Gibberellin has an inhibitory effect during initial activation of dormant grapevine buds, and at this stage its level is down-regulated. At a later stage, gibberellin level increases and enhances bud regrowth.
Keywords: Bud, dormancy, GA 2-oxidase, GA 3-oxidase, GA 20-oxidase, gibberellin, grapevine, hydrogen cyanamide
Abstract
The molecular mechanism regulating dormancy release in grapevine buds is as yet unclear. It has been hypothesized that (i) abscisic acid (ABA) represses bud–meristem activity; (ii) perturbation of respiration induces an interplay between ethylene and ABA metabolism, which leads to removal of repression; and (iii) gibberellin (GA)-mediated growth is resumed. The first two hypothesis have been formally supported. The current study examines the third hypothesis regarding the potential involvement of GA in dormancy release. We found that during natural dormancy induction, levels of VvGA3ox, VvGA20ox, and VvGASA2 transcripts and of GA1 were decreased. However, during dormancy release, expression of these genes was enhanced, accompanied by decreased expression of the bud-expressed GA-deactivating VvGA2ox. Despite indications for its positive role during natural dormancy release, GA application had inhibitory effects on bud break. Hydrogen cyanamide up-regulated VvGA2ox and down-regulated VvGA3ox and VvGA20ox expression, reduced GA1 levels, and partially rescued the negative effect of GA. GA had an inhibitory effect only when applied simultaneously with bud-forcing initiation. Given these results, we hypothesize that during initial activation of the dormant bud meristem, the level of GA must be restricted, but after meristem activation an increase in its level serves to enhance primordia regrowth.
Introduction
The molecular mechanism regulating dormancy release in grapevine buds in response to either natural or artificial stimuli is as yet unclear, and this limits its manipulation to optimize grape production (Or, 2009). We have previously proposed a working model that describes the biochemical pathways that are involved in artificially induced bud dormancy release (Ophir et al., 2009). According to this model, perturbation of cytochrome pathway activity in the mitochondria leads to respiratory stress. In response to this energy crisis, anaerobic respiration is up-regulated, which mimics hypoxia and thereby affects interplay between ethylene and abscisic acid (ABA) metabolism, which in turn leads to gibberellin (GA)-mediated growth resumption (Ophir et al., 2009). The results of various analyses have supported the predictive power of the model regarding the involvement of respiratory stress, hypoxia, ethylene, and ABA (Zheng et al., 2015, and references within; Vergara et al., 2017). The potential involvement of GA in the cascade that leads to grapevine bud dormancy release, which has not yet been addressed, is the theme of the current study.
GA12 biosynthesis from geranylgeranyl diphosphate (GGDP) proceeds in several steps, respectively regulated by ent-copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO), and ent-kaurenoic acid oxidase (KAO). Regulation of GA biosynthesis occurs mainly at the later stages of the pathway, for which the relevant enzymes are members of the GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox) families that convert GA12 to GA1 and GA4, the major bioactive GAs in plants. GA 2-oxidase (GA2ox), on the other hand, catalyzes GA1 and GA4 deactivation (Yamaguchi, 2008, and references within). Various factors modulate the levels of GA and, among those, is GA itself, which regulates its own metabolism by down-regulating GA20ox and GA3ox, and up-regulating GA2ox expression (Yamaguchi, 2008, and references within; Middleton et al., 2012).
Bioactive GA activates its response pathway by binding to its nuclear receptors, GA INSENSITIVE DWARF1s (GID1s). This complex then targets DELLAs, the major negative regulators of the GA response, for degradation via binding with SLEEPY1 (SLY1), a GA-specific F-box protein (Hirano et al., 2008; Sun, 2010, 2011; Hauvermale et al., 2012).
Bioactive GAs control many plant developmental processes (Richards et al., 2001), including seed germination (Ogawa et al., 2003) and stem elongation (Peng et al., 1997). It is well established that they promote cell expansion (Dill and Sun, 2001; van den Heuvel et al., 2001; Strader et al., 2004; Ubeda-Tomás et al., 2008; Hauvermale et al., 2012), and recently it has been demonstrated that they also control cell proliferation (Achard et al., 2009; Ubeda-Tomás et al., 2009; Hauvermale et al., 2012; Davière et al., 2014). GAs are also known for their antagonistic relationships with ABA, in regulation of various developmental processes (Weiss and Ori, 2007), including seed dormancy/germination, the most advanced model in the field of dormancy. In this case, GA enhances seed germination across a wide range of plant species, whereas ABA enhances dormancy induction and regulates its maintenance (Seo et al., 2006). This GA–ABA antagonistic relationship is depicted by the following. (i) Increased expression of genes coding for enzymes which catalyze GA catabolism and ABA synthesis (GA2ox2 and NCED6) as seed dormancy becomes deeper. The expression of these genes decreases when dormancy declines, in parallel with increased expression of genes coding for enzymes that catalyze GA synthesis (GA3ox1) and ABA catabolism (CYP707A2) in Arabidopsis seeds (Footitt et al., 2011). (ii) Bioactive GA-induced germination of lettuce seeds is accompanied by decreased levels of the LsNCED4 transcript and ABA (Gonai et al., 2004; Sawada et al., 2008). (iii) Increased expression of AtGA3ox and AtGA20ox and decreased expression of AtGA2ox6 is evident in the developing seeds of an Arabidopsis mutant having impaired ABA biosynthesis (aba2-2), as compared with wild-type plants (Seo et al., 2006). It has therefore been suggested that GA is essential for seed germination, and that ABA plays an important role in depleting bioactive GA levels during dormancy by regulation of expression of GA metabolism genes.
A role for GA during seed germination is further supported by the necessity for functional GA signaling during germination. Accordingly, mutations in SLY1 lead to increased Arabidopsis seed dormancy, and a triple knockout of GID1A, GID1B, and GID1C results in germination failure (Steber et al., 1998; Griffiths et al., 2006).
Both negative and positive effects of GA were reported on outgrowth of paradormant buds which are carried on actively growing shoots and are under the control of apical dominance. In rice, GA deficiency driven by increased expression of GA2ox or decreased expression of GA3ox resulted in increased outgrowth of tillers (Dai et al., 2007; Lo et al., 2008). Similarly, overexpression of AtGA2ox in bahiagrass resulted in reduced GA1 and enhanced outgrowth of axillary buds (Agharkar et al., 2007). In hybrid aspen, decreased bioactive GA levels in the apex and young tissues (mediated by GA2ox overexpression) resulted in loss of apical dominance and increased branching of lateral buds. Interestingly, a similar decrease in mature vasculature did not present such an effect (Mauriat et al., 2011). On the other hand, indications for positive regulation of paradormant bud outgrowth by GA was also recorded. Light-induced branching of Rosa sp. laterals was accompanied by increased expression of GA20ox and GA3ox, and decreased GA2ox, but GA could not rescue bud burst in the dark (Choubane et al., 2012). The Arabidopsis phyb mutant presented decreased branching and increased GA2ox (Su et al., 2011). GA biosynthesis inhibitors inhibited cytokinin (CK)-mediated outgrowth of axillary buds of Jatropha curcas, whereas GA promoted it, and also down-regulated expression of inhibitors of bud outgrowth from the BRC family (Ni et al., 2015). GA has also been reported to induce lateral bud outgrowth in papaya (Ni et al., 2015). Finally, in paradormant buds of hybrid aspen, the level of the GA3ox2 transcript was low during maturation, and was induced in response to decapitation (Rinne et al., 2016).
Several studies have reported an increased level of endogenous GA, or an up-regulation of GA biosynthesis genes, following exposure of perennial endodormant buds to chilling, or fluctuations in the levels of GA during the natural bud endodormancy cycle. In sweet cherry, the endogenous ABA/GA3 ratio in flower buds increased during natural dormancy induction and decreased during dormancy release (Duan et al., 2004). An increased level of GA following exposure to chilling was reported in dormant buds of peach (Frisby and Seeley, 1993). In agreement, low temperature treatments induced the expression of members of the GA20ox (Karlberg et al., 2010) and GA3ox (Rinne et al., 2011) gene families in hybrid aspen dormant buds, while down-regulating expression of paralogs of GA2ox (Karlberg et al., 2010).
Application of GA had contrasting effects (inhibition or induction) on bud break during and after winter dormancy of temperate perennial buds. It was reported that application of GA3 enhanced bursting of dormant Elberta peach buds (Donoho and Walker, 1957), and GA4 application enhanced dormancy release of Japanese apricot flower buds (Zhuang et al., 2013). In Populus, treatment with 1–100 µM GA4 induced bud burst, but treatment with GA3 resulted in bud abscission (100 µM GA3) and protrusion of embryonic leaves (0.1–10 µM GA3) (Rinne et al., 2011). In kiwi fruit, application of GA3 prior to chilling enhanced dormancy, while application after exposure to chilling promoted bud break (Lionakis and Schwabe, 1984). GA3 application also delayed dormancy release of grape and persimmon buds (Weaver, 1959; Iwasaki, 1980; Kang et al., 1998). In agreement with the above, it was formerly proposed that the observed increase in endogenous GA may be a result, rather than a cause, of bud dormancy removal, and might regulate bud expansion during regrowth (Saure, 1985; Lang, 1994).
In the current study, we describe a detailed examination of the potential involvement of GA in the artificially and naturally stimulated cascades that lead to grapevine bud dormancy release.
