Abstract
The vacuolar calmodulin (CaM)-stimulated Ca2+-ATPase, BCA1p, in cauliflower (Brassica oleracea) has an extended N terminus, which was suggested to contain a CaM-binding domain (S. Malmström, P. Askerlund, M.G. Palmgren [1997] FEBS Lett 400: 324–328). The goal of the present study was to determine the role of the N terminus in regulating BCA1p. Western analysis using three different antisera showed that the N terminus of BCA1p is cleaved off by trypsin and that the N terminus contains the CaM-binding domain. Furthermore, the expressed N terminus binds CaM in a Ca2+-dependent manner. A synthetic peptide corresponding to the CaM-binding domain of BCA1p (Ala-19 to Leu-43) strongly inhibited ATP-dependent Ca2+ pumping by BCA1p in cauliflower low-density membranes, indicating that the CaM-binding region of BCA1p also has an autoinhibitory function. The expressed N terminus of BCA1p and a synthetic peptide (Ala-19 to Met-39) were good substrates for phosphorylation by protein kinase C. Sequencing of the phosphorylated fusion protein and peptide suggested serine-16 and/or serine-28 as likely targets for phosphorylation. Phosphorylation of serine-28 had no effect on CaM binding to the alanine-19 to methionine-39 peptide. Our results demonstrate the regulatory importance of the N terminus of BCA1p as a target for CaM binding, trypsin cleavage, and phosphorylation, as well as its importance as an autoinhibitory domain.
Changes in the cytosolic free Ca2+ concentration play a central role in the transduction of external signals through the cytosol of plant cells. A wide array of physiological events such as salt stress, low temperature, heat shock, and touch and exposure to hormones, red light, or fungal elicitors has been shown to be accompanied by transient changes in the concentration of the cytosolic free Ca2+ (Bush, 1995; Sanders et al., 1999). This universal Ca2+ signal is thought to be encoded by differences in the temporal and spatial distribution of Ca2+ in the cell, as well as by the amplitude and frequency of the changes in the cytosolic Ca2+ concentration (McAinsh and Hetherington, 1998; Sanders et al., 1999; Trewavas, 1999). In response to a stimulus, Ca2+ enters from the extracellular space and/or is released from intracellular stores (in plants mainly the vacuole and/or ER) by differently activated Ca2+ channels. It is vital to the cell that excess Ca2+ is removed from the cytosol following a Ca2+ signal to bring the cell back to a resting state. Two different systems are believed to be involved in pumping Ca2+ from the cytosol over the plasma membrane (PM) or back into intracellular stores: P-type Ca2+ pumping ATPases and a Ca2+/H+ antiporter. The former has been shown to be present in the ER and the vacuolar membranes as well as in the PM, whereas the antiporter is present in the vacuolar membrane only (Askerlund and Sommarin, 1996; Evans and Williams, 1998). The ATPases have a high affinity for Ca2+ and are therefore able to bring the concentration of Ca2+ back to submicromolar levels, while the antiporters have a lower affinity for Ca2+ but a higher capacity and are thought to function under conditions of high cytosolic Ca2+.
Ca2+-ATPases have been biochemically characterized in different plant species, and several isoforms have been cloned (Wimmers et al., 1992; Huang et al., 1993; Chen et al., 1997; Liang et al., 1997; Malmström et al., 1997; Harper et al., 1998). The isoforms can be divided into two major categories depending on whether they bind calmodulin (CaM) or not (type IIB and IIA, respectively; Axelsen and Palmgren, 1998). Plants differ from animals in that type IIB CaM-stimulated Ca2+-ATPases are found not only in the PM but in internal membranes as well (Askerlund, 1997; Evans and Williams, 1998; Hong et al., 1999). Furthermore, in contrast to the animal PM-Ca2+-ATPases that bind CaM at their C terminus, we recently showed that the CaM-stimulated Ca2+-ATPase in cauliflower vacuolar membranes (BCA1p) has an extended N terminus containing a putative CaM binding domain (Malmström et al., 1997). A Ca2+-ATPase with a long, CaM-binding N terminus has also been found in Arabidopsis (Harper et al., 1998).
Our aim was to determine the role of the N terminus in regulating BCA1p. A working hypothesis is that the function of the N-terminal region of plant CaM-stimulated Ca2+-ATPases is analogous to the regulatory function of the C terminus of animal PM Ca2+-ATPases. Here we show that the N terminus of BCA1p binds CaM in a Ca2+-dependent manner and that the CaM-binding domain acts as an inhibitor to block Ca2+ transport of BCA1p. In addition, we show that the N-terminal region can be phosphorylated in vitro by protein kinase C (PKC).
MATERIALS AND METHODS
Cauliflower (Brassica oleracea L. cv Bothrytis) was grown as described below or purchased locally. DNA cloning was done in Escherichia coli strain XL1-Blue (Stratagene, La Jolla, CA) or K12 PR745 for expression of fusion protein (New England Biolabs, Beverly, MA). DNA sequencing was done at the Biomolecular Core Facilities (Lund University, Sweden) using an automated sequencer (Prism model 310, ABI, Foster City, CA). Unless otherwise noted, we used standard molecular techniques according to the method of Sambrook et al. (1989).
Northern Blots
Leaves and inflorescence were obtained from plants grown in soil in a greenhouse at 21°C. Roots were obtained from plants grown in a nutrient solution according to the method of Siegenthaler and Depéry (1976) except that the Fe concentration was doubled.
The tissues were quickly frozen in liquid nitrogen and stored at −80°C. Frozen material was pulverized in liquid nitrogen with a mortar and pestle for isolation of total RNA. Aqueous solutions and plastic material were treated with diethyl pyrocarbonate. To 1 to 2 g of ground powder was added 1.5 mL of 0.2 m Tris-HCl (pH 8.0) 0.2 m NaCl, 50 mm EDTA (pH 8.0), 2% (w/v) SDS, 1.5 mL of phenol:chloroform:isoamylalcohol, 25:24:1 (v/v/v), and 5 μL of β-mercaptoethanol. The mixture was incubated for 10 min at 48°C and then centrifuged for 10 min at 800g, followed by a second extraction with phenol:chloroform:isoamyl alcohol. The aqueous phase was aliquoted into microfuge tubes, an equal volume of 6 m LiCl was added, and RNA was allowed to precipitate overnight at 4°C. The precipitate was collected by centrifugation at 10,000g in a microfuge, and the pellets were resuspended in 100 μL of water plus 100 μL of 4 m LiCl. After 30 min on ice, the preparation was centrifuged for 10 min at maximum speed (14,000 rpm) in a microfuge. The pellet was dissolved in water and the RNA was finally precipitated with NaAc, pH 5.2. After a wash with 70% (v/v) ethanol, the pellet was dissolved in water.
