Abstract
Tissue factor (TF) is the primary activator of the blood coagulation cascade. Liver parenchymal cells (ie, hepatocytes) express TF in a molecular state that lacks procoagulant activity. Hepatocyte apoptosis is an important feature of acute and chronic liver diseases, and Fas-induced apoptosis increases hepatocyte TF procoagulant activity in vitro. We determined the impact of a pan-caspase inhibitor, IDN-7314, on hepatocyte TF activity in vitro and TF-mediated coagulation in vivo. Treatment of primary mouse hepatocytes with the Fas death receptor ligand (Jo2, 0.5 μg/ml) for 8 h increased hepatocyte TF procoagulant activity and caused release of TF-positive microvesicles. Pretreatment with 100 nM IDN-7314 abolished Jo2-induced caspase-3/7 activity and significantly reduced hepatocyte TF procoagulant activity and release of TF-positive microvesicles. Treatment of wild-type C57BL/6 mice with a sublethal dose of Jo2 (0.35 mg/kg) for 4.5 h increased coagulation, measured by a significant increase in plasma thrombin-antithrombin and TF-positive microvesicles. Total plasma microvesicle-associated TF activity was reduced in mice lacking hepatocyte TF; suggesting TF-positive microvesicles are released from the apoptotic liver. Fibrin(ogen) deposition increased in livers of Jo2-treated wild-type mice and colocalized primarily with cleaved caspase-3-positive hepatocytes. Pretreatment with IDN-7314 reduced caspase-3 activation, prevented the procoagulant changes in Jo2-treated mice, and reduced hepatocellular injury. Overall, the results indicate a central role for caspase activity in TF-mediated activation of coagulation following apoptotic liver injury. Moreover, the results suggest that liver-selective caspase inhibition may be a putative strategy to limit procoagulant and prothrombotic changes in patients with chronic liver disease.
Keywords: coagulation, caspase, liver, tissue factor, microvesicle
Apoptosis is a common feature of multiple chronic liver diseases (Malhi and Gores, 2008). Inhibition of caspases, enzymatic effectors of apoptotic signaling pathways, has shown beneficial effects in both experimental settings of liver injury and in clinical studies (Barreyro et al., 2015; Canbay et al., 2004; Hoglen et al., 2001; Linton et al., 2005; Pockros et al., 2007). Despite these benefits, the mechanisms whereby apoptosis contributes to liver disease progression are likely multifactorial. Several studies have shown that cellular changes associated with apoptosis drive the activation of the blood coagulation cascade (Greeno et al., 1996; Lopez et al., 2014; Morel et al., 2006). Interestingly, the coagulation cascade contributes to the progression of experimental liver disease in models where caspase inhibition reduces disease (Harada et al., 2006; Luyendyk et al., 2010; Weerasinghe et al., 2011). This suggests that apoptosis and coagulation may be interconnected in liver disease.
Loss of phospholipid asymmetry is proposed to induce a conformational change in the transmembrane protein tissue factor (TF) that increases its activity (Krishnaswamy et al., 1992; Rao and Pendurthi, 2012). Under conditions of cell activation or injury, microvesicles released from the plasma membrane of TF-expressing cells are thus particularly procoagulant (Owens and Mackman, 2011). TF is the primary activator of blood coagulation. Liver parenchymal cells (ie, hepatocytes) express a complex of TF and its ligand factor VII (FVII)/FVIIa in an encrypted form that lacks procoagulant activity (Sullivan et al., 2013). We have shown previously that Fas-dependent hepatocyte apoptosis increases TF procoagulant activity and that this was abolished by blocking the negatively charged phospholipid phosphatidylserine (PS) (Lopez et al., 2014). Moreover, a deficiency of TF in the liver and administration of an anticoagulant reduced coagulation and liver pathology in mice given a Fas ligand (Lopez et al., 2014; Weerasinghe et al., 2011). Despite these findings, the precise connection between caspase activation and coagulation in vivo is not entirely understood, and the effect of in vivo caspase inhibition on coagulation in liver injury has not been examined.
We tested the hypothesis that caspase activation contributes to intrahepatic coagulation in a mouse model of hepatocellular apoptosis by increasing hepatocyte TF activity. To test this hypothesis, we utilized IDN-7314, a potent pan-caspase inhibitor and close structural analog of emricasan, which is in clinical trials for the treatment of liver disease.
MATERIALS AND METHODS
Mice and treatment paradigm: Mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities at Michigan State University (MSU). Mice were housed at an ambient temperature of 22°C ± 2 °C with alternating 12-h light/dark cycles and provided ad libitum access to standard rodent chow diet and purified drinking water. All animal procedures were approved by the MSU Institutional Animal Care and Use Committees. Male, wild-type C57Bl/6 J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and used between the ages of 8–12 weeks. TFflox/flox/AlbCre mice and associated control mice were maintained at MSU and have been described previously in Sullivan et al. (2013). Mice were given endotoxin/azide-free Jo2 (hamster anti-CD95, clone Jo2, BD Biosciences, Franklin Lakes, New Jersey) at a dose of either 0.25 mg/kg (TFflox/flox/AlbCre experiments) or 0.35 mg/kg (IDN-7314 experiments) or its vehicle (saline) by intraperitoneal (i.p.) injection at 10 ml/kg. Each dose produced a similar extent of liver damage and varied based on optimization studies performed for each lot of antibody. For studies using IDN-7314 (kindly provided by Conatus Pharmaceuticals, Inc., San Diego, California), mice were given a single i.p. injection of IDN-7314 (3 mg/kg) or vehicle (50% DMSO in sterile water) at 2 ml/kg, 1 h after administration of Jo2 or vehicle. Four and half hours after Jo2 administration the mice were anesthetized with isoflurane and blood was collected from the caudal vena cava into citrate to obtain plasma. The liver was surgically removed and either fixed in 10% neutral buffered formalin or snap frozen in liquid nitrogen.
