Abstract
Suxiao Jiuxin pill (SJP) is a traditional Chinese medicine for the treatment of acute coronary syndrome in China, which contains two principal components, tetramethylpyrazine (TMP) and borneol (BOR). Thus far, however, the molecular mechanisms underlying the beneficial effects of SJP on the cardiac microenvironment are unknown. Cardiac mesenchymal stem cells (C-MSCs) communicate with cardiomyocytes (CMs) through the release of microvesicles (exosomes) to restore cardiac homeostasis and elicit repair, in part through epigenetic regulatory mechanisms. In this study, we examined whether SJP treatment altered C-MSC-derived exosomes (SJP-Exos) to cause epigenetic chromatic remodeling in recipient CMs. C-MSC isolated from mouse hearts were pretreated with SJP (SJP-Exos), TMP (TMP-Exos) or BOR (BOR-Exos). Then, HL-1 cells, a mouse cardiomyocyte line, were treated with exosomes from control C-MSCs (Ctrl-Exos), SJP-Exos, TMP-Exos or BOR-Exos. Treatment with SJP-Exos significantly increased the protein levels of histone 3 lysine 27 trimethylation (H3K27me3), a key epigenetic chromatin marker for cardiac transcriptional suppression, in the HL-1 cells. To further explore the mechanisms of SJP-Exo-mediated H3K27me3 upregulation, we assessed the mRNA expression levels of key histone methylases (EZH1, EZH2 and EED) and demethylases (JMJD3 and UTX) in the exosome-treated HL-1 cells. Treatment with SJP-Exo selectively suppressed UTX expression in the recipient HL-1 cells. Furthermore, PCNA, an endogenous marker of cell replication, was significantly higher in SJP-Exo-treated HL-1 cells than in Ctrl-Exo-treated HL-1 cells. These results show that SJP-Exos increase cardiomyocyte proliferation and demonstrate that SJP can modulate C-MSC-derived exosomes to cause epigenetic chromatin remodeling in recipient cardiomyocytes; consequently, SJP-Exos might be used to promote cardiomyocyte proliferation.
Keywords: traditional Chinese medicine, Suxiao Jiuxin pill, tetramethylpyrazine, borneol, cardiac mesenchymal stem cells, exosomes, epigenetic regulation, H3K27 methylases, H3K27 demethylases, UTX
Introduction
Suxiao Jiuxin pill (SJP) is a famous traditional Chinese medicine used for treating acute ischemic heart disease. The two major components of SJP are tetramethylpyrazine (TMP) and borneol (BOR)1,2,3. SJP has significant effects on oxidative stress and vascular reactivity that may lead to improved coronary blood flow and chest pain relief. However, the molecular mechanisms through which SJP regulates the cardiac microenvironment are unclear.
Hearts are terminally differentiated and have limited proliferative capacity. Therefore, dead cardiomyocytes are primarily replaced with scar tissue after myocardial infarction; this process leads to left ventricular remodeling and heart failure in many patients, despite guideline-directed medical therapy. Invasive therapies, such as coronary artery bypass surgery or percutaneous revascularization, improve blood supply to the ischemic myocardium but cannot regenerate cardiomyocytes. Other options for patients with end stage heart failure include heart transplantation and ventricular assist devices, but these options are limited by their cost and the limited supply of donor hearts. A recent study by Wang et al4 demonstrated that mature adult cardiomyocytes (ACMs) can reenter the cell cycle and form new cardiomyocytes through a dedifferentiation, proliferation and re-differentiation (DPR) process in the infarct border zone. This novel concept provides a promising approach for repairing injured hearts.
Stem cell therapy has consistently led to improvements in cardiac function in animal models of myocardial infarction and in humans with ischemic cardiomyopathy. The beneficial effects of stem cells have primarily been linked to the paracrine mediators they produce, which in turn act on neighboring cardiomyocytes and vascular cells to restore the microenvironment and promote functional recovery5. Cardiac mesenchymal stem cells (C-MSCs) are heart-derived mesenchymal stromal cells that express the cardiac specific transcription factor GATA-46 and have the potential to differentiate into vascular smooth muscle cells7 and endothelial cells6,8. C-MSCs play a key role in maintaining cardiac homeostasis and promoting heart repair via paracrine effects, such as inducing angiogenic cytokines9,10. C-MSC-mediated heart repair is in part mediated by exosomes, 40–100 nm microvesicles that play a critical role in cell-cell signaling communications11,12,13. Exosomes are essential for intercellular communication and are a key mechanism of stem cell-mediated heart repair11,12,13. Exosomes contain numerous biologically active components, including proteins, lipids, and microRNA; the latter of which can epigenetically regulate gene expression via cell-cell interactions. However, the role of exosomes in regulating epigenetic chromatin remodeling and how this process might be influenced by commonly prescribed medical therapies are largely unknown; in particular, the potential impact of SJP treatment on C-MSC-mediated cardiomyocyte regulation via exosomes is unclear.
