Abstract
Background
Neurotensin is a peptide that modulates central dopamine neurotransmission and dopamine-related behaviors. Methamphetamine self-administration increases neurotensin levels in the ventral tegmental area, but the consequences for self-administration behavior have not been described. Here we test the hypothesis that antagonizing neurotensin receptors in the ventral tegmental area attenuates the acquisition of methamphetamine self-administration and methamphetamine intake.
Methods
We implanted mice with an indwelling catheter in the right jugular vein and bilateral cannulae directed at the ventral tegmental area. Mice were then trained to nose-poke for i.v. infusions of methamphetamine (0.1 mg/kg/infusion) on a fixed ratio 3 schedule.
Results
Mice receiving microinfusions of the neurotensin NTS1/NTS2 receptor antagonist SR142948A in the ventral tegmental area (10 ng/side) prior to the first 5 days of methamphetamine self-administration required more sessions to reach acquisition criteria. Methamphetamine intake was decreased in SR142948A-treated mice both during training and later during maintenance of self-administration. Drug seeking during extinction, cue-induced reinstatement, and progressive ratio schedules was also reduced in the SR142948A group. The effects of SR142948A were not related to changes in basal locomotor activity or methamphetamine psychomotor properties. In both SR142948A- and saline-treated mice, a strong positive correlation between methamphetamine intake and enhanced locomotor activity was observed.
Conclusion
Our results suggest that neurotensin input in the ventral tegmental area during initial methamphetamine exposure contributes to the acquisition of methamphetamine self-administration and modulates later intake and methamphetamine-seeking behavior in mice. Furthermore, our results highlight the role of endogenous neurotensin in the ventral tegmental area in the reinforcing efficacy of methamphetamine, independent of its psychomotor effects.
Keywords: Neurotensin, methamphetamine, self-administration, mouse, VTA
Significance Statement
In this manuscript, we present the first evidence that activation of neurotensin receptors in the ventral tegmental area contributes to the acquisition of methamphetamine self-administration and has lasting effects on drug seeking. Our findings support the use of agents targeting the neurotensin system as a potential pharmacological approach to treat methamphetamine addiction.
Introduction
Methamphetamine (METH) addiction contributes a societal burden by increasing health care costs and crime, decreasing productivity, and ultimately causing loss of human life (Maxwell and Brecht, 2011). Currently, no pharmacotherapy has been approved to treat METH addiction. It is well established that the dopaminergic system plays a central role in the acute and chronic pharmacological effects of the drug. Indeed, an increase in extracellular dopamine levels is a hallmark effect of acute METH intake that is observed in the brain, and after prolonged exposure, this can lead to dysregulation of dopamine synthesis and synaptic transmission (Cadet and Krasnova, 2009; Desai et al., 2010). However, the mechanisms contributing to changes in dopamine transmission with prolonged METH use are incompletely understood.
Neurotensin is a peptide synthesized in the brain that is known to modulate dopaminergic transmission and dopamine-mediated behavior (Ferraro et al, 2016). Most actions of neurotensin are mediated by NTS1 and NTS2 receptors, which are widely distributed in the mammalian brain and expressed in the ventral mesencephalon (Quirion et al., 1987; Vincent et al., 1999). Neurotensin receptors can be found in dopamine cell bodies and dendrites in the ventral tegmental area (VTA) and the substantia nigra (SN), and in dopamine axon terminals in the nucleus accumbens and the caudate/putamen (Palacios and Kuhar, 1981; Nicot et al., 1994). Neurotensin can directly excite midbrain dopamine neurons or disinhibit firing by reducing D2 dopamine autoreceptor signaling (Jomphe et al., 2006; Piccart et al., 2015; Shi and Bunney, 1991). At the dopamine neuron terminal, neurotensin receptor signaling also reduces the activity of dopamine D2 autoreceptors (Von Euler and Fuxe, 1987). Thus, neurotensin receptor activation facilitates dopamine release by inhibiting D2 autoreceptor function, enhancing midbrain dopamine cell activity and in turn increasing dopamine release in terminal areas (Leonetti et al., 2004; Fawaz et al., 2009). In addition, we recently reported that both acute exposure to neurotensin and repeated METH self-administration depress D2 autoreceptor-mediated currents in VTA and SN dopaminergic neurons (Sharpe et al., 2014; Piccart et al., 2015), possibly suggesting a synergistic effect of METH and neurotensin on dopamine neurotransmission.
The interaction between the dopamine and neurotensin systems is speculated to modulate the reinforcing effects of psychostimulants (Binder et al., 2001). For METH, most of the functional evidence has been provided by neurochemical studies in self-administration models. Indeed, prolonged METH exposure and/or high doses of METH increase neurotensin levels in the striatum, as well as in the VTA and SN (Wagstaff et al., 1996; Frankel et al., 2011; Hanson et al., 2012). Extracellular neurotensin levels are enhanced and directly correlated to the level of METH exposure during self-administration and, to a lesser extent, to noncontingent METH administration (Hanson et al., 2012, 2013). Interestingly, elevation of neurotensin levels in the VTA has been observed in rats self-administering METH, but not in yoked rats receiving equal amounts of i.v. METH (Hanson et al., 2012). This could potentially contribute to drug-seeking behavior, since neurotensin in the VTA is itself reinforcing, supporting operant self-administration (Glimcher et al., 1987) and affecting intracranial self-stimulation responding (Rompré et al., 1992). In addition, neurotensin receptor antagonism in the VTA disrupts optogenetic self-stimulation of hypothalamic terminals (Kempadoo et al., 2013).
The increase of neurotensin levels in response to METH self-administration combined with the reinforcing properties of neurotensin suggests that the VTA is an important locus for the interaction between endogenous neurotensin and METH. Therefore, we hypothesized that endogenous neurotensin input into the VTA contributes to the reinforcing efficacy of METH during self-administration. To test this, we pharmacologically blocked neurotensin receptors by microinfusing the NTS1/NTS2 receptor antagonist SR142948A into the VTA prior to each of the first 5 days of METH self-administration training. We analyzed the effect of neurotensin receptor blockade on acquisition and stabilization of METH self-administration and METH drug-seeking behavior in mice. In addition, we analyzed the effects of SR142948A treatment on METH-induced psychomotor effects after self-administration.
