An agent-based model of C. difficile transmission found daily cleaning with a sporicidal disinfectant and screening for C. difficile at admission to be the most effective of 9 interventions. When implemented simultaneously, they reduced hospital-onset CDI by 82.3% and asymptomatic colonization by 90.6%.
Keywords: C. difficile, infection control, agent-based modeling, intervention bundles, healthcare epidemiology
Abstract
Background
Despite intensified efforts to reduce hospital-onset Clostridium difficile infection (HO-CDI), its clinical and economic impacts continue to worsen. Many institutions have adopted bundled interventions that vary considerably in composition, strength of evidence, and effectiveness. Considerable gaps remain in our knowledge of intervention effectiveness and disease transmission, which hinders HO-CDI prevention.
Methods
We developed an agent-based model of C. difficile transmission in a 200-bed adult hospital using studies from the literature, supplemented with primary data collection. The model includes an environmental component and 4 distinct agent types: patients, visitors, nurses, and physicians. We used the model to evaluate the comparative clinical effectiveness of 9 single interventions and 8 multiple-intervention bundles at reducing HO-CDI and asymptomatic C. difficile colonization.
Results
Daily cleaning with sporicidal disinfectant and C. difficile screening at admission were the most effective single-intervention strategies, reducing HO-CDI by 68.9% and 35.7%, respectively (both P < .001). Combining these interventions into a 2-intervention bundle reduced HO-CDI by 82.3% and asymptomatic hospital-onset colonization by 90.6% (both, P < .001). Adding patient hand hygiene to healthcare worker hand hygiene reduced HO-CDI rates an additional 7.9%. Visitor hand hygiene and contact precaution interventions did not reduce HO-CDI, compared with baseline. Excluding those strategies, healthcare worker contact precautions were the least effective intervention at reducing hospital-onset colonization and infection.
Conclusions
Identifying and managing the vast hospital reservoir of asymptomatic C. difficile by screening and daily cleaning with sporicidal disinfectant are high-yield strategies. These findings provide much-needed data regarding which interventions to prioritize for optimal C. difficile control.
Despite intensified efforts to reduce Clostridium difficile infection (CDI) by hospitals nationwide, its clinical and economic impacts have continued to worsen [1–3]. The rate of community-acquired [2, 4–6] and antibiotic-resistant CDI are increasing [1, 7, 8], and C. difficile has surpassed methicillin-resistant Staphylococcus aureus (MRSA) as the most common cause of healthcare-associated infections in the United States [9]. As of January 2017, hospitals with the highest CDI rates incur a financial penalty imposed by the Medicare Hospital-Acquired Condition Reduction Program [10].
In an effort to rapidly decrease CDI rates, hospitals typically implement multiple C. difficile interventions at the same time in a CDI bundle [11–15]. These bundles vary considerably in composition, strength of evidence, and effectiveness [15]. When several interventions are introduced simultaneously, it is difficult to isolate the effects of individual CDI strategies [11, 16]. The optimal bundle for CDI prevention is unknown, which hinders CDI prevention.
Unlike traditional epidemiologic studies, computer simulation modeling allows examination of counterfactual scenarios that can identify the isolated effects of individual interventions to reduce CDI. Agent-based models can account for the indirect effects and underlying complexity of hospital infection control dynamics [16, 17]. All other covariates, transmission dynamics, and assumptions are kept constant across simulation runs, so that the resulting difference between CDI rates is due to the implemented intervention or chance.
Being able to evaluate the clinical effectiveness of CDI interventions is essential to making evidence-based implementation decisions in the context of constrained hospital resources. Agent-based modeling is uniquely poised to evaluate intervention comparative effectiveness, yet this methodology has been underutilized in the field [16].
Our group published an initial agent-based model of C. difficile transmission in 2014, investigating the clinical effectiveness of vancomycin treatment, contact isolation and cohorting, healthcare worker (HCW) hand hygiene, and environmental cleaning [18]. Subsequent changes in CDI epidemiology, diagnostic testing modalities, and the rapid implementation of novel interventions aimed at CDI prevention prompted us to design a new version of that original model. Here, we developed an agent-based model of C. difficile transmission in a midsized adult hospital that reflects current CDI epidemiology and hospital practices, and evaluate the clinical effectiveness of 9 infection control interventions.
