Abstract
Inhibition of the bile salt export pump (BSEP) by a drug has been implicated as a risk factor for a drug’s potential to cause drug-induced liver injury (DILI) and is thought to be an important mechanism leading to DILI. For a wide variety of drugs a correlation has been observed between the potency of in vitro BSEP inhibition and its propensity to cause DILI in humans. These findings were interpreted to suggest that BSEP inhibition could be an important mechanism to help explain how some drugs initiate DILI. Because the Biopharmaceutics Drug Disposition Classification System (BDDCS) can be useful in characterizing and predicting some important transporter effects in terms of drug-drug interactions, we evaluated the information provided by BDDCS in order to understand the inhibition propensity of BSEP. Here we analyze the relationship between a compound’s ability to inhibit BSEP function and cause liver injury in humans using a compilation of published DILI datasets that have screened for BSEP inhibitors, other hepatic transporters and other mechanism-based toxicity key events. Our results demonstrate that there is little support for in vitro BSEP inhibition being universally DILI predictive. Rather we show that most potent BSEP inhibitors are BDDCS class 2 drugs, which we have demonstrated previously is the BDDCS class most likely to be DILI related. Since BDDCS class is not related to any proposed DILI mechanistic hypotheses, we maintain that if measures of BSEP inhibition alone or together with inhibition of other transporters cannot be differentiated from class 2 assignment, there is no support for in vitro BSEP inhibition being DILI predictive.
Keywords: drug-induced liver injury, BSEP inhibition, MRP3, MRP4, MDR3, mitochondrial toxicity
Drug-induced liver injury (DILI) encompasses a spectrum from mild biochemical abnormalities to acute liver failure. DILI is often difficult to distinguish from natural causes of liver injury such as viral hepatitis or autoimmune conditions (Khoury et al., 2015; Ogese et al., 2016). DILI often exhibits delayed onset (5 to >100 days) during continuous therapy and even may occur after cessation of therapy. Although, the underlying pathophysiological mechanism of DILI is still poorly understood, there is increasing evidence that cholestatic forms of DILI result from a drug- or metabolite-mediated inhibition of hepatobiliary transporter systems (Morgan et al., 2013). Inhibition of the bile salt export pump (BSEP) by a drug has been implicated as a risk factor for the drug’s potential to cause DILI and is thought to be an important mechanism that leads to DILI (Aleo et al., 2014, 2017; Dawson et al., 2012; Morgan et al., 2010, 2013; Schadt et al., 2015). This hypothesis results from evidence that genetic predisposition to cholestatic DILI due to BSEP gene mutations are thought to cause DILI. Patients with mutations in the ABCB11/BSEP gene that result in reduced expression levels or function of BSEP (eg, progressive familial intrahepatic cholestasis type II, PFIC2) exhibit reduced bile acid excretion compared with normal patients, and rapidly develop liver injury suspected to be due to hepatocellular accumulation of toxic bile acids (Jansen et al., 1999; Jansen and Müller, 2000; Perez and Britz, 2009). However, the extrapolation of this genetic defect to the supposition that compounds exhibiting BSEP inhibition in vitro will be DILI causative agents is tenuous.
Many drugs that cause infrequent but clinically severe liver injury in humans have been found to inhibit BSEP activity in vitro using a variety of different experimental model systems, and in vivo in experimental animals (Kis et al., 2012; Morgan et al., 2010). However, the relevance of experimental animal studies are also tenuous as studies in BSEP knockout mice indicate a milder phenotype than seen in PFIC2 humans, with the knockout mice lacking the development of progressive cholestasis (Wang et al., 2001). For a wide variety of drugs a correlation has also been observed between propensity to cause DILI in humans, potency of in vitro BSEP inhibition and their therapeutic plasma drug concentrations (Shah et al., 2015). These findings suggest that BSEP inhibition could be an important mechanism that helps explain how some drugs initiate DILI. Recently, BSEP has also been highlighted by the International Transporter Consortium as one of the emerging transporters that need to be considered when evaluating drug safety (Hillgren et al., 2013). However, the practical utility of this approach is still in its infancy and needs to be further evaluated. BSEP inhibition is just one of many possible mechanisms that can initiate idiosyncratic DILI, therefore it has been suggested that screening for in vitro BSEP inhibition is likely to be of greatest value if undertaken together with screening for other relevant adverse effects (eg, mitochondrial injury, cell cytotoxicity, metabolic bioactivation to toxic moieties) and understanding its inhibition predisposition along with some basic physicochemical properties (Aleo et al., 2014, 2017; Thompson et al., 2016). Recent research suggests that bile acids affect the mitochondria and potentially lead to mitochondrial membrane permeability transition (Schulz et al., 2013).
We have recently compared the possibility of predicting DILI potential using the Biopharmaceutics Drug Disposition Classification System (BDDCS) versus previously proposed published methods (Chan and Benet, 2017). Because BDDCS can be useful in characterizing and predicting some important transporter effects in terms of drug-drug interactions (Shugarts and Benet, 2009), we believe it would be useful to apply BSEP as a potential biomarker and evaluate the information provided by BDDCS in order to understand the inhibition propensity of BSEP. We previously compared the distribution of BSEP inhibition with the FDA hepatic liability for 264 drugs in the Chen et al. (2011) dataset and 181 drugs in the Pedersen et al. (2013) dataset with BDDCS classification of these drugs showing that drug label severity or strength of BSEP inhibition, respectively, correlated with the increase of BDDCS class 2 drugs in the drug population. For the Pedersen et al. (2013) dataset, 84.6% of strong BSEP inhibitors were BDDCS class 2 drugs. Our previous analyses suggest that comparison of proposed DILI predictive metrics with just avoiding BDDCS class 2 drugs may serve as a useful baseline in evaluating the validity of these metrics (Chan and Benet, 2017). Here, we examine further BSEP inhibition datasets (and the dose relationship in the Pedersen et al., 2013; dataset) and suggest that if a correlation of the ability of in vitro BSEP inhibition to predict DILI is not better than the correlation of the toxicity measure with BDDCS class 2 assignment, then the field can have no confidence that the measurement will usefully serve as a mechanistic predictor.
Several groups of researchers have proposed that proactive in vitro screening for BSEP during drug discovery may aid in early flagging and de-selection of compounds that exhibit a high propensity to cause idiosyncratic DILI (Aleo et al., 2017; Dawson et al., 2012; Morgan et al., 2010, 2013; Pedersen et al., 2013). Therefore, our present goal is to evaluate the potential of in vitro BSEP inhibition screening in aiding the prediction of DILI. Here we analyze the relationship between a compound’s ability to inhibit BSEP function and cause liver injury in humans using a compilation of published DILI datasets that have screened for BSEP inhibitors, other hepatic transporters, specifically MRP3, MRP4, and MDR3 inhibition and other mechanism-based toxicity key events such as the mitochondrial and cell toxicity (Aleo et al., 2014, 2017; Dawson et al., 2012; Köck et al., 2014; Morgan et al., 2013; Pedersen et al., 2013; Schadt et al., 2015).
