Abstract
Exosomes are nano-sized vesicles that serve as mediators for intercellular communication through the delivery of cargo, including protein, lipids, nucleic acids or other cellular components, to neighboring or distant cells. Exosomal cargo may vary in response to different physiological or pathological conditions. The endosomal sorting complex required for transport (ESCRT) family has been widely accepted as a key mechanism in biogenesis and cargo sorting. On the other hand, accumulating evidence show that ESCRT-independent pathways exist. Due to the critical role of exosomes in intercellular communications in delivering cargo to recipient cells, exosomes have been investigated as a vector for the delivery of endogenous or exogenous cargo for therapeutic purposes. But the number of exosomes produced by cells is limited, which hampers their application. Synthetic exosome-mimics have been fabricated and investigated as a therapeutic tool for drug delivery. This review focuses on ESCRT-independent regulation of cargo loading into exosomes, including lipid raft and ceramide-mediated mechanisms, and reported exosomes or exosome-mimics with therapeutic effects.
Keywords: exosome, extracellular vesicle, lipid raft, exosome-mimic, drug delivery
Introduction
Exosomes are membrane-bound extracellular vesicles (EVs) released from cells into the extracellular space. It is widely accepted that exosomes are generated from inward budding of the membrane in endosomes, forming intraluminal vesicles into multivesicular bodies (MVBs) that eventually fuse with the plasma membrane and release exosomes into the extracellular space1. In comparison, microvesicles, which are another subpopulation of EVs, are formed by directly budding off from the plasma membrane. Typically, exosomes are 30–100 nm in diameter, which is similar to the size of intraluminal vesicles, whereas microvesicles are larger than 100 nm2,3. Exosomes were considered to be a mechanism for discarding cell garbage until a number of studies suggested that they were nano-sized vesicles that serve as mediators for intercellular communication through the delivery of proteins, lipids, nucleic acids, or other components in or within their lipid bilayer membrane to neighboring or distant cells. Although accumulating studies have indicated the crucial role of exosomes in various physiological and pathological processes, including tumor metastasis, neurodegeneration, and tissue repair4,5,6,7,8, the regulation of biogenesis and cargo loading is not fully understood.
The endosomal sorting complex required for transport (ESCRT) family has been believed to play a crucial role in biogenesis and cargo sorting, and thus, ESCRT-dependent exosome formation has been reviewed previously1,9,10. It has been shown that ESCRT family members, including Tsg101, Hrs11, CHMP412,13, STAM113, VPS413, and VTA113, or ESCRT-associated protein, ALIX12, are involved in the regulation of exosomal biogenesis and cargo sorting in metazoan systems. Tsg10114, Vps415, and ALIX16,17 might also mediate protein or RNA loading into exosomes. However, depletion of the components of ESCRT reduced the secretion of exosomes rather than creating a complete blockade13,18, thereby indicating the presence of an ESCRT-independent pathway in exosome biogenesis and cargo sorting. Due to the critical role of exosomes in intercellular communications with respect to cargo delivery to recipient cells, exosomes or synthetic exosome-mimics have been investigated as vectors for drug delivery. The present review focuses on ESCRT-independent cargo sorting in exosome biogenesis and exosomes or exosome-mimics loaded with endogenous and/or exogenous cargo, which might serve as a potential therapeutic tool.
ESCRT-independent cargo loading into exosomes
Lipid raft and cargo sorting into exosomes
Association of proteins and molecules with lipid rafts may facilitate their secretion via exosomes
The lateral heterogeneity of the plasma membrane led to the hypothesis of lipid rafts, also known as detergent-resistant membranes (DRMs), which are membrane domains enriched in cholesterol, sphingolipids, and GPI-anchored proteins. The bilayer membrane of exosomes has also been shown to possess DRM domains, which often contain lipid raft-associated proteins19,20,21,22. The involvement of lipid rafts in protein sorting into exosomes was initially proposed in maturing reticulocytes as a putative mechanism for the selective clearance of clustered membrane receptors and lipids23. The presence of raft-associated proteins and molecules [ganglioside GM1, major histocompatibility complex (MHC) class II molecules and flotillin-1] in the DRM of exosomes in two different cell populations was demonstrated by the same research group. This phenomenon suggested that the incorporation of proteins and molecules into raft domains may facilitate their assembly into exosomes19. Therefore, an association with raft domains was proposed as a mechanism underlying the export of a transmembrane glycoprotein via exosomes in a breast carcinoma cell line21. Furthermore, in retinal pigment epithelial cells, αB-crystallin, a small heat-shock protein located in DRM, together with caveolin-1, Hsp70, and flotillin-1, has been shown to be released via exosomes. The secretion of αB-crystallin can be blocked by disruption of the lipid raft, indicating that the process is associated with the lipid raft24.
