Abstract
Bosutinib (SKI-606) is an oral, dual Src/Abl tyrosine kinase inhibitor (TKI) approved for treatment of patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) that is resistant or intolerant to prior TKI therapy or for whom other TKIs are not appropriate choices. The objective of this review is to provide a longitudinal summary of toxicities that may arise during treatment with second-line or later bosutinib in patients with Ph+ chronic phase CML and to provide strategies for managing these toxicities. As bosutinib is not currently indicated for newly diagnosed CML, toxicities associated with first-line treatment are not reviewed. Recognition and optimal management of these toxicities can facilitate patient compliance and affect treatment outcomes.
Keywords: chronic myeloid leukemia, BCR-ABL1, safety, tyrosine kinase inhibitors, bosutinib
Key Message
The prevention and optimal management of adverse events (AEs) associated with tyrosine kinase inhibitors is of increasing importance, especially given the potential for lifelong treatment. This review summarizes the toxicities associated with long-term bosutinib therapy in patients with Ph+ chronic myeloid leukemia as treatment progresses and provides strategies for managing these AEs.
Introduction
Bosutinib (SKI-606) is an orally active dual Src/Abl tyrosine kinase inhibitor (TKI) approved in the United States and Europe for the treatment of patients with Philadelphia chromosome-positive (Ph+) chronic phase (CP), accelerated phase (AP), or blast phase (BP) chronic myeloid leukemia (CML) resistant or intolerant to prior therapy or for whom other TKIs are not appropriate choices [1, 2]. Bosutinib has demonstrated efficacy and manageable tolerability based on data from trials of bosutinib as a second-line therapy in patients with CP CML resistant or intolerant to imatinib [3–5] and as third-/fourth-line treatment in patients with CP CML or advanced leukemias after failure of imatinib, dasatinib, and/or nilotinib therapy [6–9]. In the frontline setting, despite an improvement in several efficacy end points, bosutinib was not superior to imatinib for inducing cytogenetic response and was associated with increased hepatotoxicity and gastrointestinal toxicity compared with imatinib [10, 11]. Thus, bosutinib is currently not indicated for patients newly diagnosed with CML [10, 12]. However, recent results from another ongoing study of frontline bosutinib (BFORE trial) are positive, demonstrating the superiority of bosutinib over imatinib for the treatment of patients newly diagnosed with CP CML [13].
Bosutinib has a unique safety profile as compared with other TKIs, such as dasatinib, nilotinib, and ponatinib [6, 14–16]. In common with other TKIs, hematologic adverse events (AEs) such as myelosuppression are frequently associated with bosutinib treatment; the most common nonhematologic AEs associated with bosutinib across treatment lines are gastrointestinal, particularly diarrhea [6]. These toxicities occur at different times during treatment; for example, diarrhea is commonly experienced soon after treatment initiation relative to other, less common toxicities, such as liver and cardiovascular events [6, 15]. Therefore, being able to characterize and manage toxicities associated with bosutinib therapy, as well as knowing when to expect these toxicities, is critical for optimizing treatment outcomes and ensuring compliance with therapy.
The prevention and optimal management of AEs over time is of increasing importance, especially given the potential for lifelong treatment with TKIs [17, 18], and recommendations for minimizing TKI AEs from the European LeukemiaNet working party on CML were recently updated [19]. However, information regarding the timing and management of AEs associated with long-term TKI therapy is limited.
The objective of this review is to provide a summary of the toxicities associated with long-term bosutinib that may arise chronologically during therapy in patients with Ph+ CP CML as treatment progresses and to provide strategies for managing these toxicities.
Methods
A PubMed search was performed for clinical studies published in the medical literature as of 29 June 2016 (see Appendix for details of the search strategy used). Articles were eligible for inclusion if they described the results of clinical trials (phases I–III) with end points focused on toxicities associated with bosutinib in patients with Ph+ CP CML resistant or intolerant to prior TKI therapy, in accordance with the current US and EU product labels [1, 2]. Preclinical articles, review articles, health economics outcomes research articles, meeting abstracts and conference proceedings, and articles that did not directly relate to toxicities associated with TKI therapies for patients with CML were excluded. Articles were initially reviewed for relevance according to these prespecified eligibility and exclusion criteria. The bibliographies of the identified articles were also surveyed for any relevant articles. Full-text versions of articles of interest were obtained and assessed for relevance by the authors. To ensure optimal characterization of bosutinib-associated toxicities, articles reporting toxicity data with the longest treatment duration were chosen for inclusion. Information sought included AEs (frequency, grade, time to onset, and time course), rates of treatment discontinuation due to AEs, line of therapy, and AE management strategies. Articles that provided clinical trial data on cross-intolerance between bosutinib and prior TKI treatment were also identified. Data were assessed qualitatively and are summarized descriptively.
Results
A total of 18 articles were captured by the PubMed search, 7 of which reported bosutinib toxicity data from a phase I/II trial (study 200; NCT00261846) of patients with CP CML resistant or intolerant to imatinib receiving second-line bosutinib (CP 2L) [3–6, 15] and patients with CP CML resistant or intolerant to imatinib and dasatinib and/or nilotinib receiving third-/fourth-line bosutinib (CP 3L) [6, 7, 9, 15]. Another article reported bosutinib toxicity data from a similarly designed phase I/II study in patients with CP 2L and CP 3L residing in Japan [20].
