Abstract
Objectives
To study the determinants of the pharmacokinetics (PK) of rituximab (RTX) in patients with ANCA-associated vasculitis (AAV) and its association with clinical outcomes.
Methods
This study included data from 89 patients from the RTX in AAV trial who received the full dose of RTX (four weekly infusions of 375 mg/m2). RTX was quantified at weeks 2, 4, 8, 16 and 24, and summarized by computing the trapezoidal area under the curve. We explored potential determinants of the PK-RTX, and analysed its association with clinical outcomes: achievement of remission at 6 months, duration of B-cell depletion and time to relapse in patients who achieved complete remission.
Results
RTX serum levels were significantly lower in males and in newly diagnosed patients, and negatively correlated with body surface area, baseline B-cell count and degree of disease activity. In multivariate analyses, the main determinants of PK-RTX were sex and new diagnosis. Patients reaching complete remission at month 6 had similar RTX levels compared with patients who did not reach complete remission. Patients with higher RTX levels generally experienced longer B-cell depletion than patients with lower levels, but RTX levels at the different time points and area under the curve were not associated with time to relapse.
Conclusion
Despite the body-surface-area-based dosing protocol, PK-RTX is highly variable among patients with AAV, its main determinants being sex and newly diagnosed disease. We did not observe any relevant association between PK-RTX and clinical outcomes. The monitoring of serum RTX levels does not seem clinically useful in AAV.
Keywords: ANCA-associated vasculitis, rituximab, pharmacokinetics, outcomes
Rheumatology key messages
The pharmacokinetics of rituximab is highly variable in patients with ANCA-associated vasculitis.
It is associated with the duration of B cell depletion in ANCA-associated vasculitis.
It is not associated with important clinical outcomes (remission and relapses).
Introduction
ANCA-associated vasculitides (AAV) are systemic autoimmune diseases characterized by necrotizing inflammation of small- and medium-sized vessels [1]. This group includes granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), which are variably associated with ANCA targeting PR3 or MPO [2].
The rituximab in AAV (RAVE) trial showed that a single course of rituximab (RTX) has a similar efficacy to induce and maintain remission as oral CYC followed by AZA for 18 months [3], and a better efficacy in patients treated for a relapse of the disease with PR3-ANCA [4]. However, clinical responses are variable, and relapses remain frequent after remission is achieved [5]. Maintenance therapy using RTX has been shown to prevent relapses in most patients [6] but it remains unclear whether all patients require prolonged maintenance treatment and what the ideal timing of retreatment is. Consequently, the identification of early risk factors for remission-induction failure and relapse could help physicians to personalize treatment for each patient.
The pharmacokinetics (PK) of therapeutic mAbs is highly variable between individual patients and has been associated with clinical efficacy in some settings. For example, serum levels of anti-TNF drugs are associated with their efficacy in RA [7–9] and AS [10], while also affecting frequency of side effects [11]. Few studies have assessed the relationship of the PK of RTX (PK-RTX) with clinical outcomes. The strongest evidence comes from studies in patients with lymphoma, in which PK-RTX is highly associated to its efficacy [12]. In contrast, no association was found between RTX levels and clinical outcomes in RA [13, 14]. Moreover, no dose finding studies have been performed with RTX in either RA or AAV, the two autoimmune diseases for which RTX has been approved for use by regulatory agencies.
Therefore, the objectives of this study were to identify the determinants of PK-RTX [in terms of serum trough levels at different time points and global exposure represented by the area under the curve (AUC)] in patients treated for AAV and its association with clinical outcomes, aiming to assess whether PK-RTX could be used as a readily available, clinically useful biomarker for efficacy and risk of relapse with the potential of guiding decisions regarding re-dosing.
Methods
Patients
The RAVE trial was a randomized, double-blind, placebo-controlled trial comparing RTX (four weekly infusions of 375 mg/m2 each) to CYC (followed by AZA once remission was achieved) in 197 patients with severe AAV [3, 5]. Patients also received oral glucocorticoids (starting at prednisone 1 mg/kg) according to a pre-specified tapering protocol aiming at complete withdrawal around 5.5 months [15]. Patients were evaluated prospectively at baseline; at weeks 1, 2, 3 and 4; at months 2, 4 and 6; and every 3 months until month 18. At each visit, the BVAS for granulomatosis with polyangiitis (Wegener’s granulomatosis) (BVAS/WG) [16] was obtained, standard biological tests were performed, and serum samples were collected and stored at − 80 °C. In the present study, we included all patients from the RTX arm of the RAVE trial who received the full dosing of the drug, and had available serum samples for RTX quantification. Trial data are publicly available on the Immune Tolerance Network website (https://www.itntrialshare.org). Ethical approval and informed patient consent were obtained for each participant of the RAVE study. The RAVE study was approved by the institutional review boards at each participating centre, and globally by the Immune Tolerance Network.
Outcome definitions
A BVAS/WG score of ⩾1 reflects active disease within the 28 days prior to assessment, while a score of 0 indicates absence of disease activity. The primary end point of the RAVE trial was achievement of complete remission, defined by a BVAS/WG score of 0 and successful completion of the prednisone taper, at 6 months [3]. Complete response was defined as a BVAS/WG score of 0 and prednisone dose ⩽10 mg/day at the 6 months visit [3, 5].
Circulating B cell numbers were measured at each visit by FACS analysis. B cell depletion was defined as ⩽ 10 CD19+ B cells/µl. Times to B cell redetection and reconstitution were defined as the time between the first RTX infusion and the first visit when patients had B cell count ⩾10 and ⩾69/µl or baseline value, respectively [5].