Isolation of primary hepatocytes and treatment paradigm: Primary mouse hepatocytes were isolated by perfusion and collagenase digestion, as described previously in Sullivan et al. (2013). Hepatocytes were plated at a density of 5 × 105 cells per well in 6-well tissue culture-treated plates (BD Falcon) in Williams’ E medium (Sigma-Aldrich, St. Louis, Missouri) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Sigma-Aldrich). After 2 h, unattached cells were removed, fresh medium added, and the cells allowed to culture overnight at 37 °C at 5% CO2. Hepatocytes were treated in serum-free conditions with actinomycin D (ActD) (0.2 µg/ml Sigma-Aldrich) or its vehicle (DMSO, 0.1%) 30 min before addition of 0.5 µg/ml Jo2 or its vehicle (PBS) for 8 h. For studies where IDN-7314 was utilized, cells were treated with various concentrations of IDN-7314 (0.1–10 µM) or its vehicle (DMSO, 0.1%) at the same time ActD was added.
Determination of hepatocyte- and microvesicle-associated TF activity: Determination of TF-dependent factor Xa generation was performed as described previously in Sullivan et al. (2013). Briefly, the media were aspirated and replaced with 750 µl of prewarmed (37 °C) sterile HEPES-buffered saline containing albumin (HBSA; 137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1% bovine serum albumin [BSA]), and 250 µl of 600 nM factor X ([150 nM final]; Enzyme Research Laboratories, South Bend, Indiana) in HBSA with 20 mM CaCl2 [5 mM final] was added and allowed to incubate at 37 °C for 15 min (hepatocytes) or 120 min (microvesicles) (Owens et al., 2012). The reaction was stopped by the addition of 250 µl of 25 mM EDTA (pH 7.4; [5 mM final]).
Isolation and quantification of microvesicles: Microvesicles were isolated from citrated plasma or culture medium precleared of large cell fragments by centrifugation (4000 × g for 10 min at 4 °C). Supernatant and plasma samples were spun at 20 000 × g for 15 min at 4 °C and the resulting pellet washed with 1 ml of HBSA and vortexed briefly. This was spun at 20 000 × g for 15 min at 4 °C and the pellet was re-suspended in 100 µl of HBSA (Lee et al., 2012). The levels of PS-positive microvesicles in precleared (4000 × g for 5 min) cell culture supernatant were measured using the Zymuphen MP-activity kit (Aniara, West Chester, Ohio).
Determination of alanine aminotransferase (ALT) activity, plasma thrombin-antithrombin (TAT) levels, and caspase-3 activity: Serum and supernatant ALT activity was determined using commercial reagents (Thermo-Fisher, Waltham, Massachusetts). Plasma TAT levels were measured using a commercial ELISA (Siemens Healthcare Diagnostics, Malvern, Pennsylvania). Intracellular caspase-3 activity was determined by flow cytometry using commercial reagents with NucView 488 caspase-3 substrate (Biotium, Hayward, California) as described previously in Lopez et al. (2014). The results were expressed as a percent of hepatocytes gated as displaying fluorescence indicative of caspase-3 activity.
Cleaved caspase-3 and fibrin(ogen) western blots: Frozen liver samples (approximately 100 mg) were homogenized in RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% NP-40, 10 mM EDTA) containing protease and phosphatase inhibitors (G-Biosciences, St. Louis, Missouri). The homogenate was rotated end-over-end for 30 min at 4 °C, spun at 10 000× g for 10 min and supernatants were saved for determination of protein concentration using Bradford Protein Assay (Bio-Rad, Hercules, California). Cleaved caspase-3 and fibrin(ogen) levels were determined using western blotting with Criterion XT precast 4%–12% Bis-Tris gels (Bio-Rad). Following SDS-PAGE separation and semi-dry transfer, Immobilon PVDF membranes (Millipore, Billerica, Massachusetts) were blocked for 1 h with 3%–5% BSA in TBST buffer (50 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.4) and then incubated overnight with rabbit monoclonal cleaved caspase-3 antibody (Asp175, clone 5A1E, 1:1000 dilution, Cell Signaling Technology, Danvers, Massachusetts) or mouse antifibrin(ogen) β chain antibody (59D8) (1:1000 dilution, kindly provided by Dr Charles Esmon, Oklahoma Medical Research Foundation). Membranes were washed in TBST and incubated with goat antirabbit or goat antimouse horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania), as appropriate, diluted at 1:2000 for 1 h at room temperature. Membranes were washed 3 additional times and incubated with Clarity Western enhanced chemiluminescence substrate solution (Bio-Rad) and exposed to blue autoradiography film (ISC BioExpress, Kaysville, Utah). Following development of films for cleaved caspase-3 and fibrin(ogen), membranes were stripped in Restore Western Blot Stripping Buffer (Thermo-Fisher) for 20 min, washed in TBST and blocked in 3% BSA in TBST for 1 h at room temperature. Membranes were then incubated with mouse antiglyceraldehyde-3-phosphate dehydrogenase (Gapdh) antibody (Proteintech, Rosemont, Illinois) (1:10 000–1:15 000 dilution) in 1% BSA in TBST at 4 °C overnight, washed in TBST, incubated with goat antimouse HRP-conjugated secondary antibody (1:7500–1:15 000 dilution) for 1 h at room temperature and developed as described earlier. Densitometry was performed using Image J (https://imagej.net/).
Liver histopathology and immunohistochemistry: Formalin-fixed, paraffin-embedded livers were cut at 5 microns and stained for hematoxylin and eosin (H&E) by the Investigative Histopathology Laboratory at Michigan State University. Formalin-fixed sections were costained for caspase-3 and fibrin(ogen) in a sequential double immunohistochemical staining. Specifically, antigen retrieval was done by heat treatment in a citrate buffer (pH = 6) for 30 min at 100 °C. Following blocking in normal goat serum and avidin/biotin blocking system, liver sections were stained with a rabbit monoclonal anticleaved caspase-3 (Asp175, clone 5A1E) (Cell Signaling) at 1:200 dilution at 4 °C overnight. Biotinylated goat antirabbit IgG was incubated for 1 h, followed by Vectastain R.T.U. Elite ABC reagent (Vector Laboratories, Burlingame, California) incubation for 30 min, and development with Vector DAB peroxidase chromogen for 10 min. Afterwards the slides were rinsed in distilled water, placed in TBST for 15 min and blocked again as described above. Liver sections were then stained with a polyclonal rabbit antihuman fibrin(ogen) antibody (Dako, Carpinteria, California) at 1:200 dilution for 1 h at room temperature and then incubated with a biotinylated goat antirabbit IgG for 30 min, followed by incubation with Vectastain R.T.U. Elite ABC reagent (Vector Laboratories) for 30 min. Reaction was developed using Vector VIP peroxidase chromogen for 4 min followed by a quick counterstain with methyl green (Cellpoint, Rockville, Maryland), water rinse and air drying overnight. Slides were then cleared in xylene and mounted with synthetic mounting media.
