Abstract
Objective
We reviewed outcomes of 52 pregnancies in 45 women with immune thrombocytopenic purpura who delivered at Auckland Hospital with an antenatal platelet count of <100 × 109/L.
Outcome measures
Primary outcomes were maternal platelet count at delivery and treatment response. Secondary outcomes included post-partum haemorrhage (PPH).
Results
Most women had thrombocytopenia at delivery. Treatment with prednisone was given in 14 (27%) pregnancies with responses considered safe for delivery in 11 pregnancies (79%). Women in eight pregnancies also received intravenous immunoglobulin; in five pregnancies (63%) a platelet response acceptable for delivery was achieved.
Seventeen pregnancies (33%) were complicated by a PPH ≥500 mL. Ten pregnancies (19%) were complicated by a PPH ≥1000 mL. PPH was reported in all women with a platelet count <50 × 109/L at delivery.
Conclusions
There were no antenatal bleeding complications but PPH was common among women with platelet counts <50 × 109/L at the time of birth.
Keywords: Platelets, immune thrombocytopenic purpura, prednisone, intravenous immunoglobulin, pregnancy, post-partum haemorrhage
Introduction
Thrombocytopenia is the second most common haematological abnormality after anaemia encountered in pregnancy.1,2 An International Working Group defines thrombocytopenia as a platelet count of <100 × 109/L in pregnancy.1 A platelet count <100 × 109/L is observed in only 1% of pregnant women.1,2 Thrombocytopenia in pregnancy may be categorised as pre-existing, slowly progressive, which may or may not be related to pregnancy, or acute related to hypertensive disorders of pregnancy.1
Gestational thrombocytopenia accounts for 70–80% of thrombocytopenia in pregnancy and is generally characterised by a platelet count >70 × 109/L.1–4 It is a benign condition and commonly occurs in the mid second to third trimester.2,5 Platelet counts are normal outside of pregnancy. There are no confirmatory tests and the mechanism is unknown but postulated to be due to haemodilution and accelerated platelet clearance.1,2 It is not associated with neonatal thrombocytopenia.1,5
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterised by autoantibody binding to platelet antigens causing premature platelet destruction by the reticulo-endothelial system, particularly the spleen.3 The American Society of Hematology defines ITP as isolated thrombocytopenia in the absence of identifiable and specific precipitants.6 There are no diagnostic tests for ITP; antiplatelet antibody tests have poor sensitivity and specificity and are no longer recommended.2 ITP is common in women of childbearing age and affects women in 1–2 of every 1000 pregnancies.5,7 It can occur in any trimester but generally platelet counts start to decline in early pregnancy then continue to decline until delivery.2,4,5 Maternal concerns with ITP relate to bleeding risks particularly at the time of delivery. Fetal concerns relate to maternal antiplatelet antibodies crossing the placenta causing neonatal thrombocytopenia with a risk of cerebral haemorrhage.
Significant thrombocytopenia in pregnancy causes anxiety amongst clinicians. Information is limited on risks of bleeding at the time of delivery, the likelihood of needing treatment during pregnancy and response to treatments. The aim of this study was to review maternal and fetal outcomes in pregnant women with moderate to severe ITP (platelets <100 × 109/L) at Auckland Hospital over the past 3 years to gauge contemporary treatment approaches and clinical outcomes.
Methods
Patient selection
All women attending Auckland Hospital aged 15–45 years from July 2013 to July 2016 with platelet counts <100 × 109/L were identified from hospital laboratory data. Duplicate platelet counts were removed and cases were restricted to pregnant women who attended maternity services or the intensive care unit.
Clinical records were reviewed to determine potential causes of thrombocytopenia. Women were excluded if they had a known cause for thrombocytopenia other than ITP, e.g. other haematological disorders, medications, other autoimmune conditions, disseminated intravascular coagulation (DIC), liver disease and hypertensive disorders of pregnancy (e.g. haemolysis elevated liver enzymes and low platelets (HELLP) syndrome or pre-eclampsia (PET)). For women with new thrombocytopenia in pregnancy, a diagnosis of gestational thrombocytopenia was considered likely if platelets ≥70 × 109/L and platelet counts outside of pregnancy were normal. These women were excluded from the study.