From these eight articles, those by Brümmendorf et al. [5] and Cortes et al. [9] were selected to summarize most characteristics of bosutinib-associated toxicities in patients with CP 2L and CP 3L, respectively, because they report safety data with the longest duration of follow-up [median (range) duration: CP 2L, 43.6 (0.6–83.4) months; CP 3L, 32.7 (0.3–93.3) months; both are based on a snapshot ≥48 months after last subject’s first visit (LSFV)]. An article by Kantarjian et al. [6] was selected because it provides detailed descriptions of the management of bosutinib-associated toxicities, and an article by Cortes et al. [15] was selected for its comprehensive information regarding cardiac and vascular toxicities reported with bosutinib in the same study. Also included were two articles presenting data for cross-intolerance between bosutinib and prior imatinib [3] and between bosutinib and prior imatinib, dasatinib, and/or nilotinib therapy [7].
Eleven articles were excluded on the basis of the prespecified eligibility criteria and authors’ combined assessment of relevance (five pharmacokinetic studies in healthy volunteers [21–25], three studies in newly diagnosed patients [6, 10, 26], one retrospective analysis of fourth-line bosutinib as part of a compassionate use program [27], one study in patients with advanced leukemias [8], and one article summarizing the EU conditional marketing authorization for bosutinib [12]).
Bosutinib-associated toxicities in patients with CML
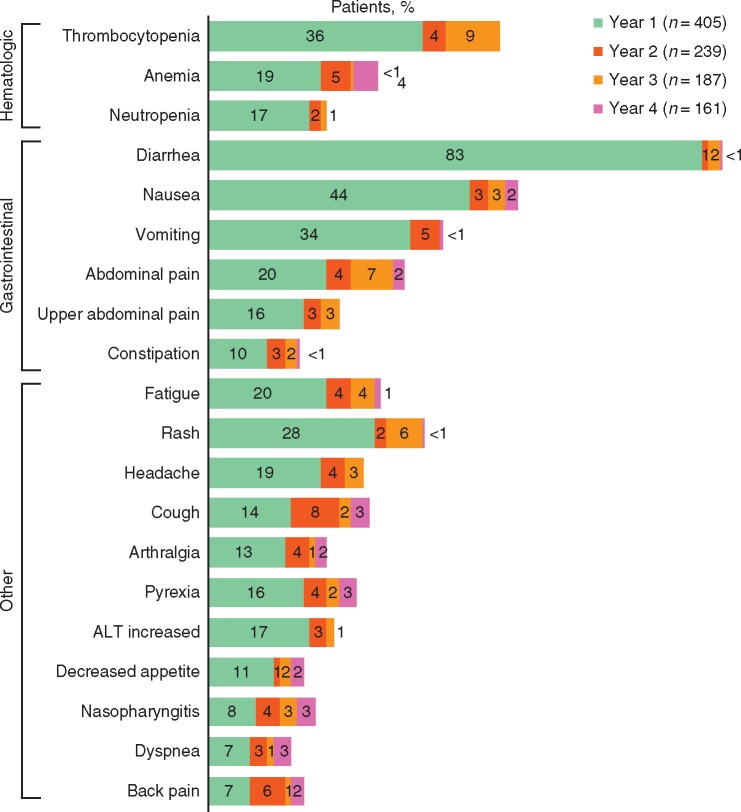
Across the CP 2L (n = 286) and CP 3L (n = 119) cohorts in the phase I/II trial of bosutinib in patients resistant/intolerant to prior imatinib and other TKIs [5, 6], the most commonly observed treatment-emergent adverse events (TEAEs) were gastrointestinal events; thrombocytopenia was the most common grade 3/4 TEAE and grade 3/4 laboratory abnormality (Table 1). The incidence of newly occurring bosutinib-associated toxicities (i.e. those not experienced by the same patient previously for those on treatment during a given year) generally decreased over time in the CP 2L (any TEAE: year 1, 99.7%; year 2, 73%; year 3, 66%; year 4, 49%) and CP 3L (any TEAE: year 1, 99%; year 2, 74%; year 3, 64%; year 4, 72%) cohorts (Figure 1) [5, 9]. Notably, in the CP 3L cohort, pleural effusions occurred more frequently in year 4 (16%) versus years 1 (5%), 2 (12%), and 3 (3%). Increased blood creatinine (any grade) had a higher incidence in years 3 and 4 (5% each) versus years 1 (2%) and 2 (1%) in the CP 2L cohort and in year 4 (13%) versus years 1 (7%), 2 (4%), and 3 (3%) in the CP 3L cohort [5, 9]. However, recently published results suggest that the decline in renal function associated with long-term bosutinib treatment is reversible and similar in frequency and characteristics to that observed with long-term imatinib [28].
Table 1.