Materials and methods
Plant material
Analyses of dormancy status and of the effects of GA, hydrogen cyanamide (HC), HC–GA, hypoxia, heat shock (HS), and chilling treatments on dormancy release were conducted using mature buds collected from cordon-trained grapevines (Vitis vinifera cv. Early sweet, grafted on 140 Ruggeri) in a commercial vineyard, planted on calcareous rendzina soil at Gilgal located in the Jordan Valley, Israel. The region is characterized by warm winters (20/10 °C mean daily maximum/minimum in January; 150 mm rainfall) and hot summers (38/23 °C in June). Vine spacing was 1.5 m within rows and 3 m between rows, and the vines were trained to a Y-shaped, open-canopy gable system. Drip irrigation with drippers spaced by 50 cm was computer controlled. The irrigation control unit was set to 40% of reference evapotranspiration (ETo) during bud break, and the amount of water applied was gradually increased to 100% of ETo at harvest. After harvest, it was gradually reduced to 40% of ETo. The meteorological data used for calculating ETo were obtained from a weather station located at Gilgal. Irrigation frequencies ranged from once a week (during bud break) to three times a week from veraison until harvest. NPK liquid fertilizer (Tuv, ICL, Haifa, Israel) was applied through the irrigation system at a rate of 1–1000 liters of water, at a ratio of 6–3–9 from bud break until veraison, and 0–0–15 from veraison until harvest. Vines were pruned to three-node spurs, and the detached canes, each carrying nine buds (in positions 4–12), were transferred to the lab. Field experiments were conducted in a commercial vineyard of the same cultivar at Argaman, in the Jordan Valley.
Natural dormancy curve
To create a dormancy curve that describes changes in dormancy status throughout the natural dormancy cycle, bud break was monitored at 1 week intervals from November to the January of the following year, as described in Zheng et al. (2015). Single-node cuttings prepared from canes were randomly mixed, and nine groups of 10 cuttings were placed in vases containing tap water. The vases were placed in a growth chamber and the cuttings forced at 22 °C under a 14/10 h light/dark regime. Bud break was defined as the stage at which green tissue became visible underneath the bud scales (Or, et al., 2002). Bud break was typically monitored at days 7, 11, 14, 18, and 21 following completion of an initial adjustment period of 48 h. Bud break percentages at 21 d were used to describe the seasonal changes in dormancy status of the bud population in the vineyard (Zheng et al., 2015).
For gene expression and hormone analyses, three groups of 100 buds were randomly sampled each week from the pool of single-node cuttings, on the day of arrival from the vineyard. These three bud pools were frozen in liquid nitrogen and stored at –80 °C.
Analyses of the effect of chemical and physical treatments on bud break
To test the effect of HC (3% Dormex®, v/v), HS (immersed in 50 °C water for 1 h), and hypoxia (forced at 1% O2 up to 48 h) on bud dormancy release, cuttings were treated as described in Zheng et al. (2015). After treatment, bud break was monitored under the forcing conditions described previously. For gene expression and hormone analyses, buds were sampled at 12, 24, 48, and 96 h for control, HC, and HS treatments, frozen in liquid nitrogen, and stored at –80 °C. Hypoxia treatments and the corresponding controls were carried out as previously described (Zheng et al., 2015), and sampled at 24 h and 48 h after sealing the jars.
Analyses of the effect of GA on bud break
To test the effect of GA on bud dormancy release, cuttings were sprayed with GA3 (stock solution: 33 g l–1 GA3, Pro-Gibb; Abbott Laboratories, Chicago, IL, USA) and placed in vases with tap water under the forcing conditions described previously. For schematic details of all the GA treatments, see Supplementary Table S1 at JXB online. Initially, two series of GA3 concentrations were used in two different experiments: 1.25, 2.5, 5, 10, 20, and 40 ppm (where 1 ppm GA3 equals 2.887 µM) for the first experiment, and 0.001, 0.01, 0.1, and 1 ppm for the second experiment. Additional experiments similarly tested the effect of application of a mix of GA4 and GA7 [10 ppm; GA4 + 7 (2:1), Duchefa, Haarlem, The Netherlands].
All GA solutions were formulated in 0.02% (v/v) Triton X-100. The control was treated similarly with 0.02% Triton X-100 solution. For the combined HC–GA treatments, cuttings were treated with a mixture of HC and 1, 5, or 10 ppm GA. To test the effect of GA on HC-triggered bud dormancy release further, GA (10 ppm) was applied at 0, 2, 6, and 10 d after HC treatment. Bud break was monitored after treatments under the forcing conditions previously described. To test the effect of GA on dormancy release of whole vines under vineyard conditions, cv. Early Sweet vines in a commercial vineyard, located in Argaman, Jordan Valley, were pruned to three-node spurs on 14 January 2014 and sprayed with 0.02% Triton X-100 without (control) or with 10 ppm GA3 (GA) to runoff (~1 liter per vine), using a 15 liter knapsack sprayer (SOLO®, VA, USA). Each treatment consisted of four blocks of three vines, in a randomized complete block design. A day after pruning, bud break was monitored for each vine in each block. The total number of buds was determined on 14 January, and the numbers of bursting buds were counted on 24 February, 4 March, and 11 March.
To test the effect of GA on pre-chilled buds, detached canes collected on 22 November 2015 were sprayed with 0.1% (w/v) fungicide (Switch® 62.5 WG; Syngenta), packed with plastic film, and stored at 4 °C for 1000 h. Canes were then used to prepare three groups of single-node cuttings, each with nine subgroups of 10 cuttings. One group was immediately treated with 1 ppm GA3. Two additional groups were treated with 0.02% Triton X-100: one was used as control and the other was treated, 7 d later, with 1 ppm GA3, when visible green tissue was detected on the first bud in the entire group. Forcing and bud break monitoring procedures were as described above.
To test the effect of GA on bud break of ecodormant buds, canes were collected from the vineyard after endodormancy release (18 February 2016), and groups of were cuttings prepared and treated as described for pre-chilled buds, except for the late GA treatment which was carried out 3 d after treatment with Triton X-100.
To test the effect of GA3 (10 ppm) on gene expression, the procedure described for analyses of the effect of chemical treatments was employed.
RNA extraction and quantitative real-time PCR (qRT-PCR) analyses
Total RNA was extracted from 20 frozen buds using cetyltrimethylammonium bromide (CTAB) as previously described (Acheampong et al., 2010). RNA was treated with RQ DNase (Promega, Madison, WI, USA) according to the manufacturer’s instructions, and dissolved in 40 μl of DNase/RNase-free water. cDNA was synthesized from total RNA using M-MLV Reverse Transcriptase (Promega) as detailed in the instruction manual.
Relative transcript levels of bud-expressed paralogs of VvGA2ox, VvGA3ox, and VvGA20ox gene families throughout the natural dormancy season and in response to HC were determined using the 96X96 Dynamic array Integrated Fluidic Circuits (96 IFCs) on the Biomark platform (Fluidigm, San Francisco, CA, USA) according to the manufacturer’s protocol. Transcript levels in buds exposed to hypoxia, HS, or GA treatments, and the relevant controls, were quantified by qRT-PCR with ABsolute Blue QPCR SYBR Green Low ROX Mix (Thermo Fisher Scientific, Waltham, MA, USA) on a Corbett Rotor-Gene 6000 (Qiagen, Hilden, Germany). VvActin and VvGAPDH primers, characterized and optimized by Reid et al. (2006), were used for normalization, while VvGA2ox, VvGA3ox, VvGA20ox, and VvGASA2 primers were previously optimized by Giacomelli et al. (2013) and Acheampong et al. (2017). All the primers are presented in Supplementary Table S2.
Quantitation of endogenous GA1 and GA4
Triplicate samples of 10 frozen buds for each biological replicate were homogenized in liquid nitrogen, and 0.5 g of the homogenized powder was used for hormone extraction and GA quantitation, as previously described (Acheampong et al., 2015). Endogenous GA levels were calculated from the ratios of the peak areas of these endogenous compounds to internal standards.
Statistical analyses
Statistical analyses were performed on a JMP 12.0.1 (SAS Institute, Cary, NC, USA) by one-way ANOVA with Student’s t-test (*P<0.05), or with Tukey’s HSD with a P-value <0.05.
Results
Profiling of VvGA3ox, VvGA20ox, and VvGA2ox transcript levels in grapevine buds during the natural dormancy cycle
Five putative VvGA20ox genes, three VvGA3ox genes, and nine VvGA2ox genes were predicted, using Arabidopsis amino acid sequences as reference (Jung et al., 2014). The number of VvGA20ox and VvGA2ox genes was adjusted to six and eight, respectively, by parallel comparisons with Glycine max and Oryza sativa, and activity tests (Giacomelli et al., 2013). Of those, two VvGA20ox genes (VvGA20ox3 and VvGA20ox6) and two VvGA3ox genes (VvGA3ox1 and VvGA3ox2) are expressed in mature woody grapevine buds. Of the VvGA2ox gene family members with previously confirmed GA deactivation activity, VvGA2ox3 is the paralog that is most highly expressed in the bud, while VvGA2ox4 is expressed at a lower level (Fasoli et al., 2012; Giacomelli et al., 2013; Khalil-Ur-Rehman et al., 2017). All these genes, except for VvGA3ox1, are under GA feedback regulation (Supplementary Fig. S1) as expected (see the Introduction).