Materials and Methods
Animals
Twenty-four male DBA mice (6–7 weeks old) purchased from Jackson Labs were group housed (3–5 per cage) in polycarbonate boxes with rodent bedding and shredding material. Animals were kept on a 12-/12-hour reverse light-dark cycle (lights off at 9:00 am) with ad libitum access to food and water. For the pilot study presented in supplementary Figure 1, male C57BL/6J mice (n=11) who were food trained on the operant task were tested for the effects of SR142948A microinfusions on METH self-administration. All procedures were approved by the Institutional Animal Care and Use Committee at UT Health San Antonio.
Drugs
SR142948A (Sigma), a well characterized nonpeptide neurotensin NTS1/NTS2 receptor antagonist (Gully et al., 1997), and METH hydrochloride (generously provided by the NIDA Drug Supply Program) were dissolved in sterile physiological saline (NaCl 0.9%).
Catheter and Cannula Implantation
At least 2 weeks after arrival, mice were implanted with an indwelling catheter in the right jugular vein as previously described (McCall et al., 2017; Sharpe et al., 2017). After the catheter was secured, mice were moved to a stereotaxic instrument (Kopf Instruments) for placement of bilateral cannulae (10.0 mm, 26-gauge stainless steel hypodermic tubing). The cannulae were aimed to terminate 1 mm above the VTA: 0.65 mm lateral, 3.0 mm caudal, and 3.6 mm ventral from Bregma (Franklin and Paxinos, 2008). The jugular catheter was flushed daily with 0.02 mL heparinized saline (30 units/mL) beginning 3 to 4 days after catheter placement. Mice were housed individually and were allowed at least 7 days to recover from surgeries. Mice (saline: n=11; SR142948A: n=13) that were successfully implanted with i.v. catheters and VTA bilateral cannulae commenced training for METH self-administration.
Intra-VTA Microinfusions
Bilateral microinfusions of SR142948A or sterile saline were given 15 minutes before self-administration training sessions for the first 5 days. The amount of SR142948A microinfused was 10 ng/side dissolved in a volume of 100 nL of saline and infused for 10 seconds, based on Reynolds et al. (2006) and a preliminary study performed in our laboratory (supplementary Figure 1). A flow diagram summarizing the behavioral procedures that follow can be seen in Figure 1A.
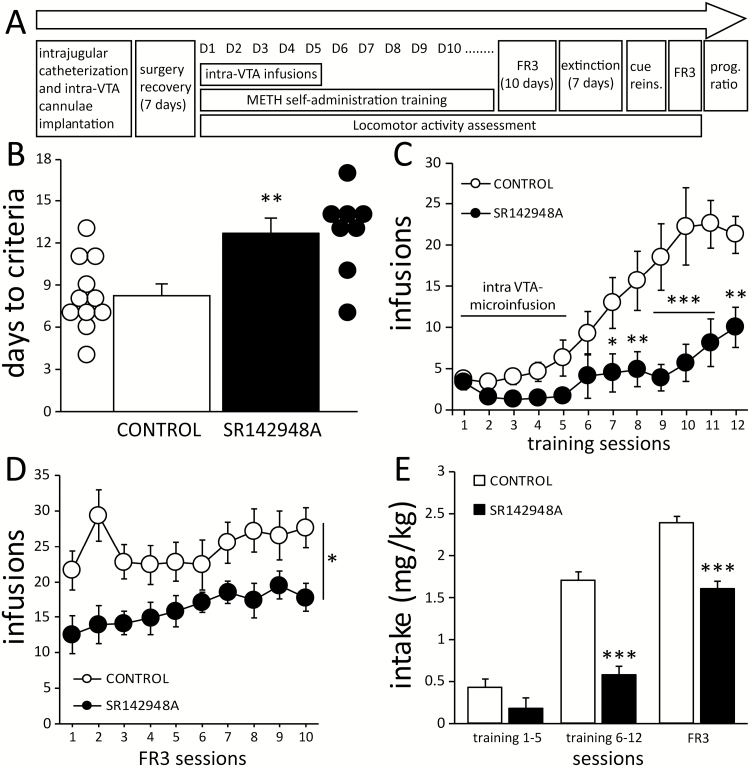
Figure 1.
Antagonism of neurotensin receptors in the ventral tegmental area (VTA) delays the acquisition of methamphetamine (METH) self-administration and decreases intake. (A) A timeline depiction of the surgical and behavioral procedures. (B) Compared with controls (n=11), mice microinfused with SR142948A (n=8) in the VTA required more training sessions to reach self-administration criteria. (C) SR142948A-treated mice self-administered a lower number of METH infusions during training and (D) later during maintenance of self-administration. (E) METH intake was reduced in SR142948A-treated mice in both training and fixed ratio 3 (FR3) sessions. Bonferroni t test posthoc comparisons: *P<.05, **P<.01, and ***P<.001 vs CONTROL.
Operant Self-Administration Training
To assess catheter patency, catheters were flushed with sterile saline before each operant session and with heparinized saline after the session. Two-hour operant sessions were conducted daily as previously described (McCall et al., 2017; Sharpe et al., 2017). Two nose-poke holes were located on one wall of the modular mouse operant chamber (Lafayette Instruments), and the correct nose poke was illuminated by a dim green light located inside of the hole. During training, responses in the correct nose poke hole were rewarded on a fixed ratio 1 schedule of reinforcement (FR1) for infusions 1 to 8, FR2 for infusions 9 to 14, and FR3 for the remainder of the session. Upon completing the response requirement, the green stimulus light was turned off, a 15-second timeout was initiated, and METH was delivered (0.1 mg/kg/infusion, 12 µL over 2 seconds, accompanied by a sound stimulus of 2 kHz). METH infusion dosage was calculated based on a typical weight of a young adult mouse (28 g) and corrected by actual body weight to obtain intake (mg/kg/session). Responding in both nose pokes holes was recorded during the timeout but was not reinforced. Self-administration was considered acquired when the number of infusions earned was ≥8, and the number of nose pokes in the correct hole represented at least 70% of the total nose pokes. Five mice (saline: n=2; SR142948A: n=3) did not acquire METH self-administration within 21 days and were removed from the study.
