Abstract
Background
This report assesses the efficacy and safety of palbociclib plus endocrine therapy (ET) in women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer (ABC) with or without visceral metastases.
Patients and methods
Pre- and postmenopausal women with disease progression following prior ET (PALOMA-3; N = 521) and postmenopausal women untreated for ABC (PALOMA-2; N = 666) were randomized 2 : 1 to ET (fulvestrant or letrozole, respectively) plus palbociclib or placebo. Progression-free survival (PFS), safety, and patient-reported quality of life (QoL) were evaluated by prior treatment and visceral involvement.
Results
Visceral metastases incidence was higher in patients with prior resistance to ET (58.3%, PALOMA-3) than in patients naive to ET in the ABC setting (48.6%, PALOMA-2). In patients with prior resistance to ET and visceral metastases, median PFS (mPFS) was 9.2 months with palbociclib plus fulvestrant versus 3.4 months with placebo plus fulvestrant [hazard ratio (HR), 0.47; 95% confidence interval (CI), 0.35–0.61], and objective response rate (ORR) was 28.0% versus 6.7%, respectively. In patients with nonvisceral metastases, mPFS was 16.6 versus 7.3 months, HR 0.53; 95% CI 0.36–0.77. In patients with visceral disease and naive to ET in the advanced disease setting, mPFS was 19.3 months with palbociclib plus letrozole versus 12.9 months with placebo plus letrozole (HR 0.63; 95% CI 0.47–0.85); ORR was 55.1% versus 40.0%; in patients with nonvisceral disease, mPFS was not reached with palbociclib plus letrozole versus 16.8 months with placebo plus letrozole (HR 0.50; 95% CI 0.36–0.70). In patients with prior resistance to ET with visceral metastases, palbociclib plus fulvestrant significantly delayed deterioration of QoL versus placebo plus fulvestrant, whereas patient-reported QoL was maintained with palbociclib plus letrozole in patients naive to endocrine-based therapy for ABC.
Conclusions
Palbociclib plus ET prolonged mPFS in patients with visceral metastases, increased ORRs, and in patients previously treated for ABC, delayed QoL deterioration, presenting a standard treatment option among patients with visceral metastases amenable to endocrine-based therapy.
Clinical trial registration
Keywords: palbociclib, metastatic breast cancer, advanced breast cancer, visceral disease, visceral metastases
Key Message
In joint exploratory subgroup analyses of patients in PALOMA-3 and PALOMA-2, median progression-free survival was significantly prolonged, objective response rate improved, and quality of life deterioration was delayed or maintained in patients with visceral metastases at baseline who were treated with palbociclib combined with endocrine therapy compared with placebo plus endocrine therapy.
Introduction
Many women with hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2−) ABC present with visceral metastases, and their prognosis is generally worse than those without visceral involvement [1]. Endocrine therapy (ET) is an effective strategy to manage HR+/HER2− ABC, and sequential ET is the preferred treatment option for patients without immediately life-threatening diseases or rapidly progressing visceral metastases [2–4]. Chemotherapy should be considered for any symptomatic visceral crisis, but otherwise treatment guidelines recommend three sequential ET regimens until no clinical benefit is evident or symptomatic visceral disease occurs [4], for the purpose of delaying the use of chemotherapy as long as possible. Despite this, many patients with visceral disease who are not in visceral crisis receive chemotherapy as early-line treatment [1, 5], although it may be generally associated with worse tolerability [6] and poorer QoL compared with ET [7].
Significant progress has been made in understanding the molecular signaling pathways that account for endocrine resistance, leading to development of various targeted therapies aimed at preventing or reversing resistance [2]. One such therapy is palbociclib, a first-in-class, selective inhibitor of cyclin-dependent kinases 4 and 6 (CDK 4/6) [8, 9]. Activation of CDK 4/6 promotes cell proliferation, and preclinical studies demonstrated that inhibition of CDK4/6 by palbociclib caused cell-cycle arrest in endocrine-resistant breast cancer cell lines [10]. In addition, combining palbociclib with ET results in synergistic effects [10]. These findings led to the design of clinical studies in which the addition of palbociclib to ET resulted in significantly improved progression-free survival (PFS) in both previously treated (PALOMA-3) and treatment-naive (PALOMA-1 and -2) women with HR+/HER2– ABC [11–14].
When evaluating new therapeutic strategies, it is important to weigh the risk and benefit for high-risk patients, such as those with visceral organ metastases. To this end, we carried out prespecified analyses in the phase III PALOMA-3 and PALOMA-2 studies and compared the efficacy and safety of palbociclib plus ET in patients with visceral versus nonvisceral metastases. These studies enrolled patients with varying degrees of ET responsiveness: premenopausal and postmenopausal women with resistance to prior ET in PALOMA-3, and postmenopausal women still treatment naive for advanced disease in PALOMA-2. Because the prevalence of visceral involvement is higher in patients who progressed on prior ET compared with patients naive to ET in the ABC setting, we first analyzed the PALOMA-3 data and then carried out the same analyses for PALOMA-2.
Methods
Patients and study designs
This analysis includes data from the phase III PALOMA-2 and PALOMA-3 trials. Patients with symptomatic visceral spread who were at risk of life-threatening complications were excluded from both studies [12, 13]. Patients remained on treatment until disease progression, unacceptable toxicity, or consent withdrawal [12, 13]. Detailed descriptions of study methodology have been described elsewhere [12, 13], and are highlighted in further detail in supplementary Table S1, available at Annals of Oncology online.
Both studies were approved by institutional review boards or ethics committees at participating sites and carried out in accordance with the International Harmonisation Good Clinical Practice guidelines and provisions of the Declaration of Helsinki.
End points
The primary end point was investigator-assessed PFS. Secondary end points for efficacy included objective response rate (ORR; defined as confirmed complete or partial response) [13], and time to response (TTR; time to first documented complete or partial response). Other secondary end points included safety and patient-reported outcomes (PROs).