Toxicities (%) associated with bosutinib 500 mg once-daily treatment in patients with CP CML with resistance or intolerance to prior imatinib and other TKIs [5, 6, 9]
| CP 2La |
CP 3Lb |
|||
|---|---|---|---|---|
| (n = 286) |
(n = 119) |
|||
| All Grade | Grade 3/4 | All Grade | Grade 3/4 | |
| TEAEsc | ||||
| Gastrointestinal | ||||
| Diarrhea | 86 | 10 | 83 | 9 |
| Nausea | 46 | 1 | 48 | 1 |
| Vomiting | 37 | 4 | 38 | 1 |
| Abdominal pain | 26 | 2 | 24 | 1 |
| Constipation | 14 | <1 | 13 | 0 |
| Musculoskeletal | ||||
| Arthralgia | 16 | 1 | 18 | 1 |
| Back pain | 14 | <1 | 12 | 3 |
| Extremity pain | 11 | 1 | – | – |
| Respiratory | ||||
| Cough | 22 | 0 | 22 | 0 |
| Pleural effusion | 8 | 2 | 17 | 5 |
| Dyspnea | 12 | 1 | 12 | 2 |
| General/other | ||||
| Rash | 36 | 9 | 28 | 3 |
| Fatigue | 26 | 1 | 24 | 2 |
| Headache | 19 | 0 | 27 | 3 |
| Pyrexia | 26 | 1 | 15 | 0 |
| Decreased appetite | 14 | 1 | 13 | 1 |
| Nasopharyngitis | 13 | 0 | 12 | 0 |
| Dizziness | 9 | 0 | 15 | 0 |
| Blood creatinine increased | 8 | <1 | 13 | 0 |
| Asthenia | 14 | 2 | − | – |
| Peripheral edema | 11 | <1 | – | – |
| Hypertension | 7 | 2 | – | – |
| Hematologicd | ||||
| Thrombocytopenia | 42 | 26 | 39 | 26 |
| Anemia | 27 | 11 | 20 | 7 |
| Neutropenia | 16 | 9 | 21 | 16 |
| Leukopenia | 12 | 5 | – | – |
| Cardiac eventse | 10 | 5 | 12 | 5 |
| Vascular eventsf | 8 | 4 | 6 | 3 |
| Laboratory abnormalities | ||||
| Nonhematologic | ||||
| Increased ALT/AST | – | 11/− | – | 6/3 |
| Increased lipase | – | 10 | – | – |
| Hyperglycemia | – | 3 | – | – |
| Hypermagnesemia | – | 11 | – | 13 |
| Hyponatremia | – | 3 | – | – |
| Hematologic | ||||
| Anemia | – | 14 | – | 8 |
| Lymphopenia | – | 15 | – | 15 |
| Neutropenia | – | 18 | – | 18 |
| Thrombocytopenia | – | 25 | – | 26 |
| Discontinuations | 60 | 76 | ||
| Due to AEs/toxicities | 22 | 24 | ||
| Due to disease progression | 18 | 20 | ||
Dashes indicate data not reported.
Data cutoff was 15 May 2013, with ≥48 months of follow-up from LSFV, except for cardiac and vascular events, which had a cutoff date of 23 May 2014, and an n = 284.
Data cutoff was 23 May 2014, with ≥48 months of follow-up from LSFV.
All-cause TEAEs were reported, regardless of relation to bosutinib treatment (includes unpublished data).
Individual hematologic TEAEs were clustered with the related terms from investigations.
Terms used to identify cardiac events excluded the HLGT pericardial disorders and included the HLGTs cardiac arrhythmias and heart failures and all subordinate terms under the SOC cardiac disorders; the PTs cardiac death, sudden cardiac death, and sudden death under the SOC general disorders and administration site conditions; and the PTs ejection fraction decreased, electrocardiogram QT interval abnormal, and electrocardiogram QT prolonged, and long QT syndrome congenital under the SOC in investigations.
Terms used to identify vascular events were the HLGTs coronary artery disorders, arteriosclerosis, stenosis, vascular insufficiency and necrosis, embolism and thrombosis, arterial therapeutic procedures (excluding aortic); the HLTs central nervous system hemorrhages and cerebrovascular accidents, central nervous system vascular disorders NEC, non-site specific vascular disorders NEC, peripheral vascular disorders NEC (excluding the PTs flushing and hot flash), transient cerebrovascular events, vascular imaging procedures NEC, and vascular therapeutic procedures NEC and all subordinate terms.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CP 2L, chronic phase, second-line treatment; CP 3L, chronic phase, third-line treatment; CP CML, chronic phase chronic myeloid leukemia; HLGT, high-level group term; HLT, high-level term; LSFV, last subject’s first visit; NEC, not elsewhere classified; PT, preferred term; SOC, system organ class; TEAE, treatment-emergent adverse event; TKI, tyrosine kinase inhibitor.
Figure 1.
TEAEs over time: percentage of patients with TEAEs occurring in year 1 and newly occurring in years 2, 3, and 4 in ≥20% of patients with CP CML (both cohorts; any grade). ALT, alanine aminotransferase; CP CML, chronic phase chronic myeloid leukemia; TEAE, treatment-emergent adverse event. Denominators are the number of patients on treatment during the indicated years (note: the incidence of certain TEAEs appears higher in later years compared with previous years owing to a lower number of patients on treatment).
In general, bosutinib-associated toxicities were of mild to moderate severity [6]. Serious AEs (SAEs) were reported in a similar percentage of patients in the CP 2L (39%) and CP 3L (34%) cohorts; across cohorts, the most common SAEs were pneumonia, pleural effusion, and pyrexia [6]. Forty (14%) patients in the CP 2L cohort died on study or within 30 days of the last bosutinib dose, most of whom had imatinib-resistant disease [5]. The most common reasons for death were disease progression (n = 24) and AEs unrelated to treatment (n = 13; as assessed by the investigator and including AEs of disease progression and death); no deaths were reported as treatment related in the CP 2L cohort [5]. In the CP 3L cohort, 26 (22%) patients died on study or within 30 days of the last dose, with disease progression (n = 12) and AEs unrelated to treatment (n = 10) also the most common reasons [9]. One death in the CP 3L cohort due to lower gastrointestinal hemorrhage in the setting of grade 4 thrombocytopenia was considered to be associated with bosutinib treatment.
Among 321 CP CML patients receiving second-line nilotinib in an open-label, phase II study, 8 (2.5%) died during treatment or within 28 days of discontinuation and an additional 36 (11.2%) patients died after 28 days following treatment discontinuation [29]. A low mortality rate was also reported with dasatinib as second-line treatment, with 2 (2.0%) deaths occurring among 101 CP CML patients, 1 within 30 days of the last dose and another 44 days after discontinuation [30]. However, the median follow-up durations reported for these studies (both 24 months) are far shorter than that of the published analysis of second-line bosutinib (43.6 months) [5, 29, 30]. Furthermore, the differences in study designs and patient populations complicate direct comparisons across studies.