METHODS
Approach
We developed an agent-based simulation model of C. difficile transmission in a 200-bed adult hospital. Agent-based modeling is an extension of discrete-event simulation in which individuals have unique attributes, are tracked individually, and interact with each other and the environment [17, 19, 20]. The hospital is divided into 10 identical wards, each containing 20 single-bed patient rooms, a visitor common area, nursing station, and physician workroom. Each model run simulates a 1-year period. The model time-step is 5 minutes.
Agents
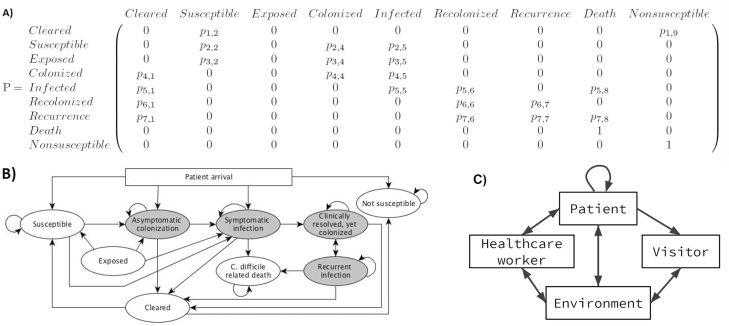
The model includes 4 agent types: patients, visitors, nurses, and physicians. Patients are assigned a room upon arrival, although intra- or interward patient transfers can occur. Each patient is categorized into 1 of 9 clinical states representing CDI status (Table 1). These states are updated every 6 hours based on probabilities in the model’s underlying discrete-time Markov chain (Figure 1), adapted from our previous agent-based C. difficile model [18]. Patients are assessed for high-risk antibiotic usage at the beginning of their second hospital day. At that time, all nonsusceptible patients using these antibiotics are moved to the susceptible state. Discussion of modifications made to our previous model and recalibration details are shown in Supplementary Materials 1 and 2, respectively.
Table 1.
Patient Clinical States
| State | Patient’s Condition |
|---|---|
| Susceptible | No symptoms or disease; at risk for C.difficile colonization |
| Nonsusceptible | Not at risk for colonization or CDI during the hospital stay |
| Exposed | Exposed to C. difficile through interactions with contagious agents or contaminated environment |
| Cleared | Prior infection or colonization has subsided |
| Death | Death due to CDI |
| Colonized | No symptoms, but gastrointestinal colonization of C. difficile |
| Infected | Symptomatic, clinically diagnosed CDI |
| Recolonized | Recovered from symptoms, but gastrointestinal colonization remains |
| Infection recurrence | Symptoms return to a previously infected patient |
Patients in the states marked with italic text are contagious and can expose others and the environment to Clostridium difficile, whereas patients in the other states cannot.
Abbreviation: CDI, Clostridium difficile infection.
Figure 1.
Matrix (A) and transition state (B) diagram representations of the discrete-time Markov chain underlying transitions between clinical states. The gray ovals represent clinical states from which C. difficile can be transmitted, while the white ovals are the noninfective states. Patient clinical states are updated every 6 hours. C, There are 10 agent:agent or agent:environmental interactions that can lead to a C. difficile transmission event. Abbreviation: C.difficile, Clostridium difficile
Visitors are assigned to 1 patient, whom they stay with until they leave the hospital, exiting through the ward’s common room. As in the existing C. difficile transmission model by Rubin et al, 2 types of HCWs are included: nurses working on a designated ward and physicians working hospital-wide [21]. HCWs and visitors can become transiently exposed to C. difficile, and therefore contagious, transmitting C. difficile via spores on their hands, clothing, or medical equipment [22]. We assume that sick visitors and HCWs do not visit the hospital and that individuals without conventional risk factors such as hospitalization and recent antibiotic usage have a low risk of colonization [23]. Therefore, HCWs and visitors in the model cannot become colonized or infected. A discussion of the overall order of events in the model and flow diagrams of patient, visitor, and HCW logic are included in Supplementary Material 3.
Transmission
There are 10 agent and environmental interactions that can result in a new C. difficile exposure (Figure 1C). The probability of C. difficile transmission during an interaction is proportional to the duration of the interaction. Each possible transmission event is coded in the model as a Bernoulli trial (Supplementary Figures 1–3). We tracked all transmissions to quantify the contributions of each agent type and the environment to C. difficile exposure.
Parameters
To maximize model generalizability, we derived input parameter estimates from relevant results in >50 peer-reviewed studies, including literature published through April 2017 (Table 2). Each parameter estimate was reviewed by content experts. The model was run using the mean parameter estimates. The distributions were used for sensitivity analyses, as described below.