Our inclusion criteria for the study were a platelet count <100 × 109/L during pregnancy presumed due to ITP with other causes of thrombocytopenia excluded. This included women with a known history of ITP prior to pregnancy and those diagnosed with ITP during pregnancy. Patients were diagnosed with new ITP in pregnancy if they had platelets <70 × 109/L during pregnancy or platelets <100 × 109/L during pregnancy with unexplained thrombocytopenia (platelets <150 × 109/L) outside of pregnancy. Women could be included in more than one pregnancy.
Clinical records were reviewed to collect the following information: age, body mass index (BMI), ethnicity, gestational age at delivery, parity, new or prior diagnosis of ITP, platelet nadir during pregnancy, platelet count at delivery, treatment given including agent and dose, response to treatment, gestation when treatment was required, mode of delivery, epidural anaesthesia use, estimated blood loss at delivery, blood product use, complications at delivery and in the post-partum period. Information collected on infants included sex, birth weight, customised birth weight centile and rates of small for gestational age babies (SGA), Apgar scores at 1 and 5 min, platelet count at birth, complications at birth and treatments received.
Outcomes
The primary outcomes for this study were maternal platelet count at delivery and response to treatment. Platelet response to treatment for ITP has been previously defined by the American Society of Hematology.6,8 A complete response is defined as a platelets ≥100 × 109/L during or after therapy.6,8 A partial response is defined as platelets 30–100 × 109/L and at least doubling of the baseline count.6,8 No response is defined as platelets <30 × 109/L or less than doubling of the baseline count. However, these definitions of platelet response to treatment relate to the general population. In the obstetric population, long-term platelet response rates are not the goal of treatment. A successful treatment outcome could be considered when the intervention enables women to reach a platelet count considered safe for either birth or neuraxial analgesia use. In this study, we have defined response to treatment as (1) attaining platelet counts considered safe for delivery (platelets ≥50 × 109/L) and (2) to enable placement of neuraxial analgesia (platelets ≥80 × 109/L).
Secondary outcomes included mode of birth and maternal bleeding complications at delivery. PPH was defined as blood loss of ≥500 mL with a vaginal delivery and ≥1000 mL with a caesarean section. Epidural use and related complications were also of interest. Fetal secondary outcomes included platelet counts and haemorrhagic complications at birth, pre-term birth (<37 weeks gestation) rates, birthweight, SGA rates and Apgar scores at 5 min <7.
Statistical analysis
Baseline characteristics of the study population were summarised using descriptive statistical methods. Most data were not normally distributed hence non parametric statistical descriptives and tests were used. Wilcoxin two independent sample tests were performed to evaluate median platelet counts at delivery and estimated blood loss in women requiring treatment for ITP compared with those not requiring treatment. Statistical significance was set at p < 0.05 and tests were two sided.
Results
Our laboratory identified a total of 5918 platelet counts <100 × 109/L in women attending Auckland Hospital aged 15–45 years from July 2013 to July 2016. Two hundred and eighty-nine women remained in the study after duplicate results for each patient were removed and data were restricted to pregnant women. Among these women, 244 were excluded from the study as thrombocytopenia was found to have a causal factor including: gestational thrombocytopenia (n = 75), PPH (n = 43), PET (n = 31), HELLP syndrome (n = 25), spurious or limited results (unable to determine cause) (n = 21), gestational thrombocytopenia and PET or PPH (n = 12), placental abruption (n = 10), haematological causes (n = 6), early pregnancy loss (n = 5), portal hypertension (n = 3), sepsis (n = 3), placenta accreta/percreta (n = 3), systemic lupus erythematosis (n = 2), acute fatty liver (n = 1), antepartum haemorrhage (n = 1), medication-induced pancytopenia (n = 1), PET and PPH (n = 1) and antiphospholipid antibody syndrome (n = 1).
