Abstract
Pulmonary arterial hypertension (PAH) is a severe complication of hereditary hemorrhagic telangiectasia (HHT); however, little is known about its clinical characteristics and prognosis. Nine newly diagnosed HHT-PAH patients were prospectively recruited between October 2007 and January 2016 and were followed up every half-year. Eighteen idiopathic pulmonary arterial hypertension (IPAH) patients, matched with HHT-PAH patients on mean pulmonary arterial pressure, pulmonary capillary wedge pressure, pulmonary vascular resistance, cardiac index, and World Health Organization (WHO) functional class (FC), were recruited. The clinical characteristics of HHT-PAH patients were described and the prognosis of these two cohorts were compared. Of HHT-PAH patients, 55.56% were WHO FC III. Kaplan–Meier survival analysis showed one- and three-year survival rates of HHT-PAH patients were 77.8% and 53.3% respectively, which were worse than matched IPAH patients (log rank: P = 0.047). HHT-PAH patients had higher red cell distribution width (14.88 ± 2.93% versus 13.19 ± 0.83%, P = 0.031), larger right ventricular anteroposterior diameter (34.67 ± 6.67 mm versus 28.56 ± 6.35 mm, P = 0.029), and lower mean corpuscular hemoglobin concentration (317.38 ± 17.71 g/L versus 335.72 ± 14.68 g/L, P = 0.011) than matched IPAH patients. Multivariate Cox proportional hazards regression analyses showed baseline total bilirubin independently predicted the mortality of HHT-PAH after adjusting by age, cardiac index, mixed venous oxygen saturation, or serum uric acid. HHT-PAH patients may have a worse prognosis than matched IPAH patients. Baseline total bilirubin may be a promising predictor for the long-term prognosis in HHT-PAH patients.
Keywords: hereditary hemorrhagic telangiectasia, pulmonary arterial hypertension, clinical characteristics, prognosis
Hereditary hemorrhagic telangiectasia (HHT), also known as Rendu-Osler-Weber syndrome, is a rare autosomal dominant vascular disorder. It is characterized by the presence of multiple arteriovenous malformations.1 HHT is reported to be caused by mutations in the genes codifying for transforming growth factor β signaling receptors2 and its estimated global prevalence was one patient per 5000–8000 inhabitants.3 The clinical diagnosis of HHT is based on the four Curaçao criteria (Table 1).4
Table 1.
Curaçao criteria for the clinical diagnosis of hereditary hemorrhagic telangiectasia (HHT).
| Curaçao criteria |
|---|
| (1) spontaneous and recurrent epistaxis |
| (2) multiple telangiectasia at characteristic sites |
| (3) visceral lesions |
| (4) a first-degree relative with HHT |
A definite diagnosis of HHT needs to meet at least three criteria; meeting two criteria is considered as a clinical “possible” HHT; only meeting one or no criterion makes the diagnosis “unlikely.”
Pulmonary arterial hypertension (PAH) is reported to be a rare manifestation of HHT, with prevalence of < 1% of cases.5 Recently, it is increasingly recognized as a severe complication of HHT. There have been a few case series describing the association between pulmonary hypertension (PH) and HHT. However, most of the studies recruited HHT patients with post-capillary PH.6 The investigations of clinical characteristics and prognosis of PAH associated with HHT (HHT-PAH) are still very limited.
A few studies have reported the beneficial treatment effect of Bosentan or the combination of Bosentan and Sidenafil on HHT-PAH patients.7,8 It also seems rational to treat HHT-PAH patients according to the PAH guidelines. However, clinical management experience for HHT-PAH patients is sparse as no long-term studies are available.
In this study, we aimed to describe the clinical and hemodynamic characteristics of a HHT-PAH cohort, which was confirmed by right heart catheterization (RHC), and to compare their long-term prognosis with a group of matched idiopathic pulmonary arterial hypertension patients (IPAH). In addition, we also wanted to illustrate the baseline characteristics which may predict the prognosis of HHT-PAH.
Methods
Patients who were diagnosed with HHT-PAH for the first time between October 2007 and January 2016 in Fuwai Hospital were prospectively recruited. The diagnosis of HHT was made according to the four Curaçao criteria.4 The diagnosis of PAH was made in accordance with standard guidelines.9 Clinical history, symptoms, signs, electrocardiograph (ECG), chest X-ray, transthoracic echocardiogram, pulmonary function test, high-resolution computed tomography (CT) of the chest, ventilation/perfusion scintigraphy lung scan, and pulmonary angiography (if necessary) were assessed to exclude PH due to left heart disease or lung diseases or chronic thromboembolism PH. The hemodynamic criteria for PAH included mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg, pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) ≥ 240 dyn·s·cm–5 measured at rest by RHC. Family HHT history was checked and additional specific diagnostic tests, including hematology, biochemistry, immunology, serology, and CT and ultrasonography of the abdomen, were performed to exclude other forms of PAH. Exclusion criteria included: (1) patients with other clinical types of PH; (2) patients who declined to participate in the study. For all HHT-PAH patients, matched IPAH patients, on cardiac index, mPAP, PVR, PCWP, and World Health Organization (WHO) functional class (FC), were recruited at the ratio of 2:1 (details about the recruitment of IPAH patients are shown in the supplementary material). Written informed consent was obtained from all enrolled patients. This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai Hospital (ethical approval no. 402).