Assessments
In PALOMA-3, visceral metastases referred to lung, liver, brain, pleural, and peritoneal involvement; in PALOMA-2, visceral referred to any lung (including pleura) and/or liver involvement. Lung involvement in PALOMA-3 is defined as the presence of a measurable lesion in the lung, whereas in PALOMA-2 lung involvement includes nonmeasurable pleura and pleural effusion in addition to a measurable lung lesion; therefore, efficacy data for the lung from only the PALOMA-3 study are reported in this study. During the treatment phase of both studies, efficacy was evaluated via tumor assessments carried out every 12 weeks—except in PALOMA-3, scans were every 8 weeks for the first year—from the date of randomization until radiographic/clinical documentation of progressive disease per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria [13]. Safety was assessed at screening, on day 1 of all treatment cycles, on day 14/15 of cycles 1 and 2, at the end of treatment/withdrawal, and post treatment follow-up by evaluating treatment-emergent adverse events (TEAEs) based on incidence, severity (graded by National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0), and the relationship to study drug. PROs were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30) in PALOMA-3 and the Functional Assessment of Cancer Therapy-Breast (FACT-B) in PALOMA-2.
Data analysis
Data cut-off dates were 23 October 2015 for PALOMA-3 (later than 5 December 2014 cut-off date for interim analysis reported by Turner et al. [12]) and 26 February 2016 for PALOMA-2. For efficacy, safety, and PROs, the data were analyzed by baseline metastatic site (visceral and nonvisceral) and treatment arm. Baseline characteristics and efficacy results were reported for patients in the intent-to-treat populations. Median PFS (mPFS) was estimated using the Kaplan–Meier (KM) method. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox proportional hazard model. In this report, the nominal P values for efficacy are presented without adjusting for multiplicity. Odds ratios and their 95% CIs were calculated for confirmed ORRs. A subpopulation treatment effect pattern plot (STEPP) analysis [15] was conducted to graph the treatment effect for PFS (HR) across a continuum of baseline treatment-free intervals (TFIs) of various subgroups in PALOMA-2; TFI was defined as the time from the end of (neo)adjuvant treatment to disease recurrence. TEAEs were reported in the as-treated population using descriptive statistics. Time to deterioration in QoL was analyzed using the KM method and P values were calculated using a one-sided unstratified log-rank test. Overall changes from baseline scores were compared using a repeated measures mixed-effects model with an intercept term, treatment, time, treatment-by-time, and baseline as covariate.
These studies are still on-going to collect survival data.
Results
Patients
The prevalence of visceral metastases was higher in the 304/521 (58.3%) patients with resistance to prior ET in PALOMA-3 than in the 324/666 (48.6%) patients in the PALOMA-2 study naive to ET in the advanced disease setting (Table 1). In PALOMA-3, the most common site for visceral metastases was the liver, 62.5% and 76.9% in the palbociclib plus fulvestrant and placebo plus fulvestrant groups, respectively (Table 1), and 50% and 41% of patients had lung involvement. In PALOMA-2, liver metastases were present in 35.0% and 41.8% of patients in the palbociclib- and placebo-containing treatment arms, respectively. Among patients with nonvisceral disease, 124/217 (57.1%) in PALOMA-3 and 151/342 (44.2%) in PALOMA-2 had bone-only disease. More than 70% of patients in PALOMA-3 with visceral or nonvisceral metastases had received previous chemotherapy for their primary diagnosis (Table 1). Of those patients, more than 30% with visceral metastases and ∼30% with nonvisceral metastases received previous chemotherapy in the advanced setting.
Table 1.
Demographic and baseline characteristics by treatment group for patients with and without visceral metastases
| Characteristics | PALOMA-3a,b |
PALOMA-2a,b |
||||||
|---|---|---|---|---|---|---|---|---|
| PAL + FUL |
PBO + FUL |
PAL + LET |
PBO + LET |
|||||
| Visceral | Nonvisceral | Visceral | Nonvisceral | Visceral | Nonvisceral | Visceral | Nonvisceral | |
| (n = 200) | (n = 147) | (n = 104) | (n = 70) | (n = 214) | (n = 230) | (n = 110) | (n = 112) | |
| Median (range) age, years | 57.0 | 57.0 | 58.5 | 54.0 | 62.0 | 62.0 | 61.0 | 62.0 |
