Abstract
4-(4-Pyridinyl methylene) curcumin (C1206) is a new derivative of curcumin that is more active than curcumin in inhibition of heat shock protein 90 (Hsp90) and antitumor action. In this study we investigated the relationship between C1206-induced inhibition of Hsp90 and its anti-leukemic effects. The fluorescence quenching experiments showed that C1206 seemed to bind the middle dimerization domain of Hsp90. The interaction between C1206 and Hsp90 was driven mainly by electrostatic interaction. In in vitro enzyme activity assay, C1206 dose-dependently inhibited Hsp90 ATPase activity with an IC50 value of 4.17 μmol/L. In both imatinib-sensitive K562 chronic myeloid leukemia cells and imatinib-resistant K562/G01 chronic myeloid leukemia cells, C1206 (0.4–3.2 μmol/L) dose-dependently caused the degradation of Hsp90 client proteins and downstream proteins (AKT, MEK, ERK, C-RAF, P-AKT, P-MEK and P-ERK). Furthermore, C1206 (0.4–3.2 μmol/L) dose-dependently induced apoptosis of K562 and K562/G01 cells through triggering mitochondrial pathway. Consistent with this result, C1206 inhibited the proliferation of K562 and K562/G01 cells with IC50 values of 1.10 and 0.60 μmol/L, respectively. These results suggest that C1206 is a novel Hsp90 inhibitor and a promising therapeutic agent for chronic myeloid leukemia.
Keywords: curcumin derivative, Hsp90 inhibitor, anticancer drug, ATPase activity, apoptosis, mitochondria, chronic myeloid leukemia, K562 cells, K562/G01 cells
Introduction
Heat shock protein 90 (Hsp90) is a highly conserved molecular chaperone involved in the maturation and stabilization of over 200 oncogenic client proteins1,2. Most Hsp90 client proteins, such as epidermal growth factor receptor (EGFR), AKT, C-Raf (also called Raf-1), Cdk4, Bcr-Abl, and p53, are essential for tumor growth, proliferation and survival3,4,5. Therefore, Hsp90 is a key protein in oncogenesis and malignancy and has recently become an emerging target for cancer therapeutics6. Hence, Hsp90 inhibitors have significant potential as antitumor compounds. Hsp90 inhibitors can cause chaperone complexes to dissociate and can lead to the degradation of Hsp90 client proteins by stimulating their presentation to proteins involved in the degradation pathway.
Curcumin is an active ingredient of the plant turmeric (Curcuma longa). In our previous work7, we found that curcumin is a lead compound of Hsp90 inhibitors. We thus synthesized a series of derivatives, some of which showed lead-like properties and were shown to be more active than curcumin in Hsp90 inhibition and antitumor action8,9. In this study, we investigate a novel curcumin derivative, 4-(4-pyridinyl methylene) curcumin (C1206), for its interaction with Hsp90 and its anti-leukemia effects. Our results show that C1206 significantly inhibits proliferation and induces apoptosis in K562 and K562/G01, which may be related to the C1206-induced effects on the molecular chaperone functions of Hsp90 and its down-regulation of Hsp90 client proteins. These data suggest that C1206 is a potent Hsp90 inhibitor with anti-leukemic effects.
Materials and methods
Materials and reagents
The bacterial strains and plasmids were obtained from the School of Life Science of Xiamen University, China. C1206 was designed and synthesized by our laboratory (Figure 1A). Ni2+-nitrilotriacetic acid (NTA) agarose was purchased from General Electric (Little Chalfont, Buckinghamshire, UK). Geldanamycin (GA) was purchased from Shanghai Sangon Biological Engineering (Lot No XP0806132012J, Shanghai, China). ATP was purchased from Sigma–Aldrich (St Louis, MO, USA). The stock solution of Hsp90, which was expressed and purified by our laboratory, was prepared in a 10 mmol/L PBS buffer at pH 7.4, and the applied concentration was fixed at 5.0 μmol/L. C1206 was dissolved in 5% DMSO for fluorescence measurements. Anti-Akt, anti-p-Akt, anti-Mek, anti-P-Mek, anti-Erk, anti-P-Erk, anti-C-Raf, anti-P-C-Raf, anti-Hsp90, anti-Hsp70, anti-Hop, anti-P23, anti-β-Actin, anti-Bax, anti-Bcl-2 (an apoptosis suppression protein) and the Apoptosis Antibody Sampler Kit (#9915, caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9, caspase-7, cleaved caspase-7) were purchased from Cell Signaling Technology, Inc (Danvers, MA, USA). The water used in the experiments was thrice-distilled using Milli-Q Biocel systern (Millipore, Biocel, MA, USA). All other reagents were of analytical reagent grade.
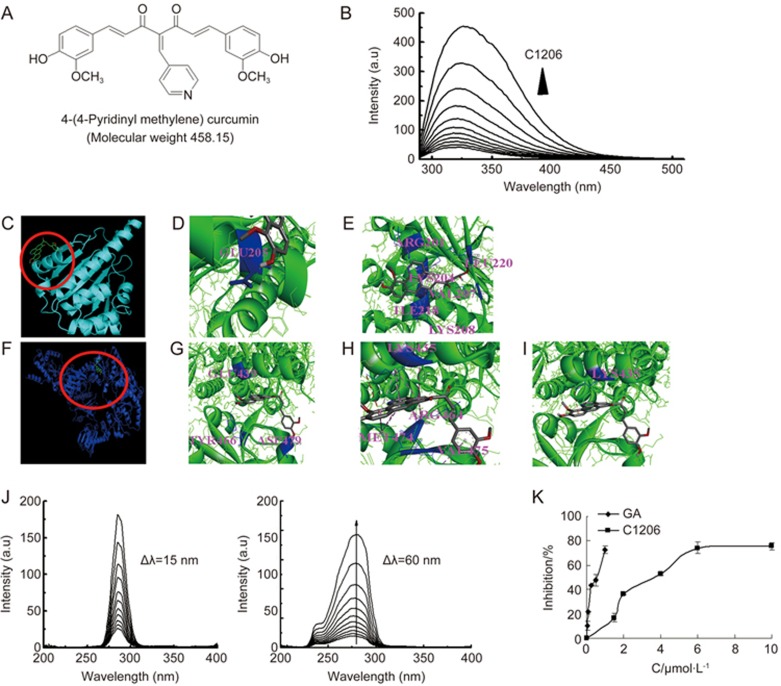
Figure 1.