Disease relapse was defined as any new disease activity, indicated by an increase in BVAS/WG of ⩾ 1 point after achievement of complete remission. A relapse was considered severe if the BVAS/WG was ⩾3, if a new major item was present, or if, in the judgement of the clinician, a new course of induction therapy using RTX and high-dose prednisone was warranted; all other relapses were considered non-severe and treated by an increase of the prednisone dose [17, 18].
Quantification of serum RTX levels
We quantified RTX in all available serum samples using two different methods: an ELISA validated in-house at Genentech; and a mass spectrometry (MS)-based assay recently developed at Mayo Clinic, referred to as monoclonal immunoglobulin Rapid Accurate Mass Measurement (miRAMM), based on serum immunoglobulin light chain molecular mass profiling [19, 20] (for details on the methods, see supplementary data, ‘Quantification of serum RTX levels’, available at Rheumatology online).
Statistical analyses
Analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Characteristics are presented as median and interquartile range (Q1, Q3) for continuous variables and frequency (percentage) for categorical variables. Separate analyses were performed for measurements obtained via miRAMM and ELISA. For each patient, PK-RTX using those two assays was summarized and analysed as week 2 (just before the third infusion of RTX) and 4 (1 week after the last infusion of RTX), and months 2, 4 and 6 serum trough levels, as well as the trapezoidal AUC integrating baseline, weeks 2 and 4, and month 2 levels. For five patients, the month 2 levels were missing and were imputed for AUC integration according to the percentile of their levels at week 4.
First, we studied which baseline factors were associated with RTX trough level at week 2 and the AUC by each assay. We then assessed the association between PK-RTX (RTX trough levels at the different visits and AUC) and important outcomes: complete remission at 6 months, time to B cell redetection, and time to relapse in patients who first reached complete remission (see for details on the methods supplementary data section ‘Statistical analysis’, available at Rheumatology online). Time to event analyses were conducted using Cox proportional hazards models. At each time point, patients were classified in tertiles according to their RTX level, and the lowest tertile (tertile 1) was considered as the reference for time to event comparison with tertiles 2 and 3 (respectively, medium and highest RTX levels).
Results
We included the 89 patients from the RAVE trial who received the full dose of RTX (four weekly infusions at 375 mg/m2 BSA) and had available serum samples for PK analyses in this study (Table 1). Among them, 51.7% were female, the median age was 55.0 years, 68.5% were PR3-ANCA positive, 75.3% had a clinical diagnosis of GPA, 47.2% were newly diagnosed at inclusion and all had active severe disease (median BVAS/WG at inclusion was 8).
Table 1.
Baseline characteristics of the study population
| Baseline characteristics | Total |
|---|---|
| (n = 89) | |
| Sex, female, n (%) | 46 (51.7) |
| Age, median (IQR), years | 55.0 (44.0–67.0) |
| BMI, median (IQR), kg/m2 | 27.3 (24.5–31.5) |
| BSA, median (IQR), m2 | 1.9 (1.8–2.1) |
| ANCA type, n (%) | |
| MPO-ANCA | 28 (31.5) |
| PR3-ANCA | 61 (68.5) |
| AAV diagnosis, n (%) | |
| Indeterminate | 1 (1.1) |
| Microscopic polyangiitis | 21 (23.6) |
| Granulomatosis with polyangiitis | 67 (75.3) |
| New diagnosis at inclusion, n (%) | 42 (47.2) |
| BVAS/WG, median (IQR) | 8.0 (6.0–10.0) |
| Renal involvement, n (%) | 59 (66.3) |
| Creatinine clearance, median (IQR), ml/min | 69.4 (42.3–98.6) |
| B cells, median (IQR), CD19+ cells/µl | 230.8 (114.8–390.5) |
| Baseline IgG level, median (IQR), g/l | 8.8 (7.5–10.2) |
BVAS/WG: Birmingham Vasculitis activity score for granulomatosis with polyangiitis (Wegener’s granulomatosis); IQR, interquartile range.
Determinants of the PK of RTX
RTX serum levels were variable between patients at the different visits. Highest values were measured at week 4, 1 week after the last RTX infusion (supplementary Fig. S1, available at Rheumatology online). ELISA measured generally lower RTX levels than miRAMM. Table 2 shows the influence of several a priori selected potential factors on PK-RTX represented as week 2 RTX levels and RTX serum level AUC, for both miRAMM and ELISA assays. Age had no effect but sex was a major determinant, with RTX levels ∼20% lower in males compared with females. The RTX dose was adapted to BSA for each individual patient. Nevertheless, we observed a negative correlation between RTX AUC by ELISA and BSA. Conversely, there was no significant correlation between BMI and week 2 RTX level or AUC. Disease classification (MPA vs GPA or MPO-ANCA vs PR3-ANCA) had no influence, but patients with newly diagnosed disease at inclusion had significantly lower RTX levels than patients included with a relapse of their AAV. Disease activity level, as measured by the BVAS/WG score, was negatively correlated with PK-RTX. We did not detect any association between renal involvement (according to BVAS/WG items) or the estimated glomerular filtration rate (using the Modification of diet in renal disease (MDRD) formula) and week 2 RTX level or AUC. We observed a negative correlation between baseline circulating B cell number and RTX levels when measured by miRAMM. Finally, baseline serum IgG level was negatively correlated with RTX serum level at week 2, but not with the AUC, nor with RTX levels at later time points (months 4 and 6). In multivariate analyses, the main determinants of week 2 RTX level and AUC were sex and new diagnosis. Patients with a new diagnosis compared with patients with relapsing disease at inclusion had higher baseline B cell counts (median 239 vs 154 cells/µl, respectively, P = 0.037), higher serum IgG levels [9.6 g/l (8.3, 11.8) vs 8.1 g/l (6.8, 9.3), P = 0.001], but a non-significantly higher BVAS/WG score (8.0 vs 7.0, respectively, P = 0.084).
