Abstract
Objectives
Excessive bone formation is an important hallmark of AS. Recently it has been demonstrated that axial bony lesions in AS patients can be visualized using 18F-fluoride PET-CT. The aim of this study was to assess whether 18F-fluoride uptake in clinically active AS patients is related to focal bone formation in spine biopsies and is sensitive to change during anti-TNF treatment.
Methods
Twelve anti-TNF-naïve AS patients [female 7/12; age 39 years (SD 11); BASDAI 5.5 ± 1.1] were included. 18 F-fluoride PET-CT scans were performed at baseline and in two patients, biopsies were obtained from PET-positive and PET-negative spine lesions. The remaining 10 patients underwent a second 18F-fluoride PET-CT scan after 12 weeks of anti-TNF treatment. PET scans were scored visually by two blinded expert readers. In addition, 18F-fluoride uptake was quantified using the standardized uptake value corrected for individual integrated whole blood activity concentration (SUVAUC). Clinical response to anti-TNF was defined according to a ⩾ 20% improvement in Assessment of SpondyloArthritis international Society criteria at 24 weeks.
Results
At baseline, all patients showed at least one axial PET-positive lesion. Histological analysis of PET-positive lesions in the spine confirmed local osteoid formation. PET-positive lesions were found in the costovertebral joints (43%), facet joints (23%), bridging syndesmophytes (20%) and non-bridging vertebral lesions (14%) and in SI joints (75%). After 12 weeks of anti-TNF treatment, 18F-fluoride uptake in clinical responders decreased significantly in the costovertebral (mean SUVAUC −1.0; P < 0.001) and SI joints (mean SUVAUC −1.2; P = 0.03) in contrast to non-responders.
Conclusions
18F-fluoride PET-CT identified bone formation, confirmed by histology, in the spine and SI joints of AS patients and demonstrated alterations in bone formation during anti-TNF treatment.
Keywords: ankylosing spondylitis, positron emission tomography, magnetic resonance imaging, bone biopsy, anti-TNF, treatment monitoring
Rheumatology key messages
18F-fluoride PET-CT visualizes bone formation in AS patients supported by histological evaluation of bone biopsies.
A significant reduction in 18F-fluoride uptake was found in the spine and SI joints of clinical responders.
18F-fluoride PET has the potential to assess short-term bone formation changes during therapy in AS.
Introduction
A hallmark of AS is new bone formation, resulting in the formation of syndesmophytes in the vertebral column and ankylosis of the SI joints and causing functional disability [1, 2]. Although anti-TNF treatment successfully decreases inflammatory activity in AS and recent studies support an inhibitory effect on bone formation, data on the inhibition of bone formation are controversial [3–5].
To date, conventional X-rays still play an important role in determining and monitoring structural bone damage in AS patients, but irreversible changes are only visible after about 5 years of complaints [6, 7]. The highest sensitivity for imaging structural bone changes is provided by CT, in particular high-resolution CT (HRCT). Recently HRCT revealed progression of osteophytes in SpA and PsA patients despite treatment with MTX or anti-TNF [8]. However, a major limitation of HRCT is its limited field of view, which only allows for evaluation of part of the axial skeleton within a single imaging session.
In contrast to CT, PET allows for whole-body imaging of changes in tissue function at a picomolar level [9]. Potentially PET can be used for early detection of disease activity, even before anatomic changes appear. In addition, this technique makes it possible to quantify disease activity, which is essential for monitoring therapeutic effects [10, 11]. Recently it has been demonstrated that PET-CT using the bone tracer 18F-fluoride was able to identify multiple lesions in the spine and SI joints of clinically active AS patients [12–16]. For imaging AS disease activity, 18F-fluoride performed better than either 18F-fluorodoexyglucose (FDG) or the macrophage tracer (R)-[11C]PK11195 [12]. 18F-fluoride uptake in bone reflects regional osteoblastic activity, as it is taken up by hydroxyapatite crystals that make up the mineral fluoroapatite within bone, especially at sites of bone remodelling [17, 18]. As such, 18F-fluoride PET visualizes sites of bone formation and may offer new perspectives of imaging disease activity and structural damage in AS as compared with MRI and conventional X-ray examinations. Pilot data have suggested that 18F-fluoride PET visualizes more lesions in the axial skeleton of AS patients than MRI [12]. In addition, as a result of its high sensitivity, 18F-fluoride uptake was seen in anterior vertebral corners 2 years before syndesmophytes were observed on conventional radiographs [14, 19].