Samples (20 μg total of RNA per lane) were separated on a 1.5% (w/v) denaturing agarose gel using standard methods. Blotting onto GeneScreen membrane (DuPont-New England Nuclear, Boston), prehybridization, hybridization, and washing were performed according to the manufacturer's instructions using the 50% (v/v) formamide/10% (v/v) dextran sulfate method. Washing was at high stringency with 2× SSC (1× SSC is 0.15 m NaCl, 0.015 m sodium citrate) and 1% (w/v) SDS at 65°C. An oligolabeling kit (Pharmacia, Uppsala) was used to prepare a 32P-labeled hybridization probe from a 341-bp PCR product corresponding to the first 113 amino acids of BCA1p. 32P-labeled hybrids were visualized with a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
Expression of Fusion Protein
Hot Start PCR was performed with Vent polymerase (New England Biolabs) on cauliflower cDNA prepared as described previously (Malmström et al., 1997). The forward primer was designed to begin with the start codon for Met-1 of BCA1 (ATGTCTAATCTCCTCAAAGATTTTCAG) and the reverse primer (ATCCAAGCTTATCATGATCTGGCTGGTTTCTCAGC) designed to make Ser-157 the last amino acid, followed by a stop codon and a HindIII site. The PCR product was fused to the 3′ end of the gene for the maltose binding protein (MBP) by cloning into the XmnI-HindIII-site of the pMAL-c2 vector (New England Biolabs). The cloned PCR product was sequenced to verify that it was in the correct reading frame and that no errors had been introduced. Expression of the N-terminal fusion protein was made with the Protein Fusion & Purification System (New England Biolabs), essentially according to the manufacturer's instructions. The fusion protein was extracted from the insoluble fraction with 0.9% (w/v) of the anionic detergent N-lauroylsarcosine (catalog no. L5777, Sigma-Aldrich, St. Louis), and 15 mL of the solubilized fraction was then dialyzed (Spectra/Por, Mr cutoff 12,000–14,000, Spectrum, Houston) against 0.8 L of 20 mm Tris-HCl, pH 7.2, 0.2 m NaCl, 1 mm Na2EDTA, 1 mm dithiothreitol (DTT), 0.5 mm phenylmethylsulfonyl fluoride (PMSF), and 1 mm benzamidine-HCl, at 4°C for about 100 h with four changes of buffer.
Membrane Preparation
Low- and high-density membranes were prepared from cauliflower microsomes by Suc gradient centrifugation as described previously (Askerlund, 1996). PMs were prepared by two-phase partitioning of microsomes, also as described previously (Askerlund and Evans, 1992).
Antibody Preparation
Anti-BCA1p (Met-1 to Ser-157) and anti-BCA1p (Ala-19 to Leu-43) rabbit polyclonal antisera were produced at Inovagen (Lund, Sweden). Anti-BCA1p (Met-1 to Ser-157) was raised against the solubilized and dialyzed fusion protein containing residues Met-1 to Ser-157 of BCA1p. Anti-BCA1p (Ala-19 to Leu-43) was raised against a synthetic peptide corresponding to residues Ala-19 to Leu-43 of BCA1p. Anti-BCA1p (Ala-19 to Leu-43) was further purified on a protein G affinity column, followed by chromatography on a column in which the antigen (peptide Ala-19 to Met-39) was coupled to the matrix. The antiserum against the intact BCA1p (anti-BCA1p) has been described previously (Askerlund, 1996).
Western Analysis and CaM Overlays
Western immunoblotting analysis was carried out essentially as described previously (Askerlund, 1996), except that secondary antibodies were detected by enhanced chemiluminescence (ECL, Amersham, Buckinghamshire, UK). CaM overlays were performed using either 125I-CaM (Askerlund, 1996) or biotin-labeled CaM coupled to streptavidin-alkaline phosphatase conjugate (Boehringer Mannheim, Mannheim, Germany).
Trypsination of Membranes and Effect of Peptides on Ca2+ Uptake
In studies of the effect of trypsin treatment on antibody binding (Fig. 3), trypsinolysis was carried out as described previously (Askerlund, 1996). To investigate the effect of peptide on Ca2+ uptake, low-density membranes from cauliflower inflorescence (0.7 mg of protein) were suspended in 0.8 mL of 25 mm 3-(N-morpholino)-propanesulfonic acid (MOPS)- 1,3-bis(Tris[hydroxymethyl]methylamino) propane (BTP), pH 7.2, 0.33 m Suc, 0.2 m KCl, 5 mm MgCl2, 0.15 mm CaCl2, 2 mm ATP, and 1 mm DTT in a centrifuge tube at 20°C. Proteolysis was started by addition of 20 μg of trypsin (product no. 109 819, Boehringer Mannheim) in 10 μL and carried out for 2.5 min, after which time, 3 mL of the same buffer supplemented with 10 mm Pefabloc SC (Biomol, Hamburg, Germany) was added and the tubes were placed on ice for 10 min. Controls received 10 μL of water instead of trypsin. The tubes were centrifuged for 45 min at 140,000g at 4°C.
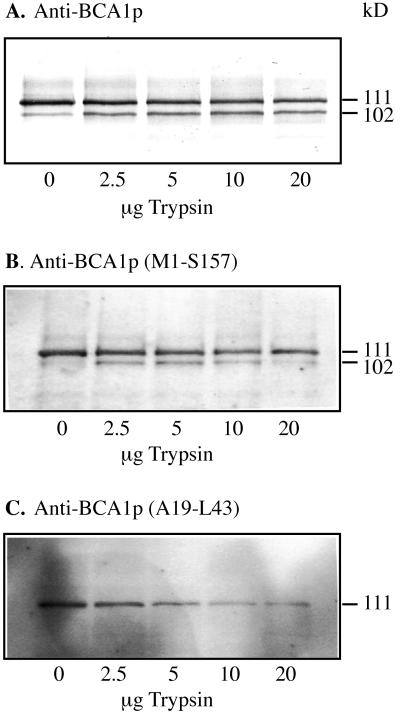
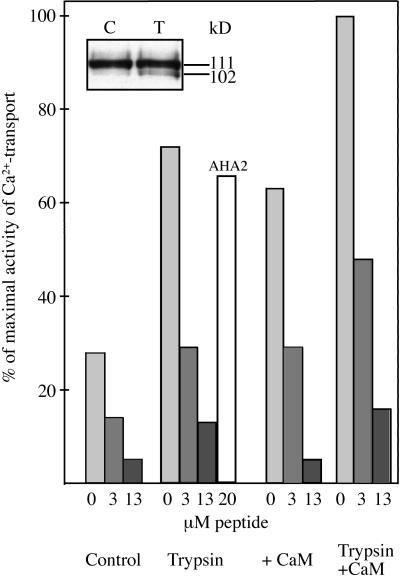
Figure 3.
Western analysis of BCA1p in low-density membranes after trypsin treatment, carried out as described in Askerlund (1996). Samples were collected from the complete Ca2+-pumping assay exactly 2 min after the addition of trypsin. The assay mix was trichloroacetic acid-precipitated, and analyzed by western blotting using anti-BCA1p (A), anti-BCA1p (Met-1 to Ser-157) (B), and anti-BCA1p (Ala-19 to Leu-43) (C). The lanes received 4 μg of protein.