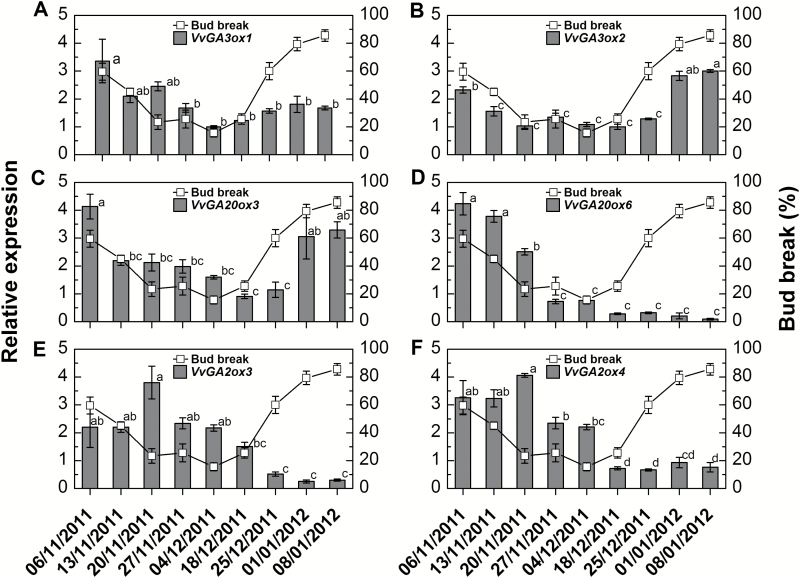
To assess the potential involvement of GA metabolism in the execution of the natural dormancy cycle, the transcript levels of the bud-expressed VvGA3ox, VvGA20ox, and VvGA2ox paralogs were quantified in buds collected from the beginning of November to the beginning of January by qRT-PCR. Transcript levels for all four GA biosynthesis genes gradually decreased during the dormancy induction period (Fig. 1A–D), and were lowest on 4 December (VvGA3ox1) or 18 December (VvGA3ox2, VvGA20ox3, and VvGA20ox6), two time points which lie within the period of dormancy maintenance. Transcript levels of VvGA3ox2 and VvGA20ox3 sharply increased during dormancy release, whereas a more gradual increase, which was not significant, was recorded for VvGA3ox1. Interestingly, VvGA20ox6 expression, which presented a profile similar to that described above during dormancy induction and maintenance, did not increase during dormancy release. It may be worth noting that the functionality of this gene as a GA20ox has not yet been validated, as it could not be amplified from Pinot Noir (Giacomelli et al., 2013).
Fig. 1.
Expression profile of genes encoding central components of GA metabolism throughout the dormancy cycle. Vines (Vitis vinifera cv. Early Sweet) from a vineyard at Gilgal, located in the Jordan Valley, were pruned to three-node spurs. The detached canes were sampled weekly throughout the dormancy cycle, cut into single-node cuttings, randomly mixed, and nine groups of 10 cuttings were prepared and placed in water-filled vases. The vases were placed in a growth chamber, at 22 °C under a 14/10 h light/dark regime. The bud break percentages at 21 d are shown (as line) in each panel. Values are averages of nine groups of replications, consisting of 10 buds each ±SE. Total RNA was extracted from buds sampled weekly, upon arrival from the vineyard, and frozen. Relative transcript levels were determined for VvGA3ox1 (A), VvGA3ox2 (B), VvGA20ox3 (C), VvGA20ox6 (D), VvGA2ox3 (E), and VvGA2ox4 (F), using qRT-PCR (see the Materials and methods) and normalized against VvActin and VvGAPDH. Values of qRT-PCR represent the mean ±SE of three biological replications, each with two technical repeats. Data points with different letters indicate significantly different values (P<0.05) according to Tukey’s HSD test.
The transcript levels for VvGA2ox3 were rather stable up to the deepest stage of dormancy/dormancy maintenance (represented by 4–18 December), with one exception on 20 November—the transition point to deep dormancy (Fig. 1E). Subsequently, transcript levels gradually decreased from 18 December to 8 January, with a corresponding increase in bud break ability. The expression profile of VvGA2ox4 (Fig. 1F) was similar to that of VvGA2ox3 (Fig. 1E), including one point of non-significant up-regulation noted for 20 November, suggesting that the mode of regulation was similar throughout the dormancy cycle.
Levels of bioactive GAs in grapevine buds during the dormancy cycle
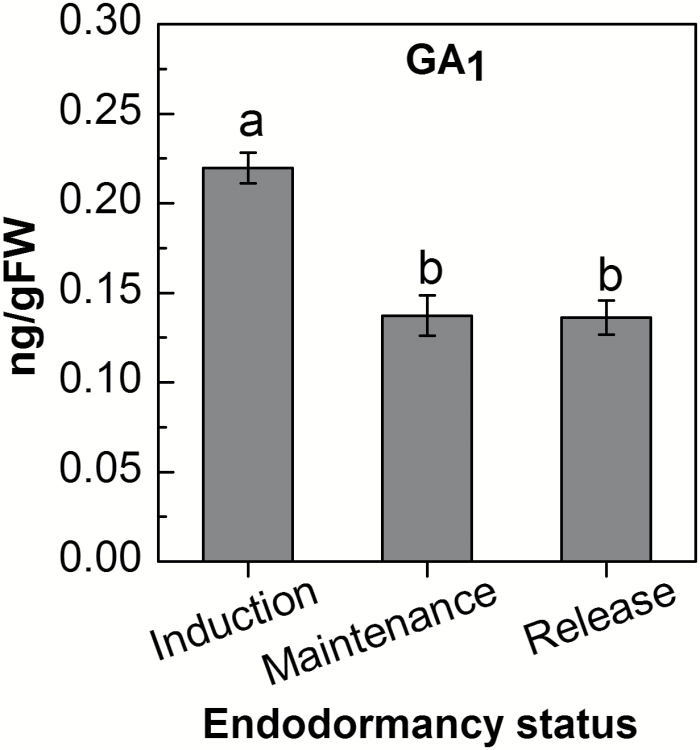
Levels of GA1 and GA4, the predominant bioactive GAs in grapevine (Giacomelli et al., 2013; Acheampong et al., 2015), were determined in grape buds which were sampled from a commercial vineyard throughout the natural dormancy cycle. Whereas GA4 was undetectable in any of the endodormancy phases, the levels of GA1 were relatively high during dormancy induction, and decreased ~40% during dormancy maintenance. No further changes were detected during endodormancy release in the ecodormant buds sampled from the vineyard for this analysis (Fig. 2).
Fig. 2.
Quantities of endogenous GA1 in grapevine buds throughout the dormancy cycle. Buds were sampled weekly from the vineyard and were frozen immediately as described in Fig. 1. Buds were homogenized, and GA1 was quantified by LC–MS/MS (Acheampong et al., 2015). 2H-labeled GA1 was spiked as internal standard. The levels of GA1 were calculated from the peak area ratios of the endogenous GA1 to its 2H-labeled internal standard. Three biological replications (10 buds per replicate) were analyzed for each time point, and means from three time points during endodormancy induction, maintenance, and release (1–13 November, 20 November–18 December, and 25 December–8 January, respectively) served to calculate GA1 levels. Data points with different letters indicate significantly different values (P<0.05) according to Tukey’s HSD test.
Profiling of the transcript level of the GA-responsive gene VvGASA2 during the natural dormancy cycle
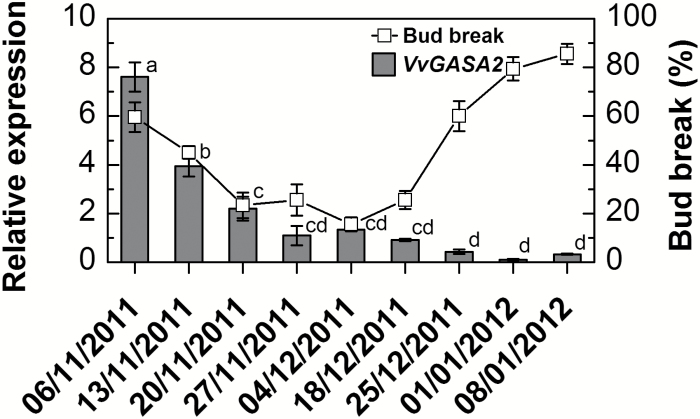
To address further GA responses during the natural dormancy cycle, we followed potential changes in the expression of VvGASA2, a grapevine homolog of the GA-responsive GAST1 PROTEIN HOMOLOG 4 (AtGASA4) (Acheampong et al., 2017). The GA-responsive nature of VvGASA2 expression in grapevine buds was initially confirmed, as shown by significant increases of its transcript level following GA3 application (1.9-and 1.6-fold increase after 48 h and 96 h from treatment, compared with control, Supplementary Fig. S1). Profiling of its transcript level throughout the natural dormancy cycle (Fig. 3) revealed the highest levels during dormancy induction, with a gradual decrease towards dormancy maintenance. No further changes were detected during endodormancy release in the ecodormant buds.
Fig. 3.
Expression profile of a gene encoding a grapevine AtGASA4 ortholog, VvGASA2, throughout the dormancy cycle. All the details are as in Fig. 1.
