Abstract
This study investigated the effects of varying photoperiodic conditions on critical life stages’ parameters of Culex quinquefasciatus. To this end, first larval stage was reared under different constant photoperiodic regimens: 0, 6 (short), 12 (equal), 13 (prevailing condition), and 18 and 24 (long) hours of light (hL). Duration of development, survivorship, emergence successes, adult longevity, caloric indices (CIs), and utilisation of teneral reserves for metamorphosis at each regimen were monitored. Analyses revealed significant negative effects of increasing photoperiod on all entomological variables measured. Short photo-phases elicited faster development times, increased life stages’ survivorship and number at emergence, adult longevity, and CI for all life stages while increasing teneral components for adult life traits. The information generated in this study is important in understanding the role played by photoperiod in disease transmission and for development of integrated vector control strategies based on environmental manipulation.
Keywords: Physiology, post-emergence, longevity, metabolic reserve, lipid, protein, sugars, vectorial fitness
Introduction
Photoperiod is the earth’s naturally recurring alternation of duration of light and darkness1; it is probably the most studied abiotic factor that influences growth and development in insects.2–4 Usually, variations in daylength serve as signals for changes in environmental conditions, and insects are capable of detecting the changes heralded long before actual arrival.5–7 These allow insects to make appropriate physiological and behavioural responses in anticipation for these conditions.8
In the Tropics, for example, especially, in the study area, short daylength precedes the onset of wet/rainy season (April to October), whereas long daylength heralds dry season (November to March), accompanying these seasons are changes in environmental conditions that affect the bionomics of insects. Meanwhile, studies have revealed differential responses of insects to photoperiodic conditions9 and even among same species located across different latitudes.8
For some insect species, growth and development is faster under short daylength (12 or fewer hours of light),9,10 whereas in others, development is almost halted. A few insects are, however, indifferent to variations in daylength, whereas for other species, the converse is true.2,11 Therefore, information on the appropriate biological and physiological responses of insect species to variation in photoperiod is valuable in developing cost-effective control protocols, especially for medical insect pests, eg, Culex quinquefasciatus mosquitoes.
Culex quinquefasciatus is the common house mosquito, medically important in the transmission of debilitating human and zoonotic diseases.12 It breeds in almost all water habitats near and far from human dwellings.13 Bites from infected females of this mosquito species transmit Wuchereria bancrofti, a pathogen responsible for over 90% of all cases of lymphatic filariasis. More than 856 million people in 52 countries worldwide and more than 120 million people in Nigeria are at risk of the disease.14
Control of the vector has been majorly using chemicals; this method, although effective, has not been potent in ameliorating the disease burden caused by this vector. Information on effects of environmental manipulation on critical entomological indices may provide an alternative strategy in the control of this vector. Critical entomological indices that determine the successes and fitness of mosquitoes include rates of development,11,12 post-emergence successes,15,16 energy availability at critical life stages,17–19 and teneral availability and utilisation for metamorphosis.20,21 Hence, empirical data on the effects of ranges of photoperiodic conditions on these indices are vital in developing vector management protocols.
Although earlier studies have reported responses of mosquitoes to various quantitative photoperiodic regimen, few or none reported responses of C quinquefasciatus to a wide range of constant photoperiodic regimen and effects on pre-imaginal development and post-imaginal fitness traits. More so, quantitative data on ideal photoperiodic regimens for development of the species are non-existent in the study area. Therefore, this study was designed to demonstrate the effects of varied photoperiodic regimen on duration of development, immature survivorship, adult emergence rates, post-emergence survivorship and longevity, energy indices, and utilisation of teneral reserve for metamorphosis in C quinquefasciatus mosquito, in a bid to develop robust and well-informed strategies for control of the disease vector.
Materials and Methods
Source and maintenance of mosquito
Freshly laid egg rafts of C quinquefasciatus mosquito were collected from a colony established in the Entomology Unit of the Department of Biological Sciences, Federal University of Technology, Minna. The insectary was maintained at 12:12 L:D hours, with mean temperature and relative humidity at 28.00°C ± 1.00°C, and 80.16% ± 4.26%, respectively. Collected egg rafts were placed in plastic hatching trays for incubation at ambient room temperature (for 24 hours) as described earlier.15 Hatched larvae were cultured according to standard protocols in well-labelled plastic trays (30 cm × 25 cm × 5 cm), at the rate of 1 larva/4 mL of water.12 The larvae were fed with fish feed (Coppens), sprinkled, gently, over on the water surface, at the rate of 0.32 mg/100 larvae every other day. Daily change of rearing water prevented accumulation of debris and formation of scum. Pupal stages were separated into labelled bowls for emergence in adult-holding cages, and the emerged adults fed on 10% sucrose solution dabbed in sterile cotton wool.22
Simulation of photoperiodic regimens
Fluorescent tubes, with intensity kept constant at 250 to 300 lx provided constant photoperiodic regimens of 0:24 (zero, 0 hours of light, hL), 6:18 (short, 6 hL), 12:12 (equal, 12 hL), 18:6 (long, 18 hL), and 24:0 (long, 24 hL) light:dark (L:D) hours.20 To obtain a standard (‘Control’) for comparison, the prevailing photoperiodic condition (13:11 L:D hours, 13 hL) during the study period was adopted. The experimental setup involved the exposure of the mosquitoes in 4 replicates of 50 larvae/bowl, and the whole study was repeated immediately after the first experimentation.
Developmental indices
Development indices adopted in this study were duration and survivorship of immature life stages (ie, Larval instars L1-4 and pupa). Duration of development of immature stages was the time taken for a life stage to change into the next and expressed in days.15 Computation of duration of development was calculated by the following formula:
where Di is the duration of life stage, Ti is the present mean age, and ti − 1 is the previous mean age at moulting.
