Abstract
Background
This study examines the effect of interstitial inflammation and interstitial fibrosis and tubular atrophy on renal survival in lupus nephritis.
Methods
Baseline characteristics, initial (n = 301) and repeat biopsies (n = 94) and clinical outcomes for patients with biopsy-proven lupus nephritis from 1998 to 2014 were retrospectively collected from the medical record. Clinical and morphologic variables were evaluated using a Cox proportional hazards model and multiple imputation to address missing data. Renal survival was defined as the time from initial biopsy to end-stage renal disease [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2], dialysis or transplant.
Results
A total of 218 patients had follow-up and Class IV had worse renal survival, especially in patients with active and chronic glomerular lesions {relative to non-IV; Class IV-A: hazard ratio [HR] 0.92 [95% confidence interval (CI) 0.41–2.04], Class IV-AC: HR 5.02 [95% CI 2.70–9.36]}. Interstitial inflammation grade [relative to interstitial inflammation <5%; interstitial inflammation 5–25%: HR 2.36 (95% CI 1.13–4.91), interstitial inflammation 25–50%: HR 3.84 (95% CI 1.53–9.62), interstitial inflammation >50%: HR 7.67 (95% CI 3.75–15.67)] and increased interstitial fibrosis and tubular atrophy (IFTA) category [relative to IFTA <5%; IFTA 5–25%: HR 3.93 (95% CI 1.58–9.75), IFTA 25–50%: HR 4.01 (95% CI 1.37–11.70), IFTA >50%: HR 13.99 (95% CI 4.91–39.83)] predicted worse renal survival among all patients and those with Class IV on initial and repeat biopsy (n = 94) in a dose-dependent manner. Interstitial inflammation grade and IFTA category were significant predictors of renal survival in a multivariable model adjusted for age, gender, race, ethnicity and serum creatinine.
Conclusions
Interstitial inflammation and IFTA independently affect renal survival and grading these lesions stratifies risk within the International Society of Nephrology and Renal Pathology Society classification of lupus nephritis.
Keywords: interstitial fibrosis, interstitial inflammation, kidney biopsy, lupus nephritis, renal pathology, tubular atrophy
Introduction
Lupus nephritis is a significant contributor to morbidity and mortality in patients with systemic lupus erythematosus (SLE) and disproportionately affects African Americans [1, 2]. Approximately 38% of patients with SLE develop lupus nephritis and at least 10% of these patients progress to end-stage renal disease (ESRD) within 10 years [3]. Lupus nephritis is separated into classes based on histologic criteria established by the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) [4]. The ISN/RPS classification focuses predominantly on glomerular lesions in defining disease activity and chronicity. Interstitial inflammation and interstitial fibrosis are graded as mild, moderate and severe, but there are no criteria for these categories and it is unclear how they affect prognosis independent of ISN/RPS class.
First-line treatment for patients with Class III or IV lupus nephritis is induction therapy with intravenous cyclophosphamide or mycophenolate mofetil in combination with steroids, followed by maintenance therapy with mycophenolate mofetil or azathioprine supported by oral steroids [5]. Alternative immunosuppressive agents, including rituximab, cyclosporine and tacrolimus may be used for refractory lupus nephritis or in patients who cannot tolerate first-line therapy [6–9]. Adjunctive therapies include renin–angiotensin system blockade, blood pressure control and statins for hyperlipidemia. The presence or absence of interstitial inflammation, interstitial fibrosis and tubular atrophy and chronic glomerular lesions is not routinely used to make therapeutic decisions and may provide insight into the risk for disease progression.
Materials and methods
Study population
This retrospective study included 301 patients with biopsy-proven lupus nephritis consecutively diagnosed at Yale New Haven Hospital, New Haven, CT, USA from 1 January 1998 to 31 December 2014. Patients biopsied prior to 1 January 1998 were excluded. All patients met at least four of the American College of Rheumatology 1997 criteria for SLE.
Histologic classification
Initial (n = 301) and repeat biopsies (n = 94) were categorized by ISN/RPS 2003 criteria by two independent pathologists [4]. Disagreements were adjudicated by consensus. All biopsies had at least 10 glomeruli for evaluation. Interstitial inflammation and interstitial fibrosis and tubular atrophy were graded semiquantitatively by hematoxylin (H&E) and eosin, periodic acid–Schiff (PAS), silver and trichrome stains using the following categories: Grade 0: ≤5%; Grade 1: 5–25%; Grade 2: 25–≤50%; Grade 3: >50% (see Supplementary data for representative images). The categories represent the percentage of nonscarred cortical area involved by mononuclear cell infiltrate and are analogous to the Banff interstitial inflammation scores for kidney allograft rejection [10]. The trichrome stain was used to help identify fibrosis. Areas that cannot be meaningfully graded for interstitial inflammation, including subcapsular cortex and the adventitia surrounding large vessels or lymphatics, were not included in the assessment. All biopsies were assessed by light, immunofluorescent and electron microscopy.
Renal survival
Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation. ESRD was defined as an eGFR <15 mL/min/1.73 m2 for at least 3 months. Renal replacement therapy (RRT) was defined as dialysis for >6 months or kidney transplant. CKD was defined as an eGFR <60 mL/min/1.73 m2 according to the Kidney Disease: Improving Global Outcomes guidelines [11]. Death was defined as all-cause mortality. Renal survival was defined as the time from initial kidney biopsy to any of the following: ESRD, dialysis for >6 months or kidney transplant.
Statistical analyses
For the composite outcome (ESRD, RRT), time-to-event analysis was performed. Patients with Class VI on initial biopsy (n = 2) were excluded. Variables were assessed by univariate proportional hazards regression and P < 0.05 was considered significant. Complete cases analysis was used to generate the first multivariable model. Multiple imputation was used to estimate missing baseline serum creatinine values before making the second model (see Supplementary data, Methods).
Results
Renal survival, race and ethnicity
In all, 301 patients with lupus nephritis were categorized by race and ISN/RPS class. The study included 94 African American, 78 non-Hispanic White, 54 Hispanic or Latino, 12 Asian and 63 patients with an unspecified race (Supplementary data, Table S1). The median duration from SLE diagnosis to renal biopsy was 3 years. Approximately 82% of patients were female and the distribution was not different between racial or ethnic groups. The proportion of African Americans increased during the study period (1998–2002, 20%; 2002–6, 32%; 2006–10, 36%; 2010–14, 38%). There was also an increase in age at the time of initial biopsy (1998–2002, 31.5; 2002–6, 33.6; 2006–10, 35.3; 2010–14, 38.0 years). However, the distribution of ISN/RPS class, tubulointerstitial lesions and laboratory values were similar between racial and ethnic groups.
A total of 218 patients had available follow-up. There were roughly similar follow-up rates for African American [78/94 (83%)], non-Hispanic White [63/78 (81%)], Hispanic [50/54 (92%)] and Asian [8/12 (66%)] groups (Supplementary data, Table S2). A significantly smaller proportion of patients with an unspecified race had available follow-up data [19/63 (30%)]. Many of these patients were referred to our institution for renal pathology services and subsequently treated at unaffiliated clinics or hospitals. Due to the small number of patients in the Asian group and the significant number of patients lost to follow-up in the group with an unspecified race, outcomes were compared between African American, Hispanic and non-Hispanic White groups in the multivariable model (n = 191). African American and Hispanic patients had a trend toward worse renal survival compared with non-Hispanic White patients {Figure 1A; African American: hazard ratio [HR] 1.86 [95% confidence interval (CI) 0.87–4.06], Hispanic: HR 1.85 [95% CI 0.83–4.13]}, predominantly due to worse outcomes in Class IV.