The membrane pellets were resuspended in 3 mL of ice-cold 25 mm MOPS-BTP, pH 7.2, 0.33 m Suc, 0.2 m KCl, 1 mm DTT, and 10 mm Pefabloc SC, homogenized in a glass-Teflon homogenizer, and again centrifuged as described above. The pellets of trypsin-treated and control membranes were homogenized in 3 mL of ice-cold 25 mm MOPS-BTP, pH 7.2, 0.33 m Suc, 0.2 m KCl, 0.15 mm CaCl2, 1 mm DTT, and 5 mm Pefabloc SC. Each fraction was then divided between two centrifuge tubes containing either 5 μm CaM or the same volume of water and incubated at 0°C for 10 min. After centrifugation (see above), the four final fractions (control ± CaM; trypsin-treated ± CaM) were resuspended in cold 25 mm MOPS-BTP, pH 7.2, 0.33 m Suc, 1 mm DTT, and 5 mm Pefabloc SC, homogenized, aliquoted, and stored at −80°C. ATP-dependent 45Ca2+ uptake was measured for 10 min at 30°C, as described previously (Askerlund, 1996). Membranes were incubated with different concentrations of BCA1p peptide (Ala-19 to Leu-43; ARQRWRSSVSIVKNRARRFRMISNL) or a control peptide derived from the C terminus of the Arabidopsis PM H+-ATPase (AHA2; EREAQWALAQRTLHGLQPK) for 15 min prior to starting the reaction by addition of ATP.
In Vitro Phosphorylation
In vitro phosphorylation of fusion protein and synthetic peptide was carried out with PKC (product no. 1459 651, Boehringer Mannheim) in 12.5 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 0.5 mm CaCl2, 1 mm DTT, 100 μg mL−1 phosphatidyl-Ser, 0.04 to 0.16 milliunit of PKC, and 0.1 to 0.2 mg of synthetic peptide (Ala-19 to Met-39) or fusion protein in 0.4 mL. The reaction was started by the addition of 0.15 mm ATP (approximately 20 μCi of [γ-32P]ATP when radioactive ATP was used), run for 0 to 90 min (as indicated in the figure legends) at 30°C, and stopped either by the addition of 0.4 mL of 2× Laemmli solubilization buffer (when samples were to be analyzed by SDS-PAGE) or 0.8 mL of 0.1% (v/v) trifluoroacetic acid (for reverse-phase HPLC).
HPLC Purification and Peptide Sequencing
The non-phosphorylated and phosphorylated peptides were separated by reverse-phase HPLC using a C18 (218 TP) column (Vydac, Hesperia, CA) and a linear gradient of water-acetonitrile containing 0.1% (v/v) trifluoroacetic acid at a flow rate of 1 mL/min and detection at A215. The eluted fractions were concentrated using ProSorb sample preparation cartridges (Perkin Elmer, Foster City, CA). N-terminal amino acid sequencing of synthetic peptides was carried out at the Biomolecular Core Facilities at Lund University using the Edman degradation procedure. Lys C digestion of fusion protein and sequencing of the resulting peptides was carried out at the Department of Plant Biology, Swedish University of Agricultural Sciences, Uppsala.
CaM Band Shift Assay
Bovine brain CaM (2.5 μg) dissolved in 100 mm Tris, pH 7.2, 4 m urea, and 0.1 mm CaCl2 was incubated for 1 h at room temperature in the presence of synthetic peptide at different molar ratios. The samples were then loaded on Ca2+-containing urea gels with 15% (w/v) acrylamide (Erikson-Vitanen and DeGrado, 1987) for the analysis of the CaM band mobility. 32P-labeled peptide-CaM bands were visualized with a phosphor imager (Molecular Dynamics).
RESULTS
The mRNA Expression of BCA1 Is Highest in Roots, Whereas the Protein Is Most Abundant in Inflorescences
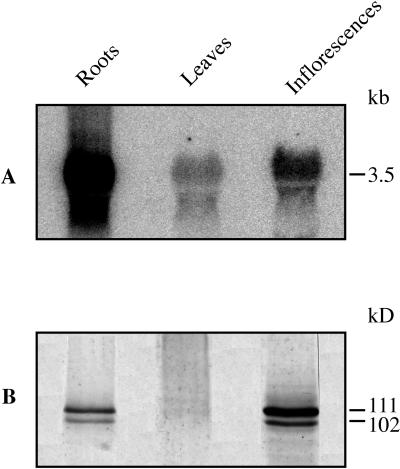
To investigate the expression levels of BCA1 in different tissues of cauliflower, northern and western analyses were performed (Fig. 1). Total RNA was extracted from cauliflower roots, leaves, and inflorescences and probed with a 32P-labeled PCR product corresponding to the first 113 amino acids of BCA1p. The N-terminal amino acid sequence is not highly conserved between different Ca2+-ATPase isoforms (Huang et al., 1993; Malmström et al., 1997; Harper et al., 1998) and the corresponding nucleotide sequence of BCA1 should therefore produce a relatively specific DNA probe. BCA1 mRNA was most abundant in roots and least abundant in leaves (Fig. 1A), in similarity with the results from analysis of both type IIA and IIB Ca2+-ATPase genes from other plants (Wimmers et al., 1992; Huang et al., 1993; Harper et al., 1998). With crude microsomal membranes, western-blot analysis showed strong staining of BCA1p only with the material from inflorescences (data not shown). With low-density microsomal membranes enriched in vacuolar membranes, a strong band at 111 kD and a weaker band at 102 kD were detected with material from both roots and inflorescences, whereas only weak bands were detected with material from leaves (Fig. 1B).
Figure 1.
Relative transcript and protein levels of BCA1p in different cauliflower tissues. A, Total RNA extracted from cauliflower roots, leaves, and inflorescences was probed with a 32P-labeled, 341-bp PCR fragment at high stringency. The PCR fragment corresponds to the first 113 amino acids of BCA1p. B, Immunoblot with low-density membranes from the same tissues probed with anti-BCA1p. Each lane received 20 μg of RNA (A) or 12.5 μg of protein (B).
Apparently, the amount of BCA1p is regulated at the translational level. Of the Ca2+-ATPases studied so far, BCA1p is the only isoform that is more expressed in flowers than in roots. BCA1p has been shown to be a vacuolar membrane protein (Askerlund, 1997) highly abundant in inflorescences, which is a relatively homogenous tissue with a large proportion of highly vacuolated cells. This may explain the higher relative expression in inflorescences in our study than in studies carried out with Arabidopsis flowers, which are in a later developmental stage and therefore more heterogeneous.
The weak bands of slightly higher molecular mass detected in western blots of membranes from leaves may represent a different isoform of the Ca2+-ATPase. Previous studies with cauliflower inflorescences showed that the PM Ca2+-ATPase has a slightly higher molecular mass than BCA1p (Askerlund, 1997), and the possibility existed that the weak bands detected in leaf membranes (Fig. 1) represented a contamination by PMs. To investigate if the bands detected in leaves were enriched in PMs, we prepared PMs from leaves by two-phase partitioning; however, the bands were detected in the intracellular membranes only (lower phase) and not in the PMs (upper phase; data not shown).