Self-Administration Stabilization, Extinction, and Progressive Ratio Procedures
After acquisition, the mice were advanced to an FR3 schedule of reinforcement (i.e., maintenance) for 10 days. Mice were then placed on an extinction schedule for 7 days, in which the animals were attached to the i.v. tether, but no infusion or sound cue were delivered during the 2-hour session. This was followed by 1 day on an FR3 cue-induced reinstatement schedule (nose pokes resulted in the sound cue, but not METH infusion). Mice were then returned to an FR3 session of drug self-administration. Finally, mice were placed on 2 days of 5-hour sessions on a progressive ratio schedule, in which the number of nose pokes necessary for an infusion was gradually increased (1, 2, 4, 6, 9, …) following the procedure described by Richardson and Roberts (1996).
Locomotor Activity
Basal locomotor activity was assessed in all mice during their habituation to the microinfusion procedure (sham microinfusions). Two days before self-administration started, mice were placed in the locomotor chambers equipped with infrared photocells for 15 minutes (pre-sham). After that, mice were gently held, without restraining, and sham injectors were descended through the cannula guides and kept in place for 1 minute. Locomotor activity was quantified again after sham microinfusion for another 15 minutes (post-sham). On the next 5 days, mice were placed in the locomotor chambers immediately after microinfusion of SR142948A or saline (training days 1–5) for 15 minutes, after which the mice were placed in the self-administration chambers for their daily session. Locomotor activity was quantified again for 15 minutes immediately following self-administration sessions. This procedure was conducted before and after each of the remaining self-administration sessions to monitor METH-induced changes in locomotion.
Cannula Placement Verification
At the end of the experiments, 100 nL of fast-green dye (2% in ethanol) was injected through the cannulae. The brains of the mice were collected, sliced into serial 200-μm sections and stored in 4% paraformaldehyde. Locations of the injection sites were determined by visual examination. Two mice infused with SR142948A had incorrectly placed cannulae (supplementary Figure 2) and were later included along with saline-infused mice in the control group as their responding was not different from the saline-treated mice (supplementary Figure 3). The remainder of the mice had both SR142948A injection sites within the boundaries of the VTA, between 3.00 and 3.30 mm posterior to Bregma, and were included in the experimental group for analysis.
Statistical Analysis
Data were analyzed using SigmaStat 4.0 statistical suite (Systat Software Inc). The 2-tailed Student’s t test was used to compare days to acquisition between groups. Two-way ANOVA (repeated measures when allowed) was used to determine interactions between treatment and daily session (treatment x session) and treatment x schedule. For posthoc comparisons after ANOVAs, Bonferroni corrected t tests were used. Correlation of METH intake with distance traveled was done using Pearson product moment correlation and the correlation coefficients (r) were then compared using Fisher’s Z-transformation. All data are reported as mean±SEM. Statistical values of P≤.05 were considered significant.
Results
Antagonism of Neurotensin Receptors in the VTA Delays and Decreases METH Self-Administration
Confirming preliminary observations obtained in a pilot experiment (supplementary Figure S1), acquisition of METH self-administration was slower in mice receiving microinfusions of SR142948A. These animals required a larger number of training sessions to meet self-administration criteria (t17=3.489, P=.002, Figure 1B). SR142948A-treated mice self-administered fewer infusions of METH during training sessions (treatment x session: F11,187=5.269, P<.001). Posthoc analysis revealed that the number of infusions in SR142948A-treated mice was significantly lower beginning on day 7 of training (Figure 1C). Following acquisition, mice that previously received SR142948A also earned fewer infusions during the subsequent 10 days of FR3 maintenance (treatment: F1,153=6.741, P=.019, Figure 1D). These differences were paralleled by decreased average METH intake during both training and maintenance (treatment x schedule: F2,371=4.603, P<.011: Figure 1E). Analysis of nose poke behavior showed similar differences between groups, with a higher number of correct responses in control animals during training (treatment x session: F11,187=6.939, P < .001: Supplementary Figure 4A) and FR3 maintenance sessions (treatment: F1,153=7.86, P<.01; Supplementary Figure 4D). In addition, mice with SR142948A treatment displayed a slower pattern of self-administration, with a significantly reduced number of bouts and a trend toward fewer infusions per bout (supplementary Figure 5).
Antagonism of Neurotensin Receptors in the VTA Decreases METH-Seeking Behavior
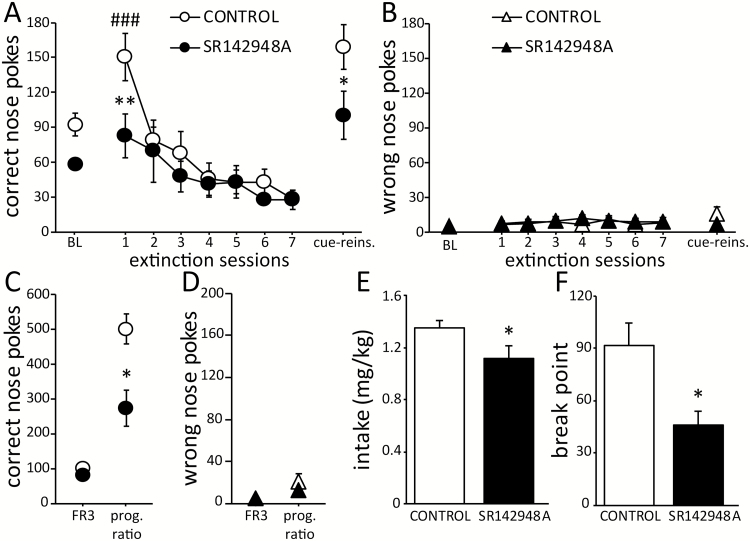
In comparison with the last FR3 session, interruption of METH availability resulted in an increased number of correct nose pokes on the first day of extinction which was significant in control mice but not in SR142948A treated animals (treatment x session: F7,105=2.653, P=.014; Figure 2A). There was no difference in the number of extinction sessions necessary to decrease the number of responses to <50% between groups (control: 3.9±0.4 days; SR142948A: 4.7±0.5 days). One animal in each group did not meet criteria for extinction within 7 extinction sessions, and these 2 mice were excluded from further analysis. In both groups, the number of correct side nose pokes increased again during the cue-induced reinstatement session (session: F1,15=16.987, P<.001 vs baseline), with a higher number of responses observed in the control group (treatment: F1,15=7.146, P=.017; Figure 2A). No differences between groups were observed in the number of wrong responses on extinction or cue-induced reinstatement (treatment: F7,105=0.086, P<.772 and F1,15=1.094, P=.312; respectively; Figure 2B).