MATERIALS AND METHODS
Compilation of BSEP Datasets
Classifying BSEP inhibition
FDA drug labels for 182 registered drugs have been evaluated by Pedersen et al. (2013) for BSEP inhibition using an in vitro membrane vesicle BSEP inhibition assay. Assignment to BSEP inhibition categories was based on the ATP dependent taurocholate (TC) transport rate when coincubated with 50 μM of test compound. Pedersen et al. (2013) defined compounds as: BSEP Inhibitors when they decreased TC transport by >50%; BSEP Weak Inhibitors when TC transport was decreased by 27.5%–50%; BSEP Noninhibitors showed a minimal decrease of TC transport by <27.5%. All compounds but L-carnitine (“No mention”, “No DILI”) could be BDDCS classified. For BDDCS Classification, only active species (eg, drug but not prodrug) were considered. In cases where DILI knowledge is limited by FDA drug labels, we have used annotations of human DILI concern collected by Chen et al. (2016). All compounds except glyburide (“Adverse Reactions”), lopinavir (“Warning and Precautions”), and sulfamethoxazole (“Warning and Precautions”) were assigned a DILI concern by Chen et al. (2016), resulting in the analysis of 178 drugs. We also reviewed the Dawson et al. (2012) dataset that investigated the relationship between in vitro human BSEP inhibition for 85 pharmaceuticals. As defined by Dawson et al. (2012), IC50 < 300 μM gave an optimal separation between drugs that causes cholestatic/mixed DILI and drugs that caused hepatocellular or no DILI. Drugs with IC50 < 300 μM were considered as BSEP Inhibitors, while all others were considered BSEP Noninhibitors (this includes BSEP Weak Inhibitors where 300 μM < IC50 < 1000 μM). All compounds except clobetasol propionate (“No DILI”) and picotamide (“No DILI”) could be BDDCS classified. Chlorpropamide was also removed from the analysis because it is a BDDCS class 0 compound (ie, BDDCS class changes as a function of urine pH). This resulted in an 82 drug dataset.
Classifying BSEP inhibition and mitochondrial toxicity
Aleo et al. (2014) selected 72 compounds from the 287 compounds reported by Chen et al. (2011) to test the hypothesis of a synergistic relationship between BSEP inhibition and mitochondrial toxicity. However, since they were testing a BSEP inhibition hypothesis, they ignored any “Most-DILI concern” molecules that did not exhibit in vitro BSEP inhibition. In our analysis here we evaluated 42 drugs in the Aleo dataset, 24 drugs that exhibited “Most DILI concern” and 18 drugs that exhibited “No DILI concern” for which BDDCS classification was available. That is, we ignored drugs classified as “Less DILI” concern. Categorization of DILI concern were derived by examining the currently approved label in the Chen et al. (2011) dataset (and thus the Aleo et al., 2014; dataset). In this dataset, compounds with IC50 > 100 μM were defined as BSEP Noninhibitors and Mitotox IC50 < 100 nmol/mg were defined as mitochondrial toxic compounds. We have also collected data from the 120 compounds investigated by Schadt et al. (2015) for a number of assays that covered various mechanisms and endpoints associated with human DILI. In that dataset 106 drugs were BDDCS classified. For the purpose of this study we chose to focus only on the results of BSEP, mitochondrial toxicity, and cytotoxicity assays. Although we analyzed the Schadt et al. (2015) dataset in our previous publication (Chan and Benet, 2017), we include a subset here for comparison with the Aleo et al. (2014) dataset. As defined by Schadt et al. (2015) drugs with BSEP IC50 > 250 μM were considered BSEP Noninhibitors; all others were considered BSEP Inhibitors. For the mitochondrial toxicity assay a ratio of IC50glucose/IC50galactose ≥3 was considered a mitochondrial toxicity flag. Compounds with TC50 <100 μM were considered positive for cellular toxicity. As noted above, different authors used different in vitro IC50 cutoffs to define DILI predictivity. Thus, each dataset is re-evaluated here independently.
Classifying BSEP, MRP3, MRP4, and MDR3 in vitro transport inhibition
The inhibitory effect of 88 drugs (100 μM) on MRP3- and MRP4- mediated substrate transport was measured in membrane vesicles by Köck et al. (2014). Drugs selected for investigation included 50 BSEP noninhibitors (24 noncholestatic; 26 cholestatic) and 38 BSEP inhibitors (16 noncholestatic; 22 cholestatic). All compounds but clobetasol propionate (“No DILI”), fluorescein (“No DILI”) and valinomycin (“No DILI”) could be BDDCS classified. Chlorpropamide was also removed because it is a BDDCS class 0 compound. Vinblastine (“Hepatocellular”) was also omitted from the dataset because no BSEP inhibition information was reported, resulting in an 83 drug dataset. Drugs were also categorized as cholestatic or hepatocellular, according to the DILI type reported in the literature. As defined by Köck et al. (2014) the compounds were further classified as active for the specified transporter if they had an IC50 ≤ 135 µM for BSEP or a percent inhibition ≥21% compared with control at 100 µM for MRP3 and MRP4; otherwise, they were classified as inactive against that transporter. The MRP4 classifications are based on findings by Köck et al. (2014) that compounds that inhibit MRP4 by at least 21% have a 50% chance of being cholestatic and the rationale for the BSEP classifications is to identify inhibitor compounds with both potent and moderate cholestatic risk, similar to Morgan et al. (2013).
We also investigated 125 pharmaceuticals (70 as Most DILI Concern and 55 as No DILI Concern) that were screened for MDR3 inhibition (Aleo et al., 2017). All compounds but triprolidine hydrochloride (No DILI concern), brompheniramine (No DILI concern), doxylamine (No DILI concern), carbetapentane citrate (No DILI concern), zimeldine (Most DILI concern) and pamabrom (No DILI concern) could be BDDCS classified, resulting in a 119 drug dataset. In our analysis, we evaluated the 2 different IC50 cutoffs (IC50 < 50 μM or IC50 < 100 μM) as proposed by Aleo et al. (2017) and a plasma exposure of >1 μM to differentiate BSEP and MDR3 inhibitors and the occurrence of DILI).
We also reanalyzed a dataset of Morgan et al. (2013) containing 109 benchmark drugs evaluated and annotated for their known association with hepatotoxicity, pharmacokinetic data in the form of area under the concentration versus time curve, indication/pharmacology, route of excretion, dose levels and frequencies, as well as other information to explore the relationship between in vitro transporter effects and evidence of liver injury in humans. An additional 21 compounds had partial annotations. The basis for selecting compounds to annotate was in vitro evidence of some level of BSEP inhibition. A detailed protocol for BSEP, MRP2, MRP3, and MRP4 membrane vesicle assays was published in Current Protocols in Toxicology (Morgan et al., 2010; van Staden et al., 2012). All compounds but epalrestat (No DILI concern), rifapentine (DILI concern), pranlukast (DILI concern), MK-571 (DILI concern), tranilast (DILI concern), valrubicin (No DILI concern), drotaverine (No DILI concern), gliquidone (DILI concern), sitaxsentan (DILI concern), loxapine succinate (DILI concern) could be BDDCS classified, resulting in a 99 drug dataset.