Fig. 1.
Kaplan–Meier estimates for renal survival by (A) race and ethnicity and (B) Class IV activity and chronicity.
Renal survival and ISN/RPS class
Patients with Class IV had increased serum creatinine and proteinuria and decreased hematocrit, serum albumin and C3 complement relative to patients without Class IV (Table 1). Class IV on initial biopsy had worse renal survival compared with non-Class IV [HR 2.19 (95% CI 1.22–3.95)], especially in patients with both active and chronic glomerular lesions [Figure 1B; Class IV-AC relative to IV-A: HR 4.41 (95% CI 2.08–9.32)]. In patients with Class IV, the proportion of glomeruli involved by active lesions [HR 0.20 (95% CI 0.04–0.95)] and the proportion of globally sclerosed glomeruli [HR 3.42 (95% CI 1.02–11.45)] were associated with worse renal survival in univariate analysis, particularly in patients with >30% global glomerulosclerosis [Class IV relative to 0% glomerulosclerosis: 0–30%, HR 1.83 (95% CI 0.66–5.84); >30%, HR 4.21 (95% CI 1.81–9.77)]. There was better renal survival in Class II [HR 0.26 (95% CI 0.08–0.85)], Class III [HR 0.37 (95% CI 0.13–1.06)] and Class V [HR 0.29 (95% CI 0.32–1.40)] and similar survival in Class III/IV + V [HR 0.94 (95% CI 0.28–3.08)] relative to Class IV (Table 2). There was no significant difference in survival between Class IV-S and IV-G [relative to IV-G: HR 1.32 (95% CI 0.40–4.36)]. The presence of necrosis in patients with Class IV was not associated with worse renal survival [relative to no necrosis: HR 1.27 (95% CI 0.62–2.63)].
Table 1.
Baseline characteristics by ISN/RPS class
| Baseline characteristics by ISN/RPS class | Class II (n = 47) | Class III-A (n = 20) | Class III-AC (n = 19) | Class IV-A (n = 70) | Class IV-AC (n = 63) | Class III/IV + V (n = 19) | Class V (n = 61) |
|---|---|---|---|---|---|---|---|
| Female (%) | 85 | 65 | 79 | 76 | 79 | 74 | 87 |
| Age (years) | 35.4 (16.1) | 30.8 (16.6) | 40.8 (17.8) | 30.1 (13.6) | 34.9 (13.3) | 36.6 (13.6) | 35.4 (13.6) |
| BMI (kg/m2) | 29.2 (17.5) | 30.0 (7.5) | 28.1 (7.9) | 28.2 (8.0) | 26.4 (5.8) | 29.2 (7.6) | 31.5 (9.2) |
| BP systolic (mmHg) | 127 (11) | 109 (17) | 121 (22) | 136 (16) | 134 (24) | 139 (18) | 117 (20) |
| BP diastolic (mmHg) | 78 (8) | 68 (11) | 72 (7) | 89 (13) | 81 (19) | 78 (15) | 71 (11) |
| SCr (mg/dL) | 1.09 (0.88) | 1.09 (0.55) | 1.34 (0.73) | 1.48 (1.11) | 1.86 (1.06) | 1.63 (1.71) | 0.87 (0.37) |
| eGFR (mL/min/1.73 m2) | 95 (40) | 83 (30) | 75 (57) | 78 (64) | 49 (34) | 79 (51) | 95 (41) |
| >90, n | 13 | 5 | 3 | 12 | 3 | 5 | 21 |
| >60–< 90, n | 9 | 3 | 2 | 8 | 8 | 3 | 11 |
| >30–< 60, n | 6 | 2 | 3 | 7 | 11 | 1 | 5 |
| <30, n | 0 | 0 | 3 | 9 | 11 | 3 | 2 |
| Unknown, n | 19 | 10 | 8 | 34 | 30 | 7 | 22 |
| Serum albumin (g/dL) | 3.2 (1.1) | 2.6 (0.9) | 3.0 (0.6) | 2.4 (0.8) | 2.5 (0.8) | 3.0 (0.8) | 2.9 (0.9) |
| WBC count (1000/ µL) | 7.3 (3.1) | 7.9 (4.2) | 4.7 (2.7) | 7.8 (5.4) | 7.0 (3.1) | 6.4 (1.9) | 5.5 (1.7) |
| Hematocrit (%) | 36.5 (6.5) | 32.6 (6.2) | 34.1 (5.0) | 30.2 (5.1) | 28.3 (5.3) | 32.3 (4.6) | 34.8 (5.0) |
| Platelet count (1000/µL) | 253 (104) | 286 (90) | 210 (101) | 214 (103) | 221 (135) | 246 (82) | 291 (99) |
| 24-h proteinuria (g) | 1.78 (1.7) | 1.86 (1.2) | 1.6 (0.3) | 5.61 (5.3) | 4.0 (3.0) | 5.16 (4.7) | 3.15 (2.4) |
| Urine protein/Cr (mg/mg) | 1.54 (2.84) | 1.46 (1.15) | 3.66 (2.80) | 2.92 (2.93) | 2.81 (2.95) | 3.13 (4.60) | 2.53 (2.26) |
| C3 (mg/dL) | 80 (43) | 65 (46) | 80 (43) | 58 (45) | 46 (23) | 59 (32) | 93 (35) |
| C4 (mg/dL) | 15 (6) | 11 (2) | 16 (16) | 15 (9) | 12 (3) | 12 (5) | 18 (9) |
| Median ANA titer | 1:160 | 1:320 | 1:320 | 1:320 | 1:320 | 1:640 | 1:480 |
| anti-dsDNA (% positive) | 47 | 90 | 86 | 73 | 86 | 90 | 61 |
| Mean proportion of globally sclerosed glomeruli (SD) | 0.08 (0.13) | 0.00 (0.03) | 0.19 (0.12) | 0.02 (0.13) | 0.18 (0.19) | 0.04 (0.09) | 0.05 (0.11) |
| IFTA, n (%) | |||||||
| <5 | 32 (68) | 13 (65) | 6 (32) | 49 (70) | 15 (24) | 9 (47) | 34 (56) |
| ≥5 to 25 | 13 (27) | 7 (35) | 6 (32) | 16 (23) | 24 (38) | 5 (26) | 20 (32) |
| ≥25 to 50 | 1 (2) | 0 | 6 (32) | 3 (4) | 13 (20) | 5 (26) | 4 (6) |
| ≥ 50 | 1 (2) | 0 | 1 (3) | 2 (3) | 11 (17) | 0 | 3 (5) |
| Interstitial inflammation, n (%) | |||||||
| <5 | 37 (79) | 13 (65) | 4 (21) | 37 (53) | 11 (17) | 9 (47) | 33 (54) |
| ≥5 to 25 | 5 (10) | 5 (25) | 10 (52) | 21 (30) | 22 (35) | 6 (31) | 24 (39) |
| ≥25 to 50 | 4 (8) | 2 (10) | 2 (10) | 3 (4) | 15 (24) | 4 (21) | 1 (2) |
| ≥50 | 1 (2) | 0 | 3 (16) | 9 (13) | 15 (24) | 0 | 3 (5) |
| Interstitial risk category, n (%) | |||||||
| Low | 41 (87) | 15 (75) | 3 (16) | 47 (67) | 16 (25) | 10 (53) | 43 (70) |
| Intermediate | 4 (9) | 5 (25) | 11 (58) | 13 (19) | 20 (32) | 7 (37) | 12 (20) |
| High | 2 (4) | 0 | 5 (26) | 10 (14) | 27 (43) | 2 (10) | 6 (10) |
Data presented as mean (SD) unless stated otherwise. ANA, antinuclear antibody; BMI, body mass index; BP, blood pressure; C3, complement component 3; C4, complement component 4; dsDNA, double-stranded DNA; SCr, serum creatinine; WBC, white blood cell.