Survival rates during the immature life stages were the percentage of mosquitoes at the beginning of a life stage that successfully entered the next stage22:
where Si is the survival rates in instar stage i; ni is the numbers of larvae entering instar stage i, and ni − 1 is the number of larva that entered the preceding instar stage.
Adult emergence sex ratio and post-emergence longevity of adult
Total number of emergent adults and sex ratio was determined by counting the numbers of adult male and female mosquitoes at eclosion. Longevity and daily survivorship were determined by allowing the adult mosquitoes to live out their lives while fed only 10% sugar and expressed in days and percentages, respectively.15
Biochemical analyses
Quantification of teneral reserve components (ie, lipid, glycogen, glucose, and protein) at the final larval instar, pupal, and adult life stages of the mosquito was carried out in triplicates according to the methods described by Van-Handel and Day23 and Kaufmann and Brown.24 Values obtained were expressed as mean ± SD in micrograms per mosquito.
Teneral reserve components used during metamorphosis in C quinquefasciatus
Teneral reserve components used for metamorphosis were calculated as difference between teneral composition at the final larval instar and pupal stages (for quantity used for pupation) and between the pupal and adult stages (for quantity used for eclosion).
Caloric indices of life stages of C quinquefasciatus
The caloric index (CI) per individual for each teneral component was determined as follows: the different contents of total proteins, lipids, and carbohydrates were converted into calories and values expressed as mean ± SD in calories. One calorie corresponds to 0.004 μg of carbohydrates and proteins, whereas for lipids, 1 calorie corresponds to 0.009 μg.17
Data analysis
A goodness of fit was used to test the data before analyses. Data from immature developmental and post-emergence success attributes, teneral reserve utilisation for metamorphosis (pupation and eclosion), and CIs were normally distributed and analysed using appropriate statistical tool. For example, differences between means of any 2 entomologic variables (eg, between sexes of mosquito in a photoperiodic treatment) were compared using Student t test, whereas those among entomologic variables (eg, among mosquitoes in photoperiodic regimens) were compared for significant difference using 1-way and 2-way analysis of variance as appropriate. All values were expressed as mean ± SD and decisions on statistical comparison of means were taken at P < .05 level of significance. The means were separated using Duncan multiple range test.
Results
Effects of varying photoperiodic conditions on duration of development of C quinquefasciatus mosquito
Table 1 shows the duration of development for immature life stages of C quinquefasciatus mosquito under different photoperiodic conditions. Duration of development of all immature stages varied considerably with change in daylength. From the table, it is evident that as daylength increased, duration of development of each larval stage and total larval duration, pupal stage, and total time (total immature duration) needed for development significantly increased.
Table 1.
Effects of varying photoperiodic conditions on duration (days) of development of Culex quinquefasciatus mosquito.
| Photoperiodic levels (light:dark hours) | Larval Instars |
Total larval duration | Pupal stage duration | Total immature duration | |||
|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||||
| 0:24 | 0.79 ± 0.14Aa | 1.15 ± 0.12Ac | 1.04 ± 0.02Ab | 1.81 ± 0.09Ad | 4.79 ± 0.09A | 0.85 ± 0.02A | 5.64 ± 0.11A |
| 6:18 | 1.11 ± 0.17Bb | 1.29 ± 0.23Ac | 1.08 ± 0.21Aa | 2.28 ± 0.26Bd | 5.77 ± 0.28B | 0.84 ± 0.01A | 6.60 ± 0.28B |
| 12:12 | 1.30 ± 0.07Ca | 1.55 ± 0.19Bb | 1.75 ± 0.30Bc | 2.57 ± 0.20BCd | 7.18 ± 0.28C | 1.03 ± 0.16B | 8.21 ± 0.52C |
| Control (13:11) | 1.20 ± 0.03Ca | 1.59 ± 0.27Bb | 1.86 ± 0.14Bc | 2.53 ± 0.25BCd | 7.19 ± 0.28C | 1.06 ± 0.16B | 8.25 ± 0.34C |
| 18:6 | 1.90 ± 0.12Da | 2.38 ± 0.16Cb | 2.62 ± 0.37Cc | 2.79 ± 0.34Cd | 9.69 ± 0.41D | 1.03 ± 0.08B | 10.73 ± 0.46D |
| 24:0 | 2.10 ± 0.05Da | 2.66 ± 0.25Db | 3.31 ± 0.30Dc | 4.06 ± 0.41Dd | 12.14 ± 0.36E | 1.39 ± 0.16C | 13.52 ± 0.35E |
Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance (ANOVA). Within a line, means (±SD) followed by same small letter are not significantly different at P < .05 according to Duncan multiple range test following ANOVA.
Shorter durations of developments were observed when the immature life stages were reared under zero and short ranges of photoperiod (0:24 to 6:18 L:D hours). However, at relatively narrow photoperiodic ranges (12:12 to 13:11 L:D hours), duration of development increased and was longest at very long photoperiods (18:6 to 24:0 L:D hours).
Effects of varying photoperiodic conditions on survivorship of immature stages of C quinquefasciatus mosquito
Table 2 shows the effects of varying photoperiods on survivorship of immature life stages of C quinquefasciatus. There was no significant variation in the survivorship of larval stages as zero (0:24), short (6:18), and narrow ranges of photoperiods (12:12 and 13:11 L:D hours). However, as photoperiod increased to 18:6 to 24:0 L:D hours, immature survivorship reduced significantly. Similar trends, as in larval stages, were observed at the pupal stage, translating the varied immature survivorship.
Table 2.