Statistics: Comparison of 2 groups was made using Student’s t-test. Comparisons of 3+ groups were made by 1- or 2-way analysis of variance, as appropriate, with Student-Newman-Keuls test for post hoc comparisons. The criterion for statistical significance was p < .05.
RESULTS
Caspase Inhibition Prevents Fas-Triggered TF Procoagulant Activity in Primary Mouse Hepatocytes
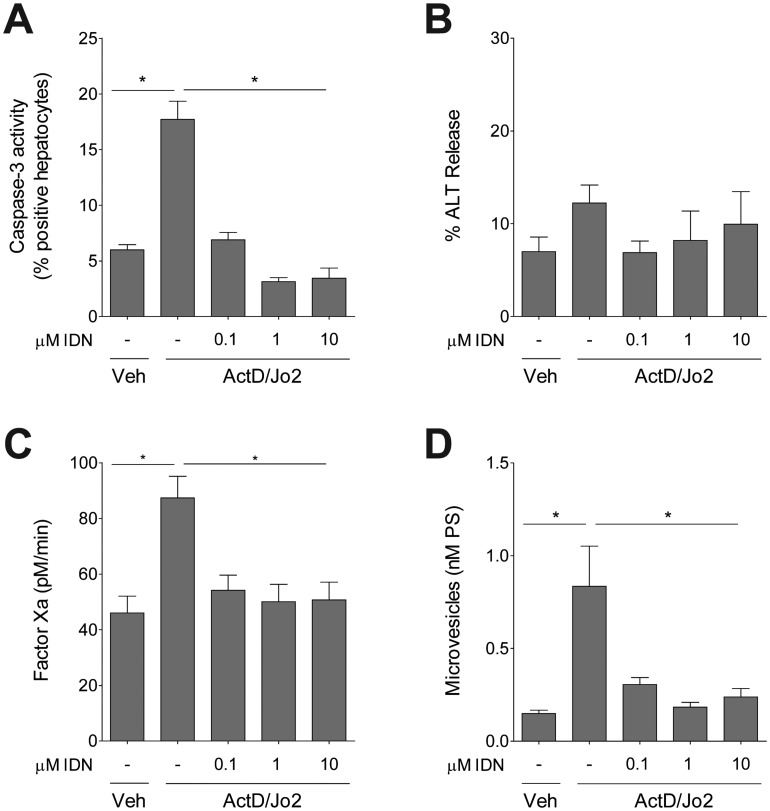
Treatment of primary mouse hepatocytes with antiCD95 antibody (clone Jo2), a Fas agonist, significantly increased the percent of hepatocytes displaying caspase-3 activity after 8 h following Jo2 treatment, indicating Jo2 induced hepatocyte apoptosis (Figure 1A). Pretreatment with IDN-7314 attenuated Fas-mediated caspase-3 activity at concentrations as low as 100 nM (Figure 1A). Jo2 treatment did not cause significant hepatocyte necrosis at this time point, indicated by a lack of change in release of ALT into the culture medium (Figure 1B). Jo2-induced apoptosis was associated with a significant increase in cell-associated TF procoagulant activity, measured by factor Xa generation, and this increase was abolished by pretreatment with IDN-7314 (Figure 1C). Jo2 treatment of hepatocytes significantly increased the release of microvesicles into the culture medium, and this was completely prevented by IDN-7314 (Figure 1D).
Figure 1.
Effect of caspase inhibition on Fas-induced hepatocyte TF activity. Primary mouse hepatocytes were treated with various concentrations of IDN-7314 or vehicle (Veh, 0.1% DMSO) prior to treatment with ActD (0.2 µg/ml) and Jo2 (0.5 µg/ml). (A) Hepatocyte caspase-3 activity was measured using a NucView 488 substrate, detected using flow cytometry. The results are expressed as % positive hepatocytes gated as positive for caspase-3 activity within the total live cell population. (B) % ALT release. (C) TF procoagulant activity measured by factor Xa generation. (D) Supernatant microvesicle levels (expressed as nM PS). All measurements were determined 8 h after Jo2 treatment. Data are expressed as mean + SEM from at least 3 independent experiments. *p < .05.
