Abstract
DNA methylation is the most studied epigenetic modification with a wide range of regulatory functions in mammalian genomes. It almost exclusively resides on CpG dinucleotides and, among others, plays important roles in early embryo development, onset, and maintenance of cancer. During the past 3 decades, many approaches have been developed to discriminate methylated from unmethylated DNA including antibody-based enrichment of methylated DNA, restriction enzyme-based, or hybridization-based methods. The conversion of unmethylated cytosines to uracils by sodium or ammonium bisulfite is regarded as golden standard as this approach requires no enzymatic reaction and provides deep and reliable insight in methylation patterns at single-base resolution. Nowadays, there are many commercial kits for bisulfite conversion available but they perform differently and also vary in protocols and chemicals used. Here, we provide the first comprehensive and comparative evaluation of bisulfite conversion kits observing major differences in conversion efficiency and DNA degradation which greatly affect the performance of downstream applications, ie, polymerase chain reactions (PCRs). Moreover, deep sequencing of amplicons containing oxidized derivates of 5ʹ-methylC shows that none of the tested kits efficiently converts 5ʹ-formylC without substantial conversion of 5ʹ-methylC or 5ʹ-hydroxymethylC. Consequently, we developed a robust and easy-to-use protocol that allows maximal discrimination between 5ʹ-formylC and 5ʹ-methylC/5ʹ-hydroxymethylC with low DNA degradation and high PCR efficiency on the bisulfite-treated DNA. We highly recommend to use our time- and cost-efficient protocol for any genome-wide or local high-resolution bisulfite sequencing application to minimize conversion-dependent error rates.
Keywords: Bisulfite conversion, DNA methylation, 5ʹ-formylcytosine
Introduction
DNA methylation is one of the best studied epigenetic mechanisms with functional implications on genome stability, cell differentiation, and transcriptional activity.1 Complex diseases such as cancer are closely linked to aberrant DNA methylation patterns characteristic for establishment and maintenance of malignant cellular features.2 Numerous approaches evolved during the past 30 years to identify and characterize DNA methylation patterns such as southern blot, methylation-specific polymerase chain reaction (PCR), and methylated DNA immunoprecipitation.3–5 The method with the highest resolution, however, is based on chemical modification of unmethylated cytosines to uracil by sodium metabisulfite leaving methylated cytosines unchanged.6 Thus, the epigenetic information can be decrypted and visualized by standard Sanger or next-generation sequencing–based approaches. However, the reliability of the method is highly dependent on the composition and the pH of the sulfonation reaction leading to variations in conversion efficiency and discriminative power of the treatment. Over the years, treatment protocols were adapted and improved, ie, increasing the conversion efficiency of unmethylated cytosines with a simultaneous decrease in DNA degradation. Several easy-to-use kits were developed with the goal to simplify and standardize bisulfite treatment to make it reliable and reproducible. With the discovery of oxidized forms of 5ʹ-methylC present in low but significant amounts in embryonic stem cells and neurons,7–10 the discrimination between different modified bases by sequencing became more challenging. It was shown that after the sulfonation reaction, 5ʹ-hydroxymethylC was not deaminated, whereas it is believed that 5ʹ-formyl- and 5ʹ-carboxyCs are fully converted to uracils.11–14 Song and colleagues developed a protocol to identify 5ʹ-formylC at single-base resolution by specifically aminating 5ʹ-formylC to protect it from being bisulfite converted.15 They used the EpiTect Bisulfite Kit (Qiagen, Hilden, Germany) and mentioned briefly in the paper’s supplementary part that they had to conduct two successive sulfonation cycles on the DNA to fully convert the unprotected 5ʹ-formylC, however, with unknown impact on DNA integrity. Hence, the behavior of oxidized 5ʹ-methylC derivates in bisulfite treatment is an issue making the comprehensive testing of commonly used commercial kits necessary. First efforts to test commercially available bisulfite conversion kits were made recently by three studies, however, either with testing only four kits or with conflicting commercial interests and no assessment of oxidized 5ʹ-methylC derivates.16–18 Here, we present a comprehensive and comparative testing of commonly used bisulfite conversion kits and assessed DNA integrity, sensitivity, and consistency in subsequent PCR reactions and conversion efficiencies on all known cytosine derivates. Due to the lack of an optimal commercially available solution, we developed a new, easy-to-use, and efficient protocol (OPTI-Bisulfite) to obtain the best discriminative power with the lowest error rate and the optimal compromise between conversion, robustness, sensitivity, and DNA degradation. Because of its consistency and reliability, OPTI-Bisulfite can be used for most of the applications, including genome-wide DNA methylation profiling.