Effects of varying photoperiod conditions on survivorship (%) of immature stages of Culex quinquefasciatus mosquito.
| Photoperiodic levels (light:dark hours) | Larval Instars |
Average larval survivorship | Pupal stage | Average immature survivorship | |||
|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||||
| 0:24 | 98.75 ± 1.16Cb | 96.24 ± 1.71Cc | 99.86 ± 0.39Cd | 97.79 ± 2.44Ca | 98.16 ± 1.44C | 99.62 ± 0.38C | 98.89 ± 1.40C |
| 6:18 | 97.13 ± 2.10Ca | 95.34 ± 4.24Ca | 96.13 ± 5.66Ca | 99.46 ± 2.25Ca | 96.26 ± 2.19C | 97.39 ± 1.89C | 96.49 ± 1.76C |
| 12:12 | 99.38 ± 0.74Cb | 91.82 ± 5.04Ca | 94.94 ± 3.14Cb | 97.19 ± 1.91Cb | 95.26 ± 2.15C | 96.28 ± 4.17C | 95.49 ± 2.03C |
| Control (13:11) | 98.13 ± 2.23Cb | 91.78 ± 5.46Ca | 93.86 ± 4.43Ca | 88.90 ± 6.47BCa | 95.24 ± 1.13C | 99.69 ± 0.86C | 96.13 ± 0.92C |
| 18:6 | 78.38 ± 5.95Bb | 63.92 ± 4.96Ba | 72.11 ± 4.89Bb | 60.39 ± 11.11Ba | 68.70 ± 3.07B | 71.60 ± 10.25B | 81.28 ± 2.37B |
| 24:0 | 51.13 ± 7.75Aa | 47.35 ± 5.87Aa | 65.32 ± 2.05Ab | 44.80 ± 3.01Aa | 52.15 ± 4.41A | 51.83 ± 2.42A | 51.99 ± 3.49A |
Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance (ANOVA). Within a line, means (±SD) followed by same small letter are not significantly different at P < .05 according to Duncan multiple range test following ANOVA.
Effects of varying photoperiodic condition on adult emergence, daily survivorship, and longevity of C quinquefasciatus mosquito
Highlighted in Tables 3 and 4 are the effects of photoperiod on adult emergence, survivorship, and longevity of C quinquefasciatus. Analyses revealed significant reduction in the values of these parameters as duration of photoperiod increased. Higher numbers of emergent adults were observed at zero (0:24), short (6:18), and narrow ranges of photoperiods (12:12 and 13:11 L:D hours), whereas very low emergence was observed at very long photoperiods (18:6 and 24:0) (Table 3). Interestingly, there were, significantly, more female than male mosquitoes in all photo-regimens (Table 4).
Table 3.
Effects of different photoperiod regimens on emergence, survivorship, and longevity of adult Culex quinquefasciatus mosquito.
| Photoperiodic levels (light:dark hours) | No. of emergent imagines |
Daily survivorship of imagines, % |
Average post-emergence longevity, d* |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Mean | Male | Female | Mean | |
| 0:24 | 23.00 ± 6.37cA | 51.00 ± 9.24cB | 74.00 ± 5.78c | 78.12 ± 4.94cA | 84.32 ± 1.24cA | 82.04 ± 1.29c | 16.07 ± 1.25cA | 21.18 ± 0.96cB | 19.54 ± 0.88c |
| 6:18 | 28.38 ± 7.41cdA | 55.25 ± 11.31cB | 83.63 ± 7.85d | 75.42 ± 4.81cA | 81.67 ± 3.98cA | 79.50 ± 3.85c | 15.05 ± 1.28bcA | 20.04 ± 2.34cB | 18.42 ± 1.91c |
| 12:12 | 30.50 ± 8.85dA | 48.38 ± 8.63cB | 78.88 ± 8.79cd | 76.04 ± 2.62cA | 81.38 ± 3.51cA | 79.90 ± 2.39c | 14.35 ± 2.13bA | 17.53 ± 1.52bB | 16.46 ± 1.55b |
| Control (13:11) | 26.50 ± 6.95cdA | 55.25 ± 5.63cB | 81.75 ± 4.17d | 77.01 ± 2.02cA | 83.70 ± 1.79cA | 81.12 ± 1.74c | 13.45 ± 0.69bA | 17.31 ± 0.64bB | 16.09 ± 0.72b |
| 18:6 | 14.88 ± 3.52bA | 19.75 ± 5.23bB | 34.63 ± 5.18b | 66.12 ± 10.65bA | 68.27 ± 8.07bA | 66.58 ± 4.78b | 8.40 ± 1.46aA | 9.93 ± 2.27aB | 9.41 ± 1.74a |
| 24:0 | 5.25 ± 2.05aA | 8.25 ± 2.38aB | 13.50 ± 3.12a | 51.69 ± 14.97aA | 56.81 ± 12.88aA | 51.50 ± 13.12a | 7.16 ± 1.97aA | 9.35 ± 1.71aB | 8.58 ± 1.83a |
Within a line, means (±SD) followed by same small letter for male and female are not significantly different at P < .05 according to Student t test analysis. Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of covariance; n = 25.
Adult fed sugar only.
Table 4.
Effects of different photoperiod regimens on percentage emergence of adult Culex quinquefasciatus mosquito.
| Photoperiodic levels (light:dark hours) | Percentage emergence |
|
|---|---|---|
| Male | Female | |
| 0:24 | 31.34 ± 9.10aA | 68.66 ± 9.10bB |
| 6:18 | 34.25 ± 10.03abA | 65.75 ± 10.03abB |
| 12:12 | 38.56 ± 9.47abA | 61.44 ± 9.47abB |
| Control (13:11) | 32.27 ± 7.73aA | 67.73 ± 7.73bB |
| 18:6 | 43.25 ± 9.53abA | 56.75 ± 9.53abB |
| 24:0 | 38.92 ± 12.33abA | 61.08 ± 12.33abB |
Within a line, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Student t test analysis. Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance.
Variation in daylength also affected daily adult survivorship of the mosquito species. For example, very long photoperiods (18:6 and 24:0) significantly reduced daily survivorship. Although there was no significant variation in daily survivorship of male and female mosquitoes within a photoperiodic treatment, there was, however, significant difference in survivorship among males and females among the photoperiodic regimens (Table 3).