Clinical evaluation
Exercise capacity was evaluated by the WHO FC. The 6-min walk test was performed according to American Thoracic Society guidelines.10 The algorithm incorporated 11 evaluable elements that were used to calculate REVEAL risk scores (RRS).11 Data for diffusing capacity of the lung for carbon monoxide were not available for some of our patients; however, the RRS only requires 7/12 evaluable elements to maintain significant predictive power and calibration.11 In this algorithm, calculated risks core could be in the range of 1 (lowest risk) to 21 (highest risk). For analysis of risk strata, patients were stratified into low (score 1–7), average (score 8) and higher stratum (score ≥ 9), based on their RRS, due to the small number of study patients. HHT-PAH and IPAH patients were also classified into low-risk, intermediate-risk, or high-risk groups according to European Respiratory Society (ERS) risk assessment.9
Right heart catheterization
Hemodynamic parameters including mean right atrial pressure (mRAP), mPAP, and PCWP were recorded during RHC. Cardiac output (CO) was measured by the thermodilution method (the mean value of three-time measurements). Body surface area (BSA) was calculated according to the Du Bois formula, i.e. BSA = 0.07184 × weight0.425 × height0.725.12 Cardiac index was calculated as CO/BSA. Pulmonary vascular resistance (PVR) was calculated using the following equation: PVR = (mPAP–PCWP)/CO. Diastolic pressure gradient (DPG) = diastolic PAP–mean PCWP. Pulmonary vasoreactivity testing was performed inhaling Iloprost. A positive acute response is defined as a reduction of mPAP ≥ 10 mmHg to reach an absolute value of mPAP ≤ 40 mmHg with an increased or unchanged CO.
Endpoint and follow-up
Each patient was followed up by telephone, outpatient, or in-hospital examinations in a six-month interval. They were included in this study from the date of signing informed consent till they had the primary outcome. The designed primary endpoint was any cause of mortality. None of the IPAH and HHT-PAH patients were lost to follow-up during the study period.
Statistics analysis
Continuous data were expressed as mean ± standard deviation (SD) and categorical data were expressed as frequency with percentage (%). Differences between the two groups were analyzed using the unpaired Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify independent variables associated with the endpoint. Results of these analyses were expressed as hazard ratio (HR) with 95% confidence interval (CI). Kaplan–Meier survival analyses were performed for the cumulative occurrence of endpoints. Between-group comparisons were made using the log-rank test. A value of P < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline demographic, clinical, and hemodynamic characteristics of HHT-PAH patients
A total of 13 newly diagnosed HHT patients, who met the hemodynamic criteria of PAH, were recruited for this study. However, four HHT had increased cardiac index (≥4.0 L/min/m2).13,14 Among these four patients, three had confirmed hepatic arteriovenous malformations (AVMs), which may lead to post-capillary PH because of high CO. Therefore, in order to be more precise and exclude the possibility of post-capillary PH, we decided to remove the four HHT patients with high CI. In the end, nine HHT patients with PAH were finally enrolled, including seven women and two men. Their mean age was 33.89 ± 10.00 years and 55.56% of them were WHO FC III. Among them, seven patients had a definite HHT diagnosis and two patients had a clinical possible HHT diagnosis (Suppl. Table S1).
The baseline characteristics of HHT-PAH patients are shown in Table 2. All the enrolled patients had confirmed PAH, with mPAP 61.22 ± 19.73 mmHg, PCWP 8.89 ± 3.26 mmHg, and PVR 848.17 ± 378.34 dyn·s·cm–5. Interestingly, one patient had a positive result of acute vasoreactivity test, which had rarely been reported before. Pericardial effusions were detected by echocardiography in three patients. Six patients received PAH-specific drugs combined with conventional therapy as soon as their PAH diagnoses were confirmed. However, the other three patients only received conventional supportive therapy including diuretics, oxygen, and digoxin because of financial issues. The median follow-up time of HHT-PAH patients was 2.70 years (range = 0.77–7.70 years). During this period, five patients died because of right heart failure. Compared with survivors, deceased HHT-PAH patients had a lower ratio of direct and total bilirubin (D/TBil, 21.11 ± 4.72 versus 14.54 ± 2.26, P = 0.046).
Table 3.
Baseline demographics, and clinical and hemodynamic characteristics of survival and deceased HHT-PAH patients.
| HHT-PAH |
|||
|---|---|---|---|
| Survivor | Non-survivor | P | |
| Patients (n) | 4 | 5 | N/A |
| Age (years) | 32.55 ± 11.58 | 34.97 ± 9.86 | 0.744 |
| Body mass index (kg/m2) | 22.18 ± 4.63 | 21.17 ± 2.83 | 0.698 |
| WHO FC I/II (n) | 2 | 2 | N/A |