Figure 2.
Methamphetamine (METH)-seeking behavior is decreased by previous intra-ventral tegmental area (VTA) treatment with SR142948A. (A) Compared with the control group, mice treated with SR142948A exhibited fewer correct side responses on the first day of extinction and in cue-induced reinstatement (“cue-reins.”). (B) No differences between groups were found in the number of wrong-side nose pokes during extinction or cue-induced reinstatement. (C, D) In the progressive ratio test (“prog. ratio”), the number of correct-side responses was decreased in SR142948A-treated mice compared with controls, with no difference between groups in the number of wrong-side responses. (E, F) METH intake and the breakpoint were lower in SR142948A-treated mice compared with controls. Control: n=10 and SR142948A: n=7, Bonferroni posthoc comparisons: *P<.05 and **P<.01 vs CONTROL; ### P<.01 vs baseline (BL, last fixed ratio 3 [FR3] session).
In the progressive ratio test, mice in both groups displayed a significant increase in the number of correct side nose poke responses (treatment x session: F1,15=5.442, P=.034). However, the number of responses was lower in SR142948A treated mice (P=.002; Figure 2C). No differences between groups were detected in the number of wrong nose pokes (treatment: F1,15=0.735, P=.405; Figure 2D). Consequently, METH intake and the maximum reinforcement ratio completed (break point) by mice treated with SR142948A was lower than that observed in control mice (intake: t15=2.11, P<.05; break point: t15=2.491, P=.024; Figure 2E–F). In addition, METH intake history exhibited a positive correlation with seeking behavior observed in the progressive ratio (r=0.606, P=.009) but not with that observed in extinction and cue-reinstatement (supplementary Figure 6).
Locomotion Is Not Affected by Intra-VTA Infusion of SR142948A, but by Changes in METH Intake
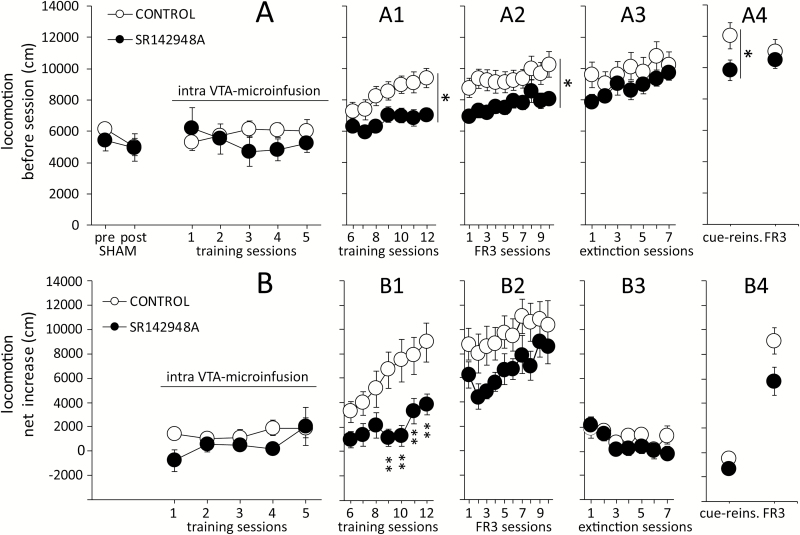
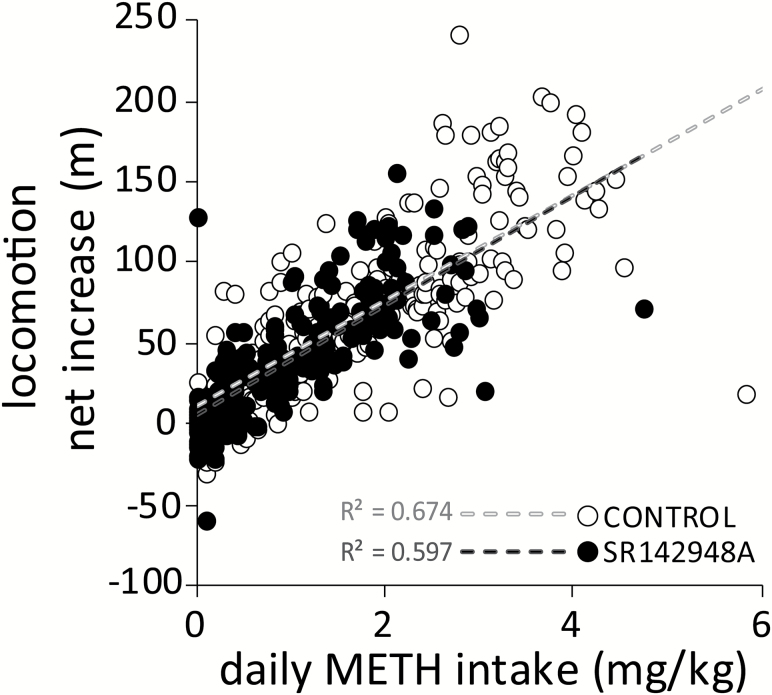
Locomotor activity was not affected by sham microinfusion or by 5 days of bilateral intra-VTA microinfusion of SR142948A or saline (treatment: F1,102=0.402, P=.535; Figure 3A). Similar to the pattern observed in the number of METH infusions, differences in basal activity between groups were detected during the subsequent training (treatment: F1,102=6.712, P=.019; Figure 3A1), maintenance (treatment: F1,153=4.539, P=.048; Figure 3A2), and cue-induced reinstatement (treatment: F1,15=5.272, P=.036; Figure 3A4) sessions, but not during extinction (treatment: F1,90=1.092, P=.313; Figure 3A3). Subtracting pre-session locomotion from post-session locomotion revealed that the net increase in locomotion was not significantly different between treatments during infusion days (treatment: F1,68=2.712, P=.118; Figure 3B), but it was significantly lower in SR142848A-treated mice during training sessions 9 to 12 (treatment x session: F6,102=2.899, P=.012; Figure 3B1). No additional differences between groups were detected in FR3 sessions (treatment: F1,153=2.523, P=.131; Figure 3B2), extinction sessions (treatment: F1,90=1.182, P=.294; Figure 3B3), or cue-induced reinstatement (treatment: F1,15=1.398, P<.255; Figure 3B4). In both groups, the net increase in locomotion was strongly correlated with the amount of daily METH self-administered (control: r=0.821, P < .001; SR142948A: r=0.773, P < .001; Figure 4), with no statistical difference in the strength of the correlation between the 2 treatment groups (Z=1.3, P=.193).