Average adult longevity was, clearly affected by photoperiod. While adults from long photoperiods (18:6 and 24:0) were short lived (<12 days), those from zero (0:24), short (6:18), and narrow ranges of photoperiods (12:12 and 13:11 L:D hours) lived longer (Table 3).
Effects of varying photoperiods on CIs of life stages of C quinquefasciatus mosquito
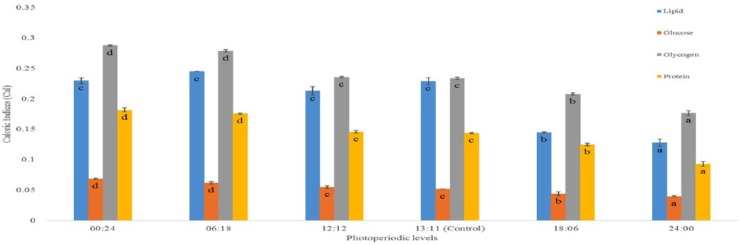
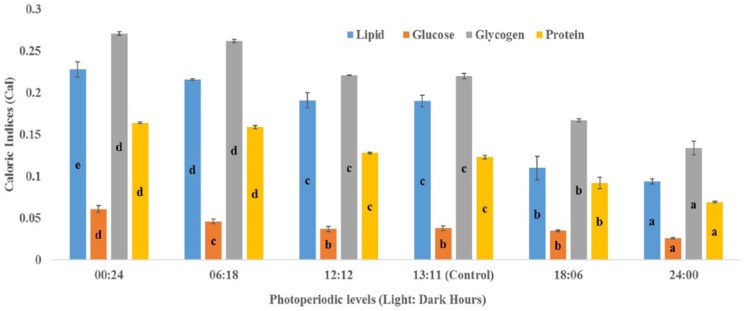
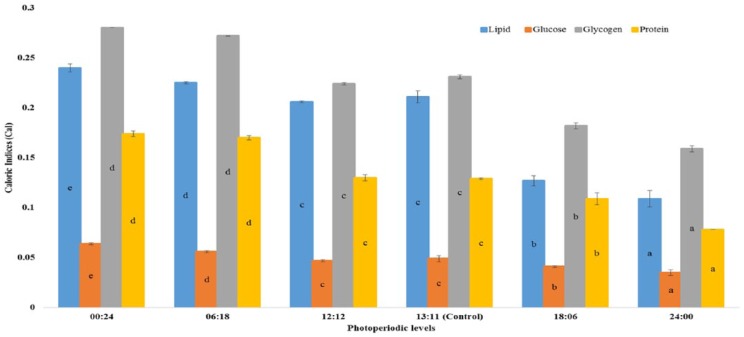
The results highlighted in Figures 1 to 3 show significant influence of photoperiod on CIs of important life stages of C quinquefasciatus. Analyses revealed that shorter photo-phase significantly increased values of CI of all teneral components in the life stages, whereas longer photoperiods reduced them.
Figure 1.
Effects of photoperiod regimen on caloric indices (cal) of fourth larval instar of Culex quinquefasciatus mosquito. Bars represent that means (±SD) followed by same letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance.
Figure 3.
Effects of photoperiod regimen on caloric indices (cal) of adult life stage of Culex quinquefasciatus mosquito. Bars represent that means (±SD) followed by same letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance.
Figure 2.
Effect of photoperiod regimen on caloric indices (cal) of pupal stage of Culex quinquefasciatus mosquito. Bars represent that means (±SD) followed by same letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance.
The CI of the final (fourth) larval instar, pupal, and adult life stages varied with change in photo-regimen. Larvae from zero (0:24), short (6:18), and narrow ranges (12:12 and 13:11 L:D hours) of photoperiods had significantly higher values for all teneral components, whereas the reverse was the case at longer photoperiods (Figure 1). In all life stages, glycogen had the highest values of CI, whereas glucose was consistently lowest (Figures 1 to 3).
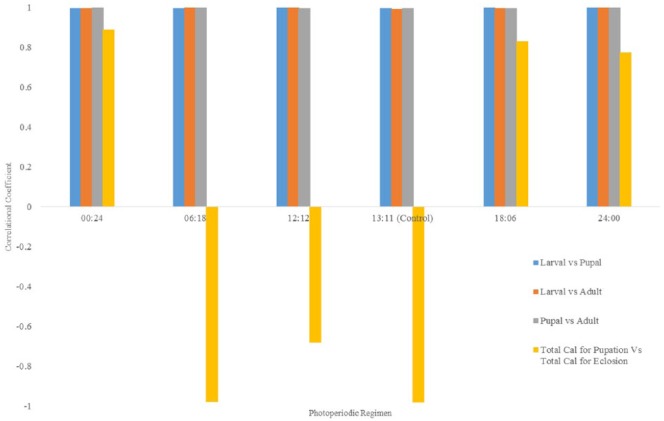
Correlations between energy values in C quinquefasciatus mosquito
Figure 4 shows the correlational relationships between energy values of teneral reserve of the life stages of C quinquefasciatus and between values for pupation and eclosion. Analyses revealed strong positive correlations between caloric values of all life stages. However, a negative but strong correlation was observed for total Cal values for pupation and eclosion at short (6:18) and narrow ranges (12:12 and 13:11 L:D hours) of photoperiods.
Figure 4.
Correlation between energy values (Cal) in Culex quinquefasciatus mosquitoes.
Effects of photoperiod on quantities of teneral reserves used during metamorphosis in C quinquefasciatus mosquito
Highlighted in Tables 5 and 6, respectively, are the effects of varying photoperiodic condition on quantities of teneral reserve used during the processes of pupation and eclosion in C quinquefasciatus. Generally, larvae reared at longer photo-phase required relatively more teneral components than those reared in shorter daylengths. More so, mosquitoes reared at zero (0:24), short (6:18), and narrow ranges (12:12 and 13:11 L:D hours) of photoperiodic treatments used lower quantities of teneral component for pupation and eclosion. For example, mosquitoes reared at these treatments had the lowest quantities of lipid, protein, glucose, and glycogen. The cohorts raised at longer photoperiods required relatively higher quantities of these teneral components for these processes.