Table 2.
Baseline determinants of the pharmacokinetics of rituximab
| miRAMM W2 | miRAMM AUC | ELISA W2 | ELISA AUC | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline determinants | Median (IQR) | P-valuea | Median (IQR) | P-value | Median (IQR) | P-value | Median (IQR) | P-value |
| Sex | ||||||||
| Male | 113 (92–144) | 0.001 | 6707 (5702–9738) | 0.008 | 103 (88–118) | <0.001 | 5792 (4613–7052) | <0.001 |
| Female | 145 (128–174) | 8918 (7132–11315) | 129 (114–145) | 7545 (6415–9254) | ||||
| ANCA | ||||||||
| PR3 | 138 (102–172) | 0.48 | 8511 (5984–10210) | 0.62 | 114 (96–135) | 0.68 | 6735 (5350–8472) | 0.59 |
| MPO | 133 (96–151) | 8322 (5680–10828) | 121(96–142) | 7313 (4989–9647) | ||||
| Disease status | ||||||||
| New diagnosis | 113 (90–141) | 0.001 | 6955 (5432–8717) | <0.001 | 101 (84–121) | <0.001 | 5637 (4474–7523) | <0.001 |
| Relapsing | 147 (130–173) | 9658 (6846–11311) | 129 (113–145) | 7434 (6166–9100) | ||||
| Diagnosis | ||||||||
| MPA | 132 (96–154) | 0.48 | 8202 (5558–9486) | 0.39 | 118 (94–131) | 0.54 | 7297 (5120–7921) | 0.82 |
| GPA | 137 (103–167) | 8535 (6005–10467) | 118 (101–141) | 6735 (5452–8715) | ||||
| Renal involvement | ||||||||
| Yes | 133 (102–159) | 0.66 | 8134 (5716–10113) | 0.39 | 119 (96–142) | 0.53 | 6888 (5089–8268) | 0.55 |
| No | 140 (102–175) | 8702 (6606–10362) | 114 (98–130) | 6792 (5861–8826) | ||||
| Correlation coefficientb | P- value | Correlation coefficient | P-value | Correlation coefficient | P-value | Correlation coefficient | P-value | |
| Age | −0.072 | 0.51 | 0.042 | 0.70 | −0.088 | 0.42 | 0.057 | 0.60 |
| BMI | 0.155 | 0.15 | 0.079 | 0.46 | 0.164 | 0.13 | 0.045 | 0.68 |
| BSA | −0.105 | 0.34 | −0.162 | 0.13 | −0.158 | 0.15 | −0.243 | 0.02 |
| BVAS/WG | −0.263 | 0.01 | −0.223 | 0.04 | −0.246 | 0.02 | −0.194 | 0.07 |
| B cells | −0.206 | 0.07 | −0.254 | 0.02 | −0.193 | 0.09 | −0.140 | 0.21 |
| Creatinine Clearance | −0.040 | 0.72 | −0.052 | 0.63 | −0.017 | 0.88 | −0.154 | 0.15 |
| Baseline IgG level | −0.227 | 0.035 | −0.122 | 0.258 | −0.233 | 0.032 | −0.153 | 0.157 |
Rituximab levels were compared between groups using Wilcoxon’s rank sum test. Rituximab levels are in μg/ml.
Correlations between rituximab level and continuous variables were assessed using Spearman’s test. AUC: area under the curve; BVAS/WG: Birmingham Vasculitis activity score for granulomatosis with polyangiitis (Wegener’s granulomatosis); GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; miRAMM: monoclonal immunoglobulin Rapid Accurate Mass Measurement; W2: week 2.
Association of the PK of RTX with remission induction and B cell depletion
Among the 89 patients included in the study, 66 met the primary end point of the RAVE trial, and 73 fulfilled the definition of a complete response at 6 months. We did not observe any difference in RTX levels, assessed by miRAMM or by ELISA, between patients who did or did not meet these two endpoints (Table 3).
Table 3.
Association between the serum levels of rituximab (μg/ml) and remission induction at month 6
| Complete response at 6 months | Complete remission at 6 months | |||||
|---|---|---|---|---|---|---|
| No (n = 16) | Yes (n = 73) | No (n = 23) | Yes (n = 66) | |||
| Rituximab quantification | Median (IQR) | Median (IQR) | P-value | Median (IQR) | Median (IQR) | P-value |
| miRAMM W2 | 134.2 (103.7–201.5) | 137.9 (101.3–157.3) | 0.89 | 136.5 (104.0–180.4) | 138.3 (100.5–161.3) | 1.00 |
| miRAMM AUC | 8254 (5591–12482) | 8511 (5945–10025) | 0.87 | 8442 (5900–11485) | 8498 (5955–10058) | 0.94 |
| ELISA W2 | 124.0 (94.0–144.5) | 115.0 (96.0–139.0) | 0.64 | 117.5 (95.3–147.0) | 118.0 (96.0–139.0) | 0.71 |
| ELISA AUC | 7241 (5441–9431) | 6746 (5144–8263) | 0.55 | 6571 (5372–8765) | 6839 (5247–8225) | 0.85 |
Complete response was defined as a BVAS/WG score of 0 and prednisone dose ≤10 mg/day at the 6 months visit, and complete remission as a BVAS/WG score of 0 and successful completion of the prednisone taper at 6 months. Rituximab levels are expressed as median (IQR), and compared between groups using the Mann–Whitney test. AUC: area under the curve; BVAS/WG: Birmingham Vasculitis activity score for granulomatosis with polyangiitis (Wegener’s granulomatosis); IQR, interquartile range; miRAMM: monoclonal immunoglobulin Rapid Accurate Mass Measurement; W2: week 2.
