Coinfected participants treated concurrently for multidrug-resistant (MDR) tuberculosis and human immunodeficiency virus had survival similar to that of participants with MDR tuberculosis alone. Mortality was higher among patients whose CD4 count was persistently ≤100 cells/mm3.
Keywords: tuberculosis, HIV, drug resistance, treatment, outcomes
Abstract
Background
Mortality in multidrug-resistant (MDR) tuberculosis–human immunodeficiency virus (HIV) coinfection has historically been high, but most studies predated the availability of antiretroviral therapy (ART). We prospectively compared survival and treatment outcomes in MDR tuberculosis–HIV-coinfected patients on ART to those in patients with MDR tuberculosis alone.
Methods
This observational study enrolled culture-confirmed MDR tuberculosis patients with and without HIV in South Africa between 2011 and 2013. Participants received standardized MDR tuberculosis and HIV regimens and were followed monthly for treatment response, adverse events, and adherence. The primary outcome was survival.
Results
Among 206 participants, 150 were HIV infected, 131 (64%) were female, and the median age was 33 years (interquartile range [IQR], 26–41). Of the 191 participants with a final MDR tuberculosis outcome, 130 (73%) were cured or completed treatment, which did not differ by HIV status (P = .50). After 2 years, CD4 count increased a median of 140 cells/mm3 (P = .005), and 64% had an undetectable HIV viral load. HIV-infected and HIV-uninfected participants had high rates of survival (86% and 94%, respectively; P = .34). The strongest risk factor for mortality was having a CD4 count ≤100 cells/mm3 (adjusted hazards ratio, 15.6; 95% confidence interval, 4.4–55.6).
Conclusions
Survival and treatment outcomes among MDR tuberculosis–HIV individuals receiving concurrent ART approached those of HIV-uninfected patients. The greatest risk of death was among HIV-infected individuals with CD4 counts ≤100 cells/mm3. These findings provide critical evidence to support concurrent treatment of MDR tuberculosis and HIV.
Multidrug-resistant (MDR) tuberculosis, defined as resistance to at least isoniazid and rifampin, is an urgent, global public health crisis that threatens the gains in tuberculosis and human immunodeficiency virus (HIV) control achieved over the past 2 decades [1]. The World Health Organization estimates that there were 480000 MDR tuberculosis cases globally in 2015, with an average treatment success rate of only 54% [1, 2]. Treatment for MDR tuberculosis involves second-line medications, which are less effective and more toxic than first-line tuberculosis medications, requiring treatment for up to 24 months [3]. As many as 20% of patients discontinue treatment before they achieve cure due to the therapy’s adverse effects, long duration, and complex regimen, which includes a daily intramuscular injection for the first 6–8 months [4].
The consequences of tuberculosis drug resistance are most profound in HIV-coinfected patients, in whom previous studies have found dramatically higher mortality rates (pooled mean mortality, 38%; 95% confidence interval [CI], 28–48) [5–8]. Many of these studies, however, predated the widespread availability of antiretroviral therapy (ART) and thus reflect MDR tuberculosis outcomes in untreated HIV [5–7]. Later studies that included ART did not systematically evaluate any bias that those who were not prescribed ART, such as those with severe concurrent illness or imminent death, may have introduced [9, 10]. Several additional small or retrospective studies suggest that survival is associated with the degree of immunosuppression and that ART use is protective [9, 11, 12]. Conversely, there has been concern that coadministration of ART with the complex MDR tuberculosis regimen would lead to additive or possibly synergistic side effects that would threaten adherence to both regimens and result in poor MDR tuberculosis outcomes, HIV virologic failure, or both. Although MDR tuberculosis treatment regimens are commonly associated with many side effects [13, 14], few studies have examined the safety of concurrent ART and MDR tuberculosis treatment. With the widespread availability and recommendation of ART for all patients with HIV [15], prospective data are needed to determine whether MDR tuberculosis patients with HIV who are treated with ART have outcomes that are similar to those experienced by patients with MDR tuberculosis alone.