C1206 physically binds to the Hsp90 and inhibits Hsp90 ATPase activity. (A) Chemical structure of C1206. The molecular weight of C1206 is 458.15. (B) Quenching effect of C1206 (0–50 μmol/L) on Hsp90 endogenous fluorescent in a concentration-dependent manner. The vertical and horizontal axes represent the fluorescent intensity and emission wavelength, respectively. The excitation wavelength is 280 nm, whereas the Hsp90 emission peak is at 337 nm. (C–E) C1206 dock to N-terminal Hsp90. (F–I) C1206 dock to C-terminal Hsp90. (J) Synchronous fluorescence spectra of C1206 (0–50 μmol/L) with Hsp90 (a) Δλ=15 nm, (b) Δλ=60 nm. The vertical and horizontal axes represent the fluorescent intensity and emission wavelength, respectively. (K) The inhibition rate of Hsp90 ATPase activity under the different concentration of GA and C1206 (0–10 μmol/L). The results represent the mean±SEM of triplicate experiments. The error bars represent the SEM.
Cloning, expression, and purification of Hsp90
The cloning, expression and purification of the various constructs of Hsp90, including the histidine (his)-tagged yeast full-length Hsp90 (1-732, 90 kDa), the N-terminal domain of Hsp90 (N-Hsp90, 1-236, 25 kDa), the middle domain of Hsp90 (M-Hsp90, 272-617, 40 kDa), and the C-terminal domain of Hsp90 (C-Hsp90, 629-732, 15 kDa), have been previously described10.
Fluorescent measurements
Samples were excited at 280 nm, and fluorescence intensities were recorded in the range of 290–500 nm at 293 K, 303 K and 310 K using a Cary Eclipse spectrofluorometer (Varian, Palo Alto, CA, USA). The fluorometric titration experiments were performed in 2.0 mL of 5.0 μmol/L Hsp90 solution (10 mmol/L PBS buffer, pH 7.6) with successive additions of C1206 solution (in 0.2% DMSO) from 5 to 50 μmol/L. All tests were performed in triplicate11.
Hsp90 ATPase activity assay
The experiments were incubated in 100 μL of 0.4 μmol/L Hsp90 and 1 mmol/L ATP and different concentrations of C1206, GA or vehicle (DMSO) in assay buffer (6 mmol/L MgCl2, 20 mmol/L KCl and 100 mmol/L Tris-HCl, pH 7.4) at 310 K for 3 h. At the end of the incubation, the ATPase activity of Hsp90 was assessed by malachite green reagent (w/v, 0.0812% malachite green, 2.32% polyvinyl alcohol, 5.72% ammonium molybdate in 6 mol/L HCl and argon water mixed in a ratio of 2:1:1:2). Cultures were analyzed in triplicate at an absorbance of 620 nm10. Kinetic analysis of Hsp90 ATPase activity was carried out using a nonlinear regression fit of the experimental points to the Michaelis–Menten equation. To obtain Km and V values, the Eadie–Hofstee linear transformation (V against V/[s]) was used, with the slope=−Km and the intercept on the x-axis=V/Km10.
Cell culture
Human K562 leukemia cells were cultured in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (medium A) at 37°C in a 5% CO2 atmosphere. K562/G01 cells were maintained in medium A containing 4 μmol/L imatinib.
Cell proliferation assays
MTT assays
Exponentially growing cells were incubated in triplicate in 96-well plates at a final concentration of 5×104 cells/mL in the presence or absence of C1206 for 24 h at 37 °C. Cell proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Company, St Louis, MO, USA) colorimetric dye reduction method. The inhibitory effect of C1206 on cell growth was expressed as an IC50 value.
CFSE staining assays
Exponentially growing cells were resuspended in the CFSE staining solution at 37 °C for 10 min. After washing with cold RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum, cells were grown in 12-well plates at a final concentration of 3×105 cells/mL in the presence or absence of C1206 for 72 h at 37 °C. The cells were resuspended in PBS and then analyzed by flow cytometry.
Apoptosis assessment by annexin-V staining
Following the drug treatments, the cells were resuspended in 100 μL of staining solution containing annexin-V-FITC/PI in HEPES buffer (10 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, and 2 mmol/L CaCl2). These reagents were supplied with the Annexin-V-FITC/PI Double Staining Kit (F Hoffmann-La Roche, Ltd, Basel, Basel-Stadt, Switzerland) and were used according to the manufacturer's instructions. After incubation at RT for 15 min in the dark, the cells were analyzed using a flow cytometer (BD FACSCanto II, BD Biosciences, Franklin, NJ, USA). Annexin-V bound to cells that expressed phosphatidylserine on the outer layer of the cell membrane. Cells that stained positive for Annexin-V were scored as apoptotic cells12.