Shift of proteins and molecules to raft domains may elicit their secretion via exosomes
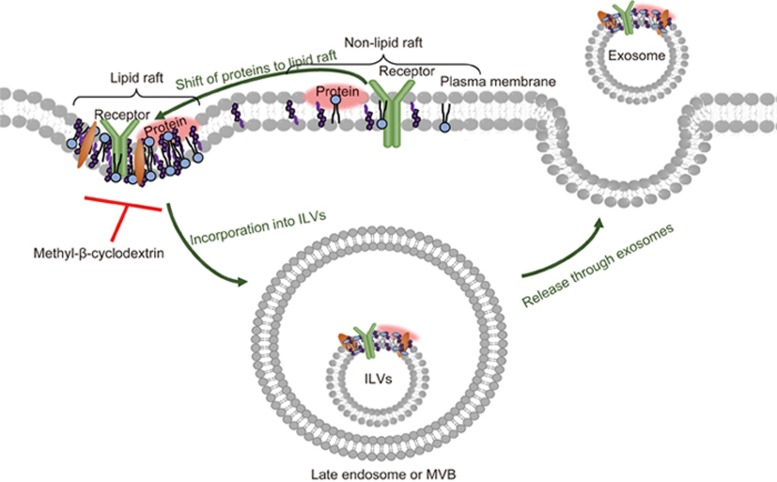
Lipid raft-mediated invagination of the plasma membrane has been well recognized as an endocytosis mechanism; therefore, the shift of proteins or molecules to lipid raft domains may facilitate their assembly into early endosomes25,26. Thus, it could be speculated that the proneness of proteins or molecules towards raft domains may be involved in protein sorting into exosomes. This raft-mediated protein sorting mechanism was observed in the loss of stemness of mammary epithelial stem cells, wherein prostaglandin E receptor 4 antagonist promoted the trafficking of membrane receptors and other signaling proteins to raft domains. Subsequently, these proteins were shuttled together with stem cell surface markers out of the cells through exosomes27. Notably, disruption of the lipid raft by cholesterol or caveolin depletion decreased the level of raft-associated proteins and stem cell surface markers in exosomes, suggesting a regulatory role of lipid rafts in protein sorting in exosomes. Similarly, heat-shock stress might induce the secretion of chemokine-containing exosomes from tumor cells via the incorporation of chemokines into lipid raft domains that can be blocked by cholesterol scavengers28. Treatment with Ca2+ ionophores triggered the recruitment of a Ca2+-dependent phospholipid-binding protein to lipid rafts and therefore increased the secretion of exosomes containing this protein29. The release of TNFα converting enzyme through EVs (80–120 nm, including exosomes and microvesicles) was also mediated by the incorporation of this enzyme into lipid rafts in 293T cells30. Thus, the association or incorporation of proteins or molecules into lipid rafts might facilitate their secretion through exosomes (Figure 1).
Figure 1.
Lipid raft-mediated cargo loading into exosomes. Lipid raft is a membrane domain enriched in cholesterol, sphingolipids, and GPI-anchored proteins. Proteins associated with lipid rafts can be sorted into intralumenal vesicles (ILVs) in multivesicular bodies (MVBs). Shift of membrane proteins in non-lipid raft domain to lipid raft might facilitate their incorporation into ILVs of MVBs. MVBs fuse with the plasma membrane and release exosomes into extracellular space. Disruption of lipid raft by methyl-β-cyclodextrin inhibits the secretion of these proteins through exosomes.
Ceramide and cargo sorting into exosomes
In a mouse oligodendroglial cell line, the knockdown of ESCRT components Tsg101 and Alix did not affect the number of secreted exosomes or the release of proteolipid proteins through exosomes, suggesting an ESCRT-independent pathway in exosome biogenesis and cargo loading31. The inhibition or deletion of neutral sphingomyelinase, an enzyme that catalyzes the formation of ceramide, reduced the release of proteolipid proteins through exosomes. Introducing sphingomyelinase promoted the budding of small vesicles from lipid-ordered domains in the artificial unilamellar vesicles that contain both lipid-ordered and lipid-disordered domains. Sphingomyelinase-induced ceramide production was required for the secretion of miRNA via exosomes32,33. C6 ceramide has been shown to stimulate exosome secretion and increase the exosomal content of tumor-suppressive miRNA in a dose-dependent manner in a multiple myeloma cell line34. Notably, ceramide-induced CD63 secretion through exosomes can be blocked by the inhibitor of sphingosine kinase, an enzyme that catalyzes the formation of S1P35, indicating that the effect of ceramide on cargo loading into exosomes may rely on its metabolite sphingosine 1 phosphate (S1P).