Effect of exogenous GA3 application on dormancy release of grapevine buds
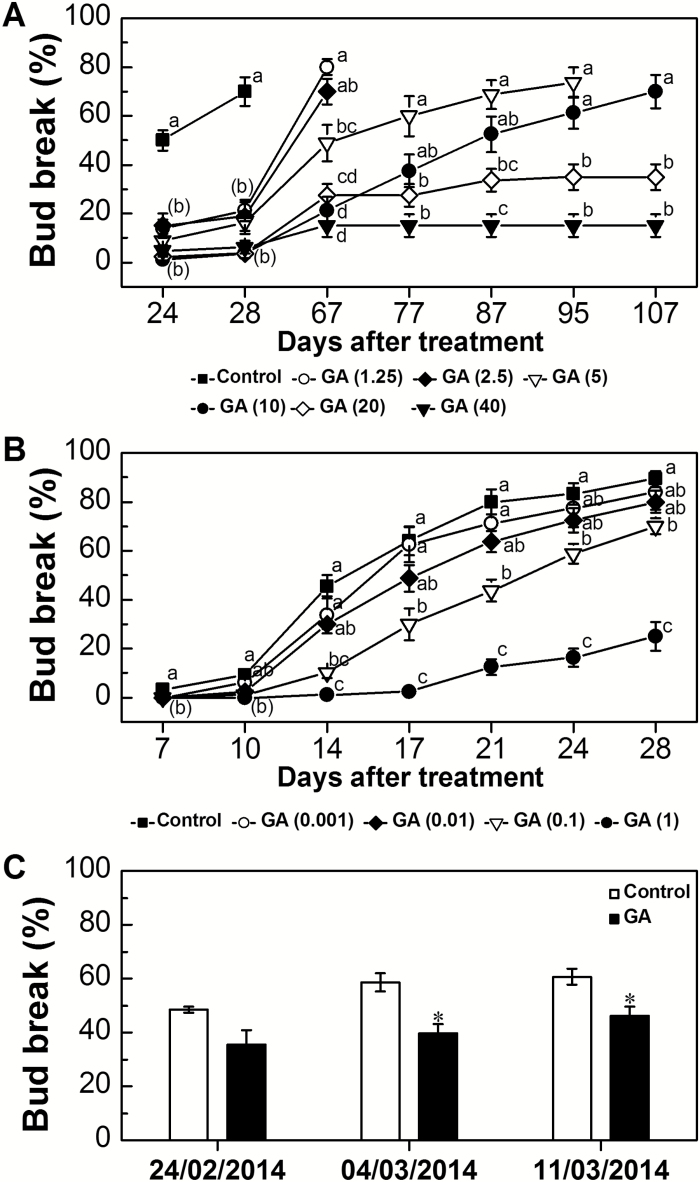
To test the hypothesis that GA enhances dormancy release of grapevine buds, the response of dormant buds to GA3 was compared with that of Triton X-100. Contrary to the initial hypothesis, application of GA to dormant buds significantly inhibited bud break (Fig. 4). The results (Fig. 4A) suggested that both time- and concentration-dependent effects exist, since: (i) up to 28 d from GA application, all analyzed concentrations of GA significantly inhibited bud break as compared with the control, which presented ~70% bud break at 28 d; and (ii) monitoring over an extended period of time allowed observation of recovery, yet the degree and the rate of recovery were concentration dependent. Low GA concentrations (1.25 ppm and 2.5 ppm) allowed recovery from the inhibitory effect after 67 d, leading to 80% and 70% bud break, respectively. Application of 5 ppm and 10 ppm GA resulted in a slower and lower recovery rate, leading to 75% and 70% bud break only after 95 d and 107 d, respectively. Higher concentrations of GA (20 ppm and 40 ppm) severely inhibited bud break, with only a slight recovery between 28 d and 67 d (reaching ~35% and 15% bud break, respectively), and complete stagnation beyond 87 d and 67 d, respectively.
Fig. 4.
The effect of GA3 application on bud break of single-node cuttings and whole vines. (A) Cuttings were sprayed with 1.25, 2.5, 5, 10, 20, and 40 ppm GA3 (where 1 ppm GA3 equals 2.887 µM) formulated with 0.02% Triton X-100, placed in vases, and monitored for 107 d. Triton X-100 (0.02%)-treated buds served as control. Each treatment was monitored until a bud break percentage similar to that of control at 28 d (of ~70%) was attained or the buds became stunted. For additional details, see Fig. 1. (B) Cuttings were sprayed with 0.001, 0.01, 0.1, and 1 ppm GA3 and monitored for 28 d. For additional details, see Fig. 1. (C) Vines were pruned to three-node spurs on 14 January 2014. GA (10 ppm GA3 with 0.02% Triton X-100 solution) and control (0.02% Triton X-100 solution) treatments were conducted on 15 January. Four blocks of three vines were used, and bud break was monitored separately for each vine in each block. The total number of buds was recorded and the number of bursting buds was counted on 24 February, 4 March, and 11 March. Bars represent the average bud break of the 12 grapevines in the four blocks for each treatment ±SE. Statistical tests indicate differences between treatments at each time point. Data points with different letters indicate significantly different values (P<0.05) according to Tukey’s HSD test. Asterisks between treatments indicate significantly differences according to Student’s t-test (*P<0.05).
A second set of experiments tested the effect of GA application at lower concentrations (0.001–1 ppm GA3) for a shorter (28 d) monitoring period (Fig. 4B). Low GA3 (0.001 ppm) had no inhibitory effect on bud break. A non-significant inhibitory effect was recorded when buds were treated with 0.01 ppm GA3, and the inhibitory effect gradually and significantly increased with increased GA3 concentrations. At a concentration of 1 ppm, severe inhibition was recorded across the entire period of analysis.
A third experiment, which tested the effect of application of a mix of GA4 and GA7 (10 ppm, ratio of 2:1) for 25 d (Supplementary Fig. S2) also resulted in significant inhibition of bud break, as compared with the control.
To test whether the negative effect of GA on bud break is also observed under natural conditions, a field experiment was conducted. Application of GA (10 ppm) on 15 January resulted in a significant decrease of 19% and 14% in bud break on 4 March and 11 March, respectively, as compared with Triton X-100-treated control buds (Fig. 4C).
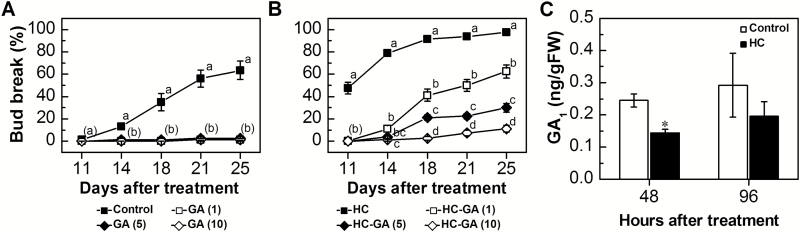
Effect of HC on the bud break inhibition induced by exogenous GA, and the endogenous GA content of grapevine buds
The effect of HC on the bud break inhibition induced by treatment with exogenous GA was tested by combined application (HC–GA; Fig. 5B) which was compared with net GA application (GA; Fig. 5A). The results were as follows. (i) Addition of HC in HC–GA treatment (Fig. 5B) rescued the inhibitory effect of 1 ppm GA (Fig. 5A), resulting in bud break that is similar to that of the untreated control (Fig. 5A). However, addition of HC failed to rescue fully the negative effect of higher concentrations of GA (5 ppm and 10 ppm), when compared with GA and control treatments. (ii) GA at 1–10 ppm significantly attenuated the enhancing effect of HC on bud break, and the degree of attenuation was concentration dependent (35, 68, and 86% decreases in bud break at 25 d in response to GA concentrations of 1, 5, and 10 ppm GA in HC–GA treatment, as compared with HC treatment, Fig. 5B).
Fig. 5.
The effect of GA3 on HC-triggered bud break. (A and B) GA buds received a single treatment of 1, 5, or 10 ppm GA3. HC–GA buds were treated with the above GA concentrations concomitantly with HC (3% Dormex®). All solutions were formulated in 0.02% Triton X-100, which served as control treatment. All other details are as in Fig. 1. Bud break was recorded 11, 14, 18, 21, and 25 d after treatment. Values represent average bud break of nine groups of 10 cuttings ±SE. (C) GA1 levels were determined in control and HC-treated buds sampled at 48 h and 96 h after treatment as described in Fig. 2. Bars represent means ±SE of three biological repeats (10 buds per repeat). Data points with different letters indicate significantly different values (P<0.05) according to Tukey’s HSD test. Asterisks between treatments indicate significant differences according to Student’s t-test (*P<0.05).
The levels of the grapevine bioactive GA molecules GA1 and GA4 were quantified in HC-treated and control buds sampled at 48 h and 96 h after treatment. GA4 was undetectable in all treated buds. GA1 was detected and its level significantly decreased (41%) at 48 h after HC treatment (Fig. 5C), as compared with control. At 96 h there was no significant difference between HC-treated and control buds.