Table 2.
Input Parameter Estimates for the Agent-Based Model
| Parameter | Mean | Distribution (Range) | Sourcea | |
|---|---|---|---|---|
| Agent parameters | ||||
| Patient | Length of stay, d | 4.8 | Lognormal (SD = 4.8) | [51–54] |
| CDI attributable length of stay increase, d | 2.3 | Exponential (mean = 2.1–2.4) | [55] | |
| Arrival rate per day | 26 | … | [51, 56] | |
| Nursing visits per 6 h | 5 | … | [21, 57–59] | |
| Doctor visits per 6 h | 1 | … | [21, 57–59] | |
| Proportion on high CDI risk antibiotics | 20% | Triangular (15–25) | [60–62] | |
| Vancomycin treatment time, d | 14 | … | [39] | |
| Vancomycin success rate | 81% | Triangular (78–83) | [63–66] | |
| Nurse | Number per ward | 4 | … | [58, 67–69] |
| Service time, min | 4.7 | Exponential (mean = 3–7) | [58, 59, 70, 71] | |
| Doctor | Number per ward | 2 | … | [51, 58] |
| Service time, min | 10.8 | Exponential (mean = 4–14) | [58, 59, 70, 71] | |
| Visitor | Daily probability of receiving visitors | 0.5 | Triangular (0.3–0.7) | [72, 73] |
| No. of visitors per visit | 2 | Triangular (1–3) | [73, 74] | |
| Service time, min | 15 | Exponential (mean = 10–30) | [58, 73–75] | |
| Admission parameters | ||||
| Proportion of susceptible patients | 39.7% | Triangular (30%–50%) | [52, 76–79] | |
| Proportion asymptomatic colonized patients | 6.1% | Triangular (4%–10%) | [40, 80–89] | |
| Proportion of patients with CDI | 0.29% | Triangular (0.25%–1%) | [80, 86, 90, 91] | |
| Proportion of nonsusceptible patients | 53.9% | … | … | |
| Transmission parameters | ||||
| Probability patient:patient contact | 5% per 30 min | Triangular (1%–15%) | EO | |
| Probability patient:nurse contact | 36% per 4.7 min | Triangular (26%–46%) | [58] | |
| Probability patient:doctor contact | 69% per 10.8 min | Triangular (59%–79%) | [58] | |
| Probability patient:visitor contact | 65% per 15 min | Triangular (55%–75%) | [58] | |
| Probability environment:nurse contact | 70% per 4.7 min | Triangular (60%–80%) | [58] | |
| Probability environment:doctor contact | 90% per 10.8 min | Triangular (80%–100%) | [58] | |
| Probability environment:visitor contact | 93% per 15 min | Triangular (83%–100%) | [58] | |
| Probability environment:patient contact | 100%; constant | … | … | |
| C. difficile transfer efficiency person:person | 30% | Triangular (15%–45%) | [92] | |
| C. difficile transfer efficiency environment:person | 44% | Triangular (29%–59%) | [93] | |
| Contamination parameters | ||||
| Colonized patient contaminated | 38% | Triangular (15%–60%) | [94–96] | |
| Active CDI patient contaminated | 70% | Triangular (60%–80%) | [96] | |
| Colonized patient room contaminated | 19% | Triangular (14%–35%) | [96–98] | |
| Active CDI patient room contaminated | 47% | Triangular (36%–60%) | [96–101] | |
| Non–C. difficile patient room contaminated | 7% | Triangular (5%–15%) | [97, 100, 101] | |
Abbreviations: CDI, Clostridium difficile infection; C.difficile, Clostridium difficile; EO, expert opinion; SD, standard deviation.
aReferences for input parameter sources are included in the Supplementary Materials.
Interventions
Nine infection control interventions were modeled, including 4 hospital centered and 5 patient centered (Table 3). Each was modeled at 3 levels, enhanced, ideal, and a baseline, nonintervention state. The baseline state served as the control and reflected standard hospital practices expected to occur without the implementation of any active intervention.
Table 3.