Histone-dependent chromatin remodeling via the trimethylation of histone H3 on Lys27 (H3K27me3) is essential in controlling stem cell and cardiomyocyte fates14, including regeneration15, cardiac reprogramming16, cell survival and proliferation17. EZH1 and EZH2, two core catalytic subunits of mammalian Polycomb repressive complex (PRC2), catalyze the addition of a methyl group to histone H3 at lysine 27, leading to epigenetic repression18. The dynamic H3K27me3 levels in cells are also controlled by UTX and JMJD319, which regulate muscle differentiation by removing H3K27me3 from the promoter of key transcription factors20.
In this study, we compared H3K27me3 levels in cardiomyocytes after C-MSC-derived exosome treatment. Compared with control C-MSC-derived exosomes (Ctrl-Exos), SJP preconditioned exosomes (SJP-Exos) significantly increased H3K27me3 levels in recipient cardiomyocytes and downregulated UTX gene expression, indicating that SJP-Exos regulate H3K27 demethylation in cardiomyocytes. Importantly, SJP-Exo treatment increased PCNA (an endogenous cell proliferation marker) expression in recipient cardiomyocytes.
Materials and methods
C-MSC isolation and cell culture
C-MSC were isolated from the hearts of 2 to 3-month-old male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) via a 2-step procedure as described previously6,8. Briefly, in step 1, ventricular cardiac tissue was minced into 1 mm3 pieces, digested with 0.1% collagenase IV and 1 U/mL dispase in DMEM/F-12, and explanted into fibronectin/gelatin-coated wells (0.5 mg of fibronectin in 100 mL of 0.1% gelatin). We maintained the cardiac explant cultures until the small, round, phase-bright cells migrated from the adherent explants and proliferated over the fibroblast layer. In step 2, Sca-1+ cells were purified from the phase-bright cells by using a mouse hematopoietic lineage-depletion cocktail kit (Stemcell Technologies, Vancouver, Canada), followed by magnetic-activated cell sorting (MACS) using Sca-1 magnetic beads (Miltenyi Biotec Inc, Auburn, CA, USA) as instructed by the manufacturers' protocols. The sorted Sca-1 cells were cultured and maintained in complete media (DMEM/F12, 10% fetal bovine serum (FBS), 200 mmol/L L-glutamine, 55 nmol/L β-mercaptoethanol, and 1% MEM non-essential amino acids).
Drug preparation, C-MSC preconditioning and exosome purification
The origin, medicinal composites, and processing technology of SJP (Tianjin Zhongxin Pharmaceutical Group Co, Ltd, Tianjin, China) were strictly standardized based on marker compounds to achieve quality control according to the Chinese Pharmacopoeia 201521. The dose selections for SJP and BOR in cell culture were based upon therapeutically effective plasma drug concentrations in patients22,23; the dosage of TMP for cell culture was based on a previous experimental report24. SJP, TMP and BOR were diluted in ethanol to 62.5 mg/mL, 25 mg/mL and 15 mg/mL, respectively, as 1000× stock solutions for the cell culture experiments. For C-MSC preconditioning, C-MSCs were treated with 0.1% ethanol (Ctrl), 62.5 μg/mL SJP (SJP), 25 μg/mL TMP or 15 μg/mL BOR in culture medium with 10% exosome-depleted FBS for 48 h. The C-MSC exosomes were purified as described previously, with minor modifications12,25,26. Briefly, supernatants were centrifuged at 1000 rounds per minute for 10 min to separate out the cells; next, the supernatants were filtered through a 0.22 μm filter to remove any cell debris. Exosomes in medium were precipitated with 5× polyethylene glycol 4000 (PEG4000, 8.5% final concentration) with 10× NaCl (0.4 mol/L final concentration) overnight at 4 °C; then, the samples were centrifuged at 2100× g for 30 min, and the pellets were resuspended in PBS and stored at −80 °C until usage.
Electron microscopy and Zeta analysis
For the transmission electron microscopy (TEM) morphology assessments, 3 μL of the exosome pellet was placed on formvar carbon-coated 200 mesh copper electron microscopy grids, incubated for 5 min at room temperature (RT), and then subjected to standard uranyl acetate staining27. The grid was washed with three aliquots of PBS and allowed to become semi-dry at room temperature before observation by transmission electron microscope (JEOL JEM 1230, Peabody, MA, USA). Micrographs were used to quantify the diameters of the exosomes. We measured the exosome particle sizes by nanoparticle tracking analysis (NTA) with ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and the corresponding software ZetaView 8.02.28. The ZetaView system was calibrated using 100 nm polystyrene particles.
Exosomal transfer to HL-1 cells
The murine cardiomyocyte cell line HL-1 (a kind gift from Prof Claycomb) was cultured in gelatin/fibronectin-coated 6 well plates with Claycomb Medium (Sigma-Aldrich) supplemented with 10% exosome-depleted FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.1 mmol/L norepinephrine (Sigma-Aldrich) and 2 mmol/L L-glutamine (Sigma-Aldrich). HL-1 cells in each 6 well plate were treated with 250 μg of Ctrl-Exos, SJP-Exos, TMP-Exos or BOR-Exos for 24 h; RNA and protein were extracted after exosome treatment. The exosome doses used in this study are in accordance with the recommendations of System Biosciences (SBI), which suggest the use of 250 μg of exosomes to treat cells in a 6 well plate format. To determine the effects of H2O2 on apoptosis, HL-1 cells were treated with 1 mmol/L H2O2 in DMEM for 2 h.