South Africa has the highest number of HIV-infected persons and among the highest incidence of drug-resistant tuberculosis worldwide [1]. Until recently, the HIV and drug-resistant tuberculosis epidemics have affected geographically disparate regions, with HIV concentrated in sub-Saharan Africa and drug-resistant tuberculosis in Eastern Europe. However, these epidemics are now converging, with HIV prevalence rising in Eastern Europe and tuberculosis drug resistance increasing in sub-Saharan Africa [16–18]. As the number of drug-resistant tuberculosis–HIV-coinfected patients rises, it is critically important to examine concurrent treatment strategies for coinfected individuals, given their high mortality rate. In this context, we conducted a prospective, observational study of patients who initiated MDR tuberculosis therapy in KwaZulu-Natal province, South Africa, where more than 70% of all tuberculosis cases are HIV infected. The objective was to compare survival, tuberculosis, and HIV treatment outcomes in MDR tuberculosis patients coinfected with HIV and receiving concurrent ART to the outcomes experienced by patients who were HIV uninfected.
METHODS
Setting
The Survival and HIV OUTcomes in MDR-TB (SHOUT MDR-TB) study was conducted between 2011 and 2015 at 3 drug-resistant tuberculosis referral hospitals in KwaZulu-Natal province, which serve urban, suburban, and rural communities. KwaZulu-Natal is South Africa’s second-largest province and accounts for nearly one-third of the country’s drug-resistant tuberculosis cases [19]. It also has the highest tuberculosis incidence (1076 per 100000 population) and HIV prevalence (16.9%) nationwide [20, 21].
During the study period, patients were diagnosed with MDR tuberculosis based on a positive culture (Mycobacterial Growth Indicator Tube, Bactec 960) and phenotypic drug-susceptibility testing (DST; 1% proportion method on Middlebrook 7H10 solid agar) to isoniazid (1 mg/L), rifampin (2 mg/L), kanamycin (16 mg/L), and ofloxacin (2 mg/L). MDR tuberculosis patients were referred to dedicated drug-resistant tuberculosis treatment centers and treated with a standardized regimen that consisted of kanamycin (15 mg/kg, maximum 1 g daily), moxifloxacin (400 mg daily), ethionamide (15–20 mg/kg, maximum 750 mg daily), terizidone (15–20 mg/kg, maximum 750 mg daily), ethambutol (15–20 mg/kg, maximum 1200 mg daily), and pyrazinamide (20–30 mg/kg, maximum 1600 mg daily). Kanamycin was given intramuscularly for at least 6 months or 4 months after culture conversion, whichever was longer. Oral medications were continued without kanamycin for an additional 12–18 months. All HIV-coinfected MDR tuberculosis patients were offered ART irrespective of their CD4 count. The first-line ART regimen included efavirenz, stavudine, and lamivudine when the study began; however, national guidelines transitioned from stavudine to tenofovir in 2013.
Study Population and Procedures
We recruited patients with culture-confirmed MDR tuberculosis who were initiating treatment between May 2011 and December 2013. Potential participants were eligible if they were aged ≥18 years and had resistance to both rifampin and isoniazid by DST. Participants were excluded if they had previous MDR tuberculosis treatment, resistance to fluoroquinolones or second-line injectable agents, or abnormal baseline creatinine (>2 times the upper limit of normal [ULN]) or alanine aminotransferase (>5 times ULN) levels. Women were excluded if pregnant because management of MDR tuberculosis requires the use of alternate regimens in pregnancy.
Participants were followed monthly during MDR tuberculosis treatment and quarterly for 1 year following successful treatment completion. Each month, participants were interviewed regarding symptoms, adverse events, and medication adherence. Sputum samples were sent monthly for fluorescent microscopy, culture, and DST. Full blood count, chemistries, and liver function tests were performed monthly. CD4 count and HIV viral load were measured every 3 months; thyroid stimulating hormone and total T4 were measured every 6 months. Pure tone audiometry was performed monthly while receiving an injectable medication and again at month 12. Color vision testing was performed at baseline and months 2, 4, 6, 9, 12, and 24.
Outcome Measures and Analyses
The primary outcome was survival, measured in days from study enrollment. Secondary outcomes included MDR tuberculosis treatment outcome and time to tuberculosis culture conversion; change in CD4 count; viral suppression, virologic failure, and resistance; incidence of adverse events; and medication adherence. MDR tuberculosis treatment outcome was defined according to Laserson et al [22], in which cure requires 5 or more negative cultures in the last 12 months of treatment. Time to culture conversion was calculated to the first of 2 consecutive negative cultures taken at least 1 month apart. Participants were considered to have developed additional resistance if 1 or more follow-up DSTs demonstrated new resistance to either fluoroquinolones or injectable medications. Change in CD4 count was assessed at 6, 12, 18, and 24 months. Virologic suppression was defined as a viral load <150 copies/mL (the highest lower limit of detection among the assays used during the study period). Virologic failure was defined as failure to achieve viral suppression within 6 months of initiating ART or 2 or more viral loads >1000 copies/mL after achieving viral suppression. Participants with virologic failure underwent HIV genotypic resistance testing.