Figure 3.
Effects of intra-ventral tegmental area (VTA) treatment with SR142948A on locomotor activity. (A) Basal locomotor activity was not affected by 5 days of SR142948A or saline microinfusions. Distance traveled before self-administration was overall higher in the control group in training days 6 to 12 (A1), in fixed ratio 3 (FR3) maintenance sessions (A2), and in cue-reinstatement (cue-reins, A4). (B) In the control group, the net increase in locomotion after methamphetamine (METH) self-administration (post-session – pre-session) was higher compared with SR142948A-treated mice only on training days 9 to 12 (B1). No significant changes between groups were detected in maintenance (B2), extinction sessions (B3), cue-reins, or the last FR3 session (B4). Bonferroni posthoc comparisons: *P<.05, **P<.01, and ***P<.001 vs CONTROL.
Figure 4.
Neurotensin receptor antagonism in the ventral tegmental area (VTA) does not alter methamphetamine (METH)-induced locomotion. In control (n=11) and SR142948A-treated (n=8) mice, the corresponding net increase in locomotion was directly correlated with the amount of METH intake, with no effect of group. In both cases, the correlation follows a strong positive linear association (P<.001).
Discussion
Here we show that treatment with a nonselective neurotensin receptor antagonist specifically in the VTA delays the acquisition of METH self-administration and decreases METH intake in mice. The long-lasting effects of SR142948A treatment did not prevent the acquisition of self-administration but did appear to decrease subsequent daily intake and the reinforcing efficacy of METH. The effects of SR142948A treatment on self-administration behavior were not associated with changes in basal locomotor activity or interference with METH psychomotor effects.
Neurotensin Modulation of METH Reinforcement Efficacy in the VTA
Previous work has established a link between VTA neurotensin and reinforcement. Activation of neurotensin receptors in the VTA (by neurotensin or neurotensin analogues) is able to sustain self-administration behavior (Glimcher et al., 1987), produces conditioned place preference (Rouibi et al., 2015), and increases locomotor activity (Kalivas et al., 1981; Kalivas and Duffy, 1990). Either acute intra-VTA pretreatment with SR142948A (single infusion, 1 week before) or systemic administration of the selective NTSR1 antagonist SR48692 prevents the induction of amphetamine behavioral sensitization but not its acute psychomotor response (Rompré and Perron, 2000; Panayi et al., 2005). This suggests that antagonism of NTS1 receptors selectively prevents amphetamine-induced sensitization. Previous behavioral work involving METH self-administration has largely focused on terminal regions or on the systemic application of neurotensinergic ligands. Interestingly, systemic administration of SR48692 does not modify METH self-administration in experienced rats (Frankel et al., 2011; Hanson et al., 2012, 2013). Work from our laboratory and others suggests that the selective NTS1 receptor agonist PD149163 reduces METH self-administration and METH drug-seeking behavior (Frankel et al., 2011; Hanson et al., 2012, 2013; Sharpe et al., 2017); however, locomotor deficits may contribute to this effect (Vadnie et al., 2014).
The data in the present study provide the first evidence that endogenous neurotensin in the VTA contributes to the reinforcing efficacy of METH. The infusion of SR142948A bilaterally in the VTA during the first 5 days of training not only delayed the acquisition of METH self-administration but had a long-lasting effect by decreasing intake and drug-seeking behavior. Examination of the number of infusions earned on day 7 and 12 of training (the average day for acquisition of self-administration by control and SR142948A groups, respectively) suggests that both groups were earning similar amounts of METH at acquisition (Figure 1c). Therefore, the time course and onset of METH self-administration in SR142948A-treated mice could suggest that the rewarding effects of METH were effectively blocked during the 5 days of treatment. It is possible that SR142948A-treated mice did not experience the reinforcing effects of METH until training day 6, which may have contributed to decreased METH intake in this group in the subsequent sessions. Interestingly, in 2 mice that received infusions of SR142948A outside the limits of the VTA, the treatment was not effective and behavior resembled the saline mice. This is consistent with the fact that SR142948A infused into the ventral pallidum of cocaine-experienced rats has no effect on cocaine self-administration (Torregrossa and Kalivas, 2008). Furthermore, since our SR142948A infusions were aimed at the rostral region of the VTA, where a subpopulation of dopamine (DA) neurons predominantly expressing NTS1 receptors is located (Woodworth et al., 2017), the persistent effects on intake observed in our experiments may be related to blockade of METH-induced sensitization through antagonism of the NTS1 receptor subtype (Rompré and Perron, 2000; Panayi et al., 2005). It should, however, be noted that there was no difference in acute locomotor stimulation caused by METH seen between treatment groups (Figure 4) as one might expect if there was a difference in sensitization between the two groups. Collectively, our data and the available literature indicate that endogenous neurotensin input selectively to the VTA contributes to the reinforcing efficacy of METH and supports drug-seeking behavior. However, it is possible that the effect of the neurotensin receptor antagonist is not specific for METH; thus, this effect on decreasing reinforcer intake and efficacy may also be true for other forms of reward (Kempadoo et al., 2013).
