum coherence filter vs MEGA-PRESS). Further, in the current study we used a 3T MRI scanner with superior spatial and temporal resolution as compared with 1.5T. Our results are also in contrast to another study reporting increased GSH levels in FEP at 3T (Wood et al., 2009). However, this study focused on the medial temporal lobes, whereas most previous studies have reported GSH levels in the mPFC, which limits their comparability. Furthermore, the mean Cramer-Rao lower bounds, a measure of quantification precision, was moderately high (~21%), which may have biased the results. In addition, phosphorous magnetic resonance spectroscopy studies have also revealed abnormalities in brain energy metabolism and redox regulation in SCZ; however, others have shown no differences (for review, see Yuksel et al., 2015). Furthermore, a recent phosphorous magnetic resonance spectroscopy study reported a significant reduction in the NAD+/NADH ratio, an index of redox state, in chronic SCZ and FEP patients, potentially reflecting oxidative stress (Kim et al., 2017). However, it is important to note that alterations in this redox pair do not suggest abnormalities in cerebral GSH levels.
In addition, there were no significant differences in peripheral GPx activity between groups, and between low-risk and high-risk genotypes. These results are consistent with the most recent 1H-MRS study that also reported no significant differences in blood GPx activity between FEP and controls (Xin et al., 2016). In the present study, CHR with history of cannabis use (>10 times lifetime) had significantly higher GPx activity compared with CHR with <10 times lifetime use, which suggests a potential effect of cannabis on oxidative stress. Cannabinoids, including cannabis, have been repeatedly shown to have antiinflammatory and antioxidant properties (Izzo et al., 2009). In fact, cannabis sativa extract was shown to increase GSH levels in saline-treated rats (Abdel-Salam et al., 2012), although another study reported decreased GSH levels following cannabis exposure (Sarafian et al., 1999). Furthermore, cannabidiol (one of the major cannabinoids in cannabis) was shown to induce GPx activity in human cell culture (Massi et al., 2006). In our study, no correlations were observed between GSH in the mPFC and peripheral GPx activity between groups. This contrasts with the Xin et al. (2016) study reporting a positive correlation between GSH in the mPFC and blood GPx activity in male controls, and the inverse relationship in male FEP patients. Further studies are needed to clarify the relationship between GSH levels in brain and peripheral oxidative markers. We did find, however, a significant positive correlation between peripheral GPx activity and RBANS total score and attention in CHR. This result, while exploratory, suggests that higher GPx activity may be associated with higher cognitive functioning.
The exploratory analysis of the effect of GCLC polymorphism (high-risk vs low-risk) on GSH levels revealed no significant differences, irrespective of disease status. This is in contrast to the recent 1H-MRS study that reported significantly lower GSH levels in mPFC in the high-risk genotype group compared with the low-risk genotype group (Xin et al., 2016). Although Gysin et al. (2007) also reported lower GSH levels in high-risk genotypes, these results were observed in fibroblasts only when challenged with oxidative stress. Therefore, further studies are needed to clarify the in vivo relationship between GCLC genotypes and cerebral GSH levels.
One of the limitations of this study is the significant difference in age between groups, although the difference in mean age between groups was <3 years. Moreover, there were no significant associations between age and cerebral GSH levels (P>0.6), and the results remained unchanged after controlling for age. Furthermore, a recent postmortem study suggests no impact of age on GSH levels in healthy human brain (range 1 day to 99 years, n=74) (Tong et al., 2016). Second, GCLC genotype and GPx results were available for only a subset of our participants (GCLC: healthy volunteers n=13, CHR n=27; GPx: healthy volunteers n=14, CHR n=25). However, as mentioned earlier, genotype had no significant effect on cerebral GSH levels in our sample, and there were no differences in GPx activity between groups. In addition, these analyses were secondary to our primary aim and exploratory. Third, all correlations reported in this study were exploratory and need to be replicated by further studies. Fourth, the 1H-MRS approach used in this study measures only MR visible signal from a specific brain region and cannot distinguish the origin of the GSH signal, white or gray matter. Therefore, the reported GSH levels represent total GSH tissue concentrations and as such do not reflect changes in specific cell types or tissues. Although this is a cross-sectional study, clinical longitudinal follow-up of our CHR sample revealed that 6 of the 30 CHR (i.e., ~20%) converted to psychosis. However, while not powered to test it, this is unlikely to affect the overall conclusion of this study, as there were no differences between CHR converters and nonconverters (F(1,28)=0.85, P=0.37). Lastly, although it would be ideal to measure peripheral GSH levels in addition to GPx activity, this was not possible in our study, as the samples were not deproteinated prior to freezing (Li et al., 2009). Future studies should investigate additional brain regions and different peripheral measures of oxidative stress across the psychosis spectrum.
In conclusion, our results show no evidence of GSH alterations in the mPFC of CHR compared with healthy volunteers, suggesting that GSH is not altered during the putative prodromal stages of psychosis.
Funding
This work was supported by an operating grant from Canadian Institutes of Health Research (CIHR) and CAMH Foundation to Dr. Mizrahi.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
The authors thank the excellent staff of the CAMH Research Imaging Centre and the Focus on Youth Psychosis Prevention clinic. The authors are grateful to Dr. D.C. Shungu and X. Mao for the XSOS software used to process the GSH data and Felix Raschke of National Center for Radiation Research in Oncology, Dresden, Germany for the voxel masking code.
