Syphilis prevalence has been declining in every region by several percentage points per year. Nevertheless, the prevalence by region continued to vary, from as little as 0.1% in the European Region to as high as 3% in the African Region.
Keywords: sexually transmitted infection, surveillance, diagnostic assay, meta-analysis, meta-regression
Abstract
Background
This study assessed levels, trends, and associations of observed syphilis prevalence in the general adult population using global pooled analyses.
Methods
A standardized database of syphilis prevalence was compiled by pooling systematically gathered data. Random-effects meta-analyses and meta-regressions were conducted using data from the period 1990–2016 to estimate pooled measures and assess predictors and trends. Countries were classified by World Health Organization region. Sensitivity analyses were conducted.
Results
The database included 1103 prevalence measures from 136 million syphilis tests across 154 countries (85% from women in antenatal care). Global pooled mean prevalence (weighted by region population size) was 1.11% (95% confidence interval [CI], .99–1.22). Prevalence predictors were region, diagnostic assay, sample size, and calendar year interacting with region. Compared to the African Region, the adjusted odds ratio (AOR) was 0.42 (95% CI, .33–.54) for the Region of the Americas, 0.13 (95% CI, .09–.19) for the Eastern Mediterranean Region, 0.05 (95% CI, .03–.07) for the European Region, 0.21 (95% CI, .16–.28) for the South-East Asia Region, and 0.41 (95% CI, .32–.53) for the Western Pacific Region. Treponema pallidum hemagglutination assay (TPHA) only or rapid plasma reagin (RPR) only, compared with dual RPR/TPHA diagnosis, produced higher prevalence (AOR >1.26), as did smaller sample-size studies (<500 persons) (AOR >2.16). Prevalence declined in all regions; the annual AORs ranged from 0.84 (95% CI, .79–.90) in the Eastern Mediterranean to 0.97 (95% CI, .97–1.01) in the Western Pacific. The pooled mean male-to-female prevalence ratio was 1.00 (95% CI, .89–1.13). Sensitivity analyses confirmed robustness of results.
Conclusions
Syphilis prevalence has declined globally over the past 3 decades. Large differences in prevalence persist among regions, with the African Region consistently the most affected.
In 2016, the World Health Assembly and its member states adopted the World Health Organization’s (WHO) Global Health Sector Strategy on Sexually Transmitted Infections (STIs), 2016–2021 [1]. The strategy aims to reduce syphilis and gonorrhea incidence over 2018–2030 by 90%, and to reduce the incidence of congenital syphilis to <50 cases per 100000 live births by 2030 [1]. However, levels and trends in adult and congenital syphilis prevalence and incidence remain uncertain [2], with recent evidence suggesting increases [3], particularly among men who have sex with men (MSM), where several countries reported the largest increases in many years [4–6].
Our study had 5 aims to inform syphilis burden estimates: (1) to create a global database of adult syphilis prevalence in the general population post-1970; (2) to pool prevalence data globally and by region; (3) to assess the association between prevalence and region, surveyed general population type, sex, and diagnostic assay; (4) to estimate the overall temporal trend in prevalence by region; and (5) to inform model assumptions and parameters, most notably the Spectrum-STI model being developed to estimate national-level STI burdens [7].
METHODS
We compiled a standardized global database of syphilis prevalence in the adult general population (Global Syphilis Prevalence Database), drawing data from the following:
Global AIDS Response Progress Reporting (GARPR) system, more recently called the Global AIDS Monitoring system [8] (last accessed in July 2016);
Institute of Health Metrics and Evaluation (IHME) database compiled for the 2015 Global Burden of Disease study [9] (last accessed in November 2016);
WHO-STI database compiled for the 2005 [10] and 2008 [11] global and regional estimates.
Other data sources included a major systematic review for STIs in sub-Saharan Africa [12]; national surveillance reports compiled by the ministries of health of Zimbabwe [7], Morocco [7], and Mongolia (unpublished data), during pilot national STI estimations using the Spectrum-STI model; and a national human immunodeficiency virus (HIV)/syphilis household survey conducted in 2016 in Zimbabwe [13].
The extracted GARPR data included prevalence measures among women screened at antenatal care (ANC), and reported by national health ministries based on either routine ANC programmatic screening or nationally representative surveys.
The extracted IHME data were based on a PubMed systematic search using broad search terms for post-1990 data, and extraction of testing data from reports in the Global Health Data Exchange [14] database maintained by IHME. Inclusion of data was restricted to those in the general population with exclusion of blood donors (because of exclusion of self-reported risks), and high-risk and other nonrepresentative populations.
The extracted WHO-STI data included nationally or subnationally representative general population surveys of sample size >100 and published after 2000. Probable active syphilis infection was defined as concurrent positive serology on both nontreponemal and treponemal assays per the WHO [15] and IHME definitions. Nontreponemal laboratory diagnosis was classified as “RPR” testing as it was done using rapid plasma reagin (RPR) or the similar Venereal Disease Research Laboratory (VDRL) assay [16]. The treponemal laboratory diagnosis was classified as “TPHA” as it was usually done using the Treponema pallidum hemagglutination assay (TPHA) or similar assay.
