Abstract
Background
Homologous recombination deficiency (HRD)-causing alterations have been reported in triple-negative breast cancer (TNBC). We hypothesized that TNBCs with HRD alterations might be more sensitive to anthracycline plus cyclophosphamide-based chemotherapy and report on HRD status and BRCA1 promoter methylation (PM) as prognostic markers in TNBC patients treated with adjuvant doxorubicin (A) and cyclophosphamide (C) in SWOG9313.
Patients and methods
In total, 425 TNBC patients were identified from S9313. HRD score, tumor BRCA1/2 sequencing, and BRCA1 PM were carried out on DNA isolated from formalin-fixed paraffin-embedded tissue. Positive HRD status was defined as either a deleterious tumor BRCA1/2 (tBRCA) mutation or a pre-defined HRD score ≥42. Markers were tested for prognostic value on disease-free survival (DFS) and overall survival (OS) using Cox regression models adjusted for treatment assignment and nodal status.
Results
HRD status was determined in 89% (379/425) of cases. Of these, 67% were HRD positive (27% with tBRCA mutation, 40% tBRCA-negative but HRD score ≥42). HRD-positive status was associated with a better DFS [hazard ratio (HR) 0.72; 95% confidence interval (CI) 0.51–1.00; P = 0.049] and non-significant trend toward better OS (HR = 0.71; 95% CI 0.48–1.03; P = 0.073). High HRD score (≥42) in tBRCA-negative patients (n = 274) was also associated with better DFS (HR = 0.64; 95% CI 0.43–0.94; P = 0.023) and OS (HR = 0.65; 95% CI 0.42–1.00; P = 0.049). BRCA1 PM was evaluated successfully in 82% (348/425) and detected in 32% of cases. The DFS HR for BRCA1 PM was similar to that for HRD but did not reach statistical significance (HR = 0.79; 95% CI 0.54–1.17; P = 0.25).
Conclusions
HRD positivity was observed in two-thirds of TNBC patients receiving adjuvant AC and was associated with better DFS. HRD status may identify TNBC patients who receive greater benefit from AC-based chemotherapy and should be evaluated further in prospective studies.
Clinical Trials Number
Int0137 (The trial pre-dates Clinicaltrial.Gov website establishment)
Keywords: triple negative breast cancer, homologous recombination deficiency (HRD), biomarker, chemotherapy, BRCA mutation
Key Message
Homologous recombination deficiency (HRD) was observed in two-thirds of triple-negative breast cancer (TNBC) patients receiving adjuvant AC chemotherapy and was associated with better outcomes. HRD status may identify TNBC patients who receive greater benefit from anthracycline chemotherapy and should be evaluated further in prospective studies.
Introduction
Adjuvant chemotherapy reduces the risk of distant recurrence and death in patients with triple-negative breast cancer (TNBC). Even so, approximately 20%–40% of patients with early stage TNBC develop metastatic disease [1–3]. The dearth of reliable response/resistance biomarkers for standard chemotherapy has slowed the development of newer agents for TNBC. Ideally, robust tumor biomarker tests would provide insight into which TNBC patients are likely to do well with anthracycline/cyclophosphamide (AC)-based adjuvant chemotherapy or, alternatively, may provide insight into mechanisms of resistance to this strategy and identification of alternative treatment approaches.
Homologous recombination (HR) is a DNA repair mechanism responsible for repair of double-strand breaks (DSBs). BRCA1/2 genes, along with other Fanconi anemia (FA) pathway genes (RAD51D, NBN, ATM, etc.), are key components of HR-mediated DNA repair. Germline BRCA1/2 mutations are prototypic molecular alterations that confer HR deficiency (HRD) and sensitivity to DNA-damaging therapy [4, 5].