The aims of this study were to assess the specificity of bone formation imaging by comparing 18F-fluoride PET accumulation in bone with histological data obtained from CT-guided biopsies of the vertebral column. Secondly, to investigate whether bone formation before and after 12 weeks of anti-TNF therapy can be detected using 18F-fluoride PET-CT.
Methods
Patients and study design
Between 2013 and 2015, 12 AS patients (between 18 and 70 years of age) who fulfilled the modified New York criteria [20] were recruited. Patients had high disease activity (BASDAI score ⩾4) [21] and were candidates for anti-TNF therapy according to standard clinical care. Clinical disease activity was assessed at baseline, including determination of CRP levels, BASDAI [21], BASMI [22] and Ankylosing Spondylitis Disease Activity Score (ASDAS) [23]. Exclusion criteria were pregnancy, lactation or treatment with investigational drugs within the previous 3 months. Stable doses of NSAIDs were continued if used at inclusion.
At baseline, before the start of anti-TNF treatment, all patients underwent an 18F-fluoride PET-CT scan of the total spine and SI joints. In two AS patients, bone biopsies were obtained of both PET-positive and PET-negative sites in the vertebral column. These patients were excluded from follow-up PET-CT scans because of potential interference of the biopsy with clinical follow-up and outcome. In the remaining 10 patients the 18F-fluoride PET-CT scan was repeated after 12 weeks. Clinical response to anti-TNF treatment was defined according to a ⩾ 20% improvement in Assessment of SpondyloArthritis international Society criteria (ASAS20) [24] at 24 weeks.
The study protocol was approved by the Medical Ethics Review Committee of the VU University Medical Center. All patients gave written informed consent prior to participation in the study.
18F-fluoride PET-CT scan
PET-CT scans were performed using Gemini TF or Ingenuity TF (Philips Healthcare, Andover, MA, USA) PET-CT scanners. Thirty minute dynamic scans of the chest (covering the left ventricle and thoracic vertebral column) were acquired, starting at the time of an i.v. injection of 18F-fluoride. After injection, the administration device was flushed with 20 ml of sodium chloride 0.9%. Subsequently, residual activity was measured to obtain the actual amount of radioactivity injected. Per patient, a mean of 111 MBq (s.d. 6) of 18F-fluoride was injected. At 45 min post-injection, whole-body scans of 5 min per bed position were acquired, covering the spine and SI joints as described previously [12].
Imaging analysis
All PET-CT data were independently assessed for PET-positive lesions (dichotomous) by two nuclear medicine specialists (O.S.H. and P.R.) blinded for clinical data and time sequence (i.e. baseline or 12 weeks) using standard 3D image viewing software. The low-dose CT was used as an anatomical reference for localization of PET findings. Standardized scoring sheets for the spine and SI joints were used based on previous 18F-fluoride PET findings in AS patients (see Appendix A, available at Rheumatology online). Discrepant readings were resolved in a consensus session in the presence of an adjudicator (C.J.L.), who also was blinded to the clinical data, but this time sequence-paired for baseline and 12 weeks. AS-like lesions were distinguished from OA lesions by radiologist expert opinion on low-dose CT and/or computed radiography (consensus J.C.J.B, B.J.H.B.). Lesions were regarded as degenerative if there no signs of AS were present close to the lesions. For example, facet joints with sclerosis but also signs of ankylosing and an open joint space were regarded as AS-like lesions and not OA lesions [25, 26].
After visual scoring of the scans, volumes of interest (VOIs) were drawn manually on top of visual PET-positive lesions for quantitative assessment of the tracer uptake using data analysis software that was developed in-house [27]. Correction for background uptake (mean uptake value of the centre of three visually unaffected vertebrae) was incorporated in the VOI analysis. Standardized uptake values of 18F-fluoride in VOIs, corrected for both body weight and individual integrated whole blood activity concentration (SUVAUC) were used for quantitative analysis. Derived from a direct comparison with full kinetic modelling, SUVAUC was the most representative semi-quantitative outcome measure for monitoring the focal tracer uptake during intervention with anti-TNF therapy in our cohort of clinically active AS patients (data not shown).
Bone biopsy procedure
In two patients, CT-guided bone biopsies were taken from PET-positive lesions in the spine on baseline 18F-fluoride PET-CT. Patients with clear PET positivity in the anterior corners of the spine were eligible. A total of four biopsies per patient were taken from these lesions at the anterior side of the vertebra. In addition, two control biopsies were taken contralaterally from the unaffected side of the same vertebra (PET-negative, uptake similar to background).