Table 2.
Follow-up characteristics and outcomes by ISN/RPS class
| Patient characteristics | Class II (n = 47) | Class III-A (n = 20) | Class III-AC (n = 19) | Class IV-A (n = 70) | Class IV-AC (n = 63) | Class III/IV + V (n = 19) | Class V (n = 61) |
|---|---|---|---|---|---|---|---|
| Renal follow-up, n (%) | 33 (70) | 13 (65) | 16 (84) | 54 (77) | 45 (71) | 17 (89) | 40 (66) |
| Mean follow-up (years) | 7.2 (4.8) | 7.4 (5.2) | 7.9 (5.1) | 8.1 (5.1) | 5.7 (4.7) | 4.1 (3.7) | 7.2 (4.3) |
| BP systolic (mmHg) | 122 (17) | 126 (17) | 128 (17) | 131 (22) | 132 (23) | 132 (23) | 125 (16) |
| BP diastolic (mmHg) | 77 (10) | 79 (10) | 78 (11) | 80 (14) | 79 (13) | 78 (12) | 78 (12) |
| C3 (mg/dL) | 98 (36) | 121 (29) | 99 (36) | 91 (31) | 76 (37) | 94 (34) | 103 (30) |
| C4 (mg/dL) | 20 (10) | 24 (11) | 25 (13) | 19 (11) | 13 (11) | 18 (6) | 23 (12) |
| Anti-dsDNA (IU/mL) | 134 (217) | 48 (69) | 135 (307) | 121 (233) | 215 (458) | 81 (72) | 96 (185) |
| Urine protein/Cr (mg/mg) | 0.51 (0.79) | 1.70 (3.25) | 1.79 (2.73) | 2.53 (4.17) | 6.0 (16.5) | 2.60 (3.48) | 1.92 (3.32) |
| SCr (mg/dL) | 1.10 (0.93) | 1.22 (1.18) | 1.25 (0.99) | 1.87 (2.95) | 2.7 (2.4) | 1.36 (1.16) | 1.54 (1.60) |
| Outcome categories in patients alive at last follow-up | |||||||
| eGFR (mL/min/1.73 m2), n | |||||||
| ≥90 | 15 | 6 | 7 | 18 | 7 | 7 | 19 |
| ≥60–< 90 | 8 | 3 | 2 | 10 | 5 | 2 | 7 |
| ≥30–< 60 | 5 | 2 | 4 | 9 | 7 | 1 | 3 |
| ≥15–< 30 | 1 | 0 | 0 | 1 | 1 | 1 | 2 |
| <15 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Dialysis living | 0 | 0 | 1 | 3 | 12 | 2 | 4 |
| Kidney transplant living | 0 | 1 | 0 | 3 | 5 | 0 | 1 |
| Outcome categories in patients that died during the study period, n | |||||||
| Death after dialysis | 2 | 1 | 0 | 3 | 6 | 1 | 2 |
| Death after transplant | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Death with CKD | 0 | 0 | 1 | 4 | 1 | 1 | 0 |
| Death without CKD | 1 | 0 | 1 | 2 | 0 | 0 | 0 |
| Death with unknown kidney function | 2 | 1 | 0 | 4 | 4 | 0 | 1 |
| Composite outcomes | |||||||
| ESRD or RRT, total n (%) | 3 (6) | 2 (10) | 2 (11) | 10 (14) | 24 (38) | 3 (16) | 9 (15) |
| All-cause death, total n (%) | 5 (11) | 2 (10) | 2 (11) | 14 (20) | 12 (19) | 2 (11) | 3 (5) |
BP, blood pressure; C3, complement component 3; C4, complement component 4; CKD, chronic kidney disease; dsDNA, double-stranded DNA; SCr, serum creatinine.
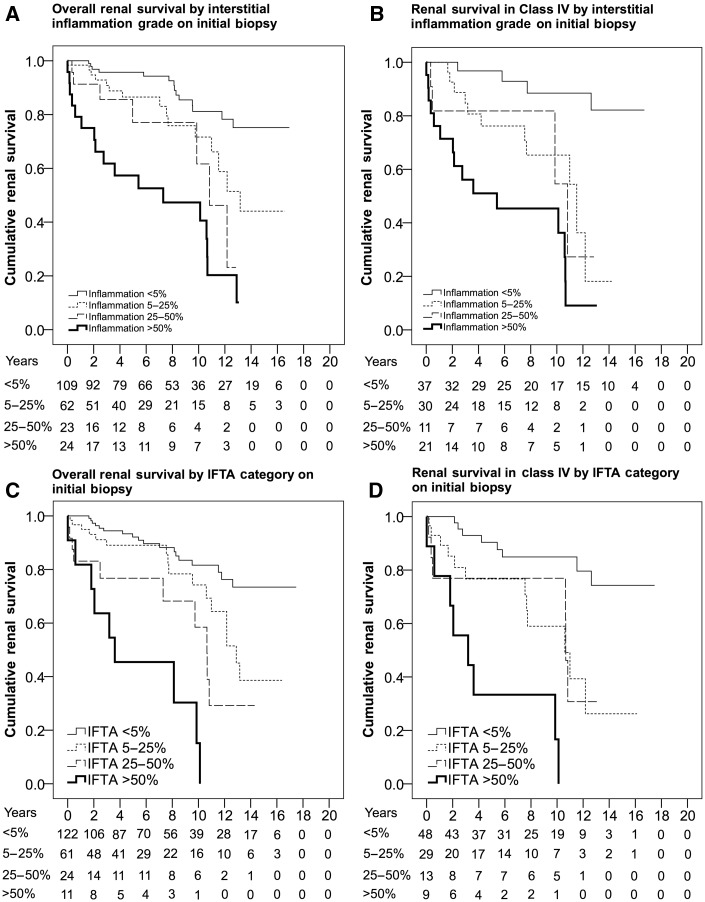
Renal survival and tubulointerstitial lesions
Interstitial inflammation was associated with worse renal survival in all patients [Figure 2A; relative to interstitial inflammation <5%: interstitial inflammation 5–25%, HR 2.36 (95% CI 1.13–4.91); interstitial inflammation 25–50%, HR 3.84 (95% CI 1.53–9.62); interstitial inflammation >50%, HR 7.67 (95% CI 3.75–15.67)] and those with Class IV [Figure 2B; interstitial inflammation 5–25%, HR 5.45 (95% CI 1.69–17.55); interstitial inflammation 25–50%, HR 5.85 (95% CI 1.44–23.77); interstitial inflammation >50%, HR 14.14 (95% CI 4.53–44.10)] in a dose-dependent manner. Interstitial inflammation grade also stratified risk in patients with Class IV with active and chronic glomerular lesions [relative to Class IV-AC with interstitial inflammation <5%; interstitial inflammation 5–25%, HR 6.92 (95% CI 0.88–54.51); interstitial inflammation 25–50%, HR 11.17 (95% CI 1.30–95.77); interstitial inflammation >50%, HR 18.36 (95% CI 2.35–143.39)]. Severe interstitial inflammation was more common in Class IV compared with Class III and was uncommon in Classes II and V (Table 1). There was a significant correlation between interstitial inflammation grade and IFTA category (r = 0.499, P < 0.001) and a weaker correlation with serum creatinine (r = 0.306, P < 0.001), 24-h protein (r = 0.292, P = 0.002) and hematocrit (r = −0.274, P = 0.002). There was a clear trend between increased interstitial inflammation grade and elevated serum creatinine in patients with Class IV (Supplementary data, Table S3; Class IV with interstitial inflammation <5%, 1.41 mg/dL; interstitial inflammation 5–25%, 1.44 mg/dL; interstitial inflammation 25–50%, 2.00 mg/dL; interstitial inflammation >50%, 2.37 mg/dL; F = 2.8, P = 0.047).