All but four patients experienced B cell depletion as soon as week 2. In those four patients, B cell depletion was achieved at week 4 in two, at month 2 in one and only at month 4 in one. All of them achieved complete remission, and two of them subsequently experienced a non-severe relapse while the two others remained in complete remission during the 18 months of the study. Their PK-RTX profile had nothing remarkable compared with patients who experienced an immediate B cell depletion, with RTX serum levels close to the median levels of the whole group.
Association of the PK of RTX with time to B cell redetection
Seven patients met the criteria for B cell depletion at week 2, had B cell count value over just 10/µl once at either week 4 or month 2, and returned below 10/µl at the next visits until later B cell redetection. These results were considered as technical variations of the FACS method, and the later time point was considered to analyse the duration of B cell depletion. Patients were censored from B cell depletion analysis if they received a new course of treatment (either cross-over therapy to cyclophosphamide for early treatment failure or best medical judgement before 6 months) or relapsed before B cell redetection.
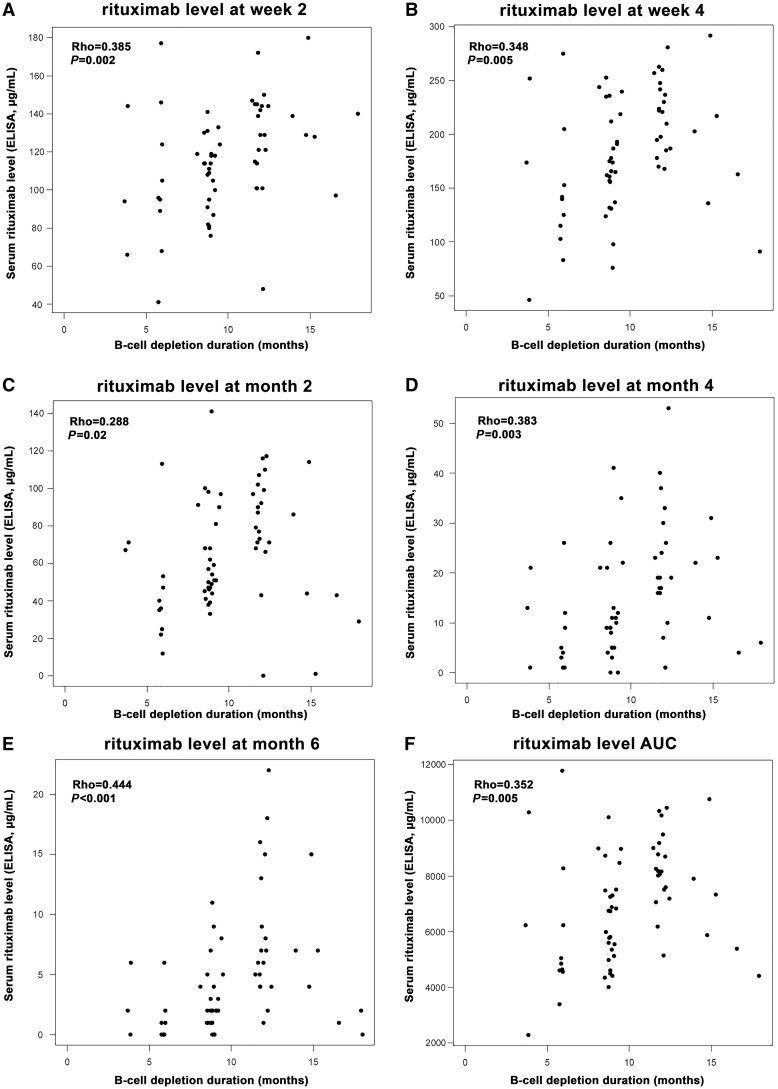
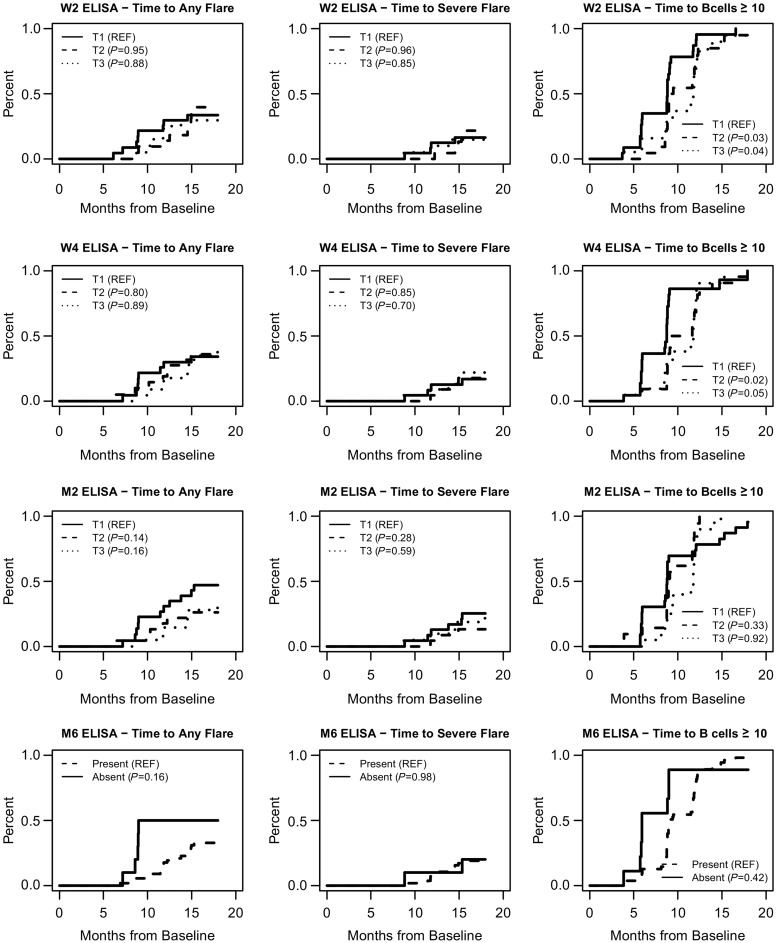
At 4, 6, 9 and 12 months, 4, 18, 57 and 88% of the patients, respectively, had detectable B cells. At 18 months, 65 of the 67 patients had experienced B cell redetection. We observed significant correlations between RTX levels at the different visits and the duration of B cell depletion (Fig. 1). We then assessed whether the level of RTX at each time point could effectively predict the time to B cell redetection. As shown in Table 4 and Fig. 2 (plots on the right of the figure), patients with lower serum RTX by ELISA (tertile 1) at early time points (weeks 2 and 4) experienced a shorter duration of B cell depletion compared with patients with higher RTX levels (tertiles 2 and 3). Similar observations were made when RTX was quantified by miRAMM (supplementary Table S1, available at Rheumatology online). We also analysed the time to B cell reconstitution (defined as B cell count ⩾69/µl or ⩾baseline count), and did not observe any significant association with PK-RTX (data not shown).
