Abstract
Mitogen-activated protein kinase (MAPK) pathways regulate many cellular functions including cell proliferation and apoptosis. We examined associations of differential gene and microRNA (miRNA) expression between carcinoma and paired normal mucosa for 241 genes in the KEGG-identified MAPK-signaling pathway among 217 colorectal cancer (CRC) cases. Gene expression data (RNA-Seq) and miRNA expression data (Agilent Human miRNA Microarray V19.0; Agilent Technologies Inc., Santa Clara, CA, USA) were analyzed. We first identified genes most strongly associated with CRC using a fold change (FC) of >1.50 or <0.67) that were statistically significant after adjustment for multiple comparisons. We then determined miRNAs associated with dysregulated genes and through miRNA:mRNA (messenger RNA) seed region matches discerned genes with a greater likelihood of having a direct biological association. Ninety-nine genes had a meaningful FC for all CRC, microsatellite unstable–specific tumors, or microsatellite stable–specific tumors. Thirteen dysregulated genes were associated with miRNAs, totaling 68 miRNA:mRNA associations. Thirteen of the miRNA:mRNA associations had seed region matches where the differential expression between the miRNA and mRNA was inversely related suggesting a direct association as a result of their binding. Several direct associations, upstream of ERK1/ERK2, JNK, and p38, were found for PDGFRA with 7 miRNAs; RASGRP3 and PRKCB with miR-203a; and TGFBR1 with miR-6071 and miR-2117. Other associations between miRNAs and mRNAs are most likely indirect, resulting from feedback and feed forward loops. Our results suggest that miRNAs may alter MAPK signaling through direct binding with key genes in this pathway. We encourage others to validate results in targeted CRC experiments that can help solidify important therapeutic targets.
Keywords: MAPK, colorectal cancer, miRNA, mRNA, ERK1/2
Introduction
Mitogen-activated protein kinases (MAPKs) are involved in the regulation of cell proliferation, differentiation, migration, and apoptosis and can influence gene expression.1 The 3 major MAPK pathways are extracellular-regulated kinases 1 and 2 (ERK1/ERK2), c-Jun-N-terminal kinases (JNKs), and p38 (MAPK14). ERK1/ERK2s are activated by growth factors and cytokines.1,2 The JNK pathway is involved in regulating responses to stress, inflammation, and apoptosis and is activated by radiation, environmental stresses, and growth factors. P38 MAPKs are involved in autoimmunity and are activated by chemical stresses, hormones, and cytokines including IL-1 (interleukin 1) and TNF (tumor necrosis factor)1,3 and targets several transcription factors (TFs) including NF-κB (nuclear factor κB) and TP53.3 Within each of these major MAPK pathways is 3-tiered and includes an MAP kinase kinase kinase (MAP3K, MEKK, or MKKK), MAP kinase kinase (MAP2K, MEK, or MKK), and the MAP kinase (MAPK). Dual-specificity MAPK phosphatases (MKPs or DUSPs) modulate the activity of MAPKs.
MAPK signaling can mediate microRNAs (miRNAs), small noncoding RNAs that regulate gene expression through translation repression or degradation of messenger RNA (mRNAs).4 One mechanism through which MAPK may affect miRNAs is through their ability to stabilize Dicer, the enzyme that cleaves double-stranded RNA and pre-miRNA into small-interfering RNA and miRNA. TRBP (HIV-1 TAR RNA-binding protein), which is regulated by the MAPK pathway ERK, stabilizes Dicer, regulating miRNA biogenesis.4,5 In addition, several miRNAs have been associated with components of MAPK signaling. MiR-101 has been shown to target MAPK phosphatase-1 (MKP-1), which is involved in inflammatory response.6 Although miR-101 has been linked to MKP-1, many other miRNAs have been associated with various components of immune response,6 suggesting that other miRNAs also might be related to MKP-1. Both miR-4728 and miR-564 have been shown to be an antagonist of MAPK signaling in breast cancer.7,8 The interactions between miRNAs and genes in the MAPK-signaling pathway in colorectal cancer (CRC) have not been explored, although studies have shown the importance of MAPK signaling in the cause and progression of CRC.9
In this study, we focused on the genes identified in Kyoto Encyclopedia of Genes and Genomes (KEGG) MAPK-signaling pathway. We identified genes within the MAPK-signaling pathway that were significantly dysregulated in CRC tissue when comparing carcinoma with adjacent normal mucosa. Evaluation of those mRNAs with a fold change (FC) of >1.50 or <0.67 with dysregulated miRNAs enabled us to identify miRNA:mRNA associations. Further evaluation of seed region matches between the miRNA and mRNA provided an indication of whether the miRNA was directly influencing gene expression or if the association was one of an indirect nature in that gene expression was altered because of binding elsewhere that influenced feedback loops in the pathway.
Methods
Study participants
Two population-based case-control studies, including all incident patients with colon and rectal cancer diagnosed between 30 and 79 years of age in Utah or who were members of Kaiser Permanente Northern California (KPNC), were used. For the colon cancer study, participants were non-Hispanic white, Hispanic, or black; for the rectal cancer study, Asian participants were included.10,11 Cases were verified by tumor registry as a first primary adenocarcinoma of the colon or rectum, diagnosed between October 1991 and September 1994 (colon study) or between May 1997 and May 2001 (rectal study).12 The study was approved by the Institutional Review Boards at the University of Utah and at KPNC.
RNA processing
Formalin-fixed paraffin embedded tissue from either the initial biopsy or surgery was used for RNA extraction. RNA was extracted, isolated, and purified from carcinoma tissue and adjacent normal mucosa as previously described.13 We observed no differences in RNA quality based on age of the tissue.