Isolation and quantification of mRNA
Total RNA from HL-1 cells was extracted by RNAzol RT (Molecular Research Center, Inc, Cincinnati, OH, USA) following the manufacturer's instructions. cDNA was synthesized from total RNA by using the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific). The synthesized cDNA was used to perform quantitative PCR on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) using PowerUp SYBR® Green Master Mix (ThermoFisher). Amplification was performed at 50 °C for 2 min, 95 °C for 2 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min with the indicated primers (Table 1).
Table 1. Prime list.
| Gene list | Sequence (5′–3′) |
|---|---|
| EZH1 FWD | AGCTTCCTCTTCAACCTCAAC |
| EZH1 REV | CACCATAACCACTTTGGCATAAC |
| EZH2 FWD | GGTTAATGGTGACCACAGGATAG |
| EZH2 REV | CGTTCGATGCCCACATACTT |
| EED FWD | CTGTGGGAAGCAACAGAGTAA |
| EED REV | TAGGTCCATGCACAAGTGTAAA |
| JMJD3 FWD | CACCCAAGAAGAGGAGAAGAAG |
| JMJD3 REV | AGAACAGAGGCCAACGATTT |
| UTX FWD | CAGCAACACCTTCTCCTAAGTC |
| UTX REV | GGGCTCTGAGATTCTTCCATTC |
| GAPDH FWD | TGACATCAAGAAGGTGGTGAAG |
| GAPDH REV | AGTGGGAGTTGCTGTTGAAG |
Western blotting assay
Purified exosomes or exosome-treated HL-1 cells were assessed for protein content using a BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins were resolved on 10% sodium dodecyl sulfate bis-tris gels and transferred onto Odyssey® nitrocellulose membranes (LI-COR Biosicences). The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) and probed with rabbit anti-CD63 (1:250, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA), rabbit anti-CD81 (1:1000, Thermo Scientific), mouse anti-Tsg101 (1:1000, Thermo Scientific), rabbit anti-H3K27me3 (1:1000, Cell Signaling Technology), rabbit anti-UTX (1:1000, Cell Signaling Technology), rabbit anti-cleaved PARP (1:1000, Cell Signaling Technology), rabbit anti-PCNA (1:1000, Cell Signaling Technology), and mouse anti-GAPDH (1:5000, Millipore) at 4 °C overnight; then, the membranes were incubated for 1 h at room temperature with IRDye 680 goat anti-rabbit IgG at 1:10 000 or IRDye 800 goat anti-mouse IgG at 1:10 000 (LICOR Biosciences). The probed blots were scanned using an Odyssey infrared imager.
Statistical analysis
All values are expressed as the mean±standard error of the mean (SEM). Student's t-test was used to compare two groups. One-way ANOVA was used for comparisons between 3 or more means. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of C-MSCs
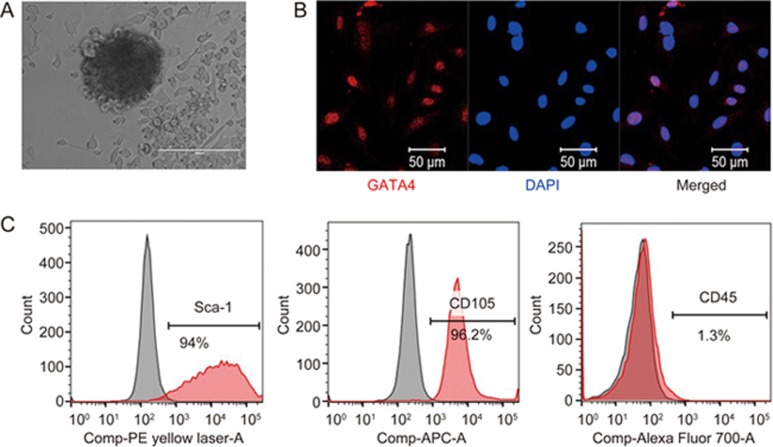
C-MSCs were isolated by using a two-step procedure that we described previously with modifications28,29,30: enzymatically digested, minced adult mouse hearts were cultured in fibronectin/gelatin coated plates. After two weeks, cardiac-derived cells were collected by light enzymatic digestion, and C-MSCs were enriched by depleting hematopoietic cells using a hematopoietic lineage-depletion cocktail; subsequently, the samples were enriched in Sca-1+ cells via MACS sorting (Figure 1A). Immunofluorescent staining demonstrated that the C-MSCs expressed GATA4, an early cardiac transcription factor, confirming their cardiac-specific origin (Figure 1B). Flow cytometry assays showed that C-MSCs expressed high levels of the MSC-specific cell surface markers Sca-1 and CD105 and very low levels of the hematopoietic maker CD45 (Figure 1C). Taken together, these data indicate that C-MSCs represent a subpopulation of cardiac-derived mesenchymal stem cells.