Adverse event severity was graded using the National Institutes of Health Division of AIDS Toxicity table [23]. Medication adherence was assessed each month using the following 3 measures: 3-day recall standardized questionnaire, 30-day recall questionnaire, and visual analog scale [24]. Adherence results were combined into a composite value, whereby any reports of adherence <100% were deemed “nonadherent.”
Participant characteristics were compared using simple frequencies, χ2, and Wilcoxon rank sum tests. Survival analysis was performed using Kaplan-Meier survival curves and log-rank tests. Baseline risk factors for mortality were analyzed using multivariate Cox proportional hazards models. We fit Cox models with CD4 modeled as baseline covariate, as well as time-dependent covariate, to account for changes in CD4 count throughout the study period.
Ethics
The institutional review boards at the University of KwaZulu-Natal, Albert Einstein College of Medicine, and Emory University and the KwaZulu-Natal Department of Health and the Centers for Disease Control and Prevention approved the study. All participants signed written informed consent forms.
RESULTS
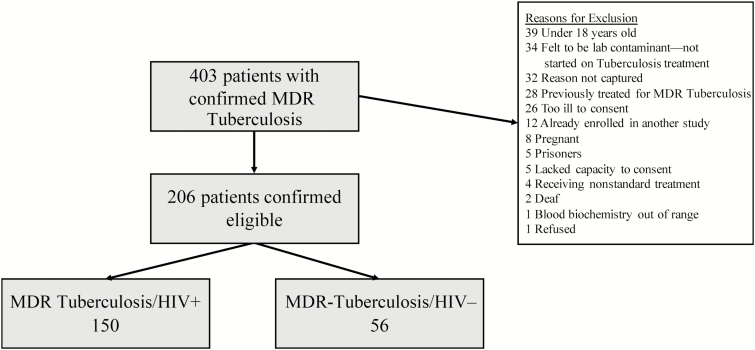
We screened 403 patients with confirmed MDR tuberculosis. Of these, 206 patients were eligible for enrollment (Figure 1). Of these enrolled participants, 150 were HIV infected and 56 were HIV uninfected (Table 1). The median age was 33 years (interquartile range [IQR], 26–41), and 131 (64%) participants were female; 133 (65%) participants had previously had tuberculosis. HIV-infected participants were more commonly female (70% vs 46%, P = .02) and older (median 34 vs 27 years), although the latter was not statistically significant (P = .91). Among HIV-infected participants, the median CD4 count at enrollment was 215 cells/mm3 (IQR, 114–378) and 60% (52/86) of those who were tested at baseline had an undetectable viral load. One hundred and twenty one (81%) HIV-infected participants were already receiving ART (median duration of 9 months; IQR, 3–30).
Figure 1.
Enrollment flowchart. Abbreviations: HIV, human immunodeficiency virus; MDR, multidrug-resistant.
Table 1.