Inherited and acquired defects in HR might serve as response biomarkers or as therapeutic targets in breast cancer. To this end, development and clinical evaluation of platforms to identify HR defects are of interest, especially in TNBC, as this subtype is considered enriched for HR pathway deficiency [6–8]. Approximately 10%–20% of TNBC patients harbor germline BRCA1/2 mutations, and another 3%–5% demonstrate somatic BRCA1/2 mutations [9, 10]. However, DNA repair capacity in the tumor may be altered through other mechanisms, such as somatic or germline mutation in other FA pathway genes, DNA methylation or attenuated mRNA expression. Hypermethylation of the BRCA1 promoter has been proposed as one of the mechanisms for silencing BRCA1 expression in sporadic TNBC, and this epigenetic inactivation of BRCA1 is associated with a gene expression profile similar to that of inherited BRCA1 mutation-associated breast cancer [9, 11–13]. Employing more global measures, rather than relying on documented changes in specific genes, may identify more patients with HR deficiency. The HRD score is an algorithmic assessment of three measures of tumor genomic instability (loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions) [6, 14, 15]. High HRD scores have been shown to be significantly associated with defects in BRCA1/2 and are associated with sensitivity to neoadjuvant platinum-based chemotherapy in TNBC [6, 16, 17].
We postulated that DNA repair deficiency phenotype can be caused and measured in different ways and could affect response to DNA-damaging or repair-inhibiting therapies like doxorubicin (which induces DNA DSBs) and cyclophosphamide (an alkylating agent which causes DNA crosslinks leading to DSBs). In this study, we sought to determine whether the HRD score and other related markers are prognostic in early stage TNBC patients who participated in SWOG adjuvant trial S9313 (Intergroup Protocol 0137). We hypothesized that HRD status and BRCA1 promoter methylation (PM) would be prognostic in TNBC patients treated with adjuvant AC.
Methods
Patients
Patient selection, assay performance, and data analysis are reported according to the REMARK criteria [18]. Breast tumor specimens prepared from paraffin blocks collected prospectively from S9313 participants were used for this study. In S9313, patients with either high-risk node-negative or low-risk node-positive breast cancer were randomly assigned to one of two equivalent dose schedules of doxorubicin (A) and cyclophosphamide (C) chemotherapy [19]. There was no difference in disease-free survival (DFS) or overall survival (OS) for patients treated on the two arms [19]. Details of the study population and treatment schedule are provided in the supplementary, available at Annals of Oncology online.
Estrogen receptor (ER) and progesterone receptor (PR) were determined both locally and centrally (Allred scoring method; ER and PR Allred score of 0 was considered negative). Human epidermal growth factor receptor 2 (HER2) was determined centrally by FISH and immunohistochemistry [20]. TNBC was defined as ER- and PR negative (on both local and central review) and HER2-negative in accordance with the 2013 ASCO-CAP HER2 testing guidelines [21]. Laboratories performing the biomarkers were blinded to patient clinical and outcome information.
Tissue processing
Genomic DNA and RNA were isolated using standard techniques and commercially available kits in research laboratory according to CLIA protocol (described in supplementary, available at Annals of Oncology online).
HRD status
Custom enrichment panel and next-generation sequencing were used to generate genome-wide single nucleotide polymorphism profiles from which the three components of the HRD score are calculated [6]. The panel also includes probes targeting the complete coding region of BRCA1 and BRCA2. A detailed description of the assay panel design, sequence alignment, and mutation detection methods has been published previously [6]. Mutations were only included in the analysis if classified as deleterious or suspected deleterious. HRD status was classified as positive if there was either a mutated tumor BRCA1/2 or a pre-defined HRD score ≥ 42 [17] HRD was classified as negative if HRD score was < 42 and tumor lacked deleterious BRCA1/2 mutation. HRD status could not be determined if HRD assay failed and tumor BRCA1/2 analysis was either negative or failed [17]. Additional details are provided in the supplementary Material, available at Annals of Oncology online.
BRCA1 PM
BRCA1 PM was assessed following bisulfite conversion of genomic DNA followed by methylation-specific PCR and agarose electrophoresis as described previously [13]. The presence of a methylated band was recorded as ‘positive’ for BRCA1 PM.
BRCA1 gene expression
BRCA1 expression was measured using NanoString Technologies gene expression assays, following the manufacturer’s protocol.