Table 5.
Effect of different photoperiod treatments on teneral components (µg/mosquito) used for pupation in Culex quinquefasciatus mosquitoes.
| Photoperiodic levels (Light:dark hours) | Teneral reserve component |
|||
|---|---|---|---|---|
| Lipid | Protein | Glucose | Glycogen | |
| 0:24 | 1.90 ± 1.00Bb | 2.00 ± 0.00ABb | 2.09 ± 0.01Bb | 1.23 ± 0.03ABa |
| 6:18 | 2.21 ± 0.09BCb | 1.67 ± 0.31Aa | 1.64 ± 0.49ABa | 1.55 ± 0.42Ba |
| 12:12 | 0.81 ± 0.81Aa | 3.87 ± 0.51Bc | 2.86 ± 0.26Cb | 1.76 ± 0.37Bb |
| 13:11 (control) | 1.99 ± 0.01BCb | 3.76 ± 0.43Bc | 0.84 ± 0.96Aa | 0.69 ± 0.70Aa |
| 18:6 | 2.77 ± 0.61Cb | 4.12 ± 1.05Bc | 6.31 ± 1.28Ed | 0.63 ± 0.83Aa |
| 24:0 | 2.16 ± 0.22BCb | 3.58 ± 1.01Bc | 4.69 ± 0.44Dd | 1.16 ± 0.37ABa |
Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of covariance (ANOVA). Within a line, means (±SD) followed by same small letter are not significantly different at P < .05 according to Duncan multiple range test following ANOVA.
Table 6.
Effect of photoperiod regimen on teneral components (µg/mosquito) used for eclosion in Culex quinquefasciatus mosquito.
| Photoperiodic levels (Light:dark hours) | Teneral reserve component |
|||
|---|---|---|---|---|
| Lipid | Protein | Glucose | Glycogen | |
| 0:24 | 1.38 ± 0.50ABb | 2.45 ± 0.43Cc | 2.30 ± 0.42Bc | 0.74 ± 0.73Aa |
| 6:18 | 0.96 ± 0.05Aa | 2.65 ± 0.09Cb | 2.59 ± 0.59Bb | 2.57 ± 0.59Cb |
| 12:12 | 1.65 ± 0.96ABb | 0.72 ± 0.52Aa | 0.76 ± 0.10Aa | 2.65 ± 0.61Cc |
| 13:11 (control) | 2.31 ± 0.10Bb | 1.48 ± 0.15Ba | 2.59 ± 0.37Bbc | 2.93 ± 0.05Cc |
| 18:6 | 1.88 ± 1.09ABb | 4.24 ± 0.20Dd | 3.87 ± 1.22Cc | 1.63 ± 0.10Ba |
| 24:0 | 1.60 ± 0.74ABa | 2.31 ± 0.48Cb | 6.09 ± 1.58Dc | 2.28 ± 0.53BCb |
Within a column, means (±SD) followed by same capital letter are not significantly different at P < .05 according to Duncan multiple range test following analysis of variance (ANOVA). Within a line, means (±SD) followed by same small letter are not significantly different at P < .05 according to Duncan multiple range test following ANOVA.
Discussion
Effects of photoperiod on duration of development of C quinquefasciatus mosquitoes
In this study, larvae of C quinquefasciatus exhibited various developmental times at different photoperiod conditions. Individuals reared at shorter daylengths (0 and 6 hL) had the shortest development times (ie, fastest growth), whereas those reared at longer daylengths (18 and 24 hL) spent the longest times in development (ie, slowest growth).
The shorter developmental time displayed by the mosquito species (in response to shorter photo-phase) could be one of either physiological or behavioural responses25 to onset of wet season, a season usually marked by short daylengths. Epidemiologically, this season favours mosquito development, as it is characterised by higher relative humidity, abundance of water bodies (for breeding), and hence mosquito species’ population outburst. Unfortunately, heavy loss of immature life stages to flooding and overflowing habitats’ banks typifies the season, hence the need for faster development in response to this ‘perceived impending season’.
In this study, shorter photo-phase may have encouraged foraging, an activity correlated with faster accumulation of teneral reserve for pupation,26 earlier attainment of threshold size for pupation,18 and faster growth. Earlier researchers in insect-photoperiod interactions have also reported similar trends. For example, Leimar25 observed similar reduction in duration of development in the common blue butterfly, Polyommatus icarus. Lopatina et al10 reported significant faster and higher growth rate at short day (12 hL) as compared with long daylength (22 hL) for carabid beetle, Amara communis, whereas Reznik and Vaghina9 reported shorter pre-imaginal development of Harmonia axyridis under short day conditions. Furthermore, longer developmental time due to exposure to longer daylengths could possibly be stress-related or diapause-related physiological response,10 although there is no documented evidence of the latter for this life stage of the mosquito species in the Tropics.
Longer daylengths in the Tropics usually herald the onset of an extensive dry season (period), a period characterised by extremely low relative humidity, shortage of water bodies for mosquito proliferation (occasioned by the extensive drought), and hence greater immature mortality. These conditions do not favour the survivorship of mosquito species, and the mosquitoes may have significantly slowed down the developmental rates to accommodate the ‘perceived non-favourable season’.
However, Bradshaw and Holzapfel11 reported rapid growth rates and metamorphosis under long days for Toxorhynchites rutilus and retarded development during short days. For Dichelops melacanthus, Chocorosqui and Panizzi2 reported longer developmental time in shorter daylength. These contradictions may be due to differential species’ responses to these photoperiod conditions.