Materials and Methods
Chromosomal DNA from human peripheral blood was extracted using the salting-out method.19 DNA aliquots containing 500, 100, 50, 25, 10, 5, and 1 ng DNA, respectively, were treated with bisulfite using different kits according to the manufacturer’s instructions. Bisulfite-treated DNA from the 500 ng aliquot was subjected to Bioanalyzer (Agilent, Santa Clara, CA, USA) using the Agilent RNA 6000 Pico Kit. About 10% of obtained bisulfite-treated DNA were used as template for PCR. The PCR conditions were as follows: 2.5 mM MgCl2, 0.2 mM of each dNTP, 200 nm each of the forward and reverse primers (A1: 5ʹ-TTGGAGAATATATGTTGGTTTAGAAGGA-3ʹ, 5ʹ-TCCCACACAAAACAACCTACAACTA-3ʹ; A2: 5ʹ-GGTAGTTTAGAGGTAAGGTGGGTTTTAT-3ʹ, 5ʹ-ACATTTACCAACCCCATTAAACTACTAA-3ʹ; A3: 5ʹ-GGGGAATTTATTTTTTTTAAGGTAGTTT-3ʹ, 5ʹ-CCTACCTCAACACTAAAACTAAAAACAA-3ʹ) in BD buffer (80 mM Tris-HCL, 20 mM (NH4)2SO4) and 3U HotFirePol (Solis BioDyne, Tartu, Estonia). For A3 primer pair, Qiagen reaction buffer and 1.5U HotStarTaq (Qiagen) were used. For each DNA lot, PCR reactions were performed in triplicates starting with 15 minutes denaturation at 95°C followed by 40 cycles of 95°C for 60 seconds, 58°C for 60 seconds, 72°C for 60 seconds, and a final 5-minute extension step at 72°C. About 10 µL PCR reaction were loaded on 1.2% agarose gels. Electrophoresis, staining, and documentation were always performed in the very same way. Evaluation of PCR product yield was achieved by measuring band intensities with the ImageJ software.
To assess the conversion efficiency, we used pGEM-T plasmid with an insert (231 bp [base pairs]) cloned into the polylinker and containing 15 cytosines in one of the DNA strands. The fragment was amplified using the primer pair matching to the flanking regions. The PCR mix contained dCTP, 5ʹ-methyl-dCTP, 5ʹ-hydroxymethyl-dCTP, 5ʹ-formyl-dCTP, and 5ʹ-carboxy-dCTP, in combination with dATP, dGTP, and dTTP. After purification, 5 ng of those amplicons were treated with bisulfite using different bisulfite conversion kits according to the manufacturer’s instructions. About 10% of each bisulfite-treated amplicon were used as template in a 30 µL PCR mix: buffer BD (80 mM Tris-HCL, 20 mM (NH4)2SO4), 2.5 mM MgCl2, 0.2 mM of each dNTP, 200 nm each of the forward and reverse primers (5ʹ-GAATTTGGGTTTTAAAGTTTTTTGTTT-3ʹ, 5ʹ-CACCCATATCCCTTACCCACTA-3ʹ, with universal Illumina adapters attached at the 5ʹ end), and 3U HotFirePol (Solis BioDyne). For each DNA lot, PCR reactions were performed in triplicates starting with 15 minutes denaturation at 95°C followed by 35 cycles of 95°C for 60 seconds, 54.5°C for 30 seconds, 72°C for 40 seconds, and a final 5-minute extension step at 72°C. About 5 µL PCR reaction were loaded on 1.2% agarose gels. Remaining 25 µL of the PCR reaction were purified with Agencourt Ampure XP beads (Beckman Coulter, Krefeld, Germany), diluted, pooled, and sequenced (Illumina v3 chemistry, 2 × 300 bp paired end) on Illumina MiSeq following the manufacturer’s instructions. Alignments and evaluation were done with the BiQ Analyzer HiMod program filtering all reads with more than 10% unrecognized CpG sites.20
A detailed protocol for OPTI-Bisulfite is provided in Supplementary File 1. Briefly, 94 µL bisulfite solution (3.075 M sodium metabisulfite (Merck, Darmstadt, Germany), 0.5379 M NaOH, and 37 µL Trolox solution (9.3435 M Trolox; Sigma-Aldrich, St. Louis, MO, USA) in 1,4-dioxane (Sigma-Aldrich)) were mixed and added to 10 µL of DNA in 0.2-mL tubes. Tubes were placed into the thermal block which accepts the reaction volumes up to 100 µL and subjected to 99°C for 15 minutes and 50°C for 30 minutes followed by 2 cycles of 99°C for 5 minutes, and 50°C for 90 minutes. Purification and desulfonation were conducted using Zymo-Spin IC columns and buffers from the EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. Standard bisulfite-DNA elution volume was set to 20 µL.