Figure 1.
Phenotypic characterization of C-MSCs. (A) Cultured C-MSCs. (B) Immunofluorescent staining of C-MSCs for the expression of the cardiac transcription factor GATA4 (red); cell nuclei were counterstained with DAPI (blue). (C) Flow cytometric analyses of C-MSCs for the expression of the cell surface markers Sca-1, CD105, and CD45.
Characterization of the C-MSC-derived exosomes
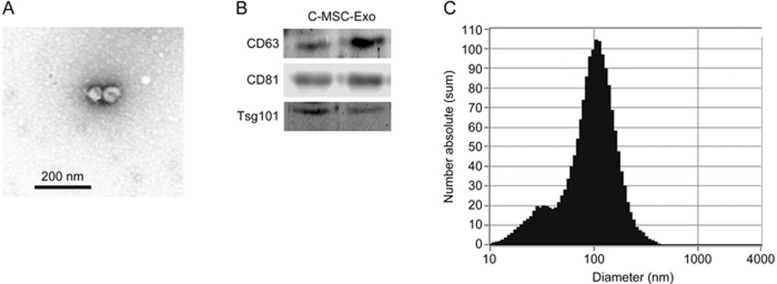
Morphological analysis of the C-MSC-derived pellets using transmission electron microscopy confirmed the presence of exosomes (Figure 2A). Western blot analysis of C-MSC-derived exosomes revealed the presence of the typical exosome markers CD63, CD81 and Tsg101 (Figure 2B). The sizes of the isolated exosomes/microvesicles were measured with dynamic light scattering (DLS) using ZetaView®, a nanoparticle tracking analyzer of hydrodynamic particle size. As shown in Figure 2C, the peak size of the purified particles was approximately 100 nm.
Figure 2.
Characterization of C-MSC-derived exosomes. (A) Transmission electron microscopic morphological analysis of exosomes isolated from C-MSCs. Scale bar=200 nm. (B) Western blot results demonstrate the expression of CD63, CD81 and Tsg101 in exosomes derived from C-MSCs. (C) Particle size distribution in purified pellets measured by ZetaView® Particle Tracking Analyzer.
Effects of C-MSC-derived exosomes on H3K27 trimethylation in cardiomyocytes
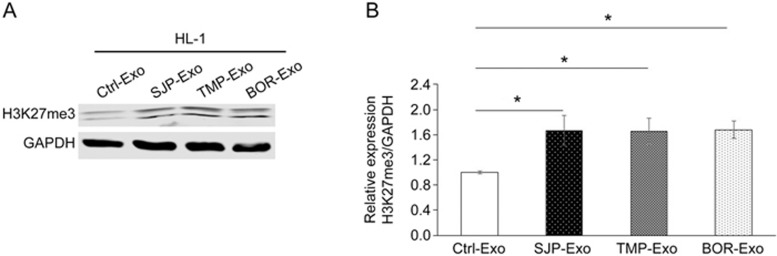
To explore the effect of SJP preconditioning on the capacity of C-MSC-derived exosomes to regulate epigenetic chromatin remodeling in cardiomyocytes, 250 μg of exosomes from the control cells (Ctrl-Exos) or SJP-treated cells (SJP-Exos) was applied to HL-1 cells in 6 well plates. SJP-Exo application, compared with Ctrl-Exo application, resulted in increased H3K27me3 levels in the HL-1 cells. To understand whether TMP and/or BOR, two major components of SJP, have effects similar to SJP preconditioning in HL-1 cells, we compared H3K27me3 levels in HL-1 cells following the application of exosomes collected from SJP-, TMP- and BOR-treated C-MSCs; we found that both TMP-Exos and BOR-Exos produced effects similar to those of SJP-Exos on H3K27me3 levels in HL-1 cells (Figure 3). Interestingly, compared with control treatment, SJP, TMP, and BOR treatment did not directly increase H3K27me3 levels in HL-1 cells (Supplemental Figure S1A, S1B).
Figure 3.
Trimethylation of H3K27 in HL-1 cells following the application of C-MSC-derived exosomes. Western blot analyses show a global increase in H3K27 trimethylation in HL-1 cells after the application of SJP-Exos, TMP-Exos or BOR-Exos in comparison with Ctrl-Exos (*P<0.05, n=3).