Statistical analyses
Disease-free survival was defined as the time from registration to first invasive recurrence (local, regional, or distant), to new primary cancer in the contralateral breast, or to death due to any cause. OS was defined as time from registration to death from any cause. Patients were censored on the date of last contact if an event had not been observed. Survival curves were assessed by the Kaplan–Meier method and unadjusted survival comparisons conducted using log-rank tests. The markers were tested for prognostic effect on DFS and OS using a Cox regression model with adjustment for randomized treatment assignment and nodal status. All reported P-values and confidence intervals (CIs) are from two-sided tests.
Results
Identification of the study population
The selection process of the 425 TNBC samples from S9313 is provided in supplementary Figure S1, available at Annals of Oncology online. We have reported previously that the DFS and OS for patients with and without archived tissue specimens were similar in this trial [22].
Patient demographics
Demographic and clinical characteristics of the 425 TNBC patients are described in supplementary Table S1, available at Annals of Oncology online. Median age was 45 years, and 33% had lymph node-positive disease. At a median follow-up of 12.6 years, there were 166 DFS and 129 OS events.
Biomarker results availability
HRD status, which depends on both BRCA mutation status and HRD score, could be determined in 89% (379/425) of patients. BRCA1 PM results were determined in 82% (348/425) of patients (see supplementary Figure S2, available at Annals of Oncology online, for details). There was no difference in DFS by HRD status known or not known (log-rank P = 0.97) or by BRCA1 PM status known or unknown (P = 0.86). Similarly, there was no difference in OS by HRD status known or not known or by BRCA1 PM status known or not known (P = 0.75 for both).
Association of HRD status with outcome
For patients with available HRD status results, 27% (105/379) demonstrated tumor BRCA mutation (BRCA1 = 81, BRCA2 = 23, BRCA1 and BRCA2 = 1) and another 40% (150/379) demonstrated HRD score ≥42 with wild-type BRCA1/2. Taken together, 67% (255/379) of patients had positive HRD status (HRD score ≥42 or presence of tumor BRCA mutation), and 33% (124/379) of patients had negative HRD status (HRD score <42 and absence of tumor BRCA mutation).
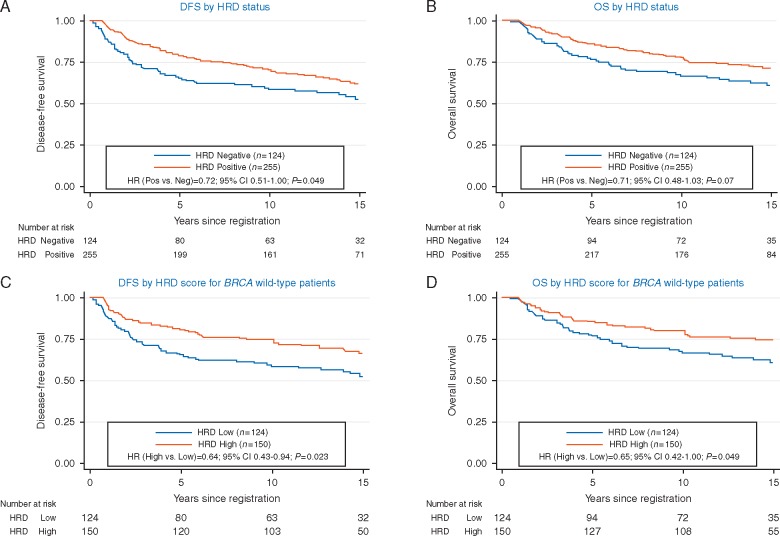
Positive HRD status was associated with a better DFS [hazard ratio (HR) = 0.72; 95% CI 0.51–1.00, P = 0.049] and non-significant trend toward better OS (HR = 0.71; 95% CI 0.48–1.03, P = 0.073), adjusting for treatment arms and nodal status (Table 1 and Figure 1A and B). We also considered whether the association of HRD status with outcomes was constant over the follow-up period. A test of the proportional hazards assumption of the Cox model suggested that all three covariate HRs (HRD status, treatment effect, and nodal status) varied over the long follow-up period. Restricting follow-up to the first 5 years showed a stronger impact of HRD status on DFS (HR = 0.57; 95% CI 0.38–0.85, P = 0.006) and non-significant trend toward better OS (HR = 0.63; 95% CI 0.38–1.03, P = 0.064). After the first 5 years, there was little impact of HRD status on DFS (HR = 1.21; 95% CI 0.65–2.28, P = 0.55) and OS (HR = 0.85; 95% CI 0.47–1.53, P = 0.59). Thus, the prognostic effect of HRD status in TNBC appeared to be more pronounced in the first 5 years.