Impact of Caspase Inhibition on Coagulation Cascade Activation in Experimental Fas-Triggered Liver Injury
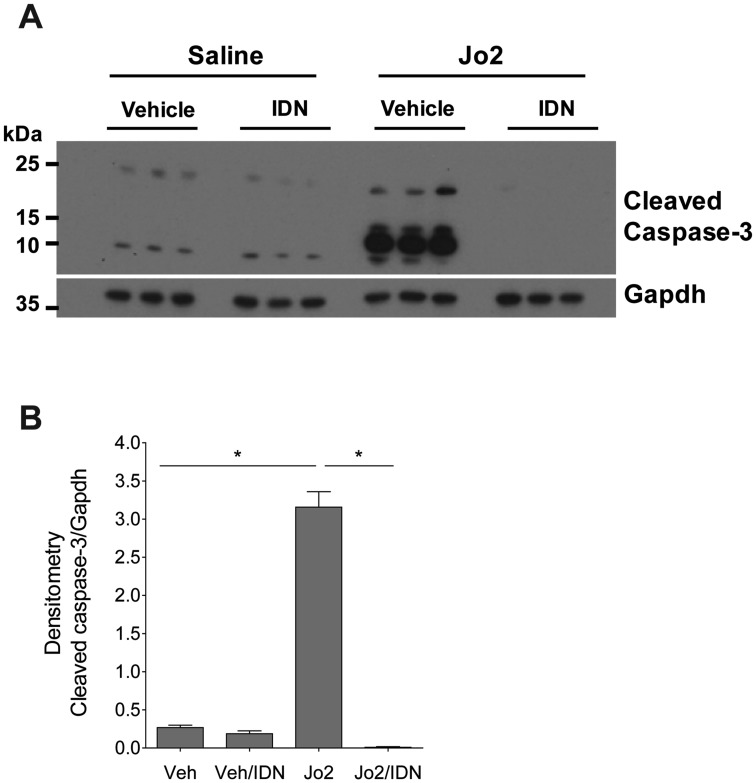
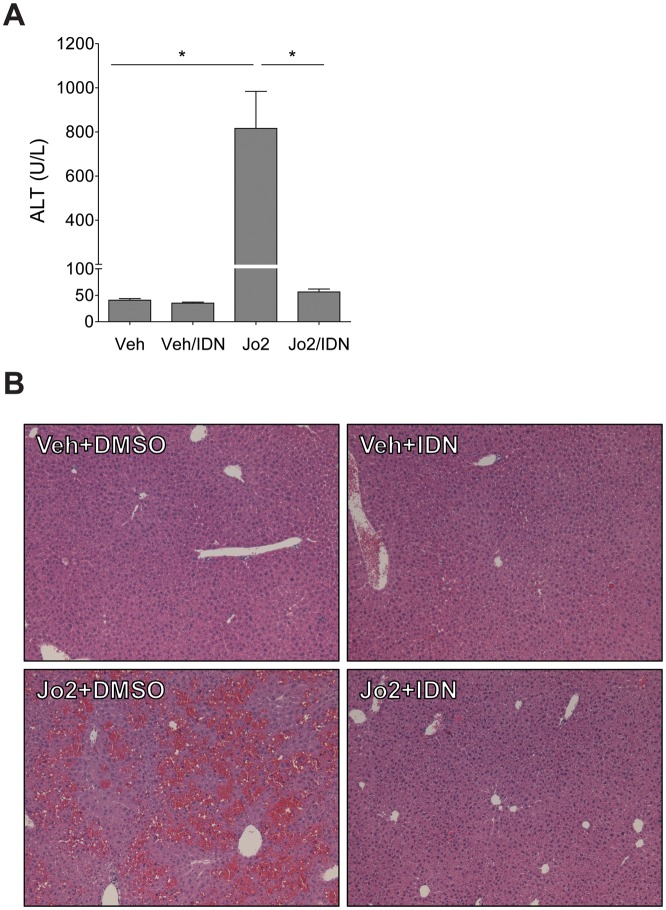
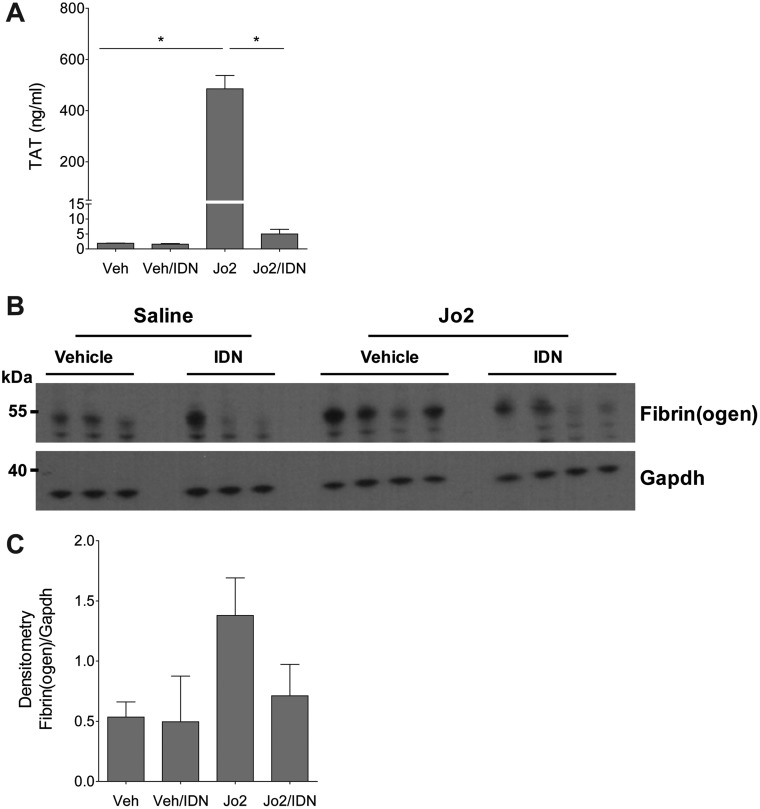
The liver is exquisitely susceptible to FasL (or Jo2)-induced liver injury owing to high Fas expression by hepatocytes (Pinkoski et al., 2000). In agreement, western blot analysis revealed a dramatic increase in cleaved caspase-3 levels in Jo2-treated mice. This was significantly attenuated in Jo2-treated mice given IDN-7314 (Figs. 2A and 2B). Treatment of mice with Jo2 significantly increased plasma ALT activity (Figure 3A) and histological evidence of sinusoidal congestion (Figure 3B), in agreement with previous studies (Weerasinghe et al., 2011). Liver histopathology was unremarkable and plasma ALT activity unaffected in mice given IDN-7314 alone (Figs. 3A and 3B). Notably, treatment with IDN-7314 abolished the Jo2-triggered increase in plasma ALT activity and eliminated congestion/hemorrhage (Figs. 3A and 3B). The results indicate that IDN-7314 effectively inhibits Fas-induced liver apoptosis in mice, an observation in agreement with other studies evaluating different caspase inhibitors in this model (Hoglen et al., 2001).
Figure 2.
Effect of IDN-7314 administration on cleaved caspase-3 levels in Fas-induced liver injury in mice. Wild-type mice were given a single injection of Jo2 (0.35 mg/kg) or vehicle (saline), then treated with 3 mg/kg IDN-7314 or vehicle (50% DMSO) 1 h later. Liver samples were collected 4½ h after Jo2 administration. (A) Western blots showing hepatic levels of cleaved caspase-3 and Gapdh protein with (B) corresponding densitometry quantification (n = 3 mice per group). Data are expressed as mean + SEM. *p < .05.