| WHO FC III/IV (n) | 2 | 3 | |
| 6MWD (m) | 424.33 ± 47.59 | 345.67 ± 44.12 | 0.104 |
| REVEAL risk score | 8.00 ± 2.83 | 9.20 ± 1.92 | 0.472 |
| Low risk (n) | 2 | 1 | 0.714 |
| Average risk (n) | 1 | 1 | |
| High risk (n) | 1 | 3 | |
| ERS risk group, low risk (n) | 2 | 0 | 0.165 |
| Intermediate risk (n) | 2 | 4 | |
| High risk (n) | 0 | 1 | |
| PAH-specific drug, yes (n) | 2 | 4 | 0.524 |
| No (n) | 2 | 1 | |
| Follow-up period (years) (Q1–Q3) | 6.26 (3.41–7.44) | 2.18 (0.78–4.37) | 0.086 |
| Hemodynamic characteristics by RHC | |||
| SvO2 (%) | 69.95 ± 10.67 | 72.92 ± 9.83 | 0.670 |
| mRAP (mmHg) | 7.25 ± 2.99 | 10.40 ± 6.95 | 0.430 |
| RVSP (mmHg) | 85.25 ± 33.41 | 96.40 ± 29.36 | 0.610 |
| RVEDP (mmHg) | 10.25 ± 1.50 | 17.00 ± 7.91 | 0.141 |
| PASP (mmHg) | 84.25 ± 31.26 | 95.60 ± 32.11 | 0.611 |
| PADP (mmHg) | 40.5 ± 10.54 | 45.60 ± 17.76 | 0.630 |
| mPAP (mmHg) | 58.00 ± 16.15 | 63.80 ± 23.75 | 0.691 |
| Cardiac index (L/min/m2) | 2.95 ± 0.55 | 3.21 ± 0.59 | 0.514 |
| PCWP (mmHg) | 9.75 ± 3.78 | 8.20 ± 3.03 | 0.515 |
| PVR (dyn·s·cm–5) | 879.61 ± 512.41 | 823.02 ± 295.95 | 0.840 |
| DPG (mmHg) | 30.75 ± 13.25 | 37.40 ± 15.88 | 0.525 |
| Pulmonary vasoreactivity test, positive (n) | 1 | 0 | 0.444 |
| Hemodynamic characteristics by echocardiography | |||
| RVAPD (mm) | 33.00 ± 4.97 | 36.00 ± 8.09 | 0.539 |
| LAAPD (mm) | 29.25 ± 7.14 | 30.75 ± 5.44 | 0.749 |
| LVEDD (mm) | 39.5 ± 7.23 | 38.00 ± 7.38 | 0.769 |
| RV/LV (%) | 87.45 ± 29.40 | 98.62 ± 31.76 | 0.605 |
| LVEF (%) | 67.65 ± 5.11 | 70.40 ± 7.09 | 0.537 |
| Pericardial effusion, yes (n) | 1 | 2 | 0.595 |
| Blood gas analysis | |||
| PH | 7.41 ± 0.02 | 7.40 ± 0.04 | 0.170 |
| pCO2 (mmHg) | 34.78 ± 2.51 | 30.00 ± 4.58 | 0.106 |
| pO2 (mmHg) | 76.18 ± 5.32 | 84.08 ± 10.11 | 0.203 |
| SaO2 (%) | 95.30 ± 0.71 | 96.50 ± 1.14 | 0.111 |
| Hematology | |||
| RBC (1012/L) | 5.28 ± 0.75 | 4.48 ± 0.92 | 0.203 |
| HGB (g/L) | 148.50 ± 35.35 | 126.20 ± 18.08 | 0.256 |
| HCT (%) | 46.53 ± 8.86 | 39.92 ± 5.97 | 0.222 |
| MCV (fl) | 87.58 ± 4.85 | 91.15 ± 7.38 | 0.449 |
| MCH (pg) | 27.83 ± 3.35 | 29.10 ± 3.40 | 0.613 |
| MCHC (g/L) | 316.25 ± 23.61 | 318.50 ± 13.08 | 0.873 |
| RDW (%) | 15.43 ± 3.88 | 14.33 ± 2.05 | 0.634 |
| PLT (109/L) | 182.75 ± 54.91 | 165.80 ± 24.25 | 0.551 |
| PDW (%) | 15.00 ± 2.17 | 12.75 ± 2.41 | 0.260 |
| Biochemistry | |||
| ALT (IU/L) | 26.75 ± 17.63 | 23.00 ± 9.22 | 0.691 |
| AST (IU/L) | 22.25 ± 4.79 | 29.20 ± 4.44 | 0.059 |
| GGT (IU/L) | 41.25 ± 28.74 | 20.75 ± 8.38 | 0.220 |
| ALP (IU/L) | 77.00 ± 29.44 | 55.50 ± 11.21 | 0.221 |
| TBil (umol/L) | 16.20 ± 4.48 | 33.35 ± 24.50 | 0.195 |
| DBil (umol/L) | 3.51 ± 2.25 | 3.28 ± 0.57 | 0.850 |
| D/TBil (%) | 21.11 ± 4.72 | 14.54 ± 2.26 | 0.046* |
| CREA (umol/L) | 65.76 ± 5.79 | 61.98 ± 16.20 | 0.674 |
| BUN (mmol/L) | 6.24 ± 1.74 | 5.64 ± 1.15 | 0.555 |
| UA (umol/L) | 371.49 ± 166.01 | 4.78 ± 6.62 | 0.285 |
| HSCRP (mg/L) | 26.90 ± 42.79 | 2.54 ± 1.45 | 0.428 |
| CRP (mg/L) | 4.78 ± 6.62 | 1.20 ± 1.24 | 0.361 |
| ESR (mm/h) | 6.00 ± 5.60 | 4.40 ± 2.30 | 0.621 |
| LDH (IU/L) | 209.25 ± 26.99 | 277.50 ± 108.60 | 0.301 |
| PT (s) | 13.73 ± 1.30 | 11.35 ± 7.08 | 0.534 |
| PTA (%) | 93.75 ± 18.03 | 66.25 ± 38.50 | 0.243 |
| INR | 1.06 ± 0.14 | 1.16 ± 0.17 | 0.374 |
| APTT (s) | 39.65 ± 3.67 | 51.35 ± 21.43 | 0.323 |
| NT-proBNP (fmol/mL) | 1387.95 ± 1425.41 | 1695.50 ± 1038.84 | 0.718 |
| Big ET (fmol/mL) | 0.64 ± 0.35 | 0.54 ± 0.24 | 0.683 |
P < 0.05.
NA, not applicable; PAH, pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; WHO FC, World Health Organization functional class; 6MWD, 6-min walk distance; ERS, European Respiratory Society; RHC, right heart catheterization; SvO2, mixed venous oxygen saturation; mRAP, mean right atrial pressure; RVSP, right ventricle systolic pressure; RVEDP, right ventricle end diastolic pressure; PASP, pulmonary arterial systolic pressure; PADP, pulmonary arterial diastolic pressure; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; DPG, diastolic pressure gradient; RVAPD, right ventricular anteroposterior diameter; LAAPD, left atrial anteroposterior diameter; LVEDD, left ventricular end diastolic diameter; RV/LV, RVAPD/ LAAPD; LVEF, left ventricular ejection fraction; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SaO2, arterial oxygen saturation; RBC, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelets count; PDW, platelet distribution width; ALT, glutamic-pyruvic transaminase; AST, aspartate amino transferase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; Tbil, total bilirubin; Dbil, direct bilirubin; D/TBil, total bilirubin/direct bilirubin; CREA, creatinine; BUN, blood urea nitrogen; UA, uric acid; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; PT, prothrombin time; PTA, prothrombin time activity percentage; INR, international normalized ratio; APTT, activated partial thromboplastin time; NT-proBNP, N terminal pro-brain natriuretic peptide; big-ET, big endothelin.
Table 2.