Participant Characteristics at Enrollment
| Characteristic | Total (n = 206) | HIV Positive (n = 150) | HIV Negative (n = 56) | P Value |
|---|---|---|---|---|
| Median age, years (IQR) | 33 (26–41) | 34 (28–40) | 27 (21–48) | .91 |
| Female | 131 (64) | 105 (70) | 26 (46) | .002 |
| Race | .073 | |||
| Black | 204 (99) | 150 (100) | 54 (96) | |
| Indian | 1 (0.5) | 0 | 1 (1.8) | |
| White | 1 (0.5) | 0 | 1 (1.8) | |
| Previous treatment for tuberculosis | 133 (65) | 107 (71) | 26 (46) | <.001 |
| Outcome of most recent tuberculosis episode | .29 | |||
| Cure/Complete | 94 (71) | 78 (73) | 16 (62) | |
| Failure | 28 (21) | 19 (18) | 9 (35) | |
| Interruption/Loss to follow-up | 8 (6.0) | 7 (6.5) | 1 (3.8) | |
| Unknown | 3 (2.3) | 3 (2.8) | 0 | |
| Smoking | 47 (23) | 35 (23) | 12 (21) | .77 |
| Alcohol use | 63 (31) | 49 (33) | 14 (25) | .29 |
| Receiving ART at MDR tuberculosis treatment initiation | 121 (81) | NA | ||
| Duration on ART at MDR tuberculosis treatment initiation, median months (IQR) | 9 (3–30) | NA | ||
| CD4 count at MDR tuberculosis treatment initiation, median cells/mm3 (IQR) | 215 (114–378) | NA | ||
| Viral load <150 copies/mL at MDR tuberculosis treatment initiationa | 52 (60) | NA | ||
| Sputum smear status positiveb | 76 (49) | 57 (49) | 19 (51) | .78 |
| Baseline chest radiographc | ||||
| Cavitary disease | 95 (47) | 71 (47) | 24 (44) | .72 |
| Bilateral disease | 122 (60) | 81 (54) | 41 (76) | .005 |
Values are presented as n (%) unless otherwise indicated. Bold denotes P < .05.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug resistant; NA, not applicable.
aViral load was available for 86 participants at baseline.
bSputum smear was available for 155 participants (117 HIV positive and 37 HIV negative).
cBaseline chest radiograph unavailable for 2 participants.
Survival
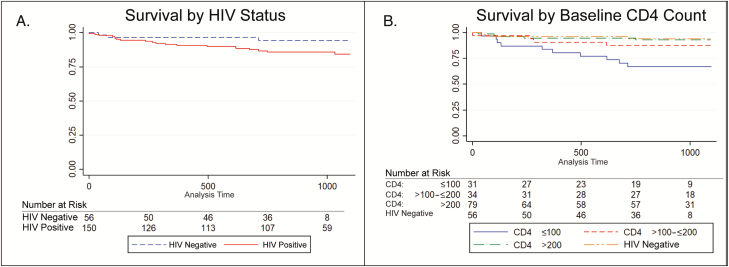
Participants were followed for a median of 32 months (IQR, 22–37; 444 person-years). Overall, survival was favorable in both groups, although there was a greater proportion of individuals in the HIV-infected group who died. A total of 24 (12%) participants died during MDR tuberculosis treatment or in the 1 year following cure (21 [14%] HIV infected and 3 [5%] HIV uninfected). Among those who died, the median survival time was 281 days (IQR, 99–618) and did not differ significantly by HIV status (P = .12, Figure 2A). Further analysis, however, revealed that participants with a baseline CD4 count ≤100 cells/mm3 had significantly worse survival (67%, P = .005) and accounted for the difference in survival between HIV-infected and HIV-uninfected participants (Figure 2B). Participants with CD4 counts of 101–200 and >200 cells/mm3 had survival similar to those who were HIV uninfected (P = .34 and P = .85, respectively). Gender, age, cavitation or bilateral disease on baseline chest radiograph, smear status, and smoking were not associated with survival (Supplementary Table S1).
Figure 2.
Kaplan-Meier survival curves. A, Comparing by human immunodeficiency virus (HIV) status. Although HIV-infected individuals had a lower survival rate (84% vs 94% at 3 year follow-up), the difference was not statistically significant (log-rank P= .12). B, Comparing HIV-infected participants in different baseline CD4 strata with HIV-uninfected participants. Individuals with a baseline CD4 count ≤100 cells/mm3 had substantially lower survival (67%) at 3 year follow-up (P= .005). HIV-infected participants with baseline CD4 counts 101–200 and >200 cells/mm3 had survival similar to HIV-uninfected multidrug-resistant tuberculosis participants (log-rank P= .34 and P= .85, respectively).
In multivariable analysis, a baseline CD4 count of ≤100 cells/mm3 was the only factor significantly associated with death (hazards ratio [HR], 6.1; 95% CI, 1.7–22.9). When we examined CD4 count as a time-varying covariate (Supplementary Table S2 and Table S3), a time-varying CD4 count ≤100 cells/mm3 was more strongly associated with decreased survival (HR, 16.7; 95% CI, 3.3–83.3) than baseline CD4 count. Survival was only 29% among individuals with a baseline CD4 count ≤100 cells/mm3 whose CD4 count did not improve beyond 100 cells/mm3 (4 of 14 survived; Supplementary Table S3); survival was substantially better (88%, 15 of 17 survived) among those whose CD4 count increased to >100 cells/mm3.