Hospital- and Patient-Centered Interventions Considered in This Study
| Intervention | Intended Effect | Timing for Potential Intervention Events | Transmission Events Directly Affected | |
|---|---|---|---|---|
| Hospital Centered | HCW hand hygiene | Improve overall HCW HH compliance; increase utilization of soap and water vs ABHR for CDI or known colonized patients | HCW entry and exit of patient room | HCW: to and from environment or patient |
| HCW contact precautions | Improve HCW contact precautions usage; provide education to reduce contact precaution contamination on donning and doffing; maintain until discharge for CDI or known colonized patients | HCW entry of patient room | HCW: to and from environment or patient | |
| Daily cleaning | Increase proportion of room cleaned daily by staff; utilize sporicidal product in all patient rooms, visitor common areas, and staff workrooms | Once every 24 h | Environment: to and from patient, HCW, and visitor | |
| Terminal cleaning | Increase proportion of room cleaned by staff at discharge or room transfer; utilize sporicidal product in all patient rooms | Patient discharge or room transfer | Environment: to and from patient, HCW, and visitor | |
| Patient Centered | Patient hand hygiene | Improve overall patient HH compliance; increases utilization of soap and water vs ABHR for CDI or known colonized patients | Once every 6 h; upon visitor and HCW exit of patient room, patient entry and exit of common room, inter- and intraward transfer, and discharge | Patient: to visitor, to and from HCW, to and from environment, and between patients |
| Patient transfer | Decrease hospital-wide patient transfer rate; restrict room transfers of CDI or known colonized patients | Between 0 and 4 times per patient per stay (maximum 2 intra- and 2 interward) | None; indirect effects via increased terminal cleaning | |
| Screening | Screen asymptomatic patients within 24 h of hospital admission via stool sample or, if necessary, rectal swab; if colonized, enact all polices as if CDI patient, except do not treat | Once, at time of admission | None; indirect effects via all 8 other interventions | |
| Visitor hand hygiene | Improve overall visitor HH compliance; increases utilization of soap and water vs ABHR for CDI or known colonized patients | Visitor exit of patient room | Visitor: from environment and patient; indirectly to environment | |
| Visitor contact precautions | Improve visitor contact precautions usage; provide education to reduce contact precaution contamination on donning and doffing; maintain until discharge for CDI or known colonized patients | Visitor exit of patient room | Visitor: from environment and patient; indirectly to environment |
Abbreviations: ABHR, alcohol-based hand rub; CDI, Clostridium difficile infection; HCW, healthcare worker; HH hand hygiene.
As with the model input parameters (Table 2), intervention effectiveness and compliance parameters were derived from an extensive literature review. The derivation of these parameters utilized an additional 50 peer-reviewed studies (Table 4). The distinction between enhanced and ideal interventions was based on intervention implementation details provided in the primary studies. The enhanced level reflected effects of typical intervention implementation. The ideal level reflected maximum possible effects of an intervention implemented under optimal conditions, such as additional financial resources, strong stakeholder support, leadership buy-in, and an expanded infection control workforce. Patient transfer data were lacking in the literature, so we derived these estimates from primary administrative data collected at the University of Wisconsin Hospital in Madison (Supplementary Material 4).
Table 4.
Intervention Parameter Estimates
| Parameter | Baseline Mean (Range) | Enhanced Mean (Range) | Ideal Mean | Sourcea | |
|---|---|---|---|---|---|
| Hand hygiene | |||||
| Soap and water effectiveness | 96 (90–100) | [102–104] | |||
| ABHR effectiveness | 29 (13–36) | [92, 103] | |||
| Standard Room Compliance | Nurse | 60 (46–68) | 79 (74–84) | 96 | [105–115] |
| Doctor | 50 (40–55) | 71 (57–80) | 91 | [105–117] | |
| Visitor | 35 (20–50) | 55 (50–67) | 84 | [106, 118–124] | |
| Patient | 33 (30–40) | 59 (55–65) | 84 | [120, 125–129] | |
| Fraction soap and water (vs ABHR) | 10 (5–25) | [110, 130] | |||
| Known C. difficile Room Compliance | Nurse | 69b | 84b | 97 | [59, 131–133] |
| Doctor | 61b | 77b | 93 | [59, 131–133] | |
| Visitor | 50b | 65b | 88 | [59, 131–133] | |
| Patient | 48b | 68b | 88 | [59, 131–133] | |
| Fraction soap and water (vs ABHR) | 80 (70–90) | 90 (80–95) | 95 | [134] | |
| Contact precautions | |||||
| Gown and glove effectiveness | 70 (60–80) | 86 (80–90) | 97 | [135–137] | |
| Healthcare worker compliance | 67 (62–72) | 77 (71–85) | 87 | [59, 118, 138–142] | |
| Visitor compliance | 50 (42–52) | 74 (70–80) | 94 | [118, 138, 139] | |
| Environmental cleaning | |||||
| Daily cleaning compliance | 46 (40–50) | 80 (70–85) | 94 | [29–33] | |
| Terminal cleaning compliance | 47 (40–50) | 77 (70–82) | 98 | [29, 143–146] | |
| Nonsporicidal effectiveness | 45 (35–50) | [147, 148] | |||
| Sporicidal effectiveness | 99.6 | [148–151] | |||
| Asymptomatic screening at admission | |||||
| Compliance | 0 | 96 (92–99) | 98 | [38, 152] | |
| PCR test sensitivity; specificity | 93 (90–94); 97 (95–99) | [153–155] | |||
| Patient transfer | |||||
| Intraward transfer rate | 5.7 (4–7.4) | 2.8 (2.2–3.5) | 1.4 | Internal data | |
| Interward transfer rate | 13.7 (10–17.4) | 6.8 (5–8.7) | 3.4 | Internal data | |
| Proportionate time between transfers | 24% (time between transfer/length of stay; 20–30) | Internal data | |||
Abbreviations: ABHR, alcohol-based hand rub; C.difficile, Clostridium difficile; PCR, polymerase chain reaction.