Patients were positioned prone, head first, and a high-resolution diagnostic CT scan (220 mAs; SOMATOM Sensation 64, Siemens Medical Solutions, Forchheim, Germany) was obtained with 1 mm slices of the region of interest. Subsequently, under local anaesthetic, sterile conditions and CT guidance, samples were obtained using a 14 G bone biopsy needle.
Analysis of bone samples
Bone biopsy samples were immediately snap frozen in liquid nitrogen and fixed in 10% formaldehyde and according to standard protocols. Samples were embedded in methyl methacrylate and 5 μM sections were cut for histological analysis, primarily haematoxylin and eosin (H&E) staining. Osteoclasts were identified by tartrate-resistant acid phosphatase staining and new bone formation (i.e. osteoblast activity) by Goldner’s Masson trichrome staining. Goldner’s Masson trichrome staining discriminates between mineralized bone (green) and immature new bone matrix (red). Bone samples were interpreted by pathologists blinded for PET-CT results.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0.0 for Windows (IBM, Armonk, NY, USA). Continuous variables are summarized using mean (s.d.) or as median and interquartile range (IQR) in case of variables that are not normally distributed. Interreader variability of PET scans was calculated using Cohen’s κ. Dichotomous variables are summarized as frequencies relative to total number. Results at the patient level were compared between responders and non-responders using the Mann–Whitney test or Fisher’s exact test and between time points using the Wilcoxon signed rank test.
Mean 18F-fluoride tracer uptake at baseline and after 12 weeks of anti-TNF therapy and differences in changes in mean 18F-fluoride tracer uptake over time were compared in the total group using generalized estimating equations with scan moment as the only independent variable. Models included time of scan (baseline or 12 weeks), response group and their interaction. A significant interaction was taken as an indication that changes in uptake during treatment differed between responders and non-responders. In case the interaction was not significant, a simpler model was fitted with only time of scan and response status to see whether mean uptake differed between responders and non-responders. An exchangeable correlation structure was used to account for multiple lesions per patient. Similarly robust standard errors were used to account for possible misspecification of the correlation structure.
A P-value <0.05 was regarded as statistically significant and a 95% CI lower bound of >0.3 was used to identify at least moderate correlations and standardized regression coefficients.
Results
Visual interpretation of baseline 18F-fluoride scan
PET-CT scans of patients (n = 2) that were subjected to spine biopsy
PET-CT scans of the two patients who underwent biopsies showed 16 and 20 PET-positive lesions in the spine, respectively, and one of them also had bilaterally enhanced 18F-fluoride uptake in the SI joints. These patients were HLA-B27+ with a BASDAI of 5.1 and 4.1, respectively.
PET-CT scans of patients (n = 10) with follow-up during anti-TNF therapy
Baseline demographics of the remaining 10 patients who underwent serial PET imaging are summarized in Table 1 and no parameters were found to be different between responders and non-responders (based on the ASAS20 response at 24 weeks). The ASAS20 response at 12 weeks did not correlate with the ASAS20 response at 24 weeks. With regard to concomitant medication use, 9 of 10 patients used stable dosages of NSAIDs and none used DMARDs during the study.
Table 1.
Patient characteristics
| Characteristic | Group (n = 10) | Responders (n = 5) | Non-responders (n = 5) |
|---|---|---|---|
| Females, % | 70 | 80 | 60 |
| HLA-B27 positive, % | 90 | 100 | 80 |
| Age, mean (s.d.), years | 36.7 (10.6) | 41.0 (10.4) | 32.6 (10.0) |
| Disease duration since diagnosisa, median (IQR), years | 5.0 (2.0–9.3) | 5.0 (5.0–15.0) | 2.0 (1.3–5.5) |
| Disease duration of symptomsb, median (IQR), years | 8.0 (4.0–15.0) | 10.0 (5–32) | 5.0 (4–10) |
| BASDAI (0–10), mean (s.d.) | 5.7 (1.1) | 5.4 (1.3) | 5.9 (1.0) |
| ASDAS (0–10), mean (s.d.) | 3.5 (0.5) | 3.4 (0.4) | 3.6 (0.7) |
| BASMI (0–10), median (IQR) | 2.0 (1.8–4.0) | 3.0 (1.5–4.0) | 2.0 (1.5–4.5) |
| CRP, median (IQR), mg/ml | 9.0 (3.5–18.5) | 5.0 (3.0–26.5) | 10.0 (5.0–19.0) |
Response is defined as ASAS20 response after 24 weeks of anti-TNF therapy.
Duration since definite AS diagnosis according to the modified New York criteria.
Duration of symptoms (years) from start of inflammatory back pain.