Characteristics of women with ITP
There were 45 women with ITP identified during the study interval. These women delivered 55 infants in 52 pregnancies, with three sets of twins. Seven women (15%) delivered on two occasions during the study period. Maternal characteristics are described in Table 1. Women received a new diagnosis of ITP in 29 pregnancies.
Table 1.
Maternal characteristics of women with ITP.
| Characteristics | Median (range) or number (%) |
|---|---|
| Age (years) | 32.2 (20–42) |
| Parity | |
| Primiparous | 29 (56%) |
| Multiparous | 23 (34%) |
| Gestational age (weeks) | 39 (27–41.6) |
| Ethnicity | |
| NZ European | 21 (47%) |
| Asian | 9 (20%) |
| Indian | 7 (16%) |
| Pacific Island | 3 (6%) |
| Maori | 1 (2%) |
| Other | 4 (11%) |
| BMI (kg/m2) | 21.35 (16.8–32) |
| Mode of delivery | |
| Caesarean section | 16 (31%) |
| Vaginal delivery | 36 (69%) |
| Diagnosis | |
| Known thrombocytopenia before pregnancy | 23 (44%) |
| During pregnancy | 29 (56%) |
| Platelet nadir during pregnancy (×109/L) | 81 (15–99) |
| Platelet count at delivery (×109/L) | 92 (23–154) |
Antenatal outcomes
Twelve women in 14 pregnancies (27%) received treatment for thrombocytopenia. The median antenatal platelet nadir in pregnancies requiring treatment was 34 × 109/L (15–75 × 109/L). All pregnancies requiring treatment were managed by an Obstetric Physician. The median gestation when treatment was given was 34 weeks (range 30–37 weeks).
Women in all 14 pregnancies were initially treated with prednisone at a median dose of 40 mg daily (range 40–60 mg). The duration of prednisone varied significantly between a one-week trial to ongoing treatment for several months until delivery. Women in 11 pregnancies (79%) achieved platelet responses safe for delivery. Prednisone alone was successful in raising the platelet count to a level to allow for neuraxial analgesia in seven pregnancies (50%).
Women in eight pregnancies received intravenous immunoglobulin (IVIG) in addition to prednisone. IVIG was given in seven pregnancies when prednisone was unsuccessful in raising the platelet count to allow for neuraxial analgesia. IVIG was also given in one pregnancy where a response to prednisone was not sustained. The median gestation women received IVIG was 36 weeks (32–39 weeks). IVIG was uniformly given at a dose of 1 g/kg in one or two doses over consecutive days. In five pregnancies (63%), platelet responses acceptable for delivery were achieved and in three pregnancies (38%) responses acceptable for regional analgesia were achieved.
Delivery outcomes
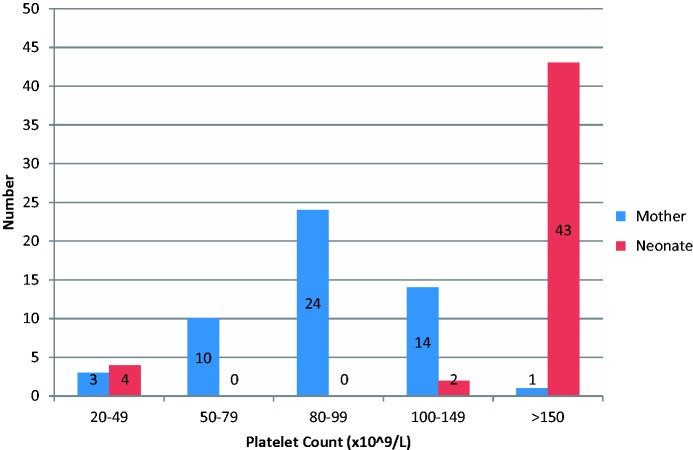
Only one woman in the study had platelets >150 × 109/L at the time of birth (Figure 1); the median platelet count was 92 × 109/L (23–154 × 109/L). In pregnancies where women were treated for ITP, the median platelet count at delivery was 79 × 109/L (23–135 × 109/L). Women in pregnancies treated for thrombocytopenia had significantly lower platelet counts at delivery than those who did not require treatment (median 79 × 109/L vs. 95 × 109/L; p = 0.012).