mRNA: RNA-Seq sequencing library preparation and data processing
Total RNA from 245 colorectal carcinoma and normal mucosa pairs was chosen for sequencing based on availability of RNA and high-quality miRNA data to have both mRNA and miRNA from the same individuals; the 217 pairs that passed quality control (QC) were used in these analyses.14 RNA library construction was performed with the Illumina TruSeq Stranded Total RNA Sample Preparation Kit with Ribo-Zero (Illumina Inc., San Diego, CA, USA). The samples were then fragmented and primed for complementary DNA (cDNA) synthesis, adapters were then ligated onto the cDNA, and the resulting samples were then amplified using polymerase chain reaction (PCR); the amplified library was then purified using Agencourt AMPure XP beads (Beckman Coulter, Inc. CA, USA). A more detailed description of the methods can be found in our previous work.15 Illumina TruSeq v3 single-read flow cell and a 50-cycle single-read sequence run were performed on an Illumina HiSeq instrument. Reads were aligned to a sequence database containing the human genome (build GRCh37/hg19, February 2009, from genome.ucsc.edu) and alignment was performed using Novoalign v2.08.01. Total gene counts were calculated for each exon and untranslated region (UTR) of the genes using gene coordinates obtained from http://genome.ucsc.edu. Genes that were not expressed in our RNA-Seq data or for which the expression was missing for most of the samples were excluded from further analysis.15
MicroRNA
The Agilent Human miRNA Microarray V19.0 (Agilent Technologies Inc., Santa Clara, CA, USA) was used. Data were required to pass QC parameters established by Agilent that included tests for excessive background fluorescence, excessive variation among probe sequence replicates on the array, and measures of the total gene signal on the array to assess low signal. Samples failing to meet quality standards were relabeled, hybridized to arrays, and rescanned. If a sample failed QC assessment a second time, the sample was excluded from analysis. The repeatability associated with this microarray was extremely high (r = .98)12; comparison of miRNA expression levels obtained from the Agilent microarray with those obtained from quantitative PCR had an agreement of 100% in terms of directionality of findings and the FCs were almost identical.16 To normalize differences in miRNA expression that could be attributed to the array, amount of RNA, location on array, or factors that could erroneously influence miRNA expression levels, total gene signal was normalized by multiplying each sample by a scaling factor which was the median of the 75th percentiles of all the samples divided by the individual 75th percentile of each sample.17
MAPK-signaling genes
The KEGG (www.genome.jp/kegg/pathway/hsa/hsa04010.html) pathway map for MAPK signaling was used to identify genes. We identified 255 genes (Supplemental Table S1) in this signaling pathway; 241 of these genes had sufficient expression in CRC tissue for statistical analysis.
Statistical methods
We performed negative binomial mixed effects modeling in SAS (accounting for carcinoma/normal status as well as subject effect) to determine which genes in the MAPK-signaling pathway had a statistically significant difference in expression between individually paired CRC and normal mucosa and their related FC. We offset the overall exposure as the log of the expression of all identified protein-coding genes in the negative binomial model (n = 17 461). The Benjamini and Hochberg18 method was used to control the false discovery rate (FDR) with a value of <0.05. An FC greater than 1 indicates a positive differential expression (ie, upregulated in carcinoma tissue); an FC between 0 and 1 indicates a negative differential expression (ie, downregulated in carcinoma tissue). We calculated the level of expression of each gene by dividing the total expression for that gene in an individual by the total expression of all protein-coding genes per million transcripts (RPMPCG or reads per million protein-coding genes). We considered overall CRC differential expression as well as differential expression for microsatellite unstable (MSI) and stable (MSS) tumors separately.
We focused on those genes and miRNAs with FCs of >1.50 or <0.67 to have find meaningful biological differences between carcinoma and normal samples. There were 814 miRNAs expressed in greater than 20% of normal colorectal mucosa samples that were analyzed; differential expression was calculated using subject-level paired data as the expression in the carcinoma tissue minus the expression in the normal mucosa. In these analyses, we fit a least squares linear regression model to the RPMPCG differential expression levels and miRNA differential expression levels. P values were generated using the bootstrap method by creating a distribution of 10 000 F statistics derived by resampling the residuals from the null hypothesis model of no association between gene expression and miRNA expression using the boot package in R. Linear models were adjusted for age and sex. Multiplicity adjustments for gene/miRNA associations were made at the gene level using the FDR by Benjamini and Hochberg.18
Bioinformatics analysis
We analyzed significantly associated genes with FC of <0.67 and >1.50 for seed region matches with miRNAs. The mRNA 3ʹ UTR FASTA as well as the seed region sequence of the associated miRNA was analyzed to determine seed region pairings between miRNA and mRNA. MicroRNA seed regions were calculated as described in our previous work19; we included seeds of 6, 7, and 8 nucleotides in length. A seed match would increase the likelihood that an identified miRNA:mRNA interaction was more likely to have a direct biological effect on expression given a higher propensity for binding. Because miRTarBase20 uses findings from many different investigations and alignments, we used FASTA sequences generated from both GRCh37 and GRCh38 Homo sapiens, using UCSC Table Browser (https://genome.ucsc.edu/cgi-bin/hgTables).21 FASTA sequences that matched our Ensembl IDs and had consensus coding sequences available were downloaded. Scripts in R 3.2.3 and in perl 5.018002 were used to conduct the analysis.
Results
The study population comprised 77.9% of participants who were diagnosed with first primary colon cancer and 22.1% with first primary rectal cancer (Table 1). Most of the study participants were men (54.4%) and non-Hispanic white (74.2%). The most common tumor mutation was in TP53 (47.5%); 13.4% had an MSI tumor.
Table 1.
Description of study population.
| No. (%) | |
|---|---|
| Site | |
| Colon | 169 (77.9) |
| Rectal | 48 (22.1) |
| Sex | |
| Male | 118 (54.4) |
| Female | 99 (45.6) |
| Age | |
| Mean (SD) | 64.8 (10.1) |
| Race | |
| Non-Hispanic white | 161 (74.2) |
| Hispanic | 14 (6.5) |
| Non-Hispanic black | 8 (3.7) |
| Unknown | 34 (15.7) |
| Tumor phenotype | |
| TP53 mutated | 103 (47.5) |
| KRAS mutated | 69 (31.8) |
| BRAF-mutated | 21 (10.1) |
| CIMP high | 45 (20.7) |
| MSI | 29 (13.4) |
Abbreviations: CIMP, CpG island methylator phenotype; MSI, microsatellite unstable.