Results
The following bisulfite conversion kits were chosen for testing: EZ DNA Methylation Kit, EZ DNA Methylation-Gold Kit, EZ DNA Methylation-Lightning Kit (all from Zymo Research); EpiTect Bisulfite Kit, EpiTect Fast Bisulfite Kit (both from Qiagen); innuCONVERT Bisulfite Basic Kit (Analytik Jena, Jena, Germany); TrueMethyl Seq Kit (Cambridge Epigenetics, CEGX, Essex, UK); and the Epi proColon 2.0 Kit (Epigenomics AG, Berlin, Germany). The tested kits were selected based on the frequency of usage in the international epigenetics community (the most commonly used kits offered by the two worldwide leading companies in this field are sold by Qiagen and Zymo Research). InnuCONVERT Bisulfite Basic Kit (Analytik Jena) was chosen based on contradictory results when amplifying larger sequence stretches. In addition, we included TrueMethyl Seq Kit (CEGX) because the bisulfite treatment procedure is coupled with an oxidative DNA treatment to identify 5-hmC at single-base resolution in whole genome sequencing experiments.12 Finally, the Epi proColon 2.0 Kit (Epigenomics) is a diagnostic kit that detects trace amounts of cell-free floating methylated DNA in blood plasma samples to support non-invasive early diagnosis of colorectal cancer. The included bisulfite treatment protocol and reagents were recently independently commercialized as Epi BiSKit (Epigenomics).
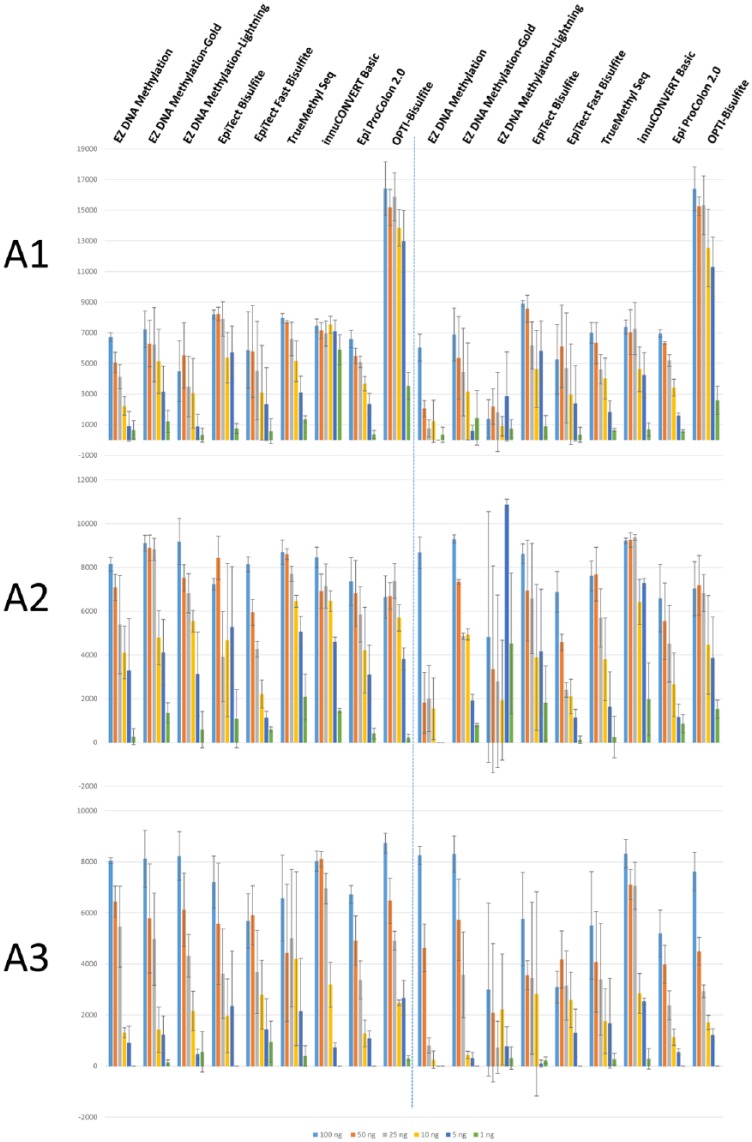
Chromosomal DNA was treated with bisulfite according to the kit manufacturer’s instructions. To obtain a comprehensive picture on genomic DNA degradation caused by low pH in the sulfonation reactions, DNA integrity was analyzed on Bioanalyzer (Figure 1). We revealed remarkable differences in the degree of DNA fragmentation caused by bisulfite treatment using different kits: rather small fragments were obtained after using EpiTect Fast (between 50 and 800 bp), EZ DNA Methylation-Lightning (50-500 bp), and innuCONVERT Bisulfite Basic (50-600 bp) kits. The lowest DNA degradation was observed with the Epi proColon 2.0 (>4000 bp) and EpiTect Bisulfite kits (equal distribution of fragments between 1000 and 5000 bp; Figure 1A). Because these surprisingly high