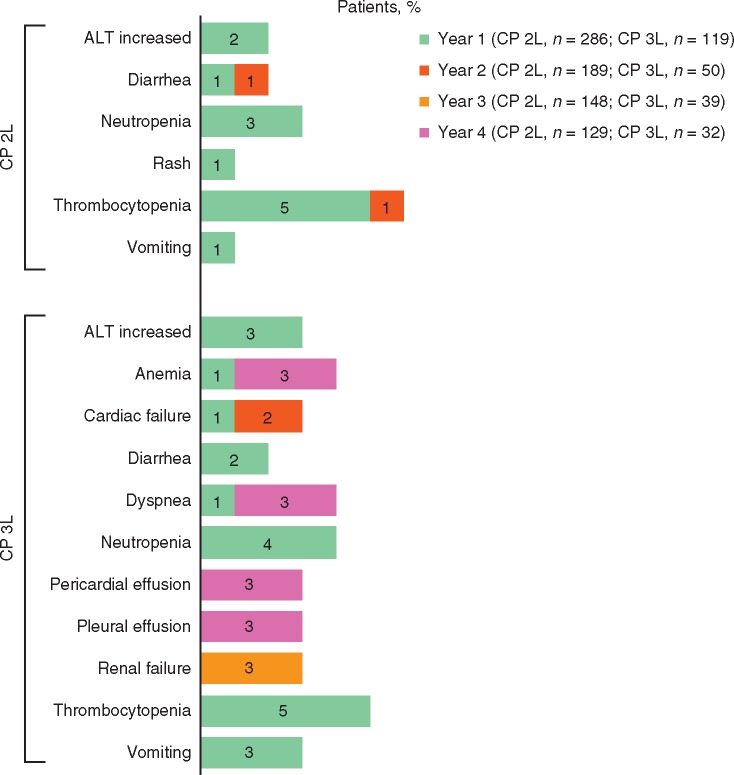
Overall, TEAEs were managed with ≥1 dose interruption or reduction in 70% and 49% of patients, respectively; these dose modifications were relatively more frequent in the CP 2L cohort (72% and 49%) compared with the CP 3L cohort (66% and 50%) [5, 9]. Across both cohorts, 23% of patients discontinued bosutinib treatment owing to AEs, the most common of which were thrombocytopenia (6%), neutropenia (3%), and increased alanine aminotransferase (ALT; 2%) [5, 9]. In both the CP 2L and CP 3L cohorts, most discontinuations due to AEs occurred during the first year of bosutinib treatment (Figure 2) [5, 9].
Figure 2.
Incidence of TEAEs resulting in treatment discontinuation in ≥1% of patients in years 1, 2, 3, and 4 by cohort. CP 2L, chronic phase, second-line treatment; CP 3L, chronic phase, third-line treatment; TEAE, treatment-emergent adverse event. Denominators are the number of patients on treatment during the specific years.
Characteristics and management of individual toxicities commonly associated with bosutinib treatment
Published guidelines for dose adjustments and recommendations for managing bosutinib-associated AEs in patients with CML are summarized in Table 2. The clinical management and characteristics of individual toxicities established in clinical trials are summarized in Table 3 and are discussed in the sections that follow.
Table 2.
| Adverse event | Management strategy |
|---|---|
Gastrointestinal
|
|
Myelosuppression
|
|
Hepatic toxicity
|
|
Dermatologic
|
|
| Fluid retention |
|
| Renal dysfunctiona |
|
Classified using KDIGO based on eGFR (ml/min/1.73 m2): normal, ≥90; mild, 60 to <90; mild to moderate, 45 to <60; moderate to severe, 30 to <45; severe, 15 to <30; kidney failure, <15.
ANC, absolute neutrophil count; CrCL, creatinine clearance; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; KDIGO, Kidney Disease: Improving Global Outcomes; NCCN, National Comprehensive Cancer Network; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PPI, proton pump inhibitor; QD, once daily; ULN, upper limit of normal.
Table 3.
| Diarrhea | ALT/AST Elevation | Myelosuppressiona | |
|---|---|---|---|
| (n = 344) | (n = 89) | (n = 216) | |
| Median (range) time to first event, days | |||
| CP 2L | 2 (1–1330) | 35 (3–1400) | 29 (1–1767) |
| CP 3L | 2 (1–210) | 81 (8–492) | 26 (1–1202) |
| Median (range) duration of an event,b days | |||
| CP 2L | 1 (1–1510) | 26 (1–1714) | 15 (1–1373) |
| CP 3L | 2 (1–413) | 15 (4–236) | 15 (1–769) |
| Event management, n (%) | |||
| Received dose reduction | 23 (7) | 17 (19) | 78 (36) |
| Received dose interruption | 58 (17) | 32 (36) | 106 (49) |
| Received concurrent medication | 231 (67) | 12 (13)c | 39 (18)d |
| Permanent treatment discontinuation due to evente | 6/405 (1)f | 9/405 (2)f | 33/405 (8) |
Myelosuppression events include anemia, hemoglobin decreased, neutropenia, neutrophil count decreased, thrombocytopenia, and platelet count decreased (includes unpublished results).
Defined as from start to stop of event with no grade change; any change in grade represents a new event.
Concurrent medications used for management of ALT and/or AST elevations included essential phospholipids, ursodiol, steroids, S-adenosylmethionine, milk thistle extract, and glycyrrhizic acid. Patients may have received ≥1 medication.
Two patients received transfusion(s) and 33 patients received growth factor(s).
Patients could report multiple TEAEs as reasons for discontinuation of treatment.