CaM Binding to the N Terminus Is Ca2+ Dependent
We previously suggested that a CaM-binding domain is present in the N terminus of BCA1p and showed that a synthetic peptide corresponding to this domain (Ala-19 to Leu-43) could bind CaM (Malmström et al., 1997). Surprisingly, CaM binding was not Ca2+ dependent. The most likely explanation for this behavior was that a flanking amino acid sequence necessary for Ca2+-dependent CaM binding was missing.
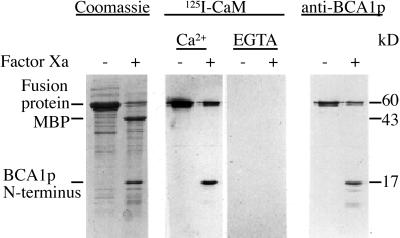
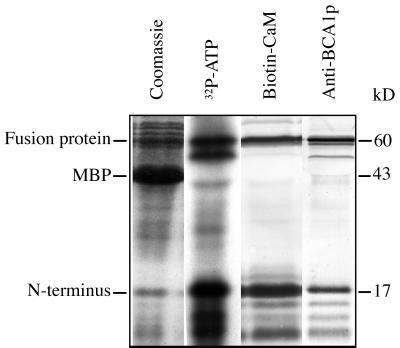
To test this hypothesis, we expressed the N terminus (the first 157 amino acids) as a fusion protein with the MBP of E. coli and analyzed its ability to bind CaM. SDS-PAGE analysis of E. coli expressing the fusion protein showed that virtually all of the fusion protein ended up in inclusion bodies and not in the cytosolic fraction (not shown). The fusion protein was extracted from the insoluble fraction with the anionic detergent N-lauroylsarcosine, dialyzed to decrease the concentration of detergent, and then used for all subsequent experiments. The purity of the dialyzed fusion protein is shown in Figure 2. Treatment of the fusion protein with factor Xa cleaved off most of the N-terminal insert (17 kD) from the MBP (Fig. 2). Fractions treated in the same way were blotted to a polyvinylidene difluoride (PVDF) membrane and overlaid with 125I-CaM in the presence or absence of Ca2+. The N-terminal fragment and the fusion protein, but not the MBP alone, were found to bind CaM. Furthermore, the binding of CaM was strictly Ca2+ dependent. The 17-kD fragment and the intact MBP-fusion protein were both detected by an anti-BCA1p (Fig. 2), confirming that the 17-kD fragment corresponds to the BCA1p N terminus.
Figure 2.
Analysis of Ca2+-dependent CaM binding to the BCA1p N terminus. The N-terminal region of BCA1p (amino acids 1–157) was expressed in E. coli as a fusion protein with MBP. Factor Xa-treated (+) or non-treated (−) fusion protein was analyzed by SDS-PAGE and Coomassie staining (left). The same fractions were blotted to PVDF membrane and overlaid with 125I-CaM in the presence or absence of Ca2+ (middle), or were probed with anti-BCA1p (right).
The Fragment Cleaved Off by Trypsin Contains the CaM-Binding Domain and Originates from the N Terminus
Earlier investigations showed that trypsin treatment of low-density membranes from cauliflower inflorescences resulted in activation of Ca2+-pumping and loss of CaM sensitivity. It was also shown that trypsin activation was accompanied by removal of a 9-kD fragment (Askerlund, 1996). The fact that the N-terminal region of BCA1p was able to bind CaM (Malmström et al., 1997, and above) strongly suggested that it was the N terminus that was cleaved off during trypsin activation.
To test this, antisera were raised against the BCA1p N-terminal fusion protein (Met-1 to Ser-157) and against the synthetic peptide (Ala-19 to Leu-43), and used to detect BCA1p in western blots of low-density membrane fractions treated with increasing amounts of trypsin (Fig. 3). As shown earlier, anti-BCA1p recognized two bands at 111 and 102 kD, corresponding to the intact and truncated Ca2+-ATPase (Askerlund, 1996; Fig. 3A). The anti-BCA1p (Met-1 to Ser-157) gave basically the same pattern as anti-BCA1p, but the 102-kD band was weaker (Fig. 3B). In contrast, anti-BCA1p (Ala-19 to Leu-43) only detected the intact 111-kD Ca2+-ATPase (Fig. 3C). Thus, trypsin digestion leads to a decrease in the binding of anti-BCA1 (Met-1 to Ser-157) to the 102-kD fragment, and anti-BCA1 (Ala-19 to Leu-43) is unable to recognize this fragment at all. Since the anti-BCA1p (Ala-19 to Leu-43) antibody is raised against the CaM-binding region (Ala-19 to Leu-43), these results clearly show that the 9-kD fragment contains the CaM-binding domain and originates from the N terminus.
The Regulatory Domains of the PM Ca2+-ATPase and BCA1p Differ in Structure
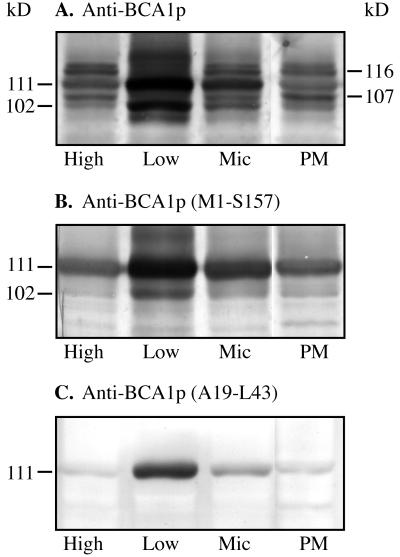
The gene encoding the PM Ca2+-ATPase has not yet been identified in any plant species. Although several studies have shown that the PM Ca2+-ATPase is CaM regulated (Rasi-Caldogno et al., 1995; Askerlund, 1997; Bonza et al., 1998; Olbe and Sommarin, 1998), nothing is known about either the location or sequence of the CaM binding region of this protein. We previously showed that anti-BCA1p cross-reacts with the PM Ca2+-ATPase (116 kD) in membrane fractions from cauliflower (Askerlund, 1996, 1997). CaM overlays suggested that the PM Ca2+-ATPase in cauliflower has a higher affinity for CaM than the vacuolar Ca2+-ATPase (Askerlund, 1997). It was therefore of interest to see if the 116-kD PM Ca2+-ATPase could be recognized by anti-BCA1p (Met-1 to Ser-157) and anti-BCA1p (Ala-19 to Leu-43), since this would give an indication of whether the regulatory domain of the PM and vacuolar Ca2+-ATPases have a similar or a different structure.
Four different membrane fractions from cauliflower inflorescence were used in western analysis: high- and low-density microsomal membrane fractions from a discontinuous Suc gradient centrifugation (Askerlund, 1996), a crude microsomal fraction, and a PM fraction obtained by two-phase partitioning. In the low-density membrane fraction, anti-BCA1p recognized two bands at 111 and 102 kD, corresponding to the intact and partially degraded BCA1p (Fig. 4A). These two bands were also weakly stained in the other membrane fractions. The reason for this is that we intentionally overloaded the gel so that we would be able to detect the weaker bands as well. As reported earlier (Askerlund, 1997), anti-BCA1p recognized two bands in the PMs at 116 and 107 kD (Fig. 4A), probably representing an intact and a degraded form of the PM Ca2+-ATPase, respectively.