differences in fragment sizes after bisulfite treatment might have a major impact on subsequent PCR reactions, we scaled down the amount of DNA prior to bisulfite reaction to 100, 50, 25, 10, 5, and 1 ng. Three different regions have been selected for bisulfite-specific PCR: (1) promoter region of BTC (A1, highly efficient PCR with high yield in PCR product), (2) intron 5 region of BTC (A2, medium efficiency and product yield), and (3) DNMT3A (A3, low efficiency and product yield). After performing PCRs in triplicates independent from each other, equal amounts of PCR reactions were loaded on agarose gels. Figure 2 shows the results of band intensity quantification. Reliable amplification of A1 was obtained with all analyzed kits when using as low as 25 ng chromosomal DNA. Efficient amplification of A1 PCR product was achieved after treatment with EpiTect Bisulfite, EZ DNA Methylation-Gold, TrueMethyl Seq, innuCONVERT Basic, and Epi proColon 2.0 kits using as low as 1 ng genomic DNA. Worst results were obtained for A1 when using EpiTect Fast Bisulfite and EZ DNA Methylation-Lightning kits which is in line with the low DNA integrity after using these kits. A2 could be reliably amplified with only 5 ng input DNA by all tested kits. With 1 ng of input DNA, a weak PCR product from the A2 region could only be consistently amplified with the EZ DNA Methylation-Gold, EpiTect Fast Bisulfite, TrueMethyl Seq, innuCONVERT Bisulfite Basic, and Epi proColon 2.0 kits. For A3, all tested kits resulted in faint or missing PCR products when 5 ng or less of DNA were used as input. Here, the best performance was obtained with EZ DNA Methylation-Gold, EpiFast Bisulfite, and TrueMethyl Seq kits (weak but consistent PCR products even with 1 ng input DNA, see also Supplementary Figure 1).
Figure 1.
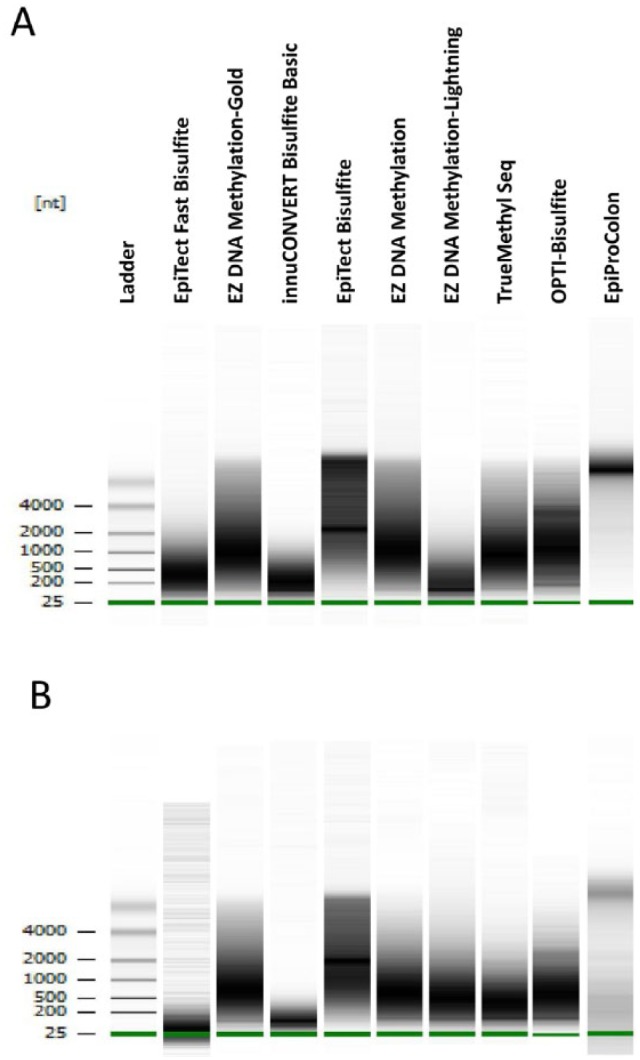
Bioanalyzer profiles of bisulfite-converted DNA after treating 500 ng of genomic DNA with different bisulfite kits/protocols; shown is the fragment size distribution in the range between 25 and more than 4000 bp depending on the kit/protocol used. Bisulfite treatment with (A) one sulfonation cycle and (B) two sulfonation cycles.