aReferences for input parameter sources are included in the Supplementary Materials.
bKnown Clostridium difficile room compliance range based on the range in standard room and standard:C. difficile infection hand hygiene noncompliance ratio (1.34).
Interventions were evaluated both individually and in CDI bundles that introduced several interventions simultaneously. Intervention bundle composition was determined via 2 mechanisms. We took a stepwise approach first, adding interventions sequentially to bundles based on their level of clinical effectiveness when introduced in isolation. We also evaluated CDI bundles composed of interventions that content experts deemed most likely to be implemented together, for example, HCW and patient hand hygiene.
Outcomes
The 2 primary outcomes were the hospital-onset CDI (HO-CDI) rate per 10000 patient-days and the asymptomatic C. difficile colonization rate per 1000 admissions. HO-CDI was defined as having both symptomatic diarrhea and a positive laboratory result on a specimen collected >3 days after admission to the hospital [24].
Simulation
The model was developed and simulated in NetLogo software version 5.3.1 [25]. We employed a model with synchronized common random numbers to reduce stochastic noise leading to variance in the results and allow for direct comparison of counterfactual scenarios [26]. Details of synchronization are included in Supplementary Material 5. Details of model verification and validation, including sensitivity analyses and a limited cross-validation, are included in Supplementary Material 6.
Ultimately, we conducted 5000 runs for 19 single-intervention scenarios: 1 at baseline, 9 with 1 enhanced-level intervention, and 9 with 1 ideal-level intervention and 8 multiple-intervention bundles (Table 5).
Table 5.
List of the Multiple-Intervention Bundle Components Considered in This Study
| Bundle Type | Intervention Components |
|---|---|
| Hand hygiene | HCW hand hygiene, patient hand hygiene |
| Cleaning | Daily cleaning, terminal cleaning |
| Patient-centered | Surveillance, patient transfer, patient hand hygiene |
| Additive maximum effectiveness bundle | Daily cleaning, surveillance |
| Daily cleaning, surveillance, HCW hand hygiene | |
| Daily cleaning, surveillance, HCW hand hygiene, patient hand hygiene | |
| Daily cleaning, surveillance, HCW hand hygiene, patient hand hygiene, terminal cleaning | |
| Daily cleaning, surveillance, HCW hand hygiene, patient hand hygiene, terminal cleaning, patient transfer |
Abbreviation: HCW, healthcare worker.
Statistical Analysis
Pairwise comparisons between baseline, enhanced single-interventions, ideal single-interventions, and enhanced level intervention bundles were conducted using the χ2 test at a significance level of α = .05, using R software (3.3.3).