Figure 1.
Maternal and neonatal platelet counts at delivery.
On the day of delivery, women in four pregnancies had platelet counts of 20–49 × 109/L. All of these women had been treated for ITP during pregnancy with prednisone and IVIG but did not achieve platelet counts ≥80 × 109/L. These women received platelet transfusions prior to or at delivery to prevent bleeding. One woman achieved a platelet response from 34 × 109/L to 86 × 109/L with two units of platelets prior to delivery and was reclassified as having platelets >50 × 109/L at delivery. Three women with platelet counts <50 × 109/L did not achieve platelet counts ≥50 × 109/L after platelet transfusions.
Analgesia
Neuraxial analgesia/anaesthesia was used in women in 28 pregnancies (54%); epidural (n = 21), combined spinal-epidural (n = 1) and spinal anaesthetic (n = 6). Two women had remifentanil patient-controlled analgesia (PCA). Women in four pregnancies had a general anaesthetic for caesarean deliveries with platelet counts between 43 and 68 × 109/L.
The median platelet count at the time of delivery in women who received a spinal or epidural was 95 × 109/L (70–154 × 109/L). Three women had platelet counts <80 × 109/L (70–78 × 109/L) at the time of epidural. There were no documented complications to the placement of an epidural.
Type of delivery
There was no significant difference in platelet counts between the modes of delivery (p = 0.58). Median platelet counts at delivery were 92 × 109/L (37–154 × 109/L) for women who delivered vaginally and 92 × 109/L (43–135 × 109/L) for women who delivered by caesarean section. There were six elective caesarean sections and ten emergency caesarean sections. Indications for emergency caesarean sections were not related to ITP. Of the three pregnancies in women with platelet counts ≤50 × 109/L at the time of delivery, one delivery was by caesarean section and there were two vaginal births.
Haemorrhagic complications
Seventeen pregnancies (33%) were complicated by a PPH. Women in eight (48%) pregnancies had received treatment for ITP. Blood loss at delivery was significantly higher in women who received treatment for ITP (median 750 mL vs. 400 mL, p = 0.03). The median platelet count at delivery for women with a PPH was 82 × 109/L (23–128 × 109/L). Women in all three pregnancies with platelet counts <50 × 109/L at delivery had a PPH.
Major PPH (blood loss ≥1000 mL) was reported in 10 pregnancies (19%). Major PPH was reported in two of the three women (67%) with platelets <50 × 109/L at the time of birth and in 8 of the 49 (16%) women with a platelet count of ≥50 × 109/L at the time of birth. In women with a safe platelet count at birth, the median blood loss was 400 mL. Women with a platelet count unsafe for birth had a median blood loss of 1750 mL (range 750–2000 mL).
Eleven vaginal births (31%) were complicated by a PPH ≥500 mL. Six caesarean sections (38%) were complicated by a PPH ≥1000 mL. The median blood loss in pregnancies with a PPH was 700 mL for vaginal births and 1190 mL for caesarean sections.
Neonatal outcomes
There were 55 infants born in this study, characteristics are shown in Table 2. One baby was stillborn; intrauterine death was diagnosed on ultrasound at 27 weeks gestation after the mother reported reduced fetal movements. The baby had intrauterine growth restriction with placental and chromosomal abnormalities.
Table 2.
Fetal characteristics and perinatal outcomes.
| Characteristics | Median (range) or number (%) |
|---|---|
| Sex | |
| Male | 24 (44%) |
| Female | 31 (56%) |
| Birth weight | 3290 g (600–5100 g) |
| Small for gestational age by customised Birth-weight centiles | 10 (18%) |
| Pre-term birth (<37 weeks) | 8 (14%) |
| Apgar score | |
| 1 min | 9 (4–10) |
| 5 min | 10 (4–10) |
| Platelet count at birth | 235 × 109/L (23–432 × 109/L) |
| Neonatal thrombocytopenia (<150 × 109/L) | 6 (12%) |
| Bleeding complications | 0 |
| Treatment given in neonatal period | 3 (50%) |
Platelet counts measured in the first few days of birth were recorded in 49 infants (Figure 1). Six babies (12%) had platelet counts <150 × 109/L, including one set of twins. Mothers of babies with neonatal thrombocytopenia also had low platelets at delivery with a median platelet count of 68 × 109/L (37–128 × 109/L). Two mothers of babies with platelet counts <150 × 109/L received treatment for ITP during pregnancy. Four babies (8%) had platelet counts <50 × 109/L and three of these babies received platelet transfusions. Two babies also received IVIG and had cranial ultrasounds which were normal. There were no neonatal haemorrhagic complications.