At least 1 reviewer evaluated each data point based on our inclusion criteria: (1) specimens collected between 1970 and 2016; (2) study population considered representative of the general population; (3) no apparent participant selection bias (eg, patients seeking care for genital symptoms were excluded); and (4) studies used nontreponemal and/or treponemal assays on serum samples. Suspected duplicates were removed.
Information extracted included prevalence, sample size, diagnostic assay, sex, and population type. The diagnostic assay was categorized as RPR/TPHA; TPHA only, in ANC or family planning (FP) population; TPHA only, in non-ANC/non-FP population; RPR only; rapid treponemal-based assay; and assay unknown. “TPHA only” was split into 2 categories because TPHA positivity is a marker of cumulative exposure that increases with age and thus should be lower in the younger ANC/FP women compared with other women [7]. Sample size was imputed for few ANC data points through linear interpolation between years with reported sample sizes.
Countries were grouped by WHO region [17]: African Region (AFRO) including most of Africa; Region of the Americas (AMRO) including North/Central/South America; South-East Asia Region (SEARO) including South Asia (eg, India) and part of South-East Asia (eg, Indonesia); European Region (EURO) including Europe and Central Asia; Eastern Mediterranean Region (EMRO) including Middle East and North Africa and part of the Horn of Africa; and Western Pacific Region (WPRO) including East Asia (eg, China), part of South-East Asia, Australia, and Oceania.
Meta-analyses
Global and regional mean syphilis prevalence (and corresponding confidence intervals [CIs]) were estimated by pooling prevalence measures. With the small number of measures pre-1990, these data were excluded from main meta-analysis as they may not be representative for 1970–1990. A meta-analysis including data over 46 years (1970–2016) was conducted as a sensitivity analysis.
The pooled means were estimated using DerSimonian and Laird random-effects models [18]. This meta-analytic approach accounts for sampling variation and heterogeneity in effect size (here syphilis prevalence) [19]. The variances of prevalence measures were stabilized using a Freeman-Tukey–type arcsine square-root transformation [20, 21], and then weighted using the inverse-variance method [19, 21]. The weights accommodate for the variance arising from sampling variation as well as distribution of true effect size [19, 21].
Cochran’s Q-test was conducted to assess the existence of heterogeneity in effect size [19, 22]. The I2 measure was estimated to assess the proportion of between-study variation in effect size that is due to actual differences in effect size across studies rather than chance. The prediction interval was estimated to assess the distribution of true effects around the estimated mean [19, 23].
The pooled mean male-to-female ratio of syphilis prevalence was assessed using studies that reported prevalence in men and women within the same population at the same time. The ratio was estimated using random-effects meta-analyses as described above.
Meta-analyses were conducted in R version 3.3.1 software [24] using the package meta [25] except for the male-to-female ratio, which was conducted using the package metafor [26].
Meta-regressions
Random-effects meta-regression models were used to identify predictors of syphilis prevalence (and male-to-female prevalence ratio) and sources of between-study heterogeneity. Pre-1990 data were excluded from main meta-regression, but a meta-regression including all data (1970–2016) was conducted as a sensitivity analysis.
The following independent variables and interaction were specified a priori because of relevance to the study’s questions: region, population type, sample size (dichotomized as ≥500 or <500 persons), diagnostic assay, time (linear measure specified by year, and then centered by mean year), and interaction. The interaction was included to measure the annual rate of decline in the odds of syphilis positivity for each region separately, as opposed to a global rate of decline. Syphilis prevalence was generally low; the annual odds ratio for syphilis positivity can be interpreted (approximately) as the average annual proportional decline in syphilis prevalence, in the given region. Factors associated with prevalence with P ≤ .1 in univariate analysis were included in the final multivariable model. Factors associated with prevalence with P ≤ .05 in the final multivariable model were considered statistically significant. Inverse variance weighting was used in all meta-regressions.
For sensitivity analyses, to confirm identified trends given the variation in data availability with time, we repeated the same meta-regression analysis plan but excluded all pre-1995 and pre-2000 data. Also to confirm identified trends, we conducted sensitivity analyses by excluding small sample-size studies (<500) and studies using an assay besides RPR/TPHA.
All meta-regressions were conducted in R version 3.3.1 software [24] using the package metafor [26].
RESULTS
Scope of Syphilis Prevalence Data
The database included 1103 prevalence measures from 154 countries carried out between 1972 and 2016. Year 2010 was the median year. Of the 136 million syphilis tests, 1.4 million (1.0%) were syphilis positive (Table 1). The median prevalence was 1.4%. Most data were from ANC women (84.8%), including both routine-care screening (44.3%) and ANC-based sentinel surveys (40.5%). Just over 50% of surveys used RPR/TPHA dual positivity for defining syphilis. AFRO had the largest number of surveys, but WPRO had the largest number of people tested. Most data were collected post-2000 (82.5%). Number of people tested increased with time, peaking in 2014.