Fig. 1.
Correlation between rituximab serum levels at different time points and duration of B cell depletion
The duration of B-cell depletion was computed in each patient as the time between the date of the first rituximab infusion and the date of the first visit with B cells ≥10/µl. The correlation with serum levels of rituximab at the different visits was assessed by Spearman’s test.
Table 4.
The pharmacokinetics of rituximab (by ELISA) and time to relapse and to B cell redetection
| Time to any flare | Time to severe flare | Time to B cells ≥ 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rituximab level tertiles | n (events) | HR (95% CI) | P-value | n (events) | HR (95% CI) | P-value | n (events) | HR (95% CI) | P-value |
| W2 Tert 1 | 23 (8) | Ref. | 23 (4) | Ref. | 23 (23) | Ref. | |||
| Tert 2 | 21 (8) | 0.971 (0.364, 2.587) | 0.95 | 21 (4) | 1.033 (0.258, 4.134) | 0.96 | 22 (20) | 0.520 (0.283, 0.955) | 0.03 |
| Tert 3 | 20 (7) | 0.923 (0.335, 2.546) | 0.88 | 20 (4) | 1, 142 (0.286, 4.568) | 0.85 | 19 (19) | 0.521 (0.280, 0.970) | 0.04 |
| W4 Tert 1 | 23 (8) | Ref. | 23 (4) | Ref. | 22 (21) | Ref. | |||
| Tert 2 | 20 (8) | 1.137 (0.426, 3.030) | 0.80 | 20 (4) | 1.143 (0.286, 4.571) | 0.85 | 22 (21) | 0.480 (0.259, 0.889) | 0.02 |
| Tert 3 | 22 (8) | 0.936 (0.351, 2.494) | 0.89 | 22 (5) | 1.299 (0.349, 4.837) | 0.70 | 21 (21) | 0.537 (0.286, 1.007) | 0.05 |
| M2 Tert 1 | 22 (11) | Ref. | 22 (6) | Ref. | 23 (22) | Ref. | |||
| Tert 2 | 22 (6) | 0.469 (0.173, 1.269) | 0.14 | 22 (3) | 0.465 (0.116, 1.862) | 0.28 | 21 (20) | 1.385 (0.716, 2.679) | 0.33 |
| Tert 3 | 20 (6) | 0.490 (0.181, 1.326) | 0.16 | 20 (4) | 0.704 (0.199, 2.496) | 0.59 | 20 (20) | 0.966 (0.507, 1.839) | 0.92 |
| M4 Tert 1 | 23 (9) | Ref. | 23 (4) | Ref. | 22 (22) | Ref. | |||
| Tert 2 | 19 (9) | 1.173 (0.465, 2.956) | 0.74 | 19 (5) | 1.522 (0.408, 5.669) | 0.53 | 19 (19) | 0.808 (0.428, 1.526) | 0.51 |
| Tert 3 | 17 (5) | 0.674 (0.226, 2.012) | 0.48 | 17 (3) | 1.009 (0.226, 4.508) | 0.99 | 17 (16) | 0.444 (0.230, 0.856) | 0.02 |
| M4 Non-detectable | 3 (1) | Ref. | 3 (0)a | 2 (2) | Ref. | ||||
| Detectable | 56 (22) | 1.106 (0.149, 8.212) | 0.92 | 56 (12) | 56 (55) | 0.549 (0.131, 2.304) | 0.41 | ||
| M6 Non-detectable | 10 (5) | Ref. | 10 (2) | Ref. | 9 (8) | Ref. | |||
| Detectable | 54 (19) | 0.490 (0.182, 1.315) | 0.16 | 54 (11) | 01.023 (0.227, 4.615) | 0.98 | 55 (55) | 0.730 (0.339, 1.571) | 0.42 |
| AUC Tert 1 | 22 (8) | Ref. | 22 (4) | Ref. | 23 (22) | Ref. | |||
| Tert 2 | 23 (10) | 1.111 (0.438, 2.816) | 0.83 | 23 (6) | 1.428 (0.403, 5.064) | 0.58 | 20 (19) | 0.575 (0.308, 1.074) | 0.08 |
| Tert 3 | 20 (6) | 0.719 (0.249, 2.072) | 0.54 | 20 (3) | 0.805 (0.180, 3.598) | 0.78 | 22 (22) | 0.695 (0.375, 1.287) | 0.25 |
Only three subjects with no RTX detectable at M4, none of which had events. AUC: area under the curve; M2: month 2; M4: month 4; M6: month 6; Tert: Teritle; W2: week 2; W4: week 4.
Fig. 2.