Figure 4.
Western analysis of different cauliflower membrane fractions. High-density membranes (High), low-density membranes (Low), microsomal membranes (Mic), and PMs from cauliflower inflorescences were separated on an 8% (w/v) SDS-polyacrylamide gel. The high- and low-density membranes were obtained from the microsomal fraction by discontinuous Suc gradient centrifugation. PMs were obtained from microsomal membranes by two-phase partitioning. Each lane received 15 μg of protein.
The anti-BCA1p (Met-1 to Ser-157) antiserum detected the bands at 111 and 102 kD in the low-density membranes and, to a lesser extent, also in the other fractions (Fig. 4B). However, in contrast to anti-BCA1p, this antiserum did not recognize the 116- and 107-kD bands in the PMs. The anti-BCA1p (Ala-19 to Leu-43) antibody only detected one band at 111 kD in all fractions (Fig. 4C). These results indicate that the regulatory domain of the PM Ca2+-ATPase differs from BCA1p in structure. Moreover, the blots show that the anti-BCA1p (Met-1 to Ser-157) and anti-BCA1p (Ala-19 to Leu-43) antisera are much more specific for BCA1p than the anti-BCA1p antiserum.
A Peptide from the N-Terminal Region Blocks Ca2+ Transport of BCA1p
If the CaM-binding domain of BCA1p also has an autoinhibitory function analogous to that of the PM Ca2+-ATPase in animals (Falchetto et al., 1991, 1992; Carafoli, 1994), the Ala-19 to Leu-43 peptide should be able to inhibit the truncated, activated enzyme. The effect of the synthetic peptide was studied in low-density membranes treated with either CaM or trypsin, as well as in untreated membrane vesicles (Fig. 5). Prior to incubation with peptide, the membrane vesicles were washed as described in “Materials and Methods” to ensure that the peptide was not complexed by excess CaM or degraded by trypsin. In the absence of peptide, trypsin and CaM treatment resulted in 160% and 125% increase in ATP-dependent Ca2+ pumping, respectively, essentially as shown previously (Askerlund, 1996). When CaM was added to trypsin-treated membranes, Ca2+ pumping was increased by 260%. Trypsin treatment was accompanied by an increased amount of the 102-kD Ca2+-ATPase fragment (inset). The peptide had a strong inhibitory effect on Ca2+ pumping (Fig. 5). At a concentration of 3 μm, the peptide inhibited to about 50% and at 13 μm to between 80% and 90%. In contrast, a synthetic peptide corresponding to amino acids Glu-870 to Lys-888 of the PM H+-ATPase from Arabidopsis (AHA2) had very little effect on Ca2+ pumping, even at 20 μm. The degree of inhibition caused by the BCA1p N-terminal peptide was about the same irrespective of whether control membranes were used, the membranes were first treated with trypsin or CaM, or both. These results suggest that the CaM-binding domain could block regions of BCA1p responsible for ATP binding, Ca2+ binding, Ca2+ translocation, or other sites that may influence the catalytic activity of the pump.
Figure 5.
Effect of synthetic peptide (Ala-19 to Leu-43) on ATP-dependent Ca2+ uptake in low-density membranes treated with trypsin, CaM, or trypsin and CaM. Precautions were taken to ensure that the peptide was not complexed by CaM or degraded by trypsin (see “Materials and Methods”). Inset, Immunoblot of control (C) and trypsin-treated (T) membranes that were used in the experiment probed with anti-BCA1p. AHA2, Synthetic peptide (Glu-870 to Lys-888) from Arabidopsis H+-ATPase isoform 2.
One or Several N-Terminal Residues Are Phosphorylated by PKC
The possibility that BCA1p is regulated by phosphorylation was investigated. Three different protein kinases were tested: protein kinase A, casein kinase II, and PKC. Of these, only PKC was able to phosphorylate the N-terminal fusion protein (Fig. 6). After cleavage with factor Xa, most of the radioactivity was detected in a 17-kD fragment, showing that the BCA1p N terminus rather than MBP was the substrate of phosphorylation. The N terminus was identified by its ability to bind CaM and to react with anti-BCA1p. The few fragments of molecular masses less than 17 kD that also bound CaM and anti-BCA1p probably represent degradation products of the 17-kD N terminus.
Figure 6.
In vitro phosphorylation of the BCA1p N terminus by PKC after cleavage with factor Xa. Polypeptides were separated by SDS-PAGE and analyzed with Coomassie-staining, autoradiography, CaM overlay, or immunoblot. The N terminus of BCA1p, as well as the uncleaved fusion protein, were strongly phosphorylated by PKC. The N terminus was identified by its ability to bind CaM and anti-BCA1p.
To determine the site of phosphorylation, the phosphorylated N terminus was cut out from the gel and cleaved with Lys C. After separation by HPLC, two radioactive peptides were identified. N-terminal sequencing of these peptides resulted in sequences both starting with Asn-14 and readable to Arg-20 or Trp-23, respectively (data not shown). In both radioactive peptides, Ser-16 could not be detected, which indicates that it was phosphorylated (Mercier et al., 1971; Wettenhall et al., 1991; see below). Both these peptides should end at Lys-31, resulting from Lys C cleavage after Lys. Three additional Ser residues are thus present in these peptides at positions 25, 26, and 28. Therefore, the phosphorylation of Ser-25, Ser-26, and Ser-28 could not be excluded as the longest sequence was only readable to Trp-23.
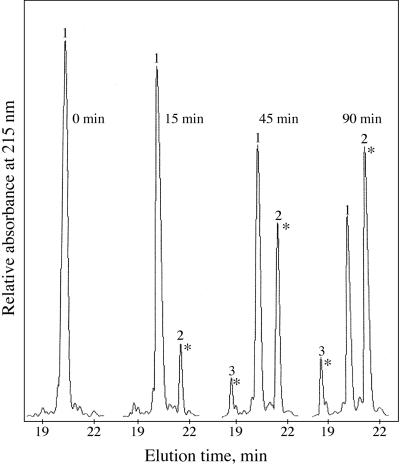
Since we were not able to exactly determine the phosphorylated amino acid in the fusion protein, a synthetic peptide (Ala-19 to Met-39) was used as a substrate for phosphorylation by PKC. The peptide was phosphorylated for different time intervals, and phosphorylated and non-phosphorylated peptides were separated by reverse-phase HPLC (Fig. 7). The non-phosphorylated peptide (peak 1) eluted at 20.2 min. After phosphorylation, a major radioactive peak appeared at 21.3 min in the chromatogram (peak 2). The more hydrophobic behavior of the phosphorylated peptide (peak 2) can be explained as neutralization of one of the several positive charges in the peptide by the phosphate group. After 90 min, more than 50% of the original peptide was phosphorylated and found under peak 2. A smaller radioactive peak (peak 3) was also visible at 18.7 min in the chromatogram after extended phosphorylation. Peaks 1 to 3 were collected, N-terminally sequenced (residues 19–31) to confirm their identity, and used for CaM band shift assays. In addition, N-terminal sequencing was exploited to determine the site of phosphorylation (Table I). A phosphorylated amino acid cannot be identified in the Edman degradation procedure, since the degradation of a phosphorylated Ser residue generates an unstable product that undergoes β elimination of the phosphoryl group (Mercier et al., 1971; Wettenhall et al., 1991). As a result, the yield of this particular Ser is much lower than the yield for a non-phosphorylated Ser residue, a fact that was exploited here to determine which Ser was phosphorylated.