| (30.0–88.0) | (33.0–82.0) | (35.0–80.0) | (29.0–74.0) | (30.0–88.0) | (36.0–89.0) | (28.0–88.0) | (32.0–88.0) | |
| Involved disease sitesc | ||||||||
| Liver | 125 (62.5) | – | 80 (76.9) | – | 75 (35.0) | – | 46 (41.8) | – |
| Lymph node | 97 (48.5) | 41 (27.9) | 39 (37.5) | 24 (34.3) | 125 (58.4) | 87 (37.8) | 65 (59.1) | 45 (40.2) |
| Bone | 136 (68.0) | 128 (87.1) | 76 (73.1) | 54 (77.1) | 126 (58.9) | 199 (86.5) | 73 (66.4) | 89 (79.5) |
| Peritoneum | 1 (<1) | – | 0 | – | 4 (1.9) | – | 0 | – |
| Number of metastatic organ sitesc | ||||||||
| 1 | 17 (8.5) | 94 (63.9) | 11 (10.6) | 49 (70.0) | 17 (7.9) | 121 (52.6) | 6 (5.5) | 60 (53.6) |
| 2 | 63 (31.5) | 32 (21.8) | 37 (35.6) | 14 (20.0) | 63 (29.4) | 54 (23.5) | 26 (23.6) | 26 (23.2) |
| 3 | 58 (29.0) | 15 (10.2) | 27 (26.0) | 6 (8.6) | 66 (30.8) | 46 (20.0) | 41 (37.3) | 20 (17.9) |
| 4 | 43 (21.5) | 3 (2.0) | 20 (19.2) | 0 | 45 (21.0) | 7 (3.0) | 23 (20.9) | 6 (5.4) |
| >4 | 18 (9.0) | 2 (1.4) | 9 (8.7) | 0 | 23 (10.7) | 2 (<1.0) | 14 (12.7) | 0 |
| ECOG performance status | ||||||||
| 0 | 114 (57.0) | 92 (62.6) | 66 (63.5) | 50 (71.4) | 113 (52.8) | 144 (62.6) | 51 (46.4) | 51 (45.5) |
| 1 | 86 (43.0) | 55 (37.4) | 38 (36.5) | 20 (28.6) | 97 (45.3) | 81 (35.2) | 59 (53.6) | 58 (51.8) |
| 2 | – | – | – | – | 4 (1.9) | 5 (2.2) | 0 | 3 (2.7) |
| Prior endocrine therapyd | ||||||||
| Tamoxifen only | 4 (2.0) | 1 (<1.0) | 3 (2.9) | 1 (1.4) | 72 (33.6) | 59 (25.7) | 40 (36.4) | 33 (29.5) |
| Aromatase inhibitor | 23 (11.5) | 21 (14.3) | 9 (8.7) | 7 (10.0) | 14 (6.5) | 19 (8.3) | 8 (7.3) | 20 (17.9) |
| Both tamoxifen and aromatase inhibitor | 96 (48.0) | 63 (42.9) | 50 (48.1) | 31 (44.3) | 38 (17.8) | 40 (17.4) | 15 (13.6) | 10 (8.9) |
| Prior chemotherapye | 149 (74.5) | 103 (70.1) | 81 (77.9) | 56 (80.0) | 106 (49.5) | 107 (46.5) | 60 (54.5) | 49 (43.8) |
| Neoadjuvant | 40 (20.0) | 26 (17.7) | 20 (19.2) | 13 (18.6) | 33 (15.4) | 21 (9.1) | 15 (13.6) | 17 (15.2) |
| Adjuvant | 85 (42.5) | 66 (44.9) | 47 (45.2) | 41 (58.6) | 88 (41.1) | 92 (40.0) | 51 (46.4) | 38 (33.9) |
| Advanced/metastatic setting | 68 (34.0) | 39 (26.5) | 41 (39.4) | 21 (30.0) | – | – | – | – |
| Liver function tests | ||||||||
| AST | ||||||||
| ≤ULN | 143 (71.5) | 124 (84.4) | 69 (66.3) | 60 (85.7) | 171 (79.9) | 197 (85.7) | 92 (83.6) | 103 (92.0) |
| >1× and ≤2× ULN | 47 (23.5) | 20 (13.6) | 24 (23.1) | 4 (5.7) | 35 (16.4) | 31 (13.5) | 15 (13.6) | 9 (8.0) |
| >2× and ≤3× ULN | 6 (3.0) | 3 (2.0) | 5 (4.8) | 2 (2.9) | 6 (2.8) | 2 (0.9) | 3 (2.7) | 0 |
| >3× ULN | 4 (2.0) | 0 | 4 (3.8) | 0 | 2 (0.9) | 0 | 0 | 0 |
| ALT | ||||||||
| ≤ULN | 170 (85.0) | 131 (89.1) | 79 (76.0) | 63 (90.0) | 180 (84.1) | 202 (87.8) | 97 (88.2) | 103 (92.0) |
| >1× and ≤2× ULN | 21 (10.5) | 14 (9.5) | 20 (19.2) | 2 (2.9) | 33 (15.4) | 27 (11.7) | 12 (10.9) | 6 (5.4) |
| >2× and ≤3× ULN | 7 (3.5) | 1 (0.7) | 3 (2.9) | 3 (4.3) | 1 (0.5) | 1 (0.4) | 1 (0.9) | 2 (1.8) |
| >3× ULN | 2 (1.0) | 0 | 2 (1.9) | 0 | 0 | 0 | 0 | 1 (0.9) |
| Alkaline phosphatase | ||||||||
| ≤ULN | 152 (76.0) | 124 (84.4) | 77 (74.0) | 58 (82.9) | 165 (77.1) | 174 (75.7) | 86 (78.2) | 92 (82.1) |
| >1× and ≤2× ULN | 35 (17.5) | 19 (12.9) | 21 (20.2) | 6 (8.6) | 37 (17.3) | 47 (20.4) | 22 (20.0) | 18 (16.1) |
| >2× and ≤3× ULN | 9 (4.5) | 1 (0.7) | 4 (3.8) | 2 (2.9) | 7 (3.3) | 5 (2.2) | 2 (1.8) | 0 |
| >3× ULN | 2 (1.0) | 3 (2.0) | 2 (1.9) | 0 | 5 (2.3) | 4 (1.7) | 0 | 2 (1.8) |
Values are n (%) unless stated otherwise.
Patients with visceral metastases were defined as those reported in the case report forms by the Principle Investigators, not those reported in the IMPALA randomization system. Involved sites include both target and nontarget sites; sites with multiple lesions are counted once.
Patients in PALOMA-2 were treatment-naive for advanced breast cancer.
Data represent prior endocrine therapy for primary diagnosis.
Data represent previous chemotherapy regimen for primary diagnosis.
–, no data; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, Eastern Cooperative Oncology Group; FUL, fulvestrant; LET, letrozole; PAL, palbociclib; PBO, placebo; ULN, upper limit of normal.
In PALOMA-2, approximately half of the patients with visceral or nonvisceral metastases had received chemotherapy previously (Table 1). The majority of patients in both studies had normal liver enzymes at baseline (i.e. aspartate aminotransferase [AST], alanine aminotransferase [ALT], and alkaline phosphatase; Table 1). Less than 2% of patients in both studies had ALT/AST >3 times the upper limit of normal.