RESULTS
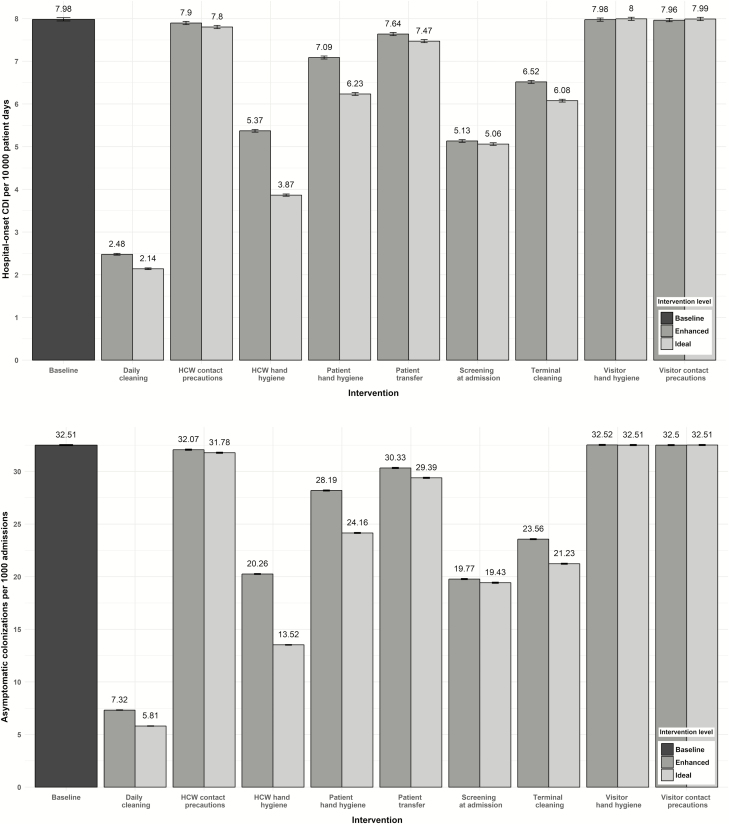
There were significant reductions in HO-CDI and asymptomatic colonization upon implementation of enhanced and ideal levels of 6 interventions: daily and terminal cleaning, HCW hand hygiene, patient hand hygiene, screening at admission, and patient transfer reduction (Figure 2 and Supplementary Table 7).
Figure 2.
Comparative effectiveness of 9 interventions at reducing hospital-onset Clostridium difficile infection (A) and asymptomatic colonization (B). Abbreviations: CDI, Clostridium difficile infection; HCW, healthcare worker.
Daily cleaning with a sporicidal disinfectant and screening at admission were the 2 most effective enhanced single interventions, reducing HO-CDI to 2.48 (95% confidence interval [CI], 2.46–2.50) and 5.13 (95% CI, 5.10–5.16) cases per 10000 patient-days, respectively. These correspond to 68.9% and 35.7% reductions in HO-CDI, compared to the baseline rate of 7.98 (95% CI, 7.95–8.02) HO-CDIs per 10000 patient days (both P < .001). They also reduced asymptomatic colonization 77.5% and 39.2%, respectively. Visitor hand hygiene and visitor contact precaution interventions did not reduce HO-CDI or asymptomatic colonization, compared to baseline. Excluding these 2 visitor strategies, HCW contact precautions was the least effective intervention at reducing hospital-onset colonization and infection.
The difference in intervention effectiveness between enhanced and ideal intervention implementation strategies varied across interventions, ranging between 0 and 18.8% additional reduction in HO-CDI rates for the ideal implementation strategy (Figure 2). Ideal strategies provided the greatest improvement for HCW hand hygiene and patient hand hygiene, the 2 interventions with the largest absolute increases in compliance between the enhanced and ideal intervention levels.
We assessed 8 CDI bundles, simulated for 5000 runs each (Table 6). All significantly reduced both HO-CDI and asymptomatic colonization rates. The most effective 2-intervention bundle was composed of daily cleaning and screening, reducing HO-CDI by 82.3% and asymptomatic colonization by 90.6%. Adding HCW and patient hand hygiene interventions resulted in a small, significant, additional decrease to HO-CDI and asymptomatic colonization rates. Visitor hand hygiene and contact precautions were not included in bundles, due to their negligible effect on reducing CDI or asymptomatic colonization and sustained instability at 5000 runs.
Table 6.
Comparative Clinical Effectiveness of 8 Multiple-Intervention Bundles
| Bundle Components | HO-CDI per 10000 Patient-days (95% CI) | Asymptomatic Colonization per 1000 Admissions (95% CI) |
|---|---|---|
| Baseline | 7.98 (7.95–8.02) | 32.51 (32.44–32.57) |
| Patient and HCW HH | 4.74 (4.71–4.77) | 17.33 (17.29–17.38) |
| Terminal and daily cleaning | 2.44 (2.41–2.46) | 6.96 (6.93–6.99) |
| Screening, patient HH, patient transfer | 3.75 (3.73–3.78) | 13.14 (13.09–13.19) |
| Daily cleaning, surveillance | 1.41 (1.39–1.43) | 3.05 (3.03–3.07) |
| Daily cleaning, surveillance, HCW HH | 1.18 (1.17–1.20) | 2.00 (1.99–2.01) |
| Daily cleaning, surveillance, HCW HH, patient HH | 1.13 (1.11–1.14) | 1.67 (1.66–1.68) |
| Daily cleaning, surveillance, HCW HH, patient HH, terminal cleaning | 1.12 (1.10–1.13) | 1.61 (1.60–1.62) |
| Daily cleaning, surveillance, HCW HH, patient HH, terminal cleaning, patient transfer | 1.11 (1.10–1.12) | 1.59 (1.57–1.60) |
Comparative effectiveness of 8 multiple-intervention combination bundles.