Figure 7.
Elution profiles from reverse-phase HPLC of the phosphorylation reaction after different times of phosphorylation of synthetic peptide Ala-19 to Met-39 (see “Materials and Methods” for details). Peaks 1, 2, and 3 eluted at 21%, 22%, and 20% acetonitrile, respectively. An asterisk indicates that the peak was radioactive when [γ-32P]ATP was used in the phosphorylation assay.
Table I.
Yield of Ser during N-terminal sequencing of a phosphorylated and non-phosphorylated synthetic peptide
| Residue No. | Amino Acid | Yield of Ser
|
||
|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | ||
| pmol | ||||
| 6 [24] | Arg | 5.6 | 3.8 | 4.3 |
| 7 [25] | Ser | 49 (100) | 54 (100) | 52 (100) |
| 8 [26] | Ser | 45 (92) | 37 (68) | 22 (44) |
| 9 [27] | Val | 6.7 | 9.2 | 7.8 |
| 10 [28] | Ser | 28 (57) | 7.8 (14) | 17 (33) |
| 11 [29] | Ile | 4.6 | 3.6 | 8.1 |
| 12 [30] | Val | 2.1 | 2.5 | 4.0 |
A synthetic peptide corresponding to A19 to M39 in BCA1p was phosphorylated with PKC, subjected to reverse-phase HPLC (Fig. 7), and the first 12 residues of peaks 1 to 3 were N-terminally sequenced. The yield of Ser-28 in radio-labeled peak 2 (underlined) was significantly lower than in peak 1 (non-radioactive), indicating that it was phosphorylated. Numbers in brackets correspond to the amino acid position in BCA1p. Numbers in parentheses are the percentage yield of Ser-26 and Ser-28 relative to the yield of Ser-25, which was set to 100%. Values for peak 3 have been multiplied with 1.7 to compensate for the lower amount of peptide subjected to sequencing.
Sequence analysis showed that the yield of Ser-25 was about the same in all three peaks, indicating that this Ser was not phosphorylated. The yield of Ser-25 was therefore used as an internal standard and set to 100%. In contrast to Ser-25, the yield of Ser-28 was considerably lower in the major phosphorylated peptide (peak 2) than in the non-phosphorylated peptide (peak 1): the percentage yield of Ser-28 compared with Ser-25 was 57% for peak 1 but only 14% for peak 2. The percentage yield of Ser-26 in peaks 1 and 2 was 92% and 68%, respectively. Consequently, the decrease in yield of Ser-26 observed for peak 2 was not so pronounced as the decrease in yield for Ser-28. This suggests that Ser-28 was phosphorylated. A similar comparison of the different Ser residues in peak 3 showed smaller differences than in peak 2, but larger than in peak 1: the percentage yield of Ser-28 was 33% and of Ser-26 44% (Table I). Thus, the decrease in yields of Ser-26 and Ser-28 in peak 3 was smaller than in peak 2, although not negligible. Possibly, this peptide was phosphorylated at Ser-26 or both Ser-26 and Ser-28.
The Phosphorylated Peptide Is Able to Bind CaM
Phosphorylation in or near the CaM-binding region has been shown to differently affect (usually decrease) the CaM binding ability of the animal PM Ca2+-ATPase and of other CaM-binding proteins (Verghese et al., 1994; Matsuoka et al., 1996; Enyedi et al., 1997; Verma et al., 1999).
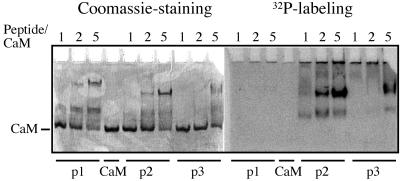
Urea gel band shift experiments have been used to detect how phosphorylation of synthetic peptides affects their CaM-binding properties (Erikson-Vitanen and DeGrado, 1987; Hofmann et al., 1994). CaM and phosphorylated or non-phosphorylated peptide (peaks 1–3; Fig. 7) were incubated together at different molar ratios and the complex formation was analyzed in a gel containing 4 m urea (Fig. 8). Both non-phosphorylated (peak 1) and phosphorylated (peaks 2 and 3) peptides retarded the migration of CaM in the gel. In all three cases, two shifted bands of different intensity were visible (Fig. 8). This suggested that all three peptides formed two different complexes, with CaM having different mobility in the urea gel. The mobility of the upper band was influenced by the introduction of a phosphate group. Up to five times more peptide than CaM was needed in the assay to obtain a nearly full shift of CaM. Since one molecule of CaM is expected to bind one molecule of peptide, this probably means that only a portion of the peptide bound to CaM under the experimental conditions used. The unbound peptide never entered the gel, since it has a large portion of positively charged amino acids (pI 12.7). The results clearly show that the non-phosphorylated (peak 1) and phosphorylated (peak 2) peptides bind CaM with the same efficiency (Fig. 8). It is possible that the minor phosphorylated peptide (peak 3) was less efficient in binding CaM since it retarded the migration of CaM to a lesser extent than the peak 2 peptide.
Figure 8.
CaM band shift assay. CaM and peptides, corresponding to peaks 1 to 3 in Figure 7, were incubated at the molar ratios indicated in the presence of urea and Ca2+. The complex formation was analyzed in a Ca2+-containing urea gel. The peptides from peaks 1, 2, and 3 are labeled p1, p2, and p3, respectively.
DISCUSSION
We earlier showed that trypsinolysis is accompanied by activation of ATP-dependent Ca2+ pumping and resulted in loss of the CaM-binding domain (Askerlund, 1996). It was not known, however, whether the cleavage occurred in the N or C terminus of BCA1p. The localization of the CaM binding region to the N terminus (Malmström et al., 1997; Fig. 2 in this paper) suggested that trypsin cleaves in the N terminus. The antibodies that were raised against the CaM-binding domain (Ala-19 to Leu-43) only detected the intact Ca2+-ATPase at 111 kD after trypsinolysis, whereas the antisera against intact BCA1p and against the whole N-terminal region (Met-1 to Ser-157) recognize the 111-kD as well as the 102-kD bands (Fig. 3). These results directly show that trypsin removes a part of the N terminus, and that the fragment removed by trypsin contains the CaM-binding domain as well as an autoinhibitory domain.