JC-1 mitochondrial membrane potential (MMP) assay
Following the drug treatments, the cells were resuspended in the staining solution provided with the JC-1 Mitochondrial Membrane Potential Assay Kit (KeyGEN Biotech, Nanjing, China) according to the manufacturer's instructions. After incubation at RT for 10 min in the dark, the cells were analyzed using a flow cytometer.
Cell cycle assessment by PI staining
Following the drug treatments, the cells were resuspended in PBS and fixed with 70% ethanol overnight at −20 °C. After washing with cold PBS, cells were incubated with DNase-free RNase and propidium iodide (PI) at 37 °C for 30 min. Cells were then analyzed by flow cytometry.
Western blot analysis
Total protein extracts were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were transferred to a PVDF membrane (150 mA, 4 °C) for 1.5 h. The membranes were blocked in blocking buffer (1% BSA, Tris-HCl 20 mmol/L, pH 7.5, NaCl 150 mmol/L, and 0.05% Tween-20) for 1 h at RT, followed by incubation with the relevant antibody overnight at 4 °C. The membranes were then incubated with anti-rabbit peroxidase-conjugated secondary IgG antibodies and developed with an enhanced chemiluminescence (ECL) substrate. The membranes were scanned on a Carestream Image Station System to visualize the bands.
Down-regulation of Hsp90 with siRNA
K562 and K562/G01 cells were seeded in antibiotic-free normal growth medium supplemented with fetal bovine serum. Single-strand siRNA oligonucleotides targeting human Hsp90α/β (sc-35608, Santa Cruz Biotechnology, Dallas, TX, USA) and control siRNA (sc-37007) were diluted in siRNA transfection medium (sc-36868) and mixed with siRNA transfection reagent (sc-29528) according to the manufacturer's protocol. K562 and K562/G01 cells were incubated with the transfection complexes for 6 h and in the normal growth medium for 24 h. The cells were allowed to grow for an additional 96 h to test cell proliferation, and the cell number of the siRNA-treated group was compared with that of the control group to calculate the inhibition rate. The knockdown of Hsp90 was confirmed by Western blotting.
Statistical analysis
All data were analyzed with two-sided unpaired t-tests using the GraphPad software package for Windows (Prism version 5.0) and Origin 8.5 software. Values are expressed as the mean values of triplicate or duplicate experiments.
Results
C1206 interacts physically with Hsp90
The binding of C1206 to Hsp90 was characterized by fluorescence quenching experiments. At an excitation wavelength 280 nm, the interaction was examined with a fluorescence spectrum from 290 nm to 510 nm. His-tagged Hsp90 displayed maximal fluorescence at 337 nm. When Hsp90 was incubated with increasing concentrations of C1206, the fluorescence intensity gradually decreased with a slight blueshift of λem (Figure 1B). We then determined which domain of Hsp90 is involved in binding C1206 by constructing three truncation segments of Hsp90: the N-terminal domain (N-Hsp90), the middle domain (M-Hsp90) and the C-terminal domain (C-Hsp90), corresponding to its ATP-binding domain, its co-chaperone binding domain and its dimerization domain, respectively. The fluorescence intensity of M-Hsp90, but not N-Hsp90 or C-Hsp90, was most obviously quenched with increasing concentrations of C1206, indicating that C1206 interacts with the middle domain.
Using the equation previously described10, the titration curves for Hsp90 and M-Hsp90 yielded estimated dissociation constants (KD) of C1206 of 15.226±0.714 μmol/L and 5.838±1.070 μmol/L, respectively, indicating that there was a binding interaction between them and that C1206 quenched the intrinsic fluorescence of Hsp9013.
The quenching process can be analyzed by Stern-Volmer equation, which has been previously described14. Values of the quenching constant (Ksv) and the quenching rate constant (Kq) were obtained from their respective slopes. For all types of quenching agents acting on biological macromolecules, the maximum diffusion-controlled dynamic collision quenching rate has a constant value of 2.0×1010L·mol−1·s−1. The quenching rate constant Kq value of C1206 ((25.383±2.078)×1012L·mol−1·s−1) was greater than 2.0×1010L·mol−1·s−1, which indicated that the quenching effect of C1206 on Hsp90 intrinsic fluorescence was not due to molecules colliding by dynamic quenching but to the formation of the complex by static quenching15.
Static quenching complies with the Lineweaver-Burk equation, which has been previously described16. Values of the apparent binding constant (KA) of C1206 (2.523×107 L/mol) and the binding site (n) of C1206 (1.475±0.041) were obtained from their intercept and slope, respectively.
The thermodynamic enthalpy change (ΔHθ), entropy change (ΔSθ) and free energy change (ΔGθ) of C1206-Hsp90 binding were also calculated from the fluorescent spectrum, yielding −5.50±2.08 kJ/mol, 74.5±6.86 J·mol−1·k−1 and −28.00±0.13 kJ/mol, respectively. The negative enthalpy change and positive entropy change suggest that electrostatic interactions predominate in stabilizing the C1206-Hsp90 complex17.
We investigated the predicted binding of C1206 to N-Hsp90 and C-Hsp90 with structural modeling. In the model of the binding of C1206 with the N-terminus of Hsp90 (Figure 1C), hydrogen bonds were formed between C1206 and residue GLU205 in N-Hsp90 (Figure 1D). Hydrophobic packing interactions were formed between C1206 and residues LEU220, LYS204, VAL207, LYS208, ILE218 and ARG201 of N-Hsp90 (Figure 1E). In comparison, in the model of the binding of C1206 with the C-terminus of Hsp90 (Figure 1F), hydrogen bonds were formed between C1206 and residues GLU439, TYR466 and ASP479 in C-Hsp90 (Figure 1G). Hydrophobic packing interactions were predicted between C1206 and residues LYS435, ARG464, MET474 and VAL475 in C-Hsp90 (Figure 1H). Specifically, van der Waals contacts formed between C1206 and residue LYS435 in C-Hsp90 (Figure 1I).