At baseline, all 10 patients showed at least one axial PET-positive lesion in the spine and/or SI joints and 6 of 10 patients had both PET-positive lesions in both the spine and SI joints. In total, 84 PET-positive lesions were observed in the spine of 6 of the 10 AS patients for whom follow-up scans were available (e.g. Fig. 1A). In 7 of the 84 PET-positive lesions, signs of OA were observed, as identified by experienced radiologists. In the spine, PET-positive lesions were found (in order of frequency) in the costovertebral joints (43%), facet joints (23%), bridging syndesmophytes (20%) and non-bridging vertebral lesions (14%). The greatest number of PET-positive lesions was found in the thoracic spine (Table 2). SI joints showed 18F-fluoride PET positivity in at least one SI joint in 9 of 10 patients, with a total of 15 of 20 PET-positive SI joints. Interobserver variability was good, with a κ = 0.7 (95% CI 0.657, 0.795).
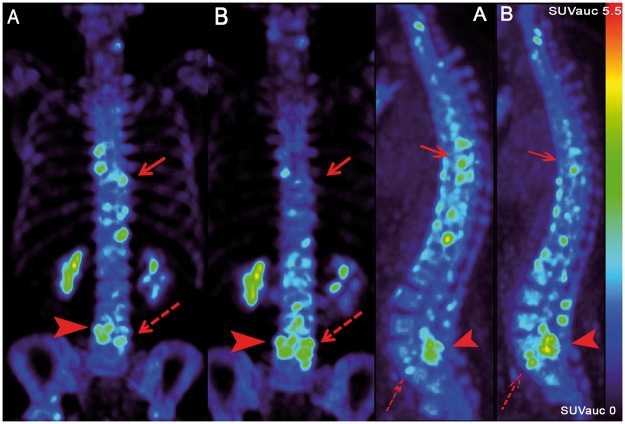
Fig. 1.
Axial and sagittal PET images before and 12 weeks after the start of anti-TNF therapy
(A) Baseline PET scan with 18F-fluoride accumulation throughout the spine. (B) After 12 weeks there was a heterogeneous effect of anti-TNF therapy on 18F-fluoride uptake: part of the AS lesions show a decrease (solid arrow) and others an increase (dashed arrow). Accumulation in the lumbar facet joints was regarded as OA (solid arrow head).
Table 2.
18F-fluoride PET-positive lesions per axial region of the vertebral column
| Lesion location | Lesions, n | Patients, n |
|---|---|---|
| Cervical | 12 | 2/10 |
| Thoracic | 55 | 6/10 |
| Lumbar | 16 | 4/10 |
| Sacral | 1 | 1/10 |
| SI joint | 15/20 | 9/10 |
Bone biopsy histology
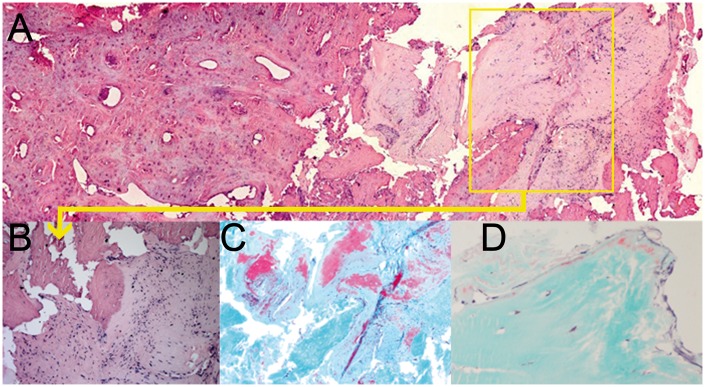
Biopsies were taken from the base of the syndesmophytes in Th12 of one patient and from L2 of the other. H&E staining showed the area of bone at the conjunction with connective tissue (anterior longitudinal ligament), with infiltration of inflammatory cells in a ‘PET-positive biopsy’ as demonstrated in Fig. 2A–C. Goldner’s Masson trichrome staining confirmed bone formation in all four PET-positive lesions of the two patients by showing osteoid near cortical bone with areas of osteoblast-like cells and an area of woven bone directly on the inside of the cortex (Fig. 2C), indicating rapid bone formation. In addition, the area of bone and connective tissue with inflammation clearly revealed calcium deposits (Fig. 2C) and tartrate-resistant acid phosphatase staining depicted a few osteoclasts in biopsies of PET-positive lesions. In contrast, control biopsies of PET-negative lesions did not show osteoid or calcium deposits in one patient and only minor ones in the other. In addition, no osteoclast or inflammatory cells were observed.