Fig. 2.
Kaplan–Meier estimates for renal survival by tubulointerstitial lesions. (A) Interstitial inflammation grade in all patients. (B) Interstitial inflammation grade in patients with Class IV. (C) IFTA category in all patients. (D) IFTA category in patients with Class IV.
Interstitial fibrosis and tubular atrophy was associated with worse renal survival in all patients (Figure 2C; IFTA 5–25% HR 3.93 (95% CI 1.58–9.75); IFTA 25–50%: HR 4.01 (95% CI 1.37–11.70); IFTA >50%, HR 13.99 (95% CI 4.91–39.83)] and those with Class IV (Figure 2D; IFTA 5–25%, HR 3.93 (95% CI 1.58–9.75); IFTA 25–50%, HR 4.01 (95% CI 1.37–11.70), IFTA >50%, HR 13.99 (95% CI 4.91–39.831)] in a dose-dependent manner. Severe IFTA was more common in Class IV compared with Class III or Class V and was uncommon in Class II (Table 1). Among patients with Class IV, IFTA category was significantly correlated with increased serum creatinine (F = 2.91, P = 0.041), increased proteinuria (F = 3.7, P = 0.017) and increased age (F = 3.7, P = 0.013) at the time of biopsy.
Multivariable proportional hazards model for renal survival
Class IV, IFTA category, interstitial inflammation grade, proportion of globally sclerosed glomeruli, serum creatinine, elevated blood pressure, serum albumin, hematocrit and 24-h protein were each associated with renal survival in univariate analysis (Table 3). Blood pressure, serum albumin, hematocrit and 24-h protein were excluded from the multivariable model due to missing data (Supplementary data, Table S4). None of the excluded variables were significant predictors of renal survival when added to the complete cases multivariable model. Complete cases analysis (n = 132) showed that interstitial inflammation grade [HR 1.58 (95% CI 1.06–2.36)], IFTA category [HR 2.20 (95% CI 1.29–3.74)], age [HR 0.96 (95% CI 0.93–0.99), male gender [HR 3.02 (95% CI 1.02–8.92)] and serum creatinine [HR 2.16 (95% CI 1.50–3.11)] were associated with worse renal survival in the multivariable model. Multiple imputation was employed to estimate missing serum creatinine values (see Supplementary data, Methods). Interstitial inflammation grade [HR 1.39 (95% CI 1.08–1.79)], IFTA category [HR 1.40 (95% CI 1.05–1.87)] and serum creatinine [HR 1.68 (95% CI 1.35–2.08)] were significantly associated with worse renal survival in the multiple imputation model (n = 191).
Table 3.
Proportional hazards model for renal survival
| Patient and biopsy characteristics | Univariate | P-value | Multivariable with complete cases (n = 132) | P-value | Multivariable after multiple imputation (n = 191) | P-value |
|---|---|---|---|---|---|---|
| Class IV | 2.19 (1.22–3.95) | 0.008 | ||||
| IFTA category (%) | Relative to IFTA <5% | <0.001 | 2.20 (1.29–3.74) | 0.004 | 1.40 (1.05–1.87) | 0.048 |
| ≥5–25 | 2.36 (1.13–4.90) | 0.021 | ||||
| ≥25–50 | 3.84 (1.53–9.62) | 0.004 | ||||
| ≥50 | 7.67 (3.75–15.67) | <0.001 | ||||
| Inflammation grade (%) | Relative to II < 5% | <0.001 | 1.58 (1.06–2.36) | 0.024 | 1.39 (1.08–1.79) | 0.028 |
| ≥5–25 | 2.14 (1.09–4.20) | 0.027 | ||||
| ≥25–50 | 4.21 (1.93–9.16) | <0.001 | ||||
| ≥50 | 11.41 (4.97–26.16) | <0.001 | ||||
| Proportion globally sclerosed glomeruli | 4.82 (1.64–14.15) | 0.004 | ||||
| Necrosis | 1.82 (1.05–3.14) | 0.031 | ||||
| Crescents | 1.47 (0.83–2.60) | 0.177 | ||||
| Arteriosclerosis | 1.28 (0.91–1.80) | 0.142 | ||||
| Age (years) | 0.99 (0.98–1.01) | 0.909 | 0.96 (0.93–0.99) | 0.036 | 0.99 (0.97–1.02) | 0.871 |
| Male | 0.89 (0.43–1.83) | 0.762 | 3.02 (1.02–8.92) | 0.044 | 1.01 (0.44–2.29) | 0.980 |
| Non-Hispanic White | 0.72 (0.40–1.30) | 0.284 | 0.69 (0.27–1.78) | 0.454 | 0.98 (0.49–1.94) | 0.958 |
| African American | 1.36 (0.75–2.48) | 0.307 | ||||
| Hispanic | 1.28 (0.68–2.39) | 0.431 | ||||
| SCr (mg/dL) | 2.05 (1.61–2.62) | <0.001 | 2.16 (1.50–3.11) | <0.001 | 1.68 (1.35–2.08) | <0.001 |
| BMI (kg/m2) | 0.96 (0.91–1.00) | 0.094 | ||||
| BP systolic (mmHg) | 1.16 (1.02–1.32) | 0.017 | ||||
| BP diastolic (mmHg) | 1.08 (1.01–1.17) | 0.024 | ||||
| Serum albumin (g/dL) | 0.59 (0.35–0.99) | 0.046 | ||||
| WBC count (1000/µL) | 0.94 (0.82–1.07) | 0.381 | ||||
| Hematocrit (%) | 0.89 (0.81–0.97) | 0.005 | ||||
| Platelet count (1000/µL) | 1.00 (0.99–1.00) | 0.470 | ||||
| 24-h protein (g) | 1.17 (1.06–1.28) | 0.001 | ||||
| Urine Pr/Cr (mg/mg) | 0.78 (0.49– 1.23) | 0.295 | ||||
| C3 (mg/dL) | 0.99 (0.98–1.00) | 0.335 | ||||
| C4 (mg/dL) | 0.99 (0.95–1.04) | 0.861 | ||||
| Anti-dsDNA positive | 0.87 (0.37–2.07) | 0.762 | ||||
| Diabetes | 1.28 (0.57–2.89) | 0.545 | ||||
| Family history of rheumatic disease | 1.61 (0.87–3.85) | 0.285 |
BMI, body mass index; BP, blood pressure; C3, complement component 3; C4, complement component 4; Cr, creatinine; dsDNA, double-stranded DNA; II, interstitial inflammation; Pr, protein; SCr, serum creatinine; WBC, white blood cells.