Abbreviations: CI, confidence interval; HCW, healthcare worker; HH hand hygiene; HO-CID, hospital-onset Clostridium difficile infection.
The patient-centered bundle comprised of screening at admission, patient hand hygiene, and reducing intra- and interward room transfers was more effective than the 2-pronged patient and HCW hand hygiene bundle. However, adding patient hand hygiene to the single HCW hand hygiene intervention significantly reduced HO-CDI rates by an additional 7.9%.
Nursing staff and the environment were the main sources of C. difficile transmission, each responsible for >40% of exposures at baseline conditions (Table 7). Transmission via direct patient-to-patient contact was minimal under all scenarios, resulting in a maximum of 0.24% of exposures.
Table 7.
Comparative Contribution of Agents and the Environment to Patients’ Clostridium difficile Exposures
| Intervention | Environment, % of Exposures (95% CI) | Nursing, bold>% of Exposures (95% CI) | Physicians, % of Exposures (95% CI) | Patient, % of Exposures (95% CI) |
|---|---|---|---|---|
| Baseline | 40.77 (40.74–40.81) | 42.79 (42.76–42.82) | 16.37 (16.35–16.40) | 0.062 (.061–.064) |
| Daily cleaning | 20.21 (20.16–20.27) | 56.13 (56.05–56.20) | 23.42 (23.36–23.49) | 0.236 (.230–.243) |
| HCW contact precautions | 40.94 (40.91–40.97) | 42.73 (42.70–42.76) | 16.27 (16.24–16.29) | 0.062 (.061–.064) |
| HCW hand hygiene | 46.39 (46.35–46.43) | 39.32 (39.28–39.36) | 14.20 (14.17–14.23) | 0.090 (.088–.093) |
| Patient hand hygiene | 41.27 (41.23–41.30) | 42.66 (42.62–42.69) | 16.02 (15.99–16.05) | 0.056 (.054–.057) |
| Patient transfer | 39.74 (39.70–39.77) | 43.57 (43.54–43.61) | 16.63 (16.60–16.65) | 0.065 (.064–.067) |
| Screening | 43.58 (43.53–43.62) | 41.66 (41.61–41.70) | 14.73 (14.70–14.77) | 0.033 (.032–.035) |
| Terminal cleaning | 34.97 (34.94–35.01) | 46.92 (46.88–46.96) | 18.03 (18.00–18.06) | 0.079 (.077–.081) |
| Visitor hand hygiene | 40.77 (40.74–40.81) | 42.78 (42.74–42.81) | 16.39 (16.36–16.41) | 0.062 (.060–.064) |
| Visitor contact precautions | 40.77 (40.74–40.81) | 42.80 (42.77–42.83) | 16.37 (16.34–16.39) | 0.062 (.060–.063) |
Abbreviations: CI, confidence interval; HCW, healthcare worker.
Full sensitivity analysis results are shown in Supplementary Material 7. Trends in relative clinical effectiveness of the 7 evaluated interventions changed slightly under parameter estimate variation. Cross-validation results are included in Supplementary Material 8.
DISCUSSION
Because prevalence of asymptomatic C. difficile carriage is much higher than active CDI, previous studies have postulated that asymptomatic colonization may be responsible for a considerable proportion of new CDI cases [21, 27]. Consistent with this, our 2 most effective single-intervention strategies were daily cleaning with a sporicidal disinfectant and screening at admission. These largely act by reducing transmission of C. difficile from asymptomatically colonized patients.
The daily cleaning intervention utilized a sporicidal agent in all patient rooms and common areas. The substitution of sporicidal for nonsporicidal agents in the rooms of patients without a known CDI requires little additional time for cleaning services staff [28] and, once implemented, necessitates few workflow changes. Previous studies of daily cleaning interventions have reported drastically increased compliance, resulting in >75% average daily cleaning rates for high-touch surfaces [29–33]. Sustaining this level of compliance can be challenging and requires continued administrative support, yet the potential benefits are substantial. In addition to C. difficile reduction, hospital-wide use of sporicidal products may reduce vancomycin-resistant Enterococcus colonization rates by nearly 25% [34].