Includes patients with no rechallenge or unsuccessful rechallenge following dose interruption, as well as those who discontinued treatment because of an event without dose interruption.
Gastrointestinal AEs
Management guidelines recommend that all patients receiving bosutinib should be assessed for diarrhea and signs of dehydration; the characteristics of these events, including onset, duration, stool composition, and frequency, should be monitored (Table 2). Nonpharmacologic management strategies include dose modification [2, 31]; adding fiber to the diet; and avoiding alcohol, lactose-containing products, laxatives/stool softeners, raw fruits and vegetables, spicy or fatty foods, and caffeine. Pharmacologic approaches include antidiarrheals, antiemetics, and/or fluid replacement; proton pump inhibitors should be avoided because they may decrease bosutinib exposure [2].
Diarrhea was common in the CP 2L and CP 3L cohorts (86% and 83%, respectively), but the occurrence of grade 3/4 diarrhea events was generally low (10% and 9%; Table 1) [5, 9]. Other gastrointestinal TEAEs [any grade (grade 3/4)] reported with bosutinib included nausea [CP 2L, 46% (1%); CP 3L, 48% (1%)], vomiting [37% (4%); 38% (1%)], and abdominal pain [26% (1%); 24% (1%)] [5, 9].
Despite being the most commonly reported TEAE, diarrhea was responsible for only 1% of discontinuations across the CP 2L and CP 3L cohorts [5, 9]. Diarrhea typically occurred within 1 week of treatment initiation [median (range) time to onset: CP 2L, 2 (1–1330) days; CP 3L, 2 (1–210) days], although events were generally transient (median duration/event: CP 2L, 1 day; CP 3L, 2 days). Diarrhea management was effective, with the majority (67%) of affected patients receiving concomitant antidiarrheal medications, most commonly loperamide. Dose interruptions and dose reductions were required in 14% and 6% of patients with diarrhea, respectively.
Liver toxicities
Management guidelines recommend that patients should be assessed for signs of hepatotoxicity, such as elevated ALT and aspartate aminotransferase (AST), based on the appearance of jaundice and/or dark or ‘tea-colored’ urine. These patients should be monitored monthly using hepatic enzyme tests for the first 3 months of bosutinib administration (more frequently in patients with preexisting transaminase elevations) [2]. There are currently no pharmacologic interventions for ALT/AST elevations, although concomitant medications, including essential phospholipids, ursodiol, steroids, S-adenosylmethionine, milk thistle extract, and glycyrrhizic acid, have been used in clinical trials [6]. Hepatic toxicity management is commonly achieved using dose modification (Table 3).
Hepatotoxicity was more commonly observed in the CP 2L versus the CP 3L cohort, with elevated ALT/AST TEAEs (any grade) occurring in 25% and 15% of patients, respectively, and the grade 3/4 laboratory abnormality increased ALT occurring in 11% and 6% (Table 1) [5, 9]. Across the CP 2L and CP 3L cohorts, the first ALT/AST TEAEs with bosutinib occurred early after treatment initiation [median (range) time to onset, 35 (3–1400) and 81 (8–492) days, respectively] and events were typically transient [median (range) event duration among patients who resumed treatment, 26 (1–1714) and 15 (4–236) days]. Patients in these cohorts with ALT/AST TEAEs were managed with transient dose interruptions (37% and 32%, respectively), dose reductions (17% and 26%), or concomitant medications (16% and 5%). In earlier reports among patients who were rechallenged with bosutinib after dose interruption due to ALT/AST elevations, 74% did not experience further ALT/AST events or did not permanently discontinue treatment because of ALT/AST elevations [6].
Cardiac and vascular AEs
Overall, cardiac toxicities were infrequent with bosutinib and occurred mostly in patients with preexisting cardiac conditions [15]. In a comprehensive analysis of cardiac and vascular toxicities among all patients enrolled in the phase I/II study [including 167 patients with advanced-phase leukemia (AP CML, BP CML, or acute lymphoblastic leukemia)], the overall incidence of cardiac TEAEs (any grade) was 10% (grade ≥3, 5%); serious cardiac TEAEs occurred in 4% of patients, most commonly congestive cardiac failure and atrial fibrillation (1.0% each) [15]. The incidence of cardiac events was similar in the CP 2L (any grade, 10%; grade 3/4, 5%) and CP 3L (12%; 5%) cohorts (unpublished data). Overall, five patients in the phase I/II study discontinued bosutinib because of cardiac toxicity, and three deaths occurred owing to cardiac TEAEs [cardiac failure (n = 1) and congestive cardiac failure (n = 2)]; none of these were considered treatment related. Most events occurred during the first year of treatment [median (range) time to first event: 91 (1–2229) days] and were of short duration [median (range) cumulative duration: 8 (1–706) days]. Cardiac TEAEs were managed with dose interruption in 12 (20%) patients, among whom 10 were rechallenged with bosutinib, 9 successfully. Other management strategies included dose reduction (5%) or concomitant medication (47%).
Across all cohorts, including patients with advanced leukemias, the occurrence of vascular TEAEs (any grade) was 8% (grade ≥3, 5%), of which 1% were considered treatment related [15]. Vascular TEAEs occurred in 8% of CP 2L patients and 6% of CP 3L patients with the incidence of grade 3/4 events being similar in both cohorts (4% and 3%, respectively) (unpublished data). Five patients discontinued bosutinib because of vascular toxicity, and nine deaths due to vascular TEAEs were reported, seven of which occurred in patients with advanced disease. Of the nine deaths, one was considered probably related to bosutinib treatment (myocardial infarction in a patient with AP CML). The median (range) time to first vascular event was 327 (8–2452) days, and most events were of short duration [median (range) cumulative duration: 9 (1–318) days]. Vascular TEAEs were managed with concomitant medication in 15 (34%) patients. Of the six (14%) patients who experienced dose interruptions because of vascular toxicity, all were rechallenged successfully; one additional patient had a dose reduction.