Efficacy
PALOMA-3
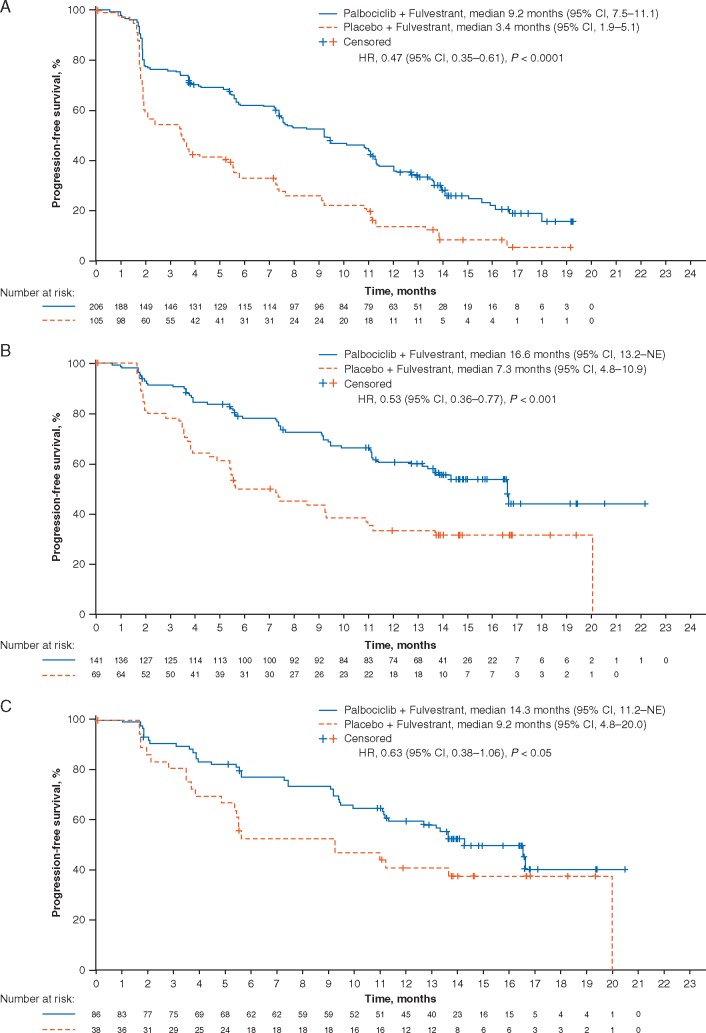
At data cut-off (23 October 2015), mPFS was significantly longer in patients treated with palbociclib plus fulvestrant than with placebo plus fulvestrant in the presence of visceral metastases (9.2 months, 95% CI 7.5–11.1 versus 3.4 months, 1.9–5.1, respectively; HR 0.47; 95% CI 0.35–0.61) and in the absence of visceral metastases (16.6 months, 13.2–NE versus 7.3 months, 4.8–10.9; HR 0.53; 0.36–0.77) (Figure 1A and B; Table 2). In patients with liver metastases, mPFS was significantly longer in the palbociclib plus fulvestrant group versus the placebo plus fulvestrant group (Table 2). In patients with lung metastases, mPFS was significantly longer in the palbociclib plus fulvestrant group versus the placebo plus fulvestrant group (11.1 months, 95% CI 9.3–12.0 versus 3.6 months 2.1–7.2, respectively, HR 0.45 (95% CI 0.29–0.68). In patients with bone-only disease, mPFS was longer with palbociclib plus fulvestrant compared with placebo plus fulvestrant (14.3 months, 95% CI 11.2–NE versus 9.2 months, 4.8–20.0, respectively; HR 0.63; 95% CI 0.38–1.06) (Figure 1C).
Figure 1.
Kaplan–Meier plots of progression-free survival in patients in the PALOMA-3 study with visceral metastases (A), nonvisceral metastases (B), and bone-only disease (C), as well as in patients in the PALOMA-2 study with visceral metastases (D), nonvisceral metastases (E), and bone-only disease (F). HR, hazard ratio; NE, not estimable; NR, not reached.
Table 2.
Median PFS, ORR, and TTR by treatment group for patients with and without visceral metastases
| PALOMA-3 |
PALOMA-2 |
|||
|---|---|---|---|---|
| (N = 521) |
(N = 666) |
|||
| Visceral | Nonvisceral | Visceral | Nonvisceral | |
| PAL + FUL versus PBO + FUL | PAL + FUL versus PBO + FUL | PAL + LET versus PBO + LET | PAL + LET versus PBO+ LET | |
| ITT population, n | 200 versus 104 | 147 versus 70 | 214 versus 110 | 230 versus 112 |
| mPFS, months | 9.2 versus 3.4 | 16.6 versus 7.3 | 19.3 versus 12.9 | NR versus 16.8 |
| HR (95% CI) | 0.47 (0.35–0.61) | 0.53 (0.36–0.77) | 0.63 (0.47–0.85) | 0.50 (0.36–0.70) |
| ORR, % | 28.0 versus 6.7 | 11.6 versus 11.4 | 55.1 versus 40.0 | 30.0 versus 29.5 |
| OR (95% CI) | 5.39 (2.31–14.55) | 1.01 (0.39–2.87) | 1.84 (1.13–3.03) | 1.03 (0.61–1.74) |
| mTTR, months | 3.8 and 3.6 | 3.7 and 3.6 | 4.3 and 5.3 | 2.9 and 5.4 |
| Patients with liver metastases, n | 125 versus 80 | – | 75 versus 46 | – |
| mPFS, months | 7.5 versus 2.4 | – | 13.7 versus 8.4 | – |
| HR (95% CI) | 0.49 (0.36–0.68) | – | 0.62 (0.41–0.95) | – |
| ORR, % | 27.2 versus 3.8 | – | 41.3 versus 37.0 | – |
| mTTR, months | 3.8 and 5.6 | – | 3.0 and 2.9 | – |
| Patients without visceral metastases and excluding bone-only, n | 61 versus 32 | 128 versus 64 | ||
| mPFS, months | – | 16.6 versus 5.6 | – | 27.6 versus 21.9 |
| HR (95% CI) | – | 0.43 (0.24–0.76) | – | 0.65 (0.42–1.03) |
| Patients with visceral metastases and ECOG performance status 0, n | 114 versus 66 | – | 113 versus 51 | – |
| mPFS, months | 9.4 versus 3.6 | – | 19.4 versus 16.4 | – |
| HR (95% CI) | 0.52 (0.37–0.74) | – | 0.89 (0.56–1.40) | – |
| ORR, % | 32.5 versus 10.6 | – | 54.0 versus 47.1 | – |
| Patients with visceral metastases and ECOG performance status 1 or 2, n | 86 versus 38 | – | 101 versus 59 | – |
| mPFS, months | 9.2 versus 2.0 | – | 16.8 versus 11.0 | – |
| HR (95% CI) | 0.37 (0.24–0.57) | – | 0.48 (0.32–0.71) | – |
| ORR, % | 22.1 versus 0 | – | 56.4 versus 33.9 | – |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FUL, fulvestrant; HR, hazard ratio; ITT, intent-to-treat; LET, letrozole; m, median; NR, not reached, not estimable; OR, odds ratio; ORR, overall response rate; PAL, palbociclib; PBO, placebo; PFS, progression-free survival; TTR, time to response.