Figure 3.
Effect of IDN-7314 administration on Fas-induced liver injury in mice. Wild-type mice were given a single injection of Jo2 (0.35 mg/kg) or vehicle (saline), then treated with 3 mg/kg IDN-7314 or vehicle (50% DMSO) 1 h later. Plasma and liver samples were collected 4½ h after Jo2 administration. (A) Plasma ALT activity. (B) Representative photomicrographs (100×) show H&E-stained liver sections (n = 5–10 mice per group). Data are expressed as mean + SEM. *p < .05.
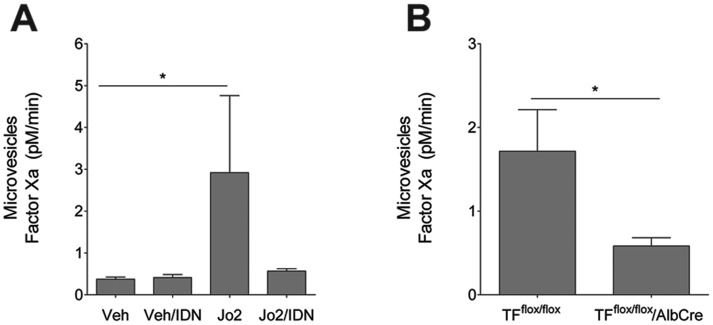
Our in vitro studies showed that primary mouse hepatocytes release microvesicles upon stimulation with Jo2 and that this was prevented by IDN-7314 (Figure 1D). Administration of Jo2 significantly increased plasma levels of TF-positive microvesicles (Figure 4A). Consistent with our in vitro studies, this increase was entirely prevented by administration of IDN-7314 (Figure 4A). The cellular source of TF-positive microvesicles in Jo2-treated mice is not known, although the liver specificity of IDN-7314 mode of action implies the liver as a potential source. Indeed, a recent study suggested that hepatocytes were a source of TF-positive microvesicles in the plasma of mice subjected to chronic bile duct ligation (BDL) (Rautou et al., 2016). We have shown previously that hepatocyte TF deficiency is associated with reduced coagulation in mice treated with Jo2 (Lopez et al., 2014). Interestingly, after Jo2 administration, and compared with Jo2-treated control mice (TFflox/flox mice), plasma TF-positive microvesicle levels were significantly lower in mice lacking TF in hepatocytes (TFflox/flox/AlbCre mice) (Figure 4B). Collectively, the results suggest that Jo2-induced caspase activation drives the release of TF-positive microvesicles from hepatocytes.
Figure 4.
Impact of caspase inhibition and hepatocyte TF deficiency on plasma TF-positive microvesicle levels. For (A), wild-type mice were given a single injection of Jo2 (0.35 mg/kg) or vehicle (saline), then treated with 3 mg/kg IDN-7314 or vehicle (50% DMSO) 1 h later. For (B), control mice (TFflox/flox mice) and mice deficient in liver TF (TFflox/flox/AlbCre mice) were given a single injection of Jo2 (0.25 mg/kg). Plasma TF-positive microvesicle procoagulant activity was determined 4½ h after Jo2 administration as described in Methods. Data are expressed as mean + SEM. *p < .05.
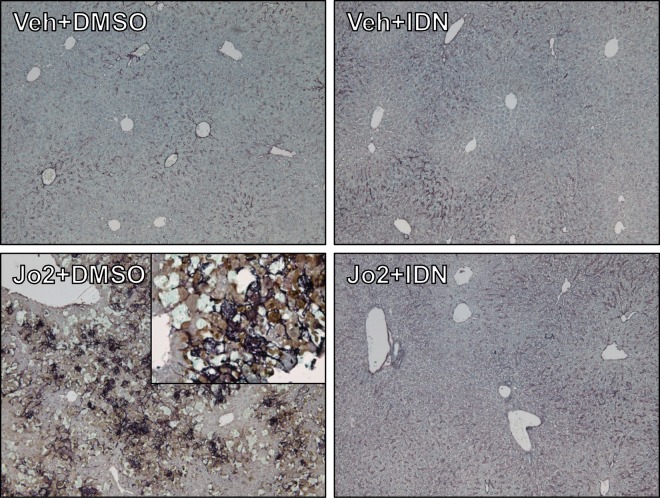
To determine the effect of IDN-7314 on Jo2-induced coagulation cascade activation we measured TAT levels, a stable biomarker of thrombin generation, and hepatic fibrin(ogen) deposition, an indicator of intrahepatic coagulation. Jo2 administration significantly increased plasma TAT levels and this marked coagulation cascade activation was blocked by IDN-7314 administration (Figure 5A). To measure hepatic fibrin(ogen) deposition, we utilized 2 approaches. Western blot analysis revealed hepatic fibrin(ogen) β chain accumulation in Jo2-treated mice that was attenuated by administration of IDN-7314 (Figs. 5B and 5C). This increase was corroborated using a 2-color immunohistochemical approach to examine intrahepatic fibrin(ogen) deposition and its localization with cleaved (activated) caspase-3. Jo2 administration dramatically increased staining for cleaved caspase-3 (brown, Figure 6). Fibrin(ogen) deposits (purple, Figure 6) co-localized to areas of caspase-3 activation and appeared as spider-like fibrils often wrapped around hepatocytes displaying apoptotic morphology (Figure 6, high-magnification inset). IDN-7314 abolished cleaved caspase-3 staining and dramatically reduced hepatic fibrin(ogen) deposition (Figure 6), indicating a robust suppression of intrahepatic coagulation by caspase inhibition in this experimental setting.
Figure 5.
Effect of caspase-inhibition on Fas-induced coagulation. Wild-type mice were given a single injection of Jo2 (0.35 mg/kg) or vehicle (saline), then treated with 3 mg/kg IDN-7314 or vehicle (50% DMSO) 1 h later. Plasma and liver samples were collected 4½ h after Jo2 administration. (A) Plasma TAT levels. (B) Western blots showing hepatic levels of fibrin(ogen) β chain and Gapdh protein with (C) corresponding densitometry quantification (n = 3–10 mice per group). Data are expressed as mean + SEM. *p < .05.