RNA sorting into exosomes
RNA sorting into exosomes is unlikely to be random
The RNAs enriched in exosomes include coding mRNAs and non-coding RNAs such as ribosomal RNA (rRNA), micro RNAs (miRNAs), long noncoding RNAs (lncRNAs), and recently identified circular RNAs (circRNAs)36,37,38,39. Accumulating evidence has shown that miRNA can be shuttled via exosomes to neighboring cells, where they regulate gene expression and biological functions32,40,41,42,43,44. Therefore, the mechanisms underlying miRNA sorting into exosomes has gained increasing attention and are the focus of this review.
Early miRNA array analysis indicated that a sorting mechanism of miRNAs into exosomes might exist since the level of miRNAs with smaller numbers was lower than that of miRNA with larger numbers in prostate cancer cell-derived exosomes45. Further studies have shown that the miRNA profiles of secreted exosomes are distinct from those of cells15,44,46,47,48,49, indicating that miRNAs are sorted into exosomes under specific mechanisms rather than being sorted randomly.
Cellular abundance and miRNA sorting into exosomes
It has been shown that sorting of miRNAs into exosomes can be regulated by the levels of miRNAs and endogenous target sequences47. Deletion of Dicer in bone marrow-derived cells induced an obvious reduction of miRNAs in secreted exosomes compared to their levels in the cells. Overexpression of miRNA-511-3P (miR-511-3p) in marrow-derived macrophages increased the level of this miRNA in exosomes to a greater extent than its level in cells. Conversely, knockout of an miRNA target gene or an increase in an miRNA target sequence has been shown to increase or decrease the miRNA in exosomes. In silico analysis also indicated that exosomal miRNAs that shared the same seed sequence exhibited similar fold changes compared to random miRNA pairs. These results indicated that sorting of miRNAs into exosomes might depend on their sequence and could be affected by their cellular abundance and competition with miRNAs targets.
Exo-motifs and miRNA sorting into exosomes
RNA-binding proteins that play a major role in intracellular RNA trafficking have been suggested to be involved in RNA sorting into exosomes. It has been suggested that annexin A2, a non-canonical RNA-binding protein50,51, may be required for the viral RNA sorting into exosomes that are released from infected cells to trigger the immune response in dendritic cells40. Mutation of RNA-binding protein Y-box protein 1 was found to impair the sorting of two exosome-enriched miRNAs (miR-144 and miR-233) into exosomes in HEK293T cells, leading to intracellular accumulation of these two miRNAs49.
Analysis of the miRNAs sequences presented in exosomes identified common seed sequences, termed EXO-motifs, that facilitated binding to RNA-binding proteins, such as hnRNPA2B1 and SYNCRIP52,53. SUMOylated hnRNPA2B1 has been shown to control the loading of miR-198 into exosomes, which was blocked by mutation of the EXO-motif of miR-19852. In hepatocytes, suppression of SYNCRIP by short hairpin RNAs induced the retention of exosomal miRNAs within cells, and GGCU was identified as an EXO-motif for sorting of miRNAs into exosomes by SYNCRIP53. Interestingly, knockdown of hnRNPA2B1 did not affect the loading of GGCU-containing miRNAs into exosomes in hepatocytes. Analyses of binding activities of SYNCRIP and hnRNPA2B1 revealed different binding capacities to EXO-motifs, suggesting that RNA-binding proteins may cooperate with each other during miRNA sorting into exosomes via specific binding to EXO-motifs53.
However, the mechanisms underlying the sorting of RNA-binding protein-miRNA complexes into exosomes have not yet been clarified. Most likely, RNA-binding protein-miRNA complexes may be loaded into exosomes via mechanisms of protein sorting, such as the ESCRT-dependent pathway and lipid raft-dependent mechanisms. Lipid rafts have been shown to facilitate the trafficking of annexin A2 to intralumenal vesicles in MVBs29. Ubiquitinated Y-box protein 1 can be secreted into the extracellular space through interactions with TSG101, a component of the ESCRT-1 complex14. hnRNPA2B1 has been shown to colocalize with ceramide, which might facilitate its secretion into exosomes52.