Identification of the underlying mechanism of SJP-Exo-mediated H3K27 trimethylation in cardiomyocytes
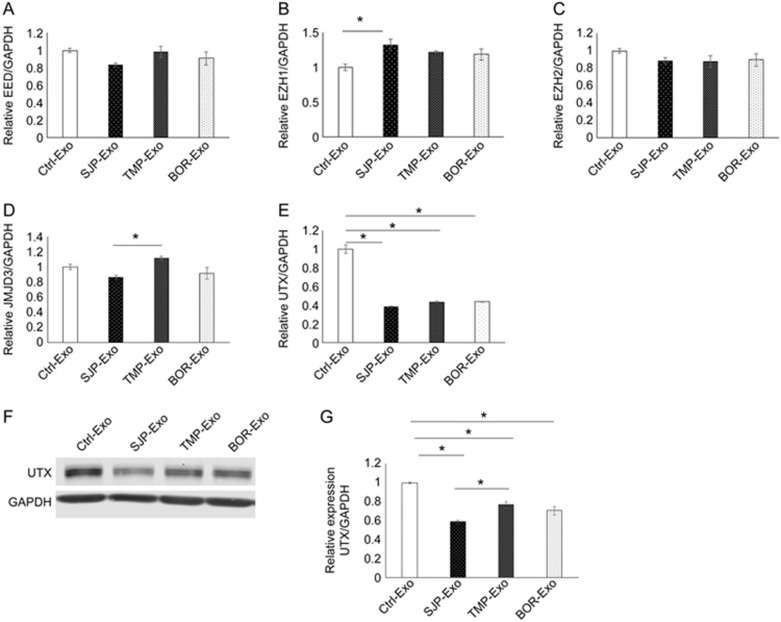
Both histone methylases and histone demethylases can control H3K27me3 levels in cardiomyocytes. To identify how SJP-Exo application increased H3K27me3 levels in HL-1 cells, we measured the expression of H3K27 methylation-related genes (EZH1, EZH2 and EED) and H3K27 demethylation-related genes (JMJD3 and UTX). Using qRT-PCR assays, we found that SJP-Exo application had minimal effects on the expression of all three H3K27 methylation-related genes (Figure 4A–4C) and the H3K27 demethylase JMJD3 (Figure 4D) in HL-1 cells. However, SJP-Exo application significantly reduced the expression of the H3K27 demethylase UTX (by approximately 60%) (Figure 4E). In agreement with the qRT-PCR data, Western blots showed that SJP-Exos significantly reduced UTX protein levels in HL-1 cells (Figure 4F, 4G). This finding suggests that the application of SJP-Exos increases H3K27me3 in HL-1 cells by inhibiting the histone demethylase UTX. Moreover, we compared the effects of SJP-Exos with those of TMP-Exos and BOR-Exos on UTX expression in HL-1 cells. We found that compared with TMP-Exo treatment, SJP-Exos significantly reduced the UTX levels in cardiomyocytes (Figure 4F, 4G). We further measured the dose-response and time-response of UTX expression levels to SJP-Exo treatment in HL-1 cells. As shown in Supplemental Figure S2A, SJP-Exo-mediated UTX reductions were detected by Western blot at 125 μg, and the 250 μg and 500 μg doses significantly reduced the UTX levels in HL-1 cells (P<0.05, n=3). Next, we studied the time-response of UTX expression levels to SJP-Exo treatment in HL-1 cells. We observed that in comparison with 12 h treatment, 24 h SJP-Exo treatment significantly reduced the UTX levels in HL-1 cells, but 48 h treatment did not further decrease these levels (Supplemental Figure S2B).
Figure 4.
Expression of histone methylases and demethylases in HL-1 cells following exosome treatment. (A-E) Gene expression (qRT-PCR assay) of histone methylases (EED, EZH1 and EZH2) and histone demethylases (JMJD3 and UTX) in HL-1 cells following the application of Ctrl-Exos, SJP-Exos, TMP-Exos and BOR-Exos (*P<0.05, n=3); (F, G) Western blot results show the UTX protein levels in HL-1 cells following the application of Ctrl-Exos, SJP-Exos, TMP-Exos and BOR-Exos. Densitometric arbitrary units were normalized to GAPDH abundance and are expressed as the mean±SEM (*P<0.05, n=3).
Identification of the functional consequences of SJP-Exo treatment in cardiomyocytes
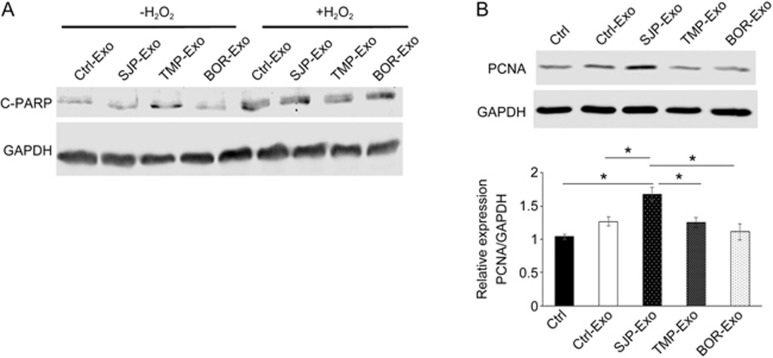
To determine the function of SJP-Exos in cardiomyocytes, we first tested whether SJP-Exo treatment affects H2O2-induced apoptosis in cardiomyocytes. To do this, we compared apoptosis in cardiomyocytes with/without oxidative stress. As shown in Figure 5A, compared with Ctrl-Exo treatment, SJP-Exo treatment did not reduce the levels of cleaved PARP (a marker for apoptosis) either at baseline or following treatment with H2O2. We next studied whether SJP-Exo treatment affected cardiomyocyte proliferation; to do this, we measured the levels of PCNA (an endogenous marker for cell proliferation) expression in HL-1 cells. We observed that compared with Ctrl or Ctrl-Exo groups, SJP-Exo treatment significantly increased the PCNA protein levels in HL-1 cells (Figure 5B), indicating that SJP-Exo treatment promoted cardiac proliferation.