Other toxicities
Management strategies for other toxicities associated with bosutinib treatment include the use of antiemetics for nausea and vomiting and the use of hypoallergenic moisturizing creams, topical/systemic steroids, and antihistamines for rash (Table 3) [2, 31]. Myelosuppression should be monitored in patients receiving bosutinib based on signs of easy bruising, unexpected bleeding, and blood in urine or stool. Complete blood counts should be assessed in all patients weekly for the first month and then monthly thereafter or as indicated clinically.
Nonhematologic TEAEs (any grade) reported in the phase I/II trial of bosutinib also included fatigue (CP 2L, 25%; CP 3L, 24%), rash (35%; 28%), headache (19%; 27%), pyrexia (26%; 15%), and peripheral edema (10%; 8%), most of which were low grade [5, 9]. Pleural effusion was reported in 43 patients with CP CML (CP 2L, 8%; CP 3L, 17%), more commonly in patients aged ≥65 years. Among the 20 patients in the CP 3L cohort who experienced pleural effusion, all but 1 had received prior dasatinib and 14 had a medical history of these events [9]. One death due to pleural effusion was reported, occurring after >6 years on study in a patient receiving second-line bosutinib (congestive heart failure was also listed as an AE leading to death in this patient; both considered to be unrelated to treatment). Pleural effusions typically first occurred >1 year after initiation of bosutinib treatment and were transient.
Hematologic toxicities reported in the CP 2L and CP 3L cohorts of the phase I/II trial included anemia (27% and 20%, respectively), thrombocytopenia (42% and 39%), and neutropenia (16% and 21%) [5]. Hematologic TEAEs were the most common grade 3/4 events [anemia (11% and 7%), thrombocytopenia (26% and 26%), and neutropenia (9% and 16%)] and the most common reason for treatment discontinuation (Figure 2). Myelosuppression generally first occurred early after treatment initiation and was transient.
Cross-intolerance between bosutinib and other TKIs
A retrospective evaluation of cross-intolerance (defined here as discontinuing bosutinib due to the same AE that led to discontinuation of a prior TKI) between second-line bosutinib and imatinib (based on a snapshot <24 months from LSFV) found that 27% of imatinib-intolerant patients experienced the same grade 3/4 AE with bosutinib, and 6% discontinued because of the same AE [3]. Similarly, a study of cross-intolerance between third-/fourth-line bosutinib and dasatinib (based on a snapshot ≥12 months from LSFV) found that 22% of dasatinib-intolerant patients experienced the same grade 3/4 AE with bosutinib, and 8% discontinued bosutinib because of the same AE [7].
Among patients receiving second-line bosutinib who were intolerant to prior imatinib treatment because of myelosuppression, approximately half (n = 15/31) experienced grade 3/4 myelosuppression with bosutinib, with myelosuppression resulting in discontinuation of bosutinib in 13% of these patients [3]. Similarly, 40% (n = 8/20) of patients with prior dasatinib intolerance related to myelosuppression experienced grade 3/4 myelosuppression with bosutinib when administered in the third line; 10% of these patients discontinued bosutinib owing to myelosuppression [7]. Among patients with intolerance to prior imatinib therapy due to diarrhea, only one experienced grade 3/4 diarrhea with bosutinib [3]. None of the patients intolerant to prior dasatinib treatment owing to diarrhea reported grade 3/4 diarrhea with bosutinib [7]. Additionally, no patient with intolerance to prior imatinib [3] or dasatinib [7] therapy owing to nausea, vomiting, or abdominal pain experienced the same grade 3/4 AE with bosutinib. Three of 10 patients receiving second-line bosutinib with intolerance to prior imatinib treatment due to rash experienced grade 3/4 rash with bosutinib [3]; however, no patient with intolerance to dasatinib due to skin disorders experienced the same grade 3/4 AE with bosutinib [7]. Moreover, none of the second-line bosutinib patients with intolerance to imatinib due to edema [3], fatigue [3], or hepatobiliary disorders [3] had cross-intolerance or experienced the same grade 3/4 AE with bosutinib. Only 2 of 19 patients with intolerance to prior dasatinib treatment due to pleural effusion experienced the same grade 3/4 AE with bosutinib, neither of whom discontinued bosutinib treatment because of pleural effusion [7].
Clinical perspectives and management strategies
To manage toxicities and facilitate adherence to bosutinib, patients should receive information on how to mitigate potential toxicities and also be asked to report all side effects at first onset, if possible. To fulfill this request, patients must be educated on the early identification of toxicities to allow for the possibility of early intervention. In addition to this, close monitoring and follow-up are required, particularly among patients with comorbidities or other risk factors. Given the risk of nonadherence with an oral cancer therapy such as bosutinib, open communication between healthcare providers and the patient is of utmost importance for prompt and appropriate management of toxicities during bosutinib treatment.
Gastrointestinal toxicities
Across treatment lines, gastrointestinal events (particularly diarrhea) are the most common AEs associated with bosutinib treatment. Although common, diarrhea is predominantly of low severity and is generally transient [6, 26]. In addition to results from clinical trials, clinical experience has shown that gastrointestinal AEs are manageable and rarely result in discontinuation of bosutinib. These events not only respond well to supportive measures, such as antiemetics and antidiarrheal medication, but also often improve spontaneously. Although dose modifications owing to these AEs are uncommon, nearly all patients who undergo dose interruption for diarrhea are successfully rechallenged with bosutinib.