Fig. 2.
H&E and Goldner stained histology images of vertebral bone biopsies
(A) H&E staining overview of a PET-positive bone biopsy with the conjunction of connective tissue and bone matrix (20×). (B) H&E staining (detailed view of a PET-positive lesion; 200×) with cell infiltration in connective tissue. (C) Goldner staining of the same area with osteoid depositions (red) in areas with inflammation (40×). (D) Goldner staining of a PET-negative bone biopsy with minor osteoid depositions.
18F-fluoride uptake during anti-TNF treatment
Quantitative analysis of all PET-positive lesions in the spine and SI joints, pooled for the 10 included patients, revealed heterogeneity in 18F-fluoride uptake changes over time in axial lesions both between patients and between PET-positive lesions within patients. The mean SUVAUC of all 84 spine lesions and SI joints at baseline and at 12 weeks and the change in the SUVAUC are tabulated separately for clinical responders and non-responders after 24 weeks in Table 3. No correlation was found between PET outcome and the ASAS20 response after 12 weeks of anti-TNF treatment.
Table 3.
18F-fluoride SUVAUC for spine and SI joints of AS patients
| 18F-fluoride accumulation in: | Baseline, mean (95% CI) | 12 weeks, mean (95% CI) | Change, mean (95% CI) |
|---|---|---|---|
| Spine | |||
| Responders | 3.9 (3.6, 4.1) | 4.0 (3.7, 3.2) | 0.1 (−0.3, 0.4) |
| Non-responders | 4.5 (4.0, 4.9) | 3.9 (2.9, 4.8) | −0.6 (−1.9, 0.8) |
| Total group | 4.2 (3.8, 4.7) | 3.9 (3.4, 4.5) | −0.3 (−1.3, 0.7) |
| Spine: thoracic level only | |||
| Responders | 3.9 (3.4, 4.4) | 3.4 (3.1, 3.6) | −0.5 (−0.8, −0.2) |
| Non-responders | 4.9 (3.8, 5.9) | 4.5 (3.2, 5.9) | −0.3 (−2.0, 1.4) |
| Total group | 4.4 (3.7, 5.2) | 4.0 (3.2, 4.9) | −0.3 (−1.1, 0.5) |
| Spine: costovertebral at the thoracic level | |||
| Responder | 4.1 (4.0, 4.3) | 3.1 (3.0, 3.2) | −1.0 (−1.3, −0.7) |
| Non-responders | 4.5 (3.5, 5.4) | 4.1 (3.1, 5.1) | −0.4 (−2.3, 1.6) |
| Total group | 4.3 (3.7, 4.9) | 3.7 (2.9, 4.5) | −0.7 (−1.8, 0.5) |
| SI joints | |||
| Responders | 5.6 (4.7, 6.5) | 4.3 (3.6, 5.0) | −1.2 (−2.3, −0.2) |
| Non-responders | 5.2 (4.6, 5.7) | 5.6 (4.0, 7.1) | 0.4 (−0.6, 1.4) |
| Total group | 5.4 (4.9, 6.0) | 4.9 (4.0, 5.8) | −0.6 (−1.5, 0.3) |
Spine
Grouping all lesions of the spine for all patients, the mean 18F-fluoride uptake as measured by the SUVAUC did not change during 12 weeks of anti-TNF therapy [mean change in SUVAUC: −0.3 (95% CI −1.3, 0.7); P = 0.5]. Moreover, the mean change in the SUVAUC was similar for responders and non-responders (group by time interaction P = 0.4). Interestingly, 34 of 54 (63%) of the lesions at the thoracic level were located in costovertebral joints and responders showed a significant decrease in 18F-fluoride uptake in costovertebral joints (P < 0.001), in contrast to a non-significant decrease in non-responders (Table 3). Other lesion types, such as bridging syndesmophytes, non-bridging vertebral lesions and facet joints, showed a heterogeneous response to anti-TNF treatment (e.g. Fig. 2B). Pooling SUVAUC results of all facet joints, bridging syndesmophytes and non-bridging vertebral lesions showed stable 18F-fluoride accumulation during 12 weeks of anti-TNF therapy [mean change in SUVAUC 0.04 (95% CI −0.9, 0.9); P = 0.9]. Lesions identified as OA showed either stable or slightly increased 18F-fluoride uptake over time (Fig. 2B).