Discussion
In this study, women in 14 pregnancies (27%) received prednisone for ITP. Women in eight pregnancies additionally received IVIG due to lack of optimal response with prednisone. Women in most pregnancies achieved platelet responses safe for delivery with treatment (79% with prednisone and 63% with IVIG). Women in some pregnancies achieved a platelet response considered safe for regional analgesia with treatment (50% with prednisone and 38% with IVIG). Neonatal thrombocytopenia was reported in 12% of infants. This rate is similar to that reported in other cases series. There were no infant bleeding complications.
Major PPH was reported in 10 pregnancies (19%) compared to a rate of 10% in the general obstetric cohort at Auckland Hospital in 2015.9 Major PPH was reported in two of the three women (67%) with platelet counts <50 × 109/L at the time of birth and in 8 of the 49 (16%) women with platelet counts >50 × 109/L at the time of birth. The median blood loss at delivery was 750 mL in women who were given treatment for ITP compared with 400 mL (p = 0.03) in those who did not receive treatment. In women with a platelet count ≥50 × 109/L at delivery the median blood loss was 400 mL. Women with a platelet count <50 × 109/L at delivery had a median blood loss of 1750 mL.
A limitation of this study was its retrospective design. Data collection was limited to information recorded in clinical notes. However, we were able to retrieve complete information for most patients due to well-documented electronic clinical records. Our study had a small sample size but this was appropriate for our obstetric population. There are around 7000 births at Auckland Hospital per year so we could anticipate 21–42 pregnancies in women with ITP over a 3-year period (assuming an incidence of ITP in pregnancy of 1–2: 1000 pregnancies5,7). Further prospective studies with larger sample sizes are needed to confirm if our findings reflect outcomes in this patient population.
The clinical management of ITP in pregnancy is complex. The American Society of Hematology recommends treatment antenatally in severe thrombocytopenia with platelet counts <30 × 109/L or if there are bleeding manifestations.1,6 In the absence of bleeding and a platelet count ≥30 × 109/L, treatment is not considered to be required until 36 weeks gestation or sooner if delivery is expected.1 First-line treatment for ITP is similar to that outside of pregnancy with oral corticosteroids or IVIG.6,10 There are concerns regarding corticosteroid use in pregnancy with increased risks of gestational diabetes, weight gain, accelerated bone loss, hypertension, placental abruption and premature labour.5 Some studies have shown an increased risk of congenital anomalies with corticosteroid use in the first trimester.11 It has been suggested that low-dose prednisone 0.25–0.5 mg/kg daily or IVIG (2 g/kg over 2 to 5 days) should be given first line, rather than conventional doses of prednisone 1 mg/kg outside of pregnancy.1 Regarding the choice of agent, only one study has directly compared IVIG with prednisone in the management of ITP during pregnancy and results showed no difference in maternal platelet counts at delivery between the treatments.11 Corticosteroids are easy to administer and lower in cost, and hence are usually given first line. IVIG is expensive, costing over $12000 NZD for the recommended dose in an 80 kg woman.
Previous studies have shown that around 30–35% of women receive treatment for their ITP during pregnancy.12 The primary considerations for the management during pregnancy are achieving a platelet count sufficient to reduce bleeding risks and allow epidural use at delivery. Recommendations based on expert opinion suggest aiming for a platelet count ≥50 × 109/L for delivery and ≥80 × 109/L for neuraxial analgesia/anaesthesia.1 It is our practice at Auckland Hospital to trial a course of prednisone at 32–34 weeks gestation to assess platelet response. Women in our study received prednisone at a median of 34 weeks gestation. Treatment can then be stopped and re-introduced closer to delivery if there was a response. This also allows sufficient time prior to delivery to trial IVIG if prednisone was not successful in attaining an acceptable platelet response. Clinical guidelines for the management of ITP during pregnancy are lacking.