Figure 2.
Amplicon yield after treating different amounts of genomic DNA (100, 50, 25, 10, 5, 1 ng) with different bisulfite conversion kits/protocols and subsequent bisulfite-specific PCR; shown is the averaged band intensity over background with standard deviation from 3 independent PCR reactions; left: one sulfonation cycle, right: two sulfonation cycles; (A1) high PCR efficiency with high product yield, (A2) medium PCR efficiency and product yield, and (A3) low efficiency and product yield.
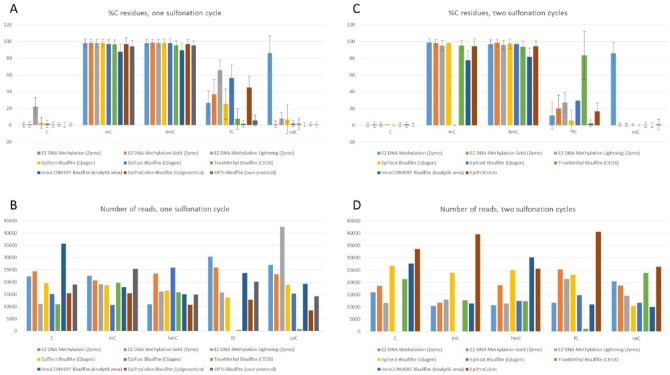
Next, we studied the chemical impact of the bisulfite reagents on unmethylated and methylated cytosines taking also the oxidized 5ʹ-methylC derivates into account. We created a bisulfite amplicon that harbors 15 cytosines in the top strand and cloned it into pGEM-T vector. The plasmid was used as template for PCRs replacing the dCTP nucleotide by 5ʹ-methyl-dCTP, 5ʹ-hydroxymethyl-dCTP, 5ʹ-formyl-dCTP, or 5ʹ-carboxy-dCTP. Purified amplicons were treated with different bisulfite kits, reamplified, and deep sequenced on the Illumina MiSeq machine. According to published reports, the full conversion of 5ʹ-formylC was only achieved when using the EpiTect Bisulfite Kit and two successive sulfonation cycles.15 We therefore included an experimental series on all selected kits performing two sulfonation reactions prior to desulfonation and purification. As shown in Figure 3, even after single sulfonation treatment, most of the kits managed to convert the unmethylated cytosine to uracil with high efficiency (~1% cytosines left after treatment). EpiTect Bisulfite and EpiTect Fast Bisulfite kits were less efficient (3% and 2% cytosines left, respectively), and EZ DNA Methylation-Lightning Kit leaves 23% of cytosines within our test template unconverted. When performing two sulfonation cycles, all tested kits made it down to approximately 1% remaining (unconverted) cytosines. According to manufacturer’s data, 5ʹ-methylC and 5ʹ-hydroxymethylC should not be affected by the treatment, which was actually not the case for any kit. Approximately 2% of 5ʹ-methylC and 5ʹ-hydroxymethyC were converted with the EZ DNA Methylation, EZ DNA Methylation-Gold, EZ DNA Methylation-Lightning, and EpiTect Bisulfite kits. Unwanted conversion was even higher when EpiTect Fast Bisulfite, TrueMethyl Seq, and Epi proColon 2.0 kits were used (~3% converted 5ʹ-methyl-/5ʹ-hydroxymethylCs). With 13% of unwanted conversion of 5ʹ-methyl-/5ʹ-hydroxymethylCs, the innuCONVERT Bisulfite Basic Kit (single sulfonation) performs worst. With two sulfonation cycles, the unwanted conversion of 5ʹ-methyl- and 5ʹ-hydroxymethylC ranges between 22% (innuCONVERT Bisulfite Basic Kit) and 1% (EZ DNA Methylation Kit). The most heterogeneous results were obtained when 5ʹ-formylC–containing fragments served as templates. Highest conversion rates were obtained with innuCONVERT Bisulfite Basic and TrueMethyl Seq kits (2% and 7% remaining 5ʹ-formylCs, respectively) or with the EpiTect Bisulfite Kit when running two sulfonation cycles (5% remaining 5ʹ-formylCs). EZ DNA Methylation-Lightning and EpiTect Fast Bisulfite kits performed worst (65% and 56% remaining 5ʹ-formylC residues). The conversion of 5ʹ-carboxyCs to uracils appears to be more efficient. Only EZ DNA Methylation-Lightning and EpiTect Bisulfite kits show 8% and 7% remaining 5ʹ-carboxyCs, respectively, which are gone after two sulfonation cycles. Interestingly, the EZ DNA Methylation Kit was able to convert only 14% of 5ʹ-carboxyCs which