Tubulointerstitial lesions and risk categorization
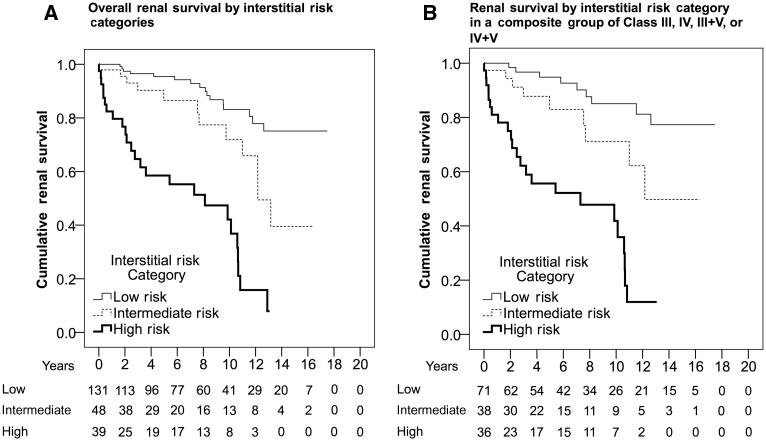
To visualize how interstitial inflammation and IFTA affect renal survival, patients were separated into three risk categories based on biopsy findings (Table 4) and outcomes (Supplementary data, Tables S6 and S7). Patients with the lowest risk for renal failure and mortality had the least severe tubulointerstitial lesions (green category in Table 4), whereas patients with the highest risk for renal failure and mortality had the most severe tubulointerstitial lesions (red category in Table 4). An increased proportion of patients with Class III and IV were in the intermediate- and high-risk categories compared with patients with Class II and V (Table 1). Interstitial risk category [area under the curve (AUC) = 0.739 (95% CI 0.655–0.823), P < 0.001] was a comparable predictor of renal survival compared with interstitial inflammation grade [AUC 0.709 (95% CI 0.624–0.794), P < 0.001] or IFTA category [AUC 0.686 (95% CI 0.598–0.774), P < 0.001] and simplifies the number of potential interstitial inflammation grade – IFTA category combinations. The interstitial risk category partially resolves the difficulty in distinguishing between the 5–25% and 25–50% groups because in many instances either estimate will place the patient in the same risk category. Notably, Hsieh et al. [12] reported that staining for CD45 is more sensitive for identifying interstitial inflammation and helps to distinguish between the intermediate categories.
Table 4.
Risk categories for renal survival by interstitial inflammation and interstitial fibrosis and tubular atrophy on initial biopsy (n = 301)
| Number of patients (% total) | Interstitial inflammation <5%, n (%) | Interstitial inflammation ≥5–25%, n (%) | Interstitial inflammation ≥25–50%, n (%) | Interstitial inflammation ≥50%, n (%) |
|---|---|---|---|---|
| IFTA <5% |
|
|
|
|
| IFTA ≥5–25% |
|
|
|
|
| IFTA ≥25–50% |
|
|
|
|
| IFTA ≥50% |
|
|
|
|
Green, low risk (n = 176, 9% ESRD or RRT, 8% mortality); yellow, intermediate risk (n = 72, 17% ESRD or RRT, 18% mortality); red, high risk (n = 53, 51% ESRD or RRT, 26% mortality).
The interstitial risk category was a significant predictor of renal survival in all patients [Figure 3A; relative to low risk: intermediate risk, HR 2.48 (95% CI 1.17–5.26); high risk, HR 8.41 (95% CI 4.48–15.78)], patients with Class IV (relative to Class IV low risk: Class IV with intermediate risk, HR 3.89 (95% CI 1.28–11.77); Class IV with high risk, HR 12.01 (95% CI 4.64–31.09)] and after separating Class IV into Class IV-A (relative to Class IV-A low risk: Class IV-A with intermediate risk, HR 1.12 (95% CI 0.13–9.69); Class IV-A with high risk, HR 5.35 (95% CI 1.40–20.34)] and Class IV-AC [relative to class IV-AC low risk, Class IV-AC with intermediate risk: HR 8.60 (95% CI 1.00–73.65); Class IV-AC with high risk, HR 27.40 (95% CI 3.15–238.01)]. Patients were also separated into groups based on the presence and absence of Class III or IV lesions. Group 1 consisted of patients with Class II or V [relative to Class II or V with low risk: intermediate risk, HR 1.71 (95% CI 0.43–6.69); high risk, HR 4.71 (95% CI 0.93–23.65)] and group 2 consisted of Class III, IV, III + V and IV + V (Figure 3B; relative to III/IV or III/IV + V with low risk: intermediate risk, HR 2.53 (95% CI 1.00–6.41); high risk, HR 8.71 (95% CI 3.96–19.12)].
Fig. 3.
Kaplan–Meier estimates for renal survival by interstitial risk categories: (A) all patients and (B) patients with Class III, IV, III + V and IV + V.
The value of repeat biopsy
A total of 94 patients underwent repeat biopsy and the median time between biopsies was 2.6 years. All repeat biopsies were indication biopsies for persistent or worsening renal insufficiency and proteinuria. A ‘clinically significant’ class switch from a non–Class III/IV lesion to Class III/IV or III/IV + V occurred in 6 of 11 patients with Class II and 6 of 17 patients with Class V on the first biopsy (Table 5). In contrast, 17 of 66 patients with Class III/IV on the first biopsy switched to Class II or V. A minority of these patients (6/17) had globally sclerosed glomeruli on repeat biopsy but no evidence of fibrous crescents or adhesions. Most patients had an increase in IFTA category on repeat biopsy [Tables 6 and 7; 51/94 (54%)] and the remaining patients either stayed in the same category (36/94) or showed a decrease (7/94). Similarly, many patients had increased (36/94) or persistent (20/94) interstitial inflammation, with a minority of patients showing a decrease (17/94) or no inflammation (21/94). A total of 11 patients reached the primary endpoint (ESRD or RRT) prior to repeat biopsy and were excluded from survival analysis. Approximately half of these patients were in the intermediate- (2/11) or high-risk (3/11) categories on initial biopsy and nearly all of these patients were in the intermediate- (3/11) and high-risk (7/11) categories on repeat biopsy.
Table 5.
ISN/RPS class on index and repeat biopsy (n = 94)
| ISN/RPS class | Index II (n = 11) | Index III (n = 12) | Index III + V (n = 3) | Index IV-S (n = 5) | Index IV-G (n = 43) | Index IV + V (n = 3) | Index V (n = 17) | Repeat biopsies (n = 94), n (%) |
|---|---|---|---|---|---|---|---|---|
| Repeat II | 3 | 2 | 1 | 2 | 8 (9) | |||
| Repeat III | 2 (AC = 1) | 5 (AC = 3) | 1 (AC = 1) | 2 (AC = 2) | 5 (AC = 4) | 15 (16) | ||
| Repeat III + V | 1 (AC = 1) | 2 (AC = 2) | 1 (AC = 1) | 4 (4) | ||||
| Repeat IV-S | 1 | 1 | 2 (2) | |||||
| Repeat IV-G | 3 (AC = 2) | 2 (AC = 2) | 23 (AC = 17, C = 1) | 1 (AC = 1) | 3 (AC = 2) | 32 (34) | ||
| Repeat IV + V | 1 (AC = 1) | 4 (AC = 4) | 2 (AC = 2) | 7 (7) | ||||
| Repeat V | 1 | 2 | 2 | 6 | 2 | 11 | 24 (26) | |
| Repeat VI | 1 | 1 | 2 (2) |
AC, number of biopsies with active and chronic lesions on repeat biopsy; C, number of biopsies with chronic lesions only.
Table 6.