Effects of photoperiod on survivorship of immature stages C quinquefasciatus mosquito
Insects’ immature survivorship plays an important role in the number of adults at eclosion,15 and success at this will determine the population status of the species. This study revealed significant effects of daylength on survivorship of pre-imaginal life stages of C quinquefasciatus.
Generally, mosquitoes exposed to zero, short (6 hL), and narrow range (12 and 13 hL) of daylengths recorded higher survivorship and were, statistically, higher values than those reared at longer daylengths. The higher survivorship at zero, short (6 hL), and narrow range (12 and 13 hL) of daylengths suggests that these photo-conditions may be among those favourable for survivorship of the mosquito species. This photo-condition is species specific. For example, Mathias et al,5 Leimar,25 Carmine and Ronald,27 and Lanciani and Anderson28 have reported different responses to photo-conditions by Wyeomyia smithii, Polyommatus icarus, and Anopheles quadrimaculatus, respectively. However, Kollberg et al26 reported that daylength conditions do not affect the survivorship of pre-imaginal European pine sawfly, Neodiprion sertifer.
Effects of photoperiod on adult eclosion, daily survivorship and post-emergence longevity of C quinquefasciatus
The total number of adults at eclosion, their survivorship, and longevity are indices of biological fitness of mosquito species and hence capacity to transmit disease pathogen. In this study, zero, short (6 hL), and narrow ranges (12 and 13 hL) of photoperiodic conditions favoured high adult emergence, whereas longer photo-phase reduced eclosion. These higher numbers of emergent at zero, short (6 hL), and narrow ranges (12 and 13 hL) of photoperiodic conditions could be due to the higher pre-imaginal survivorship observed and reported earlier in this study. Interestingly, in all photoperiodic regimens, there were higher proportions of female to male mosquitoes, a possible indicator that photoperiodic conditions may not play significant role in determining sex in this mosquito species as observed in other insect species, eg, aphids.3
Furthermore, daily adult survivorship of the mosquito species reduced significantly at longer photo-conditions (ie, at 18 and 24 hL). Photoperiod did not affect survivorship between male and female within a treatment but significantly reduce survivorship among sexes (eg, among males or females) of all treatment as photoperiod increased. Earlier studies by Haddow et al29 and Nayar and Sauerman16 reported similar sex-linked variation in survivorship in response to photoperiod.
In this study, average post-emergence longevity was significantly affected by photoperiod, as those reared under shorter photo-phase (0 and 6 hL) lived longer days than those raised at narrow ranges and longer daylengths. These results may imply that shorter photo-phases may enhance vectorial fitness of this mosquito species, as studies have revealed that longer-lived adults have increased chances of host-vector contacts and increased biting rates with resultant increase in cycles of infection and re-infection of hosts. Longevity also determines the probability of disease parasites development in vectors.30
However, increasing photoperiod significantly reduced longevity among males and females. For example, male and female adult mosquitoes from shorter daylength conditions were positively associated with increased life span, whereas those from long daylength were short lived. More so, between male and female mosquitoes of a photoperiod treatment, female survived longer than the male. This could probably be a reflection of nature’s investment in female sex of many species, especially, insects, for physiological and metabolic processes of egg production and continuity of progeny.19 Studies by Lanciani and Anderson28 and Lanciani31 also described the same relationship between photoperiod and longevity for Anopheles crucians and A quadrimaculatus adult mosquitoes, respectively.
Effects of photoperiod on CIs of critical life stages of C quinquefasciatus mosquito
According to Bouabida et al,32 the ability of mosquitoes to survive and, therefore, live long enough to transmit disease pathogens is hugely dependent on its caloric reserves. The caloric reserves as expressed from CIs give an indication to the amount of energy available for peculiar major life stage activities. For example, the greater the reserve at the last phagoperiod, the greater the energy available for metamorphosis to pupae,17 pupal tissue re-organisation,33 adult flight,21,34 and egg development.35
In this study, analyses revealed that photoperiod significantly affected the energy available at the last phagoperiod (L4), pupae, and adult stages of C quinquefasciatus. Mosquitoes reared at shorter photoperiodic regimen had greater CI at these life stages than their counterparts raised at longer photoperiod. This is, perhaps, an indication that short photoperiod, eg, ≤6 hL, increases the amount of energy available during development. This may also connote readily availability of metabolic resources needed for growth, development, and other life activities and hence greater potential for higher success rates for such cohorts with a resultant efficiency at disease pathogen transmission.
Among teneral components, lipid and glycogen consistently had the highest CI at all life stages. The reason for this was not clear but could reflect the relative greater importance of these to energy availability and utilisation.36,37 Furthermore, the fourth larval stages had the highest values of CI for all teneral components, although further development resulted in progressive reduction in CI. This was not surprising, as teneral reserve accumulation has been reported to climax at this life stage (L4)17 and reduce during metamorphosis. Physiologically, the increased metabolic demand during metamorphosis (pupation and eclosion) and non-feeding characteristics of the pupal stage could have accounted for the reduced CI observed in this study. The high CI values at L4 also make it a suitable life stage choice for mosquito laboratory investigations (eg, insecticidal resistance bioassays).38
Effects of photoperiod on metabolic reserve for pupation and eclosion of C quinquefasciatus mosquitoes
The successes at pupation (breaking out from the larval exuvae) and eclosion (breaking out from pupal exuvae) are critical for the survival of mosquito species15 and correlated with population upsurge of mosquito species.39 Furthermore, the quantities of metabolic reserves used by insects for these processes are important, as they determine the amount of reserves available at adulthood for life activities.19,21,40 For example, greater utilisation of metabolic reserves for pupation and eclosion has been correlated with reduction in quantities available to adult life stages at emergence34 and hence the quantity and quality of teneral reserve available for egg development19 and energy for other critical adult life’s traits.