BDDCS classification
As we previously reported in Chan and Benet (2017), the assignment of BDDCS class of each drug was performed by evaluating the available solubility data, maximum dose strength (mg), and extent of metabolism (Benet et al., 2011). There was a recent expansion on the list of BDDCS drug classification to >1100 drugs, including many drugs that have been removed from the market as a result of toxic manifestations (Hosey et al., 2016). This latter BDDCS classification list was particularly challenging since for many drugs that came onto the market a number of years ago, and then removed because of toxicity, little reliable information both in terms of metabolism and solubility can be found in the literature. Therefore, when a drug is on the border of 2 classes, the BDDCS class was selected based on expected or known drug interactions.
Hosey and Benet (2015) noted a marked distinction between extensively and poorly metabolized compounds and this can be well predicted based on an in vitro measure of drug permeability. Recently, Dave and Morris (2016) showed that the solubility classification could be evaluated using a 0.3 mg/ml cutoff, thus not requiring knowledge of the clinical dose.
Classifying DILI severity of drugs in the dataset
The DILI severity assessment was categorized as follows: “Most DILI Concern”, “Less DILI Concern” and “No DILI Concern”, ordered by decreasing severity as described by Chen et al. (2011, 2016).
Data Analysis
The distribution of the different in vitro BSEP inhibition groups was evaluated against the Chen DILI assessment. Next proportions of each of the assays: Hepatobiliary transporters, cell cytotoxicity, or mitochondrial toxicity, as well BDDCS class 2 classification were tabulated. positive predictive value (PPV), false negative rate (FNR), and Accuracy (ACC) were calculated in order to analyze the ability of these in vitro assays to predict DILI. None of the datasets are provided here or in Supplementary Tables since all of the values are given in the Supplementary Tables of the publications cited.
RESULTS
Relationship Between BSEP Inhibition and FDA Drug Labels and FDA DILI Severity Assignment
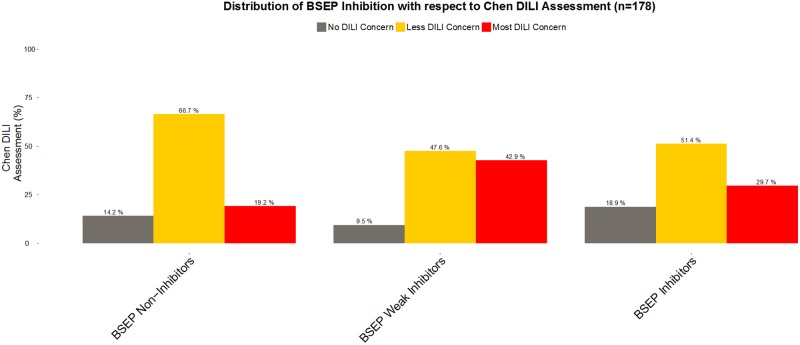
In Figure 1, using the FDA DILI severity assessment, we analyze the Chen et al. (2016) dataset as we previously reported for the Pedersen et al. (2013) dataset in Chan and Benet (2017). We observe in Figure 1 that among the BSEP inhibitors only 29.7% were characterized in the “Most DILI concern” category, while BSEP weak inhibitors show an even higher proportion of 42.9% for “Most DILI concern.” In addition, when we look at the distribution between BSEP Noninhibitors vs. BSEP Inhibitors in terms of DILI severity assessed, we observe 14.2% among BSEP Noninhibitors versus 18.9% among BSEP Inhibitors in the “No DILI” group. Similarly to this point, 29.7% of BSEP Inhibitors versus 19.2% of BSEP Noninhibitors are associated with “Most DILI Concern.” Using in vitro BSEP inhibition alone is not an adequate biomarker given the poor differentiation that we observe in the analysis of this dataset.
Figure 1.
Distribution of BSEP Inhibition with respect to the Chen et al. (2016) DILI Assessment. (120 drugs BSEP Noninhibitors, 21 BSEP Weak Inhibitors, and 37 BSEP Inhibitors).
Relationship Between BSEP Inhibition and Daily Dosage
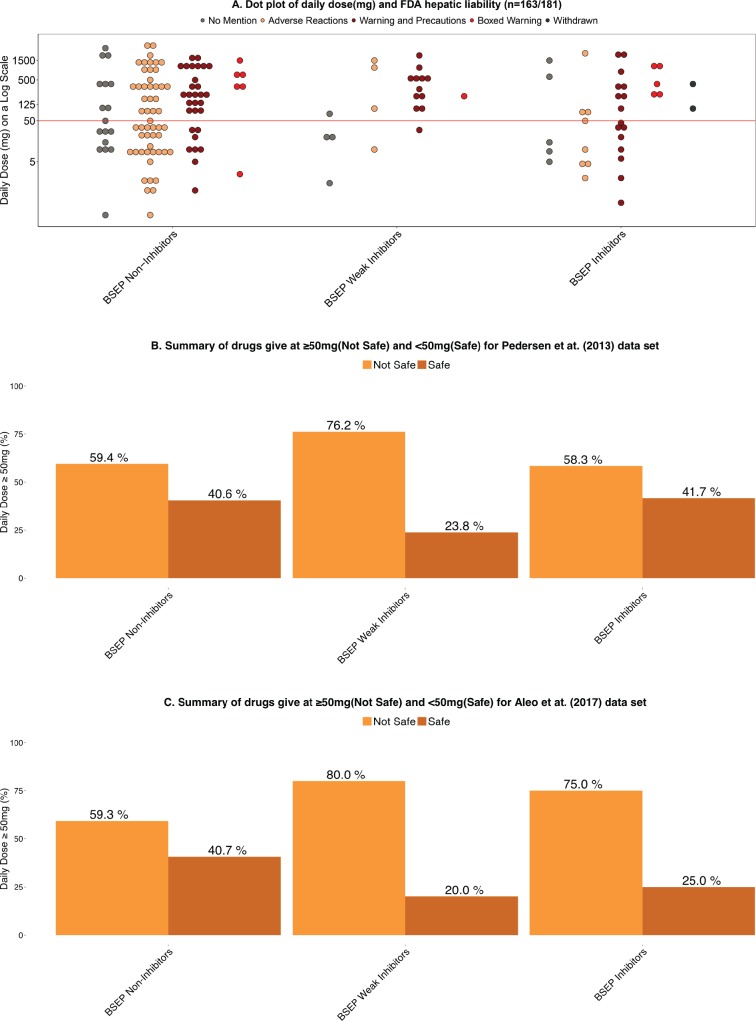
Lammert et al. (2008) have attributed hepatic adverse events to compounds with significant hepatic metabolism and daily dose ≥50 mg. We confirmed that daily dose provided the best DILI predictability (Chan and Benet, 2017). Here we have examined the relationship between daily dosages against the FDA hepatic liability categories according to the 3 BSEP inhibition groups for 163 drugs from the Pedersen et al. (2013) dataset where daily drug dosage was available (see Figure 2A). As seen in the dot plot, we observe no difference in the spread of the drugs and the distribution of BSEP inhibition group. We would expect BSEP inhibitors to exhibit a differentiation at dose ≥50 mg, but no shift is observed. In Figure 2B, we see no difference in terms of dose distribution between BSEP Noninhibitors (40.6%) and for BSEP Inhibitors (41.7%) given at “Safe” doses of <50 mg for the Pedersen et al. (2013) dataset. A different conclusion is seen with the Aleo et al. (2017) data as depicted in Figure 2C. The daily dose distribution for BSEP Noninhibitors and Weak Inhibitors is almost identical to that observed by Pedersen et al. (2013). However, a marked distinction for BSEP Inhibitors is seen in the Aleo et al. (2017) 125 drug dataset, where 75% of BSEP Inhibitors are given at daily doses ≥50 mg (Figure 2C), although the safe dosage distribution for BSEP Noninhibitors is similar for both datasets.