Table 1.
Biomarkers and outcomes
| Biomarker | n (%) | 5-year DFS (95% CI) | 5-year OS (95% CI) | 10-year DFS (95% CI) | 10-year OS (95% CI) |
|---|---|---|---|---|---|
| HRD status (N = 379) | |||||
| Negativea | 124 (33) | 65.2% (56.0% to 72.8%) | 76.5% (68.0% to 83.0%) | 58.3% (49.0% to 66.5%) | 66.4% (57.2% to 74.0%) |
| Positiveb | 255 (67) | 78.7% (73.2% to 83.3%) | 85.8% (80.9% to 89.6%) | 70.5% (64.4% to 75.7%) | 77.5% (71.8% to 82.3%) |
| HRD score in BRCA1/2 wild-type (N = 274) | |||||
| <42 | 124 (45) | 65.2% (56.0% to 72.8%) | 76.5% (68.0% to 83.0%) | 58.3% (49.0% to 66.5%) | 66.4% (57.2% to 74.0%) |
| ≥42 | 150 (55) | 80.5% (73.2% to 86.1%) | 85.2% (78.5% to 90.0%) | 74.4% (66.6% to 80.7%) | 79.7% (72.2% to 85.3%) |
| BRCA1 PM (N = 348) | |||||
| Present | 111 (32) | 76.4% (67.3% to 83.3%) | 80.9% (72.3% to 87.1%) | 70.8% (61.3% to 78.4%) | 77.1% (68.0% to 83.9%) |
| Absent | 237 (68) | 73.8% (67.7% to 78.9%) | 82.6% (77.2% to 86.9%) | 64.5% (58.0% to 70.3%) | 72.2% (65.9% to 77.5%) |
CI, confidence interval; DFS, disease-free survival; HRD, homologous recombination deficiency; OS, overall survival.
aHRD negative status=HRD score < 42 and absence of tumor BRCA mutation.
bHRD positive status=HRD score ≥ 42 or presence of tumor BRCA mutation.
Figure 1.
(A) Disease-free survival (DFS) by homologous recombination deficiency (HRD) status. (B) Overall survival (OS) by HRD status. (C) DFS by HRD score for BRCA wild-type patients. (D) OS by HRD score for BRCA wild-type patients.
Association of HRD score with outcome in patients with BRCA1/2 wild-type tumors
Of the 274 patients with BRCA1/2 wild-type tumors and known HRD score, 55% (150/274) demonstrated HRD score of ≥42. High HRD score (≥42) in patients with BRCA1/2 wild-type tumors was associated with better DFS (HR = 0.64; 95% CI 0.43–0.94; P = 0.023) and OS (HR = 0.65; 95% CI 0.42–1.00; P = 0.049), adjusting for treatment and nodal status (Figure 1C and D and Table 1).
Association of tumor mutation status with outcome
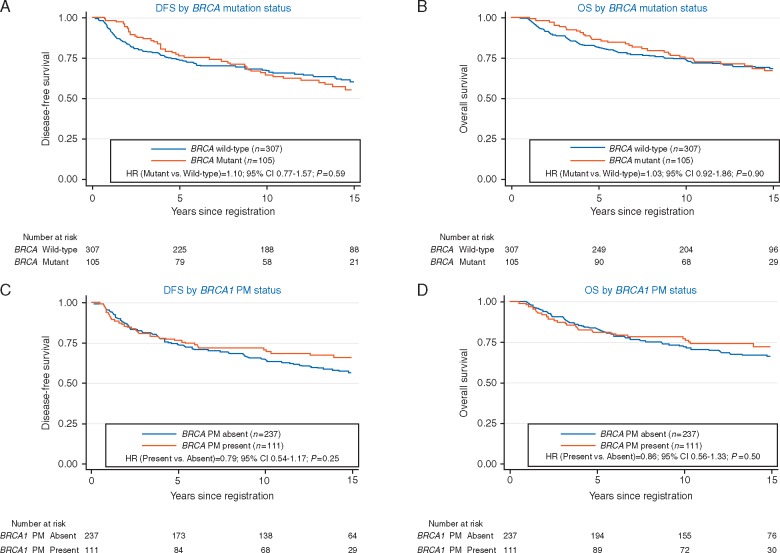
Tumor BRCA mutation status was positive in 25% (105/412) of patients. Tumor BRCA mutation status (mutant or wild-type) did not impact DFS (P = 0.59) or OS (P = 0.90), adjusting for nodal status and treatment (Figure 2A and B).