Tyrosine and tryptophan are the main sources of protein fluorescence. When the D-value (Δλ) between excitation and emission wavelengths was stabilized at 15 or 60 nm, the characteristic patterns in the synchronous fluorescence spectra can provide information on the Tyr or Trp residues18. When Δλ was 15 nm, increasing concentrations of C1206 did not cause the fluorescence peak position of Tyr residues to shift (Figure 1J), which meant that the microenvironment of Tyr residues did not change after binding and that microenvironment hydrophobicity did not obviously decrease. The conformation of the Tyr residue of Hsp90 did not change. As shown in Figure 1J, the addition of C1206 led to a dramatic decrease in fluorescence intensity with a slight blueshift of λem. The blueshift indicated that the polarity around the tryptophan residues decreased and that hydrophobicity increased.
C1206 inhibits the ATPase activity of Hsp90
To characterize the inhibition of Hsp90 by C1206 binding, a colorimetric assay for inorganic phosphate based on the formation of a phosphomolybdate complex and subsequent reaction with malachite green were used to measure the inhibitory effects of C1206 on the ATPase activity of Hsp9010. When the concentration of ATP was 1 mmol/L, the inhibition of Hsp90 ATPase activity of C1206 with the IC50 values was 4.17 μmol/L. C1206 inhibits Hsp90 ATPase activity potently (Figure 1K).
C1206 damages the molecular chaperone functions of Hsp90 and down-regulates Hsp90 client proteins in K562 and K562/G01 cells
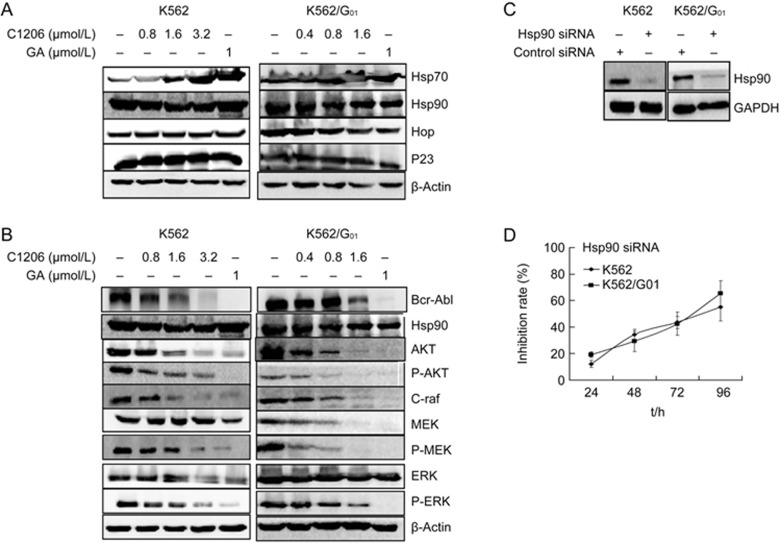
C1206 inhibited the molecular chaperone functions of Hsp90 and induced the degradation of Hsp90 client proteins, as shown by Western blotting. The results presented in Figure 2A and 2B indicate that C1206 altered the composition of molecular chaperone complexes associated with Hsp90 (Figure 2A) and that increasing concentrations of C1206 reduced the levels of Hsp90 client proteins, such as AKT, Raf, MEK, and ERK, in both imatinib-sensitive K562 cells and imatinib-resistant K562/G01 cells. Their phosphorylated forms also decreased in a similar manner with increasing doses of C1206 (Figure 2B). Interestingly, the inhibition efficiency of C1206 is of the same order of magnitude as that of GA, as measured by the degradation of a majority of client proteins.
Figure 2.
C1206 affected the molecular chaperone functions of Hsp90 and down-regulated client proteins level of Hsp90 in imatinib-sensitive or imatinib-resistant CML cells. K562 and K562/G01 cells were treated with indicated concentrations of C1206 for 24 h. The expression levels of (A) molecular chaperone of Hsp90 and (B) client proteins of Hsp90 were monitored by Western blot analysis. RNA interference in CML cells. (C) siRNA reduced the expression of Hsp90 in K562 and K562/G01. (D) Effects of siRNA on cell proliferation. Viable cells were counted by typan blue exclusion assays. The inhibition rate was calculated by comparing the cell number of the siRNA-treated group with that of the control group. The results represent the mean±SEM of triplicate experiments. The error bars represent the SEM.
C1206 induces apoptosis in K562 and K562/G01 cells by triggering the mitochondrial pathway
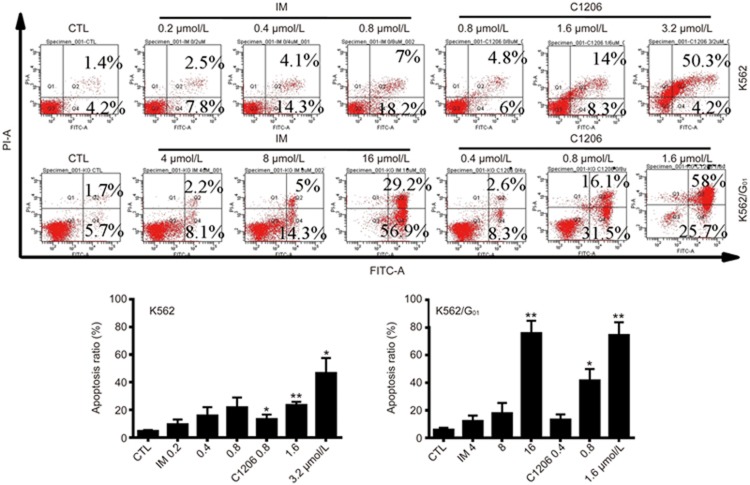
To determine whether the growth inhibition of imatinib-sensitive and imatinib-resistant leukemic cells by C1206 is associated with the induction of apoptosis, we used annexin-V–FITC/PI staining and quantified the number of C1206-induced apoptotic K562 and K562/G01 cells. Consistent with the cellular proliferation assay results, apoptosis was induced in a concentration-dependent manner in both the imatinib-sensitive K562 cells and imatinib-resistant K562/G01 cells (Figure 3).