Figure 2.
A, Dot plot of daily dose (mg) and FDA hepatic liability (n = 163/181) (106 drugs BSEP Noninhibitors, 21 BSEP Weak Inhibitors, and 36 BSEP Inhibitors). B, Summary of drugs given at ≥ 50 mg (Not Safe) and <50 mg (Safe) for Pedersen et al. (2013) dataset (n = 163/181) (106 drugs BSEP Noninhibitors, 21 BSEP Weak Inhibitors, and 36 BSEP Inhibitors). C, Summary of drugs given at ≥ 50 mg (Not Safe) and <50 mg (Safe) for Aleo et al. (2017) dataset (n = 125) (91 drugs BSEP Noninhibitors, 10 BSEP Weak Inhibitors, and 24 BSEP Inhibitors).
Relationship Between Type of Liver Toxicity and MRP3, MRP4, and BSEP Inhibition
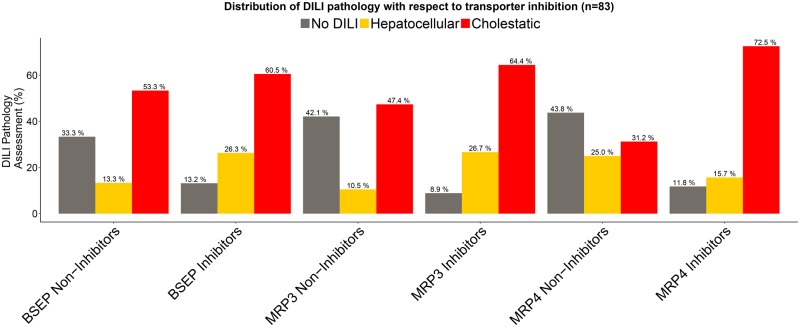
With respect to type of liver toxicity, looking at the relationship between BSEP inhibitors vs. BSEP noninhibitors in Figure 3 for the Köck dataset, we observe the least differentiation between cholestatic type of injury (60.5% of BSEP Inhibitors vs 53.3% of BSEP Noninhibitors). When we looked at the relationship between MRP3 Inhibitors versus MRP3 Noninhibitors we observe an increase in MRP3 inhibitors being associated with cholestatic type of injury (64.4% vs 47.4%). However, examining the relationship for MRP4, we observe that MRP4 had the highest differentiation in terms of cholestatic type of liver injury between MRP4 Inhibitors (72.5%) versus MRP4 Noninhibitors (31.2%) (see Figure 3).
Figure 3.
Distribution of DILI pathology with respect to transporter inhibition for the Köck et al. (2014) dataset (45 BSEP Noninhibitors, 38 BSEP Inhibitors; 38 MRP3 Noninhibitors, 45 MRP3 Inhibitors; 32 MRP4 Noninhibitors, 51 MRP4 Inhibitors).
For extent of hepatocellular injury in Figure 3, we note a 2-fold increase in BSEP inhibitors that are associated with hepatocellular injury versus BSEP noninhibitors. An even greater differentiation is seen for MRP3 Noninhibitors 10.5% versus 26.7% for MRP3 Inhibitors. This differentiation in hepatocellular injury goes the opposite way for MRP4, 25% MRP4 Noninhibitors versus 15.7% MRP4 Inhibitors.
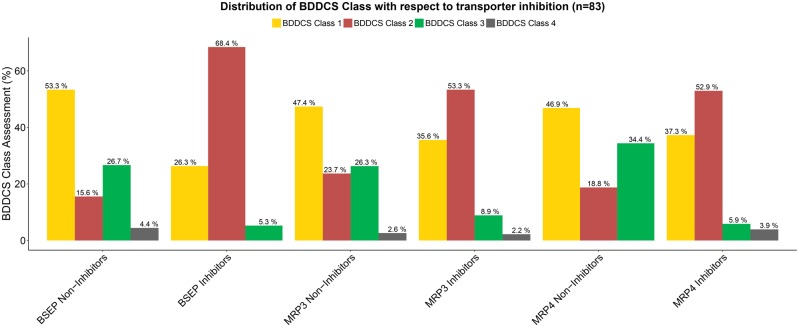
As seen in Figure 4, there is marked difference in BDDCS distribution of BSEP inhibition for the Köck et al. (2014) dataset. That is, 68.4% of BSEP Inhibitors are BDDCS class 2 drugs versus. 15.6% of BSEP Noninhibitors, concomitant with the marked decrease for classes 1 and 3 drug percentages for BSEP inhibitors. For MRP3 and MRP4 Inhibitors, we observe that the distribution of BDDCS classes 1 and 2 is very similar, and we do not observe as much of a decrease between MRP3 and MRP4 noninhibitors and inhibitors for BDDCS class 1 drugs as seen for BSEP. However for all 3 transporters BDDCS class 2 compounds constitute the majority of inhibitors.
Figure 4.
Distribution of BDDCS Class with respect to transporter inhibition for the Köck et al. (2014) dataset as listed in the legend for Figure 3.
We note that the highest groups associated with “No DILI” were MRP3 Noninhibitors and MRP4 Noninhibitors (Figure 3); they also have the highest percentage of BDDCS class 3 drugs (Figure 4). In terms of the BDDCS assessment, we observe a trend that BDDCS class 3 drugs are much less likely to cause transporter inhibition for BSEP, MRP3, and MRP4.
Comparative Analysis of Mitochondrial Toxicity and BSEP Inhibition Assay
Aleo et al. (2014) have proposed a synergetic relationship between BSEP and mitochondrial toxicity. They suggest that the involvement of mitochondrial dysfunction appears to be an additional mechanistic liability for DILI. Mitochondrial dysfunction can de-energize a cell and lead to oxidative stress, apoptosis, and hepatocellular injury. Moreover, the accumulation of cytotoxic bile acids within hepatocytes; has long been known to disrupt mitochondrial function. It has been hypothesized that the combination of these attributes of potent inhibition of mitochondrial function and BSEP transport may be more frequently associated with drugs that cause more severe forms of human DILI. It should also be noted that in certain disease states, like type 2 diabetes and nonalcoholic steatohepatitis, there are significant deficits in normal mitochondrial function, which in turn may further predispose individual patients to DILI through this mechanism.