Figure 2.
(A) Disease-free survival (DFS) by BRCA mutation status. (B) Overall survival (OS) by BRCA mutation status. (C) DFS by BRCA1 promoter methylation (PM) status. (D) OS by BRCA1 PM status. BRCA mutation information was available for 312 patients, and BRCA1 PM was available for 348 subjects.
Association of BRCA1 PM with outcome
The presence of BRCA1 PM was detected in 32% (111/348) of patients. Although the DFS HR for BRCA1 PM was similar to that for HRD, it was not statistically significant (HR = 0.79; P = 0.25). OS had similar results (Figure 2C and D).
Association of BRCA1 PM with BRCA1 mRNA expression
BRCA1 mRNA expression results were available for 396/425 (87%) samples, and both BRCA1 PM and BRCA1 expression data were available from 330 samples. As expected, the presence of BRCA1 PM was associated with lower BRCA1 transcript expression (Wilcoxon P < 0.0001) (supplementary Figure S3, available at Annals of Oncology online).
Association of HRD score with tumor BRCA1/2 mutation and BRCA1 promoter methylation
Supplementary Table S2, available at Annals of Oncology online, provides the overlap between HRD score, BRCA1 PM, and tumor BRCA mutation. Compared with tumors without BRCA1/2 mutation, tumors with BRCA1/2 mutation demonstrated higher HRD scores (median HRD score was 61 for tumors with BRCA mutation vs. 47 for tumors without BRCA mutation, P < 0.0001) (supplementary Figure S4A, available at Annals of Oncology online). Similarly, compared with tumors without BRCA1 PM, tumors with BRCA1 PM demonstrated higher HRD scores (median HRD score 66 for tumors with BRCA1 PM versus 43 for tumors without BRCA1 PM, P < 0.0001) (supplementary Figure S4B, available at Annals of Oncology online). BRCA1/2 mutation and BRCA1 PM collectively accounted for 83% (187/255) of patients with positive HRD status. There was very little overlap between BRCA1/2 mutation and BRCA1 PM. Out of 346 samples for which both BRCA1/2 mutation and BRCA1 PM data were available, only 3% (n = 11) demonstrated both mutation and methylation (all 11 demonstrated HRD score ≥ 42).
Discussion
In this study, we observed that two-thirds of TNBC patients treated with adjuvant AC in S9313 exhibited HRD positivity (based on the HRD score and tumor BRCA mutation). Patients with positive HRD status had better 10-year DFS compared to those with negative HRD status (HR = 0.72). The prognostic impact of HRD status was independent of nodal status and seemed to be more pronounced for the first 5 years (5-year DFS HR = 0.57). We further observed high HRD score (≥42) in more than half (55%) of tBRCA wild-type patients, and high HRD score in these patients was independently associated with better DFS (HR = 0.64) and OS (HR = 0.65), thus confirming that HR deficiency mediated by mechanisms other than BRCA mutation is present in a substantial proportion of TNBC and is likely to be biologically, and perhaps clinically, important.
Tumor BRCA1/2 mutation was noted in 25% of our cohort. Due to lack of availability of germline DNA, we could not determine whether these mutations were germline or somatic in nature. However, this BRCA mutation prevalence is consistent with known literature. Previous studies have demonstrated germline BRCA1/2 mutations in 15%–20% and somatic BRCA1/2 mutations in 3%–5% of unselected TNBC [9, 10, 23]. Tumor BRCA mutation status was not prognostic in our cohort, (perhaps due to relatively modest number of patients with BRCA mutation), a finding which is also consistent with previous studies [24, 25]. Given the prevalence of BRCA mutation, this patient cohort was probably at high risk for other BRCA-related cancers. However, information on contralateral breast cancer or other non-breast cancer (e.g. ovarian) related deaths are not currently available, thus we cannot comment on the potential impact of these events on long-term DFS.