Figure 6.
Effect of IDN-7413 administration on Fas-induced fibrin(ogen) and cleaved caspase-3 costain. Wild-type mice were given a single injection of Jo2 (0.35 mg/kg) or vehicle (saline), then treated with 3 mg/kg IDN-7314 or vehicle (50% DMSO) 1 h later. Liver samples were collected 4½ h after Jo2 administration. Representative photomicrographs (100×; inset 400×) show 2-color immunohistochemical staining for fibrin(ogen) (dark purple) and cleaved (activated) caspase-3 (brown); counterstain is light blue. For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article.
DISCUSSION
Liver apoptosis is a common feature of multiple liver diseases including viral hepatitis and fatty liver disease (Feldstein et al., 2003; Hayashi and Mita, 1999). Strong evidence suggests that this form of cell death contributes to the progression of liver disease and that caspases play a central role (Barreyro et al., 2015; Canbay et al., 2004; Hoglen et al., 2001; Linton et al., 2005; Pockros et al., 2007). Analogous to previous studies (Hoglen et al., 2001, 2004), we found that IDN-7314 very effectively inhibited Fas-induced hepatocellular apoptosis in vitro and in vivo. Notably, beyond experimental Fas-induced apoptosis, caspase inhibitors have shown beneficial effects in experimental hepatic ischemia-reperfusion injury, cholestasis, and nonalcoholic steatohepatitis. Of interest, in these settings the hemostatic system is activated and inhibition of coagulation reduces liver disease severity (Harada et al., 2006; Luyendyk et al., 2010; Sullivan et al., 2010; Weerasinghe et al., 2011). Utilizing a straight-forward model of Fas-induced liver apoptosis, we demonstrate here that inhibiting caspase activity effectively abolishes intrahepatic coagulation in vivo. Thus, one mechanism whereby apoptosis could exacerbate liver disease is by acting as a driving force for the procoagulant response.
TF is the primary activator of blood coagulation cascade (Tilley and Mackman, 2006). TF activity is regulated by separation from its ligand FVII/FVIIa, which circulates in plasma (Mackman et al., 2007). We have shown previously that hepatocytes express TF, most likely complexed with coagulation FVII/FVIIa; however, this complex lacks procoagulant activity due to its conformation (Sullivan et al., 2013). Liver TF deficiency reduced coagulation in mice with Fas-induced liver injury (Lopez et al., 2014). By examining apoptosis both in vitro and in vivo, our studies with IDN-7314 establish caspase-driven apoptosis as a mechanism of hepatocyte TF decryption in vitro and a trigger of coagulation in vivo. The mechanism whereby hepatocyte apoptosis increases TF procoagulant activity most likely relates to a loss of phospholipid asymmetry of the plasma membrane that changes TF into an active conformation (Lopez et al., 2014). Anionic phospholipids, such as PS, greatly increase the procoagulant activity of the TF: FVIIa complex (Morrissey et al., 1997), and we have shown that the PS-binding protein lactadherin reduces hepatocyte TF activity (Lopez et al., 2014). Collectively, our studies here establish hepatocyte apoptosis as an in vivo trigger of TF decryption and intrahepatic coagulation. Future studies should consider exploring this pathway in other experimental settings, such as liver injury induced by lipopolysaccharide (LPS) and D-galactosamine, another liver injury model associated with increased coagulation (Miyazaki et al., 2012). Notably, however, in addition to the potential for coagulation triggered by hepatocyte injury, LPS is known to drive hematopoietic cell TF expression and coagulation (Pawlinski et al., 2010), potentially complicating interpretation. Notably, in the LPS/D-galactosamine model, prolonged caspase inhibition with z-VAD-fmk also caused apoptosis to convert to necrotic cell death (Ni et al., 2016). This observation may be dependent on both the caspase inhibitor selected and experimental model, as other studies have demonstrated benefit of long-term caspase inhibition without evidence of increased hepatocellular injury (Anstee et al., 2010; Barreyro et al., 2015). Nonetheless, hepatocellular necrosis is also linked with coagulation cascade activation (Rautou et al., 2016; Sullivan et al., 2013). Thus, necrotic injury, if it were to occur, would also likely be associated with a procoagulant response.
An increasing number of studies suggest that microvesicles, particularly TF-positive microvesicles, contribute to a procoagulant state in various conditions (Owens and Mackman, 2011). The loss of phospholipid asymmetry makes TF-positive microvesicles highly procoagulant (Owens and Mackman, 2011), and increased plasma levels of TF-positive microvesicles associates with several thrombotic conditions (Hisada et al., 2016; Manly et al., 2010; Rautou and Mackman, 2013). Although platelets and monocytes are primary sources of plasma microvesicles, there is increasing evidence that multiple cell types contribute to the pool of circulating microvesicles. For example, plasma microvesicle-associated TF activity is significantly reduced after BDL in mice with liver-specific TF deficiency (Rautou et al., 2016). Corroborating our observation that caspase inhibition prevented microvesicle release from Jo2-stimulated hepatocytes in vitro, we found that liver TF deficiency also reduced plasma levels of TF-positive microvesicles. This suggests that the liver is a source of procoagulant microvesicles in this experimental setting of apoptosis. It is difficult to discern the exact contribution of TF-positive microvesicles in this experimental setting. We did not observe any obvious association between plasma TAT and TF-positive microvesicle levels, suggesting cell-, not microvesicle-associated TF, is driving acute coagulation in Jo2-treated mice. Indeed, this is consistent with the observation that fibrin deposits co-localize with apoptotic hepatocytes in the livers of Jo2-treated mice.