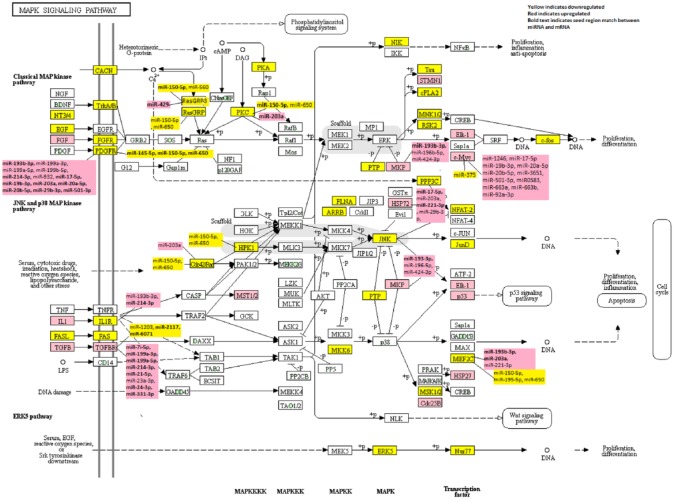
Of the 241 genes analyzed in the KEGG MAPK pathway, 83 (34.4%) were statistically significantly dysregulated in CRC overall with an FC of >1.50 or <0.67 (Table 2; Supplemental Table S2 has a list of all genes in the pathway). Of these 83 dysregulated genes, 60 were downregulated and 23 were upregulated. The most downregulated genes were fibroblast growth factor 9 (FGF9) (FC 0.18), protein phosphatase 3 regulatory subunit B (PPP3R2) (FC 0.21), and 2 calcium channel voltage-dependent genes subunit gamma 3 and 7 (CACNG3 and CACNG7) (FC 0.25). Interestingly, 2 of the most upregulated genes were also FGFs (FGF20 FC 5.29 and FGF19 FC 9.42). The gene that was most upregulated in carcinomas was CACNG4 (FC 3.30). Figure 1 illustrates the up- and downregulated genes in the MAPK pathway.
Table 2.
Significant differentially expressed genes in the MAPK-signaling pathway where fold change is <0.67 or >1.50.
| Gene name | Tumor mean | Normal mean | Fold change | P value | Adjusted P value |
|---|---|---|---|---|---|
| Downregulated | |||||
| FGF9 | 1.66 | 9.08 | 0.18 | 4.27E−30 | 6.44E−29 |
| PPP3R2 | 0.20 | 0.93 | 0.21 | 3.56E−07 | 8.02E−07 |
| CACNG3 | 0.16 | 0.62 | 0.25 | 4.46E−04 | 7.32E−04 |
| CACNG7 | 0.17 | 0.67 | 0.25 | 1.91E−05 | 3.48E−05 |
| CACNA1A | 3.27 | 12.58 | 0.26 | 2.27E−32 | 4.57E−31 |
| CACNA1G | 1.26 | 4.80 | 0.26 | 4.08E−16 | 1.79E−15 |
| CACNA1F | 1.68 | 6.25 | 0.27 | 1.60E−21 | 1.01E−20 |
| RASGRP2 | 6.72 | 24.73 | 0.27 | 1.04E−31 | 1.93E−30 |
| PRKCB | 14.65 | 53.12 | 0.28 | 1.52E−41 | 7.31E−40 |
| CACNA1I | 1.91 | 6.56 | 0.29 | 8.80E−19 | 4.51E−18 |
| MAPK10 | 9.26 | 28.10 | 0.33 | 8.93E−34 | 1.96E−32 |
| MAP4K1 | 7.24 | 21.46 | 0.34 | 1.58E−26 | 1.74E−25 |
| CACNG2 | 0.21 | 0.57 | 0.37 | 2.28E−03 | 3.41E−03 |
| PRKACB | 62.60 | 162.73 | 0.38 | 5.72E−40 | 1.97E−38 |
| FGFR2 | 26.51 | 68.82 | 0.39 | 2.32E−21 | 1.40E−20 |
| MEF2C | 27.15 | 66.82 | 0.41 | 1.00E−41 | 6.02E−40 |
| FOS | 185.52 | 453.52 | 0.41 | 9.65E−31 | 1.66E−29 |
| CACNG1 | 0.27 | 0.65 | 0.42 | 3.35E−05 | 5.89E−05 |
| FGF10 | 1.70 | 4.06 | 0.42 | 1.85E−10 | 5.45E−10 |
| PTPN5 | 0.89 | 2.09 | 0.42 | 7.08E−07 | 1.55E−06 |
| DUSP1 | 67.66 | 154.53 | 0.44 | 1.02E−34 | 2.46E−33 |
| EGF | 2.83 | 6.34 | 0.45 | 1.71E−05 | 3.15E−05 |
| CACNB2 | 26.71 | 59.55 | 0.45 | 1.00E−38 | 3.03E−37 |
| IL1R2 | 7.02 | 15.61 | 0.45 | 1.00E−13 | 3.77E−13 |
| NTRK1 | 1.10 | 2.43 | 0.45 | 3.24E−07 | 7.36E−07 |
| CACNA1B | 1.79 | 3.91 | 0.46 | 1.99E−06 | 4.14E−06 |
| NTRK2 | 17.81 | 38.41 | 0.46 | 1.04E−08 | 2.67E−08 |
| DUSP5 | 28.27 | 59.40 | 0.48 | 1.39E−22 | 9.28E−22 |
| CACNA1H | 53.11 | 106.08 | 0.50 | 5.22E−27 | 5.99E−26 |
| FGFR3 | 36.15 | 70.78 | 0.51 | 1.13E−18 | 5.54E−18 |
| MAPT | 4.51 | 8.74 | 0.52 | 1.01E−12 | 3.40E−12 |
| NFATC1 | 12.01 | 22.97 | 0.52 | 3.23E−17 | 1.50E−16 |
| PDGFRA | 88.47 | 166.61 | 0.53 | 5.08E−26 | 5.11E−25 |
| CACNA2D3 | 1.56 | 2.91 | 0.53 | 1.46E−04 | 2.46E−04 |
| MAPK7 | 21.28 | 38.75 | 0.55 | 6.78E−29 | 9.08E−28 |
| FAS | 28.16 | 50.95 | 0.55 | 2.75E−24 | 2.46E−23 |
| CACNA1S | 0.56 | 1.00 | 0.56 | 6.90E−03 | 1.00E−02 |
| RASGRP1 | 21.77 | 38.90 | 0.56 | 1.20E−22 | 8.23E−22 |
| PRKACG | 0.17 | 0.31 | 0.56 | 1.84E−03 | 2.79E−03 |
| RASGRP3 | 25.53 | 45.22 | 0.56 | 1.16E−20 | 6.81E−20 |
| FGF2 | 10.04 | 17.66 | 0.57 | 2.79E−13 | 9.89E−13 |
| FGF13 | 2.42 | 4.18 | 0.58 | 2.84E−05 | 5.06E−05 |
| vRAC2 | 22.11 | 37.39 | 0.59 | 4.70E−16 | 2.02E−15 |
| FGF7 | 8.17 | 13.70 | 0.60 | 1.49E−08 | 3.69E−08 |
| NTF3 | 0.84 | 1.41 | 0.60 | 1.04E−03 | 1.61E−03 |