Association between rituximab serum levels and time to relapse and to B cell redetection
Serum rituximab levels by ELISA at each visit were analysed as tertiles, the lowest tertile (tertile 1) considered as the reference for time to event comparison with tertiles 2 and 3 (respectively, medium and highest rituximab levels) using Cox proportional hazards models. Time to any flare and time to severe flare were analysed in patients who reached complete remission, and time to B cell redetection (defined as B cells ≥10/µl) was analysed considering flare as a competing event (since treatments administered for flares could influence the duration of B cell depletion).
Association of the PK of RTX with relapse risk
Relapses were analysed in the 72 patients who first met the definition of complete remission, independently of the 6 month time point. During the 18 months of follow-up, 26 patients experienced relapses, and 15 experienced a severe relapse. We did not detect any significant association between time to relapse and RTX levels at any visit or using the AUC by ELISA (Table 4 and Fig. 2) or by miRAMM (supplementary Table S1, available at Rheumatology online). We also compared RTX levels at all visits and AUC between patients who relapsed and those who did not relapse as a categorical outcome, using both definitions of severe and any relapses during the 18-month period of the study, and did not observe any significant difference (data not shown).
The only divergence between survival curves was observed at the month 2 time point for the risk of any flare, with patients in the first tertile (i.e. with the lowest RTX levels by ELISA) experiencing numerically more relapses than patients with higher RTX serum levels, but this association did not reach statistical significance (Table 4 and Fig. 2). Similarly, patients with undetectable RTX by ELISA at 6 months had numerically more relapses in the following year than patients in whom RTX was still detectable (5 flares in 10 patients without detectable levels vs 19 relapses in 54 patients with detectable levels, P = 0.16 by Cox analysis, Table 4 and Fig. 2).
Discussion
In this study, we report that PK-RTX (analysed as serum trough levels at different time points and AUC) was highly variable among patients with AAV despite a dosing protocol adjusted for BSA, its main determinants being sex and receiving RTX for a disease relapse (vs for a newly diagnosed disease). However, the serum level of RTX obtained after the infusions was not associated with important clinical outcomes, such as reaching complete remission, relapse risk or time to relapse. The absence of associations between PK-RTX and clinical outcomes shows that drug level monitoring is not a clinically useful tool in the context of therapy with RTX for AAV. Our findings question the need for an individual dose regimen, based on individual characteristics.
A clinically useful tool to predict response to treatment and ideal RTX redosing to prevent relapses in AAV patients has yet to be discovered. Several factors have been previously associated with an increased risk of relapse: history of previous relapses, diagnosis of GPA (vs MPA), PR3-ANCA positivity (vs MPO-ANCA) [5], ANCA titre increases [21, 22], HLA-DPB1*04:01 allele carriage [23], variations in serum levels of S100A8/A9 (calprotectin) [24] and potentially urinary soluble CD163 which is associated with disease activity [25]. However, until now none of these markers have proven their utility in guiding treatment in individual patients, and there is still an unmet need of clinically useful biomarkers.
To address the issue of the clinical utility of RTX level monitoring in patients with AAV, we used two different assays to measure the drug serum levels. Therapeutic mAbs may exist in free, partially bound and fully bound forms in the bloodstream, and the choice of which form to measure and how to measure them may be important [26, 27]. Despite analytical differences between the ELISA and miRAMM assays, no clinically meaningful association with clinical outcome was detected using either method.
Lower serum levels of RTX in men have been reported in patients with RA [28] and patients with lymphoma [29–31], probably due to a higher volume of distribution of the drug in men. BSA and sex were the most significant covariates in patients with RA, but BSA accounted for only a small part of PK-RTX variability, whereas men had ∼30% lower RTX serum levels [28]. Despite the BSA-based dose-adjustment protocol used in the RAVE study, we still observed a small negative inverse correlation between BSA and serum RTX levels, that is, patients with higher BSA achieved slightly lower serum RTX levels. However, this effect was small and was not significant in multivariate analyses.
The higher RTX levels obtained in patients included for a relapse of AAV may be related to previous exposure to immunosuppressants and especially to CYC, which causes a well-documented, long-lasting B cell lymphopenia [5], and potentially hypogammaglobulinaemia. Patients entering the trial for relapsing disease indeed had lower baseline B cell counts than patients enrolled with newly diagnosed disease, but the effect of B cell count on PK-RTX was small. Conflicting observations on this matter have been made in RA. In one study, PK-RTX was not influenced by pre-treatment B cell numbers [28]. In another recent study, authors reported a significant inverse relationship between pre-treatment B cell counts and PK-RTX, supporting the notion of the importance of the ‘antigen burden’ for the PK of RTX: the more circulating B cells expressing CD20, the faster RTX is consumed [32]. In the same study, total serum IgG level was significantly correlated with a faster clearance of RTX and therefore with lower serum RTX levels, potentially explained by a saturation of the neonatal Fc receptor system that is important in the recycling of circulating IgG. In our study, we observed a small effect (negative correlation) of baseline IgG level on the serum levels of RTX at early (week 2) but not at later time points. Patients included for a relapse of AAV had both lower B cell counts and lower serum IgG levels compared with newly diagnosed patients, both factors potentially explaining the higher RTX levels obtained in those patients. In the context of lymphoma and chronic lymphocytic leukaemia, multiple studies reported that RTX clearance was faster (and therefore RTX serum levels were lower) in patients with high tumour burden represented by the size of the tumour bulk [33] or by the number of leukaemic B cells [34], respectively.
The biological effect of RTX (i.e. the duration of B cell depletion) is associated with its PK in patients with autoimmune diseases, but this association does not translate into an association with clinical outcomes. The current study found a significant association of RTX levels at early time points on the time to B cell redetection: patients with lower serum RTX levels at weeks 2 and 4 experienced a shorter B cell depletion than patients with higher levels. Similarly, in primary SS, serum RTX levels are significantly correlated with the duration of B cell depletion, but not with the drug’s clinical efficacy [35].