Exosomes or synthetic exosome-mimics serve as a potential therapeutic tool
Naturally occurring exosomes
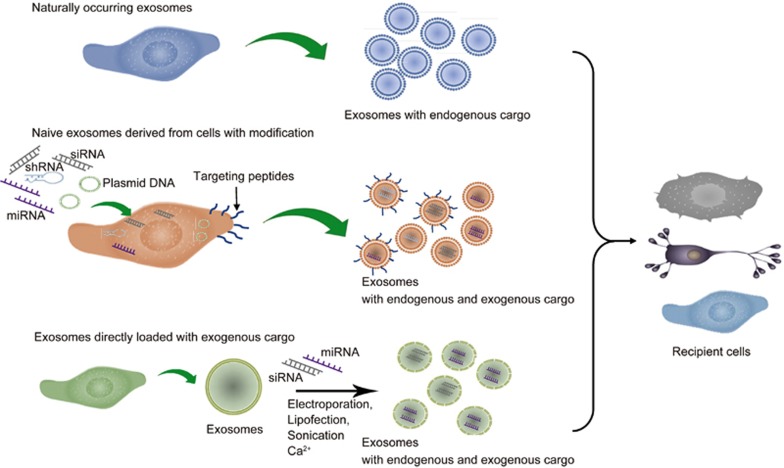
Exosomes with therapeutic effects include naturally occurring exosomes, exosomes secreted by cells with modifications, and exosomes loaded directly with exogenous cargo (Figure 2). Naturally occurring exosomes derived from stem cells have been suggested to exert protective effects on neighboring cell populations54,55 (Table 1). Intramyocardial injection of exosomes derived from embryonic stem cells (ESCs) promoted the proliferation and survival of cardiomyocytes and reduced fibrosis in a mouse model of myocardial infarction54. These exosomes also increased the number of resident cardiac progenitor cells, and cardiac progenitor cells pretreated with exosomes from ESCs showed enhanced capacity for survival and proliferation. These protective effects might result from miR-294, which was enriched in ESC-derived exosomes. Amniotic fluid stem cells secreted exosomes that prevented ovarian granulosa cells from damage during chemotherapy through the delivery of anti-apoptotic miRNAs55.
Figure 2.
Naturally occurring and engineered exosomes for therapeutic purposes. Naturally occurring exosomes from stem cells have shown the protective effects on the survival and proliferation of cells such as cardiomyocytes. Cells loaded with exogenous cargo including siRNAs, miRNAs, and shRNAs secrete exosomes containing these siRNAs, miRNAs, or shRNA-derived miRNAs. Cells expressing target peptides by plasmid transfection produce exosomes that can target specific cell populations. Exogenous cargo can be directly loaded into exosomes by electroporation, lipofection, sonication or Ca2+. Exosomes loaded with endogenous and/or exogenous cargo can be taken up by recipient cells for the regulation of gene expression.
Table 1. Exosomes or exosome-mimics as a therapeutic tool.
| Group | Sub-group | Delivered cargo | Modification for targeted delivery | In vivo administration route |
|---|---|---|---|---|
| Naturally occurring exosomes | miR290-29554 | – | in situ injection54,55 | |
| miR-10a55 | ||||
| Naïve exosomes from cells with modification | Stress-treated cells | miR-2256, | – | in situ injection56 |
| miR-10a57 | ||||
| Heat shock factor 158 | ||||
| Genetic modified cells | miR-14359,66 | Tumor-targeting peptide60 | in situ injection64 | |
| miR-let7a60 | iv injection59,60 | |||
| miR-146b61 | ||||
| miR-21062 | ||||
| anti-miR-963 | ||||
| miR-12264 | ||||
| miR-let7c65 | ||||
| miR-155 and miR-125b291 | ||||
| Exosomes loaded with exogenous cargo | Electroporation | BACE1 siRNA67 | Neuron-targeting peptide67 | iv injection67,74 |
| Doxorubicin (drug)74 | Tumor-targeting peptide74 | |||
| Lipofection | RAD51 siRNA68 | – | – | |
| Sonication | HER2 siRNA69 | – | – | |
| CaCl2 | miR-15a and anti-miR-15a70 | – | inhalation70 (function not tested) | |
| Coincubation | Withaferin A75 (drug) | Tumor-targeting ligand75 | ip injection75 oral delivery75 | |
| Exosome-mimics | Cell extrusion | Doxorubicin (drug)76 | – | iv injection76 |
| c-Myc shRNA77 | ||||
| Cell membrane-cloaked nanoparticles | sTRAIL81 (drug) | – | iv injection81,83 | |
| Emtansine83 (drug) | ||||
| Monophosphoryl lipid A84 (drug) |
Naïve exosomes derived from cells with modifications
Exosomal cargo can vary in response to different physiological and pathological conditions47,56,57,58. Exosomal miRNAs and proteins from cells with modifications (pathological factors, transfection and drug loading) can be delivered to recipient cells as a therapeutic approach (Table 1). Our previous studies have investigated the role of stress-treated stem cell-derived exosomes in cardiac protection. Ischemic preconditioning triggered the secretion of miR-22-enriched exosomes from mesenchymal stem cells (MSCs), and these exosomes protected cardiomyocytes from ischemic injury56. Exosomes derived from H2O2-preincubated cardiac progenitor cells exhibited an increased level of miR-21 and attenuated H2O2-induced apoptosis in cardiomyocytes57. Moreover, heat-shock pretreated cardiac stem cells produced heat shock factor 1 enriched exosomes that could be taken up by cardiomyocytes and reduced apoptosis58.