In this study, there were variations in the quantities and component type of teneral used by the mosquito species for the processes of pupation and eclosion at different photoperiod regimens. Mosquito larvae reared at shorter photo-phase (0 and 6 hL) and at narrow ranges (12 and 13 hL) of photoperiod required relatively lower quantities of teneral components for these processes than their siblings reared in longer daylength. These could have been responsible for the higher quantities of total teneral reserves used by the latter group of this mosquito species for these metamorphic processes as reported earlier.20 The reason for this is not clear yet but may suggest that longer photoperiods increase physiological and developmental demands of these nutrients in the mosquito species.
More so, the reduction in teneral expenditure at short photo-phases may be a physiologic to increase energy available for adult in response to ‘perceived impending season’ (ie, rainy season condition), which favours breeding and therefore demands greater metabolic reserves for adult activities. Rainy season is usually characterised by heightened adult life activities (such as flight after eclosion, searching for mate, blood meal foraging, egg development and maturation, and oviposition) which are energy demanding. Therefore, greater retention (by reducing expenditure) may guarantee successful breeding activities. These results may also suggest that the mosquitoes developing at short photo-phases may be of greater epidemiologic threat.
Physiologically, the standard mosquitoes (ie, control) used more protein than any other teneral components for pupation, probably, because this process (pupation) entails more of formation and reorganisation of new tissues. More so, not surprising and in confirmation of the reason stated earlier, the reference mosquitoes (ie, control) used more of energy precursors (ie, lipid, glucose, and glycogen) and very little protein for eclosion. This is probably because new tissue formation is not a key feature of this process but, rather, intense energy demands for respiratory metabolism and fledging of already formed body parts.
Conclusions
From the foregoing, variations in length of daylight significantly affect important aspects of biological fitness of C quinquefasciatus mosquito. Short daylength significantly reduced duration of development while increasing survivorship. It also increased adult emergence, post-emergence survivorship, and longevity of the species. This study also provides information on the bionomics of mosquitoes inhabiting dark crevices and habitats (eg, septic tanks), and the epidemiologic importance of such mosquito species and habitat, as both are usually situated close to human dwellings. The information can serve as baseline information in the development of control protocols based on environmental manipulation.
Acknowledgments
The authors’ deepest appreciation goes to the management and staff members of the Department of Biological Sciences, Federal University of Technology, for providing a conducive environment for the study. They also thank the University Management for facilitating the US Agency for International Development (USAID), USA, Higher Education Partnership/University of Mississippi (UM) for the sponsorship and grant, without which this study would not have been feasible.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A grant from the United State Agency for International Development/ University of Mississippi (USAID/UM; Sub award NO. 15-12-024) was used to fund the study The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ACU, IKO, FOA, and ICJO conceived and designed the experiment. ACU, BMB, and CCU performed the experiments. ACU, and CCU analysed the data. ACU and CCU wrote the first draft of the manuscript. IKO, FOA, and ICJO contributed to writing of the manuscript. All authors agreed with manuscript results and conclusion. All authors made critical revisions and approved final version of the manuscript.
References
- 1. MacRae TH. Diapause: diverse states of developmental and metabolic arrest. J Biol Res. 2005;3:3–14. [Google Scholar]
- 2. Chocorosqui VR, Panizzi AR. Photoperiod influence on the biology and phenological characteristics of Dichelops melacanthus (Dallas, 1851) (Heteroptera: Pentatomidae). Braz J Biol. 2003;63:655–664. [DOI] [PubMed] [Google Scholar]
- 3. Tauber E, Kyriacou BP. Insect photoperiodism and circadian clocks: models and mechanisms. J Biol Rhythms. 2001;16:381–390. [DOI] [PubMed] [Google Scholar]
- 4. MacRae TH. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci. 2010;67:2405–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathias D, Laura KR, William EB, Holzapfel CM. Evolutionary divergence of circadian and photoperiodic phenotypes in the pitcher-plant mosquito, Wyeomyia smithii. J Biol Rhythms. 2006;21:132–139. [DOI] [PubMed] [Google Scholar]
- 6. Urbaneja A, Llacer E, Garrido A, Jacas J. Effect of variable photoperiod on development and survival of Cirrospilus sp. Nr. Lyncus (Hymenoptera: Eulophidae), an ectoparasitoid of Phyllocnistis citrella (Lepidoptera: Gracillariidae). Flor Entom. 2001;84:305–307. [Google Scholar]
- 7. Danks HV. Studying insect photoperiodism and rhythmicity: components, approaches and lessons. Eur J Entomol. 2003;100:209–221. [Google Scholar]
- 8. Śniegula S, Nilsson-Örtman V, Johansson F. Growth pattern responses to photoperiod across latitudes in a Northern Damselfly. PLoS ONE. 2012;7:e46024. doi:101371journalpone0046024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reznik SY, Vaghina NP. Photoperiodic control of development and reproduction in Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol. 2011;108:385–390. [Google Scholar]
- 10. Lopatina EB, Kipyatkov VE, Balashov SV, Kutcherov DA. Photoperiod-temperature interaction: a new form of seasonal control of growth and development in insects and in particular carabid beetle, Amara communis (Coleoptera: Carabidae). J Evol Biochem Physiol. 2011;47:578–592. [PubMed] [Google Scholar]