Figure 5.
Effect of SJP-Exos on H2O2-induced apoptosis and proliferation in HL-1 cells. (A) Western blot of cleaved PARP (C-PARP) expression in HL-1 cells following overnight treatment with Ctrl-Exos, SJP-Exos, TMP-Exos and BOR-Exos and with/without 1 mmol/L H2O2 treatment for 2 h; (B) Western blot of the expression of the proliferation marker PCNA in HL-1 cells following the application of Ctrl, Ctrl-Exos, SJP-Exos, TMP-Exos and BOR-Exos (*P<0.05, n=3).
Discussion
Suxiao Jiuxin pill (Supplemental Figure S3) has multiple beneficial effects on vascular function and is widely used to treat heart disease. Here, we demonstrate a novel function of SJP through which it can mediate the ability of cardiac mesenchymal stem cell-derived exosomes to regulate the levels of H3K27me3, a key epigenetic chromatin marker for cardiac transcriptional suppression, in HL-1 cardiomyocytes and to promote cardiomyocyte proliferation. The major mechanism appears to be related to the downregulation of the expression of the demethylase UTX. These functions of SJP are shared by its two major herbal components, TMP and BOR. Our findings have implications regarding the mechanisms of the beneficial effects of SJP in treating heart disease patients.
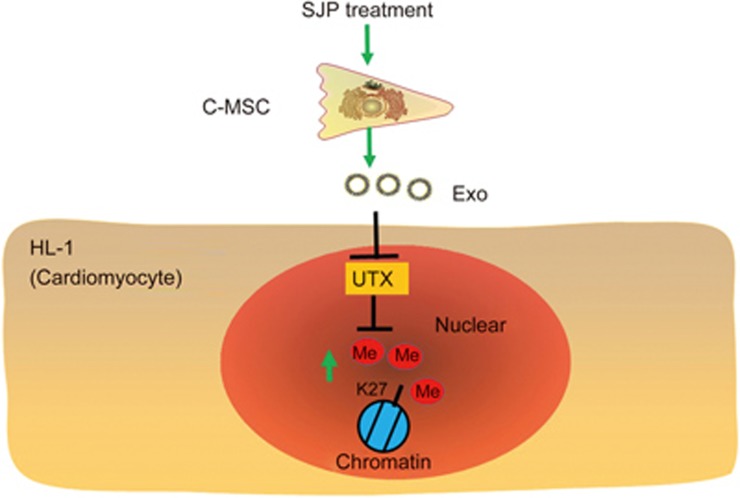
The trimethylation of histone 3 lysine 27 modulates cardiomyocyte proliferation. H3K27me3 levels are controlled by balancing H3K27 methyltransferases (EZH1, EZH2, EED) and demethylases (UTX and JMJD3). The enhancer of zeste homolog 2 (EZH2) is an essential catalytic subunit of Polycomb repressive complex 2 (PRC2), which is responsible for H3K27me331 and the transcriptional repression of targeted genes by inhibiting the binding of acetyltransferases, such as p300 and CBP, to Polycomb group (PcG) target genes32. Recent studies demonstrate that EZH2-mediated H3K27me3 plays a critical role in heart development, and Nkx2.5-specific EZH2 knockout leads to lethal congenital heart defects via the inhibition of cardiomyocyte proliferation33. EZH2 overexpression increases H3K27me3 levels and promotes the proliferation and migration of stem and progenitor cells34. UTX is one of two H3K27me3 demethylases that remove the suppressive H3K27me3 marks. In this study, we found that SJP functionally alters the exosomes produced by C-MSCs; when these exosomes are applied to HL-1 cardiomyocytes, they cause significant increases in the protein levels of H3K27me3 (Figure 6). Mechanistic studies demonstrated that the SJP–Exos had little effect on the transcripts of key histone methyltransferases (EED, EZH1 and EZH2) or the histone demethylase JMJD3. In contrast, expression of the histone demethylase UTX was dramatically reduced by the SJP-Exos, which may explain why the application of these exosomes increased H3K27me3 levels in HL-1 cardiomyocytes. The specific mechanism for these exosomes in regulating the expression of UTX is unclear. Since exosomes contain numerous non-coding RNAs, including miRNAs, lncRNAs and circRNAs, that might modulate UTX expression via the gene network, the final effect may result from both the direct and indirect effects of cross talk among multiple signaling and noncoding RNAs35; therefore, integrated analyses of these noncoding RNAs are needed to understand their regulatory machinery.
Figure 6.
Schematic overview of SJP preconditioned C-MSC-derived exosomes in regulating H3K27me3 levels in recipient cardiomyocytes. Mechanistically, SJP-Exo application leads to decreased expression of the histone demethylase UTX in the recipient cardiomyocytes.