Figure 3.
Effect of C1206 on the induction of apoptosis in imatinib-sensitive or imatinib-resistant CML cells. K562 and K562/G01 cells were cultured in the presence of C1206 at the indicated concentrations for 24 h, harvested, double stained with annexin V and PI and subsequently analysed by flow cytometry. The results represent the mean±SEM of triplicate experiments. (n=3, *P<0.05, **P<0.01).
The mitochondrial pathway of apoptosis functions in response to various types of stress. A variety of chemotherapeutic agents trigger apoptosis in susceptible cells by inducing MMP disruption, followed by the release of cytochrome c, which interacts with cytosolic docking proteins19,20,21, thereby facilitating the activation of procaspase-9 and the subsequent proteolytic processing of procaspase-3 and procaspase-7. Once activated, these caspases cleave and activate downstream effector caspases, including caspases 3, 6 and 7, which in turn cleave nuclear proteins, such as PARP, and induce apoptosis. MMP is an important parameter of mitochondrial function, which is used as an indicator of cell health. JC-1 selectively enters mitochondria and reversibly changes color from green to red as the MMP increases in healthy cells. By contrast, in apoptotic or unhealthy cells with a low MMP, JC-1 remains in the monomeric form, which only fluoresces green. The ratio of red to green represents the number of healthy cells 22.
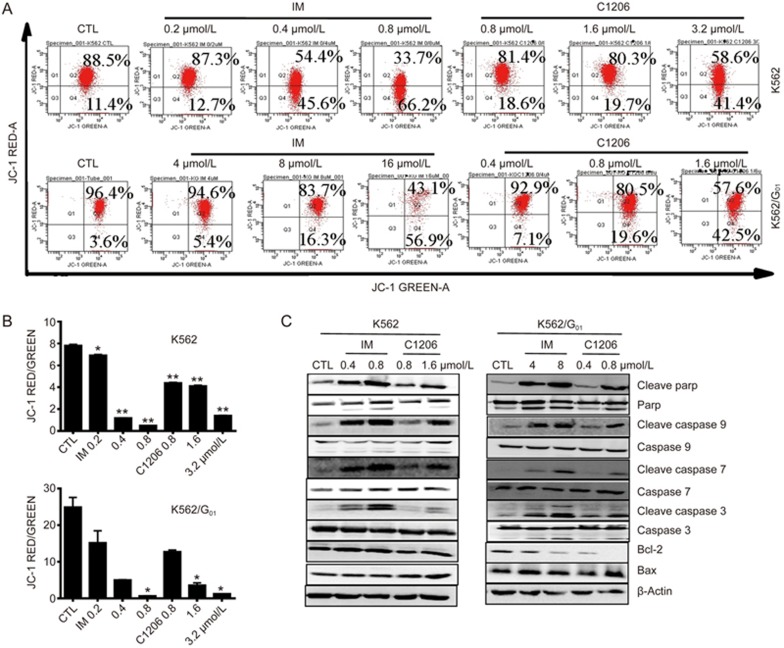
These experiments demonstrated that treatment with C1206 led to a significant reduction in MMP, as demonstrated by an increase in the green color of JC-1 and a decrease in the red color of JC-1 (Figure 4A). Therefore, the red/green ratio significantly decreased after C1206 treatment (Figure 4B). A decrease in the MMP promotes the release of cytochrome c, which in turn cleaves procaspases 9, 7, and 3 to produce the activate caspases 9, 7, and 3 (Figure 4C). These active caspases then cleave PARP and induce apoptosis.
Figure 4.
C1206 triggers the mitochondrial pathway of apoptosis in imatinib-sensitive or imatinib-resistant CML cells. (A) Treatment of K562 or K562/G01 cells with C1206 at the indicated concentrations for 24 h significantly reduced the MMP as demonstrated by JC-1 staining. (B) Quantification of the JC-1 red/green ratio. The results represent the mean±SEM of triplicate experiments. The error bars represent the SEM (n=3, *P<0.05, **P<0.01). (C) Treatment of imatinib-sensitive or imatinib-resistant K562 cells with C1206 for 24 h activated caspases 9, 7, and 3 and the PARP pathway as determined by Western blot. β-Actin served as the protein loading control.