As reviewed above, sorting of miRNAs into exosomes may increase in response to elevations in their cellular levels47. Therefore, exosomes may serve as vesicles to export overloaded miRNAs from donor cells to recipient cells. THP-1 macrophages transfected with modified miR-143 increased the secretion of miR-143-containing microvesicles59. After uptake by recipient cells, these exogenous miRNAs were demonstrated to be functionally active. Exosomes derived from human embryonic kidney cell line 293 (HEK293) cells transfected with siRNA or miRNA together with plasmids containing GE11 peptide DNA (targets tumor cells by binding to a surface receptor) can target tumors and suppress tumor growth60. Exosomes secreted from marrow stromal cells that were transfected with miR-146b delivered this miRNA to tumor cells in vitro, and intra-tumor injection of these exosomes suppressed the xenograft growth of rat primary brain tumors61. Neural progenitor cells transfected with miR-210 produced exosomes that protected endothelial cells from angiotensin II-induced apoptosis62. Anti-miR-9 oligonucleotides can be delivered by exosomes from MSCs to glioblastoma multiforme cells, where they sensitize the cells to anti-glioblastoma multiforme drug treatment63. Adipose tissue-derived MSCs transfected with miR-122-expressing plasmids produced miR-122-enriched exosomes64. Exosomes with miR-122 could be delivered to hepatocellular carcinoma cells and sensitized cancer cells to chemotherapeutic therapy. Exosomes derived from MSCs overexpressing miR-let7c delivered miR-let7c to kidney cells and regulated the expression of genes related to fibrosis65.
A comparison of the efficiency of exosomes from miR-143-loaded MSCs with direct lipofection of miRNAs into recipient cells revealed that exosomes exhibited similar inhibition of cell migration with lipofection; however, the loading efficiency of exosomes was much lower than that of direct lipofection66. The authors proposed that miRNAs in naïve exosomes were incorporated with RNA-induced silencing complexes that might facilitate the appropriate location of miRNAs to target mRNAs.
Exosomes directly loaded with exogenous nucleic acids or drugs
Exogenous RNAs can be directly loaded into exosomes by electroporation, lipofection, sonication, and calcium chloride (Table 1). Purified exosomes from dendritic cells that were transfected with a neuron-targeting peptide-encoding plasmid were loaded with siRNA by electroporation67. SiRNA-containing exosomes could be delivered to neurons in the brain and knocked down specific genes without inducing an obvious immune response. Naked siRNA could not be delivered to the brain, and unmodified exosomes could not induce gene silencing. Exosomes originating from HeLa cells were transfected with siRNA by lipofection, and these exosomes could silence specific genes in recipient cells68. siRNA loaded into EVs by sonication could be delivered to breast cancer cells, which induced a 50% knockdown of an oncogene, although only a limited number of siRNAs were incorporated into the recipient cells69. It has been shown that calcium chloride can mediate the transfection of miRNAs or their inhibitors into exosomes in the case of heat shock, and these RNAs were functionally active after delivery to recipient cells70.
However, several studies showed that nucleic acids directly loaded into EVs might not be functionally active when taken up by recipient cells. It has been suggested that transfection of siRNA into EVs by electroporation might induce the formation of insoluble siRNA aggregates71. EVs loaded with plasmid DNAs by electroporation delivered DNAs to recipient cells; however, these DNAs were not functionally active72. It has been shown that mRNAs, siRNAs and plasmid DNAs transfected into HEK293FT cell-derived exosomes by lipofection could not induce or downregulate protein expression in recipient cells73. It has been shown that siRNA loaded into EVs by sonication induced less siRNA aggregation than electroporation; however, the amount of siRNA incorporated into recipient cells by exosomes was still limited69.
Tumor-targeting exosomes were generated by the expression iRGD peptide together with a membrane protein in dendritic cells and were loaded with doxorubicin by electroporation; these exosomes showed anti-tumor effects in vivo with targeting capability and no apparent tissue damage74. Bovine milk-derived exosomes loaded with a chemopreventive drug (withaferin A) by direct coincubation showed enhanced anti-tumor effects via intraperitoneal (ip) injection in lung cancer-bearing mouse compared to the naked drug75. The additional loading of folic acid, a tumor-targeting ligand, into exosomes augmented the inhibition of tumor growth in mice when it was administered orally compared to exosomes loaded with the drug alone.