VTA Neurotensin Does Not Alter the Psychomotor Effects of METH
We did not observe an effect of SR142948A infused into the VTA on basal locomotor activity. This corroborates previous reports of a lack of observable motor deficits when SR142948A is administered either into the VTA or systemically (Reynolds et al., 2006; Marie-Claire et al., 2008; Caceda et al., 2012). Furthermore, intra-VTA infusion of SR142948A does not block the acute psychomotor response to amphetamine (Panayi et al., 2005). Our results also show that SR142948A-treated mice gradually develop a lower level of basal hyperlocomotion; however, this finding could be explained by the lower METH intake in this group across sessions. As is shown in our correlation analysis, enhanced locomotor activity exhibits a strong linear relationship with METH intake in both groups. In addition, in all mice, the net increase in locomotion was negligible in sessions when METH was not available (extinction and cue-induced reinstatement). Since there is no difference between treatment groups in the locomotor response (net change in activity) when plotted against METH intake during individual sessions, these data suggest that treatment with SR142948A does not affect the locomotor stimulant properties of METH. Certainly, the lower METH intake in SR142948A-treated mice may have influenced their subsequent locomotor response and drug-seeking behavior (see below). Taken together, we conclude that selective antagonism of neurotensin receptors in the VTA affects the reinforcing efficacy (measured by intake) but not the psychomotor effects of METH self-administration (locomotor output).
Integrative Effects of Neurotensin and METH in the VTA
The VTA contains the cell bodies of dopaminergic neurons that have been heavily implicated in reward and expresses NTS1 and possibly NTS2 receptors (Deutch and Zahm, 1992; Binder et al., 2001; Sarret et al., 2003). The rewarding and psychostimulant effects of neurotensin in the VTA may be contingent on its capability to elicit an increase in dopamine levels in terminal areas such as the nucleus accumbens (Kalivas and Duffy, 1990; Leonetti et al., 2004). This seems to largely depend on activation of NTS1 receptors in VTA DA neurons and is less likely to involve activation of NTS2 receptors, which are predominantly expressed in astrocytes (Leonetti et al., 2004; Woodworth et al., 2017). In the VTA, neurotensin levels increase in response to METH self-administration, and this is observable as early as 5 days after initial exposure to the drug but is not seen in noncontingent METH-exposed animals (Hanson et al., 2012).
It is possible that endogenous neurotensin actions in the VTA contribute to increased dopamine levels in accumbens after METH self-administration (Le Cozannet et al., 2013). Interestingly, neurotensin-evoked dopamine release in the nucleus accumbens is not blocked by systemic administration of either SR142948A or SR48692 (Gully et al., 1997); however, co-infusion of SR142948A with neurotensin in the VTA reduces the rise in dopamine at the terminal (Leonetti et al., 2002). Given this, we hypothesize that the decrease in METH self-administration observed in our results may be due, at least in part, to NTS1 receptor blockage in VTA DA neurons interfering with a METH-induced increase of dopamine in the accumbens. Theoretically, this decreased the incentive value of METH infusions, at least during the first days of training, thus delaying the acquisition of METH self-administration (as we observed). Furthermore, METH intake history can influence drug-seeking behavior under certain experimental conditions (Yang et al., 2007), and our observations suggest that this is dependent on the availability of METH during the test. Indeed, METH intake history correlated with behavior in a progressive ratio but not during extinction or cue reinstatement, when METH was not available (supplementary Figure 6).
Although neurotensin produces multiple cellular effects in the VTA, its interactions with METH are most likely dopaminergic. Acutely, METH and other amphetamines increase extracellular dopamine levels by competing as substrates for uptake through dopamine transporters located on the plasma membrane. These compounds then decrease vesicular dopamine content by acting as substrates at vesicular transporters and/or disrupting vesicular pH gradients and ultimately produce DA efflux into the extracellular space through dopamine transporter reversal (Sulzer, 2011). Elevated extracellular dopamine is observed at both axon terminal and somatodendritic areas (Di Chiara and Imperato, 1988; Kalivas et al., 1989). In the VTA, acute METH application modulates dopamine neurons such that lower concentrations of METH increase firing activity (through a transporter mechanism), while higher concentrations decrease it in a D2 receptor-dependent manner (Branch and Beckstead, 2012). Activating neurotensin receptors in the VTA attenuates the inhibitory effects of D2 autoreceptor activation and can produce excitatory effects on VTA dopamine neuron firing activity (Shi and Bunney, 1991; Piccart et al., 2015; Stuhrman and Roseberry, 2015). METH self-administration is also able to chronically decrease D2 autoreceptor function (Sharpe et al., 2014), and at doses already reached during the initial days of training in our present experiments (0.18–0.42 mg/kg), METH strongly reduces the firing rate of dopamine neurons in vivo (Kamata and Kameyama, 1985). Considering that endogenous neurotensin levels in the VTA increase in response to METH self-administration (Hanson et al., 2012), it is possible that this is a compensatory mechanism to restore normal dopamine neuron activity. The neurotensin receptor antagonists SR142948A and SR48692 do not have direct effects on dopamine neuron firing or D2 autoreceptor function in the VTA, although SR142948A can reverse one form of neurotensin-induced plasticity (Piccart et al., 2015).