That PK-RTX has a clear association with clinical outcomes in patients with lymphoma could be explained by the fact that lower serum RTX levels are measured in patients with a high tumour bulk, who have an intrinsically worse prognosis compared with patients with a low tumour burden [12]. This association between PK-RTX and its efficacy, mediated by the effect of tumour mass on PK-RTX, has also been demonstrated in mouse models of haematological malignancies [36]. This study did not observe any meaningful association between PK-RTX and clinical outcome in AAV, similar to what has been reported previously in patients with RA [13, 14]. RTX induces a complete blood B cell depletion in almost all patients with autoimmune diseases regardless of the RTX serum level obtained. While higher RTX exposure increases the duration of B cell depletion slightly, it does not seem to influence the clinical efficacy. This could indicate that the beneficial effects of RTX in autoimmune diseases are mediated by a broader modulation of the immune system homeostasis than is apparent from the mere destruction of CD20-positive B cells. Since there is no association between PK-RTX and clinical outcomes (i.e. patients reaching lower RTX levels experiencing similar benefits from the drug than patients reaching higher serum levels), our observations do not support the utility of BSA-based individualized dosing protocols as initially developed for the treatment of lymphoma. In most patients who relapse after remission, disease flares are not concomitant with B cell repopulation but happen several months later. This observation could also explain the efficacy of lower RTX doses to prevent relapses in patients in complete remission, as shown for AAV [6] as well as RA [37, 38].
A limitation of our study is that protocol-driven treatment was limited to the first 18 months after remission-induction therapy. Previous studies have shown that many patients will relapse after this period, and as many as 75% of the patients may eventually relapse during the 10-year period following initial diagnosis [39]. We cannot exclude that initial PK-RTX has an influence on B cell subset profile after B cell repopulation, which in turn may influence long-term relapse risk. A recent study suggested that the repopulation of naïve B cells 6 months after RTX infusions was associated with a reduced risk of relapse during the next year in AAV patients [40], and studies in RA and SS have reported that RTX has long-lasting effects on B cell subset profile, for >2 years in some patients, mainly characterized by a decrease in memory B cells compared with pre-treatment values [41–43]. Further studies investigating long-term influence of PK-RTX on B cell subsets and clinical outcomes are warranted.
In summary, studying a well-characterized prospective population of patients with AAV treated with RTX, using well-defined clinical outcomes and two different assays to quantify the drug, we did not detect clinically meaningful associations between PK-RTX and clinical outcomes. The monitoring of RTX levels does not seem useful in this context. Further studies are needed to determine the optimal dose of RTX in AAV, and to define the best timing of retreatment of at-risk patients in remission to prevent relapse, without exposing other patients to unnecessary immunosuppression.
Supplementary Material
Acknowledgements
The rituximab vs cyclophosphamide for ANCA-associated vasculitis (RAVE) trial, from which these data were obtained, was supported by a grant from the National Institute of Allergy and Infectious Diseases to the Immune Tolerance Network (N01-AI-15416; protocol no. ITN021AI). Genentech, Inc. and Biogen IDEC, Inc. provided the study medications and partial funding for the trial. Genentech, Inc. performed the rituximab concentration measurements by ELISA for the trial. D.C. received fellowship grants from the French Society of Rheumatology and from Brest University Hospital, France. U.S.’s laboratory is supported by funds from the Connor Group Foundation and the Mayo Foundation.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: W.S.C. has received support from Biogen for a clinical trial of baminercept therapy for primary Sjogren’s syndrome funded by a grant from National Institutes of Allergy and Infectious Diseases (NIAID), Autoimmunity Centers of Excellence 5U19-AI056363 and consulting fees from Bristol-Myers Squibb (<$5000) and from Abbvie (<$5000). F.F. has received unrestricted research grants from Genentech Inc. the maker of rituximab. P.B. is an employee of Genentech, Inc. U.S. has received consulting fees from Genetech. C.A.L. has received grant/research support from Genentech. M.D.C. is an employee of Genentech, a member of the Roche group. P.A.M. has received, within the past 3 years, funds from consulting and/or research grants from AbbVie, Actelion, Bristol-Myers Squibb, Boeringher-Ingelheim, Celgene, ChemoCentryx, Genentech/Roche, GlaxoSmithKline, InflaRx, Kypha, MedImmune, PrincipioBio, Seattle Genetics and TerumoBCT. R.F.S. has consulted for and received research support from Roche/Genetech. J.H.S. has received grants and research support from Roche/Genentech and performed consulting for Roche/Genentech. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U.. ANCA-associated vasculitis – clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016;12:570–9. [DOI] [PubMed] [Google Scholar]
- 3. Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Unizony S, Villarreal M, Miloslavsky EM. et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis 2016;75:1166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Specks U, Merkel PA, Seo P. et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013;369:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guillevin L, Pagnoux C, Karras A. et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. [DOI] [PubMed] [Google Scholar]
- 7. Ternant D, Bejan-Angoulvant T, Passot C, Mulleman D, Paintaud G.. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet 2015;54:1107–23. [DOI] [PubMed] [Google Scholar]
- 8. Pouw MF, Krieckaert CL, Nurmohamed MT. et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513–8. [DOI] [PubMed] [Google Scholar]