To study this effect we analyzed the Aleo et al. (2014) dataset (Table 1) together with our previous analysis of the Schadt et al. (2015) dataset (Table 2) to see if there is indeed a strong correlation between mitochondria mitotoxicity and BSEP inhibition acting synergistically. When comparing the correlation between BSEP inhibition and DILI comparable PPV values are observed for the Aleo and Schadt datasets. The FNR for the Aleo dataset is zero because as noted earlier, Aleo eliminated any drug showing Most-DILI concern that was not a BSEP inhibitor. Thus, the ACC of the Aleo dataset for BSEP inhibition is greater than that for BSEP inhibition in the Schadt dataset. For the mitotoxicity assay, Aleo report a higher PPV, lower FNR and higher ACC than Schadt. However, in both Tables 1 and 2, BDDCS class 2 characterization shows comparable results to both BSEP and mitotoxicity. Thus, we believe there is no support for either of these measures being useful predictors of DILI potential. As seen in Table 2, Schadt et al. (2015) also investigated the relationship with cellular toxicity yielding even poorer correlations. Both Aleo et al. (2014) and Dawson et al. (2012) differentiated BSEP Inhibitors, as noted earlier, as Weak and Strong Inhibitors. In the analyses here we combined both Weak and Strong as BSEP Inhibitors. However, we also tested each relationship reported using the data for only Strong Inhibitors. No marked differences from the reported results were seen between the sets of analyses as we show here in Table 3 for the Dawson et al. (2012) dataset.
Table 1.
Comparison of BSEP and Mitochondrial Toxicity Assays Associated With DILI (Aleo et al., 2014; Dataset)
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 42) |
|---|---|---|---|
| BSEP | 72.7% | 0.0% | 78.6% |
| Mitotox | 94.1% | 33.3% | 78.6% |
| BDDCS class 2 | 80.0% | 16.7% | 78.6% |
Table 2.
Comparison of BSEP and Mitochondrial Toxicity Assays Associated With DILI (Schadt et al., 2015; Dataset)
| Criteria | % Correct (PPV) | % DILI Missing (FNR) | % ACC (True Positive + True Negative/106) |
|---|---|---|---|
| BSEP | 69.2% | 62.5% | 65.5% |
| Mitotox | 71.4% | 79.2% | 61.8% |
| Cellular toxicity | 48.3% | 70.8% | 55.5% |
| BDDCS class 2 | 64.6% | 35.4% | 69.1% |
Table 3.
BSEP Inhibition Assay Associated With DILI (Dawson et al., 2012; Dataset)
| Weak Inhibitors Included as BSEP-Inhibitors | |||
|---|---|---|---|
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 82) |
| BSEP (Strong and Weak Inhibitors) | 84.2% | 49.2% | 54.9% |
| BDDCS class 2 | 88.2% | 52.4% | 54.9% |
|
Weak Inhibitors Excluded | |||
| Criteria | % of Drugs with DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 76) |
| BSEP (Strong Inhibitors) | 87.5% | 52.5% | 53.9% |
| BDDCS class 2 | 89.7% | 55.9% | 52.6% |
Comparison of BSEP, MRP3, MRP4, and MDR3 In Vitro Transport Inhibition
In Table 4, we report the results of our analysis on the effect of transporter inhibition in the prediction of DILI using the Köck et al. (2014) compilation defined earlier. Here again for this dataset, comparable results are obtained for BSEP inhibition and BDDCS class 2 categorization. However, better predictability values are seen for the correlation with MRP3 and MRP4 inhibition, with MRP3 or MRP4 Inhibitors giving the best predictability. Adding BSEP inhibition to these measures decreases predictability back to BDDCS class 2 values.
Table 4.
Summary of BSEP, MRP3, and MRP4 In Vitro Transport Inhibition and DILI Assessment for the Köck et al. (2014) Dataset
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 83) |
|---|---|---|---|
| BSEP inhibitor | 86.8% | 47.6% | 57.8% |
| MRP3 inhibitor | 91.1% | 34.9% | 68.7% |
| MRP4 inhibitor | 88.2% | 28.6% | 71.1% |
| MRP3 or MRP4 inhibitor | 88.3% | 15.9% | 79.5% |
| BDDCS class 2 | 87.9% | 54.0% | 54.2% |
| BSEP and MRP3 inhibitor | 92.6% | 60.3% | 51.8% |
| BSEP and MRP4 inhibitor | 90.3% | 55.6% | 54.2% |
| BSEP and MRP3 or MRP4 inhibitor | 91.7% | 47.6% | 60.2% |
In Tables 5 and 6, we carry out the same assessment with the Aleo et al. (2017) dataset. Because of the marked increases in FNR when BSEP and MDR3 inhibition are combined, either for strong (IC50 < 50 μM) in Table 5 or weaker (IC50 < 100 μM) inhibition criteria in Table 6, or when these inhibition criteria are further combined with Cmax, total > 1 μM, none of these metrics provide better accuracy than just BDDCS class 2 alone. It is interesting that a statistical comparison of just Cmax, total > 1 μM, that Aleo et al. (2017) did not provide, gives the best accuracy. Of note, combining this metric with BDDCS class 2 assignment increase PPV, but also FNR, leading to decrease accuracy. This is consistent with our proposition that BDDCS class 2 assignment is not a useful predictor of DILI, just as none of the other Criteria in Tables 1–6, but for a DILI metric to be useful in drug development, it must provide better accuracy than BDDCS class 2 assignment alone.” Although we don’t disagree with the Aleo et al. (2017) contention that avoiding dual BSEP and MDR3 inhibitors could lead to less likelihood of causing clinical DILI, or that avoiding NMEs that inhibit these transporters in addition to MRP2, MRP3, and MRP4 inhibitors will result in less DILI, the same can be said for avoiding BDDCS class 2 drugs. These are not useful recommendations in drug development since FNR values are never <82% when only transporters with IC50 < 100 μM are considered (see Table 2 in Aleo et al., 2017).
Table 5.
Summary of BSEP and MDR3 In Vitro Transport Inhibition and DILI Assessment for the Aleo et al. (2017) Dataset (Using IC50 cutoff < 50 μM to Define BSEP and MDR3 Inhibitors)
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 119) |
|---|---|---|---|
| BSEP inhibitor | 73.9% | 75.4% | 51.3% |
| MDR3 inhibitor | 57.6% | 44.9% | 50.4% |
| BDDCS class 2 | 87.2% | 50.7% | 66.4% |
| Cmax, total > 1 μM | 75.7% | 18.8% | 73.9% |
| BDDCS class 2 + Cmax, total > 1 μM | 93.9% | 55.1% | 66.4% |
| BSEP and MDR3 inhibitor | 77.8% | 79.7% | 50.4% |
| BSEP and MDR3 + Cmax, total > 1 μM | 100.0% | 79.7% | 53.8% |
Table 6.