MDR Tuberculosis Treatment Outcomes
Among the 206 enrolled participants, 184 (89%) achieved sputum culture conversion; 62 (30%) converted to negative on first-line treatment before MDR tuberculosis treatment initiation. The median time to conversion in the remaining 122 participants was 62 days (IQR, 51–108). There was no difference in the proportion of individuals who achieved culture conversion or in the time to conversion based on HIV status, death, or baseline CD4 count ≤100 cells/mm3 (data not shown). Fifteen (7.3%) participants withdrew consent or moved before completing MDR tuberculosis treatment. Among the remaining 191 participants, 140 (73%) had a successful tuberculosis treatment outcome (cure, n = 130 [68%]; completed, n = 10 [5%]; Table 2). Treatment failed in 6 (3%) participants, 23 (12%) interrupted treatment prematurely, and 22 (12%) died while on treatment. Treatment outcomes did not differ between HIV-infected and HIV-uninfected participants (P = .50, Table 2), aside from the difference in survival noted previously. Two participants died in the 1 year after completing MDR tuberculosis treatment, having been deemed cured. One of these participants had a negative sputum culture prior to death, but the details of the second patient were not available. Eight (4%) participants developed additional tuberculosis resistance during the course of treatment, of whom 5 had new resistance to a fluoroquinolone, 2 with resistance to kanamycin, and 1 with resistance to both (ie, extensively drug-resistant tuberculosis).
Table 2.
Final Tuberculosis Treatment Outcome Compared Between Human Immunodeficiency Virus (HIV)–Infected and HIV-Uninfected Multidrug-Resistant Tuberculosis Participants
| Multidrug-Resistant Tuberculosis Treatment Outcome | All Patients (n = 191a) (%) | HIV Infected (n = 138) (%) | HIV Uninfected (n = 53) (%) |
|---|---|---|---|
| Cure | 130b (68.1) | 90 (65.2) | 40 (75.5) |
| Treatment completion | 10 (5.2) | 7 (5.1) | 3 (5.7) |
| Treatment failure | 6 (3.1) | 5 (3.6) | 1 (1.9) |
| Default | 23 (12.0) | 17 (12.3) | 6 (11.3) |
| Died | 22 (11.5) | 19 (13.8) | 3 (5.7) |
Treatment outcomes did not differ significantly between HIV-infected and HIV-uninfected participants (P= .50).
Abbreviation: HIV, human immunodeficiency virus.
aExcludes 13 participants who withdrew from study (10 HIV positive and 3 HIV negative) and 2 participants who moved out of the province during the study and for whom final treatment outcomes were not available.
bTwo patients died after cure and discontinuation of multidrug-resistant tuberculosis treatment.
P = .50 for omnibus χ2 comparison between HIV-infected and HIV-uninfected participants.
HIV Outcomes
All HIV-infected participants were offered ART; 121 (81%) participants were already receiving ART at enrollment, 23 (15%) started after enrollment, and 6 (4%) did not start ART while in study follow-up (1 died, 1 withdrew, 1 refused, and 3 were lost to follow-up before initiating ART). Median time to initiation among those who started ART after enrollment was 28 days (IQR, 16–72). Overall, the median CD4 count rose from 215 cells/mm3 at baseline to 321 and 386 cells/mm3 at 12 and 24 months, respectively (P < .0001 and P = .005 for paired comparison to baseline) with concurrent ART and MDR tuberculosis treatment. The proportion of participants with an undetectable viral load was 61% at baseline, 76% at 12 months, and 64% at 24 months. Twenty-seven (18%) HIV-infected participants had virologic failure. Of these, 19 had plasma available for HIV viral resistance testing and 13 had evidence of viral resistance (details of mutations in Supplementary Table S4). Eighteen participants had either a CD4 count ≤100 cells/mm3 at 2 or more time points or only 1 available CD4 count that was ≤100 cells/mm3. Of these, 14 (78%) had virologic failure or had incomplete viral suppression without meeting the criteria for failure. Of these 14 participants, 10 (71%) died.