Effect of HC treatment on expression of central components of GA metabolism in grapevine buds
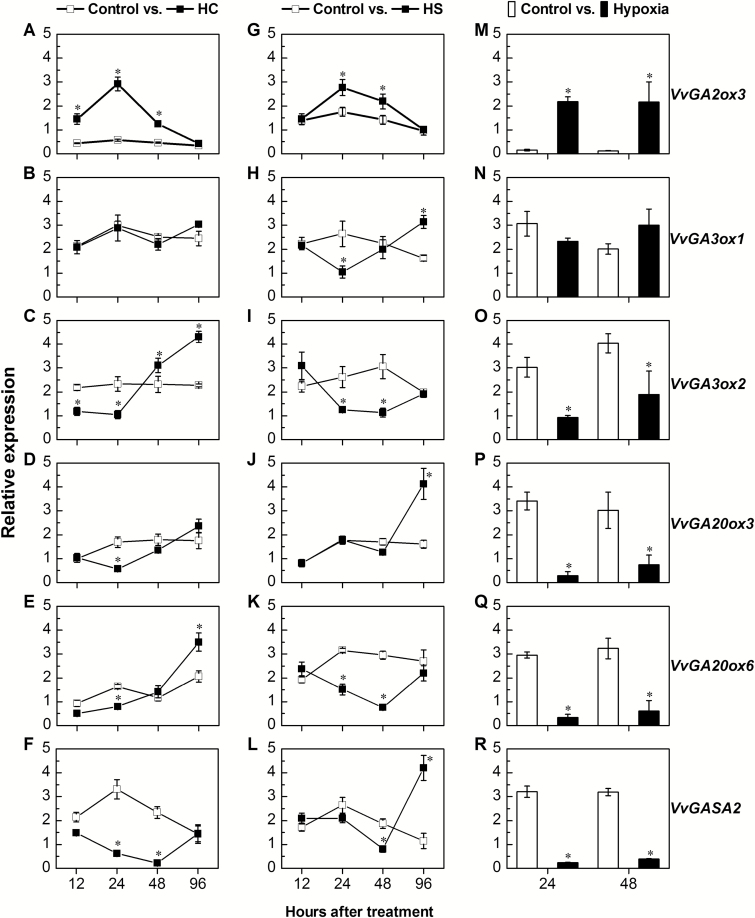
It was formerly suggested that: (i) the increased level of GA in both seeds and buds is a result, rather than a cause, of dormancy removal; and (ii) the decrease in ABA levels may be a prerequisite for elevations in GA levels and in GA sensitivity within seeds (see the Introduction). Integration of these assumptions with the results described above provides the speculation that GA might have a negative effect on grape bud dormancy release, but, nonetheless, a positive effect on regrowth after dormancy release. Following this rationale, stimuli of dormancy release might be involved in relieving the GA repression during dormancy release via modification of GA metabolism. To test this assumption, comparative transcript profiling of central regulators of GA biosynthesis and degradation was carried out, using HC-treated and control buds.
HC treatment significantly up-regulated the expression of the GA deactivation gene VvGA2ox3 at 12, 24, and 48 h, with a maximum difference of 5.2-fold at 24 h and no difference at 96 h (Fig. 6A). In contrast, the expression of the GA biosynthesis genes VvGA3ox2, VvGA20ox3, and VvGA20ox6 was significantly decreased at 24 h after HC application (Fig. 6C–E). The expression levels of VvGA3ox2 and VvGA20ox6 were also significantly increased at 96 h after HC application (Fig. 6C, E). VvGA3ox1 expression was not significantly regulated by HC at any of the time points (Fig. 6B).
Fig. 6.
The effect of artificial stimuli of dormancy release on the expression profile of central components of GA metabolism and the GA-responsive VvGASA2 gene. Total RNA was extracted from control- (0.02% Triton X-100), HC- (3% Dormex® with 0.02% Triton X-100), and HS- (50 °C water for 1 h) treated buds sampled at 12, 24, 48, and 96 h after treatment, and from hypoxia- (1% O2) treated buds at 48 h and 96 h after treatment. Relative expression of VvGA2ox3 (A, G, and M), VvGA3ox1 (B, H, and N), VvGA3ox2 (C, I, and O), VvGA20ox3 (D, J, and P), VvGA20ox6 (E, K, and Q), and VvGASA2 (F, L, and R) was determined by qRT-PCR in HC- (A–F), HS- (G–L), and hypoxia- (M–R) treated buds, as described in the Materials and methods, and normalized against VvActin and VvGAPDH. Values represent the mean expression ±SE of three biological replications, each with two technical repeats. Asterisks between treatments indicate significant differences according to Student’s t-test (*P<0.05).
Analyses of the effect of other dormancy release stimuli, hypoxia and HS, were carried out similarly (Fig. 6G–K, M–Q). As seen for treatment with HC, both HS and hypoxia significantly up-regulated VvGA2ox3 expression at 24 h and 48 h after treatment (Fig. 6G, M), but down-regulated VvGA3ox2 and VvGA20ox6 expression (Fig. 6I, K, O, Q). VvGA3ox1, which was unaffected by HC, was significantly down-regulated by HS at 24 h, and up-regulated at 96 h (Fig. 6H). Similar differences, also non-significant, were detected in VvGA3ox1 expression in response to hypoxia (Fig. 6N). In the case of VvGA20ox3, significant late (96 h) up-regulation was shown in response to HS (Fig. 6J), and significant down-regulation was evident in response to hypoxia at 24 h and 48 h (Fig. 6P).
Interestingly, expression of the GA-responsive VvGASA2 was significantly decreased in response to HC, HS, and hypoxia at 24–48 h after treatment (Fig. 6F, L, R), in accordance with the decrease in the levels of VvGA3ox and VvGA20ox transcripts (Fig. 6C–E, H–I, K, O–Q) and GA1 (Fig. 5C), and the increase in levels of the VvGA2ox transcript described above (Fig. 6A, G, M). Increases in VvGASA2 expression were recorded between 48 h and 96 h after HC and HS treatments, but this increase was significantly higher than its level in the control only at 96 h after HS treatment.
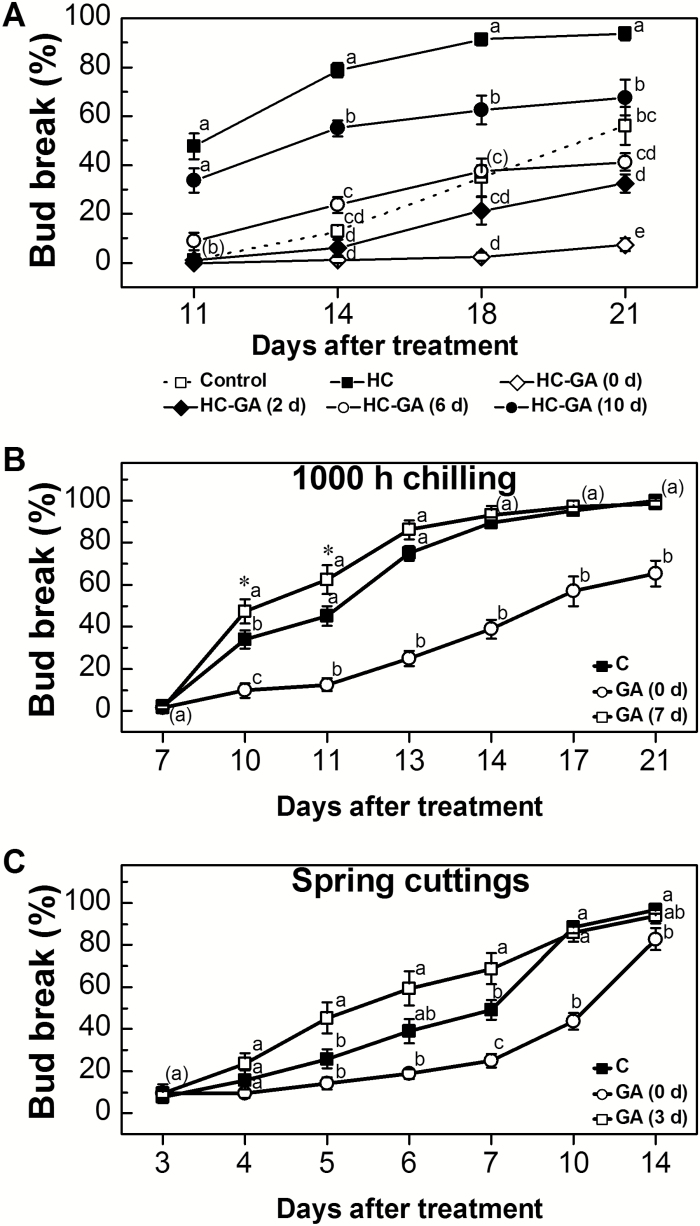
The effect of timing of GA application on bud break inhibition capacity
To understand further the nature of the inhibition exerted by GA, we monitored the effect of timing of GA3 application after stimulation of bud break by either artificial or natural means. The inhibitory effect of GA on HC-treated buds at the stage of dormancy release (sampled on 27 December) decreased significantly with increasing time lapsed between HC and GA applications (Fig. 7A). Application of GA concomitantly with HC resulted in 7.5% bud break after 21 d, whereas GA application 2, 6, and 10 d after HC treatment resulted in 32.5, 41.3, and 67.5% bud break, respectively, after 21 d. Similar results were obtained when buds, previously exposed to 1000 h of chilling, were treated with GA (Fig. 7B). Treating pre-chilled buds with GA at the beginning of the forcing process resulted in 24.3, 32.6, 50.0, 50.7, 38.2, and 34.7% inhibition of bud break, at 10, 11, 13, 14, 17, and 21 d, compared with untreated chilled buds. However, application of GA 7 d later enhanced bud break by 13.2, 17.4, and 11.1% at 10, 11, and 13 d, respectively (Fig. 7B). The bud break values of the GA (0 d) treatment were statistically different from those of the untreated chilled buds and the GA- (7 d) treated buds. The GA- (7 d) treated buds exhibited significant differences from the untreated buds only at 10 d (or at 10 d and 11 d, when analyzed separately by Student’s t-test). In a third analysis (Fig. 7C), GA was applied to bud populations after the natural completion of the endodormancy cycle (on 28 February). Application of GA at the beginning of the forcing process resulted in significant inhibition of bud break, as compared with the untreated control (24.2% and 44.5% inhibition of bud break at 7 d and 10 d, respectively), whereas application of GA 3 d after forcing had a significant enhancing effect, as compared with the untreated control (19.5% and 19.5% enhancement of bud break at 5 d and 7 d). Significant differences were evident between the two GA treatments at 5, 6, 7, and 10 d (31.3, 40.6, 43.8, and 42.2%, respectively).
Fig. 7.