- 11. Bradshaw WE, Holzapfel CM. Biology of tree-hole mosquitoes: photoperiodic control of development in northern Toxorhynchites rutilus (Coq.). Can J Zool. 1975;53:889–893. [Google Scholar]
- 12. Ukubuiwe AC, Olayemi IK, Omalu ICJ, Odeyemi MO, Jibrin AI, Oyibo-Usman KA. Comparative assessment of immature survivorship and developmental duration of Culex pipiens pipiens (Diptera: Culicidae) populations in north central Nigeria. Biomed Cent Epidemiol. 2012;3:WMC003753. [Google Scholar]
- 13. Olayemi IK, Ukubuiwe AC, Oyibo-Usman KA. Mosquito species occurrence and diversity in conventional larval breeding sites in Minna metropolis, Nigeria. Int J Innov Sci Res. 2014;9:86–93. [Google Scholar]
- 14. World Health Organization. Annual Report on Lymphatic filariasis 2017. Geneva, Switzerland: World Health Organization; 2017. www.who.int/mediacentre/factsheets/fs102/en. Accessed January 1, 2018. [Google Scholar]
- 15. Ukubuiwe AC, Olayemi IK, Jibrin AI. Genetic variations in bionomics of Culex quinquefasciatus (Diptera: Culicidae) mosquito population in Minna, North Central Nigeria. Int J Insect Sci. 2016;8:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nayar JK, Sauerman DM. A comparative study of growth and development in Florida mosquitoes. I. Effects of environmental factors on ontogenetic timings, endogenous diurnal rhythm and synchrony of pupation and emergence. J Med Entomol. 1970;7:163–174. [DOI] [PubMed] [Google Scholar]
- 17. Timmermann SE, Briegel H. Larval growth and biosynthesis of reserves in mosquitoes. J Insect Physiol. 1999;45:461–470. [DOI] [PubMed] [Google Scholar]
- 18. Timmermann SE, Briegel H. Moulting and metamorphosis in mosquito larvae: a morphometric analysis. Mitthe Schweiz Entomol Gesel. 1998;71:373–387. [Google Scholar]
- 19. Briegel H. Physiological bases of mosquito ecology. J Vector Ecol. 2003;28:1–11. [PubMed] [Google Scholar]
- 20. Ukubuiwe AC, Olayemi IK, Omalu ICJ, et al. Influence of photoperiod on larval growth indices and energy budget for metamorphosis in Culex quinquefasciatus mosquito (Diptera: Culicidae): its implication in integrated vector management. In: Proceedings from the 30th International Conference of the Biotechnology Society of Nigeria (BSN); August 27-30, 2017:167–176; Federal University of Technology, Minna, Minna. [Google Scholar]
- 21. Clements AN. The source of energy for flight in mosquitoes. J Exp Biol. 1995;32:547–554. [Google Scholar]
- 22. Olayemi IK, Ande AT. Life table analysis of Anopheles gambiae (Diptera: Culicidae) in relation to malaria transmission. J Vector Borne Dis. 2009;46:295–298. [PubMed] [Google Scholar]
- 23. Van-Handel E, Day JF. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J Am Mosq Control Assoc. 1988;4:549–550. [PubMed] [Google Scholar]
- 24. Kaufmann C, Brown MR. Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 2008;54:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leimar O. Life history plasticity: influence of photoperiod on growth and development in the common blue butterfly. Oikos. 1996;76:228–234. [Google Scholar]
- 26. Kollberg I, Bylund H, Schmidt A, Gershenzon J, Björkman C. Multiple effects of temperature, photoperiod and food quality on the performance of a pine sawfly. Ecol Entomol. 2013;38:201–208. [Google Scholar]
- 27. Carmine LA, Ronald E. Effect of photoperiods on Anopheles quadrimaculatus. Flor Entom. 1993;76:622. [Google Scholar]
- 28. Lanciani CA, Anderson JF. Effect of photoperiod on longevity and metabolic rate in Anopheles quadrimaculatus. J Am Mosq Control Assoc. 1993;9:158–163. [PubMed] [Google Scholar]
- 29. Haddow AJ, Van-Smeren ECC, Lumsden M. The mosquitoes of two rural sites in Eastern Sudan. Bull Ent Res. 2002;42:207–238. [Google Scholar]
- 30. Lyimo EO, Koella JC. Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology. 1992;104:233–237. [DOI] [PubMed] [Google Scholar]
- 31. Lanciani CA. Photoperiod and longevity in Anopheles crucians. J Am Mosq Control Assoc. 1993;9:308–312. [PubMed] [Google Scholar]
- 32. Bouabida H, Tine-Djebbar F, Tine S, Soltani N. Activity of spiromesifen on growth and development of Culex pipiens (Diptera: Culicidae): toxicological, biometrical and biochemical aspects. J Entomol Zool Stud. 2017;5:572–577. [Google Scholar]
- 33. Chambers GM, Klowden MJ. Correlation of nutritional reserves with a critical weight for pupation in larval Aedes aegypti mosquitoes. J Am Mosq Control Assoc. 1990;6:394–399. [PubMed] [Google Scholar]
- 34. Kaufmann C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J Vector Ecol. 2004;29:140–153. [PubMed] [Google Scholar]
- 35. Aparna T, Yiping L, Fernando GN, Mark RB. Effects of larval nutrition on the endocrinology of mosquito egg development. J Exp Biol. 2006;209:645–655. [DOI] [PubMed] [Google Scholar]
- 36. Jensen PV, Børgesen LW. Regional and functional differentiation in the fat body of Pharaoh’s ant queens, Monomorium pharaonis (L.). Arthropod Struct Dev. 2000;29:171–184. [DOI] [PubMed] [Google Scholar]
- 37. Kerkut GA, Gilbert LI, eds. Comprehensive Insect Physiology, Biochemistry and Pharmacology. New York, NY: Pergamon Press; 1985:200. [Google Scholar]
- 38. World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. http://WHO/CDS/WHOPES/GCDPP/2005.13. Published 2005. Accessed September 24, 2017.
- 39. Focks DA, Alexander N. Multicountry study of Aedes aegypti Pupal Productivity Survey Methodology: Findings and Recommendations (TDR/IRM/DEN/06.1). Geneva, Switzerland: Special Programme for Research and Training in Tropical Diseases; (TDR); 2006. http://apps.who.int/tdr/publications/tdr-researchpublications/multicountry-study-aedes-aegypti/pdf/aedes_aegypti.pdf. Accessed September 19, 2017. [Google Scholar]
- 40. Lang JT. Relationship of fecundity to the nutritional quality of larval and adult diets of Wyeomyia smithii. Mosq News. 1978;38:396–403. [Google Scholar]