Summary of BSEP and MDR3 In Vitro Transport Inhibition and DILI Assessment for the Aleo et al. (2017) Dataset (Using IC50 cutoff <100 μM to Define BSEP and MDR3 Inhibitors)
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 119) |
|---|---|---|---|
| BSEP Inhibitor | 73.5% | 63.8% | 55.5% |
| MDR3 Inhibitor | 57.6% | 44.9% | 50.4% |
| BDDCS class 2 | 87.2% | 50.7% | 66.4% |
| Cmax, total > 1 μM | 75.7% | 18.8% | 73.9% |
| BDDCS class 2 + Cmax, total > 1 μM | 93.9% | 55.1% | 66.4% |
| BSEP and MDR3 Inhibitor | 69.2% | 73.9% | 50.4% |
| BSEP and MDR3 + Cmax, total > 1 μM | 94.4% | 75.4% | 55.5% |
In Tables 7 and 8, we report the results of our analysis on the effect of transporter inhibition in the prediction of DILI using the Morgan et al. (2013) dataset. We observe that the PPV of combining steady state drug levels divided by the IC50 for BSEP inhibition and MRP 2, 3, or 4 inhibition gives very high PPVs. However, the FNR of utilizing these measures is just >50%, and much worse (>80%) when combining the 2 measures proposed by Morgan et al. Similarly, we show that the compilation of BDDCS class 2 drugs in the Morgan dataset provides slightly better accuracy than their suggested BSEP metric. Thus, we question the validity to their recommendation. We maintain that none of these measures or combining of these measures adequately predicts DILI, just as BDDCS does not predict DILI.
Table 7.
Summary of BSEP and MRP3 In Vitro Transport Inhibition and DILI Assessment for the Morgan et al. (2013) Dataset
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 109) |
|---|---|---|---|
| Css/IC50 BSEP Inhibition ≥ 0.1 | 94.4% | 51.4% | 65.1% |
| Above + Css/IC50 MRP 2, 3, or 4 Inhibition ≥ 0.1 | 92.9% | 81.4% | 46.8% |
Table 8.
Summary of BSEP and MRP3 In Vitro Transport Inhibition and DILI Assessment for the Morgan et al. (2013) Dataset for Only BDDCS Classifiable Drugs
| Criteria | % of Drugs With DILI Predicted Correctly, PPV | % of DILI Missing in the Prediction, FNR | % of DILI Predicted Accurately, ACC (n = 99) |
|---|---|---|---|
| Css/IC50 BSEP Inhibition ≥ 0.1 | 93.5% | 54.0% | 63.6% |
| Above + Css/IC50 MRP 2, 3 or 4 Inhibition ≥ 0.1 | 90.0% | 85.7% | 44.4% |
| BDDCS Class 2 | 70.7% | 15.9% | 67.7% |
DISCUSSION
It is thought that BSEP inhibition results in intrahepatic bile acid accumulation and this may lead to DILI. However, few reports indicate that drug-induced BSEP dysfunction actually leads to hepatotoxicity, and the relationship between drug-induced BSEP dysfunction and liver injury risk is yet to be determined. Here we show that pharmacological BSEP interference by small molecules is not a strong susceptibility factor. BSEP inhibition alone cannot accurately predict hepatotoxic potential of drugs as depicted by Figure 1. It is unclear as to what extent BSEP inhibition is functionally significant in vivo. We observe that the great majority of compounds that have been associated with DILI and are BSEP inhibitors are also BDDCS class 2. Because we are able to make similar predictions based on BDDCS determinant characteristics, this leads us to discount the predictive ability of mechanistic association of BSEP and DILI. We have previously observed that as hepatic warning severity increases, the proportion of BDDCS class 2 drugs increases and the proportions of both BDDCS classes 1 and 3 drugs decrease (Chan and Benet, 2017).
The translation of in vitro potency of a small molecule on inhibiting BSEP to human risk of liver injury is problematic for many reasons. Drug concentrations within human hepatocytes in vivo are unknown. It is likely that they are much higher than plasma concentrations. The apparent IC50 values assume all added drug is available in solution. True values are likely to be much lower, due to binding to proteins and lipids. BSEP inhibition by drug metabolites not evaluated in the assay also may be markedly more potent than parent drug. Furthermore, bile flow is present in vivo, bile acid concentrations in serum and cholestatic serum are much higher than in in vitro media, and cholestatic DILI typically occurs over an extended time period versus the time period of in vitro inhibition studies.
Aleo et al. (2014) suggest that mitochondrial toxicity together with BSEP inhibition may provide improved DILI predictability. When we analyzed the predictability of BSEP inhibition together with mitochondrial toxicity, we observe that BDDCS class 2 characterization shows comparable results. Thus, we believe that neither BSEP inhibition nor mitochondrial toxicity are useful independent predictors of DILI (see Tables 1 and 2).
The activities of a compound on other related transporters, such as the multidrug resistance-associated proteins MRP3, MRP4, and potentially others, may show a greater effect on overall liver injury. Köck et al. (2014) demonstrated that inhibition of MRP4, in addition to BSEP, may be a risk factor for the development of cholestatic DILI. In Table 4, we report comparable results for BSEP inhibition and BDDCS class 2 categorization. However, MRP4 inhibition gives the best performance amongst MRP3, MRP4, and BSEP inhibition. Our data analysis suggests that screening for MRP4 or MRP3 could lead to higher accuracy than BSEP, but that addition of BSEP inhibition to measures of MRP4 and MRP3 inhibition gives less predictability, back to values similar to those for BDDCS class 2 only.
In 2013, Morgan et al. stated that “integration of exposure data, and knowledge of an effect to not only BSEP but also one or more of the MRPs is a useful tool for informing the potential for liver injury due to altered BA transport.” Aleo et al. (2017) proposed a similar evaluation methodology for MDR3. In Tables 5 and 6, we evaluate this hypothesis for the Aleo et al. (2017) dataset showing that BSEP inhibition together with MDR3 transporter inhibition, and when Cmax, total > 1 μM, provides no better predictability than just looking at BDDCS class 2 (like that for BSEP + MRP3 or MRP4 inhibition for the Köck et al. (2014) dataset in Table 4).
When we evaluated the 2013 Morgan et al. dataset, although they utilized a different criteria than just BSEP inhibition, we show that the addition of MRP 2, 3, or 4 inhibition to BSEP yielded poorer accuracy (Table 7) than their measurement for BSEP inhibition alone due to the increased FNR. In Table 8, we show that for BDDCS classifiable drugs the Morgan et al. hypothesis leads to higher PPVs in comparison to BDDCS class 2 drugs, but also markedly higher FNR percentages, so that the accuracy of the Morgan et al. metric of combining transporter inhibitors is markedly less than for BSEP inhibition alone, which is slightly less than the accuracy obtained by just avoiding BDDCS class 2 drugs. Thus, we believe the results of Tables 7 and 8 do not support the Morgan et al. (2013) recommendation.
Furthermore, we are surprised that Morgan et al. (2013) would recommend a flow scheme for hazard identification that states “If Css/BSEP IC50 ratio ≪ 0.1 and little effect or no effect on MRPs” sponsors should “Proceed with minimal risk”. By taking ≪0.1 to be < 0.01, we find that there are 41 drugs that meet these criteria in the Morgan et al. listing, of which at least 16 (39%) according to Morgan et al. have a known association with liver injury. Thus, we find the Morgan et al. (2013) recommendation to be unsupported.