To proactively address gastrointestinal side effects, it is important to inform patients that diarrhea is a common side effect of bosutinib treatment, and that it is typically transient and of low severity. Antidiarrheal medication at initiation of bosutinib treatment may be considered. Patients should be advised to report any gastrointestinal toxicity at the first sign of such events to allow for prompt initiation of the standard interventions (e.g. concomitant medication and oral hydration).
Run-in dosing with bi-weekly increases from a starting dose of 300 mg daily is currently being investigated in the German Bosutinib Dose Optimization study (BODO; EudraCT: 2014-005531-13) in CML patients receiving second-line bosutinib after failure of nilotinib or dasatinib. The Bosutinib Efficacy, Safety, Tolerability study (BEST, NCT02810990) is investigating run-in dosing of second-line bosutinib in elderly patients with CML, with a starting dose of 200 mg that is increased to 300 mg with an option to further titrate up to 400 mg, depending on molecular response. A recent report described the effects of a bosutinib dose escalation schedule on diarrhea [32]. In this study, 20 patients received a starting dose of 200 mg per day followed by an increase of 100 mg every 5–7 days until a target dose of 400 or 500 mg per day was reached. With this regimen, only 40% of patients developed diarrhea and no patients experienced grade 3/4 diarrhea. Another study aimed at determining if a lower starting dose (300 mg daily) of second-line bosutinib will reduce the occurrence or severity of diarrhea and other side effects while maintaining efficacy is currently planned (NCT02906696).
Hematologic toxicities
Myelosuppression is frequently reported within the first month of initiating bosutinib, with thrombocytopenia, anemia, and neutropenia being the most common hematologic AEs associated with bosutinib treatment and the most frequent reason for cross-intolerance. Hematologic AEs are most often managed by dose interruptions and reductions, usually successfully; most patients are able to maintain treatment adherence and few discontinue bosutinib treatment due to these events [6]. Other interventions for myelosuppression include growth factor support and transfusions, when clinically indicated [6].
Cardiac and vascular events
Cardiac and vascular AEs are of increasing concern among patients receiving TKI therapy. Notably, an increased risk of cardiovascular events has been reported with newer-generation TKIs, resulting in the implementation of additional safety measures to monitor and manage these events [33]. Given the low incidence of cardiac and vascular toxicities observed with bosutinib relative to other newer-generation TKIs, bosutinib may be considered the preferable treatment option for patients with cardiac or vascular comorbidities. The most common strategy for managing cardiac AEs during bosutinib therapy is concomitant medication followed by dose interruption and dose reduction. Most patients who experience cardiac or vascular events are able to remain on bosutinib treatment.
Although bosutinib is not associated with an increased risk of cardiac or vascular AEs, healthcare providers should be aware of the potential for these events, particularly in patients with comorbidities. It is, therefore, important to perform health assessments before initiating treatment to help determine cardiac and vascular risk status and identify patients in need of closer monitoring.
Discussion
Conclusions
Optimizing outcomes and ensuring compliance with bosutinib treatment requires the knowledge, close monitoring and appropriate management of AEs, along with education to alert patients to potential treatment-associated toxicities. Although there is currently an unmet need for published guidelines for monitoring and optimizing long-term compliance, current recommendations include frequent monitoring of BCR-ABL1 gene transcript levels (three to four times per year) to improve TKI treatment adherence among patients with CML [34]. A multi-team approach that involves hematologists, pharmacists, physician assistants, and nurses working closely with one another and with referring oncologists and other specialists provides patients with knowledge about their disease, treatment side-effects, and easy access for early reporting, which may enhance adherence and optimize outcomes [35].
Acknowledgements
Editorial support was provided by Simon J. Slater, PhD, and Johna Van Stelten, PhD, of Complete Healthcare Communications, LLC, and was funded by Pfizer.
Funding
The phase I/II study (study 200; NCT00261846), for which previously unpublished data have been reported in the text and Tables 1 and 3, was sponsored by Pfizer (no grant number applies).
Disclosure
HJK served as an advisor for Pfizer Inc, Bristol-Myers Squibb, Novartis, and Ariad. CG-P received research funding from Pfizer Inc. and has served as a consultant for Pfizer Inc and Bristol-Myers Squibb. THB received research funding from Novartis; served as a consultant for Novartis, Ariad, Pfizer Inc, and Bristol-Myers Squibb; and holds a patent on the use of imatinib and hypusination inhibitors.
Appendix
PubMed search terms used for toxicity-related articles in patients with Chronic Myeloid Leukemia:
(Chronic Myeloid Leukemia[Title/Abstract] OR Chronic Myelogenous Leukemia[Title/Abstract] OR CML[Title/Abstract] OR Philadelphia chromosome-positive leukemias[Title/Abstract]) AND bosutinib[Title] AND (Toxicity[Text Word] OR adverse events[Text Word] OR Safety[Text Word] OR Tolerability[Text Word]) AND English[Language] AND (Clinical Trial, Phase III[Publication Type] OR Clinical Trial, Phase II[Publication Type] OR Clinical Trial, Phase I[Publication Type] OR Clinical Trial[Publication Type] OR Phase III[Title/Abstract] OR Phase II[Title/Abstract] OR Phase I[Title/Abstract] OR Phase 3[Title/Abstract] OR Phase 2[Title/Abstract] OR Phase 1[Title/Abstract] OR Phase 1/2[Title/Abstract]) NOT Review[Publication Type].
References
- 1.BosulifTM (bosutinib). Summary of Product Characteristics. Pfizer Inc, Sandwich, Kent, UK, 2013.
- 2.Bosulif® (bosutinib). Full Prescribing Information, Pfizer Labs, Pfizer Inc, New York, NY, USA, 2017.