remained even after the second sulfonation cycle. Figure 1B shows that two successive sulfonation cycles induce more DNA degradation because of longer incubation times at low pH being strongest when using EpiTect Fast Bisulfite and innuCONVERT Bisulfite Basic kits. This potentially leads to low efficiencies in downstream applications (eg, PCRs; Figure 2) with an impact on sequencing depth and equal read distribution. In fact, read numbers deviate between 10 000 and 20 000 for most of the sequenced amplicons, however, with TrueMethyl Seq and innuCONVERT Bisulfite Basic kits among those with comparably low read numbers. With Epi proColon 2.0 and EpiTect Bisulfite kits, in average, high read numbers were obtained (Figure 3). Although all amplicons are measured and normalized after purification and before sequencing, a priori lower amounts of PCR product led to less efficient cluster formation on the MiSeq flow cell.
Figure 3.
Local deep sequencing of a bisulfite amplicon containing 15 unmethylated (C), methylated (mC), hydroxymethylated (hC), formylated (fC), or carboxylated (cC) cytosines, respectively. (A) Average amplicon methylation given as percent of remaining C residues after bisulfite treatment with one sulfonation cycle; (B) number of analyzed reads obtained with one sulfonation cycle; (C) A with two sulfonation cycles; and (D) B with two sulfonation cycles. Standard deviation represents the variation in percent of remaining C residues across the 15 respective positions. Amplicon data of the EpiTect Fast Bisulfite Kit with two sulfonation cycles are missing for cytosine and 5ʹ-methylC because no amplicon was obtained after polymerase chain reaction.
In conclusion, the efficient conversion of 5ʹ-formylC was always achieved at the cost of significant unwanted conversion of 5ʹ-methylC and 5ʹ-hydroxymethylC and higher DNA degradation. The best results were obtained with the EpiTect Bisulfite Kit with two rounds of sulfonation, however, harming DNA integrity and time-/cost-efficiency. We decided to put effort into the development of a new bisulfite conversion protocol, which we named OPTI-Bisulfite aiming at the best compromise between conversion efficiency, DNA degradation, and time-/cost-efficiency. The sulfonation reaction according to the OPTI-Bisulfite protocol is performed in 1.922 M sodium metabisulfite, 0.3362 M NaOH and 2.92 M Trolox (antioxidant) to reach the optimal pH of 5.2. This reaction mix is subjected to the temperature cycling as follows: 15 minutes at 99°C, 30 minutes at 50°C followed by two cycles of 5 minutes at 99°C, and 90 minutes at 50°C. Purification and desulfonation are conducted using Zymo-Spin IC columns and buffers from the EZ DNA Methylation Kit to reach the highest treatment consistency and sample purity. Thanks to the added antioxidant Trolox, DNA integrity in OPTI-Bisulfite was improved and proved to be superior to most of the kits tested, with DNA fragment sizes varying between 200 and 4000 bp peaking at approximately 1500 bp (Figure 1A). Consistency in amplification was achieved even when using as low as 1 ng of genomic DNA for A1, A2, and A3. Conversion efficiency of unmethylated cytosines was similar to most of the analyzed commercial kits (less than 1% cytosines remaining), conversion of 5ʹ-formylC was achieved with highest possible efficiency (3% 5ʹ-formylCs remaining) with rather low unwanted conversion of 5ʹ-methyl and 5ʹ-hydroxymethylC (3%-5% converted 5ʹ-methyl/5ʹ-hydroxymethylCs). 5ʹ-carboxyCs were fully converted to uracils.