| RPS6KA1 | 89.25 | 148.07 | 0.60 | 1.24E−30 | 2.00E−29 |
| JUND | 127.78 | 210.71 | 0.61 | 1.88E−36 | 5.04E−35 |
| PLA2G4F | 22.98 | 37.48 | 0.61 | 2.67E−10 | 7.76E−10 |
| CACNA2D2 | 10.30 | 16.63 | 0.62 | 1.02E−08 | 2.63E−08 |
| RPS6KA5 | 18.57 | 29.87 | 0.62 | 1.84E−15 | 7.76E−15 |
| FLNC | 46.98 | 75.44 | 0.62 | 3.90E−09 | 1.02E−08 |
| ARRB1 | 52.99 | 84.74 | 0.63 | 4.44E−24 | 3.61E−23 |
| NR4A1 | 201.17 | 319.66 | 0.63 | 5.51E−11 | 1.72E−10 |
| FASLG | 1.07 | 1.70 | 0.63 | 4.96E−03 | 7.29E−03 |
| IL1R1 | 57.71 | 89.40 | 0.65 | 9.55E−17 | 4.34E−16 |
| HSPA2 | 7.29 | 11.21 | 0.65 | 5.51E−06 | 1.08E−05 |
| PTPN7 | 16.22 | 24.78 | 0.65 | 3.08E−08 | 7.57E−08 |
| MAP3K14 | 33.86 | 51.73 | 0.65 | 1.46E−24 | 1.35E−23 |
| MAP2K6 | 25.29 | 38.55 | 0.66 | 1.60E−10 | 4.77E−10 |
| MKNK1 | 63.58 | 95.95 | 0.66 | 2.04E−27 | 2.46E−26 |
| Upregulated | |||||
| HSPB1 | 60.24 | 40.01 | 1.51 | 3.87E−12 | 1.24E−11 |
| TGFBR1 | 106.44 | 70.13 | 1.52 | 6.14E−19 | 3.21E−18 |
| STK3 | 41.26 | 26.65 | 1.55 | 1.64E−18 | 7.88E−18 |
| HSPA6 | 5.49 | 3.43 | 1.60 | 3.67E−05 | 6.40E−05 |
| ELK1 | 49.96 | 30.79 | 1.62 | 4.90E−24 | 3.81E−23 |
| DUSP10 | 27.04 | 16.22 | 1.67 | 3.95E−14 | 1.59E−13 |
| FGF1 | 4.77 | 2.79 | 1.71 | 9.30E−06 | 1.75E−05 |
| TP53 | 105.07 | 59.63 | 1.76 | 3.25E−24 | 2.79E−23 |
| HSPA1B | 102.12 | 55.34 | 1.85 | 4.49E−24 | 3.61E−23 |
| PDGFRB | 166.24 | 87.76 | 1.89 | 8.03E−28 | 1.02E−26 |
| TGFB2 | 9.23 | 4.52 | 2.04 | 8.04E−14 | 3.13E−13 |
| FGF18 | 1.90 | 0.85 | 2.22 | 1.27E−05 | 2.36E−05 |
| HSPA8 | 631.28 | 273.40 | 2.31 | 5.34E−56 | 1.29E−53 |
| CACNG8 | 7.57 | 3.27 | 2.32 | 7.89E−10 | 2.19E−09 |
| STMN1 | 77.81 | 31.07 | 2.50 | 1.15E−40 | 4.63E−39 |
| DUSP4 | 56.69 | 21.44 | 2.64 | 2.53E−22 | 1.65E−21 |
| CDC25B | 169.88 | 60.96 | 2.79 | 9.71E−54 | 7.80E−52 |
| IL1A | 7.56 | 2.39 | 3.17 | 2.02E−19 | 1.08E−18 |
| NTF4 | 0.55 | 0.17 | 3.23 | 3.80E−06 | 7.57E−06 |
| CACNG4 | 3.41 | 1.03 | 3.30 | 8.11E−10 | 2.22E−09 |
| MYC | 181.11 | 49.00 | 3.70 | 5.76E−55 | 6.94E−53 |
| FGF20 | 0.72 | 0.14 | 5.29 | 2.29E−05 | 4.15E−05 |
| FGF19 | 1.39 | 0.15 | 9.42 | 6.08E−13 | 2.12E−12 |
Figure 1.
Dysregulated genes and their associated microRNAs in the KEGG MAPK-signaling pathway in colorectal cancer.
Because more than 85% of tumors were considered MSS, it is not surprising that findings for mRNA expression in CRCs and for MSS-specific cancers were almost identical (see Supplemental Table S3 for MSS-specific associations). Five genes, CACNB4 (FCMSS 0.65), MAP3K6 (FCMSS 0.66), CD14 (FCMSS 0.66), CACNA2D4 (FCMSS 0.66), and MAP4K4 (FCMSS 1.52) had slightly stronger FC for MSS-specific tumors than for all tumor combined (FCoverall 0.67, 0.73, 0.68, 0.67, and 1.47, respectively). More differences between MSI-specific tumors and all tumors were observed as might be expected (see Supplemental Table S4 for MSI-specific associations). Although CACNB4, MAP3K6, CD14, and CACNA2D4 were not associated with differential expression in MSI tumors, 7 genes were significantly downregulated for MSI-specific tumors only (RPS6KA6 FC 0.20, PLA2G4E FC 0.30, RASGFR1 FC 0.39, PLA2G4C FC 0.47, MAPK8IP1 FC 0.47, PDGFA FC 0.53, and TGFBR2 FC 0.61). Four other genes were significantly upregulated only for MSI-specific tumors (DUSP7 FC 1.57, HRAS FC 1.92, IL1B FC 2.06, and PLA2G4A FC 2.70). However, 13 genes associated with significant higher expression in carcinoma compared with normal mucosa were not significantly upregulated in MSI tumors (HSPB1, TGFBR1, ELK1, FGF1, PDGFRB, TGFBR2, FDF18, CACNG8, NTF4, CACNG4, FGF20, ILA1A, and FGF19).