Newly developed pan-caspase inhibitors with high liver retention and low systemic plasma levels are the subject of several completed and ongoing clinical trials, with early studies suggesting reduction in metrics of disease (Pockros et al., 2007; Shiffman et al., 2010). Of particular interest is the potential for caspase inhibition to demonstrate benefit in patients with liver fibrosis, a condition for which therapeutic options are very limited. In addition to reducing the severity of liver-related morbidity, our results suggest another potential benefit of caspase inhibition. Patients with severe liver fibrosis (ie, cirrhosis) are increasingly appreciated to display a delicately rebalanced hemostatic system (Lisman et al., 2010). That is, patients with severe liver disease exhibit both bleeding and thrombosis as complications, with subtle changes capable of pushing the hemostatic system into disarray. Notably, microvesicle levels are increased in the plasma of patients with liver cirrhosis (Campello et al., 2016; Hisada et al., 2016; Rautou et al., 2014). In agreement with findings in mice subjected to BDL (Rautou et al., 2016), our results indicate that TF-positive microvesicles are likely derived from hepatocytes. Moreover, we demonstrate that TF-positive microvesicle release is associated with caspase-dependent hepatocyte apoptosis in vivo. Although a definitive connection between TF-positive microvesicles and thrombosis risk in cirrhosis has not been demonstrated, it is intriguing to consider the hypothesis that hepatic apoptosis could contribute to a procoagulant state through the release of microvesicles. By preventing apoptosis-associated microvesicle release, caspase inhibitors may have the unexpected benefit of reducing thrombosis risk in patients with severe liver disease. Although such proof-of-concept clinical studies have not yet been done, a positive step forward could be in determining the association of plasma-cleaved cytokeratin 18 (CK18-Asp396), a marker of hepatocyte apoptosis, and TF-positive microvesicles in patients with cirrhosis.
In summary, we found that pan-caspase inhibition very effectively inhibits apoptosis-associated TF decryption in vitro and in vivo. Notably, caspase inhibition largely abolished intrahepatic coagulation and the release of hepatocyte-derived TF-positive microvesicles in mice with Fas-induced liver damage. The results indicate that hepatocyte apoptosis is a potent trigger of intrahepatic coagulation in vivo. Although additional studies are warranted, this suggests that the severity of liver apoptosis may contribute to a procoagulant state in patients with liver disease.
ACKNOWLEDGMENTS
The authors would like to thank Amy Porter and Kathy Joseph from the Histopathology Laboratory for their assistance with immunohistochemistry. Dr Alfred Spada and Dr Patricia Contreras are employees of Conatus Pharmaceuticals, Inc.
FUNDING
This work was supported by a sponsored research contract from Conatus Pharmaceuticals, Inc. and National Institutes of Health (NIH) grant (R01 ES017537). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the NIH.
REFERENCES
- Anstee Q. M., Concas D., Kudo H., Levene A., Pollard J., Charlton P., Thomas H. C., Thursz M. R., Goldin R. D. (2010). Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J. Hepatol. 53, 542–550. [DOI] [PubMed] [Google Scholar]
- Barreyro F. J., Holod S., Finocchietto P. V., Camino A. M., Aquino J. B., Avagnina A., Carreras M. C., Poderoso J. J., Gores G. J. (2015). The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 35, 953–966. [DOI] [PubMed] [Google Scholar]
- Campello E., Zanetto A., Spiezia L., Radu C. M., Gavasso S., Ferrarese A., Farinati F., Senzolo M., Simioni P. (2016). Hypercoagulability detected by circulating microparticles in patients with hepatocellular carcinoma and cirrhosis. Thromb. Res. 143, 118–121. [DOI] [PubMed] [Google Scholar]
- Canbay A., Feldstein A., Baskin-Bey E., Bronk S. F., Gores G. J. (2004). The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther 308, 1191–1196. [DOI] [PubMed] [Google Scholar]
- Feldstein A. E., Canbay A., Angulo P., Taniai M., Burgart L. J., Lindor K. D., Gores G. J. (2003). Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443. [DOI] [PubMed] [Google Scholar]
- Greeno E. W., Bach R. R., Moldow C. F. (1996). Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab. Invest. 75, 281–289. [PubMed] [Google Scholar]
- Harada N., Okajima K., Uchiba M. (2006). Dalteparin, a low molecular weight heparin, attenuates inflammatory responses and reduces ischemia-reperfusion-induced liver injury in rats. Crit. Care Med. 34, 1883–1891.http://dx.doi.org/10.1097/01.CCM.0000220764.10155.03 [DOI] [PubMed] [Google Scholar]
- Hayashi N., Mita E. (1999). Involvement of Fas system-mediated apoptosis in pathogenesis of viral hepatitis. J Viral Hepat 6, 357–365.http://dx.doi.org/10.1046/j.1365-2893.1999.00175.x [DOI] [PubMed] [Google Scholar]
- Hisada Y., Alexander W., Kasthuri R., Voorhees P., Mobarrez F., Taylor A., McNamara C., Wallen H., Witkowski M., Key N. S. et al. , (2016). Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays. Thromb. Res. 139, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglen N. C., Chen L. S., Fisher C. D., Hirakawa B. P., Groessl T., Contreras P. C. (2004). Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]-4-oxo-5-(2, 3, 5, 6-tetrafluoro-phenoxy)-pentanoic acid): A liver-targeted caspase inhibitor. J. Pharmacol. Exp. Ther. 309, 634–640. [DOI] [PubMed] [Google Scholar]
- Hoglen N. C., Hirakawa B. P., Fisher C. D., Weeks S., Srinivasan A., Wong A. M., Valentino K. L., Tomaselli K. J., Bai X., Karanewsky D. S. et al. , (2001). Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J. Pharmacol. Exp. Ther. 297, 811–818. [PubMed] [Google Scholar]
- Krishnaswamy S., Field K. A., Edgington T. S., Morrissey J. H., Mann K. G. (1992). Role of the membrane surface in the activation of human coagulation factor X. J. Biol. Chem. 267, 26110–26120. [PubMed] [Google Scholar]