Table 1.
Summary of the Data Characteristics of the Global Syphilis Prevalence Database
| Characteristic | No. (%) of Studies, Surveys, and Years of Routine ANC Screening | No. of Samples Tested | No. of Positive Samples | Median Prevalence, % | Prevalence Range, % |
|---|---|---|---|---|---|
| WHO region | |||||
| AFRO | 488 (44.2) | 30 390 450 | 963 400 | 2.7 | 0.0–22.1 |
| AMRO | 206 (18.7) | 22 455 430 | 194 161 | 0.8 | 0.0–15.1 |
| EMRO | 63 (5.7) | 20 24 670 | 3822 | 0.8 | 0.0–16.0 |
| EURO | 86 (7.8) | 14 134 541 | 19 154 | 0.7 | 0.0–2.2 |
| SEARO | 110 (10.0) | 15 258 147 | 43 169 | 0.6 | 0.0–5.8 |
| WPRO | 150 (13.6) | 51 280 258 | 137 149 | 1.2 | 0.0–16.7 |
| Global | 1103 (100) | 135 543 496 | 1 365 885 | 1.4 | 0.0–22.1 |
| Sample size | |||||
| ≥500 | 998 (90.5) | 135 514 522 | 1 359 562 | 1.25 | 0.0–20.3 |
| <500 | 105 (9.5) | 28 974 | 1293 | 3.3 | 0.0–22.1 |
| Data type | |||||
| ANC routine | 489 (44.3) | 130 148 057 | 1 264 180 | 0.6 | 0.0–16.7 |
| ANC survey | 447 (40.5) | 4 579 718 | 86 327 | 1.8 | 0.0–22.1 |
| Women survey | 85 (7.7) | 458 887 | 4770 | 1.9 | 0.0–21.8 |
| Men survey | 74 (6.7) | 230 351 | 4956 | 2.0 | 0.0–20.3 |
| Women and men survey | 8 (0.7) | 126 483 | 622 | 0.5 | 0.1–2.5 |
| Diagnostic assay | |||||
| RPR/TPHA | 564 (51.1) | 82 698 827 | 400 024 | 1.1 | 0.0–18.7 |
| TPHA in ANC or FP | 21 (1.9) | 1 527 823 | 33 292 | 1.1 | 0.0–9.3 |
| TPHA in non-ANC or non-FP | 20 (1.8) | 29 755 | 2899 | 10.6 | 3.3–20.3 |
| RPR | 285 (25.8) | 25 247 224 | 519 171 | 1.9 | 0.0–22.1 |
| Rapid treponemal-based assay | 36 (3.3) | 6 649 547 | 72 051 | 1.4 | 0.1–8.75 |
| Assay unknown | 177 (16.0) | 19 390 320 | 333 418 | 0.9 | 0.0–14.1 |
| Data collection period | |||||
| 1972–1985 | 6 (0.5) | 27 353 | 585 | 3.1 | 1.0–8.7 |
| 1986–1990 | 15 (1.4) | 34 536 | 478 | 7.5 | 0.4–16.0 |
| 1991–1995 | 43 (3.9) | 190 258 | 8142 | 8.7 | 0.1–21.8 |
| 1996–2000 | 113 (10.2) | 425 807 | 13 520 | 2.7 | 0.0–20.3 |
| 2001–2005 | 178 (16.1) | 1 209 566 | 26 584 | 1.7 | 0.0–17.2 |
| 2006–2010 | 336 (30.6) | 30 364 908 | 459 509 | 1.1 | 0.0–22.1 |
| 2011 | 71 (6.4) | 13 116 304 | 128 226 | 0.9 | 0.0–9.3 |
| 2012 | 84 (7.6) | 21 040 667 | 147 297 | 0.6 | 0.0–10.3 |
| 2013 | 87 (7.9) | 24 546 802 | 167134 | 0.4 | 0.0–13.7 |
| 2014 | 86 (7.8) | 27 467 301 | 229 868 | 0.9 | 0.0–13.5 |
| 2015 | 81 (7.3) | 13 791 174 | 175 548 | 1.1 | 0.1–16.7 |
| 2016 | 2 (0.2) | 80 000 | 640 | 0.8 | 0.6–1.0 |
| All years | 1103 (100) | 135 543 496 | 1 360 855 | 1.4 | 0.0–22.1 |
Abbreviations: AFRO, African Region; AMRO, Region of the Americas; ANC, antenatal care; EMRO, Eastern Mediterranean Region; EURO, European Region; FP, family planning; RPR, rapid plasma reagin; SEARO, South-East Asia Region; TPHA, Treponema pallidum hemagglutination assay; WHO, World Health Organization; WPRO, Western Pacific Region.