- 9. Jani M, Chinoy H, Warren RB. et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kneepkens EL, Krieckaert CL, van der Kleij D. et al. Lower etanercept levels are associated with high disease activity in ankylosing spondylitis patients at 24 weeks of follow-up. Ann Rheum Dis 2015;74:1825–9. [DOI] [PubMed] [Google Scholar]
- 11. Bejan-Angoulvant T, Ternant D, Daoued F. et al. Brief Report: Relationship between serum infliximab concentrations and risk of infections in patients treated for spondyloarthritis. Arthritis Rheumatol 2017;69:108–13. [DOI] [PubMed] [Google Scholar]
- 12. Berinstein NL, Grillo-Lopez AJ, White CA. et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol 1998;9:995–1001. [DOI] [PubMed] [Google Scholar]
- 13. Breedveld F, Agarwal S, Yin M. et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol 2007;47:1119–28. [DOI] [PubMed] [Google Scholar]
- 14. Thurlings RM, Teng O, Vos K. et al. Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:409–12. [DOI] [PubMed] [Google Scholar]
- 15. Wallace ZS, Miloslavsky EM, Cascino M. et al. Disease activity, glucocorticoid exposure, and rituximab determine body composition changes during induction treatment of ANCA-associated vasculitis. Arthritis Care Res 2017;69:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone JH, Hoffman GS, Merkel PA. et al. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001;44:912–20. [DOI] [PubMed] [Google Scholar]
- 17. Miloslavsky EM, Specks U, Merkel PA. et al. Outcomes of nonsevere relapses in antineutrophil cytoplasmic antibody-associated vasculitis treated with glucocorticoids. Arthritis Rheumatol 2015;67:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miloslavsky EM, Specks U, Merkel PA. et al. Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2014;66:3151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills JR, Cornec D, Dasari S. et al. Using mass spectrometry to quantify rituximab and perform individualized immunoglobulin phenotyping in ANCA-associated vasculitis. Anal Chem 2016;88:6317–25. [DOI] [PubMed] [Google Scholar]
- 20. Mills JR, Barnidge DR, Murray DL.. Detecting monoclonal immunoglobulins in human serum using mass spectrometry. Methods 2015;81:56–65. [DOI] [PubMed] [Google Scholar]
- 21. Fussner LA, Hummel AM, Schroeder DR. et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol 2016;68:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kemna MJ, Damoiseaux J, Austen J. et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol 2015;26:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hilhorst M, Arndt F, Joseph Kemna M. et al. HLA-DPB1 as a risk factor for relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a cohort study. Arthritis Rheumatol 2016;68:1721–30. [DOI] [PubMed] [Google Scholar]
- 24. Pepper RJ, Draibe JB, Caplin B. et al. Association of serum calprotectin (S100A8/A9) level with disease relapse in proteinase 3-antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2017;69:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Reilly VP, Wong L, Kennedy C. et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016;27:2906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JW, Kelley M, King LE. et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J 2011;13:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuang B, King L, Wang HF.. Therapeutic monoclonal antibody concentration monitoring: free or total? Bioanalysis 2010;2:1125–40. [DOI] [PubMed] [Google Scholar]
- 28. Ng CM, Bruno R, Combs D, Davies B.. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol 2005;45:792–801. [DOI] [PubMed] [Google Scholar]
- 29. Muller C, Murawski N, Wiesen MH. et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012;119:3276–84. [DOI] [PubMed] [Google Scholar]
- 30. Cornec D, Tempescul A, Querellou S. et al. Identification of patients with indolent B cell lymphoma sensitive to rituximab monotherapy. Ann Hematol 2012;91:715–21. [DOI] [PubMed] [Google Scholar]
- 31. Lunning MA, Armitage JO.. Rituximab maintenance therapy in diffuse large B-cell lymphoma: is XY the most important variable? Haematologica 2015;100:853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lioger B, Edupuganti SR, Mulleman D. et al. Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol 2017;83:1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tout M, Casasnovas O, Meignan M. et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a LYSA study. Blood 2017;129:2616–23. [DOI] [PubMed] [Google Scholar]
- 34. Tout M, Gagez AL, Lepretre S. et al. Influence of FCGR3A-158V/F genotype and baseline CD20 antigen count on target-mediated elimination of rituximab in patients with chronic lymphocytic leukemia: a study of FILO Group. Clin Pharmacokinet 2017;56:635–47. [DOI] [PubMed] [Google Scholar]
- 35. Cornec D, Costa S, Devauchelle-Pensec V. et al. Blood and salivary-gland BAFF-driven B-cell hyperactivity is associated to rituximab inefficacy in primary Sjogren's syndrome. J Autoimmun 2016;67:102–10. [DOI] [PubMed] [Google Scholar]
- 36. Dayde D, Ternant D, Ohresser M. et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood 2009;113:3765–72. [DOI] [PubMed] [Google Scholar]
- 37. Mariette X, Rouanet S, Sibilia J. et al. Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis 2014;73:1508–14. [DOI] [PubMed] [Google Scholar]
- 38. Bredemeier M, de Oliveira FK, Rocha CM.. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:228–35. [DOI] [PubMed] [Google Scholar]
- 39. Puechal X, Pagnoux C, Perrodeau E. et al. Long-term outcomes among participants in the WEGENT Trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener's) or Microscopic Polyangiitis. Arthritis Rheumatol 2016;68:690–701. [DOI] [PubMed] [Google Scholar]
- 40. Md Yusof MY, Vital EM, Das S. et al. Repeat cycles of rituximab on clinical relapse in ANCA-associated vasculitis: identifying B cell biomarkers for relapse to guide retreatment decisions. Ann Rheum Dis 2015;74:1734–8. [DOI] [PubMed] [Google Scholar]
- 41. Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC.. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:613–20. [DOI] [PubMed] [Google Scholar]
- 42. Pers JO, Devauchelle V, Daridon C. et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjogren's syndrome. Arthritis Rheum 2007;56:1464–77. [DOI] [PubMed] [Google Scholar]
- 43. Roll P, Dorner T, Tony HP.. Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum 2008;58:1566–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.