Comparisons of baseline demographics, and clinical and hemodynamic characteristics between HHT-PAH and IPAH patients.
| HHT-PAH | IPAH | P | |
|---|---|---|---|
| Patients (n) | 9 | 18 | N/A |
| Age (years) | 33.89 ± 10.00 | 32.06 ± 9.18 | 0.638 |
| Female (%) | 7 (77.78) | 13 (72.22) | N/A |
| Body mass index (kg/m2) | 21.62 ± 3.51 | 21.85 ± 3.69 | 0.876 |
| WHO FC I/II | 4 | 8 | N/A |
| WHO FC III/IV | 5 | 10 | |
| 6MWD (m) | 385.00 ± 59.5 | 469.27 ± 62.39 | 0.011* |
| REVEAL risk score | 8.67 ± 2.29 | 7.11 ± 1.57 | 0.048* |
| Low risk (n) | 3 | 11 | 0.417 |
| Average risk (n) | 2 | 3 | |
| High risk (n) | 4 | 4 | |
| ERS risk group, low risk (n) | 2 | 5 | 0.951 |
| Intermediate risk (n) | 6 | 11 | |
| High risk (n) | 1 | 2 | |
| PAH-specific drug, yes (n) | 6 | 17 | 0.093 |
| No (n) | 3 | 6 | |
| Follow-up period (years) (Q1–Q3) | 2.70 (1.49–6.35) | 4.36 (2.70–5.99) | 0.537 |
| Deceased patient (n) | 5 | 3 | 0.072 |
| Hemodynamic characteristics by RHC | |||
| SvO2 (%) | 71.60 ± 9.67 | 73.63 ± 5.88 | 0.503 |
| mRAP (mmHg) | 9.00 ± 5.50 | 6.72 ± 3.71 | 0.421 |
| RVSP (mmHg) | 91.44 ± 29.73 | 96.56 ± 28.81 | 0.671 |
| RVEDP (mmHg) | 14.00 ± 6.69 | 11.67 ± 6.67 | 0.400 |
| PASP (mmHg) | 90.56 ± 30.30 | 95.72 ± 27.04 | 0.657 |
| PADP (mmHg) | 43.33 ± 14.37 | 41.00 ± 16.02 | 0.716 |
| mPAP (mmHg) | 61.22 ± 19.73 | 61.06 ± 18.44 | 0.983 |
| Cardiac index (L/min/m2) | 3.09 ± 0.55 | 3.08 ± 0.54 | 0.939 |
| PCWP (mmHg) | 8.89 ± 3.26 | 7.78 ± 3.00 | 0.386 |
| PVR (dyn·s·cm–5) | 848.17 ± 378.34 | 966.08 ± 398.35 | 0.468 |
| DPG (mmHg) | 34.44 ± 14.29 | 33.22 ± 15.82 | 0.847 |
| Pulmonary vasoreactivity test, positive (n) | 1 | 5 | 0.628 |
| Negative (n) | 8 | 13 | |
| Hemodynamic characteristics by echocardiography | |||
| RVAPD (mm) | 34.67 ± 6.67 | 28.56 ± 6.35 | 0.029* |
| LAAPD (mm) | 30.00 ± 5.93 | 27.00 ± 2.54 | 0.205 |
| LVEDD (mm) | 38.67 ± 6.89 | 37.06 ± 5.02 | 0.494 |
| RV/LV (%) | 94.66 ± 29.38 | 79.45 ± 24.12 | 0.191 |
| LVEF (%) | 69.18 ± 6.09 | 66.82 ± 5.63 | 0.328 |
| Pericardial effusion, yes (n) | 3 | 3 | 0.367 |
| No (n) | 6 | 15 | |
| Blood gas analysis | |||
| PH | 7.42 ± 0.03 | 7.42 ± 0.03 | 0.746 |
| pCO2 (mmHg) | 32.12 ± 4.38 | 35.11 ± 3.85 | 0.081 |
| pO2 (mmHg) | 80.57 ± 8.89 | 85.21 ± 17.76 | 0.470 |
| SaO2 (%) | 95.97 ± 1.11 | 95.91 ± 1.74 | 0.925 |
| Hematology | |||
| RBC (1012/L) | 4.84 ± 0.90 | 5.06 ± 0.55 | 0.513 |
| HGB (g/L) | 136.11 ± 27.75 | 152.06 ± 14.76 | 0.137 |
| HCT (%) | 42.86 ± 7.71 | 45.11 ± 4.34 | 0.433 |
| MCV (fl) | 89.36 ± 6.09 | 89.79 ± 4.48 | 0.841 |
| MCH (pg) | 28.46 ± 3.20 | 30.12 ± 1.36 | 0.196 |
| MCHC (g/L) | 317.38 ± 17.71 | 335.72 ± 14.68 | 0.011* |
| RDW (%) | 14.88 ± 2.93 | 13.19 ± 0.83 | 0.031* |
| PLT (109/L) | 173.33 ± 38.79 | 180.33 ± 54.11 | 0.733 |
| PDW (%) | 13.71 ± 2.43 | 14.34 ± 3.19 | 0.643 |
| Biochemistry | |||
| ALT (IU/L) | 24.67 ± 12.77 | 39.94 ± 32.07 | 0.091 |
| AST (IU/L) | 26.11 ± 5.65 | 27.78 ± 15.67 | 0.691 |
| GGT (IU/L) | 31.00 ± 22.46 | 39.83 ± 28.24 | 0.444 |
| ALP (IU/L) | 66.25 ± 23.61 | 82.33 ± 60.96 | 0.481 |
| TBil (umol/L) | 25.73 ± 19.73 | 20.00 ± 10.62 | 0.332 |
| DBil (umol/L) | 3.39 ± 1.53 | 3.97 ± 2.65 | 0.569 |
| D/TBil (%) | 17.82 ± 4.91 | 19.39 ± 4.35 | 0.422 |
| CREA (umol/L) | 63.66 ± 12.16 | 66.40 ± 12.83 | 0.598 |
| BUN (mmol/L) | 5.91 ± 1.38 | 5.06 ± 1.21 | 0.114 |
| UA (umol/L) | 314.35 ± 135.3058 | 363.17 ± 99.96 | 0.298 |
| HSCRP (mg/L) | 2.79 ± 4.56 | 2.54 ± 3.14 | 0.870 |
| CRP (mg/L) | 14.72 ± 30.19 | 3.67 ± 4.76 | 0.412 |
| ESR (mm/h) | 5.11 ± 3.89 | 5.56 ± 5.06 | 0.819 |
| LDH (IU/L) | 243.38 ± 81.84 | 218.06 ± 47.99 | 0.338 |
| PT (s) | 12.54 ± 4.88 | 13.88 ± 1.40 | 0.283 |
| PTA (%) | 80.00 ± 31.47 | 92.11 ± 15.65 | 0.198 |
| INR | 1.12 ± 0.16 | 7.06 ± 25.44 | 0.494 |
| APTT (s) | 45.50 ± 15.55 | 36.73 ± 3.56 | 0.157 |
| NT-proBNP (fmol/mL) | 1558.81 ± 1152.30 | 1194.15 ± 776.98 | 0.409 |
| Big ET (fmol/mL) | 0.58 ± 0.27 | 0.55 ± 0.51 | 0.887 |
P < 0.05.