Adverse Events and Adherence
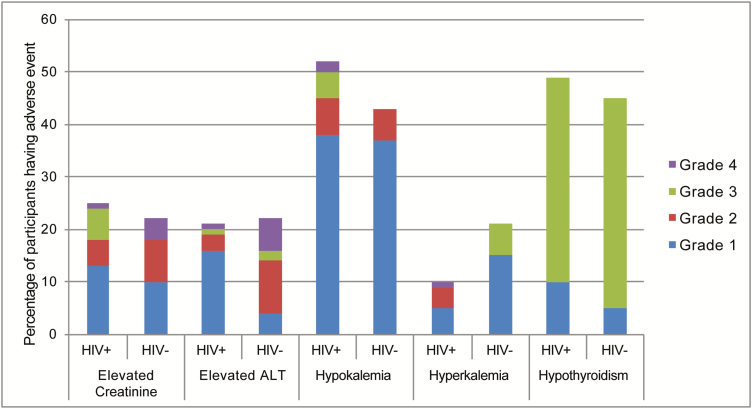
Overall, adverse events were common but mild, with 187 (91%) participants experiencing a laboratory-, hearing-, or vision-related adverse event (Figure 3). Of these, 36%, 8%, and 8% experienced a severe adverse event (SAE), respectively. However, the majority of laboratory SAEs were transient and resolved spontaneously. Importantly, HIV-infected participants were no more likely to experience SAEs than those who were HIV uninfected (Figure 3).
Figure 3.
Percentage of participants who experienced each laboratory adverse event by severity grade and human immunodeficiency virus (HIV) status. p = NS for all comparisons between HIV positive and HIV negative. Abbreviations: HIV, human immunodeficiency virus; ALT, alanine aminotransferase.
Participants reported a high degree of medication adherence (81% fully adherent to both tuberculosis and HIV medications). Despite the substantially higher pill burden, adherence among HIV-infected participants was not significantly different from adherence for those who were HIV uninfected (81% vs 82% fully adherent, P = .84).
DISCUSSION
In this study, we examined whether MDR tuberculosis–HIV coinfected patients receiving ART could achieve survival and treatment outcomes that were comparable to those achieved by patients with MDR tuberculosis alone. While we found that concurrently treated patients had high survival and MDR tuberculosis cure rates, the notable exception was individuals whose CD4 counts remained persistently <100 cells/mm3 despite receiving ART; these participants remained at a substantially higher risk of death throughout follow-up. We also found that concurrent MDR tuberculosis and ART treatment was well tolerated, with no significant differences in frequency of adverse events or medication adherence, despite the potential for additive toxicity and the additional pill burden. These results provide critical, prospective evidence to support the recommendation to treat HIV in the context of MDR tuberculosis coinfection.
While several randomized clinical trials have shown that early initiation of ART with drug-susceptible tuberculosis is associated with improved survival [25–27], there are no comparable data for treatment of MDR tuberculosis and HIV coinfection. Multiple retrospective studies have shown a strong association between mortality and HIV in patients with MDR tuberculosis [2, 8], and some have specifically found higher mortality in those with a baseline CD4 count <100 cells/mm3 [28, 29]. Many of the earlier studies, however, predated the availability of ART and analyzed only baseline CD4 count as a static variable. To our knowledge, no study has examined the effect of improvements in CD4 count over time while receiving ART.
Our data provide compelling evidence that successful outcomes are possible in patients with MDR tuberculosis and HIV coinfection and that ART should be initiated promptly. HIV-infected patients who received ART had high MDR tuberculosis cure rates and substantially better survival compared to patients in the pre-ART era [2, 7, 30]. Further analysis by CD4 count showed that participants with a baseline CD4 count ≤100 cells/mm3 had a higher risk of mortality, mirroring the results of a recent retrospective study [31], while those with a baseline CD4 count >100 cells/mm3 had a hazard of death that was similar to that for participants who were HIV uninfected. Our time- varying analysis of CD4 count, however, provided further insights. Although a baseline CD4 count ≤100 cells/mm3 was associated with mortality, the risk was limited to those whose CD4 count did not improve while on therapy, often because of virologic failure. Individuals who began with a CD4 count ≤100 cells/mm3 but whose CD4 count rose above 100 cells/mm3 did not carry an increased hazard of death when compared to HIV-uninfected participants. This finding should serve as an impetus for drug-resistant tuberculosis programs to ensure fully integrated care of HIV, aggressive initiation of ART, and monitoring to detect virologic failure. The goal of such programs must be to achieve immunologic recovery as soon as possible and to maintain virologic suppression throughout tuberculosis treatment and beyond.