Risk categories for renal survival on initial biopsy (n = 94)
| Number of patients (% total) | Interstitial inflammation <5%, n (%) | Interstitial inflammation ≥5–25%, n (%) | Interstitial inflammation ≥25–50%, n (%) | Interstitial inflammation ≥50%, n (%) |
|---|---|---|---|---|
| IFTA <5% | 38 (40) | 13 (13) | 2 (2) | 3 (3) |
| IFTA ≥5–25% | 8 (8) | 11 (12) | 3 (3) | 6 (6) |
| IFTA ≥25–50% | 1 (1) | 1 (1) | 3 (3) | 2 (2) |
| IFTA ≥50% | 1 (1) | 1 (1) | 0 | 1 (1) |
Green, low risk (n = 59, 25% ESRD or RRT, 12% mortality); yellow, intermediate risk (n = 18, 44% ESRD or RRT, 17% mortality); red, high risk (n = 17, 59% ESRD or RRT, 18% mortality).
Table 7.
Risk categories for renal survival on repeat biopsy (n = 94)
| Number of patients (% total) | Interstitial inflammation <5%, n (%) | Interstitial inflammation ≥5–25%, n (%) | Interstitial inflammation ≥25–50%, n (%) | Interstitial inflammation ≥50%, n (%) |
|---|---|---|---|---|
| IFTA <5% | 21 (22) | 7 (7) | 0 | 0 |
| IFTA ≥5–25% | 5 (5) | 13 (14) | 4 (4) | 3 (3) |
| IFTA ≥25–50% | 1 (1) | 6 (6) | 8 (9) | 4 (4) |
| IFTA ≥50% | 2 (2) | 8 (9) | 1 (1) | 11 (12) |
Green, low risk (n = 33, 15% ESRD or RRT, 3% mortality); yellow, intermediate risk (n = 24, 38% ESRD or RRT, 9% mortality); red, high risk (n = 37, 57% ESRD or RRT, 24% mortality).
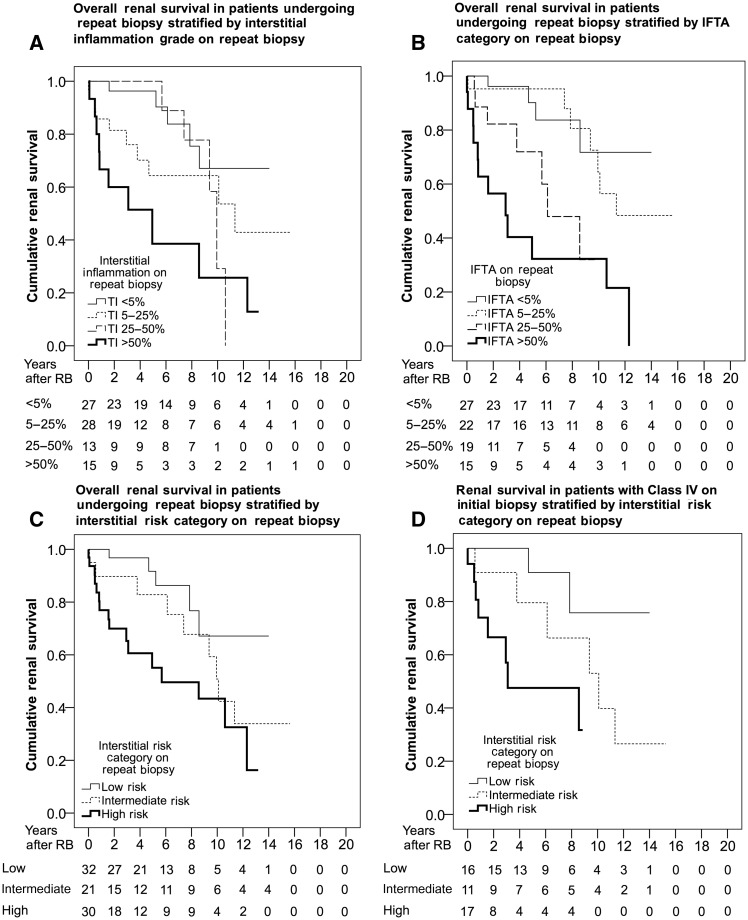
Interstitial inflammation grade in the second biopsy was associated with worse renal survival in all patients [Figure 4A; HR 1.66 (95% CI 1.20–2.29)] and in patients with Class IV on initial biopsy [HR 2.36 (95% CI 1.41–3.94)]. Similarly, the IFTA category was associated with worse renal survival in all patients [Figure 4B; HR 1.96 (95% CI 1.40–2.75)] and in those with Class IV on initial biopsy [HR 3.24 (95% CI 1.73–6.07)]. To determine if the interstitial risk category was predictive of renal survival at repeat biopsy, patients were grouped into categories as previously described (Table 7). The interstitial risk category was a significant predictor of renal survival at repeat biopsy in all patients [Figure 4C; relative to low risk: intermediate risk, HR 2.44 (95% CI 0.81–7.31); high risk, HR 4.32 (95% CI 1.57–11.82)] and in those with Class IV on initial biopsy [Figure 4D; relative to low risk: intermediate risk, HR 4.03 (95% CI 0.81–20.09); high risk, HR 9.93 (95% CI 1.94–50.68)].
Fig. 4.
Kaplan–Meier estimates for renal survival in patients undergoing repeat biopsy. (A) Interstitial inflammation grade in all patients. (B) IFTA category in all patients. (C) Interstitial risk category in all patients. (D) Interstitial risk category in patients with Class IV on the initial biopsy.
There was a correlation between interstitial inflammation on the first biopsy and IFTA on the second biopsy (r = 0.433, P < 0.001). Interstitial inflammation grade on the first biopsy was significantly associated with IFTA category on the second biopsy independent of age, gender, race, ethnicity and IFTA category on the first biopsy [interstitial inflammation on the first biopsy: odds ratio (OR) 1.61 (95% CI 1.04–2.47); IFTA on the first biopsy: OR 2.23 (95% CI 1.24–3.99)]. In other words, for a unit increase in interstitial inflammation grade on the initial biopsy, a patient is approximately 1.6 times as likely to have an increase in IFTA category on repeat biopsy.
Discussion
The utility of kidney biopsy has been questioned since increasingly sensitive and specific laboratory testing is available for the diagnosis of lupus nephritis. This study demonstrates the independent prognostic value of interstitial inflammation and IFTA after adjusting for established risk factors for renal survival, including race, ethnicity, age and elevated serum creatinine. Increased interstitial inflammation grade or IFTA category stratified risk for renal survival in all patients and those with ISN/RPS Class IV on initial and repeat biopsy. For a unit increase in inflammation grade or IFTA category, there was an ∼1.5- to 2-fold increased rate of renal death during the study period.
The ISN/RPS classification has been criticized for focusing predominantly on glomerular lesions when tubulointerstitial lesions better predict renal survival [12–19]. Many studies have relied on the National Institutes of Health (NIH) chronicity index to demonstrate the effect of renal scarring on outcomes, however, the NIH chronicity index is a composite score of glomerular and tubulointerstitial injury that does not convey the dominant effect of IFTA [15]. In our study, ISN/RPS Class IV was a significant predictor of renal survival in univariate analysis and patients with both active and chronic glomerular lesions (IV-AC) had worse renal survival than patients with only active lesions (IV-A). These data complement the report by Hiramatsu et al. [20], which showed that chronic glomerular lesions are a key determinant of response to therapy in Class IV [20]. However, Hiramatsu et al. did not systematically evaluate tubulointerstitial lesions. In fact, neither Class IV nor the proportion of glomeruli with chronic lesions was a significant predictor of renal survival after adjusting for interstitial inflammation grade and IFTA category in our multivariable models. One potential explanation is that a significant portion of the risk attributable to ISN/RPS Class IV is due to the increased frequency of severe tubulointerstitial lesions (Table 1). Similar to our study, Yu et al. [21] showed that patients with Class IV have an increased proportion of moderate to severe tubulointerstitial lesions and that glomerular sclerosis was not a significant predictor of renal outcome after adjusting for interstitial inflammation, interstitial fibrosis or tubular atrophy [21]. In our experience, there were very few patients (4%) with significant glomerulosclerosis (>30%) that did not have any IFTA (<5%), which suggests that IFTA may be a more sensitive indicator of kidney injury.