NA, not applicable; PAH, pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; WHO FC, World Health Organization functional class; 6MWD, 6-min walk distance; ERS, European Respiratory Society; RHC, right heart catheterization; SvO2, mixed venous oxygen saturation; mRAP, mean right atrial pressure; RVSP, right ventricle systolic pressure; RVEDP, right ventricle end diastolic pressure; PASP, pulmonary arterial systolic pressure; PADP, pulmonary arterial diastolic pressure; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; DPG, diastolic pressure gradient; RVAPD, right ventricular anteroposterior diameter; LAAPD, left atrial anteroposterior diameter; LVEDD, left ventricular end diastolic diameter; RV/LV, RVAPD/ LAAPD; LVEF, left ventricular ejection fraction; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SaO2, arterial oxygen saturation; RBC, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelets count; PDW, platelet distribution width; ALT, glutamic-pyruvic transaminase; AST, aspartate amino transferase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; Tbil, total bilirubin; Dbil, direct bilirubin; D/TBil, total bilirubin/direct bilirubin; CREA, creatinine; BUN, blood urea nitrogen; UA, uric acid; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; PT, prothrombin time; PTA, prothrombin time activity percentage; INR, international normalized ratio; APTT, activated partial thromboplastin time; NT-proBNP, N terminal pro-brain natriuretic peptide; big-ET, big endothelin.
Predictive value of TBil on the survival of HHT-PAH patients
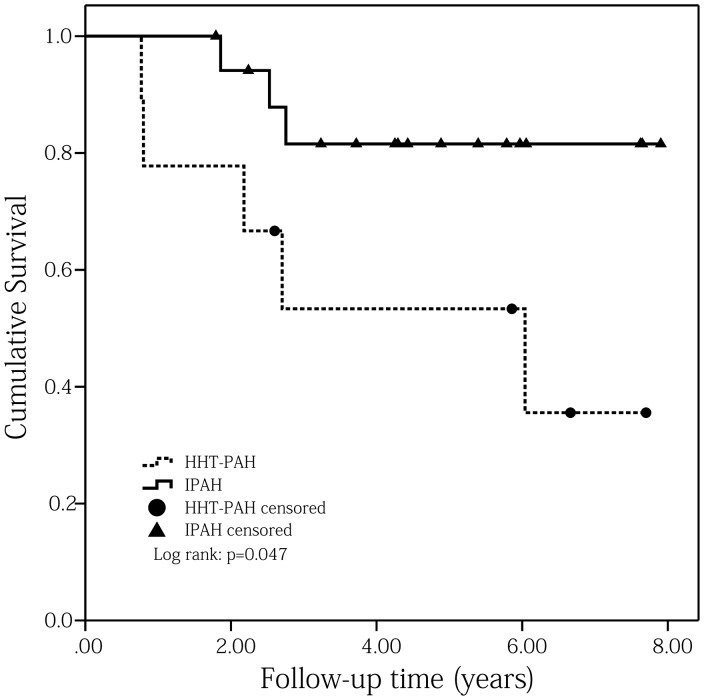
Kaplan–Meier survival analysis showed the one- and three- year survival rates of HHT-PAH patients were 77.8% and 53.3%, respectively (Fig. 1). Univariate Cox proportional hazards regression analyses determined that baseline total bilirubin (TBil) was a predictive factor for the primary endpoint (HR = 1.067, 95% CI = 1.003–1.135, P = 0.041; Table 4). Considering the possible predictive values of age, WHO FC ≥ III, 6-min walk distance (6MWD), NT-proBNP, pericardial effusion, mRAP, cardiac index, mixed venous oxygen saturation (SvO2), and serum uric acid on the prognosis of PH patients,9,15 these parameters were each examined with TBil using bivariate Cox proportional hazards regression analyses. Results showed that TBil still had the ability to independently predict the mortality of HHT-PAH patients after adjusting by age or SvO2 or cardiac index or serum uric acid (Table 5).
Fig. 1.
Comparisons of Kaplan–Meier survival curves for PAH associated with HHT and matched IPAH patients (log rank: P = 0.047).
Table 4.
Univariate Cox proportional hazards regression analyses for primary endpoint of HHT-PAH patients.
| Variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Age | 0.982 (0.888–1.085) | 0.716 |
| WHO FC I/II | 1.708 (0.277–10.546) | 0.564 |
| REVEAL risk score | 1.548 (0.940–2.650) | 0.112 |
| ERS risk group | 93.99 (0.081–108827.28) | 0.207 |
| 6MWD | 0.950 (0.888–1.018) | 0.145 |
| PAH-specific drug | 3.370 (0.357–31.853) | 0.289 |
| SvO2 | 1.006 (0.912–1.109) | 0.907 |
| mRAP | 1.186 (0.933–1.508) | 0.163 |
| RVSP | 1.007 (0.974–1.042) | 0.664 |
| RVEDP | 1.204 (0.983–1.475) | 0.073 |
| PASP | 1.010 (0.977–1.044) | 0.572 |
| PADP | 1.042 (0.971–1.118) | 0.259 |
| mPAP | 1.020 (0.968–1.075) | 0.463 |
| PCWP | 0.792 (0.562–1.115) | 0.181 |
| PVR | 1.000 (0.998–1.003) | 0.718 |
| Cardiac index | 1.074 (0.175–6.594) | 0.938 |
| DPG | 1.054 (0.978–1.136) | 0.166 |
| RVAPD | 1.182 (0.960–1.455) | 0.115 |
| LAAPD | 0.984 (0.817–1.185) | 0.865 |
| LVEDD | 0.945 (0.821–1.087) | 0.427 |
| RV/LV | 1.026 (0.986–1.067) | 0.203 |
| LVEF | 1.164 (0.899–1.507) | 0.249 |
| Pericardial effusion | 0.147 (0.013–1.710) | 0.126 |
| pCO2 | 0.796 (0.601–1.055) | 0.113 |
| pO2 | 1.049 (0.956–1.150) | 0.313 |
| SaO2 | 1.756 (0.750–4.113) | 0.195 |
| RBC | 0.546 (0.152–1.964) | 0.354 |
| HGB | 0.985 (0.952–1.018) | 0.365 |
| HCT | 0.830 (0.609–1.131) | 0.239 |
| MCV | 1.033 (0.862–1.238) | 0.725 |
| MCH | 1.057 (0.755–1.478) | 0.748 |
| MCHC | 1.010 (0.950–1.073) | 0.756 |
| RDW | 0.910 (0.629–1.316) | 0.616 |
| PLT | 0.995 (0.971–1.020) | 0.708 |
| PDW | 0.820 (0.524–1.284) | 0.386 |
| ALT | 0.999 (0.930–1.074) | 0.989 |
| AST | 1.355 (0.987–1.861) | 0.060 |
| GGT | 0.936 (0.788–1.112) | 0.452 |
| ALP | 0.921 (0.821–1.033) | 0.162 |
| TBil | 1.067 (1.003–1.135) | 0.041* |
| DBil | 1.178 (0.628–2.209) | 0.609 |
| D/TBil | 0.666 (0.381–1.162) | 0.152 |
| CREA | 0.989 (0.884–1.106) | 0.843 |
| BUN | 0.643 (0.269–1.540) | 0.322 |
| CRP | 0.950 (0.819–1.103) | 0.501 |
| HSCRP | 0.816 (0.503–1.323) | 0.410 |
| UA | 0.998 (0.990–1.007) | 0.700 |
| LDH | 1.009 (0.997–1.021) | 0.156 |
| PT | 0.986 (0.830–1.171) | 0.870 |
| PTA | 0.995 (0.966–1.025) | 0.742 |
| INR | 3.944 (0.013–1241.199) | 0.640 |
| APTT | 0.954 (0.806–1.129) | 0.582 |
| ESR | 0.910 (0.712–1.163) | 0.450 |
| NT-proBNP | 1.000 (0.999–1.001) | 0.641 |
P < 0.05.
Table 5.
Multivariate Cox proportional hazards regression analyses for baseline characteristics to predict primary endpoints.
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| TBil | 1.068 (1.002–1.137) | 0.042* |
| Age | 1.009 (0.879–1.158) | 0.900 |
| TBil | 1.076 (1.002–1.155) | 0.044* |
| SvO2 | 1.045 (0.896–1.219) | 0.573 |
| TBil | 1.068 (1.004–1.136) | 0.038* |
| CI | 1.300 (0.157–10.759) | 0.807 |
| TBil | 1.066 (1.001–1.136) | 0.048* |
| UA | 1.000 (0.991–1.008) | 0.947 |
| TBil | 1.074 (0.995–1.160) | 0.068 |
| WHO functional class ≥ III | 0.655 (0.054–7.912) | 0.740 |
| TBil | 1.055 (0.987–1.127) | 0.118 |
| REVEAL risk score | 1.382 (0.732–2.610) | 0.319 |
| TBil | 1.214 (0.710–2.075) | 0.479 |
| 6MWD | 0.885 (0.643–1.218) | 0.454 |
| TBil | 1.070 (0.997–1.149) | 0.062 |
| NT-proBNP | 1.000 (0.999–1.001) | 0.847 |
| TBil | 1.070 (0.991–1.156) | 0.084 |
| Pericardial effusion | 0.147 (0.009–2.505) | 0.185 |
| TBil | 1.082 (0.971–1.206) | 0.152 |
| mRAP | 0.933 (0.621–1.402) | 0.739 |
P < 0.05.
Comparisons between HHT-PAH and matched IPAH patients
A total of 18 matched IPAH patients were enrolled in this study and their median follow-up time was 4.36 years (range = 1.79–7.90 years). During this period, three of them died because of right heart failure. Kaplan–Meier survival analyses showed that HHT-PAH patients had a worse prognosis than matched IPAH patients (log rank: P = 0.047; Fig. 1). Comparisons between these two cohorts showed that HHT-PAH patients had higher RRS score (8.67 ± 2.29 versus 7.11 ± 1.57, P = 0.048), worse 6MWD (385.00 ± 59.5 m versus 469.27 ± 62.39 m, P = 0.011), larger right ventricular anteroposterior diameter (RVAPD; 34.67 ± 6.67 mm versus 28.56 ± 6.35 mm, P = 0.029), wider red cell distribution width (RDW; 14.88 ± 2.93% versus 13.19 ± 0.83%, P = 0.031), and lower mean corpuscular hemoglobin concentration (MCHC; 317.38 ± 17.71 g/L versus 335.72 ± 14.68 g/L, P = 0.011) than matched IPAH patients (Table 2).
Reassessment of HHT-PAH patients after long-term specific drug therapy
Four HHT-PAH patients had detailed clinical reassessments after receiving PAH-specific drug therapy (Suppl. Table S2). Among them, two received Sildenafil, one received Bosentan, and one had Diltiazem because of positive pulmonary vasoreactivity result. After treatment, one patient had improved WHO FC, two remained in the same condition, and one had deteriorated WHO FC. As for echocardiographic parameters, two patients had improved RV/LV, while two showed decreased RV/LV.
Discussion
Novel findings from the present study are that: (1) we reported the one- and three-year survival rates of HHT-PAH for the first time; (2) HHT-PAH patients might have a worse prognosis than matched IPAH patients; and (3) TBil might be a promising predictor for the long-term prognosis in HHT-PAH patients.