C1206 inhibits proliferation and induces cell cycle arrest in both imatinib-sensitive K562 and imatinib-resistant K562/G01 cells
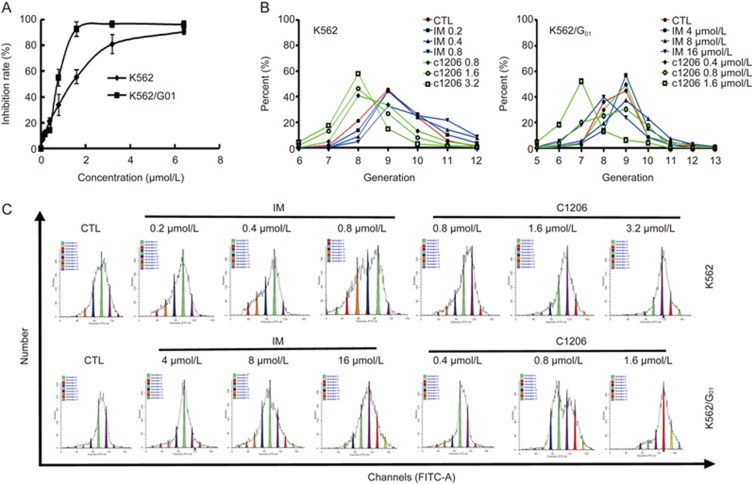
To evaluate its effect on cell proliferation, C1206 was administered to K562 or K562/G01 cells for 24 h, after which the cells were subjected to an MTT assay. A significant inhibition of cell proliferation was observed in a concentration-dependent manner (Figure 5A). The IC50 values of C1206 for the K562 and K562/G01 cell lines were 1.10 μmol/L and 0.60 μmol/L, respectively. The dose-dependent inhibition of K562 or K562/G01 cell proliferation by C1206 was also shown by CFSE staining assay (Figure 5B and 5C). These results indicated that imatinib-resistant K562/G01 cells were also sensitive to C1206.
Figure 5.
C1206 inhibits the proliferation of K562 and K562/G01 cells. (A) K562 and K562/G01 cell lines ware treated with increasing concentrations of C1206 (0–6.4 μmol/L) for 24 h and subjected to MTT assay. The results represent the mean±SEM of triplicate experiments. The error bars represent the SEM. (B, C) K562 and K562/G01 cells were stained with CFSE and then were treated with C1206 at the indicated concentrations for 72 h, harvested and subsequently analysed by flow cytometry.
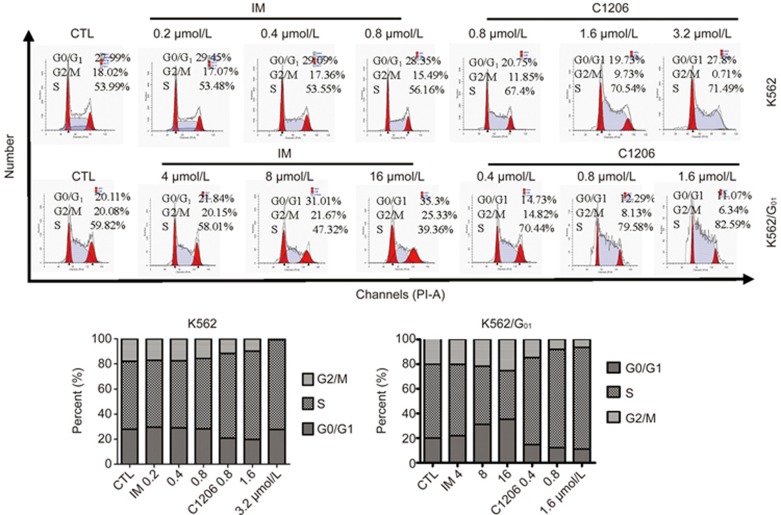
To gain further insight into the effects of C1206 on cell proliferation, we next studied the effects of C1206 on cell cycle distribution. When compared to vehicle controls, C1206-treated cells displayed marked S phase arrest after 24 h of treatment. The increase in the S phase cell population induced by C1206 was accompanied by a concomitant decrease in cells in the G1 and G2/M phases. These results indicated that C1206-treated cells were more efficient at inducing S arrest both in K562 and K562/G01 cells (Figure 6).
Figure 6.
C1206 induces the S phase cell cycle arrest in imatinib-sensitive or imatinib-resistant CML cells. K562 and K562/G01 cells were treated with C1206 at the indicated concentrations for 24 h, harvested, and fixed with 70% ethanol overnight at −20 °C,harvested, stained with PI and subsequently analysed by flow cytometry.
Down-regulation of Hsp90 with siRNA confirms the essential role of Hsp90 targets in CML cell survival
To further validate the essential role of Hsp90 in CML cell survival, we transfected siRNAs targeting Hsp90 into K562 or K562/G01 cells. The expression of transgenes was confirmed by Western blotting (Figure 2C). Down-regulation of Hsp90 expression significantly inhibited the growth of CML cells (Figure 2D). These results along with data from the C1206 experiments confirmed the essential role of Hsp90 in the anti-leukemia effects of C1206.
Discussion
Hsp90 is required for the proper folding and maturation of most oncogenic proteins, which can then aberrantly activate multiple signaling pathways23,24,25. Therefore, the molecular chaperone Hsp90 is an attractive target for cancer therapy. Most Hsp90 inhibitors have been developed to inhibit Hsp90 chaperone function by binding to Hsp9026. Benzoquinone ansamycin antibiotics, such as geldanamycin (GA) and its derivative 17-allylamino-geldanamycin (17AAG), were the first identified Hsp90 inhibitors27. Although GA and its derivatives have exhibited potent anticancer effects, severe hepatotoxicity has prevented their clinical development28. Curcumin showed protective effects against liver injury, could obviously improve the hepatic function and protect the liver29,30. Further investigation is required to determine whether C1206 can protect the liver as its parent compound does.
In our previous work7,31, we found that curcumin inhibited the proliferation of K562 and K562/G01 cells especially and specifically targeted P210bcr/abl, which initiates several signal pathways that are responsible for the resistance of CML cells to several chemotherapeutic agents. Curcumin inhibited the proliferation of K562 and K562/G01 cells and the inhibitory effect was correlated with down-regulation of P210bcr/abl by curcumin involves disrupting the molecular chaperone functions of Hsp90. Although the parent compound curcumin is a multitargeted anticancer agent, interfering with the effects of tumor necrosis factor, HER2, EGFR, and Bcr-abl, the poor bioavailability of curcumin has limited its clinical application32,33. To improve the solubility and activity of curcumin, we designed and synthesized C1206. However, further investigation is required to determine whether C1206 inhibits multiple signaling pathways as its parent compound does.