Meta-analyses of Syphilis Prevalence
Meta-analyses (for 1990–2016 data) estimated the pooled mean regional syphilis prevalence at 3.04% (95% CI, 2.84%–3.24%) in AFRO, 0.97% (95% CI, .82%–1.13%) in AMRO, 0.63% (95% CI, .46%–.82%) in EMRO, 0.12% (95% CI, .09%–.15%) in EURO, 0.65% (95% CI, .56%–.75%) in SEARO, and 1.27% (95% CI, 1.18%–1.36%) in WPRO (Table 2). The global (unweighted) mean prevalence was 1.61% (95% CI, 1.51%–1.71%). Weighted by region population size, the global mean prevalence was lower at 1.11% (95% CI, .99%–1.22%), reflecting lower prevalence in populous regions such as SEARO. The meta-analysis including all data (1970–2016) arrived at similar results (Supplementary Table 1).
Table 2.
Pooled Mean Estimates for Syphilis Prevalence Globally and by Region, 1990–2016
| WHO Region | Studies, Surveys, and Years of Routine ANC Screening | Samples Tested | Prevalence, % | Heterogeneity Measures | |||
|---|---|---|---|---|---|---|---|
| Total No. | Total No. | Mean | (95% CI) | Q a (P Value) | I 2 , % b (95% CI) | Prediction Interval, % c (95% CI) | |
| AFRO | 480 | 30 386 735 | 3.04 | (2.84–3.24) | 497 920.82 (<.0001) | 99.9 (99.9–99.9) | (.2–8.9) |
| AMRO | 205 | 22 453 896 | 0.97 | (.82–1.13) | 283 166.71 (<.0001) | 99.9 (99.9–99.9) | (.0–4.4) |
| EMRO | 60 | 2 024 180 | 0.63 | (.46–.82) | 12 481.22 (<.0001) | 99.5 (99.5–99.6) | (.0–2.7) |
| EURO | 86 | 14 134 541 | 0.12 | (.09–.15) | 23 597.66 (<.0001) | 99.6 (99.6–99.7) | (.0–0.6) |
| SEARO | 110 | 15 258 147 | 0.65 | (.56–.75) | 48 374.37 (<.0001) | 99.8 (99.8–99.8) | (.·0–2.0) |
| WPRO | 147 | 51 243 687 | 1.27 | (1.18–1.36) | 77 534.39 (<.0001) | 99.8 (99.8–99.8) | (.5–2.4) |
| Global | 1088 | 135 501 186 | 1.61 | (1.51–1.71) | 2 326 538.45 (<.0001) | 100.0 (100.0–100.0) | (.0–6.5) |
Abbreviations: AFRO, African Region; AMRO, Region of the Americas; ANC, antenatal care; CI, confidence interval; EMRO, Eastern Mediterranean Region; EURO, European Region; SEARO, South-East Asia Region; WHO, World Health Organization; WPRO, Western Pacific Region.
aQ is the Cochran Q statistic assessing the existence of heterogeneity in effect size (syphilis prevalence).
b I 2 is a measure assessing the magnitude of between-study variation that is due to differences in effect size across studies rather than chance.
cPrediction interval estimates the 95% interval in which the true effect size in a new study would lie.
There was significant evidence for heterogeneity in effect size (syphilis prevalence) within all regions; the P value was always <.0001. Most variability was attributed to differences in effect size rather than chance (I2 > 99.5%). However, the prediction intervals were relatively narrow, indicating only moderate variation in prevalence across studies.
Meta-regression for Syphilis Prevalence
Univariate meta-regression analyses of syphilis prevalence (1990–2016) data selected the variables region, sample size, population type, diagnostic assay, and interaction for inclusion in the final multivariable model (Table 3). The final model’s adjusted R2 was 47.16%. AFRO had the highest prevalence. Relative to AFRO, the adjusted odds ratios (AORs) were 0.42 (95% CI, .33–.54) for AMRO, 0.13 (95% CI, .09–.19) for EMRO, 0.05 (95% CI, .03–.07) for EURO, 0.21 (95% CI, .16–.28) for SEARO, and 0.41 (95% CI, .32–.53) for WPRO.
Table 3.