In the context of HHT, PH is categorized into two distinct types: pre- and post-capillary PH.5,16 Post-capillary PH is considered to be associated with liver vascular malformations and secondary to high CO, typically after the onset of left heart failure,5 while pre-capillary PH, which can also be called PAH in the context of HHT, is considered to be caused by pulmonary arteriopathy. HHT-PAH was thought to have largely increased PAP and PVR, increased transpulmonary gradient (TPG), decreased CO, and normal PCWP.5 However, HHT with post-capillary PH was considered to have largely increased CO, increased PAP and PCWP, and normal TPG and PVR.5 Recently, TPG has been replaced by DPG according to the new guideline.9
In this study, we initially enrolled 13 HHT patients who met the hemodynamic criteria of PAH, with high mPAP, high PVR, and normal PCWP. However, four patients of this cohort had increased cardiac index (≥4.0 L/min/m2)13 and three of the four patients had definite hepatic AVMs detected by abdominal CT. In order to exclude the possibility of PH induced by high CO, we decided to remove the four HHT patients with high cardiac index to get a pure HHT-PAH cohort.
PAH was considered as a rare complication of HHT and its exact prevalence in the HHT population has not been elucidated. Sopeña et al. found that combining with PH could significantly reduce survival rates of HHT patients.2 There are previous studies which reported the long-term survival of individual HHT patients with PH17 or showed the survival of patients who had coexisting HHT and PH from any causes.14 However, these studies did not report the survival rates of HHT-PAH patients. In this study, we found that the one- and three-year survival rates of HHT-PAH patients were 77.8% and 53.3%, respectively, which were much lower than the reported idiopathic/familial PAH survival rates by the REVEAL Registry study (the one- and three-year survival rates were 91% ± 2% and 74% ± 2%, respectively).18 We then introduced a cohort of IPAH patients who matched with HHT-PAH patients on cardiac index, mPAP, PVR, PCWP, and WHO FC. Kaplan–Meier survival analyses between these two cohorts showed that HHT-PAH patients have a worse prognosis regardless of the same cardiopulmonary hemodynamic characteristics. By comparing the baseline characteristics of HHT-PAH and matched IPAH patients, we found that HHT-PAH patients had higher RRS, worse 6MWD, and larger RVAPD and RDW than matched IPAH patients. This explained why HHT-PAH patients had a worse prognosis on the one hand9,11,19–21 and also indicated that HHT-PAH patients might have worse exercise tolerance, severe symptoms, and worse clinical condition even with the same cardiopulmonary hemodynamic characteristics as IPAH patients.
Another point we want to highlight here is that HHT-PAH patients had wider RDW and lower MCHC than matched IPAH patients and deceased HHT-PAH had lower D/TBil than survivors. These all suggested the possible existence of subclinical hemolysis in HHT-PAH, though the comparisons of hemoglobin, hematocrit, bilirubin, and lactate dehydrogenase (LDH) did not show statistical significances between these two groups. Over the last decade, there has been increasing interest in hemolysis as a potential cause of PH. Free hemoglobin, when released from the erythrocyte, reduces the bioavailability of nitric oxide (NO), and promotes endothelial dysfunction with thrombosis, inflammation, vasoconstriction, and smooth muscle proliferation in the capillaries.22 Some studies have found that subclinical hemolysis plays important roles in the pathogenesis or pathophysiology of PAH23,24 and may be associated with the severity of pulmonary vascular disease and clinical outcomes.22 Though it is not possible to determine from the current study whether the subclinical hemolysis present in HHT-PAH patients represents a cause, effect, or epi-phenomenon of the illness, we suggested that subclinical hemolysis might be a feature of the HHT-PAH phenotype. Additional tests of hemolysis, including RBC lifespan or half-life (the golden standard), plasma-free hemoglobin, reticulocyte counts, erythrocyte creatine, and haptoglobin, are still needed to further confirm the values of subclinical hemolysis in HHT-PAH. Besides that, wider RDW and lower MCHC in HHT-PAH patients also suggested iron deficiency, which is highly prevalent in PAH and HHT and is associated with worse disease severity and clinical outcomes.13,25 However, we did not measure markers of iron metabolism in this study.
Comparisons of baseline characters between survival and deceased HHT-PAH showed that non-survivors had lower D/TBil than survivors. Univariate and multivariate Cox proportional hazards regression analyses found that baseline TBil could predict the mortality of HHT-PAH patients independently, even after adjusting by age or SvO2 or cardiac index or serum uric acid. So HHT-PAH patients with higher TBil indicated worse prognosis. We thought this was partially due to hemolysis and previous studies have also identified TBil as a predictive risk factor for both systolic and diastolic heart failure and PAH because of hemodynamic alterations, including elevated central venous pressure and decreased cardiac index;26–28 therefore, the recognition of liver dysfunction as a reflection of end-organ damage in right heart failure should also be paid much attention in HHT-PAH patients.
Even though there is no systematic evidence for the treatment of HHT-PAH at present, it seems rational to treat these patients with PAH-specific medication. Cases have reported the short-term or long-term effects of PAH-specific drugs (including bosentan, sildenafil, and epoprostenol) on HHT-PAH patients. It seemed that PAH-specific medication could improve the symptoms, exercise capacity, and cardiopulmonary hemodynamics of HHT-PAH patients.7,16,29,30 However, in this study, reassessments of four HHT-PAH patients after receiving long-term PAH-specific drugs did not show significant clinical improvement in every patient. Besides that, these patients did not undergo a second RHC after receiving long-term PAH-specific drugs, so post-treatment cardiac index and PVR were unavailable. Therefore, strictly designed randomized controlled trials and pre- and post-treatment RHCs are still needed.
Our study had several limitations. First, this study consisted of patients referred to a single tertiary center, which may constitute a referral bias. Second, our center is a specialized hospital for cardiovascular diseases and all the recruited patients were suspected with PH, unknowing the diagnosis of HHT when they first came to us. Therefore, we cannot ascertain the exact prevalence of PAH in HHT. Third, gene mutation screening was not performed because of the expensive cost which was beyond most of the patients’ affordability. Forth, detailed information of post PAH-specific drug therapy were only available in four patients, but no second RHC record. Consequently, we could not confirm the therapeutic effect of PAH specific drug on HHT-PAH patients. Fifth, the study population of HHT-PAH was limited and may reflect a relatively small sample of the entire HHT-PAH population. Given that PAH is a rare manifestation of HHT, with prevalence of < 1% of HHT cases, it may be challenging to conduct large-scale studies. Finally, important parameters of hemolysis, including red blood cell lifespan or half-life (the golden standard test), plasma-free hemoglobin, reticulocyte counts, erythrocyte creatine, haptoglobin, and parameters reflecting iron metabolism, were also needed to confirm the values of iron deficiency and subclinical hemolysis in HHT-PAH patients.