The effect of the timing of application of GA3 on bud break. (A) Buds were treated with GA (10 ppm) 0, 2, 6, and 10 d after HC treatment. For additional details, see Figs 4A and 1. (B) Canes were collected on 22 November 2015, pre-chilled at 4 °C for 1000 h, and used to prepare cuttings. A set of nine groups of 10 cuttings were immediately treated with 1 ppm GA3, and another two sets (one serving as control treatment, and the other for the delayed 7 d treatment) were treated with 0.02% Triton X-100. Buds were forced for 7 d. At 7 d, one of the two Triton X-100-treated sets was treated with 1 ppm GA3. For additional details, see Fig. 1. (C) Canes were collected in the vineyard after endodormancy release (18 February 2016). The experiment was designed and undertaken as described in (B). The delayed GA treatment was carried out after 3 d. Values are averages of the nine groups in each treatment ±SE. Data points with different letters indicate significantly different values (P<0.05) according to Tukey’s HSD test. Asterisks between treatments indicate significantly differences according to Student’s t-test (*P<0.05).
Discussion
Changes in regulation of GA metabolism during the natural dormancy cycle of grapevine buds
The transcript profiles of GA metabolism genes recorded in the buds throughout the natural dormancy cycle generally agree with the changes in the level of endogenous active GA and the GA-responsive VvGASA2 transcript, with the assumptions of the proposed cascade that leads to dormancy release (Ophir et al., 2009), and with a recent study in Arabidopsis seeds (Footitt et al., 2011).
The period of dormancy induction was accompanied by a gradual decrease in transcript levels for the bud-expressed VvGA3ox and VvGA20ox genes (coding for GA biosynthetic enzymes), and their levels were lowest during deepest dormancy. On the other hand, the level of the highest bud-expressed VvGA2ox paralog, VvGA2ox3, was relatively high during this same period (Fig. 1). Similar behavior was recorded for Arabidopsis seed development under natural conditions, where increased dormancy was accompanied by decreased expression of GA3ox1 and increased expression of GA2ox2 (Footitt et al., 2011). The reduced GA biosynthesis capacity and the maintenance of stable GA inactivation ability during dormancy induction of grapevine mature buds correlates with the decrease in level of endogenous active GA1 following the period of dormancy induction, and its lower level during dormancy maintenance (Fig. 2). Overall, the presented results support the assumption that the cascade of events leading to natural bud dormancy induction involves a decrease in GA biosynthesis capacity and a maintenance of GA degradation ability, which results in decreased levels of bioactive GA following dormancy induction. Maintenance of low levels of GA may therefore be required during deep dormancy. Such an assumption was recently considered regarding hybrid aspen bud dormancy (Rinne et al., 2016), being justified in terms of the need to restrain growth of the packed embryonic shoot.
During the period of gradual dormancy release, a similar scenario was evident, but with the trends reversed. The level of VvGA2ox3 transcripts markedly decreased during the transition from deep dormancy (4 December) to the stage of dormancy release (18 December–1 January). In contrast, expression levels of the GA biosynthesis genes generally increased during the period of dormancy release. The most notable increase accompanied acquirement of maximal bud break ability within the bud population under forcing conditions (1–8 January). Similar behavior was recorded for Arabidopsis seeds developing under natural conditions, where the transition from deep dormancy to shallow dormancy was accompanied by increased expression of GA3ox1 and decreased expression of GA2ox2 (Footitt et al., 2011). Increased levels of GA3ox in response to stimuli of outgrowth have been reported in light-induced paradormant Rosa sp. buds (Choubane et al., 2012), and in decapitated paradormant hybrid aspen buds (Rinne et al., 2016).
The pattern observed in the transcript level of GA-responsive VvGASA2 (Fig. 3), with highest levels during dormancy induction, a decrease towards dormancy maintenance, and no further changes during endodormancy release, is similar to the changes detected in the GA1 level. Assuming that VvGASA2 transcription reflects the endogenous levels of GA (Herzog et al., 1995; Aubert et al., 1998), this similarity confirms the validity of the GA1 levels detected and their functional relevance. Of importance is the support for the absence of a significant increase in GA1 within the analyzed period (Fig. 2), in parallel with the increased GA biosynthesis ability and decreased GA inactivation ability during stages of dormancy release, as reflected by the transcript level.
The contradiction between the recorded increases in GA biosynthesis capacity—which supports the assumption that increased GA synthesis capacity is required either during or after endodormancy release—and the absence of accompanying increase in the GA level should be addressed. Here, we wish to note that while analyses under forcing conditions in growth chambers show that endodormancy is released, the bud population sampled in the vineyard for GA analysis is still ecodormant during January, and thus may not yet utilize its increased GA synthesis capacity. This may simply stem from the absence of a temperature appropriate for synthesis or may reflect the need for meristem activation for GA synthesis, as part of the regulated developmental program. In this regard, it is worth noting similar changes in levels of bioactive GAs recorded across the potato dormancy cycle in storage, using tubers that were transferred to forcing conditions (20o C for 7 d) prior to GA analysis. In agreement with our finding, it was shown that (i) GA4 was not detected in potato buds; (ii) GA1 levels decreased during dormancy in storage; and (iii) GA1 levels increased only in tubers that had already exhibited actively elongating sprouts (Suttle, 2004).
The parallel decreases observed in VvGA3ox and VvGA20ox transcript levels and in the GA level following dormancy induction count against negative feedback regulation by GA, and suggest the involvement of another mode of regulation. The relatively high level of VvGA2ox may be a consequence of the high GA level at the beginning of the cycle. However, the absence of changes in its level during dormancy maintenance despite significant decreases in GA level question simple regulation, again suggesting contributions by other regulators. In both cases, ABA appears to be an appropriate candidate (Seo et al., 2006; Toh et al., 2008). In agreement with this, an increased synthetic capacity of ABA and an increase in its levels were previously recorded in grapevine buds during dormancy induction (Zheng et al., 2015).
During dormancy release, the increases in VvGA3ox and VvGA20ox transcript levels and decreased VvGA2ox transcripts levels in principle agree with a GA feedback mechanism. Yet, some observations indicate that additional regulators may be involved in these changes: (i) a decreased GA level is evident from the end of November (the stage of dormancy maintenance, Fig. 2), but a sharp induction of VvGA3ox2 and VvGA20ox3 occurs only at the beginning of January; (ii) VvGA20ox6, which is also regulated by GA (Supplementary Fig. S1), is not induced in parallel with VvGA3ox2 and VvGA20ox3; and (iii) expression levels of VvGA2ox3 and VvGA2ox4 significantly decrease at the onset of dormancy release, whereas the GA level remains stably low from the end of November. Again, in both cases, ABA is an appropriate candidate for regulation of these changes (Seo et al., 2006). In agreement with this, increased degradation capacity of ABA and levels of its degradation products were previously recorded in grapevine buds during dormancy release (Zheng et al., 2015).
Exogenous GA delays bud dormancy release and limits the enhancing effect of HC
Based on the assumptions of the working model, and the above-described finding regarding changes in GA metabolism throughout the natural dormancy cycle, it was assumed that application of GA would bypass the need for regulated activation of GA biosynthesis during dormancy release, and serve as a direct stimulus of dormancy release. This assumption was supported by several reports in the literature: (i) GA3 application enhanced bursting of dormant Elberta peach buds (Donoho and Walker, 1957); and (ii) GA4 enhanced dormancy release of Japanese apricot flower buds (Zhuang et al., 2013) and poplar buds (Rinne et al., 2011).
The data presented in the current study do not support this prediction, as addition of exogenous GA3 did not stimulate grapevine bud break. On the contrary, a concentration-dependent inhibitory effect of GA3 was documented, both in the laboratory and in field trials (Fig. 4). Significant inhibition was also documented in response to GA4 + 7 (Supplementary Fig. S2). This inhibitory effect corroborates data reported in peach (Reinoso et al., 2002), kiwi fruit (Lionakis and Schwabe, 1984), and persimmon (Kang et al., 1998). A more complicated scenario has been described in poplar, where GA3 application led to bud abscission and protrusion of embryonic leaves, whereas GA4 induced bud burst (Rinne et al., 2011). Indeed, it was previously reported that GA application prolonged grapevine dormancy (Weaver, 1959; Iwasaki, 1980). The delay exerted by exogenous GA on the advancing effect of HC (Fig. 5), which appeared to be concentration dependent as well, further suggests that GA somehow interferes with the cascade of biochemical changes required for bud break. Examples of a negative effect of GA on outgrowth of paradormant buds also appear in the literature, as described in the Introduction.
The changes recorded throughout the natural dormancy cycle and in response to exogenous GA treatments may seem contradictory at first glance. However, the contradiction may be reconciled by the hypothesis that the effects of GA treatment are a complex function that specifically depends on bud dormancy status. According to this hypothesis, increasing the level of GA by exogenous application prior to removal of the inhibition of meristem activity, an increase that is naturally inhibited in the buds at that stage, has a negative effect on dormancy release, whereas increasing its level after dormancy release has a positive effect on growth of bud primordia. This hypothesis is supported by the results of analysis of mature Arabidopsis seeds subjected to 7 d of imbibition in moist-chilled conditions, in which levels of GA4 (the biologically active GA detected in mature Arabidopsis seeds) decreased ~3-fold after 48 h, and increased only 18 h after transfer to germination conditions (Chiwocha et al., 2005). The hypothesis may also be supported by the results of analysis of stored potato tubers, where (i) application of GA did not promote sprouting of deeply dormant tubers; (ii) exposure of tubers to inhibitors of GA biosynthesis did not extend tuber dormancy but rather hastened dormancy release; (ii) endogenous GA1 levels increased only in tubers that already exhibit actively elongating sprouts; and (iv) GA induces sprouting of non-dormant tubers (Suttle, 2004).