Meta-regression Results for the Predictors of Adult Syphilis Prevalence Levels and Sources of Between-Study Heterogeneity, 1990–2016
| Characteristic | Variable | No. of Studies, Surveys, and Years of Routine ANC Screening | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| WHO region | AFRO | 480 | 1 | 1 | ||
| AMRO | 205 | 0.28 (.22–.35) | <.0001 | 0.42 (.33–.54) | <.0001 | |
| EMRO | 60 | 0.13 (.09–.19) | <.0001 | 0.13 (.09–.19) | <.0001 | |
| EURO | 86 | 0.03 (.02–.04) | <.0001 | 0.05 (.03–.07) | <.0001 | |
| SEARO | 110 | 0.20 (.15–.27) | <.0001 | 0.21 (.16–.28) | <.0001 | |
| WPRO | 147 | 0.35 (.27–.45) | <.0001 | 0.41 (.32–.53) | <.0001 | |
| Sample size | ≥500 | 991 | 1 | 1 | ||
| <500 | 97 | 3.68 (2.55–5.31) | <.0001 | 2.16 (1.60–2.92) | <.0001 | |
| Population | ANC survey | 441 | 1 | 1 | ||
| ANC routine | 489 | 0.34 (.28–.43) | <.0001 | 0.95 (.76–1.18) | .6361 | |
| Women survey | 82 | 1.00 (.67–1.47) | .9647 | 0.69 (.50–.97) | .0312 | |
| Men survey | 71 | 1.60 (1.05–2.42) | .0250 | 1.21 (.86–1.70) | .2843 | |
| Women and men survey | 6 | 0.13 (.03–.55) | .0057 | 0.19 (.06–.63) | .0064 | |
| Diagnostic test | RPR/TPHA | 553 | 1 | 1 | ||
| TPHA in ANC or FP population | 21 | 1.96 (.94–4.10) | .0730 | 2.41 (1.36–4.22) | .0026 | |
| TPHA in non-ANC or non-FP population | 18 | 15.30 (6.89–33.78) | <.0001 | 2.71 (1.39–5.27) | .0033 | |
| RPR | 283 | 1.78 (1.39–2.27) | <.0001 | 1.26 (1.04–1.53) | .0178 | |
| Rapid treponemal-based assay | 36 | 0.79 (.45–1.39) | .4199 | 1.39 (.90–2.15) | .1416 | |
| Assay unknown | 177 | 0.93 (.70–1.25) | .6258 | 0.93 (.74–1.18) | .5570 | |
| Region and year | AFRO and year | 480 | 0.91 (.89–.93) | <.0001 | 0.95 (.93–.97) | <.0001 |
| AMRO and year | 205 | 0.90 (.86–.95) | .0001 | 0.92 (.88–.97) | .0007 | |
| EMRO and year | 60 | 0.83 (.76–.90) | <.0001 | 0.84 (.79–.90) | <.0001 | |
| EURO and year | 86 | 0.64 (.60–.69) | <.0001 | 0.94 (.87–1.03) | .1904 | |
| SEARO and year | 110 | 0.91 (.86–.97) | .0015 | 0.90 (.86–.94) | <.0001 | |
| WPRO and year | 147 | 0.98 (.94–1.04) | .5687 | 0.97 (.93–1.01) | .2298 | |
R 2 for the multivariable meta-regression model was 47.2%.
Abbreviations: AOR, adjusted odds ratio; AFRO, African Region; AMRO, Region of the Americas; ANC, antenatal care; CI, confidence interval; EMRO, Eastern Mediterranean Region; EURO, European Region; FP, family planning; OR, odds ratio; RPR, rapid plasma reagin; SEARO, South-East Asia Region; TPHA, Treponema pallidum hemagglutination assay; WHO, World Health Organization; WPRO, Western Pacific Region.
The meta-regression yielded adjustment factors for the diagnostic assays. Compared to studies using RPR/TPHA for diagnosis, studies diagnosing with TPHA only in the ANC/FP populations had >2-fold higher odds of test positivity (AOR, 2.41 [95% CI, 1.36–4.22]). Studies diagnosing with TPHA only in non-ANC/non-FP populations had nearly 3-fold higher odds (AOR, 2.71 [95% CI, 1.39–5.27]). Studies utilizing RPR only also had higher odds (AOR, 1.26 [95% CI, 1.04–1.53]).
Small study sample size (<500) was associated with higher prevalence. The AOR was 2.16 (95% CI, 1.60–2.92).
The AORs for the interaction term were all <1, indicating declining syphilis prevalence in all regions, but this decline was not statistically significant for EURO and WPRO. AORs (per year) were 0.95 (95% CI, .93–.97) for AFRO, 0.92 (95% CI, .88–.97) for AMRO, 0.84 (95% CI, .79–.90) for EMRO, 0.94 (95% CI, .87–1.03) for EURO, 0.90 (95% CI, .86–.94) for SEARO, and 0.97 (95% CI, .93–1.01) for WPRO. An AOR of 0.95 implies (approximately) a 5% annual decline in syphilis prevalence.
For sensitivity analyses, the above meta-regressions were redone on data collected for all times (1970–2016; Supplementary Table 2), post-1995 (Supplementary Table 3), and post-2000 (Supplementary Table 4). These yielded similar results for the trends and predictors, affirming the inherent consistency of the results and confirming the temporal declines in all regions. A meta-regression excluding studies with <500 sample size, and another excluding studies using an assay besides RPR/TPHA, both yielded also similar trends and predictors (Supplementary Tables 5 and 6).