Of the 99 dysregulated genes identified overall or for MSS- or MSI-specific tumors, 13 were associated with miRNAs for a total of 68 miRNA:mRNA associations after adjustment for multiple comparisons (Table 3). Several genes were associated with 2 or 3 miRNAs, such as RASGRP2, MAP4K1, IL1R1, DUSP4, RAC2, RASGRP3, and PRKCB. HSPA8 was associated with 5 miRNAs, whereas PDGFRB and MEF2C were associated with 6 miRNAs. TGFBR1 (11 miRNAs), PDGFRA (10 miRNAs), and MYC (12 miRNAs) had the most associations with miRNAs. Thirteen of the miRNAs:mRNA seed region matches (MEF2C with miR-203a; TGFBR1 with miR-2117 and miR-6071; DUSP4 with miR-193b-3p; PDGFRA with miR-17-5p, miR-19b-3p, miR-203a, miR-20a-5p, miR-20b-5p, miR-29b-3p, miR-501-3p; RASGRP3 with miR-429; and PRKCB and miR-203a) were inversely associated suggesting a greater likelihood of a direct effect between the miRNA and mRNA. Notably, none of the miRNAs associated with MYC had a seed region match, whereas all of the miRNAs associated with PDGFRA had a seed region match. Many miRNAs were associated with multiple mRNAs (Table 4). Both miR-150-5p and miR-650 were associated with the same 7 genes. Three of these genes had a seed region match for miR-150-5p (PDGFRA, RASGRP3, and PRKCB), whereas miR-650 only had a seed region match with PDGFRA. MiR-203a was associated with 5 mRNAs, 3 of which (MEF2C, PDGFRA, and PRKCB) had a seed region match. MiR-193b-3p was associated with 4 genes, 3 of which had a seed region match (MEF2C, PDGFRB, and DUSP4). Figure 1 also shows these miRNA:mRNA associations in the KEGG MAPK-signaling pathway.
Table 3.
Associations between MAPK pathway–dysregulated mRNA and miRNAs.
| Gene name | Tumor mean | Normal mean | Fold change | MiRNA | Tumor mean | Normal mean | Fold change | β | Raw P value | FDR P value |
|---|---|---|---|---|---|---|---|---|---|---|
| RASGRP2 | 6.72 | 24.73 | 0.27 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .31 | <.0001 | .0407 |
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .29 | .0002 | .0407 | ||||
| MEF2C | 27.15 | 66.82 | 0.41 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .35 | <.0001 | .0116 |
| hsa-miR-193b-3p | 9.12 | 5.42 | 1.68 | .28 | <.0001 | .0116 | ||||
| hsa-miR-195-5p | 3.59 | 12.18 | 0.29 | .25 | .0004 | .025 | ||||
| hsa-miR-203a | 12.52 | 3.70 | 3.38 | −.30 | <.0001 | .0116 | ||||
| hsa-miR-221-3p | 13.53 | 4.12 | 3.28 | −.26 | .0002 | .0148 | ||||
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .34 | <.0001 | .0116 | ||||
| MAP4K1 | 7.24 | 21.46 | 0.34 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .30 | <.0001 | .0203 |
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .30 | .0003 | .0488 | ||||
| TGFBR1 | 106.44 | 70.13 | 1.52 | hsa-let-7i-5p | 62.16 | 39.97 | 1.56 | .25 | .0006 | .031 |
| hsa-miR-1203 | 1.76 | 2.83 | 0.62 | −.23 | .0007 | .031 | ||||
| hsa-miR-199a-3p | 44.83 | 22.53 | 1.99 | .24 | .0007 | .031 | ||||
| hsa-miR-199a-5p | 20.18 | 9.28 | 2.17 | .22 | .0016 | .0458 | ||||
| hsa-miR-2117 | 1.50 | 4.09 | 0.37 | −.22 | .0011 | .0358 | ||||
| hsa-miR-214-3p | 13.24 | 6.13 | 2.16 | .26 | .0005 | .031 | ||||
| hsa-miR-21-5p | 463.11 | 167.37 | 2.77 | .25 | .0006 | .031 | ||||
| hsa-miR-23a-3p | 174.68 | 87.53 | 2.00 | .25 | .0008 | .031 | ||||
| hsa-miR-24-3p | 106.75 | 62.39 | 1.71 | .24 | .0009 | .0318 | ||||
| hsa-miR-331-3p | 14.64 | 9.30 | 1.57 | .24 | .0006 | .031 | ||||
| hsa-miR-6071 | 0.97 | 1.70 | 0.57 | −.22 | .0017 | .0458 | ||||
| HSPA8 | 631.28 | 273.40 | 2.31 | hsa-miR-17-5p | 61.04 | 16.38 | 3.73 | .27 | .0003 | .0407 |
| hsa-miR-203a | 12.52 | 3.70 | 3.38 | .35 | <.0001 | .0271 | ||||
| hsa-miR-221-3p | 13.53 | 4.12 | 3.28 | .28 | <.0001 | .0271 | ||||
| hsa-miR-29b-3p | 24.31 | 9.83 | 2.47 | .27 | .0003 | .0407 | ||||
| hsa-miR-93-5p | 41.72 | 15.20 | 2.74 | .25 | .0003 | .0407 | ||||
| PDGFRB | 166.24 | 87.76 | 1.89 | hsa-miR-193b-3p | 9.12 | 5.42 | 1.68 | .27 | .0002 | .0181 |
| hsa-miR-199a-3p | 44.83 | 22.53 | 1.99 | .33 | <.0001 | .0102 | ||||
| hsa-miR-199a-5p | 20.18 | 9.28 | 2.17 | .35 | <.0001 | .0102 | ||||
| hsa-miR-199b-5p | 4.69 | 1.53 | 3.07 | .34 | <.0001 | .0102 | ||||
| hsa-miR-214-3p | 13.24 | 6.13 | 2.16 | .38 | <.0001 | .0102 | ||||
| hsa-miR-934 | 4.36 | 0.94 | 4.66 | .46 | <.0001 | .0102 | ||||
| IL1R1 | 57.71 | 89.40 | 0.65 | hsa-miR-193b-3p | 9.12 | 5.42 | 1.68 | .31 | <.0001 | .0203 |