In this study, we observed that both TMP-Exos and BOR-Exos produced effects similar to those of SJP-Exo on H3K27me3 levels in HL-1 cells; these results suggest that the major components of SJP are equally effective at regulating the function of C-MSC-derived exosomes to elicit epigenetic chromatin modifications in recipient cardiomyocytes. We also observed that compared with TMP-Exo treatment, SJP-Exos significantly reduced the UTX levels in cardiomyocytes, suggesting that the multi-component herbal compound might be more effective at regulating exosome-mediated epigenetic chromatin remodeling than each individual herb component. The concept of “synergy” proposed by Traditional Chinese Medicine refers to the synergistic effects of multiple-component herbal combinations that lead to enhanced pharmacological responses. In traditional Chinese medicine practice, multi-herb medicines are prescribed to patients with cardiovascular diseases based on their synergistic, multi-targeted interactive effects36.
As shown by our experiments, SJP-Exo treatment does not protect cardiomyocytes from apoptosis caused by H2O2-mediated oxidative stress; however, SJP-Exo treatment can increase the levels of PCNA, a proliferation marker expressed in cardiomyocytes, suggesting that SJP-Exos might be used to stimulate cardiac proliferation. In future studies, we might use SJP-Exos to treat heart failure by delivering the exosomes directly to the cardiomyocytes. This strategy for promoting cardiomyocyte proliferation might improve cardiac repair.
In this study, we used a polyethylene glycol (PEG)-based method that we described previously to purify exosomes12. Compared with the gold-standard differential centrifugation and sucrose gradient purification methods, PEG-based exosome purification has been demonstrated to have the advantages of preserving RNA cargo, not inhibiting biological activity, and enriching highly pure exosomes37. Our enriched exosomes were confirmed to express the CD63, CD81 and Tsg101 markers by Western blot analyses (Figure 2B); however, PEG-based methods cannot differentiate the exosomes from microvesicles.
In summary, we demonstrated that SJP can regulate C-MSC-derived exosome functions to downregulate the expression of the demethylase UTX, thus increasing H3K27me3 levels in recipient cardiomyocytes. Most importantly, SJP-Exos increased PCNA expression in cardiomyocytes. This finding broadens our current understanding of the molecular mechanisms of SJP in treating ischemic heart diseases and highlights for the first time the role of stem cell-derived exosomes in epigenetically remodeling chromatin in recipient cells.
Author contribution
Conception and design: Xiao-long WANG, Yaoliang TANG; Data collection: Xiao-fen RUAN, Yong-jun LI, Cheng-wei JU, Yan SHEN; Data analysis: Xiao-fen RUAN, Xiao-long WANG; Data interpretation: Xiao-fen RUAN, Wei LEI, Can CHEN, Xiao-long WANG, Yaoliang TANG; Manuscript writing: Il-man KIM, Hong YU, Neal L WEINTRAUB, Yaoliang TANG.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81403352, 81573647 to Xiao-long WANG and Xiao-fen RUAN, and 81528002 to Yaoliang TANG), the Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (No 14DZ2273200); this study was also partially supported by the Tianjin Zhongxin Pharmaceutical Group Co, Ltd, Tianjin, China.
Il-man KIM, Neal L WEINTRAUB, and Yaoliang TANG were partially supported by the American Heart Association GRNT31430008, NIH-AR070029, NIH-HL086555, NIH-HL134354, and NIH-HL12425.
Footnotes
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary Information
Trimethylation of H3K27 in HL-1 cells following application of Ctrl, SJP, TMP and BOR.
Dose and time effect of SJP-Exo on UTX expression in HL-1.
Fingerprint chromatograms of SJP.