Compared with the known Hsp90 inhibitors, this study showed that C1206 is a novel scaffold unrelated to that of any Hsp90 inhibitor published to date. In the present study, we have shown a strong interaction between C1206 and full-length Hsp90, N-Hsp90, M-Hsp90 and C-Hsp90. From the binding energy and dissociation constants, we deduce that C1206 is an inhibitor of Hsp90 and is able to bind to the middle domain co-chaperone binding domain of Hsp90. The quenching effect of C1206 on Hsp90 intrinsic fluorescence shows static quenching34. The thermodynamic parameters and the pattern of synchronous fluorescence suggest that electrostatic interactions predominate in stabilizing the C1206-Hsp90 complex. C1206 inhibits the ATPase activity of Hsp90, the binding of C1206 inhibits the catalysis of ATP hydrolysis.
The wide-ranging functions of Hsp90 require a series of co-chaperones to drive the chaperone cycle to completion26. We have shown that C1206 is an Hsp90 inhibitor that directly binds to the middle domain of Hsp90 and inhibits the ATPase activity of Hsp90. This leads to the degradation of multiple Hsp90 client proteins, which may be the primary mechanism mediating the anticancer effects of C1206. Hsp90 and co-chaperone proteins interact with client proteins in an ordered pathway that involves sequential ATP-dependent interactions of the client proteins. The patterns of interacting co-chaperones is likely client protein specific35,36.
Hsp90 affects the activity of client proteins critical for multiple steps of tumor progression, and it is an important target of cancer therapeutics37,38. Hsp90 client proteins have varying sensitivity to Hsp90 inhibitors. Hsp90 inhibitors are able to induce degradation of multiple Hsp90 client proteins. For example, proteins levels of the Hsp90 client protein Akt are known to be decreased by Hsp90 inhibition39. The antagonistic efficacy of C1206 against chronic myeloid leukemia (CML) lines has been investigated at both the molecular and cellular levels. It has been demonstrated that C1206 inhibits CML cell lines K562 and K562/G01 in a dose-dependent manner. Compared with curcumin, which has IC50 values of 11.67 μmol/L and 6.50 μmol/L for K562 and K562/G01 cell lines, respectively, C1206 has the advantage. C1206 only slightly inhibited the proliferation of human peripheral blood mononuclear cells (PBMCs). The inhibition rate of PBMCs treated with 100 μmol/L C1206 for 24 h was only 47.32%. K562 and K562/G01 cells treated with different concentrations of C1206 clearly showed that the levels of Akt, P-Akt, Raf, Mek, P-Mek, Erk and P-Erk (Figure 2B) decreased in response to C1206. C1206 was shown to inhibit the proliferation of K562 and K562/G01 cells through the down-regulation of AKT and Raf/MEK/ERK signal transduction pathway31. Hsp90 is able to inhibit apoptosis, which aids in tumor cell maintenance. Hsp90 inhibitors targeted to the mitochondria can selectively kill tumor cells40. C1206 triggers activation of the mitochondrial pathway of apoptosis in K562 and K562/G01 cells (Figure 4). Moreover, it was shown that C1206 arrests cell cycle progression.
In conclusion, as a novel Hsp90 inhibitor, C1206 binds to the middle domain of Hsp90 and inhibits Hsp90 ATPase activity, resulting in the degradation of Hsp90 client proteins. C1206 displays promising antitumor activity against cancer cells in vitro. Our observations provide a basis for the further development of Hsp90-targeted therapy for patients with CML.
Author contribution
Jian-hua XU designed research; Ying-juan FAN, Ping-zhang GAO, Fang CAI, Li-ping ZHU and Bi LIU performed research; Lian-ru ZHANG, Yi-xiang ZHOU, Qiao-fa LIN contributed new reagents; Ying-juan FAN, Ping-zhang GAO, Fang CAI, Li-ping ZHU and Bi LIU analyzed data; Ying-juan Fan wrote the paper.
Acknowledgments
This work was funded by Fujian Provincial Health and Family Planning Commission of China (2015-1-72), the Natural Science Foundation of Fujian Province of China (2017J01821), the National Science and Technology Foundation of China for Key Projects of “Major New Drugs Innovation and Development” (2012ZX09103-101-028) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (2016Y9059).