Exosome-mimics
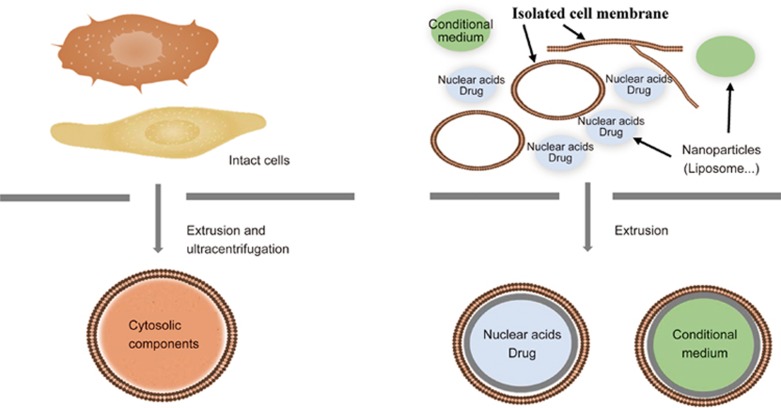
Cells secrete a limited number of exosomes. A large number of nanovesicles can be produced by cell extrusion or polymer-nanoparticles coated with cell membranes (Figure 3). These nanovesicles may serve as exosome-mimics for drug delivery (Table 1).
Figure 3.
Fabrication of exosome-mimics. The passage of intact cells through micro-sized filters or tubes generates cell membrane-enclosed nanovesicles (left). These nanovesicles may be used as exosome-mimics for the delivery of nucleic acids and drugs to recipient cells. Cell membrane-cloaked nanoparticles can be fabricated by extruding polymer-coated nanoparticles together with the cell membrane (right). These nanoparticles may be used for the targeted delivery of nucleic acids, drugs and conditioned medium from cells.
Nanovesicles generated by cell extrusion
Two Korean groups fabricated exosome-mimetic nanovesicles by extruding cells. The forceful and sequential passage of monocytes or macrophages through 10-, 5-, and 1-μm filters led to the generation of a large amount of nanovesicles with a peak diameter of approximately 120–130 nm. Unlike liposomes, these nanovesicles loaded with doxorubicin were targeted to tumors and showed anti-tumor effects similar to exosomes in vivo76. Furthermore, GFP-silencing siRNA loaded into monocyte-derived nanovesicles by electroporation could be taken up by endothelial cells and eventually knocked down GFP77. Nanovesicles generated by extruding fibroblasts transfected with shRNA could also be harnessed as a vector to deliver functionally active miRNAs to recipient cells. The other group used multiple microchannels to break down murine ESCs into membrane-bound nanovesicles with a size of 60–120 nm in diameter78. These nanovesicles could be incorporated into fibroblasts to deliver endogenous RNAs and proteins of stem cells to fibroblasts. The same group developed a device that can generate large-scale nanovesicles using centrifugal force to extrude cells through 10-μm and 5-μm filters79.
Cell membrane-cloaked nanoparticles
Recently, cell membrane-cloaked nanoparticles have emerged as a potential tool for drug delivery with the advantages of immunocompatibility, stability and targeting capability80,81,82,83,84. Erythrocyte membrane-encapsulated polymeric nanoparticles have been shown to have long-circulating properties80. The membrane of erythrocytes was ruptured in hypotonic conditions, and erythrocyte membrane-enclosed nanovesicles were generated by extrusion through a 100-nm porous membrane. Compared to conventional PEG-coated nanoparticles, these membrane-bounded vesicles were fused with PLGA particles, which were 70-nm in diameter, to produce erythrocyte membrane-coated nanoparticles with an increased half-life in the blood and prolonged circulation retention. Cell membrane coating has been used for targeted drug delivery. Platelet membrane-cloaked nanoparticles showed reduced uptake by macrophages and could selectively adhere to damaged vasculature82. MSC membrane-generated nanoparticles delivered the anti-tumor drug sTRAIL to tumor tissue and suppressed tumor growth in vivo, whereas liposome-encapsulating sTRAIL did not show any anti-tumor effect81. Cancer cell membrane-coated nanoparticles showed homotypic targeting to tumors with immunocompatibility83,84 and therefore may serve as a potential vector for anti-tumor drug delivery or immunotherapy.
Recently, a Chinese group fabricated stem cell-mimics using stem cell membrane-cloaked PLGA microparticles comprised of conditional medium from stem cells85,86. Intramyocardial injection of these stem cell-mimics reduced infarction size and increased left ventricle ejection fraction in a mouse model of myocardial infarction. These protective effects were equivalent to those of stem cells. Therefore, cell membrane-coated nano-sized PLGA particles containing conditional medium may serve as exosome-mimics for drug delivery.