| hsa-miR-214-3p | 13.24 | 6.13 | 2.16 | .27 | .0003 | .0407 | ||||
| DUSP4 | 56.69 | 21.44 | 2.64 | hsa-miR-193b-3p | 9.12 | 5.42 | 1.68 | −.27 | <.0001 | .0163 |
| hsa-miR-196b-5p | 17.89 | 5.53 | 3.24 | −.31 | <.0001 | .0163 | ||||
| hsa-miR-424-3p | 39.81 | 25.37 | 1.57 | −.29 | .0003 | .0271 | ||||
| RAC2 | 22.11 | 37.39 | 0.59 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .39 | <.0001 | .0203 |
| hsa-miR-203a | 12.52 | 3.70 | 3.38 | −.28 | <.0001 | .0203 | ||||
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .36 | <.0001 | .0203 | ||||
| PDGFRA | 88.47 | 166.61 | 0.53 | hsa-miR-145-5p | 132.97 | 223.14 | 0.60 | .26 | .0002 | .0163 |
| hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .24 | .0004 | .0233 | ||||
| hsa-miR-17-5p | 61.04 | 16.38 | 3.73 | −.28 | <.0001 | .0136 | ||||
| hsa-miR-19b-3p | 29.80 | 10.42 | 2.86 | −.29 | <.0001 | .0136 | ||||
| hsa-miR-203a | 12.52 | 3.70 | 3.38 | −.30 | <.0001 | .0136 | ||||
| hsa-miR-20a-5p | 70.78 | 17.61 | 4.02 | −.28 | .0002 | .0163 | ||||
| hsa-miR-20b-5p | 17.65 | 3.30 | 5.35 | −.30 | <.0001 | .0136 | ||||
| hsa-miR-29b-3p | 24.31 | 9.83 | 2.47 | −.27 | .0003 | .0222 | ||||
| hsa-miR-501-3p | 7.07 | 2.95 | 2.39 | −.25 | .0004 | .0233 | ||||
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .32 | <.0001 | .0136 | ||||
| MYC | 181.11 | 49.00 | 3.70 | hsa-miR-1246 | 629.21 | 412.81 | 1.52 | .27 | .0002 | .0163 |
| hsa-miR-17-5p | 61.04 | 16.38 | 3.73 | .35 | <.0001 | .0136 | ||||
| hsa-miR-19b-3p | 29.80 | 10.42 | 2.86 | .27 | .0002 | .0163 | ||||
| hsa-miR-20a-5p | 70.78 | 17.61 | 4.02 | .33 | <.0001 | .0136 | ||||
| hsa-miR-20b-5p | 17.65 | 3.30 | 5.35 | .31 | .0002 | .0163 | ||||
| hsa-miR-3651 | 58.66 | 25.92 | 2.26 | .28 | .0003 | .0188 | ||||
| hsa-miR-375 | 20.50 | 54.53 | 0.38 | −.29 | <.0001 | .0136 | ||||
| hsa-miR-501-3p | 7.07 | 2.95 | 2.39 | .26 | .0003 | .0188 | ||||
| hsa-miR-583 | 6.61 | 3.22 | 2.05 | .26 | .0004 | .0233 | ||||
| hsa-miR-663a | 374.83 | 234.91 | 1.60 | .28 | .0003 | .0188 | ||||
| hsa-miR-663b | 65.50 | 32.21 | 2.03 | .33 | <.0001 | .0136 | ||||
| hsa-miR-92a-3p | 121.60 | 41.18 | 2.95 | .32 | <.0001 | .0136 | ||||
| RASGRP3 | 25.53 | 45.22 | 0.56 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .35 | <.0001 | .0203 |
| hsa-miR-429 | 13.33 | 8.29 | 1.61 | −.25 | .0003 | .0407 | ||||
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .35 | <.0001 | .0203 | ||||
| PRKCB | 14.65 | 53.12 | 0.28 | hsa-miR-150-5p | 14.90 | 39.17 | 0.38 | .38 | <.0001 | .0102 |
| hsa-miR-203a | 12.52 | 3.70 | 3.38 | −.30 | <.0001 | .0102 | ||||
| hsa-miR-650 | 4.51 | 16.60 | 0.27 | .35 | <.0001 | .0102 |
Abbreviations: FDR, false discovery rate; mRNA, messenger RNA; miRNA, microRNA.
Bold text indicates seed region match between miRNA and mRNA.
Table 4.
MiRNAs in MAPK pathway associated with mRNA by seed region match.
| MiRNA | With seed region match | Without seed region match |
|---|---|---|
| hsa-let-7i-5p | TGFBR1 | |
| hsa-miR-1203 | TGFBR1 | |
| hsa-miR-1246 | MYC | |
| hsa-miR-145-5p | PDGFRA | |
| hsa-miR-150-5p (7) | PDGFRA, RASGRP3, PRKCB | RASGRP2, MEF2C, MAP4K1, RAC2, |
| hsa-miR-17-5p (3) | HSPA8, PDGFRA | MYC |
| hsa-miR-193b-3p (4) | MEF2C, PDGFRB, DUSP4 | IL1R |
| hsa-miR-195-5p | MEF2C | |
| hsa-miR-196b-5p | DUSP4 | |
| hsa-miR-199a-3p (2) | TGFBR1 | PDGFRB |
| has-miR-199a-5p | TGFBR1 | PDGFRB |
| hsa-miR-199b-5p | PDGFRB | |
| hsa-miR-19b-3p (2) | PDGFRA | MYC |
| hsa-miR-203a (5) | MEF2C, PDGFRA, PRKCB | HSPA8, RAC2 |
| hsa-miR-20a-5p (2) | PDGFRA | MYC |
| hsa-miR-20b-5p (2) | PDGFRA | MYC |
| hsa-miR-2117 | TGFBR1 | |
| hsa-miR-214-3p (3) | TGFBR1, PDGFRB, IL1R1 | |
| hsa-miR-21-5p | TGFBR1 | |
| hsa-miR-221-3p (2) | HSPA8 | MEF2C |
| hsa-miR-23a-3p | TGFBR1 | |
| hsa-miR-24-3p | TGFBR1 | |
| hsa-miR-29b-3p (2) | PDGFRA | HSPA8, |
| hsa-miR-331-3p | TGFBR1 | |
| hsa-miR-3651 | MYC | |
| hsa-miR-375 | MYC | |
| hsa-miR-424-3p | DUSP4 | |
| hsa-miR-429 | RASGRP3 | |
| hsa-miR-501-3p (2) | PDGFRA | MYC |
| hsa-miR-583 | MYC | |
| hsa-miR-6071 | TGFBR1 | |
| hsa-miR-650 (7) | PDGFRA | RASGRP1, MEF2C, MAP4K1, RAC2, RASGRP3, PRKCB |
| hsa-miR-663a | MYC | |
| hsa-miR-663b | MYC | |
| hsa-miR-92a-3p | MYC | |
| hsa-miR-934 | PDGFRB | |
| hsa-miR-93-5p | HSPA8 |
Abbreviations: mRNA, messenger RNA; miRNA, microRNA.