Idiosyncratic DILI presents with an array of clinical symptoms and can vary in severity from a mild increase in liver enzymes (alanine aminotransferase ALT), bilirubin, and alkaline phosphatase [ALP]) to acute liver failure and death. Assessment is based on clinical and biochemical findings, and accurate diagnosis with drug causality requires detailed case patient records reviewed by multiple expert hepatologists. On the basis of biochemical measures, 3 types of DILI can occur: “hepatocellular” caused by damage predominantly to hepatocytes, where serum ALT at the time of maximum elevation is >3 times the upper limit of normal (ULN) and the ratio (R) of the ULN for ALT/ALP is ≥5, “cholestatic” caused by disruption in bile flow, where serum bilirubin is elevated and ALP at the time of maximum elevation is 2 ≥ ULN while R is ≤ 2, and “mixed”, where ALT > 3 ULN, ALP > 2ULN, and bilirubin are elevated, while R > 2 but < 5 (Verma and Kaplowitz, 2009). A concomitant rise in ALT (>3× the ULN range) and bilirubin (>2× ULN) is suggestive of severe liver injury where hepatocyte damage is coupled with disrupted biliary excretion, increased serum bilirubin, and jaundice (Ogese et al., 2016). With respect to liver type of toxicity, when we looked at the relationship between BSEP inhibitors and noninhibitors, we observe that there is no significant difference between cholestatic type of injury, although there was an increase in hepatocellular injury (Figure 3). The BSEP inhibitor and noninhibitor data from Köck et al. (2014) in Figure 3 were compiled from the previous reports of Dawson et al. (2012) and Morgan et al. (2010). In Dawson et al. (2012), one can determine that cholestatic DILI was caused by 68.4% of strong BSEP Inhibitors while hepatocellular DILI was caused by only 15.8% of strong BSEP Inhibitors. Such as analysis from the Morgan et al. (2010) paper is not readily available, but was carried out by Köck et al. (2014). Yet the data for BSEP inhibition in Figure 3 would suggest that the Morgan et al. (2010) results contradict the Dawson et al. (2012) report. Thus, we view with skepticism any utility of in vitro BSEP inhibition screening for predicting DILI since such predictions as indicated in Tables 1–8 show no differentiation with drugs being BDDCS class 2.
DILI is multifactorial; inhibition of multiple hepatic efflux transporters could confer additional risk. DILI for many drugs involves cholestasis and accumulation of bile acids within hepatocytes. The adaptive response by the liver is an important component in predicting the potential for cholestatic hepatotoxicity. Bile acid disposition is tightly regulated by the Farnesoid X Receptor (FXR). FXR activation leads to increased fibroblast growth factor 19, suppression of cytochrome P450 7A1, induction of BSEP, MRP3, and organic solute transporter alpha/beta (OSTα/β). Chenodeoxycholic acid (CDCA), an endogenous FXR agonist, upregulates BSEP in human sandwich culture hepatocytes (Jackson et al., 2016). Increased function of basolateral efflux transporters can be an important “safety valve” if BSEP-mediated efflux is compromised. CDCA upregulates OSTα/β. Adaptation to the harmful effects of such accumulation can mean the difference between hepatocyte death and survival (Chalasani et al., 2008). Basolateral and canalicular efflux transporters play a critical role in hepatic and systemic exposure for some drugs, endogenous compounds, and metabolites. Inhibition of hepatic efflux transporters may increase hepatocyte exposure and cause toxicity. Induction of basolateral efflux transporters may decrease intracellular concentrations and increase systemic exposure. At this stage our analysis suggests that in vitro BSEP inhibition itself is not an adequate or useful predictor of DILI potential.
Although mutations in BSEP have been associated with liver disease in a univariate manner (Morgan et al., 2010), it is not yet fully understood how pharmacological inhibition of BSEP in humans in vivo relates to the familial dysfunction of this transporter. The case examples where autoantibodies to BSEP led to post transplant liver failure in patients with PFIC2 (Jara et al., 2009; Reinehr et al., 2005) offer a glimpse at how complete shutdown of BSEP might manifest when exposed to an unlimited challenge. However, this is an example of extreme pharmacology and not necessarily representative of what occurs with small molecules.
There is a general acceptance that inhibitors of BSEP are a source of toxicity. However, according to our analysis of DILI this is not true. What we find is that most DILI occurs with BDDCS class 2 compounds and almost all BSEP inhibitors are class 2 compounds, but we do not see a relationship with the strength of BSEP inhibition and toxicity, which makes us believe that the generally held hypothesis is incorrect.
For the purposes of early screening, binning compounds based on their relative BSEP-mediated inhibition does not limit the possibility of liver liabilities in humans. Our data suggest that compounds that are BDDCS class 2 are as likely as BSEP inhibitors to lead to DILI. As we noted earlier, if potential drug characteristics, such as in vitro BSEP inhibition (or mitochondrial toxicity) provide no better prediction than BDDCS class 2 categorization one cannot have faith in the proposed toxicology screen. The majority of in vitro BSEP inhibition analyses have concentrated on high PPV, ignoring the high FNR percentages and the resulting inadequate accuracy measures.
FUNDING
R.C. was supported by the American Association of Pharmaceutical Scientists Graduate Fellowship, American Foundation for Pharmaceutical Education Predoctoral Fellowship, North American Graduate Fellowship from the American College of Toxicology, Supplemental Training for Education Program from the Society of Toxicology, and NIGMS (grant R25 GM56847). Dr Benet is a member of the UCSF Liver Center supported by the National Institutes of Health (NIH) (grant P30 DK026743). Dr Benet is a founder, holds stock and is chairman of the scientific advisory board of Hμrel Corporation.