Discussion
Here, we present the first comprehensive comparison of widely used commercially available bisulfite conversion kits. We assessed DNA degradation, sensitivity, and robustness of subsequent PCR reactions and cytosine to uracil conversion efficiencies on all cytosine derivates which are present in mammalian DNA. For standard bisulfite sequencing applications with unlimited sample material, DNA conversion, integrity, and amplifyability were acceptable with most of the kits, showing the best results with EZ DNA Methylation-Gold and the diagnostic kit Epi proColon 2.0. As all the commercial kits were shown to have their pros and cons, the choice which kit to use is a matter of downstream application. Of particular importance is the very heterogeneous conversion efficiency of 5ʹ-formylC that can give rise to high false-positive rates in high-throughput deep bisulfite sequencing, in particular, when analyzing embryonic stem cells or certain brain areas.7,10 Remarkably, 5ʹ-carboxyC is almost not converted by the EZ DNA Methylation Kit which may also lead to false-positive 5ʹ-methylC detection.7–10 5ʹ-formylC was well converted with the EpiTect Bisulfite Kit when conducting two successive sulfonation cycles with an acceptable compromise between conversion of 5ʹ-formylC and presence of unconverted 5ʹ-methylC and 5ʹ-hydroxymethylC. This result confirms EpiTect Bisulfite Kit as the best commercially available choice to be used in fCAB and MAB-Seq experiments.7,13,15 However, longer exposure of DNA to low pH leads to increased DNA degradation (Figure 1) and less reliable PCR outcomes (Figure 2) making a shorter and more protective treatment eligible. Our fast, cost-efficient, reliable, and easy-to-use protocol converts cytosines, 5ʹ-carboxyCs, and even 5ʹ-formylCs efficiently and, at the same time, protects DNA from being massively degraded. Consistent amplification of PCR products with as low as 1 ng of input DNA was achieved, even when difficult genomic regions were targeted. Reducing the number of false positives and false negatives in future high-resolution sequencing approaches is of particular importance when sample input is low.21–23 The reliable detection of rare cytosine derivates in oxBs or CAB-Seq experiments is highly dependent on the discriminatory power between convertible and protected cytosine derivates during bisulfite treatment.12,15,24 In this regard, OPTI-Bisulfite may allow the most reliable detection of rare bases with lowest error rates.
Supplemental Material
Supplemental material, 766097_Supplemental_Fig.1 for Comprehensive Evaluation of Commercial Bisulfite-Based DNA Methylation Kits and Development of an Alternative Protocol With Improved Conversion Performance by Sascha Tierling, Beate Schmitt and Jörn Walter in Genetics & Epigenetics
Supplemental Material
Supplemental material, 766097_supplementary_file_1 for Comprehensive Evaluation of Commercial Bisulfite-Based DNA Methylation Kits and Development of an Alternative Protocol With Improved Conversion Performance by Sascha Tierling, Beate Schmitt and Jörn Walter in Genetics & Epigenetics
Acknowledgments
The authors thank Christina Lo Porto and Jasmin Kirch for excellent technical assistance and specially thank Konstantin Lepikhov for critical reading of the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the German Epigenome Programme (DEEP) of the Federal Ministry of Education and Research in Germany (BMBF) (01KU1216).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ST conceived and designed the study, analyzed all PCR and deep sequencing-related data, and drafted the manuscript. BS performed the wet lab experiments and helped with study design. JW participated in the design and the research coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Disclosures and Ethics: The material presented in this paper has not been published before nor has it been submitted for publication to another scientific journal or is being considered for publication elsewhere. This work has been approved by all co-authors.