Tubulointerstitial lesions progress on repeat biopsy. In our study, 54% of patients had increased IFTA on their second biopsy. Furthermore, interstitial inflammation on the first biopsy was independently associated with increased IFTA on the second biopsy and approximately two-thirds of the patients with moderate to severe interstitial inflammation on the second biopsy (interstitial inflammation 25–50% or interstitial inflammation >50%) had mild or absent inflammation on the first biopsy (interstitial inflammation 5–25% or interstitial inflammation <5%). In a large repeat biopsy study (n = 142), Pagni et al. [22] showed that patients with Class IV are more likely to have interstitial inflammation on the first biopsy and increased interstitial fibrosis on the second biopsy. These data suggest that increased inflammation leads to fibrosis but are limited by the fact that many patients were biopsied for worsening renal insufficiency or proteinuria. Some authors advocate for protocol biopsies after induction or maintenance therapy to assess response to treatment, whereas others prefer to reserve repeat biopsy for renal flares [23–30]. In general, these studies have shown that clinical variables do not predict histologic remission, a change in ISN/RPS class during renal flare is not uncommon and most patients show progression of the chronicity index. However, few studies have critically examined interstitial inflammation as a predictor of long-term renal outcome. Notably, Alsuwaida et al. [31] showed that increased interstitial inflammation on repeat biopsy at 12–18 months was a poor predictor of renal survival. These findings are similar to our study, which showed that both interstitial inflammation and IFTA are significant predictors of renal survival in all patients undergoing repeat biopsy and in those with Class IV on the first biopsy.
Tubulointerstitial lesions are important predictors of renal survival in diseases other than lupus nephritis. The Oxford Classification of immunoglobulin A (IgA) nephropathy uses semiquantitative grading of interstitial fibrosis and tubular (T)-score, which has been shown to strongly predict renal survival in meta-analysis [32, 33]. Increased interstitial inflammation has also been shown to predict disease progression in IgA nephropathy [34]. Inflammatory cells in the interstitium assemble into aggregates that may worsen tissue injury by enhancing antigen presentation and autoantibody production [35]. Interstitial inflammation has been implicated in membranous nephropathy, acute and chronic interstitial nephritis, allograft rejection and lupus nephritis [36–38]. Interestingly, interstitial inflammation in lupus nephritis does not appear to be related to tubulointerstitial immune deposits but may be caused by autoantibodies to interstitial antigens like vimentin [39, 40]. Interstitial inflammation can occur in the absence of glomerular inflammation and these studies suggest that preventing the development of tubulointerstitial lesions may be a therapeutic target in lupus nephritis (recently reviewed by Clark et al. [13]).
The reproducibility of interstitial lesion grading is a significant limitation [32, 41]. On the one hand, intragrade correlation coefficients (ICCs) for interstitial inflammation (ICC = 0.58) and IFTA (ICC = 0.78) were considered ‘good’ or ‘very good’ by the Oxford IgA Nephropathy Working Group and are similar to values from our study (interstitial inflammation ICC = 0.67, IFTA ICC = 0.65). The values from our study represent an inter-observer agreement of approximately 77% [41]. On the other hand, this level of reproducibility has been criticized as too unreliable for clinical use. The majority of disagreements in our study were the result of a grading change from absent to mild or mild to moderate and infrequently resulted in a change in risk category (∼19%). Reproducibility of ISN/RPS class further complicates risk stratification and the clinical utility of grading interstitial lesions has not been established [42, 43].
Recent studies by Hsieh et al., [12] Yu et al. [21] and Alsuwaida et al. [24] have shown that semiquantitative grading of IFTA and interstitial inflammation are the key predictors of renal survival over and above the NIH activity and chronicity index. Hsieh et al. [12] compared CD45 staining to modified light microscopy, which uses standard immunohistochemistry to quantify interstitial inflammation in areas away from fibrosis, and found that CD45 staining helps distinguish between the intermediate categories of interstitial inflammation. Renal survival analysis was similar between methods, but the increased sensitivity of CD45 staining nearly eliminated patients from the lower tiers of interstitial inflammation (0% and <10%) compared with modified light microscopy where patients were more evenly spread across groups. Nevertheless, our study showed similar results using modified light microscopy and expanded on these findings by examining how tubulointerstitial lesions affect renal survival in a subset of patients with ISN/RPS Class IV. This is an important distinction because patients with Class IV have a larger proportion of moderate to severe tubulointerstitial lesions and are more likely to receive immunosuppressive therapy, which could potentially confound the survival analysis. Hsieh et al. [12], Yu et al. [21] and Alsuwaida et al. [24] grouped all ISN/RPS classes together when they reported the effect of tubulointerstitial lesions on renal survival. Hsieh et al. (n = 68) and Alsuwaida et al. (n = 73) were limited by sample size, whereas Yu et al. (n = 313) published the largest study looking at IFTA and interstitial inflammation in lupus nephritis [21]. When comparing our study with Yu et al. [21], there was a similar-sized patient population, age at initial biopsy and mean follow-up time. Our study had fewer patients with Class III/IV [172/301 (57%) versus 225/313 (72%); P < 0.001], an increased proportion of patients with chronic glomerular lesions [III/IV-AC: 82/172 (47%) versus 76/225 (34%); P = 0.005] and a similar proportion of severe tubulointerstitial lesions [interstitial inflammation >50%: 31/301 (10%) versus 24/313 (8%); IFTA >50%: 18/301 (6%) versus 17/313 (5%)], which led to modestly worse renal survival [ESRD or RRT: 54/301 (18%) versus 37/313 (12%); P = 0.04] and more patients that died in long-term follow-up [40/301 (13%) versus 3/301 (1%); P < 0.001]. Our study consisted of a mix of African American, Hispanic and non-Hispanic Whites, whereas their study was predominantly Asian. Yu et al. [21] placed patients into groups based on the severity of active glomerular lesions and active or chronic tubulointerstitial lesions, which makes it difficult to compare the independent effects of interstitial inflammation and IFTA on renal survival. Furthermore, they did not incorporate both of these variables into any of their multivariable models. However, despite significant differences, all of these studies showed a dose-dependent decrease in renal survival as interstitial inflammation or IFTA increased and may help to define the contribution of tubulointerstitial lesions within the ISN/RPS classification of lupus nephritis.
Supplementary Material
Acknowledgements
P.C.W., G.M. and M.K. were part of ‘Increasing incidence of Class V membranous nephritis: a single institution biopsy study’. American Society of Nephrology Annual Meeting, 5–8 November 2015, San Diego, CA, USA. J Am Soc Nephrol; October 2015; abstract 491.
P.C.W., M.K. and G.M. were part of ‘Immunotherapy mitigates racial disparities in lupus nephritis outcomes’. United States and Canadian Academy of Pathology Annual Meeting, 3–8 March 2016, Seattle, WA, USA. Mod Pathol February 2016, abstract 1628.