REFERENCES
- Aleo M. D., Luo Y., Swiss R., Bonin P. D., Potter D. M., Will Y. (2014). Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology 60, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Aleo M. D., Shah F., He K., Bonin P. D., Rodrigues A. D. (2017). Evaluating the role of multidrug resistance protein 3 (MDR3) inhibition in predicting drug induced liver injury using 125 pharmaceuticals. Chem. Res. Toxicol. 30, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Benet L. Z., Broccatelli F., Oprea T. I. (2011). BDDCS applied to over 900 drugs. AAPS J. 13, 519–547.http://dx.doi.org/10.1208/s12248-011-9290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N., Fontana R. J., Bonkovsky H. L., Watkins P. B., Davern T., Serrano J., Yang H., Rochon J. (2008). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135, 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Benet L. Z. (2017). Evaluation of DILI predictive hypotheses in early drug development. Chem. Res. Toxicol. 30, 1017–1029.http://dx.doi.org/10.1021/acs.chemrestox.7b00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Suzuki A., Thakkar S., Yu K., Hu C., Tong W. (2016). DILIrank: The largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov. Today 21, 648–653. [DOI] [PubMed] [Google Scholar]
- Chen M., Vijay V., Shi Q., Liu Z., Fang H., Tong W. (2011). FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov. Today 16, 697–703.http://dx.doi.org/10.1016/j.drudis.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Dave R. A., Morris M. E. (2016). Novel high/low solubility classification methods for new molecular entities. Int. J. Pharm. 511, 111–126.http://dx.doi.org/10.1016/j.ijpharm.2016.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S., Stahl S., Paul N., Barber J., Kenna J. G. (2012). In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab. Dispos. 40, 130–138. [DOI] [PubMed] [Google Scholar]
- Hillgren K. M., Keppler D., Zur A. A., Giacomini K. M., Stieger B., Cass C. E., Zhang L. (2013). Emerging transporters of clinical importance: An update from the International Transporter Consortium. Clin. Pharmacol. Ther. 94, 52–63. [DOI] [PubMed] [Google Scholar]
- Hosey C. M., Benet L. Z. (2015). Predicting the extent of metabolism using in vitro permeability rate measurements and in silico permeability rate predictions. Mol. Pharm. 12, 1456–1466.http://dx.doi.org/10.1021/mp500783g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey C. M., Chan R., Benet L. Z. (2016). BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs. AAPS J. 18, 251–260.http://dx.doi.org/10.1208/s12248-015-9845-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. P., Freeman K. M., Friley W. W., St. Claire R. L., Black C., Brouwer K. R. (2016). Basolateral efflux transporters: A potentially important pathway for the prevention of cholestatic hepatotoxicity. Appl. In Vitr. Toxicol. 2, 207–216. [Google Scholar]
- Jansen P. L., Strautnieks S. S., Jacquemin E., Hadchouel M., Sokal E. M., Hooiveld G. J., Koning J. H., De Jager-Krikken A., Kuipers F., Stellaard F. et al. , (1999). Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117, 1370–1379. [DOI] [PubMed] [Google Scholar]
- Jansen P. L. M., Müller M. (2000). Genetic cholestasis: Lessons from the molecular physiology of bile formation. Can. J. Gastroenterol 14, 233–238. [DOI] [PubMed] [Google Scholar]
- Jara P., Hierro L., Martínez-Fernández P., Alvarez-Doforno R., Yánez F., Diaz M. C., Camarena C., De la Vega A., Frauca E., Muñoz-Bartolo G. (2009). Recurrence of bile salt export pump deficiency after liver transplantation. N. Engl. J. Med. 361, 1359–1367. [DOI] [PubMed] [Google Scholar]
- Khoury T., Abu A., Yosha L., Benson A. A., Daher S., Mizrahi M. (2015). Drug induced liver injury: Review with a focus on genetic factors, tissue diagnosis, and treatment options. J. Clin. Transl. Hepatol. 3, 99–108.http://dx.doi.org/10.14218/JCTH.2015.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis E., Ioja E., Rajnai Z., Jani M., Méhn D., Herédi-Szabó K., Krajcsi P. (2012). BSEP inhibition - In vitro screens to assess cholestatic potential of drugs. Toxicol. In Vitro 26, 1294–1299. [DOI] [PubMed] [Google Scholar]
- Köck K., Ferslew B. C., Netterberg I., Yang K., Urban T. J., Swaan P. W., Stewart P. W., Brouwer K. L. (2014). Risk factors for development of cholestatic drug-induced liver injury: Inhibition of hepatic basolateral bile acid transporters MRP3 and MRP4. Drug Metab. Dispos. 42, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert C., Einarsson S., Saha C., Niklasson A., Bjornsson E., Chalasani N. (2008). Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology 47, 2003–2009. [DOI] [PubMed] [Google Scholar]
- Morgan R. E., Trauner M., van Staden C. J., Lee P. H., Ramachandran B., Eschenberg M., Afshari C. A., Qualls C. W., Lightfoot-Dunn R., Hamadeh H. K. (2010). Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 118, 485–500. [DOI] [PubMed] [Google Scholar]
- Morgan R. E., van Staden C. J., Chen Y., Kalyanaraman N., Kalanzi J., Dunn R. T., Afshari C. A., Hamadeh H. K. (2013). A multifactorial hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol. Sci. 136, 216–241. [DOI] [PubMed] [Google Scholar]
- Ogese M. O., Ahmed S., Alfirevic A., Betts C. J., Dickinson A., Faulkner L., French N., Gibson A., Hirschfield G. M., Kammüller M. et al. , (2016). New approaches to investigate drug-induced hypersensitivity. Chem. Res. Toxicol. 30, 239–259. [DOI] [PubMed] [Google Scholar]
- Pedersen J. M., Matsson P., Bergström C. A., Hoogstraate J., Norén A., LeCluyse E. L., Artursson P. (2013). Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol. Sci. 136, 328–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M.-J., Briz O. (2009). Bile-acid-induced cell injury and protection. World J. Gastroenterol. 15, 1677–1689.http://dx.doi.org/10.3748/wjg.15.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R., Becker S., Keitel V., Eberle A., Grether-Beck S., Häussinger D. (2005). Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology 129, 2009–2031. [DOI] [PubMed] [Google Scholar]
- Schadt S., Simon S., Kustermann S., Boess F., McGinnis C., Brink A., Lieven R., Fowler S., Youdim K., Ullah M. et al. , (2015). Minimizing DILI risk in drug discovery - a screening tool for drug candidates. Toxicol. In Vitro 30, 429–437. [DOI] [PubMed] [Google Scholar]
- Schulz S., Schmitt S., Wimmer R., Aichler M., Eisenhofer S., Lichtmannegger J., Eberhagen C., Artmann R., Tookos F., Walch A. et al. , (2013). Progressive stages of mitochondrial destruction caused by cell toxic bile salts. Biochim. Biophys. Acta Biomembr. 1828, 2121–2133. [DOI] [PubMed] [Google Scholar]
- Shah F., Leung L., Barton H. A., Will Y., Rodrigues A. D., Greene N., Aleo M. D. (2015). Setting clinical exposure levels of concern for drug-induced liver injury (DILI) using mechanistic in vitro assays. Toxicol. Sci. 147, 500–514. [DOI] [PubMed] [Google Scholar]
- Shugarts S., Benet L. Z. (2009). The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 26, 2039–2054.http://dx.doi.org/10.1007/s11095-009-9924-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. A., Isin E. M., Ogese M. O., Mettetal J. T., Williams D. P. (2016). Reactive metabolites: Current and emerging risk and hazard assessments. Chem. Res. Toxicol. 29, 505–533. [DOI] [PubMed] [Google Scholar]
- van Staden C. J., Morgan R. E., Ramachandran B., Chen Y., Lee P. H., Hamadeh H. K. (2012). Membrane vesicle ABC transporter assays for drug safety assessment. Curr. Protoc. Toxicol. 23.5.1–23.5.24. [DOI] [PubMed] [Google Scholar]
- Verma S., Kaplowitz N. (2009). Diagnosis, management and prevention of drug-induced liver injury. Gut 58, 1555–1564.http://dx.doi.org/10.1136/gut.2008.163675 [DOI] [PubMed] [Google Scholar]
- Wang R., Salem M., Yousef I. M., Tuchweber B., Lam P., Childs S. J., Helgason C. D., Ackerley C., Phillips M. J., Ling V. (2001). Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. U.S.A. 98, 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]