Previously published and ongoing work indicate multiple alternative cellular and circuit mechanisms of action for neurotensin. Systemic administration of SR142948A or SR48692 increases the number of spontaneously active dopamine neurons in the VTA without affecting their firing properties (Gully et al., 1997; Santucci et al., 1997). Further, prolonged systemic administration of SR48692 decreases the number of spontaneously active dopamine cells in the VTA (Santucci et al., 1997). Other neurotransmitter systems in the VTA may also be recruited by modulation of local neurotensin receptors. For example, the inhibitory effects of GABA on dopamine neurons, through GABAB receptor activation, are decreased by neurotensin (Stuhrman and Roseberry, 2015; Tschumi and Beckstead, 2018). Neurotensin can also modulate glutamatergic activity in dopaminergic and nondopaminergic neurons in the VTA and affect electrical brain self-stimulation behavior (Kempadoo et al., 2013; Bose et al., 2015; Rouibi et al., 2015).
The current studies were conducted solely in male mice. Sex differences have previously been reported in the context of dopamine and other neuropeptides such as oxytocin (Cox et al., 2013); however, this has not been explored regarding neurotensin. Indeed, a recent report suggests that METH-induced locomotor activity and stereotypy behavior may be enhanced in female compared with male rodents (Milesi-Hallé et al., 2007; Ohia-Kwoko et al., 2017). Further research will be needed to address sex differences in the acquisition of METH self-administration and METH-seeking behavior (Roth and Carroll, 2004; Ruda-Kucerova et al., 2015) and the possible implications of a role for neurotensin in METH self-administration.
CONCLUSION
Our data indicate that antagonism of neurotensin receptors in the VTA delays the onset of METH self-administration and produces a lasting decrease in METH intake. Neurotensin receptor activation in the VTA is not required for the psychomotor effects of METH, but rather may contribute to the early adaptations occurring in the VTA during the transition to compulsive METH intake. Our results also indicate that neurotensin receptors in the VTA contribute to the early reinforcing efficacy of METH, thus facilitating METH self-administration. The long-lasting effects of SR142948A treatment imply that neurotensin input into the VTA during initial METH exposure modulates later drug intake and drug-seeking behavior. Further studies will be necessary to fully elucidate the cellular mechanisms accompanying neurotensin receptor activation in the VTA during METH self-administration to determine the utility of manipulating the neurotensin system as a possible treatment for METH addiction.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Supplementary Material
Acknowledgments
This work was supported by a National Institute of Health grant to M.J.B. (R01DA032701) and funds obtained through the Presbyterian Health Foundation and the Oklahoma Center for Adult Stem Cell Research.
Statement of Interest
None.
References
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB(2001)Neurotensin and dopamine interactions. Pharmacol Rev 53:453–486. [PubMed] [Google Scholar]
- Bose P, Rompré PP, Warren RA(2015)Neurotensin enhances glutamatergic EPSCs in VTA neurons by acting on different neurotensin receptors. Peptides 73:43–50. [DOI] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ(2012)Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol 108:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceda R, Binder EB, Kinkead B, Nemeroff CB(2012)The role of endogenous neurotensin in psychostimulant-induced disruption of prepulse inhibition and locomotion. Schizophr Res 136:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN(2009)Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol 88:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM(2013)Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J(2010)Monoaminergic psychomotor stimulants: discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther 333:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Zahm DS(1992)The current status of neurotensin-dopamine interactions. Issues and speculations. Ann N Y Acad Sci 668:232–252. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A(1988)Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D, Trudeau LE(2009)Presynaptic action of neurotensin on dopamine release through inhibition of D(2) receptor function. BMC Neurosci 10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tiozzo Fasiolo L, Beggiato S, Borelli AC, Pomierny-Chamiolo L, Frankowska M, Antonelli T, Tomasini MC, Fuxe K, Filip M(2016)Neurotensin: a role in substance use disorder?J Psychopharmacol 30:112–127. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR(2011)Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther 336:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G(2008)The mouse brain in stereotaxic coordinates. Compact 3rd ed San Diego: Academic Press/Elsevier. [Google Scholar]
- Glimcher PW, Giovino AA, Hoebel BG(1987)Neurotensin self-injection in the ventral tegmental area. Brain Res 403:147–150. [DOI] [PubMed] [Google Scholar]
- Gully D, Labeeuw B, Boigegrain R, Oury-Donat F, Bachy A, Poncelet M, Steinberg R, Suaud-Chagny MF, Santucci V, Vita N, Pecceu F, Labbé-Jullié C, Kitabgi P, Soubrié P, Le Fur G, Maffrand JP(1997)Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J Pharmacol Exp Ther 280:802–812. [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, Frankel PS(2012)Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience 203:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Robson CM, McFadden LM, Frankel PS, Alburges ME(2013)Response of neurotensin basal ganglia systems during extinction of methamphetamine self-administration in rat. J Pharmacol Exp Ther 346:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Lemelin PL, Okano H, Kobayashi K, Trudeau LE(2006)Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur J Neurosci 24:2789–2800. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Bourdelais A, Abhold R, Abbott L(1989)Somatodendritic release of endogenous dopamine: in vivo dialysis in the A10 dopamine region. Neurosci Lett 100:215–220. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P(1990)Effect of acute and daily neurotensin and enkephalin treatments on extracellular dopamine in the nucleus accumbens. J Neurosci 10:2940–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ Jr(1981)Increase in spontaneous motor activity following infusion of neurotensin into the ventral tegmental area. Brain Res 229:525–529. [DOI] [PubMed] [Google Scholar]