In conclusion, the one- and three-year survival rates of HHT-PAH patients were 77.8% and 53.3%, respectively, which were worse than matched IPAH patients. Total bilirubin might be a promising predictor for the long-term prognosis in HHT-PAH patients.
Supplemental Material
Supplemental material for The clinical characteristics and long-term prognosis of pulmonary arterial hypertension associated with hereditary hemorrhagic telangiectasia by Wen Li, Chang-ming Xiong, Qing Gu, Xiao-tong Wang, Xiao-ling Cheng, Li Huang, Tao Yang, Qin Luo, Zhi-hui Zhao, Xin-hai Ni, Zhi-hong Liu and Jian-guo He: ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was supported by grants from Chinese Ministry of Science and Technology, National Key Technology R&D Program (Project no. 2016YFC1304400); Chinese Ministry of Science and Technology, National Key Technology R&D Program (Project no. 2011BAI11B15); Chinese National Natural Science Foundation (Project no. 81570048), and Peking Union Medical College Youth Fund (Project no. 33320140128).
References
- 1.Garg N, Khunger M, Gupta A, et al. Optimal management of hereditary hemorrhagic telangiectasia. J Blood Med 2014; 5: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sopeña B, Pérez-Rodríguez MT, Portela D, et al. High prevalence of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. Eur J Intern Med 2013; 24: e30–34. [DOI] [PubMed] [Google Scholar]
- 3.Zarrabeitia R, Albiñana V, Salcedo M, et al. A review on clinical management and pharmacological therapy on hereditary haemorrhagic telangiectasia (HHT). Curr Vasc Pharmacol 2010; 8: 473–481. [DOI] [PubMed] [Google Scholar]
- 4.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011; 48: 73–87. [DOI] [PubMed] [Google Scholar]
- 5.Faughnan ME, Granton JT, Young LH. The pulmonary vascular complications of hereditary haemorrhagic telangiectasia. Eur Respir J 2009; 33: 1186–1194. [DOI] [PubMed] [Google Scholar]
- 6.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003; 40: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SA, Jang SY, Ki CS, et al. Successful bosentan therapy for pulmonary arterial hypertension associated with hereditary hemorrhagic telangiectasia. Heart Vessels 2011; 26: 231–234. [DOI] [PubMed] [Google Scholar]
- 8.Providência R, Cachulo Mdo C, Costa GV, et al. Hereditary hemorrhagic telangiectasia: rare cause of pulmonary hypertension? Arq Bras Cardiol 2010; 94: e34–36. e94–96. [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 12.Verbraecken J, Van de Heyning P, De Backer W, et al. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 2006; 55: 515–524. [DOI] [PubMed] [Google Scholar]
- 13.Shovlin CL. Circulatory contributors to the phenotype in hereditary hemorrhagic telangiectasia. Front Genet 2015; 6: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyle MA, Fenstad ER, McGoon MD, et al. Pulmonary hypertension in hereditary hemorrhagic telangiectasia. Chest 2016; 149: 362–371. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med 1999; 160: 487–492. [DOI] [PubMed] [Google Scholar]
- 16.Vorselaars VMM, Velthuis S, Snijder RJ, et al. Pulmonary hypertension in hereditary haemorrhagic telangiectasia. World J Cardiol 2015; 7: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minai OA, Rigelsky C, Eng C, et al. Long-term outcome in a patient with pulmonary hypertension and hereditary hemorrhagic telangiectasia. Chest 2007; 131: 984–987. [DOI] [PubMed] [Google Scholar]
- 18.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes CJ, Wharton J, Howard LS, et al. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart 2011; 97: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 21.Benza RL, Miller DP, Foreman AJ, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant 2015; 34: 356–361. [DOI] [PubMed] [Google Scholar]
- 22.Brittain EL, Janz DR, Austin ED, et al. Elevation of plasma cell-free hemoglobin in pulmonary arterial hypertension. Chest 2014; 146: 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox BD, Okumiya T, Attas-Fox L, et al. Raised erythrocyte creatine in patients with pulmonary arterial hypertension–evidence for subclinical hemolysis. Respir Med 2012; 106: 594–598. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Kato M, Nakaya T, et al. Decreased haptoglobin levels inversely correlated with pulmonary artery pressure in patients with pulmonary arterial hypertension: A cross-sectional study. Medicine (Baltimore) 2017; 96: e8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes CJ, Wharton J, Howard L, et al. Iron deficiency in pulmonary arterial hypertension: a potential therapeutic target. Eur Respir J 2011; 38: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosy AP, Vaduganathan M, Huffman MD, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 2012; 14: 302–311. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H, Li Y, Xie N. Association of serum total bilirubin levels with diastolic dysfunction in heart failure with preserved ejection fraction. Biol Res 2014; 47: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda Y, Takeda Y, Tomimoto S, et al. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyake R, Fujino T, Abe K, et al. Pulmonary arterial hypertension associated with hereditary hemorrhagic telangiectasia successfully treated with sildenafil. Int J Cardiol 2016; 214: 275–276. [DOI] [PubMed] [Google Scholar]
- 30.Chadha D, Handa A, Kumar A. Pulmonary hypertension in a patient with hereditary haemorrhagic telangiectasia. BMJ Case Rep 2013; 2013: bcr2012008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for The clinical characteristics and long-term prognosis of pulmonary arterial hypertension associated with hereditary hemorrhagic telangiectasia by Wen Li, Chang-ming Xiong, Qing Gu, Xiao-tong Wang, Xiao-ling Cheng, Li Huang, Tao Yang, Qin Luo, Zhi-hui Zhao, Xin-hai Ni, Zhi-hong Liu and Jian-guo He: ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories in Pulmonary Circulation