Meta-analysis and Meta-regression for the Male-to-Female Prevalence Ratio
Forty-three studies were identified (from 4 of the 6 regions) that included data from the same population at the same time for both men and women. The majority were from AFRO (67.4%), 72% had ≥500 sample size, and 72% used RPR/TPHA dual positivity for diagnosis. Most studies were drawn from the published literature.
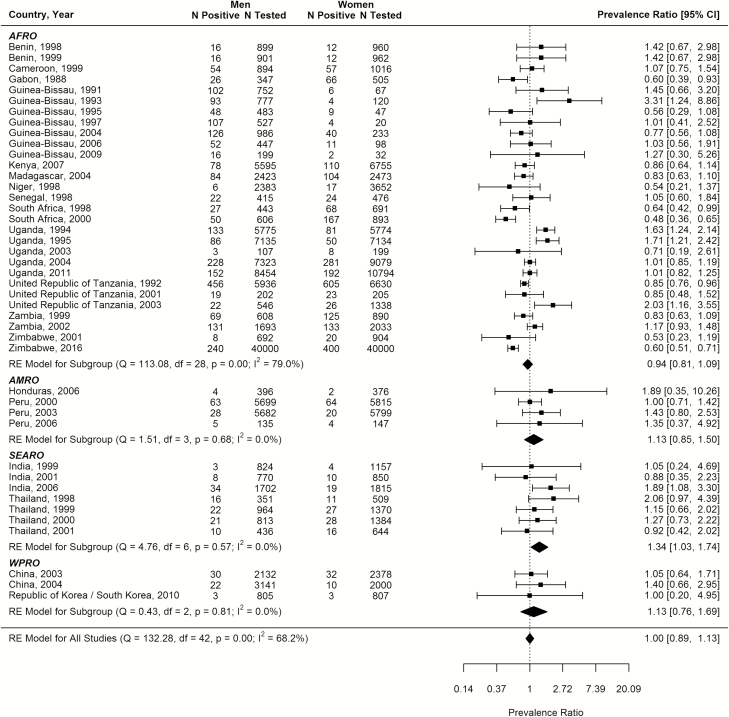
The pooled mean male-to-female prevalence ratio was 1.00 (95% CI, .89–1.13; Figure 1). The meta-regression analyses did not identify any significant predictor for this ratio (Supplementary Table 7).
Figure 1. .
Meta-analysis of male-to-female syphilis prevalence ratio. Abbreviations: AFRO, African Region; AMRO, Region of the Americas; CI, confidence interval; df, degrees of freedom; RE, random effects; SEARO, South-East Asia Region; WPRO, Western Pacific Region.
DISCUSSION
We presented analyses of 1103 syphilis prevalence measures representing 136 million tests from 154 countries over 4 decades. Prevalence has been declining for 3 decades, if not more, by several percentage points per year across the regions. While the global mean weighted (1990–2016) prevalence was 1.11%, regional prevalence varied widely, from 0.12% in EURO to 3.04% in AFRO. The analyses yielded adjustment factors for the different diagnostic assays; diagnosis by other than RPR/TPHA dual-positivity overestimated prevalence. Small sample-size studies (<500) also overstated prevalence estimates. Prevalence did not differ between men and women in the subanalysis that investigated the male-to-female prevalence ratio.
The downward trends in prevalence in all regions were remarkable. Though there were differences in the decline rates, the decline was consistent in all regions, suggesting a global phenomenon. Whether these declines reflect falls in incidence and/or shorter durations of active infection is unclear. The incidence may have fallen due to the expansion of HIV/STI response including primary prevention interventions [1, 27], declines in sexual risk behavior in response to the threat of HIV infection [28], increased HIV-associated mortality that may have disproportionally affected people at higher STI risk [29], shorter duration of active infection in sex partners [30, 31], and possibly demographic, sociocultural, and socioeconomic changes. Factors that may have contributed to a shorter active-infection duration include progressive improvements in coverage of syphilis screening and treatment (notably in ANC), or more widespread use of antibiotics in general (including for non-STI infections, which sometimes cure concurrent syphilis). It was noteworthy that there are considerable and persistent differences in prevalence by region, and that the prevalence in the EURO region appears to be very low (and declining), two findings that warrant further investigation.
While the evidence for the declines at the aggregate regional level is robust, this may not necessarily reflect prevalence declines in specific countries or specific subpopulations. Surveillance data indicate that syphilis prevalence is increasing among MSM [4–6]. It is possible that the declines in the general population may reflect changes taking place in specific sexual networks, such as in commercial heterosexual sex networks, while prevalence could be increasing in other sexual networks, such as among MSM. There is even some evidence in few countries for increased incidence among reproductive-age women, along with increases in congenital syphilis incidence [32, 33]. This highlights the need for continued vigilance in syphilis testing and treatment as overall population prevalence declines.