The ORR was significantly higher in patients with visceral metastases treated with palbociclib plus fulvestrant versus placebo plus fulvestrant (28.0% versus 6.7%, respectively, Table 2). ORRs were significantly higher in patients with liver metastases (27.2% versus 3.8%) (Table 2) and numerically higher in patients with lung metastases (25.0% versus 11.6%) in the palbociclib plus fulvestrant group compared with the placebo plus fulvestrant group. For patients with liver metastases, median TTR (mTTR) was also shorter with palbociclib plus fulvestrant treatment than with placebo plus fulvestrant (3.8 versus 5.6 months) (Table 2) and the mTTR in lung tumoral lesions was 3.9 and 3.6 months, respectively.
PALOMA-2
At data cut-off (26 February 2016), mPFS for patients with visceral metastases was significantly longer in those treated with palbociclib plus letrozole compared with placebo plus letrozole (19.3 months, 95% CI 16.4–22.2 versus 12.9 months, 8.4–16.6, respectively; HR 0.63; 95% CI 0.47–0.85) (Figure 1D). In patients without visceral metastases, mPFS was also significantly longer in patients treated with palbociclib plus letrozole versus those treated with placebo plus letrozole with the mPFS not yet reached for patients in the palbociclib plus letrozole group (Figure 1E). In patients with liver metastases, mPFS was significantly longer in patients treated with palbociclib plus letrozole compared with placebo plus letrozole (13.7 months, 95% CI 10.9–16.6 versus 8.4 months, 5.5–12.9, respectively; HR 0.62; 95% CI 0.41–0.95). For patients with bone-only disease, mPFS was significantly prolonged with palbociclib plus letrozole compared with placebo plus letrozole (not reached, 95% CI 24.8–NE versus 11.2 months, 8.2–22.0, respectively; HR 0.36; 95% CI 0.22–0.59) (Figure 1F).
The ORR was significantly higher in patients with visceral metastases treated with palbociclib plus letrozole compared with placebo plus letrozole (55.1% versus 40.0%, respectively; Table 2). The ORR and mTTR were similar between palbociclib plus letrozole and placebo plus letrozole treatment groups in patients with liver metastases (ORR: 41.3% versus 37.0%, respectively; mTTR: 3.0 and 2.9 months; Table 2).
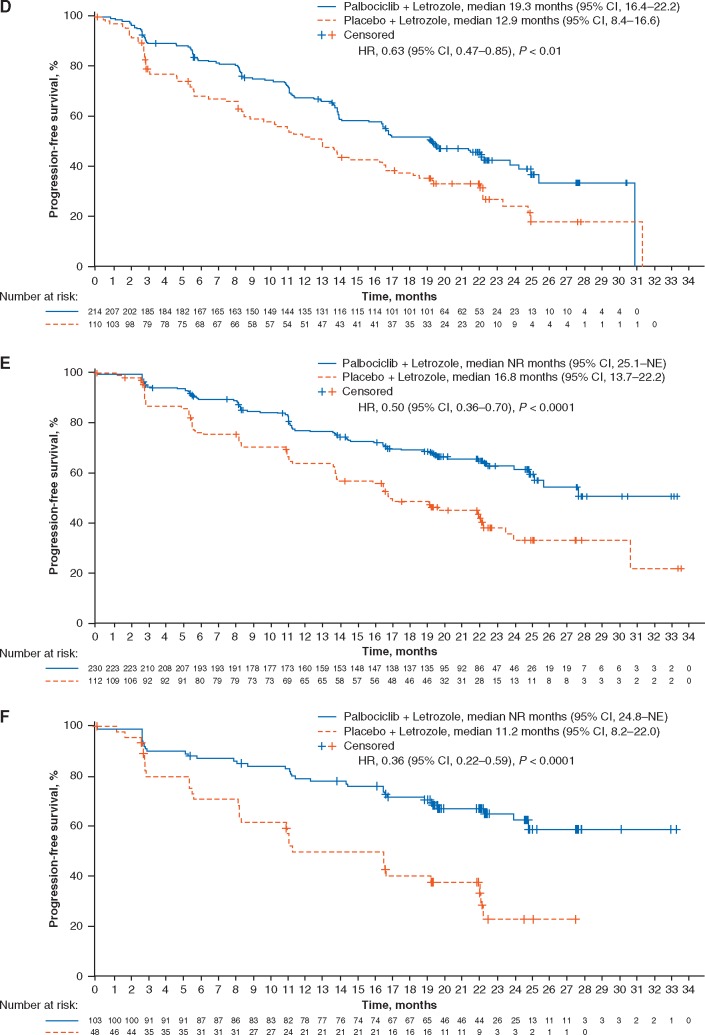
The exploratory STEPP analysis carried out on PALOMA-2 baseline TFI data revealed that the treatment effect on PFS (in terms of HR) remained relatively constant across study subpopulations regardless of the length of baseline TFI (Figure 2).
Figure 2.
STEPP analysis results of the PFS treatment effect and baseline TFI in subpopulations of patients in the PALOMA-2 study who received adjuvant therapy (A), patients who received adjuvant therapy and had visceral metastases (B), and patients who received adjuvant therapy and had nonvisceral metastases (C). TFI, treatment-free interval (for the purposes of this study, TFI was considered equivalent to disease-free interval [prespecified] in the PALOMA-2 study and was defined as the time from the end of [neo]adjuvant treatment to disease recurrence); PFS, progression-free survival; STEPP, subpopulation treatment effect pattern plot.