SI joints
There was no significant change in tracer uptake over time at the group level when pooling responders and non-responders (mean change in SUVAUC −0.6 (95% CI −1.5, 0.3); P = 0.2]. However, clinical responders showed a significant decrease in SI 18F-fluoride uptake [mean change in SUVAUC −1.2 (95% CI −2.3, −0.2); P = 0.03; group by time interaction P = 0.02], whereas non-responders showed a non-significant increase in SUVAUC [mean change in SUVAUC 0.4 (95% CI −0.6, 1.4; P = 0.4].
Discussion
This study provides data that support 18F-fluoride PET as a novel monitoring technique to visualize and quantify axial lesions with bone formation in clinically active AS patients before and after anti-TNF treatment. Specific imaging of bone formation using this PET approach is supported by unique pilot histologic data of the anterior spine. The study presents novel data showing the ability of 18F-fluoride PET-CT to monitor changes in bone formation in AS patients during anti-TNF treatment. Part of the lesions (costovertebral lesions and SI joints) with identified bone formation on 18F-fluoride PET-CT at baseline decreased during 12 weeks of anti-TNF therapy in clinical responders but not in non-responders. Other lesion types, including typical OA lesions and advanced bridging syndesmophytes, did not alter during treatment at a group level.
The histological data in this study support in vivo identification of bone formation activity in active AS lesion sites by 18F-fluoride PET even though in this study histological analysis was descriptive and obtained in only two patients. Although this is the first study describing the histological substrate of 18F-fluoride PET-positive lesions in AS, similar findings have been reported in multiple myeloma patients. In those patients, 18F-fluoride uptake correlated highly with the mineral apposition rate in iliac bone marrow biopsies, which is the gold standard for investigating bone formation [28]. Our study indicates that 18F-fluoride uptake is also a valid method for quantification of osteoblastic activity in AS patients. PET-CT-guided bone biopsies could further provide opportunities for pathogenetic research on AS, in particular with regard to the relationship between inflammation and bone formation. It could reveal novel targets for new treatments that inhibit or ultimately prevent the disabling bone formation of AS.
The present findings of multiple axial 18F-fluoride PET-positive lesions in the axial skeleton of clinically active AS patients are in concordance with most results of previously performed cross-sectional studies [12–16]. These studies demonstrated that 18F-fluoride PET-CT showed more axial lesions than 18F-FDG and macrophage targeting [12], 18F-fluoride PET-CT outcomes were associated with clinical parameters such as BASDAI [13] and the technique has promising diagnostic test values (sensitivity, specificity) for detecting sacroiliitis [15, 16]. One study investigated the value of baseline 18F-fluoride PET-CT in predicting anti-TNF response in suspected SpA patients. In this study, no predictive value was found, which may be related to inclusion of a heterogeneous population, because these patients did not meet the ASAS classification criteria and had a negative MRI of SI joints. In addition, the study did not include follow-up monitoring with 18F-fluoride PET-CT to investigate the response of lesions to anti-TNF treatment [29]. A potential confounder for detection of AS lesions by 18F-fluoride imaging may be the co-presence of OA, since degenerative changes may also cause a positive 18F-fluoride signal [30, 31]. In the present study, 7 of 84 PET-positive lesions showed definite signs of OA as identified by two expert radiologists. By excluding these lesions from part of the analyses, it was possible to further analyse the impact of anti-TNF in typical AS lesions. Interestingly, 18F-fluoride uptake in definite OA lesions showed an increasing trend during anti-TNF treatment, while typical AS lesions, in particular costovertebral lesions and SI joints of responders, showed a decrease in 18F-fluoride uptake during treatment. An increase in 18F-fluoride accumulation in OA lesions was also observed by Kobayashi et al. [32], with significantly greater uptake in advanced OA lesions than in early OA lesions. In AS, lesions with more advanced structural changes, such as syndesmophyte bridges and facet joints, responded heterogeneously to anti-TNF treatment. The latter is probably also dependent on the stage of reversibility of the bone formation at these sites. This indicates that 18F-fluoride PET monitoring of anti-TNF in AS may be most valuable in early AS lesions when structural changes are still limited.