Our study has limitations. Although our database covered all regions and 154 countries, availability of data varied by region and country. While nearly all large countries contributed data, there were exceptions (eg, Russia in EURO). Surveys may have intentionally oversampled higher-STI or higher-risk areas and populations for reasons of public health surveillance. The availability of data increased with time, and the vast majority of data were collected after 2000. This may have biased trend estimates if earlier data were less representative. We could not, given available data, assess possible effects of age and urban–rural differences on prevalence.
The higher prevalence in AFRO may in part be inflated by the higher rates of nonvenereal treponematoses infections in this region [34]. Serologic methods (RPR or TPHA and combination) cannot differentiate syphilis from other treponematoses [34]. More generally, syphilis diagnostic methods are imperfect. TPHA only and RPR only provide inflated prevalence estimates. TPHA positivity reflects ever exposure, and therefore not necessarily current infection. RPR-only diagnosis can overestimate prevalence with false positivity with conditions such as HIV infection and pregnancy [35]. RPR/TPHA dual positivity, the gold standard, unavoidably includes a small fraction of false positives due to people whose syphilis infection was successfully treated but who remain “serofast” [36]. We attempted to address the diagnostic biases by including diagnostic type as a variable, and showing similar results in a sensitivity analysis that used only RPR/TPHA studies; nevertheless, the regional prevalence estimates and time trends are still subject to some bias associated with geographical and temporal variations in test types used across the surveys.
While we assessed the average linear trends in prevalence, the declines may have varied in intensity with time. We attempted to assess the variation in the decline rates through a sensitivity analysis (not shown) by incorporating a year-squared term in the multivariable regression, but this did not result in a superior model fit.
The male-to-female prevalence ratio was assessed based on a relatively small database (43 studies) from specific countries from 4 of the 6 regions—the estimated ratio may not be representative of the global ratio. There could be also variations in this ratio by setting or region depending on the type of syphilis epidemic dynamics.
Despite these limitations, our study has key strengths. This syphilis database is, to our knowledge, the largest and most comprehensive ever assembled. Much of included data was collected through standardized protocols over years, enhancing our ability to assess trends. Our sensitivity analyses confirmed the consistency and robustness of predictors and trends across all regions and regardless of survey characteristics.
Our findings inform global and country-level STI surveillance, burden estimation, and program target setting. The results provided key parameter inputs for modeling, such as for the Spectrum-STI surveillance tool [7]. First, our results affirmed, based on empirical data, a 1:1 male-to-female prevalence ratio, a key modeling assumption. Second, our results provided diagnostic-assay adjustment factors. We found larger biases of TPHA-only and RPR-only screening algorithms (Table 3) than previously assumed (Supplementary Table 8) [7, 37]. In contrast, the adjustment factor for the rapid, treponemal-based assays was not statistically different from 1. This supports WHO’s recommendation to use this assay in settings where RPR/TPHA testing is not feasible or not indicated [38]. Third, after adjustment for confounders, we found no difference in prevalence between ANC/FP and other women (Table 3).
The WHO’s Global Health Sector Strategy on STIs, 2016–2021 target of 90% reduction in syphilis incidence over 2018–2030 [2] corresponds to an average annual reduction of 17%. This is substantially greater than the estimated annual declines in prevalence (Table 3), the Spectrum-STI estimates from national applications [7, 31], and the 2015 Global Burden of Disease estimates [9]. This suggests that the Global STI Strategy target may be ambitious or that insufficient resources have been made available to achieve the target. It is clear also that major public health and programmatic challenges remain on the road to elimination of congenital syphilis by 2030.
CONCLUSIONS
Syphilis prevalence in the general population appears to be declining in all regions. However, large differences across regions persist, with sub-Saharan Africa continuing to be the most affected region. The drivers and determinants of these declines and heterogeneities merit further study, especially the role that syphilis- and other STI-specific programs played.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. L. K., L. A. R., and N. N. conceived the study and developed the analysis plan. E. L. K., J. R., N. K., R. M. C., and A. S. collated data. N. N., E. L. K., A. S., and L. A. R. designed the analysis methodology. A. S. with L. A. R. conducted the analyses. A. S. and L. A. R. with E. L. K. wrote the first draft of the article. All authors contributed to the interpretation of the results and final version of the article.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the position of Avenir Health, World Health Organization (WHO), or other affiliated organization. The findings achieved herein are solely the responsibility of the authors.