The Ca2+ transport activity of trypsin-treated low-density membranes was markedly reduced by the addition of low amounts of the CaM-binding peptide (Fig. 5). This suggested that the autoinhibitory domain and the CaM-binding region at least partially overlaps. The concentration needed for 50% inhibition did not vary between control membranes and membranes treated with trypsin, CaM, or both. Trypsin and CaM are thought to have the same effect, that is to remove the autoinhibitory N terminus from the active site of the Ca2+-ATPase. Both these treatments can be expected to have the effect of making one or several binding sites accessible for the peptide, explaining the similar degree of inhibition. The fact that control membranes were similarly inhibited by the same concentration of peptide may be due to a certain endogenous degradation of the Ca2+-ATPase and/or to stimulation by endogenous CaM. Recent studies with full-length and N-terminally truncated Ca2+-ATPase expressed in yeast suggest that the enzyme is virtually inactive in the absence of CaM or proteolysis (Harper et al., 1998). This may explain why Ca2+ transport in control membranes was inhibited by the peptide in the present study—most of the activity would then be due to the small fraction of already activated Ca2+-ATPase. Part of the peptide inhibition in control and CaM-treated membranes may also be due to a direct interaction between endogenous or added CaM and peptide (Malmström et al., 1997). The competition for CaM between the N terminus of the native protein and the synthetic peptide may decrease the amount of CaM bound to the N terminus, facilitating it to act as an autoinhibitor of Ca2+ pumping.
CaM also stimulated to a certain degree the Ca2+ transport in trypsin-treated membranes (Fig. 5). This is in agreement with the western blot of trypsin-treated membranes, which shows that only a small portion of the Ca2+-ATPase was degraded (Fig. 5, inset); the remaining part of the Ca2+-ATPase should thus retain its responsiveness to CaM.
The extensively studied animal PM Ca2+ pump has a long C terminus that is the target for regulation of the pump by CaM, phosphorylation, and protease cleavage (Carafoli, 1994). A CaM-binding peptide has been shown to inhibit the activated, protease-treated Ca2+ pump (Enyedi et al., 1989; Falchetto et al., 1991, 1992). Several studies in which a CaM-binding peptide was cross-linked to the truncated animal PM Ca2+-ATPase have indicated that the peptide may bind at two different domains: one in the first cytosolic loop (between transmembrane helices 2 and 3) and the other in the second cytosolic loop (between transmembrane helices 4 and 5) (Falchetto et al., 1991, 1992). The binding site (Ile-206 to Val-271) in the first cytosolic loop of the mammalian Ca2+ pump shares sequence homology with the corresponding region of BCA1p (Leu-251 to Val-315: 55% identity, 71% similarity). This first cytoplasmic unit has been proposed to be a transduction domain of the animal PM Ca2+-ATPase, coupling ATP hydrolysis to Ca2+ transport (Falchetto et al., 1992). Future experiments will show exactly where on BCA1p the N terminus interacts to cause inhibition of Ca2+ pumping.
Depending on the isoform, phosphorylation of the animal PM Ca2+-ATPase either activates the pump or prevents CaM binding. In the cases where binding is not affected, phosphorylation takes place in an autoinhibitory domain that is close to or overlapping with the CaM-binding domain or in a short loop connecting two CaM-binding lobes (Verma et al., 1999).
The N-terminal fusion protein was a good substrate for phosphorylation by PKC (Fig. 6). Amino acid sequencing suggests that the phosphorylation site(s) is located between Asn-14 and Lys-31. Since no Tyr or Thr is present in this region, the most likely targets for phosphorylation are the Ser residues in positions 16, 25, 26, and 28. Ser-16 was not detected in any of the two radioactive peptides obtained from the Lys C cleavage of the fusion protein, indicating that this residue may have been phosphorylated (Mercier et al., 1971; Wettenhall et al., 1991). However, this experiment did not give any clue as to whether Ser-25, Ser-26, or Ser-28 were phosphorylated. In addition, experiments where the synthetic peptide (Ala-19 to Met-39) was used as a substrate for phosphorylation showed that one or several Ser residues (Ser-25, Ser-26, and Ser-28) could be phosphorylated. Edman degradation of phosphorylated and non-phosphorylated synthetic peptide (peaks 1–3; Fig. 7) suggested that primarily Ser-28 was phosphorylated by PKC, as the yield of this residue was considerably lower in the phosphorylated peptide (peak 2, Table I) than in the non-phosphorylated peptide (peak 1, Table I). This position fits nicely with the consensus sequence of PKC phosphorylation sites (Pinna and Ruzzene, 1996). The most likely explanation of why there are two phosphorylated peaks in the chromatogram is that the peptide can be phosphorylated at two different positions simultaneously but at different rates. The faster of these two reactions would then result in phosphorylation of Ser-28 giving rise to peak 2, whereas the slower reaction would phosphorylate a different residue and result in peak 3. We were not able to determine the phosphorylation site in peak 3, but a likely position is Ser-26 or both Ser-26 and Ser-28 (Table I).
The urea gel shift experiments (Fig. 8) suggested that phosphorylation in the CaM-binding domain of BCA1p does not prevent CaM binding. In agreement with the data obtained from the urea gel experiments, we were not able to detect any difference in the ability of the phosphorylated or non-phosphorylated fusion protein to bind CaM in CaM overlay experiments, or in their ability to bind to a CaM-agarose column (data not shown). Thus, N-terminal phosphorylation of BCA1p does not seem to affect CaM binding, but may instead regulate the activity of the pump in a more direct fashion.
CONCLUDING REMARKS
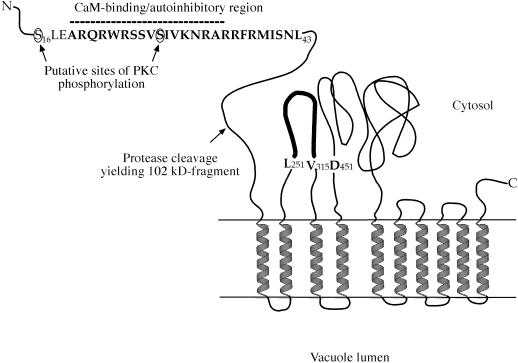
Our results demonstrate the regulatory importance of the N terminus of BCA1p, its autoinhibitory function, and as a site for CaM binding, trypsin cleavage, and phosphorylation, all summarized in Figure 9. It is known that Ca2+ is important in flower development (Peng and Iwahori, 1995; Furuyama and Dzelzkalns, 1999, and refs. therein). The fact that BCA1p is most abundant in inflorescences could implicate a role for CaM-regulated Ca2+ transport in flower development. Future studies will determine the relative importance of the different possible modes of regulation, as well as when and how they are utilized.
Figure 9.
Schematic model of the regulation of BCA1p at its N terminus. The autoinhibitory region (Ala-19 to Leu-43) is in bold, and the dotted line indicates the putative sequence necessary for CaM binding, as demonstrated for the homologous sequence of Arabidopsis Ca2+-ATPase ACA2 (Harper et al., 1998). The loop in bold represents the putative peptide interaction domain (Leu-251 to Val-315) of BCA1p. The model is not drawn to scale.
ACKNOWLEDGMENTS
We wish to thank Christer Larsson for the gift of the AHA2 peptide and for helpful suggestions on the manuscript, and Stephanie Agius for correction of the English language.