To date there is still debate about the relationship between inflammation and bone formation in AS [33, 34]. In large clinical trials with follow-up data after up to 2–4 years of anti-TNF treatment, no effect was found on bone formation despite successful suppression of inflammation in the majority of AS patients [35, 36]. This raised the question whether inflammation and bone formation are TNF-linked processes or regulated by different pathways. Although the present biopsy data from PET-positive lesions suggest that inflammation and bone formation can both be found at the site of enthesis in anterior spine lesions, their linkage and involved molecular pathways need further investigation. Recent data have shown that early and continuous anti-TNF treatment for up to 8 years seems to arrest new bone formation [4, 5]. This suggests that suppression of inflammation by anti-TNF treatment may result in a decrease in bone formation if the treatment is applied at an early phase of AS disease, that is, before substantial structural changes are present. For assessing the effects of AS therapies such as anti-TNF and other recently introduced biologics on bone formation, sensitive imaging techniques such as 18F-fluoride PET-CT may be helpful in monitoring changes of bone formation in an early stage of treatment. With regard to the radiation burden of PET-CT, the effective radiation dose for one 18F-fluoride PET-CT scan is about 5 mSv, compared with 10–11 mSv for a diagnostic CT of the abdomen; in the near future this could be lowered because of new developments in hybrid imaging techniques, such as optimization of low-dose CT scans and also the development of PET-MRI [37]. Conventional radiography does not provide a means to study short-term therapeutic effects on bone formation since changes in bone formation usually only become apparent after 2 years of treatment [35]. Moreover, our data also showed that the 12 week changes in bone formation in specified lesions on 18F-fluoride PET-CT correlated with the clinical evaluation later in time, at 24 weeks. In contrast, clinical findings at 12 weeks did not correlate with later therapeutic outcome. This underlines the distinctive value of 18F-fluoride PET-CT to discriminate clinical responders from non-responders at 12 weeks of treatment, based on efficacy of (molecular) bone formation.
Conclusions
18F-fluoride PET-CT clearly visualizes (changes in) axial lesions with bone formation in the spine and SI joints of AS patients, which is supported by histological signs of osteoid formation at PET-positive sites. ASAS20 responders at 24 weeks showed a significant reduction in 18F-fluoride uptake in costovertebral lesions and SI joints after 12 weeks of anti-TNF treatment. The technique therefore holds promise for diagnosis and therapy monitoring of AS, and PET-CT-guided bone biopsies could further provide opportunities for pathogenetic research of AS. These preliminary results with 18F-fluoride PET-CT in 10 AS patients are promising but need to be validated in future studies with larger cohorts of AS patients.
Supplementary Material
Acknowledgements
We would like to thank F.A. van Gaalen for his contributions to this study and we would like to thank M. van Dijk-Baak (research nurse) for excellent patient care during the study.
Funding: This is an investigator-initiated study that was partially funded by the Dutch Arthritis Association.
Disclosure statement: B.L.P.B. is an employee of UCB. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Maksymowych WP, Mallon C, Morrow S. et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 2. Rezvani A, Bodur H, Ataman S. et al. Correlations among enthesitis, clinical, radiographic and quality of life parameters in patients with ankylosing spondylitis. Mod Rheumatol 2014;24:651–6. [DOI] [PubMed] [Google Scholar]
- 3. van der Heijde D, Salonen D, Weissman BN. et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haroon N, Inman RD, Learch TJ. et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baraliakos X, Haibel H, Listing J, Sieper J, Braun J.. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710–5. [DOI] [PubMed] [Google Scholar]
- 6. Forestier J, Deslous-Paoli P.. Radiological study of sacro-iliac joints in ankylosing spondylitis with reference to the evolution of the disease. Ann Rheum Dis 1957;16:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudwaleit M, Khan MA, Sieper J.. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. [DOI] [PubMed] [Google Scholar]
- 8. Finzel S, Kraus S, Schmidt S. et al. Bone anabolic changes progress in psoriatic arthritis patients despite treatment with methotrexate or tumour necrosis factor inhibitors. Ann Rheum Dis 2013;72:1176–81. [DOI] [PubMed] [Google Scholar]
- 9. Jones T. The role of positron emission tomography within the spectrum of medical imaging. Eur J Nucl Med 1996;23:207–11. [DOI] [PubMed] [Google Scholar]
- 10. Elzinga EH, van der Laken CJ, Comans EF. et al. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med 2011;52:77–80. [DOI] [PubMed] [Google Scholar]