Conclusion and perspectives
Cargo loading into exosomes is regulated by multiple mechanisms, such as the ESCRT-dependent pathway, lipid raft-mediated pathway, and ceramide-dependent pathway. Exosomes serve as mediators that modify the condition of recipient cells by delivering protein, lipid, and RNA cargo. Stem cells (with or without the treatment of pathological factors) secrete exosomes that promote cell survival and proliferation54,55,56,57,58. Modifications of exosome cargo can confer additional benefits, such as enhanced effects and targeting capability, to exosomes. As the function of exosomal cargo has become more widely recognized, exosomes have been proposed as a potential alternative to cell-based therapies87. A phase I trial (NCT02565264) has been registered to study autologous plasma-derived exosomes in cutaneous wound healing. Exosomes have also emerged as a nanoplatform for drug delivery88. An ongoing phase I trial (NCT01294072) uses exosomes as a vehicle to deliver an anti-tumor drug, curcumin, for the treatment of colon cancer. Exosomes containing therapeutic cargo could be generated by loading exogenous cargo into cells or by directly loading cargo into exosomes. However, the direct loading of nucleic acids into exosomes may not deliver functionally active cargo into recipient cells efficiently. This phenomenon may result from the low efficiency of transfection by exosomes66,69,88 or the aggregation and degradation of nucleic acids during loading69,71. Thus, RNAs or proteins passively loaded into exosomes by lipofection or electroporation without cellular cargo sorting regulation might be less favorable than RNAs or proteins loaded into naturally occurring or preconditioned cell-derived naïve exosomes.
Cells secrete a limited number of exosomes, which significantly hampers the development of basic research and clinical trials using exosomes. Synthetic exosome-mimics by cell extrusion or cell membrane-cloaked nanoparticles, which can be fabricated on a large-scale, provide novel platforms for drug delivery. MiRNAs have been widely studied as a functional constituent of exosomes. However, anionic miRNAs with a short half-life could not pass through cell membranes easily and were prone to accumulation in the liver and kidney89,90. Exosome-mimics generated by cell extrusion or cell membrane-cloaked nanoparticles could serve as a vector for functional miRNA and drug delivery, although they are different from exosomes since cargo loading is not selected by specific mechanisms, such as the ESCRT-dependent and lipid raft-dependent pathways. Nevertheless, these exosome-mimics showed similarities to exosomes in their zeta-potential, size distribution, and morphology and possessed the immunocompatibility and stability of exosomes due to coating of the plasma membrane76,78,80. Furthermore, the incorporation of specific peptides into cell membranes60,67 could effectuate these membrane-bounded exosome-mimics in the targeted drug delivery.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No 81330007 and No U1601227 to Xi-yong YU), the Science and Technology Programs of Guangdong Province (No 2014A050503047 and No 2015B020225006 to Xi-yong YU) and Guangzhou Science and Technology Program (No 201604010087 to Xi-yong YU)
References
- Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014; 29: 116–25. [DOI] [PubMed] [Google Scholar]
- Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res 2016; 118: 330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 2015; 219: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbichler TB, Dudas J, Riechelmann H, Skvortsova, II. The role of exosomes in cancer metastasis. Semin Cancer Biol 2017; 44: 170–81. [DOI] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 2014; 49: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Li Q, Zhao B, Wang Y. Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Transl Med 2017; 6: 1753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Wang XH, Fan GC. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin 2017; 39: 514–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao FL, Tan L, Liu H, Wang JJ, Ma XT, Zhao B, et al. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharmacol Sin 2018; 39: 552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 2017; 74: 66–77. [DOI] [PubMed] [Google Scholar]
- Baixauli F, Lopez-Otin C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 2014; 5: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun 2010; 399: 384–90. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14: 677–85. [DOI] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126: 5553–65. [DOI] [PubMed] [Google Scholar]
- Palicharla VR, Maddika S. HACE1 mediated K27 ubiquitin linkage leads to YB-1 protein secretion. Cell Signal 2015; 27: 2355–62. [DOI] [PubMed] [Google Scholar]
- Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015; 61: 1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geminard C, De Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004; 5: 181–93. [DOI] [PubMed] [Google Scholar]
- Iavello A, Frech VS, Gai C, Deregibus MC, Quesenberry PJ, Camussi G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int J Mol Med 2016; 37: 958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009; 10: 925–37. [DOI] [PubMed] [Google Scholar]
- de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood 2003; 102: 4336–44. [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003; 278: 10963–72. [DOI] [PubMed] [Google Scholar]
- Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 2009; 9: 2820–35. [DOI] [PubMed] [Google Scholar]
- Dubois L, Ronquist KK, Ek B, Ronquist G, Larsson A. Proteomic profiling of detergent resistant membranes (Lipid Rafts) of prostasomes. Mol Cell Proteomics 2015; 14: 3015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 1997; 110: 1867–77. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem 2011; 286: 3261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 2007; 8: 128–40. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Jana NR. Clathrin to lipid raft-endocytosis via controlled surface chemistry and efficient perinuclear targeting of nanoparticle. J Phys Chem Lett 2015; 6: 3688–97. [DOI] [PubMed] [Google Scholar]
- Lin MC, Chen SY, Tsai HM, He PL, Lin YC, Herschman H, et al. PGE2 /EP4 signaling controls the transfer of the mammary stem cell state by lipid rafts in extracellular vesicles. Stem Cells 2017; 35: 425–44. [DOI] [PubMed] [Google Scholar]