Statement of Interest
Dr. Mizrahi has received (once) speaker and consultant fees from Otsuka Lundbeck Canada. There are no other conflicts of interests related to this work.
References
- Abdel-Salam OM, El-Sayed El-Shamarka M, Salem NA, El-Din MGA(2012)Effects of Cannabis sativa extract on haloperidol-induced catalepsy and oxidative stress in the mice. EXCLI J 11:45–58. [PMC free article] [PubMed] [Google Scholar]
- Altuntas I, Aksoy H, Coskun I, Çayköylü A, Akçay F(2000)Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med 38:1277–1281. [DOI] [PubMed] [Google Scholar]
- Barron H, Hafizi S, Andreazza AC, Mizrahi R(2017)Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci 18:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T(2008)Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014)Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K, Trabesinger A, Kirsten-Krüger M, Lauer C, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M(2000)Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 12:3721–3728. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB(2002)Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. In: SCID-I/P. New York, NY: Biometrics Research, New York State Psychiatric Research. [Google Scholar]
- Flatow J, Buckley P, Miller BJ(2013)Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JW, Wang J- F, Andreazza AC, Shao L, Young LT(2011)Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P(2007)Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci 104:16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA(2015)Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging 42:1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R(2009)Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F, Öngür D(2017)Redox dysregulation in schizophrenia revealed by in vivo NAD+/NADH measurement. Schizophr Bull 43:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus KA, Gabbay V, Mao X, Johnson A, Murrough JW, Mathew SJ, Shungu DC(2014)In vivo 1 H MRS study of potential associations between glutathione, oxidative stress and anhedonia in major depressive disorder. Neurosci Lett 569:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Piao C, Wongpanit K, Manabe N(2009)Delayed deproteinization causes methodological errors in amino acid levels in plasma stored at room temperature. Asian-Aust J Anim Sci 22:1703–1708. [Google Scholar]
- Markwardt CB.(2009)Non-linear least squares fitting in IDL with MPFIT. arXiv preprint arXiv:09022850.
- Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, Seckl J(2000)Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magn Reson Med 44:646–649. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D(2006)The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci 63:2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, Takanashi J, Matsuda T, Shimizu E, Ikehira H(2008)Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1 H-MRS study. PloS One 3:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R(1998)Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266–272. [DOI] [PubMed] [Google Scholar]
- Micó JA, Rojas-Corrales MO, Gibert-Rahola J, Parellada M, Moreno D, Fraguas D, Graell M, Gil J, Irazusta J, Castro-Fornieles J(2011)Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC psychiatry 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW(2003)Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29:703. [DOI] [PubMed] [Google Scholar]
- Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A(2011)Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC psychiatry 11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A(2009)Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 33:1178–1183. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN(1998)The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20:310–319. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, LeRoux P, Hurd R, Wang P, Sachs N, Ketter T editors (2001)Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proc Intl Soc Magn Reson Med 9:1011. [Google Scholar]
- Sarafian TA, Magallanes JAM, Shau H, Tashkin D, Roth MD(1999)Oxidative stress produced by marijuana smoke: an adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol 20:1286–1293. [DOI] [PubMed] [Google Scholar]
- Sawa A, Seidman LJ(2014)Is prophylactic psychiatry around the corner? Combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron 83:991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu DC.(2012)N-acetylcysteine for the treatment of glutathione deficiency and oxidative stress in schizophrenia. Biol Psychiatry 71:937–938. [DOI] [PubMed] [Google Scholar]
- Shungu DC, Mao X, Gonzales R, Soones TN, Dyke JP, Veen JW, Kegeles LS(2016)Brain γ- aminobutyric acid (GABA) detection in vivo with the J-editing 1H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test–retest reliability. NMR Biomed 29:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS(2013)Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull:sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Vaughan T, Ugurbil K, Lim KO, Schulz SC, Gruetter R(2005)Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. Magn Reson Mater Phy 18:276. [DOI] [PubMed] [Google Scholar]
- Tipple TE, Rogers LK(2012)Methods for the determination of plasma or tissue glutathione levels. Methods Mol Biol 889:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice PS, Moszczynska A, Mattina K, Ang L- C, Boileau I, Furukawa Y, Sailasuta N, Kish SJ(2016)Do glutathione levels decline in aging human brain?Free Radic Biol Med 93:110–117. [DOI] [PubMed] [Google Scholar]
- Wang PW, Sailasuta N, Chandler RA, Ketter TA(2006)Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr 18:120–126. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange D, Mitsumoto H, Shungu D(2014)Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett 570:102–107. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt T-M, McConchie M, Berk M, McGorry PD, Pantelis C(2009)Medial temporal lobe glutathione concentration in first episode psychosis: a 1 H-MRS investigation. Neurobiol Dis 33:354–357. [DOI] [PubMed] [Google Scholar]
- Wright SM, Wald LL(1997)Theory and application of array coils in MR spectroscopy. NMR Biomed 10:394–410. [DOI] [PubMed] [Google Scholar]
- Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L, Jenni R, Lu H, Schaller B, Cuenod M(2016)Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull 42:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Keshavan MS(2011)Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxidants & redox signaling 15:2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R(2006)Altered glutathione redox state in schizophrenia. Dis Markers 22:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, Tegin C, O’Connor L, Du F, Ahat E, Cohen BM, Ongur D(2015)Phosphorus magnetic resonance spectroscopy studies in schizophrenia. J Psychiatr Res 68:157–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.