Bold text indicates negative β coefficient between miRNA and mRNA for those with a seed region match; numbers in parentheses indicate number of associations identified.
Discussion
Roughly 41% of genes in the MAPK-signaling pathway were dysregulated in CRCs when considering all cancers as well as MSI and MSS tumors. Genes both upstream and downstream of ERK1/ERK2, JNK, p38, and ERK5 were dysregulated, implying that all MAPK-signaling pathway arms may be involved in CRC. Of the 99 dysregulated genes, 13 were associated with miRNAs and 13 of the 68 miRNA:mRNA associations had both a seed region match with a negative β coefficient between the differential expression of the miRNA and mRNA, suggesting a greater likelihood that these genes were directly influenced by miRNA binding. We focus our discussion mainly on those genes and their associated miRNAs given their greater probability of being useful targets for examining prognosis or therapeutic agents.
Given the number of associations observed between miRNAs and mRNAs in the MAPK-signaling pathway, interpretation of results can be difficult. Seed region matches between the miRNA and the 3ʹUTR of the mRNA can increase the likelihood that binding occurs that can alter the gene expression. However, other associations between miRNAs and mRNAs in the MAPK-signaling pathway, such as those in which there is a positive β coefficient or no identified seed match, suggest an indirect biological effect. Indirect effects most likely operate through a feedback or feed forward loop.22–24 In feedback loops, regulators such as miRNAs can have either the same effect (repression of expression) or opposite effects on each other.23 In feed forward loops, a TF regulates the miRNA and the target gene (TG), which in turn is regulated by the miRNA. In this instance, the miRNA may regulate the TG directly, through seed region binding leading to mRNA degradation or translational repression, or indirectly, through repression of the TF that is influencing transcription of the same TG. Studies suggest that regulatory pathways involving miRNAs are prevalent mechanisms of altering gene expression.23
Examination of the MAPK-signaling pathway focusing on those miRNA:mRNA associations that have a greater likelihood of a direct effect, given both a seed region match and an inverse association between the differentially expressed miRNA and mRNA, may help provide focus on targets that may be the most rewarding in terms of future research relating to targeted therapeutics. Within each of the major components of the signaling pathway, ie, ERK1/2, JNK, and p38, we identified miRNA:mRNA associations of potential importance. In the classical arm of the pathway (see Figure 1), several factors including the growth hormone PDGFRA, RASGRP3 which can alter immune function as well as activate RAS genes, and PRKCB, a protein kinase involved in numerous cell functions including apoptosis and cell proliferation, had associations with miRNAs that suggested a direct biological effect. PDGFRA was inversely associated with 7 miRNAs, 5 of which were in the miR17-92 cluster as well as with miR-203a and miR-501-3p. Differential expression of PRKCB was inversely associated with differential expression of miR-203a. Downstream of these associations, several of these miRNAs were associated with DUSP4 and MYC, suggesting an indirect effect between miRNAs and these genes. Upregulation of miR-203, as in our CRC data, has been shown to inhibit proliferation and metastasis in CRC.25 Studies support that miR-150 and the miR17-92 cluster are functionally involved in immune function and cell survival and proliferation.26
Others have reviewed components of the ERK1/ERK2 MAPK pathway that are associated with various cancers.27 Several miRNAs were identified as targeting EGFR and RAS genes. Of these miRNAs, miR-145 previously was associated with EGFR, and we identified this miRNA as also being associated with PDGFRA. As our analysis of miRNA:mRNA interactions focused only on those genes that were significantly differently expressed at levels >1.50 or <0.67, we did not assess miRNAs with EGFR (FC 0.81) or any of the RAS genes specifically. Our findings instead focused on miRNA:mRNA associations that could alter RAS gene function because of downstream effects. Our findings along with those of Masliah-Planchon et al27 suggest the need for further research into miRNA:mRNA interactions that appear to have a direct effect and may yield useful biomarkers that can be used for prognosis as well as therapeutic purposes. Previous studies have shown that higher TRBP expression increased expression of miR-17 and miR-20a, both which were upregulated in our data4; TRBP is regulated by the MAPK/ERK pathway4 suggesting another indirect effect between genes in this signaling pathway and miRNAs.
The JNK/p38 arm of the KEGG MAPK-signaling pathway also had miRNA:mRNA associations that would imply a direct effect. Some associations, including IL1R1 with miR-214-3p and TFGBR1 with miR-2217 and miR-6071, were upstream of JNK and p38, implying an impact on factors that alter activation of the pathway. Other direct biological associations were observed between MEF2C and miR-193b-3p and miR-203a, downstream of p38. Again these direct biological associations suggest potential targets for future research. IL1R has previously been associated with miR-378,27 although we did not see an association with this miRNA in our CRC data. Other studies have shown that miR-125a-3p can upregulate p38 in orofacial tissue28; however, we also did not see an association between this miRNA and any genes within the p38 arm of the pathway.
Our study represents one of the largest reported to date that includes paired expression levels for both miRNAs and mRNA; however, the sample size is still considered small to examine several factors including their effects on prognosis. A strength is our paired samples of carcinoma and normal mucosa, although it should be recognized that normal mucosa may not be truly “normal.” Despite this limitation, the normal mucosa samples we used were the closest normal mucosa that could be obtained for a matched paired analysis. In addition, normal colonic mucosa was taken from the same colonic site as the tumor and the miRNA and mRNA data were generated from the same RNA prep. We did not examine all statistically significant genes that were differentially expressed and miRNAs, but focused on those that also had an FC of >1.50 or <0.67. Using these cut points to define further analysis with miRNAs, we could have missed genes associated with miRNAs. We exclusively used the KEGG pathway database to identify signaling pathway genes, and genes not identified in KEGG that may be related to the pathway and have an impact of expression of both miRNA and mRNA were not examined. An important limitation in our data in evaluating the impact of miRNA on genes in the pathway was our use of mRNA expression data. Because miRNAs could have part or even most of their impact posttranscriptionally, we could have missed important associations. However, much of the current information on miRNA TGs comes from gene expression data. At any rate, observed associations may still have important biological meaning, but it must be acknowledged that important associations could have been missed.20,29
In conclusion, we show that the MAPK-signaling pathway is dysregulated in CRC and that several dysregulated genes in this pathway are associated with miRNAs. Some inverse associations were noted with negative β coefficients and seed region matches between the miRNA and mRNA, suggesting that these miRNAs directly repress gene expression and thus alter MAPK signaling. Other associations, that were both downstream or upstream of these direct biological miRNA:mRNA associations, may further result in disruption of the pathway. We encourage others to both replicate these findings and validate results in targeted laboratory experiments that can help solidify important therapeutic targets.