Patient-reported outcomes
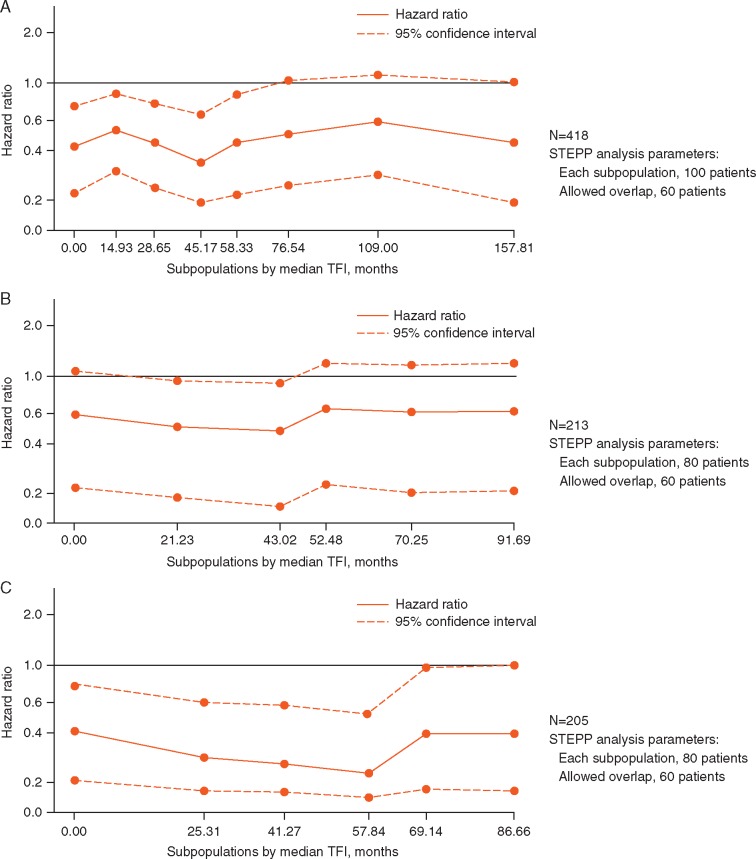
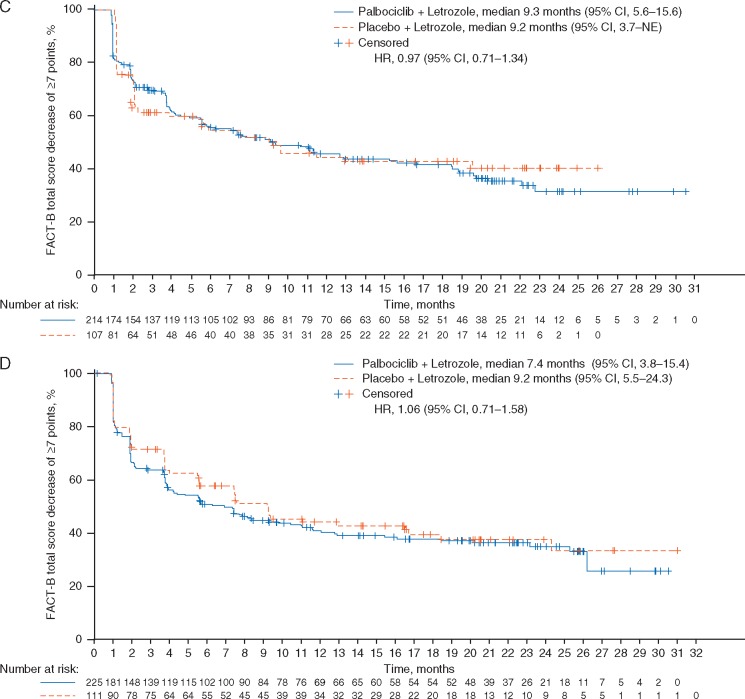
In PALOMA-3, a statistically significantly greater delay in time to deterioration in global QoL was observed in the subgroup with visceral metastases, who were treated with palbociclib plus fulvestrant compared with placebo plus fulvestrant (HR 0.54; P < 0.001; Figure 3A). A similar trend was observed between treatments in patients without visceral metastases but it was not statistically significant (Figure 3B). In patients with visceral metastases, the overall change in QLQ-C30 scales from baseline scores favored the palbociclib plus fulvestrant group for global health/QoL, emotional functioning, role functioning (supplementary Figure S1, available at Annals of Oncology online) and symptoms like pain, nausea and vomiting, and insomnia (all P < 0.05). No significant differences were observed between treatment arms in the nonvisceral metastases group. In PALOMA-2, patient-reported QoL, as assessed by the FACT-B total score, was maintained with the addition of palbociclib to letrozole; no significant differences were observed between treatment groups in the visceral and nonvisceral metastases subgroups (Figure 3C and D). No significant difference in time to deterioration in QoL was observed between treatment arms in the patients with and without visceral metastases.
Figure 3.
Kaplan–Meier plots of time to deterioration of QoL based on the EORTC QLQ-C30 in patients with visceral metastases (A) and in patients without visceral metastases (B) in the PALOMA-3 study and time to deterioration of FACT-B total score in patients with visceral metastases (C) and without visceral metastases (D) in the PALOMA-2 study. EORTC, European Organisation for Research and Treatment of Cancer; HR, hazard ratio; QoL, health-related quality of life; NE, not estimable; NR, not reached; QLQ-C30, EORTC quality-of-life questionnaire.
Safety
In both PALOMA studies, most patients experienced ≥1 TEAE regardless of visceral metastatic status and treatment group (supplementary Table S2, available at Annals of Oncology online), and the rates of TEAEs leading to palbociclib or placebo permanent discontinuation were similarly low between patients with and without visceral metastases (PALOMA-3, all patients ≤6.2%; PALOMA-2, all ≤7%, except 11.3% in nonvisceral patients receiving palbociclib plus letrozole). For palbociclib plus ET, the rate of grade 3 TEAEs >5%, as well as the rates of TEAEs leading to temporary treatment discontinuations or dose reductions in PALOMA-3 and -2 were comparable across groups, despite the presence or absence of visceral metastases (supplementary Tables S2 and S3, available at Annals of Oncology online). Permanent discontinuations due to AEs among all subgroups of patients were low, particularly in relation to reports of neutropenia and regardless of the presence or absence of visceral disease. Among patients with visceral metastases, the most commonly reported grade 3 TEAEs of >5% incidence were neutropenia (54.8%) and leukopenia (43.2%) in patients treated with palbociclib plus fulvestrant in PALOMA-3 and neutropenia (57.5%) and leukopenia (22.4%) in patients treated with palbociclib plus letrozole in PALOMA-2 (supplementary Table S3, available at Annals of Oncology online). The incidence of infections of any grade was similar between visceral and nonvisceral palbociclib-treated patients across both studies in PALOMA-3 (42.2% and 46.6%, respectively) and PALOMA-2 (56.1% and 63.0%) (supplementary Table S3, available at Annals of Oncology online). Infections of any grade in patients who received placebo plus fulvestrant were lower in patients with visceral metastases (28.8%) due to the very short duration of treatment than with nonvisceral metastases (35.3%), and similar to the pattern reported in patients who received placebo plus letrozole (38.2% and 46.4%, respectively) (supplementary Table S3, available at Annals of Oncology online). Within PALOMA-3 and -2, grade 3 and 4 AEs of >5% incidence were similar between patients receiving palbociclib and ET. Serious AEs of >1% incidence for any grade and grades 3 and 4 (palbociclib- or placebo-related) were low in patients receiving palbociclib and ET regardless of visceral metastases, and similarly low between patients treated with placebo plus ET, regardless of visceral metastases (supplementary Table S3, available at Annals of Oncology online).