References
- Wegele H, Muller L, Buchner J. Hsp70 and Hsp90-a relay team for protein folding. Rev Physiol Biochem Pharmacol 2004; 151: 1–44. [DOI] [PubMed] [Google Scholar]
- Richter K, Hendershot LM, Freeman BC. The cellular world according to Hsp90. Nat Struct Mol Biol 2007; 14: 90–4. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17- demethoxygeldanamycin. Biochem Biophys Res Commun 2006; 351: 658–63. [DOI] [PubMed] [Google Scholar]
- Soga S, Neckers LM, Schulte TW, Shiotsu Y, Akasaka K, Narumi H, et al. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res 1999; 59: 2931–8. [PubMed] [Google Scholar]
- Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90)chaperone complex inhibitor, radicicol potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. Int J Radiat Biol 2005; 81: 63–76. [DOI] [PubMed] [Google Scholar]
- Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci 2007; 32: 517–30. [DOI] [PubMed] [Google Scholar]
- Wu LX, Xu JH, Huang XW, Zhang KZ, Wen CX, Chen YZ. Down-regulation of P210bcr/abl by curcumin involves disrupting the molecular chaperone functions of Hsp90. Acta Pharmacol Sin 2006; 27: 694–9. [DOI] [PubMed] [Google Scholar]
- Chen C, Lui Y, Chen YZ, Xu JH. C086, a novel analog of curcumin, induces growth inhibition and ownregulation of NFκB in colon cancer cells and xenograft tumors. Cancer Biol Ther 2011; 12: 797–807. [DOI] [PubMed] [Google Scholar]
- Wu LX, Yu J, Chen RJ, Liu Y, Lou LG, Wu Y, et al. Dual inhibition of Bcr-Abl and Hsp90 by C086 potently inhibits the proliferation of imatinib-resistant CML Cells. Clin Cancer Res 2015; 21: 833–43. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Guo QJ, He XM, Yang SX, Chen C, Zhang LR. Study on a screening model for inhibitor of Hsp90 ATPase activity. J Xiamen Univ 2010; 49: 711–6. [Google Scholar]
- Gao PZ, Wu H, Guo J, Xu YM. Study on the interaction between breviscapinum and bovine serum albumin by fluorescence spectrometry. Chin JMAP 2012; 29: 106–9. [Google Scholar]
- Meng A, Wang Y, Brown SA, Van ZG, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol 2003; 31: 1348–56. [DOI] [PubMed] [Google Scholar]
- Zhang G, Keita B, Brochon JC, Oliveira PD, Nadjo L, Craescu CT, et al. Molecular interaction and energy transfer between human serum albumin and polyoxometalates. J Phys Chem B 2007; 111: 1809–14. [DOI] [PubMed] [Google Scholar]
- Chakraborty B, Basu S. Interaction of BSA with proflavin: A spectroscopic approach. J Lumin 2009; 129: 34–9. [Google Scholar]
- Pastukhov AV, Levchenko LA, Sadkov AP. Spectroscopic study on binding of rutin to human serum albumin. Mol Struct 2006; 842: 60–6. [Google Scholar]
- Yan CN, Tong JQ, Xiong D, Liu Y, Pan ZT. Studies on the binding reaction features between pefloxacin and bovine serum albumin by fluorescence spectrophtometry. Chin J Anal Chem 2006; 6: 796–800. [Google Scholar]
- Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 1981; 20: 3096–102. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kou ZN, Shi YJ, Zhu JB. Investigation on effect of fluorescence enhancement of Danshensu-BSA system by synchronous fluorescence spectrometry. J Instrument Anal 2011; 30: 444–7. [Google Scholar]
- Tretiakova I, Blaesius D, Maxia L, Wesselborg S, Schulze-Osthoff K, Cinatl J Jr, et al. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis 2008; 13: 119–31. [DOI] [PubMed] [Google Scholar]
- Tian Z, Shen J, Moseman AP, Yang QL, Yang JS, Xiao PG, et al. Dulxanthone A induces cell cycle arrest and apoptosis via up-regulation of p53 through mitochondrial pathway in HepG2 cells. Int J Cancer 2008; 122: 31–8. [DOI] [PubMed] [Google Scholar]
- Del BB, Valentini MA, Comporti M, Maellaro E. Cisplatin-induced apoptosis in melanoma cells: role of caspase-3 and caspase-7 in Apaf-1 proteolytic cleavage and in execution of the degradative phases. Ann N Y Acad Sci 2003; 1010: 200–4. [DOI] [PubMed] [Google Scholar]
- Troiano L, Ferraresi R, Lugli E, Nemes E, Roat E, Nasi M, et al. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat Protoc 2007; 2: 2719–27. [DOI] [PubMed] [Google Scholar]
- Falsone SF, Gesslbauer B, Tirk F, Piccinini AM, Kungl AJ. A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett 2005; 579: 6350–4. [DOI] [PubMed] [Google Scholar]
- Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 2004; 10: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 2005; 120: 715–27. [DOI] [PubMed] [Google Scholar]
- Neckers L. Development of small molecule Hsp90 inhibitors: utilizing both forward and reverse chemical genomics for drug identification. Curr Med Chem 2003; 10: 733–9. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 1994; 91: 8324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol 1995; 36: 305–15. [DOI] [PubMed] [Google Scholar]
- Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int 2009; 29: 1457–66. [DOI] [PubMed] [Google Scholar]
- Wei JS, Yu ZW, Chiang YC, Yang Y, Chai TY, Folt W, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 2012; 7: e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LX, Xu JH, Wu GH, Chen YZ. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of P210bcr-abl-initiated Ras signal transduction pathway. Acta Pharmacol Sin 2003; 24: 1155–60. [PubMed] [Google Scholar]
- Qi J, Peng H, Gu ZL, Liang ZQ, Yang CZ. Establishment of an imatinib resistant cell line K562/G01 and its characterization. Zhonghua Xue Ye Xue Za Zhi 2004; 25: 337–41. [PubMed] [Google Scholar]
- Hossain DM, Bhattacharyya S, Das T, Sa G. Curcumin: the multitargeted therapy for cancer regression. Front Biosci (Schol Ed) 2012; 4: 335–55. [DOI] [PubMed] [Google Scholar]
- Pastukhov AV, Levchenko LA, Sadkov AP. Spectroscopic study on binding of rutin to human serum albumin. Mol Struct 2006; 842: 60–6. [Google Scholar]
- Felts SJ, Karnitz LM, Toft DO. Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR). Cell Stress Chaperones 2007; 12: 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D, Cox M, Cheung-Flynn J, Prapapanich V, Carrigan P, Smith D. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol 2004; 39: 279–95. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J 2008; 410: 439–53. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5: 761–72. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 2000; 97: 10832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell 2007; 131: 257–70. [DOI] [PubMed] [Google Scholar]