BRCA1 PM was associated with lower BRCA1 mRNA expression (corresponding to epigenetic silencing of BRCA1 gene) and was associated with higher HRD scores. Several prior studies have evaluated BRCA1 PM in TNBC patients treated with various chemotherapy regimens, showing conflicting prognostic impact [13, 26, 27]. In this large, uniformly treated TNBC patient population, we did not observe a prognostic impact of BRCA1 PM on outcome. There was a notable lack of overlap between BRCA mutation and BRCA1 PM, supporting the notion that mechanisms of gene function loss appear to be non-redundant and invoke the principle of complementarity.
In an exploratory analysis, the combined effect of BRCA mutation and BRCA1 PM on outcome was not found to be prognostic (data not shown). Thus, HRD score/status continues to be a more robust prognostic factor even if BRCA mutation and methylation status are known, indicating that BRCA mutation and methylation do not capture all of the patients with HR deficiency.
These data were derived from a mature, prospective randomized clinical trial, obviating concerns about bias in outcome ascertainment. Further, our results demonstrate that formalin-fixed paraffin-embedded tumor tissue collected > 20 years ago as part of an intergroup trial can be successfully used for DNA- and RNA-based biomarkers. However, the study does have certain limitations. All patients received adjuvant AC chemotherapy, without an untreated or alternatively treated comparator arm. Thus, we cannot determine whether HRD is prognostic in spite of or predictive of benefit from AC chemotherapy. Furthermore, we cannot remark on whether HRD would predict benefit from taxanes, which are currently part of all standard neo/adjuvant chemotherapy for breast cancer. Recent data do suggest that positive HRD status is associated with improved pathological complete response to neoadjuvant anthracycline plus taxane chemotherapy and also to platinum-based chemotherapy [17, 28]. Although we show that the prognosis of patients with high HRD is superior to those with low HRD, currently there are insufficient data to with hold or select other treatments, such as taxanes or platinum agents, based on HRD status.
In summary, HRD status is prognostic in TNBC patients who were uniformly treated with AC chemotherapy. The clinical utility of HRD in the presence of DNA-damaging therapy like anthracyclines, platinum salts, and poly(ADP-ribose) polymerase (PARP) inhibitors is the subject of ongoing investigations. Neoadjuvant clinical trials (NCT01982448 and NCT02032277) are evaluating the ability of this HRD assay to predict pathological complete response with platinum, taxane, or AC/taxane chemotherapy in TNBC. SWOG S1416 (NCT02595905) is using multiple HRD biomarkers to predict benefit from addition of a PARP inhibitor to platinum chemotherapy in metastatic TNBC. Our study demonstrates the clinical validity of the HRD assay; additional studies are warranted to further refine and establish the clinical utility of HR deficiency in TNBC [29].
Supplementary Material
Acknowledgement
The authors wish to gratefully acknowledge the late Dr Robert Livingston for his important contributions to SWOG and to study S9313.
Funding
National Cancer Institute/National Clinical Trials Network grants U10CA180888, U10CA180819, U10CA180801, U10CA180858 and in part by Amgen, American Society of Clinical Oncology Advanced Clinical Cancer Research Award by Conquer Cancer Foundation (PS), the Eileen Stein Jacoby Fund, University of Kansas Cancer Center's Cancer Center Support Grant (P30 CA168524) Biospecimen Repository Core Facility, Breast Cancer Research Foundation (DFH and PS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
DW reports employment by and ownership interest (stock) in Myriad Genetics, LLC. RW reports employment by, ownership interest (stock) in, and a leadership role in Myriad Genetics, LLC. KMT reports employment by, ownership interest (stock) in, and patents, royalties, other Intellectual Property, and other expenses from Myriad Genetics, LLC. A-RH reports employment by and ownership interest (stock) in Myriad Genetics, LLC. All remaining authors have declared no conflicts of interest.