This hypothesis also serves to expand previous assumptions that an increase in endogenous GA may be a result rather than a cause of bud dormancy removal, and that GA may be needed for bud expansion after dormancy release (Saure, 1985; Lang, 1994).
The negative effect of GA treatments on grapevine bud break depends on bud dormancy status
Treatment with GA had no negative effect on bud burst when applied 10 d after HC application (Fig. 7A). This suggests that: (i) the inhibitory effect of exogenous GA is not a non-specific, wide-ranging suppressive effect on bud primordial growth activity; and (ii) the inhibitory effect of GA on HC-treated buds is greatest when GA is applied either when the meristem activity is still repressed or during the very early stages of removal of repression. However, when applied after dormancy is released, GA would not have an inhibitory effect. In that regard, it is instructive to note that HC-induced biochemical changes start as early as 24 h after treatment, level off at 96 h, and lead to actual burst after 14 d or earlier, depending on dormancy status (Ophir et al., 2009). Hence, at 10 d, the bud meristem activity may already have resumed.
The inhibitory effect of GA, when applied to pre-chilled buds or to naturally ecodormant buds in parallel with forcing initiation, and its enhancing effect when applied 7 d and 3 d later, respectively (Fig. 7B, C), further suggest that GA application during initial activation of the endo- or ecodormant woody bud meristem has a negative effect on meristem activation, while its application to an already activated meristem enhances regrowth.
Support for this idea comes from (i) the inhibitory effect of GA3 on kiwi fruit bud break when applied before chilling accumulation, and its promoting effect when applied after exposure to optimal chilling hours which enhance dormancy release (Lionakis and Schwabe, 1984); and (ii) the observation that application of biologically active GAs was markedly less effective than application of fluridone, an inhibitor of ABA synthesis, for enhancement of dormancy release of Arabidopsis seeds. Interestingly, combined application of fluridone with GA3 improved the enhancement of dormancy release, compared with that of fluridone alone. It was therefore suggested that a decrease in ABA level and dormancy intensity may be a prerequisite for a positive effect of elevated levels of GA and sensitivity to GA during seed germination (Ali-Rachedi et al., 2004).
The expression profiles of GA metabolism regulators in response to artificial stimuli of bud break suggest complex and dormancy status-dependent regulation
The improved bud break of combined HC–GA-treated buds, as compared with buds treated with GA alone (Fig. 5A, B), suggests that exposure to HC may facilitate manipulation of the artificially increased levels of GA. In a wider view, HC may enhance bud break through precise regulation, over time, of the levels of bioactive GA in the buds: preventing a rise of GA levels in the buds prior to meristem growth, whilst enhancing GA biosynthesis at or after resumption of meristem growth.
In line with the above, HC application resulted in decreases and increases, respectively, of bud-expressed, functionally characterized GA biosynthesis and deactivation genes (Giacomelli et al., 2013) 12–24 h following HC application (Fig. 6A, C–E). Interestingly, a similar scenario was recently described during natural dormancy release of Japanese pear flower buds (Bai et al., 2013). This provides support for the suggestion that temporary limitation of GA availability is particularly important at the time of removal of the dormancy block. The significant decrease in GA level at 48 h after HC application (Fig. 5C) supports the idea that the effect of HC on transcription indeed leads to a temporary decrease in GA availability. The decreased expression of the GA-responsive VvGASA2 24–48 h after application of HC (Fig. 6F) further supports the suggestion that treatment with HC reduces the GA level within this time window.
The effect of HC was multifaceted and dependent on time of application, as a reverse effect was evident at 96 h (i.e. an increased transcript level of GA biosynthesis enzymes and VvGASA2, and a decrease of GA deactivation enzymes, Fig 6A, C–F). These opposing effects of HC, which depend on dormancy status (assuming that at 96 h repression was removed), suggest that the effect of HC over time may involve optimized co-ordination of hormonal interactions during the cascade of events that start with dormancy release and continue to meristem activation. According to the suggested scenario, HC initially ‘guarantees’ that GA availability is limited until dormancy is released, and later increases in GA availability enhance primordial regrowth. The validity of the timing-dependent reverse effects of HC on expression of GA biosynthesis and catabolism regulators is supported by similar effects recorded in response to other dormancy release stimuli, such as hypoxia and HS (Fig. 6G–K, M–Q). It is also supported by the expression profiles of VvGASA2 (Fig. 6L, R). The decreased GA level observed in Arabidopsis seeds during imbibition, and its increase only after transfer to conditions that favor germination, also agrees with the hypothesis (Chiwocha et al., 2005).
Does the GA inhibitory effect operate via inhibition of CK function during meristem activation?
The results that have been presented clearly demonstrate an inhibitory effect of GA when applied to dormant grapevine buds, and there are indications for its relevance in natural situations. However, the detailed nature of this inhibitory effect is not yet clear.
Interestingly, GA is known to repress numerous CK responses in various developmental processes in different plants (Greenboim-Wainberg et al., 2005; Fleishon et al., 2011). Among various antagonistic effects, it was shown that GA constitutive signaling has a detrimental effect on shoot apical meristem (SAM) function, which requires CK for establishment and maintenance of meristematic identity (Jasinski et al., 2005; Skylar and Wu, 2011, and references within). A positive role for CK in regulation of paradormant bud outgrowth is also well established (Domagalska and Leyser, 2011; Müller and Leyser, 2011; Braun et al., 2012; Dun et al., 2012). In light of the above, we propose a speculative scenario in which the GA inhibitory effect on grapevine bud break results from its antagonistic effect on CK responses required for activation of the bud meristem. In agreement with this, decreased CK levels in potato tubers by overexpression of cytokinin oxidase/dehydrogenase1 (CKX) resulted in a prolonged dormancy period which was not affected by GA3. It was therefore suggested that CK has an essential role in terminating tuber dormancy, whereas GA supports sprout growth after dormant meristem activation (Hartmann et al., 2011). Interestingly, preliminary analysis revealed that CK levels are dramatically increased during natural and artificial dormancy release, supporting the assumption that CK plays a role during grape woody bud meristem activation. The suggested scenario, and its underlying assumptions, will require future detailed study.
In light of the above, a speculative working model is suggested, as a foundation for further study. According to this model, the GA level decreases during dormancy induction, due to down-regulated expression of GA biosynthesis genes. A parallel increase of ABA biosynthesis capacity may be involved in regulation of such decreased GA biosynthetic capacity. GA is then maintained at a low level until the meristem is activated, since its premature increase will inhibit meristem activation. Inhibition may be mediated by repression of CK responses which are involved in regulation of meristem activation. Once the meristem is activated, GA is required to support primordial growth and bud burst. Increased ABA degradation capacity during dormancy release may participate in up-regulation of GA biosynthetic capacity, by induction of GA3ox and GA20ox expression. Low levels of GA during dormancy release may also contribute to such up-regulation of expression by a negative feedback mechanism.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Schematic details of all the GA treatments.
Table S2. Primers used for gene expression analyses by qRT-PCR.
Fig. S1. The effect of GA3 application on the expression profiles of the GA-responsive VvGASA2 gene and the central components of GA metabolism.
Fig. S2. The effect of GA4 + 7 application on bud break of single-node cuttings.
Acknowledgements
This research was supported by the United States–Israel Binational Agricultural Research and Development Fund (BARD grant no. IS-4639- 13 to EO, DG, and RO).