Discussion
Patients with HR+/HER2– ABC with visceral metastases represent a clinical challenge as their prognosis is generally worse than that of patients without visceral involvement [1]. In this subgroup analysis of patients from PALOMA-3 and PALOMA-2, mPFS was significantly prolonged in patients with and without visceral metastases at baseline when they were treated with palbociclib plus fulvestrant or letrozole compared with placebo plus fulvestrant or letrozole. These results are consistent with the smaller randomized phase II PALOMA-1 study [16]. The percentage of patients with visceral metastases was higher in PALOMA-3 than in PALOMA-2, which is consistent with evidence from clinical practice in which patients receiving later lines of therapy and/or with prior endocrine resistance were more likely to have visceral metastases compared with patients with de novo metastatic disease.
In the PALOMA-2 study, a STEPP analysis did not identify an interaction between the PFS treatment effect and baseline TFI in patients who had received adjuvant therapy and in subgroups based on visceral or nonvisceral metastases. This is in contrast to the results from the double-blind, randomized, phase III MONARCH 3 study, which compared the addition of abemaciclib or placebo with a nonsteroidal aromatase inhibitor to evaluate investigator-assessed PFS, the primary study end point, in postmenopausal women with HR+/HER2− ABC [17]. In the overall population in MONARCH 3, PFS HRs were lower in subpopulations with a baseline TFI <36 months (an adverse prognostic factor) and increased in subpopulations with TFIs >36 months (a more favorable prognostic factor) [18]. In MONARCH 3, TFI, defined as the time from the end of adjuvant ET until informed consent [17], is similar to the definition for TFI in our analysis of PALOMA-2. However, the median follow-up duration was shorter in the MONARCH 3 study than in PALOMA-2, with few progression events in patients with TFI ≥36 months [13, 17]; the difference in findings between the studies is potentially explained by the different durations of follow-up.
In the PALOMA-3 and PALOMA-2 studies, the improvement in PFS with palbociclib plus ET was consistent in patients with bone-only disease at baseline versus placebo plus ET, with substantial absolute benefit from the addition of palbociclib most evident in PALOMA-2 (Figure 1). The addition of abemaciclib to ET also likely provided PFS benefit over endocrine monotherapy in patients with bone-only disease at baseline in the MONARCH 3 study (HR 0.58, 95% CI 0.27–1.25) [17], although the statistical analysis of this result may be affected by the shorter duration of follow-up time.
In the phase III, randomized, double-blind, MONARCH 2 study, 373 out of the 669 (55.8%) women of any menopausal status with HR+/HER2− ABC who had progressed on (neo)adjuvant ET within 12 months from the end of adjuvant ET, or while receiving front-line treatment of metastatic disease, had visceral disease at baseline [19]. The patients in this study were treated with either abemaciclib (150 mg twice daily, on a continuous schedule) plus fulvestrant (500 mg) or placebo plus fulvestrant. Although the patient population in MONARCH 2 had not received prior chemotherapy and were less heavily pretreated than patients in PALOMA-3, investigator-assessed PFS (primary end point) in patients with visceral disease was significantly more favorable following treatment with abemaciclib plus fulvestrant than with ET alone (HR 0.481; 95% CI 0.369–0.627), demonstrating the favorable effect of CDK 4/6 inhibitors across a spectrum of patients [19]. The time to deterioration in global QoL was significantly delayed in patients with visceral metastases who were treated with palbociclib plus fulvestrant compared with the placebo arm.
Chemotherapy is often used in patients with visceral metastases who are not in visceral crisis, despite worse clinical outcomes [20]. A network meta-analysis comparing palbociclib treatment with chemotherapy agents for the treatment of HR+/HER2− ABC showed that palbociclib plus fulvestrant and palbociclib plus letrozole demonstrate a trend in incremental efficacy compared with chemotherapy agents for the first-line and previously treated patients [21]. However, a head-to-head comparison between ET combined with a CDK4/6 inhibitor versus chemotherapy is currently lacking and as such the results of the network meta-analysis reported by Wilson et al. should be interpreted with caution [21]. Our findings highlight the benefit of palbociclib-containing combination therapy for patients with visceral metastases without the cytotoxic effects of chemotherapy. Furthermore, no new safety findings were observed relative to the AE profiles reported in the primary PALOMA-3 and PALOMA-2 studies [12, 13]. Of importance is the analysis of patients with liver metastases. Our studies do not suggest that liver metastases should be treated differently, despite the fact that overall outcomes for such patients may be inferior with respect to the whole study population.
Comparisons with other studies should be interpreted with caution. In the recent phase III FALCON study in endocrine-naive patients with HR+ ABC, patients with visceral metastases treated with fulvestrant had a mPFS that was similar to patients treated with anastrozole (13.8 versus 15.9 months, respectively) [22]. A strategy to improve clinical outcomes in endocrine-naive patients is related to the introduction of targeted therapies, as was evident in PALOMA-2, in which mPFS was significantly prolonged with palbociclib plus letrozole in patients with visceral metastases versus placebo plus letrozole treatment (19.3 versus 12.9 months, respectively). In patients with nonvisceral disease in the FALCON study, mPFS was significantly longer in patients treated with fulvestrant (22.3 months) compared with anastrozole (13.8 months); however, the number of patients with locally advanced stage IIIb or IIIc disease included in that study was not reported [22]. In PALOMA-2, the mPFS of palbociclib plus letrozole has not yet been reached in patients without visceral metastases after a median follow-up of 23 months.