References
- 1. Liedtke C, Mazouni C, Hess KR. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26(8): 1275–1281. [DOI] [PubMed] [Google Scholar]
- 2. Haffty BG, Yang Q, Reiss M. et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. JCO 2006; 24: 5652–5657. [DOI] [PubMed] [Google Scholar]
- 3. Tan DS, Marchio C, Jones RL. et al. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat 2008; 111(1): 27–44. [DOI] [PubMed] [Google Scholar]
- 4. Isakoff SJ, Goss PE, Mayer EL. et al. Impact of BRCA1/2 mutation status in TBCRC009: A multicenter phase II study of cisplatin or carboplatin for metastatic triple negative breast cancer. Cancer Res 2012; 72(24 Suppl): 140s–141s. [Google Scholar]
- 5. Tutt A, Robson M, Garber JE. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376(9737): 235–244. [DOI] [PubMed] [Google Scholar]
- 6. Timms KM, Abkevich V, Hughes E. et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 2014; 16(6): 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma P, Stecklein SR, Kimler BF. et al. Efficacy of neoadjuvant carboplatin/docetaxel chemotherapy in sporadic and BRCA-associated triple-negative breast cancer (TNBC). J Clin Oncol 2014; 32(5s):abstr 1022. [Google Scholar]
- 8. Watkins J, Weekes D, Shah V. et al. Genomic complexity profiling reveals that HORMAD1 overexpression contributes to homologous recombination deficiency in triple-negative breast cancers. Cancer Discov 2015; 5(5): 488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma P, Klemp JR, Kimler BF. et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat 2014; 145(3): 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedenfalk I, Duggan D, Chen Y. et al. Gene-expression profiles in hereditary breast cancer. N Engl J Med 2001; 344(8): 539–548. [DOI] [PubMed] [Google Scholar]
- 12. Esteller M, Silva JM, Dominguez G. et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst 2000; 92(7): 564–569. [DOI] [PubMed] [Google Scholar]
- 13. Sharma P, Stecklein SR, Kimler BF. et al. The prognostic value of promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res 2014; 3(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abkevich V, Timms KM, Hennessy BT. et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer 2012; 107(10): 1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birkbak NJ, Wang ZC, Kim JY. et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov 2012; 2(4): 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Telli ML, Jensen KC, Vinayak S. et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. JCO 2015; 33: 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Telli ML, Timms KM, Reid J. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 2016; 22(15): 3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McShane LM, Altman DG, Sauerbrei W. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97(16): 1180–1184. [DOI] [PubMed] [Google Scholar]
- 19. Linden HM, Haskell CM, Green SJ. et al. Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). JCO 2007; 25: 656–661. [DOI] [PubMed] [Google Scholar]
- 20. Tubbs R, Barlow WE, Budd GT. et al. Outcome of patients with early-stage breast cancer treated with doxorubicin-based adjuvant chemotherapy as a function of HER2 and TOP2A status. JCO 2009; 27: 3881–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolff AC, Hammond MEH, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol 2013; 31(31): 3997–4013. [DOI] [PubMed] [Google Scholar]
- 22. Porter PL, Barlow WE, Yeh IT. et al. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst 2006; 98(23): 1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Couch FJ, Hart SN, Sharma P. et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. JCO 2015; 33: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C. et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 2007; 43(5): 867–876. [DOI] [PubMed] [Google Scholar]
- 25. Robson ME, Chappuis PO, Satagopan J. et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 2004; 6: R8–R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Diao L, Chen Y. et al. Promoter methylation of BRCA1 in triple-negative breast cancer predicts sensitivity to adjuvant chemotherapy. Ann Oncol 2013; 24(6): 1498–1505. [DOI] [PubMed] [Google Scholar]
- 27. Ignatov T, Poehlmann A, Ignatov A. et al. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat 2013; 141(2): 205–212. [DOI] [PubMed] [Google Scholar]
- 28. von Minckwitz G, Timms K, Untch M.. Prediction of pathological complete response (pCR) by Homologous Recombination Deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: Results from GeparSixto. J Clin Oncol 2015; 33: 1004. [Google Scholar]
- 29. Simon RM, Paik S, Hayes DF.. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009; 101(21): 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.