References
- 1. Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. [DOI] [PubMed] [Google Scholar]
- 2. Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. [DOI] [PubMed] [Google Scholar]
- 3. Ponzetto-Zimmerman C, Wolgemuth DJ. Methylation of satellite sequences in mouse spermatogenic and somatic DNAs. Nucleic Acids Res. 1984;12:2807–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson IM, Davies JJ, Weber M, et al. Epigenomics: mapping the methylome. Cell Cycle. 2006;5:155–158. [DOI] [PubMed] [Google Scholar]
- 6. Frommer M, McDonald LE, Millar DS, et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iurlaro M, McInroy GR, Burgess HE, et al. In vivo genome-wide profiling reveals a tissue-specific role for 5-formylcytosine. Genome Biol. 2016;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi I, Kim R, Lim HW, Kaestner KH, Won KJ. 5-hydroxymethylcytosine represses the activity of enhancers in embryonic stem cells: a new epigenetic signature for gene regulation. BMC Genomics. 2014;15:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner M, Steinbacher J, Kraus TF, et al. Age-dependent levels of 5-methyl-, 5-hydroxymethyl-, and 5-formylcytosine in human and mouse brain tissues. Angew Chem Int Ed Engl. 2015;54:12511–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONE. 2010;5:e8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Booth MJ, Branco MR, Ficz G, et al. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science. 2012;336:934–937. [DOI] [PubMed] [Google Scholar]
- 13. Wu H, Wu X, Shen L, Zhang Y. Single-base resolution analysis of active DNA demethylation using methylase-assisted bisulfite sequencing. Nat Biotechnol. 2014;32:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neri F, Incarnato D, Krepelova A, Parlato C, Oliviero S. Methylation-assisted bisulfite sequencing to simultaneously map 5fC and 5caC on a genome-wide scale for DNA demethylation analysis. Nat Protoc. 2016;11:1191–1205. [DOI] [PubMed] [Google Scholar]
- 15. Song CX, Szulwach KE, Dai Q , et al. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell. 2013;153:678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes EE, Jung M, Meller S, et al. Performance evaluation of kits for bisulfite-conversion of DNA from tissues, cell lines, FFPE tissues, aspirates, lavages, effusions, plasma, serum, and urine. PLoS ONE. 2014;9:e93933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leontiou CA, Hadjidaniel MD, Mina P, Antoniou P, Ioannides M, Patsalis PC. Bisulfite conversion of DNA: performance comparison of different kits and methylation quantitation of epigenetic biomarkers that have the potential to be used in non-invasive prenatal testing. PLoS ONE. 2015;10:e0135058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dietrich D. DNA methylation analysis from body fluids. Methods Mol Biol. 2018;1655:239–249. [DOI] [PubMed] [Google Scholar]
- 19. Lahiri DK, Bye S, Nurnberger JI, Hodes ME, Crisp M. A non-organic and non-enzymatic extraction method gives higher yields of genomic DNA from whole-blood samples than do nine other methods tested. J Biochem Biophys Methods. 1992;25:193–205. [DOI] [PubMed] [Google Scholar]
- 20. Becker D, Lutsik P, Ebert P, Bock C, Lengauer T, Walter J. BiQ analyzer HiMod: an interactive software tool for high-throughput locus-specific analysis of 5-methylcytosine and its oxidized derivatives. Nucleic Acids Res. 2014;42:W501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smallwood SA, Lee HJ, Angermueller C, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11:817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gravina S, Dong X, Yu B, Vijg J. Single-cell genome-wide bisulfite sequencing uncovers extensive heterogeneity in the mouse liver methylome. Genome Biol. 2016;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang K, Li X, Dong S, et al. Q-RRBS: a quantitative reduced representation bisulfite sequencing method for single-cell methylome analyses. Epigenetics. 2015;10:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Booth MJ, Ost TW, Beraldi D, et al. Oxidative bisulfite sequencing of 5-methylcytosine and 5-hydroxymethylcytosine. Nat Protoc. 2013;8:1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 766097_Supplemental_Fig.1 for Comprehensive Evaluation of Commercial Bisulfite-Based DNA Methylation Kits and Development of an Alternative Protocol With Improved Conversion Performance by Sascha Tierling, Beate Schmitt and Jörn Walter in Genetics & Epigenetics
Supplemental material, 766097_supplementary_file_1 for Comprehensive Evaluation of Commercial Bisulfite-Based DNA Methylation Kits and Development of an Alternative Protocol With Improved Conversion Performance by Sascha Tierling, Beate Schmitt and Jörn Walter in Genetics & Epigenetics