- Lee R. D., Barcel D. A., Williams J. C., Wang J. G., Boles J. C., Manly D. A., Key N. S., Mackman N. (2012). Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb. Res. 129, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton S. D., Aja T., Armstrong R. A., Bai X., Chen L. S., Chen N., Ching B., Contreras P., Diaz J. L., Fisher C. D. et al. , (2005). First-in-class pan caspase inhibitor developed for the treatment of liver disease. J. Med. Chem. 48, 6779–6782. [DOI] [PubMed] [Google Scholar]
- Lisman T., Caldwell S. H., Burroughs A. K., Northup P. G., Senzolo M., Stravitz R. T., Tripodi A., Trotter J. F., Valla D. C., Porte R. J. (2010). Hemostasis and thrombosis in patients with liver disease: The ups and downs. J. Hepatol. 53, 362–371. [DOI] [PubMed] [Google Scholar]
- Lopez M., Kopec A. K., Joshi N., Geddings J. E., Cline H., Towery K. L., Rockwell C. E., Mackman N., Luyendyk J. P. (2014). Fas-induced apoptosis increases hepatocyte tissue factor procoagulant activity in vitro and in vivo. Toxicol. Sci. 141, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk J. P., Sullivan B. P., Guo G. L., Wang R. (2010). Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am. J. Pathol. 176, 177–186.http://dx.doi.org/10.2353/ajpath.2010.090672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Tilley R. E., Key N. S. (2007). Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 27, 1687–1693.http://dx.doi.org/10.1161/ATVBAHA.107.141911 [DOI] [PubMed] [Google Scholar]
- Malhi H., Gores G. J. (2008). Cellular and molecular mechanisms of liver injury. Gastroenterology 134, 1641–1654.http://dx.doi.org/10.1053/j.gastro.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly D. A., Wang J., Glover S. L., Kasthuri R., Liebman H. A., Key N. S., Mackman N. (2010). Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb. Res. 125, 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M., Kato M., Tanaka M., Tanaka K., Takao S., Kohjima M., Ito T., Enjoji M., Nakamuta M., Kotoh K. et al. , (2012). Antithrombin III injection via the portal vein suppresses liver damage. World J. Gastroenterol. 18, 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel O., Toti F., Hugel B., Bakouboula B., Camoin-Jau L., Dignat-George F., Freyssinet J. M. (2006). Procoagulant microparticles: Disrupting the vascular homeostasis equation? Arterioscler. Thromb. Vasc. Biol. 26, 2594–2604. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Neuenschwander P. F., Huang Q., McCallum C. D., Su B., Johnson A. E. (1997). Factor VIIa-tissue factor: Functional importance of protein-membrane interactions. Thromb. Haemost. 78, 112–116. [PubMed] [Google Scholar]
- Ni H. M., McGill M. R., Chao X., Woolbright B. L., Jaeschke H., Ding W. X. (2016). Caspase inhibition prevents tumor necrosis factor-alpha-induced apoptosis and promotes necrotic cell death in mouse hepatocytes in vivo and in vitro. Am. J. Pathol. 186, 2623–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens A. P., Mackman N. (2011). Microparticles in hemostasis and thrombosis. Circ. Res. 108, 1284–1297.http://dx.doi.org/10.1161/CIRCRESAHA.110.233056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens A. P., Passam F. H., Antoniak S., Marshall S. M., McDaniel A. L., Rudel L., Williams J. C., Hubbard B. K., Dutton J. A., Wang J. et al. , (2012). Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J. Clin. Invest. 122, 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlinski R., Wang J. G., Owens A. P. 3rd, Williams J., Antoniak S., Tencati M., Luther T., Rowley J. W., Low E. N., Weyrich A. S. et al. , (2010). Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkoski M. J., Brunner T., Green D. R., Lin T. (2000). Fas and Fas ligand in gut and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G354–G366. [DOI] [PubMed] [Google Scholar]
- Pockros P. J., Schiff E. R., Shiffman M. L., McHutchison J. G., Gish R. G., Afdhal N. H., Makhviladze M., Huyghe M., Hecht D., Oltersdorf T. et al. , (2007). Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology 46, 324–329. [DOI] [PubMed] [Google Scholar]
- Rao L. V., Pendurthi U. R. (2012). Regulation of tissue factor coagulant activity on cell surfaces. J. Thromb. Haemost. 10, 2242–2253.http://dx.doi.org/10.1111/jth.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou P. E., Mackman N. (2013). Microvesicles as risk markers for venous thrombosis. Expert Rev. Hematol. 6, 91–101.http://dx.doi.org/10.1586/ehm.12.74 [DOI] [PubMed] [Google Scholar]
- Rautou P.-E., Tatsumi K., Antoniak S., Owens A. P., Sparkenbaugh E., Holle L. A., Wolberg A. S., Kopec A. K., Pawlinski R., Luyendyk J. P. et al. , (2016). Hepatocyte tissue factor contributes to the hypercoagulable state in a mouse model of chronic liver injury. J. Hepatol. 64, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou P. E., Vion A. C., Luyendyk J. P., Mackman N. (2014). Circulating microparticle tissue factor activity is increased in patients with cirrhosis. Hepatology 60, 1793–1795.http://dx.doi.org/10.1002/hep.27033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman M. L., Pockros P., McHutchison J. G., Schiff E. R., Morris M., Burgess G. (2010). Clinical trial: The efficacy and safety of oral PF-03491390, a pancaspase inhibitor - A randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol. Ther. 31, 969–978. [DOI] [PubMed] [Google Scholar]
- Sullivan B. P., Kopec A. K., Joshi N., Cline H., Brown J. A., Bishop S. C., Kassel K. M., Rockwell C., Mackman N., Luyendyk J. P. (2013). Hepatocyte tissue factor activates the coagulation cascade in mice. Blood 121, 1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B. P., Weinreb P. H., Violette S. M., Luyendyk J. P. (2010). The coagulation system contributes to alphaVbeta6 integrin expression and liver fibrosis induced by cholestasis. Am. J. Pathol. 177, 2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley R., Mackman N. (2006). Tissue factor in hemostasis and thrombosis. Semin. Thromb. Hemost. 32, 5–10.http://dx.doi.org/10.1055/s-2006-933335 [DOI] [PubMed] [Google Scholar]
- Weerasinghe S. V., Moons D. S., Altshuler P. J., Shah Y. M., Omary M. B. (2011). Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: Heparin prevents and treats liver damage. Hepatology 53, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]