Supplemental Material
Supplemental material, Supplemental_Tables for The MAPK-Signaling Pathway in Colorectal Cancer: Dysregulated Genes and Their Association With MicroRNAs by Martha L Slattery, Lila E Mullany, Lori C Sakoda, Roger K Wolff, Wade S Samowitz and Jennifer S Herrick in Cancer Informatics
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute. The authors acknowledge Sandra Edwards for data oversight and study management and Michael Hoffman and Erica Wolff for miRNA analysis. They also acknowledge Dr Bette Caan and the staff at Kaiser Permanente Northern California for sample and data collection.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NCI grant CA163683.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MLS obtained funding, oversaw grant, developed analysis plan, and was responsible for writing the paper. LEM conducted bioinformatics analysis and helped write the paper. LCS contributed data and edited manuscript. RKW oversaw genomic analysis and approved final copy of paper. WS conducted pathology analysis and provided edits to paper and approved final copy of manuscript. JSH managed data and conducted statistical analysis and approved final version of the manuscript.
References
- 1. Imajo M, Tsuchiya Y, Nishida E. Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life. 2006;58:312–317. [DOI] [PubMed] [Google Scholar]
- 2. Marshall MS. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. [DOI] [PubMed] [Google Scholar]
- 3. Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118:3569–3572. [DOI] [PubMed] [Google Scholar]
- 4. Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heo I, Kim VN. Regulating the regulators: posttranslational modifications of RNA silencing factors. Cell. 2009;139:28–31. [DOI] [PubMed] [Google Scholar]
- 6. Zhu QY, Liu Q, Chen JX, Lan K, Ge BX. MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010;185:7435–7442. [DOI] [PubMed] [Google Scholar]
- 7. Schmitt DC, Madeira da, Silva L, Zhang W, et al. ErbB2-intronic microRNA-4728: a novel tumor suppressor and antagonist of oncogenic MAPK signaling. Cell Death Dis. 2015;6:e1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mutlu M, Saatci O, Ansari SA, et al. miR-564 acts as a dual inhibitor of PI3K and MAPK signaling networks and inhibits proliferation and invasion in breast cancer. Sci Rep. 2016;6:32541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer—beyond physical activity. Cancer Res. 1997;57:75–80. [PubMed] [Google Scholar]
- 11. Slattery ML, Caan BJ, Benson J, Murtaugh M. Energy balance and rectal cancer: an evaluation of energy intake, energy expenditure, and body mass index. Nutr Cancer. 2003;46:166–171. [DOI] [PubMed] [Google Scholar]
- 12. Slattery ML, Herrick JS, Pellatt DF, et al. MicroRNA profiles in colorectal carcinomas, adenomas and normal colonic mucosa: variations in miRNA expression and disease progression. Carcinogenesis. 2016;37:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slattery ML, Herrick JS, Mullany LE, et al. An evaluation and replication of miRNAs with disease stage and colorectal cancer-specific mortality. Int J Cancer. 2015;137:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pellatt AJ, Slattery ML, Mullany LE, Wolff RK, Pellatt DF. Dietary intake alters gene expression in colon tissue: possible underlying mechanism for the influence of diet on disease. Pharmacogenet Genomics. 2016;26:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slattery ML, Pellatt DF, Mullany LE, Wolff RK, Herrick JS. Gene expression in colon cancer: A focus on tumor site and molecular phenotype. Gene Chromosome Canc. 2015;54:527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pellatt DF, Stevens JR, Wolff RK, et al. Expression profiles of miRNA subsets distinguish human colorectal carcinoma and normal colonic mucosa. Clin Transl Gastroenterol. 2016;7:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agilent Technologies Inc. Agilent GeneSpring User Manual. Santa Clara, CA: Agilent Technologies Inc; 2013. [Google Scholar]
- 18. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 19. Mullany LE, Herrick JS, Wolff RK, Slattery ML. MicroRNA seed region length impact on target messenger RNA expression and survival in colorectal cancer. PLoS ONE. 2016;11:e0154177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou CH, Chang NW, Shrestha S, et al. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016;44:D239–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karolchik D, Hinrichs AS, Furey TS, et al. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin Y, Zhang Q, Zhang HM, et al. Transcription factor and miRNA co-regulatory network reveals shared and specific regulators in the development of B cell and T cell. Sci Rep. 2015;5:15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez NJ, Walhout AJ. The interplay between transcription factors and microRNAs in genome-scale regulatory networks. Bioessays. 2009;31:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003;100:11980–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao B, Yu T, Xue D, et al. A multidimensional integration analysis reveals potential bridging targets in the process of colorectal cancer liver metastasis. PLoS ONE. 2017;12:e0178760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoefig KP, Heissmeyer V. MicroRNAs grow up in the immune system. Curr Opin Immunol. 2008;20:281–287. [DOI] [PubMed] [Google Scholar]
- 27. Masliah-Planchon J, Garinet S, Pasmant E. RAS-MAPK pathway epigenetic activation in cancer: miRNAs in action. Oncotarget. 2016;7:38892–38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong Y, Li P, Ni Y, Zhao J, Liu Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS ONE. 2014;9:e111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slattery ML, Herrick JS, Stevens JR, Wolff RK, Mullany LE. An assessment of database-validated microRNA target genes in normal colonic mucosa: implications for pathway analysis. Cancer Inform. 2017;16. doi: 10.1177/1176935117716405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Tables for The MAPK-Signaling Pathway in Colorectal Cancer: Dysregulated Genes and Their Association With MicroRNAs by Martha L Slattery, Lila E Mullany, Lori C Sakoda, Roger K Wolff, Wade S Samowitz and Jennifer S Herrick in Cancer Informatics