Although the comparisons between patients with and without visceral metastases were prespecified analyses in PALOMA-3 and PALOMA-2, patients with advanced visceral metastases (i.e. patients with symptomatic visceral spread who were at risk of life-threatening complications) were excluded from both studies [12, 13]. Therefore, our findings should not be extrapolated to this subpopulation. Guidelines limit the use of ET to patients without life-threatening visceral metastases; however, our findings lend further support to recommendations for use of ET for the management of HR+/HER2– ABC as initial or salvage therapy, even in the presence of visceral metastases [2–4].
Conclusions
The findings of this prespecified subgroup analysis demonstrate the clinical benefit of palbociclib in combination with ET in patients with HR+/HER2– ABC with visceral metastases. Median PFS and response rate compare favorably with that seen with chemotherapy. The deterioration in symptoms for global QoL assessment were delayed in patients with prior resistance to ET and visceral disease and maintained by the addition of palbociclib to ET in patients with visceral disease who were previously untreated with systemic ET for ABC.
Supplementary Material
Acknowledgements
Editorial support for development of this manuscript was provided by Susan Reinwald, PhD, and Amy Roth Shaberman, PhD, of Complete Healthcare Communications, LLC.
Funding
This study was sponsored by Pfizer. This study was not supported by a grant.
Disclosure
S-AI received honoraria from Novartis and has a consulting/advisory role with Novartis, Hanmi, and Spectrum. MCo received honoraria from Novartis and has a consulting/advisory role with AstraZeneca, Celldex, OBI Pharma, Pfizer, Pierre Fabre, and Puma Biotechnology. MCr received honoraria from Pfizer, Agendia, Celgene, Dompé Farmaceutici, and Pfizer and has a consulting/advisory role with Cynvenio Biosystems, Dompé Farmaceutici, Newomics, and Vortex Biosciences. AD received honoraria from Pfizer and AD’s institution has received research funding from Bayer, Calithera Biosciences, Genentech, GlaxoSmithKline, Incyte, Millennium, Pfizer, Veridex, and Wyeth; JE received honoraria from Pfizer, Novartis, and Roche, has a consulting/advisory role with Pfizer and Novartis Pharma KK, and has served on a speakers’ bureau for Pfizer, Novartis, Roche, and Celgene. KAG has a consulting/advisory role with Pfizer, Novartis, AstraZeneca, NanoString Technologies, and Merck. NCT received honoraria from Pfizer and has a consulting/advisory role with Pfizer and his institution has received research funding from Servier, Pfizer, Lily, Roche, and AstraZeneca. RSF received honoraria and his institution has received funding from Bayer, Pfizer, Bristol-Myers Squibb, Novartis, and Eisai. SM has received honoraria and accommodation and travel expenses from Novartis; SM’s institution has received research funding from Oncothyreon, Pfizer, Novartis, Genentech, Takeda, and Bayer. VD has a consulting/advisory role with Genentech, Lilly, Pfizer, AbbVie, Novartis Pharma KK, and Roche-Peru and has served on a speakers’ bureau for Pfizer, Novartis Pharma KK, and Roche-Peru. OL has nothing to disclose. AM, CHB, CG, DRL, and SI are employees of and own stock in Pfizer.
References
- 1. Harb WA. Management of patients with hormone receptor-positive breast cancer with visceral disease: challenges and treatment options. Cancer Manag Res 2015; 7: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rugo H, Rumble B, Macrae E. et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. JCO 2016; 34: 3069–3103. [DOI] [PubMed] [Google Scholar]
- 3. Cardoso F, Costa A, Senkus E. et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017; 31: 244–259. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 2.2017. https://www.nccn.org/store/login/login.aspx? ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (5 July 2017, date last accessed).
- 5. Lin PL, Hao Y, Xie J. et al. Physician experiences and preferences in the treatment of HR+/HER2− metastatic breast cancer in the United States: a physician survey. Cancer Med 2016; 5(2): 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilcken N, Hornbuckle J, Ghersi D.. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003; (2): CD002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, Zhang J, Jerusalem G.. The association of chemotherapy versus hormonal therapy and health outcomes among patients with hormone receptor-positive, HER2-negative metastatic breast cancer: experience from the patient perspective. Expert Rev Pharmacoecon Outcomes Res 2014; 14(6): 929–940. [DOI] [PubMed] [Google Scholar]
- 8. Fry DW, Harvey PJ, Keller PR. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3: 1427–1438. [PubMed] [Google Scholar]
- 9. Toogood PL, Harvey PJ, Repine JT. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem 2005; 48(7): 2388–2406. [DOI] [PubMed] [Google Scholar]
- 10. Finn R, Dering J, Conklin D. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11(5): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cristofanilli M, Turner NC, Bondarenko I. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17(4): 425–439. [DOI] [PubMed] [Google Scholar]
- 12. Turner NC, Ro J, Andre F. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373(3): 209–219. [DOI] [PubMed] [Google Scholar]
- 13. Finn RS, Martin M, Rugo HS. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375(20): 1925–1936. [DOI] [PubMed] [Google Scholar]
- 14. Finn RS, Crown JP, Lang I. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16(1): 25–35. [DOI] [PubMed] [Google Scholar]
- 15. Bonetti M, Gelber RD.. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Statist Med 2000; 19: 2595–2609. [DOI] [PubMed] [Google Scholar]
- 16. Finn RS, Crown JP, Ettl J. et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 2016; 18(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goetz MP, Toi M, Campone M. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35(32): 3638–3646. [DOI] [PubMed] [Google Scholar]
- 18. Di Leo A, Toi M, Campone M. et al. MONARCH 3: abemaciclib as initial therapy for patients with HR+, HER2- advanced breast cancer. Presented at European Society of Medical Oncology Annual Meeting, 8–12 September 2017; Madrid, Spain. [Google Scholar]
- 19. Sledge GW Jr, Toi M, Neven P. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. JCO 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 20. Lobbezoo DJ, van Kampen RJ, Voogd AC. et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol 2016; 27(2): 256–262. [DOI] [PubMed] [Google Scholar]
- 21. Wilson F, Varu A, Mitra D. et al. Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res Treat 2017; 166(1): 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson JFR, Bondarenko IM, Trishkina E. et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388(10063): 2997–3005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.