- 11. Roivainen A, Hautaniemi S, Mottonen T. et al. Correlation of 18F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J Nucl Med Mol Imaging 2013;40:403–10. [DOI] [PubMed] [Google Scholar]
- 12. Bruijnen ST, van der Weijden MA, Klein JP. et al. Bone formation rather than inflammation reflects ankylosing spondylitis activity on PET-CT: a pilot study. Arthritis Res Ther 2012;14:R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idolazzi L, Salgarello M, Gatti D. et al. 18F-fluoride PET/CT for detection of axial involvement in ankylosing spondylitis: correlation with disease activity. Ann Nucl Med 2016;30:430–4. [DOI] [PubMed] [Google Scholar]
- 14. Lee SG, Kim IJ, Kim KY. et al. Assessment of bone synthetic activity in inflammatory lesions and syndesmophytes in patients with ankylosing spondylitis: the potential role of 18F-fluoride positron emission tomography-magnetic resonance imaging. Clin Exp Rheumatol 2015;33:90–7. [PubMed] [Google Scholar]
- 15. Strobel K, Fischer DR, Tamborrini G. et al. 18F-fluoride PET/CT for detection of sacroiliitis in ankylosing spondylitis. Eur J Nucl Med Mol Imaging 2010;37:1760–5. [DOI] [PubMed] [Google Scholar]
- 16. Zilber K, Gorenberg M, Rimar D. et al. Radionuclide methods in the diagnosis of sacroiliitis in patients with spondyloarthritis: an update. Rambam Maimonides Med J 2016;7:e0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook GJ, Fogelman I.. The role of positron emission tomography in the management of bone metastases. Cancer 2000;88(12 Suppl):2927–33. [DOI] [PubMed] [Google Scholar]
- 18. Fischer DR, Maquieira GJ, Espinosa N. et al. Therapeutic impact of [18F]fluoride positron-emission tomography/computed tomography on patients with unclear foot pain. Skeletal Radiol 2010;39:987–97. [DOI] [PubMed] [Google Scholar]
- 19. Park EK, Pak K, Park JH. et al. Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: a 2-year longitudinal study. Rheumatol Int 2017;37:765–73. [DOI] [PubMed] [Google Scholar]
- 20. van der LS, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 21. Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 22. Jenkinson TR, Mallorie PA, Whitelock HC. et al. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994;21:1694–8. [PubMed] [Google Scholar]
- 23. Lukas C, Landewe R, Sieper J. et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 24. Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M.. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44:1876–86. [DOI] [PubMed] [Google Scholar]
- 25. Bleil J, Maier R, Hempfing A. et al. Histomorphologic and histomorphometric characteristics of zygapophyseal joint remodeling in ankylosing spondylitis. Arthritis Rheumatol 2014;66:1745–54. [DOI] [PubMed] [Google Scholar]
- 26. Bennett AN, Rehman A, Hensor EM. et al. Evaluation of the diagnostic utility of spinal magnetic resonance imaging in axial spondylarthritis. Arthritis Rheum 2009;60:1331–41. [DOI] [PubMed] [Google Scholar]
- 27. Boellaard R, Hoekstra O, Lammertsma A.. Software tools for standardized analysis of FDG whole body studies in multi-center trials. J Nucl Med 2008;49(Suppl 1):159. [Google Scholar]
- 28. Regelink JC, Raijmakers PG, Bravenboer N. et al. 18F-fluoride-PET for dynamic in vivo monitoring of bone formation in multiple myeloma. EJNMMI Res 2016;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darrieutort-Laffite C, Ansquer C, Maugars Y. et al. Sodium 18F-sodium fluoride PET failed to predict responses to TNFα antagonist therapy in 31 patients with possible spondyloarthritis not meeting ASAS criteria. Joint Bone Spine 2015;82:411–6. [DOI] [PubMed] [Google Scholar]
- 30. Jadvar H, Desai B, Conti PS.. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin Nucl Med 2015;45:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Temmerman OP, Raijmakers PG, Kloet R. et al. In vivo measurements of blood flow and bone metabolism in osteoarthritis. Rheumatol Int 2013;33:959–63. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi N, Inaba Y, Tateishi U. et al. New application of 18F-fluoride PET for the detection of bone remodeling in early-stage osteoarthritis of the hip. Clin Nucl Med 2013;38:e379–83. [DOI] [PubMed] [Google Scholar]
- 33. Sieper J, Poddubnyy D.. Inflammation, new bone formation and treatment options in axial spondyloarthritis. Ann Rheum Dis 2014;73:1439–41. [DOI] [PubMed] [Google Scholar]
- 34. Rios Rodriguez V, Poddubnyy D.. Old and new treatment targets in axial spondyloarthritis. RMD Open 2015;1(Suppl 1):e000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Heijde D, Landewe R, Baraliakos X. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. [DOI] [PubMed] [Google Scholar]
- 36. Braun J, Baraliakos X, Hermann KG. et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchbender C, Ostendorf B, Ruhlmann V. et al. Hybrid 18F-labeled fluoride positron emission tomography/magnetic resonance (MR) imaging of the sacroiliac joints and the spine in patients with axial spondyloarthritis: a pilot study exploring the link of MR bone pathologies and increased osteoblastic activity. J Rheumatol 2015;42:1631–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.