References
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology 19, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Acheampong AK, Hu J, Rotman A et al. 2015. Functional characterization and developmental expression profiling of gibberellin signalling components in Vitis vinifera. Journal of Experimental Botany 66, 1463–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheampong AK, Rotman A, Zheng C, Keren A, Halaly T, Crane O, Ogrodovitch A, Or E. 2010. A method for isolating total RNA from mature buds and other woody tissues of Vitis vinifera. In: Delrot S, Medrano H, Or E, Bavaresco L, Grando S, eds. Methodologies and results in grapevine research. Dordrecht: Springer Netherlands, 301–307. [Google Scholar]
- Acheampong AK, Zheng C, Halaly T, Giacomelli L, Takebayashi Y, Jikumaru Y, Kamiya Y, Lichter A, Or E. 2017. Abnormal endogenous repression of GA signaling in a seedless table grape cultivar with high berry growth response to GA application. Frontiers in Plant Science 8, 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agharkar M, Lomba P, Altpeter F, Zhang H, Kenworthy K, Lange T. 2007. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnology Journal 5, 791–801. [DOI] [PubMed] [Google Scholar]
- Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M. 2004. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219, 479–488. [DOI] [PubMed] [Google Scholar]
- Aubert D, Chevillard M, Dorne AM, Arlaud G, Herzog M. 1998. Expression patterns of GASA genes in Arabidopsis thaliana: the GASA4 gene is up-regulated by gibberellins in meristematic regions. Plant Molecular Biology 36, 871–883. [DOI] [PubMed] [Google Scholar]
- Bai S, Saito T, Sakamoto D, Ito A, Fujii H, Moriguchi T. 2013. Transcriptome analysis of Japanese pear (Pyrus pyrifolia Nakai) flower buds transitioning through endodormancy. Plant and Cell Physiology 54, 1132–1151. [DOI] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP et al. 2012. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiology 158, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR. 2005. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. The Plant Journal 42, 35–48. [DOI] [PubMed] [Google Scholar]
- Choubane D, Rabot A, Mortreau E et al. 2012. Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. Journal of Plant Physiology 169, 1271–1280. [DOI] [PubMed] [Google Scholar]
- Dai M, Zhao Y, Ma Q, Hu Y, Hedden P, Zhang Q, Zhou DX. 2007. The rice YABBY1 gene is involved in the feedback regulation of gibberellin metabolism. Plant Physiology 144, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière JM, Wild M, Regnault T, Baumberger N, Eisler H, Genschik P, Achard P. 2014. class I TCP–DELLA interactions in inflorescence shoot apex determine plant height. Current Biology 24, 1923–1928. [DOI] [PubMed] [Google Scholar]
- Dill A, Sun T. 2001. Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews. Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Donoho CW, Walker DR. 1957. Effect of gibberellic acid on breaking of rest period in Elberta peach. Science 126, 1178–1179. [DOI] [PubMed] [Google Scholar]
- Duan C, Li X, Gao D, Liu H, Li M. 2004. Studies on regulations of endogenous ABA and GA3 in sweet cherry flower buds on dormancy. Acta Horticulturae Sinica 31, 149–154. [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasoli M, Dal Santo S, Zenoni S et al. 2012. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. The Plant Cell 24, 3489–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleishon S, Shani E, Ori N, Weiss D. 2011. Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytologist 190, 609–617. [DOI] [PubMed] [Google Scholar]
- Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. 2011. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proceedings of the National Academy of Sciences, USA 108, 20236–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby JW, Seeley SD. 1993. Chilling of endodormant peach propagules: IV. Terminal shoot growth of cuttings, including gibberellic acid treatments. Journal of the American Society for Horticultural Science 118, 263–268. [Google Scholar]
- Giacomelli L, Rota-Stabelli O, Masuero D, Acheampong AK, Moretto M, Caputi L, Vrhovsek U, Moser C. 2013. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: functional characterization and evolution of grapevine gibberellin oxidases. Journal of Experimental Botany 64, 4403–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, Kawaide H, Kamiya Y, Yoshioka T. 2004. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. Journal of Experimental Botany 55, 111–118. [DOI] [PubMed] [Google Scholar]
- Greenboim-Wainberg Y, Maymon I, Borochov R, Alvarez J, Olszewski N, Ori N, Eshed Y, Weiss D. 2005. Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. The Plant Cell 17, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I et al. 2006. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. The Plant Cell 18, 3399–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S. 2011. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiology 155, 776–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauvermale AL, Ariizumi T, Steber CM. 2012. Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiology 160, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Dorne AM, Grellet F. 1995. GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Molecular Biology 27, 743–752. [DOI] [PubMed] [Google Scholar]
- Hirano K, Ueguchi-Tanaka M, Matsuoka M. 2008. GID1-mediated gibberellin signaling in plants. Trends in Plant Science 13, 192–199. [DOI] [PubMed] [Google Scholar]
- Iwasaki K. 1980. Effects of bud scale removal, calcium cyanamide, GA3, and ethephon on bud break of ‘Muscat of Alexandria’ grape (Vitis vinifera L.). Journal of the Japanese Society for Horticultural Science 48, 395–398. [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M. 2005. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Current Biology 15, 1560–1565. [DOI] [PubMed] [Google Scholar]
- Jung CJ, Hur YY, Jung SM et al. 2014. Transcriptional changes of gibberellin oxidase genes in grapevines with or without gibberellin application during inflorescence development. Journal of Plant Research 127, 359–371. [DOI] [PubMed] [Google Scholar]
- Kang S, Motosugi H, Yonemori K, Sugiura A. 1998. Cold hardiness of persimmon (Diospyros kaki Thunb.) buds in relation to dormancy release and temperature conditioning. Journal of the Japanese Society for Horticultural Science 67, 153–160. [Google Scholar]
- Karlberg A, Englund M, Petterle A, Molnar G, Sjödin A, Bako L, Bhalerao RP. 2010. Analysis of global changes in gene expression during activity–dormancy cycle in hybrid aspen apex. Plant Biotechnology 27, 1–16. [Google Scholar]
- Khalil-Ur-Rehman M, Sun L, Li C-X, Faheem M, Wang W, Tao J-M. 2017. Comparative RNA-seq based transcriptomic analysis of bud dormancy in grape. BMC Plant Biology 17, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA. 1994. Dormancy—the missing link: molecular studies and integration of regulatory plant and environmental interactions. HortScience 29, 1255–1263. [Google Scholar]
- Lionakis SM, Schwabe WW. 1984. Bud dormancy in the kiwi fruit, Actinidia chinensis Planch. Annals of Botany 54, 467–484. [Google Scholar]
- Lo SF, Yang SY, Chen KT, Hsing YI, Zeevaart JA, Chen LJ, Yu SM. 2008. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. The Plant Cell 20, 2603–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Sandberg LG, Moritz T. 2011. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. The Plant Journal 67, 805–816. [DOI] [PubMed] [Google Scholar]
- Middleton AM, Ubeda-Tomás S, Griffiths J et al. 2012. Mathematical modeling elucidates the role of transcriptional feedback in gibberellin signaling. Proceedings of the National Academy of Sciences, USA 109, 7571–7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O. 2011. Auxin, cytokinin and the control of shoot branching. Annals of Botany 107, 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Gao C, Chen MS, Pan BZ, Ye K, Xu ZF. 2015. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant and Cell Physiology 56, 1655–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. 2003. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell 15, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir R, Pang X, Halaly T, Venkateswari J, Lavee S, Galbraith D, Or E. 2009. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene–ABA interplay and cell enlargement. Plant Molecular Biology 71, 403–423. [DOI] [PubMed] [Google Scholar]
- Or E. 2009. Grape bud dormancy release—the molecular aspect. In: Roubelakis-Angelakis KA, ed. Grapevine molecular physiology & biotechnology. Dordrecht: Springer, 1–29. [Google Scholar]
- Or E, Vilozny I, Fennell A, Eyal Y, Ogrodovitch A. 2002. Dormancy in grape buds: isolation and characterization of catalase cDNA and analysis of its expression following chemical induction of bud dormancy release. Plant Science 162, 121–130. [Google Scholar]
- Peng J, Harberd NP. 1997. Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiology 113, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. 2006. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso H, Luna V, Dauría C, Pharis RP, Bottini R. 2002. Dormancy in peach (Prunus persica) flower buds. VI. Effects of gibberellins and an acylcyclohexanedione (trinexapac-ethyl) on bud morphogenesis in field experiments with orchard trees and on cuttings. Canadian Journal of Botany 80, 664–674. [Google Scholar]
- Richards DE, King KE, Ait-ali T, Harberd NP. 2001. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annual Review of Plant Biology 52, 67–88. [DOI] [PubMed] [Google Scholar]
- Rinne PL, Paul LK, Vahala J, Kangasjärvi J, van der Schoot C. 2016. Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen. Journal of Experimental Botany 67, 5975–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne PL, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjärvi J, van der Schoot C. 2011. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. The Plant Cell 23, 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saure MC. 1985. Dormancy release in deciduous fruit trees. Horticultural Reviews 7, 239–300. [Google Scholar]
- Sawada Y, Aoki M, Nakaminami K et al. 2008. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiology 146, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A et al. 2006. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. The Plant Journal 48, 354–366. [DOI] [PubMed] [Google Scholar]
- Skylar A, Wu X. 2011. Regulation of meristem size by cytokinin signaling. Journal of Integrative Plant Biology 53, 446–454. [DOI] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P. 1998. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Ritchie S, Soule JD, McGinnis KM, Steber CM. 2004. Recessive-interfering mutations in the gibberellin signaling gene SLEEPY1 are rescued by overexpression of its homologue, SNEEZY. Proceedings of the National Academy of Sciences, USA 101, 12771–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Abernathy SD, White RH, Finlayson SA. 2011. Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant, Cell and Environment 34, 1986–1998. [DOI] [PubMed] [Google Scholar]
- Sun TP. 2010. Gibberellin–GID1–DELLA: a pivotal regulatory module for plant growth and development. Plant Physiology 154, 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP. 2011. The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Current Biology 21, R338–R345. [DOI] [PubMed] [Google Scholar]
- Suttle JC. 2004. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. Journal of Plant Physiology 161, 157–164. [DOI] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A et al. 2008. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology 146, 1368–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Federici F, Casimiro I, Beemster GT, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ. 2009. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Current Biology 19, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GT, Hedden P, Bhalerao R, Bennett MJ. 2008. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nature Cell Biology 10, 625–628. [DOI] [PubMed] [Google Scholar]
- van den Heuvel K, Barendse GWM, Wullems GJ. 2001. Effect of gibberellic acid on cell division and cell elongation in anthers of the gibberellin deficient gib-1 mutant of tomato. Plant Biology 3, 124–131. [Google Scholar]
- Vergara R, Noriega X, Aravena K, Prieto H, Pérez FJ. 2017. ABA represses the expression of cell cycle genes and may modulate the development of endodormancy in grapevine buds. Frontiers in Plant Science 8, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RJ. 1959. Prolonging dormancy in Vitis vinifera with gibberellin. Nature 183, 1198–1199. [DOI] [PubMed] [Google Scholar]
- Weiss D, Ori N. 2007. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiology 144, 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annual Review of Plant Physiology 59, 225–251. [DOI] [PubMed] [Google Scholar]
- Zheng C, Halaly T, Acheampong AK, Takebayashi Y, Jikumaru Y, Kamiya Y, Or E. 2015. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. Journal of Experimental Botany 66, 1527–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W, Gao Z, Wang L, Zhong W, Ni Z, Zhang Z. 2013. Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. Journal of Experimental Botany 64, 4953–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.