This study confirms findings of other trials that severe thrombocytopenia is uncommon in infants born to mothers with ITP. It is estimated that thrombocytopenia occurs in 10% of babies born to mothers with ITP.5 In our study, six babies (12%) had platelet counts <150 × 109/L; four babies (8%) had platelet counts <50 × 109/L. It is recommended that babies with platelet counts at birth <50 × 109/L have cranial ultrasounds and babies with platelet counts <30 be treated with IVIG and platelet transfusions.2,4 Three babies in our study received platelet transfusions and two babies also received IVIG. Studies have shown that the risk of intracranial haemorrhage is <1.5% with mortality <1%.1,2 Two babies in our study had cranial ultrasounds with no neonatal haemorrhages. The correlation between maternal and fetal platelet counts in previous studies is poor. In our study, the median platelet count in mothers of babies with thrombocytopenia was low but this may reflect our small sample size.
Conclusion
Women with ITP in pregnancy require specialist monitoring and may need treatment to improve platelet counts for delivery. Most women with ITP have an uncomplicated pregnancy. We report an increase in the risk of PPH in women with ITP and a platelet count <50 × 109/L at the time of birth. Women who required treatment for ITP had a significantly higher estimated blood loss at delivery. Our study raises the possibility that we should aim for a higher platelet count at delivery. A clinical guideline would help standardise our approach for managing ITP during pregnancy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Ethics approval for the review of medical records was obtained.
Guarantor
KG.
Contributorship
KG collected data, carried out the analysis and drafted the initial manuscript. CM helped with study design, assisted with interpretation of results and reviewed and revised the initial manuscript.
References
- 1.Rajasekhar A, Gernsheimer T, Stasi R, et al. 2013 Clinical practice guide on thrombocytopenia in pregnancy. Washington DC: American Society of Hematology. http://www.hematology.org.
- 2.Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood 2013; 121: 38–47. [DOI] [PubMed] [Google Scholar]
- 3.Wyszynski DF, Carman WJ, Cantor AB, et al. Pregnancy and birth outcomes among women with idiopathic thrombocytopenic purpura. J Pregnancy 2016; Article ID 8297407, 8 pages, http://dx.doi.org/10.1155/2016/8297407. [DOI] [PMC free article] [PubMed]
- 4.Gernsheimer T, Mcrae KR. Immune thrombocytopenic purpura in pregnancy. Curr Opin Hematol 2007; 14: 574–580. [DOI] [PubMed] [Google Scholar]
- 5.Yuce T, Acar D, Kalafat E, et al. Thrombocytopenia in pregnancy: Do the time of diagnosis and delivery route affect pregnancy outcome in parturients with idiopathic thrombocytopenic purpura? Int J Haematol 2014; 100: 540–544. [DOI] [PubMed] [Google Scholar]
- 6.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011; 117: 4190–4207. [DOI] [PubMed] [Google Scholar]
- 7.Gill JJ, Kelton JG. Management of idiopathic thrombocytopenic purpura in pregnancy. Semin Hematol 2000; 37: 275–289. [DOI] [PubMed] [Google Scholar]
- 8.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardisation of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009; 113: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 9.National Women’s Hospital. National Women’s Annual Clinical Report 2015. National Women’s Hospital, Auckland. http://nationalwomenshealth.adhb.govt.nz/health-professionals/annual-clinical-report/yearly-annual-clinical-reports.
- 10.Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment for immune thrombocytopenia in pregnancy. Blood 2016; 128: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 11.Stavrou E, McCrae KR. Immune thrombocytopenia in pregnancy. Haematol Oncol Clin N Am 2009; 23: 1299–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webert KE, Mittal R, Sigouin C, et al. A retrospective 11 year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003; 102: 4306–4311. [DOI] [PubMed] [Google Scholar]