References
- Lu Z, Zhang Y, Zhuang P, Zhang J, Zhou H, Zhang M, et al. Protective effect of Suxiao jiuxin pill, a traditional Chinese medicine, against acute myocardial ischemia in dogs. BMC Complement Altern Med 2015; 15: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Li D, Zheng H, Lv J, Leng J, Zhang L, et al. Acupoint application in patients with chronic stable angina pectoris: study protocol of a randomized, double-blind, controlled trial. Evid Based Complement Alternat Med 2014; 2014: 619706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhuang P, Lu Z, Zhang M, Zhang T, Zhang Y, et al. Suxiaojiuxin pill enhances atherosclerotic plaque stability by modulating the MMPs/TIMPs balance in ApoE-deficient mice. J Cardiovasc Pharmacol 2014; 64: 120–6. [DOI] [PubMed] [Google Scholar]
- Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, et al. Dedifferentiation, proliferation and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 2017; 136: 834–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Wang YJ, Chen LJ, Pan YH, Zhang L, Weintraub NL. Cardiac-derived stem cell-based therapy for heart failure: progress and clinical applications. Exp Biol Med (Maywood) 2013; 238: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Shen L, Qian K, Phillips MI. A novel two-step procedure to expand cardiac Sca-1+ cells clonally. Biochem Biophys Res Commun 2007; 359: 877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ashraf M, Wang Y, Zhou M, Zhang J, Qin G, et al. The role of notch 1 activation in cardiosphere derived cell differentiation. Stem Cells Dev 2012; 21: 2122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res 2009; 104: 1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 2005; 80: 229–36. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept 2004; 117: 3–10. [DOI] [PubMed] [Google Scholar]
- Campbell CR, Berman AE, Weintraub NL, Tang YL. Electrical stimulation to optimize cardioprotective exosomes from cardiac stem cells. Med Hypotheses 2016; 88: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 2015; 192: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 2013; 431: 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan AH, Wang S, Ko KD, Zare H, Tsai PF, Feng X, et al. Roles of H3K27me2 and H3K27me3 examined during fate specification of embryonic stem cells. Cell Rep 2016; 17: 1369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai S, Yu X, Li Y, Peng Y, Li C, Yue Y, et al. Divergent requirements for EZH1 in heart development versus regeneration. Circ Res 2017; 121: 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkhabi M, Zonooz ER, Baharvand H. Boosters and barriers for direct cardiac reprogramming. Life Sci 2017; 178: 70–86. [DOI] [PubMed] [Google Scholar]
- Chen L, Ma Y, Kim EY, Yu W, Schwartz RJ, Qian L, et al. Conditional ablation of Ezh2 in murine hearts reveals its essential roles in endocardial cushion formation, cardiomyocyte proliferation and survival. PLoS One 2012; 7: e31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodega B, Marasca F, Ranzani V, Cherubini A, Della Valle F, Neguembor MV, et al. A cytosolic Ezh1 isoform modulates a PRC2-Ezh1 epigenetic adaptive response in postmitotic cells. Nat Struct Mol Biol 2017; 24: 444–52. [DOI] [PubMed] [Google Scholar]
- Dal-Pra S, Hodgkinson CP, Mirotsou M, Kirste I, Dzau VJ. Demethylation of H3K27 is essential for the induction of direct cardiac reprogramming by miR combo. Circ Res 2017; 120: 1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rando TA. UTX in muscle regeneration--the right dose and the right time. J Clin Invest 2016; 126: 1233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pharmacopoeia Committee. Chinese Pharmacopoeia 2015, 2015.
- Wang XL, Liu YM, Zhu GJ. Effects of Suxiao jiuxin pill on patients with acute coronary syndrome undergoing early percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012; 32: 1483–7. [PubMed] [Google Scholar]
- Guo J, Meng H, Wang L, Zhang L, Hang X, Xu R. Effect of blood drug level change of Borneol in Suxiaojiuxin pill on patients with myocardial ischemia. Modern J Integr Tradit Chin Western Med 2004; 13: 2387–402. [Google Scholar]
- Yu L, Li M, She T, Han L. Effects of Tetramethylpyrazine on Ang II-induced cardiomyocyte hypertrophy and the underlying mechanisms. Chin J Exp Tradit Med Form 2013; 19: 154–7. [Google Scholar]
- Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012; 126: 2601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li Y, Yu H, Shen Y, Ju C, Ma G, et al. Isolation of extracellular vesicles from stem cells. Methods Mol Biol 2017; 1660: 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis 2012; 3: e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Phillips MI, Miao HL, Zeng R, Qin G, Kim IM, et al. Infrared fluorescent protein 1.4 genetic labeling tracks engrafted cardiac progenitor cells in mouse ischemic hearts. PLoS One 2014; 9: e107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou M, Wang X, Qin G, Weintraub NL, Tang Y. Assessing in vitro stem-cell function and tracking engraftment of stem cells in ischaemic hearts by using novel iRFP gene labelling. J Cell Mol Med 2014; 18: 1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Pan Y, Zhang L, Wang Y, Weintraub N, Tang Y. Two-step protocol for isolation and culture of cardiospheres. Methods Mol Biol 2013; 1036: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev 2002; 16: 2893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Malatesta M, Jung HR, Walfridsson J, Willer A, Olsson L, et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res 2010; 38: 4958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 2012; 110: 406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Crowe DL. The histone methyltransferase EZH2 promotes mammary stem and luminal progenitor cell expansion, metastasis and inhibits estrogen receptor-positive cellular differentiation in a model of basal breast cancer. Oncol Rep 2015; 34: 455–60. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang Y, Chen L, Pan Y, Weintraub N. Cross talk between the Notch signaling and noncoding RNA on the fate of stem cells. Prog Mol Biol Transl Sci 2012; 111: 175–93. [DOI] [PubMed] [Google Scholar]
- Che CT, Wang ZJ, Chow MS, Lam CW. Herb-herb combination for therapeutic enhancement and advancement: theory, practice and future perspectives. Molecules 2013; 18: 5125–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider MA, Hurwitz SN, Meckes DG Jr. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep 2016; 6: 23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trimethylation of H3K27 in HL-1 cells following application of Ctrl, SJP, TMP and BOR.
Dose and time effect of SJP-Exo on UTX expression in HL-1.
Fingerprint chromatograms of SJP.