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 2011; 186: 2219–28. [DOI] [PubMed] [Google Scholar]
- Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem 2011; 286: 30911–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wittki S, Brau T, Dreyer FS, Kratzel K, Dindorf J, et al. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol Cell 2013; 49: 668–79. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319: 1244–7. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285: 17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Li X, Wang Y, Dong M, Zhan FH, Liu J. The ceramide pathway is involved in the survival, apoptosis and exosome functions of human multiple myeloma cells in vitro. Acta Pharmacol Sin 2017; 39: 561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler JS. Regulation of transport in cultured epithelia. Biol Cell 1985; 55: 173–5. [DOI] [PubMed] [Google Scholar]
- Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 2012; 7: e46874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 2013; 14: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res 2012; 40: 10937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 2015; 25: 981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 2012; 12: 558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–9. [DOI] [PubMed] [Google Scholar]
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 2009; 11: 1143–9. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, et al. KRAS-dependent sorting of miRNA to exosomes. Elife 2015; 4: e07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta 2012; 1819: 1154–63. [DOI] [PubMed] [Google Scholar]
- Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 2013; 8: e58502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep 2014; 8: 1432–46. [DOI] [PubMed] [Google Scholar]
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 2015; 6: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 2016; 5. [DOI] [PMC free article] [PubMed]
- Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM. Annexin A2 is a novel RNA-binding protein. J Biol Chem 2004; 279: 8723–31. [DOI] [PubMed] [Google Scholar]
- Mickleburgh I, Burtle B, Hollas H, Campbell G, Chrzanowska-Lightowlers Z, Vedeler A, et al. Annexin A2 binds to the localization signal in the 3' untranslated region of c-myc mRNA. FEBS J 2005; 272: 413–21. [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013; 4: 2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, et al. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep 2016; 17: 799–808. [DOI] [PubMed] [Google Scholar]
- Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015; 117: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep 2016; 6: 23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 2014; 9: e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Disease 2016; 7: e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 2014; 32: 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, et al. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol Ther 2011; 19: 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013; 21: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 2013; 335: 201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang J, Chen Y, Chen Y, Ma X, Bihl JC, et al. NPC-EXs alleviate endothelial oxidative stress and dysfunction through the miR-210 downstream Nox2 and VEGFR2 pathways. Oxid Med Cell Longev 2017; 2017: 9397631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids 2013; 2: e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 2015; 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther 2016; 24: 1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 2014; 445: 381–7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–5. [DOI] [PubMed] [Google Scholar]
- Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 2013; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 2016; 9: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 2017; 312: L110–L21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 2013; 172: 229–38. [DOI] [PubMed] [Google Scholar]
- Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm 2015; 12: 3650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 2015; 112: E1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014; 35: 2383–90. [DOI] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 2016; 371: 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013; 7: 7698–710. [DOI] [PubMed] [Google Scholar]
- Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, et al. RNAi delivery by exosome-mimetic nanovesicles–implications for targeting c-Myc in cancer. Biomaterials 2016; 102: 231–8. [DOI] [PubMed] [Google Scholar]
- Jo W, Jeong D, Kim J, Cho S, Jang SC, Han C, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip 2014; 14: 1261–9. [DOI] [PubMed] [Google Scholar]
- Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, et al. Large-scale generation of cell-derived nanovesicles. Nanoscale 2014; 6: 12056–64. [DOI] [PubMed] [Google Scholar]
- Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 2011; 108: 10980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano Furman NE, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, et al. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett 2013; 13: 3248–55. [DOI] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015; 526: 118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016; 10: 7738–48. [DOI] [PubMed] [Google Scholar]
- Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 2014; 14: 2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 2017; 8: 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takov K, Yellon DM, Davidson SM. Regarding Article, “Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice”. Circ Res 2017; 120: e46–e7. [DOI] [PubMed] [Google Scholar]
- Sluijter JPG, Davidson SM, Boulanger CM, Iren Buzas E, de Kleijn DPV, Engel FB, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2017; 114: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 2017; 38: 754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto AG. The road toward microRNA therapeutics. Int J Biochem Cell Biol 2010; 42: 1298–305. [DOI] [PubMed] [Google Scholar]
- O'Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther 2012; 12: 262–74. [DOI] [PubMed] [Google Scholar]
- Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of microRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep 2016; 6: 30110. [DOI] [PMC free article] [PubMed] [Google Scholar]