Conflict of interest statement
None declared.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
References
- 1. Korbet SM, Schwartz MM, Evans J. et al. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007; 18: 244–254 [DOI] [PubMed] [Google Scholar]
- 2. Nee R, Martinez-Osorio J, Yuan CM. et al. Survival disparity of African-American versus non-African-American patients with ESRD due to SLE. Am J Kidney Dis 2015; 66: 630–637 [DOI] [PubMed] [Google Scholar]
- 3. Hanly JG, O'Keeffe AG, Su L. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weening JJ, D'Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004; 15: 241–250 [DOI] [PubMed] [Google Scholar]
- 5. Wilhelmus S, Bajema IM, Bertsias GK. et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant 2016; 31: 904–913 [DOI] [PubMed] [Google Scholar]
- 6. Lightstone L. The landscape after LUNAR: rituximab's crater-filled path. Arthritis Rheum 2012; 64: 962–965 [DOI] [PubMed] [Google Scholar]
- 7. Moroni G, Doria A, Mosca M. et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 2006; 1: 925–932 [DOI] [PubMed] [Google Scholar]
- 8. Henderson LK, Masson P, Craig JC. et al. Induction and maintenance treatment of proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2013; 61: 74–87 [DOI] [PubMed] [Google Scholar]
- 9. Deng J, Huo D, Wu Q. et al. A meta-analysis of randomized controlled trials comparing tacrolimus with intravenous cyclophosphamide in the induction treatment for lupus nephritis. Tohoku J Exp Med 2012; 227: 281–288 [DOI] [PubMed] [Google Scholar]
- 10. Racusen LC, Solez K, Colvin RB. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO). Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011) 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsieh C, Chang A, Brandt D. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011; 63: 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark MR, Trotter K, Chang A.. The pathogenesis and therapeutic implications of tubulointerstitial inflammation in human lupus nephritis. Semin Nephrol 2015; 35: 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neumann K, Wallace DJ, Azen C. et al. Lupus in the 1980s: III. Influence of clinical variables, biopsy, and treatment on the outcome in 150 patients with lupus nephritis seen at a single center. Semin Arthritis Rheum 1995; 25: 47–55 [DOI] [PubMed] [Google Scholar]
- 15. Austin HA 3rd, Muenz LR, Joyce KM. et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 1983; 75: 382–391 [DOI] [PubMed] [Google Scholar]
- 16. Appel GB, Cohen DJ, Pirani CL. et al. Long-term follow-up of patients with lupus nephritis. a study based on the classification of the World Health Organization. Am J Med 1987; 83: 877–885 [DOI] [PubMed] [Google Scholar]
- 17. Parichatikanond P, Francis ND, Malasit P. et al. Lupus nephritis: clinicopathological study of 162 cases in Thailand. J Clin Pathol 1986; 39: 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esdaile JM, Levinton C, Federgreen W. et al. The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: a study of 87 patients and review of the literature. Q J Med 1989; 72: 779–833 [PubMed] [Google Scholar]
- 19. Williams W, Sargeant LA, Smikle M. et al. The outcome of lupus nephritis in Jamaican patients. Am J Med Sci 2007; 334: 426–430 [DOI] [PubMed] [Google Scholar]
- 20. Hiramatsu N, Kuroiwa T, Ikeuchi H. et al. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford) 2008; 47: 702–707 [DOI] [PubMed] [Google Scholar]
- 21. Yu F, Wu LH, Tan Y. et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int 2010; 77: 820–829 [DOI] [PubMed] [Google Scholar]
- 22. Pagni F, Galimberti S, Galbiati E. et al. Tubulointerstitial lesions in lupus nephritis: International multicentre study in a large cohort of patients with repeat biopsy. Nephrology (Carlton) 2016; 21: 35–45 [DOI] [PubMed] [Google Scholar]
- 23. Pagni F, Galimberti S, Goffredo P. et al. The value of repeat biopsy in the management of lupus nephritis: an international multicentre study in a large cohort of patients. Nephrol Dial Transplant 2013; 28: 3014–3023 [DOI] [PubMed] [Google Scholar]
- 24. Alsuwaida A, Husain S, Alghonaim M. et al. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrol Dial Transplant 2012; 27: 1472–1478 [DOI] [PubMed] [Google Scholar]
- 25. Daleboudt GM, Bajema IM, Goemaere NN. et al. The clinical relevance of a repeat biopsy in lupus nephritis flares. Nephrol Dial Transplant 2009; 24: 3712–3717 [DOI] [PubMed] [Google Scholar]
- 26. Lu J, Tam LS, Lai FM. et al. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. Am J Nephrol 2011; 34: 220–225 [DOI] [PubMed] [Google Scholar]
- 27. Hill GS, Delahousse M, Nochy D. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int 2001; 59: 304–316 [DOI] [PubMed] [Google Scholar]
- 28. Zickert A, Sundelin B, Svenungsson E. et al. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014; 1: e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh A, Ghosh R, Kaur P. et al. Protocol renal biopsy in patients with lupus nephritis: a single center experience. Saudi J Kidney Dis Transpl 2014; 25: 801–807 [DOI] [PubMed] [Google Scholar]
- 30. Esdaile JM, Joseph L, MacKenzie T. et al. The pathogenesis and prognosis of lupus nephritis: information from repeat renal biopsy. Semin Arthritis Rheum 1993; 23: 135–148 [DOI] [PubMed] [Google Scholar]
- 31. Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 2013; 22: 1446–1454 [DOI] [PubMed] [Google Scholar]
- 32. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 33. Lv J, Shi S, Xu D. et al. Evaluation of the Oxford classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 2013; 62: 891–899 [DOI] [PubMed] [Google Scholar]
- 34. Myllymaki JM, Honkanen TT, Syrjanen JT. et al. Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int 2007; 71: 343–348 [DOI] [PubMed] [Google Scholar]
- 35. Pei G, Zeng R, Han M. et al. Renal interstitial infiltration and tertiary lymphoid organ neogenesis in IgA nephropathy. Clin J Am Soc Nephrol 2014; 9: 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steinmetz OM, Velden J, Kneissler U. et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int 2008; 74: 448–457 [DOI] [PubMed] [Google Scholar]
- 37. Chang A, Henderson SG, Brandt D. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 2011; 186: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Segerer S, Schlondorff D.. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int 2008; 73: 533–537 [DOI] [PubMed] [Google Scholar]
- 39. Park MH, D'Agati V, Appel GB. et al. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron 1986; 44: 309–319 [DOI] [PubMed] [Google Scholar]
- 40. Kinloch AJ, Chang A, Ko K. et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol 2014; 66: 3359–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hisano S, Joh K, Katafuchi R. et al. Reproducibility for pathological prognostic parameters of the Oxford classification of IgA nephropathy: a Japanese cohort study of the Ministry of Health, Labor and Welfare. Clin Exp Nephrol 2017; 21: 92–96 [DOI] [PubMed] [Google Scholar]
- 42. Furness PN, Taub N.. Interobserver reproducibility and application of the ISN/RPS classification of lupus nephritis-a UK-wide study. Am J Surg Pathol 2006; 30: 1030–1035 [DOI] [PubMed] [Google Scholar]
- 43. Restrepo-Escobar M, Granda-Carvajal PA, Jaimes F.. Systematic review of the literature on reproducibility of the interpretation of renal biopsy in lupus nephritis. Lupus 2017. doi: 10.1177/0961203317706556 (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.