Footnotes
This work was supported by the Swedish Natural Science Research Council and the European Union Biotechnology Program.
LITERATURE CITED
- Askerlund P. Modulation of an intracellular calmodulin-stimulated Ca2+-pumping ATPase in cauliflower by trypsin: the use of calcium green-5N to measure Ca2+ transport in membrane vesicles. Plant Physiol. 1996;110:913–922. doi: 10.1104/pp.110.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P. Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower. Plant Physiol. 1997;114:999–1007. doi: 10.1104/pp.114.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P, Evans DE. Reconstitution and characterization of a calmodulin-stimulated Ca2+-pumping ATPase purified from Brassica oleracea L. Plant Physiol. 1992;100:1670–1681. doi: 10.1104/pp.100.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P, Sommarin M. Calcium efflux transporters in higher plants. In: Smallwood M, Knox JP, Bowles DJ, editors. Membranes: Specialised Functions in Plants. Oxford: Bios Scientific Publisher; 1996. pp. 281–299. [Google Scholar]
- Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- Bonza C, Carnelli A, De Michaelis MI, Rasi-Caldogno F. Purification of the plasma membrane Ca2+-ATPase from radish seedlings by calmodulin-agarose affinity chromatography. Plant Physiol. 1998;116:845–851. doi: 10.1104/pp.116.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993–1002. [PubMed] [Google Scholar]
- Chen X, Chang M, Wang B, Wu R. Cloning of a Ca2+-ATPase gene and the role of cytosolic Ca2+ in the gibberellin-dependent signaling pathway in aleurone cells. Plant J. 1997;11:363–371. doi: 10.1046/j.1365-313x.1997.11030363.x. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Elwess NL, Filoteo AG, Verma AK, Paszty K, Penniston JT. Protein kinase C phosphorylates the “a” forms of plasma membrane Ca2+ pump isoforms 2 and 3 and prevents binding of calmodulin. J Biol Chem. 1997;272:27525–27528. doi: 10.1074/jbc.272.44.27525. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Vorherr T, James P, McCormick DJ, Filoteo AG, Carafoli E, Penniston JT. The calmodulin binding domain of the plasma membrane Ca2+ pump interacts both with calmodulin and with another part of the pump. J Biol Chem. 1989;264:12313–12321. [PubMed] [Google Scholar]
- Erikson-Vitanen S, DeGrado W. Calmodulin-binding sequences. Methods Enzymol. 1987;139:455–478. doi: 10.1016/0076-6879(87)39106-2. [DOI] [PubMed] [Google Scholar]
- Evans DE, Williams LE. P-type calcium ATPases in higher plants: biochemical, molecular and functional properties. Biochim Biophys Acta. 1998;1376:1–25. doi: 10.1016/s0304-4157(97)00009-9. [DOI] [PubMed] [Google Scholar]
- Falchetto R, Vorherr T, Brunner J, Carafoli E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J Biol Chem. 1991;266:2930–2936. [PubMed] [Google Scholar]
- Falchetto R, Vorherr T, Carafoli E. The calmodulin-binding site of the plasma membrane Ca2+ pump interacts with the transduction domain of the enzyme. Protein Sci. 1992;1:1613–1621. doi: 10.1002/pro.5560011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Dzelzkalns VA. A novel calcium-binding protein is expressed in Brassica pistils and anthers late in flower development. Plant Mol Biol. 1999;39:729–737. doi: 10.1023/a:1006169808171. [DOI] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem. 1998;273:1099–1106. doi: 10.1074/jbc.273.2.1099. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Anagli J, Carafoli E, Vorherr T. Phosphorylation of the calmodulin binding domain of the plasma membrane Ca2+ pump by protein kinase C reduces its interaction with calmodulin and with its pump receptor site. J Biol Chem. 1994;269:24298–24303. [PubMed] [Google Scholar]
- Hong B, Ichida A, Wang Y, Gens JS, Pickard BG, Harper JF. Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 1999;119:1165–1176. doi: 10.1104/pp.119.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Berkelman T, Franklin AE, Hoffman NE. Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope (abstract) (published erratum: Proc Natl Acad Sci USA [1994] 91: 9664) Proc Natl Acad Sci USA. 1993;90:10066–10070. doi: 10.1073/pnas.90.21.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström S, Askerlund P, Palmgren MG. A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Lett. 1997;400:324–328. doi: 10.1016/s0014-5793(96)01448-2. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Hughes CA, Bennett V. Adducin regulation: definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271:25157–25166. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- Mercier J-C, Grosclaude F, Ribadeau-Dumas B. Structure primaire de la caséine αs1-bovine: séquence complète. Eur J Biochem. 1971;23:41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Olbe M, Sommarin M. The spinach plasma membrane Ca2+ pump is a 120-kDa polypeptide regulated by calmodulin-binding to a terminal region. Physiol Plant. 1998;103:35–44. [Google Scholar]
- Peng SA, Iwahori S. Localization of calcium in the cells of apical meristem during flower differentiation of Japanese pear, Pyrus pyrifolia Nakai. J Jpn Soc Hortic Sci. 1995;63:725–738. [Google Scholar]
- Pinna LA, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F, Carnelli A, De Michaelis MI. Identification of the plasma membrane Ca2+-ATPase and of its autoinhibitory domain. Plant Physiol. 1995;108:105–113. doi: 10.1104/pp.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler PA, Depéry F. Influence of unsaturated fatty acids in chloroplasts. Eur J Biochem. 1976;61:573–580. doi: 10.1111/j.1432-1033.1976.tb10052.x. [DOI] [PubMed] [Google Scholar]
- Trewavas A. Le calcium, c'est la vie: calcium makes waves. Plant Physiol. 1999;120:1–6. doi: 10.1104/pp.120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese GM, Johnson JD, Vasulka C, Haupt DM, Stumpo DJ, Blackshear PJ. Protein kinase C-mediated phosphorylation and calmodulin binding of recombinant myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein. J Biol Chem. 1994;269:9361–9367. [PubMed] [Google Scholar]
- Verma AK, Paszty K, Filoteo AG, Penniston JT, Enyedi A. Protein kinase C phosphorylates plasma membrane Ca2+ pump isoform 4a at its calmodulin binding domain. J Biol Chem. 1999;274:527–531. doi: 10.1074/jbc.274.1.527. [DOI] [PubMed] [Google Scholar]
- Wettenhall REH, Aebersold RH, Hood LE. Solid-phase sequencing of 32P-labeled phosphopeptides at picomole and subpicomole levels. Methods Enzymol. 1991;201:186–199. doi: 10.1016/0076-6879(91)01017-v. [DOI] [PubMed] [Google Scholar]
- Wimmers LE, Ewing NN, Bennett AB. Higher plant Ca2+-ATPase: primary structure and regulation of mRNA abundance by salt. Proc Natl Acad Sci USA. 1992;89:9205–9209. doi: 10.1073/pnas.89.19.9205. [DOI] [PMC free article] [PubMed] [Google Scholar]