Both MDR tuberculosis and HIV outcomes in our study were quite favorable. We attribute this to our excellent study retention, high medication adherence, and, most importantly, the treatment of HIV. HIV-infected participants in our study had a consistent rise in CD4 count throughout the study, even though a majority of them had already been on ART at the time of MDR tuberculosis treatment initiation. Although the proportion of participants had end-of-treatment viral suppression that was lower than the international target of 90% [32], we are reassured that despite the complexity and toxicity of MDR tuberculosis treatment, as well as the added pill burden, HIV medication adherence and control of HIV were not dramatically compromised.
Our study also confirms the safety of concurrent ART and MDR tuberculosis treatment. Despite initial concerns about additive or synergistic side effects, we found no significant differences in laboratory adverse events between patients with and without HIV coinfection. These results confirm findings from our earlier cohort [33] that although adverse events are common, they are generally mild, with no significant differences in SAEs between HIV-infected and HIV-uninfected patients. The absence of serious nephrotoxicity is particularly notable, given that nearly all of our HIV-infected participants received concurrent tenofovir (disoproxil fumarate) and kanamycin.
This study has a number of limitations. First, patients who were started on MDR tuberculosis treatment at the specialty centers have an inherent survival bias, having had to survive long enough to be diagnosed and referred. Such patients may thus have better survival overall. Nevertheless, the participants in our study are a representative sample of the patients who present to the MDR tuberculosis program for care, and our data provide evidence of how they should be managed. Second, because participants were seen for study visits at their routine clinic visits and often traveled great distances (>100 km), they were sometimes unable to complete all screening procedures such as blood draws and audiometry testing, resulting in missing data. Third, reliable assessment of immune reconstitution inflammatory syndrome (IRIS) is challenging as there is no diagnostic test or pathognomonic finding. We created a structured screening tool to assist clinic physicians in identifying cases of IRIS. In an interim review, however, we found that many possible cases of IRIS had been missed. We therefore felt that our data were incomplete and may not be informative. Although the ideal timing of ART initiation in HIV-infected patients with MDR tuberculosis is not known, most of our HIV-infected participants were already on ART at the time of study enrollment and would thus not have been at risk of paradoxical IRIS. Fourth, our medication adherence measures were all self-reported and may have underestimated nonadherence. Finally, although a study investigator reviewed the medical records of all participants who died, the records, themselves were often incomplete or lacked sufficient detail to determine a cause of death in most cases. Our analysis, however, uses death from any cause and is thus the most conservative estimate.
MDR tuberculosis remains a dire threat to public health worldwide, and treatment options remain suboptimal. With the MDR tuberculosis and HIV epidemics converging, treatment of coinfected patients will increasingly pose a unique treatment dilemma for providers in sub-Saharan Africa. Until now, the decision to treat both MDR tuberculosis and HIV concurrently has been based on expert opinion and retrospective studies. Our study is the first prospective demonstration that HIV-infected patients who receive concurrent ART can achieve comparable survival, MDR tuberculosis cure rates, and HIV outcomes as HIV-uninfected MDR tuberculosis patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial Support. This study was funded by the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; R01AI087465 and R01AI089349, both to N. R. G.). It was also supported in part by grants from NIH/NIAID to J. C. M. B. (K23AI083088), N. R. G. (K24AI114444), the Emory University CFAR (P30AI050409) and TBRU (U19AI111211), Albert Einstein College of Medicine (CFAR P30AI051519), Albert Einstein College of Medicine CFAR (P30AI051519), Albert Einstein College of Medicine and Montefiore Medical Center ICTR (UL1TR001073), and the Atlanta CTSI (UL1TR000454).
Acknowledgments. We are grateful to the study team at the University of KwaZulu-Natal and South African Medical Research Council for their tireless efforts in data collection, record abstraction, participant recruitment, and interviews. We thank Drs Alois Mngadi and Francois Eksteen, as well as the doctors and staff of King Dinuzulu Hospital, Murchison Hospital, and Greytown Hospital for their outstanding clinical management of the study participants. We also thank Rebecca Goldstein, John Gharbin, Fay Stephens, and Kristin Nelson for their contributions to data cleaning and analysis. Finally, we thank the participants and their families who consented to participate in this study.