- Kamata K, Kameyama T(1985)Effects of methamphetamine on dopamine cells in the substantia nigra pars compacta and the ventral tegmental area. Jpn J Pharmacol 38:231–234. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A(2013)Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci 33:7618–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cozannet R, Markou A, Kuczenski R(2013)Extended-access, but not limited-access, methamphetamine self-administration induces behavioral and nucleus accumbens dopamine response changes in rats. Eur J Neurosci 38:3487–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti M, Brun P, Sotty F, Steinberg R, Soubrie P, Bert L, Renaud B, Suaud-Chagny MF(2002)The neurotensin receptor antagonist SR 142948A blocks the efflux of dopamine evoked in nucleus accumbens by neurotensin ejection into the ventral tegmental area. Naunyn Schmiedebergs Arch Pharmacol 365:427–433. [DOI] [PubMed] [Google Scholar]
- Leonetti M, Brun P, Clerget M, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny MF(2004)Specific involvement of neurotensin type 1 receptor in the neurotensin-mediated in vivo dopamine efflux using knock-out mice. J Neurochem 89:1–6. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Palminteri S, Romualdi P, Noble F(2008)Effects of the selective neurotensin antagonist SR 142948A on 3,4-methylenedioxymethamphetamine-induced behaviours in mice. Neuropharmacology 54:1107–1111. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, Brecht ML(2011)Methamphetamine: here we go again?Addict Behav 36:1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall NM, Kotecki L, Dominguez-Lopez S, Marron Fernandez de Velasco E, Carlblom N, Sharpe AL, Beckstead MJ, Wickman K(2017)Selective ablation of GIRK channels in dopamine neurons alters behavioral effects of cocaine in mice. Neuropsychopharmacology 42:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM(2007)Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav 86:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot A, Rostene W, Berod A(1994)Neurotensin receptor expression in the rat forebrain and midbrain: a combined analysis by in situ hybridization and receptor autoradiography. J Comp Neurol 341:407–419. [DOI] [PubMed] [Google Scholar]
- Ohia-Kwoko O, Haile CN, Kosten TA(2017)Sex differences in the acute locomotor response to methamphetamine in the BALB/c mice. Behav Brain Res 327:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ(1981)Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294:587–589. [DOI] [PubMed] [Google Scholar]
- Panayi F, Colussi-Mas J, Lambas-Senas L, Renaud B, Scarna H, Berod A(2005)Endogenous neurotensin in the ventral tegmental area contributes to amphetamine behavioral sensitization. Neuropsychopharmacology 30:871–879. [DOI] [PubMed] [Google Scholar]
- Piccart E, Courtney NA, Branch SY, Ford CP, Beckstead MJ(2015)Neurotensin induces presynaptic depression of D2 dopamine autoreceptor-mediated neurotransmission in midbrain dopaminergic neurons. J Neurosci 35:11144–11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R, Welner S, Gauthier S, Bedard P(1987)Neurotensin receptor binding sites in monkey and human brain: autoradiographic distribution and effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Synapse 1:559–566. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Geisler S, Berod A, Zahm DS(2006)Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur J Neurosci 24:188–196. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC(1996)Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Rompré PP, Bauco P, Gratton A(1992)Facilitation of brain stimulation reward by mesencephalic injections of neurotensin-(1–13). Eur J Pharmacol 211:295–303. [DOI] [PubMed] [Google Scholar]
- Rompré PP, Perron S(2000)Evidence for a role of endogenous neurotensin in the initiation of amphetamine sensitization. Neuropharmacology 39:1880–1892. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME(2004)Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 172:443–449. [DOI] [PubMed] [Google Scholar]
- Rouibi K, Bose P, Rompré PP, Warren RA(2015)Ventral midbrain NTS1 receptors mediate conditioned reward induced by the neurotensin analog, D-Tyr[11]neurotensin. Front Neurosci 9:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A(2015)Sex Differences in the reinstatement of methamphetamine seeking after forced abstinence in sprague-dawley rats. Front Psychiatry 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci V, Gueudet C, Steinberg R, Le Fur G, Soubrie P(1997)Involvement of cortical neurotensin in the regulation of rat meso-cortico-limbic dopamine neurons: evidence from changes in the number of spontaneously active A10 cells after neurotensin receptor blockade. Synapse 26:370–380. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A(2003)Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol 461:520–538. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Beckstead MJ(2017)PD149163, a neurotensin receptor 1 agonist, decreases methamphetamine self-administration in DBA/2J mice without causing excessive sedation. PLoS One 12:e0180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ(2014)Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Bunney BS(1991)Neurotensin modulates autoreceptor mediated dopamine effects on midbrain dopamine cell activity. Brain Res 543:315–321. [DOI] [PubMed] [Google Scholar]
- Stuhrman K, Roseberry AG(2015)Neurotensin inhibits both dopamine- and GABA-mediated inhibition of ventral tegmental area dopamine neurons. J Neurophysiol 114:1734–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D.(2011)How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW(2008)Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. J Pharmacol Exp Ther 325:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumi CW, Beckstead MJ(2018)Neurotensin speeds inhibition of dopaminergic neurons through temporal modulation of GABAA and GABAB synaptic input. Neuropharmacology In press. [DOI] [PMC free article] [PubMed]
- Vadnie CA, Hinton DJ, Choi S, Choi Y, Ruby CL, Oliveros A, Prieto ML, Park JH, Choi DS(2014)Activation of neurotensin receptor type 1 attenuates locomotor activity. Neuropharmacology 85:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P(1999)Neurotensin and neurotensin receptors. Trends Pharmacol Sci 20:302–309. [DOI] [PubMed] [Google Scholar]
- Von Euler G, Fuxe K(1987)Neurotensin reduces the affinity of D-2 dopamine receptors in rat striatal membranes. Acta Physiol Scand 131:625–626. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR(1996)Microdialysis assessment of methamphetamine-induced changes in extracellular neurotensin in the striatum and nucleus accumbens. J Pharmacol Exp Ther 278:547–554. [PubMed] [Google Scholar]
- Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, Fuller PM, Leinninger GM(2017)Neurotensin receptor-1 identifies a subset of ventral tegmental dopamine neurons that coordinates energy balance. Cell Rep 20:1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yamada K, Nitta A, Nabeshima T(2007)Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res 177:261–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.