Financial support. The study was funded partially by WHO, Department of Reproductive Health and Research, STI Program. We thank Dr Teodora Wi and Dr Melanie Taylor, both from WHO, for facilitation in compiling data reported through the Global AIDS Response Progress Reporting system. This publication was partially made possible by the National Priorities Research Program grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The authors are also grateful for support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global health sector strategy on sexually transmitted infections 2016–2021. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. World Health Organization. The global elimination of congenital syphilis: rationale and strategy for action. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 3. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2015. Atlanta, GA: CDC, 2016. [Google Scholar]
- 4. Tucker JD, Cohen MS. China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis 2011; 24:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stamm LV. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother 2010; 54:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohammed H, Mitchell H, Sile B, Duffell S, Nardone A, Hughes G. Increase in sexually transmitted infections among men who have sex with men, England, 2014. Emerg Infect Dis 2016; 22: 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korenromp EL, Mahiané G, Rowley J et al. Estimating prevalence trends in adult gonorrhoea and syphilis in low- and middle-income countries with the Spectrum-STI model: results for Zimbabwe and Morocco from 1995 to 2016 [manuscript published online ahead of print 21 March 2017]. Sex Transm Infect 2017. doi:10.1136/sextrans-2016-052953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joint United Nations Programme on HIV/AIDS. Global AIDS response progress reporting 2015—guidance. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 9. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis, and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 11. World Health Organization. Global incidence and prevalence of selected sexually transmitted infections—2008. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 12. Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 2012; 307:2079–86. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control, ICAP at Columbia University, Zimbabwe National AIDS Council (NAC), Zimbabwe National Statistics Agency (ZIMSTAT), Zimbabwe Biomedical Research and Training Institute (BRTI). Zimbabwe population-based HIV impact assessment ZIMPHIA 2015–2016. Fact sheet. Washington, DC: PEPFAR; 2016 Available at: http://phia.icap.columbia.edu/wp-content/uploads/2016/11/ZIMBABWE-Factsheet.FIN_.pdf. Accessed 17 November 2017. [Google Scholar]
- 14. Global Health Data Exchange (GHDx) database. 2017. Available at: http://ghdx.healthdata.org/. Accessed 17 November 2017.
- 15. Newman L, Rowley J, Vander Hoorn S et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10: e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes KK. Sexually transmitted diseases. 4th ed New York: McGraw-Hill Medical, 2008. [Google Scholar]
- 17. World Health Organization. WHO regional offices Available at: http://www.who.int/about/regions/en/. Accessed 1 October 2017.
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 19. Borenstein M. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons, 2009. [Google Scholar]
- 20. Freeman MF, Tukey JW.. Transformations related to the angular and the square root. The Annals of Mathematical Statistics 1950; 21:607–11. [Google Scholar]
- 21. Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat 1978; 32: 138. [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 25. Schwarzer G. meta: An R package for meta-analysis. R News 2007; 7: 40–5. [Google Scholar]
- 26. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010; 36:48. [Google Scholar]
- 27. Wijesooriya NS, Rochat RW, Kamb ML et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Health 2016; 4: e525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Awad SF, Abu-Raddad LJ. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s?Epidemics 2014; 8:9–17. [DOI] [PubMed] [Google Scholar]
- 29. Kenyon CR, Osbak K, Buyze J, Chico RM. The changing relationship between bacterial STIs and HIV prevalence in South Africa—an ecological study. Int J STD AIDS 2015; 26:556–64. [DOI] [PubMed] [Google Scholar]
- 30. Osbak KK, Rowley JT, Kassebaum NJ, Kenyon CR. The prevalence of syphilis from the early HIV period is correlated with peak HIV prevalence at a country level. Sex Transm Dis 2016; 43:255–7. [DOI] [PubMed] [Google Scholar]
- 31. Kenyon CR, Osbak K, Tsoumanis A. The global epidemiology of syphilis in the past century—a systematic review based on antenatal syphilis prevalence. PLoS Negl Trop Dis 2016; 10:e0004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. Increase in incidence of congenital syphilis—United States, 2012–2014. Atlanta, GA: CDC, 2015. [Google Scholar]
- 33. Chen ZQ, Zhang GC, Gong XD et al. Syphilis in China: results of a national surveillance programme. Lancet 2007; 369:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitjà O, Šmajs D, Bassat Q. Advances in the diagnosis of endemic treponematoses: yaws, bejel, and pinta. PLoS Negl Trop Dis 2013; 7:e2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nandwani R, Evans DT. Are you sure it’s syphilis? A review of false positive serology. Int J STD AIDS 1995; 6:241–8. [DOI] [PubMed] [Google Scholar]
- 36. Hook EW 3rd, Marra CM. Acquired syphilis in adults. N Engl J Med 1992; 326:1060–9. [DOI] [PubMed] [Google Scholar]
- 37. Ham DC, Lin C, Newman L, Wijesooriya NS, Kamb M. Improving global estimates of syphilis in pregnancy by diagnostic test type: a systematic review and meta-analysis. Int J Gynaecol Obstet 2015; 130(Suppl 1):S10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. The use of rapid syphilis tests. Special Programme for Research and Training in Tropical Diseases (TDR) sponsored by UNICEF/UNDP/World Bank/WHO. Geneva, Switzerland: Sexually Transmitted Diseases Diagnostics Initiative, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.