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2016. Document no WHO/HTM/TB/2016.13, Geneva. [Google Scholar]
- 2. Ahuja SD, Ashkin D, Avendano M et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012; 9:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. WHO/HTM/TB/201604; Geneva, Switzerland, 2016. [PubMed] [Google Scholar]
- 4. Falzon D, Jaramillo E, Schünemann HJ et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38:516–28. [DOI] [PubMed] [Google Scholar]
- 5. Wells CD, Cegielski JP, Nelson LJ et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 2007; 196(Suppl 1):S86–107. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi NR, Shah NS, Andrews JR et al. ; Tugela Ferry Care and Research Collaboration HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010; 181:80–6. [DOI] [PubMed] [Google Scholar]
- 7. Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis 2010; 14:413–9. [PMC free article] [PubMed] [Google Scholar]
- 8. Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis 2015; 19:969–78. [DOI] [PubMed] [Google Scholar]
- 9. O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR Jr. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis 2013; 19:416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pietersen E, Ignatius E, Streicher EM et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383:1230–9. [DOI] [PubMed] [Google Scholar]
- 11. Dheda K, Shean K, Zumla A et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010; 375:1798–807. [DOI] [PubMed] [Google Scholar]
- 12. Gandhi NR, Andrews JR, Brust JC et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis 2012; 16:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bloss E, Kuksa L, Holtz TH et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis 2010; 14:275–81. [PubMed] [Google Scholar]
- 14. Furin JJ, Mitnick CD, Shin SS et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2001; 5:648–55. [PubMed] [Google Scholar]
- 15. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. September 2015 Geneva: Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1 [PubMed] [Google Scholar]
- 16. Nunes EA, De Capitani EM, Coelho E et al. Patterns of anti-tuberculosis drug resistance among HIV-infected patients in Maputo, Mozambique, 2002–2003. Int J Tuberc Lung Dis 2005; 9:494–500. [PubMed] [Google Scholar]
- 17. Nelson LJ, Talbot EA, Mwasekaga MJ et al. Antituberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet 2005; 366:488–90. [DOI] [PubMed] [Google Scholar]
- 18. Dubrovina I, Miskinis K, Lyepshina S et al. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics?Int J Tuberc Lung Dis 2008; 12:756–62. [PubMed] [Google Scholar]
- 19. Conradie F, Meintjes G, Hughes J et al. Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. S Afr Med J 2014; 104:164–6. [DOI] [PubMed] [Google Scholar]
- 20. Shisana O, Rehle T, Simbayi LC et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press, 2014. [Google Scholar]
- 21. Ndjeka N. Multi-Drug Resistant Tuberculosis: Strategic Overview on MDR-TB Care in South Africa. Republic of South Africa: Department of Health, 2014. [Google Scholar]
- 22. Laserson KF, Thorpe LE, Leimane V et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9:640–5. [PubMed] [Google Scholar]
- 23. Division of AIDS (DAIDS). Table for grading the severity of adult and pediatric adverse events Version 1.0/Clarification 1. Available at: http://rcc.tech-res.com/safetyandpharmacovigilance. Accessed 28 November 2013.
- 24. Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials 2004; 5:74–9. [DOI] [PubMed] [Google Scholar]
- 25. Blanc FX, Sok T, Laureillard D et al. ; CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Havlir DV, Kendall MA, Ive P et al. ; AIDS Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abdool Karim SS, Naidoo K, Grobler A et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohr E, Cox V, Wilkinson L et al. Programmatic treatment outcomes in HIV-infected and uninfected drug-resistant TB patients in Khayelitsha, South Africa. Trans R Soc Trop Med Hyg 2015; 109:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daniels JF, Khogali M, Mohr E et al. Time to ART initiation among patients treated for rifampicin-resistant tuberculosis in Khayelitsha, South Africa: impact on mortality and treatment success. PLoS One 2015; 10:e0142873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farley JE, Ram M, Pan W et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One 2011; 6:e20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin SS, Modongo C, Boyd R et al. High treatment success rates among HIV-infected multidrug-resistant tuberculosis patients after expansion of antiretroviral therapy in Botswana, 2006–2013. J Acquir Immune Defic Syndr 2017; 74:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. UNAIDS. 90–90–90: An ambitious treatment target to help end the AIDS epidemic. JC2684 2014. [Google Scholar]
- 33. Brust JC, Shah NS, van der Merwe TL et al. Adverse events in an integrated home-based treatment program for MDR-TB and HIV in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2013; 62:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.