- 3. Cortes JE, Kantarjian HM, Brummendorf TH. et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118(17): 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gambacorti-Passerini C, Brummendorf TH, Kim DW. et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24-month follow-up. Am J Hematol 2014; 89(7): 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brummendorf TH, Cortes JE, Khoury HJ. et al. Factors influencing long-term efficacy and tolerability of bosutinib in chronic phase chronic myeloid leukaemia resistant or intolerant to imatinib. Br J Haematol 2016; 172(1): 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantarjian HM, Cortes JE, Kim DW. et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood 2014; 123(9): 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoury HJ, Cortes JE, Kantarjian HM. et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood 2012; 119(15): 3403–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gambacorti-Passerini C, Kantarjian HM, Kim DW. et al. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors. Am J Hematol 2015; 90(9): 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cortes JE, Khoury HJ, Kantarjian HM. et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol 2016; 91(12): 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cortes JE, Kim DW, Kantarjian HM. et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol 2012; 30(28): 3486–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brümmendorf TH, Cortes JE, de Souza CA. et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol 2015; 168(1): 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanaizi Z, Unkrig C, Enzmann H. et al. The European Medicines Agency review of bosutinib for the treatment of adult patients with chronic myelogenous leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2014; 19(4): 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortes JE, Gambacorti-Passerini C, Deininger MW. et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol 2017; 17: S316. JCO.2017.74.7162 (epub Nov 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amsberg GK, Koschmieder S.. Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia. Onco Targets Ther 2013; 6: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortes JE, Khoury HJ, Kantarjian H. et al. Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am J Hematol 2016; 91(6): 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moslehi JJ, Deininger M.. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015; 33(35): 4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gambacorti-Passerini C, Antolini L, Mahon FX. et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 2011; 103(7): 553–561. [DOI] [PubMed] [Google Scholar]
- 18. Vigano I, Di Giacomo N, Bozzani S. et al. First-line treatment of 102 chronic myeloid leukemia patients with imatinib: a long-term single institution analysis. Am J Hematol 2014; 89: E184–E187. [DOI] [PubMed] [Google Scholar]
- 19. Steegmann JL, Baccarani M, Breccia M. et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016; 30(8): 1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakaseko C, Takahashi N, Ishizawa K. et al. A phase 1/2 study of bosutinib in Japanese adults with Philadelphia chromosome-positive chronic myeloid leukemia. Int J Hematol 2015; 101(2): 154–164. [DOI] [PubMed] [Google Scholar]
- 21. Abbas R, Chalon S, Leister C. et al. Evaluation of the pharmacokinetics and safety of bosutinib in patients with chronic hepatic impairment and matched healthy subjects. Cancer Chemother Pharmacol 2013; 71(1): 123–132. [DOI] [PubMed] [Google Scholar]
- 22. Abbas R, Hug BA, Leister C. et al. Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol 2011; 51(12): 1721–1727. [DOI] [PubMed] [Google Scholar]
- 23. Abbas R, Hug BA, Leister C. et al. A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother Pharmacol 2012; 69(1): 221–227. [DOI] [PubMed] [Google Scholar]
- 24. Abbas R, Leister C, El Gaaloul M. et al. Ascending single-dose study of the safety profile, tolerability, and pharmacokinetics of bosutinib coadministered with ketoconazole to healthy adult subjects. Clin Ther 2012; 34(9): 2011–2019. [DOI] [PubMed] [Google Scholar]
- 25. Abbas R, Leister C, Sonnichsen D.. A clinical study to examine the potential effect of lansoprazole on the pharmacokinetics of bosutinib when administered concomitantly to healthy subjects. Clin Drug Investig 2013; 33(8): 589–595. [DOI] [PubMed] [Google Scholar]
- 26. Gambacorti-Passerini C, Cortes JE, Lipton JH. et al. Safety of bosutinib versus imatinib in the phase 3 BELA trial in newly diagnosed chronic phase chronic myeloid leukemia. Am J Hematol 2014; 89(10): 947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Gutierrez V, Martinez-Trillos A, Lopez Lorenzo JL. et al. Bosutinib shows low cross intolerance, in chronic myeloid leukemia patients treated in fourth line. Results of the Spanish compassionate use program. Am J Hematol 2015; 90: 429–433. [DOI] [PubMed] [Google Scholar]
- 28. Cortes JE, Gambacorti-Passerini C, Kim DW. et al. Effects of bosutinib treatment on renal function in patients with Philadelphia chromosome-positive leukemias. Clin Lymphoma Myeloma Leuk 2017; 17(10): 684–695. [DOI] [PubMed] [Google Scholar]
- 29. Kantarjian HM, Giles FJ, Bhalla KN. et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011; 117(4): 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kantarjian HM, Shah NP, Cortes JE. et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 2012; 119(5): 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: chronic myelogenous leukemia. Ft. Washington, PA: NCCN; 2018. [DOI] [PubMed]
- 32. Kota VK, El Rassi F, Arellano M. et al. Effects of bosutinib dose escalation on gastrointestinal adverse-events. In: 17th Annual John Goldman Conference on Chronic Myleloid Leukemia: Biology and Therapy, Estoril, Portugal, October 1-4, 2015.
- 33. US Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. Rockville, MD: US FDA; 2013.
- 34. Guerin A, Chen L, Dea K. et al. Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Curr Med Res Opin 2014; 30(7): 1345–1352. [DOI] [PubMed] [Google Scholar]
- 35. Holloway S, Lord K, Bethelmie-Bryan B. et al. Managing chronic myeloid leukemia: a coordinated team care perspective. Clin Lymphoma Myeloma Leuk 2012; 12(2): 88–93. [DOI] [PubMed] [Google Scholar]