In the context of implementation, screening patients at admission requires fewer stakeholders and behavioral changes than more complex interventions such as HCW hand hygiene or contact precautions [35–37]. The intervention can be targeted to a subset of hospital employees, namely, front-line nursing staff and laboratory services. A work systems study of a pilot C. difficile screening intervention currently in place on 1 unit at our facility found the intervention to be well received by stakeholders, including patients (unpublished data). Screening for MRSA, a similarly transmitted nosocomial pathogen, has been successfully implemented at Veterans Affairs hospitals nationwide [38]. This screening intervention had a 96% participation rate and reduced MRSA by 45% among non–intensive care unit patients. This reduction is similar to the 35.7% reduction in HO-CDI we simulated due to C. difficile screening.
While asymptomatic C. difficile screening is not routinely recommended [39], the single large existing study in which screening was implemented as a single intervention found a 56% reduction in HO-CDIs [40]. This reduction is likely higher than our model because of a concomitant, unintended increase in HCW hand hygiene during the study period. In the study, HCWs caring for asymptomatic carriers were required to use gloves and to wash their hands with soap and water. Daily disinfection of patient rooms was conducted using a chorine-based, sporicidal product.
Patient hand hygiene was another highly effective patient-centered intervention. Adding patient hand hygiene to HCW hand hygiene reduced HO-CDI rates an additional 7.9%. Typical patient hand hygiene interventions focus on patient empowerment as a strategy for increasing HCW hand hygiene, but improving compliance among patients themselves has rarely been a goal [41]. However, patients’ hand hygiene rates typically decline in the hospital, and key opportunities are missed for washing hands before eating and after toileting [42]. Patients are central to the C. difficile transmission pathway as they experience direct physical contact with HCWs, visitors, and the environment, and should be a focus for hand hygiene interventions.
Visitor hand hygiene and contact precaution interventions had no effect on HO-CDI rates. This is likely due in part to the short duration of time that visitors spent with patients. The impact of visitor interventions may vary in settings with extensive visitor contact, such as pediatric hospitals and long-term care facilities. Future modeling studies are needed to evaluate CDI interventions in these contexts.
Another reason for the null effect of visitor contact precautions may be related to limited effectiveness of contact precaution interventions in general. HCW contact precautions showed only a small effect, even though precautions were continued for the duration of a known C. difficile patient’s stay. Contact precaution use is not without costs and may be associated with increased adverse effects [43]. These include higher rates of anxiety and depression [44] and increases in preventable adverse events, such as falls and pressure ulcers [45]. Current infection control guidelines state that in areas where MRSA and vancomycin-resistant enterococci are endemic, visitors may not be required to use contact precautions for these pathogens [46]. While hospitals are still recommended to consider contact precautions for visitors of CDI patients, the evidence for this recommendation is weak.
Three other agent-based models of C. difficile transmission have previously evaluated intervention effectiveness, including an admissions screening model [47], 6-intervention model [21], and our group’s initial 4-intervention model [18]. Lanzas and Dubberke reported that screening reduced HO-CDI by 25% and new colonizations by 52%, under the conditions that most closely replicate our model [47]. In comparison, the screening intervention rates of HO-CDI reduction (35.7%) and asymptomatic colonization reduction (39.2%) were highly correlated in our model. The smaller reduction in HO-CDI in the Lanzas and Dubberke model compared to the asymptomatic colonization rate may be due to modeling decisions and underlying assumptions regarding transitions between different patient clinical states.
The 6-intervention model by Rubin et al found that HCW hand hygiene had the greatest single-intervention impact on CDI rate [21]. Environmental cleaning was not effective, although it did not include sporicidal agents for terminal cleaning of non–C. difficile rooms or daily cleaning of any room and thus is not comparable to our study interventions. Similar to our findings, Rubin et al found HCW contact precautions to be ineffective at reducing HO-CDI. Our group’s original model simulated treatment, HCW hand hygiene, environmental cleaning, and contact isolation [18]. While considerable changes have been introduced to the current model, it is notable that the environmental cleaning strategy was the most effective in both models.
Predictive validity, or a model’s ability to predict future outcomes in real-life scenarios, has not been assessed for any C. difficile agent-based model in the literature. Our model is easily customizable to an individual hospital’s infection control context. By inputting its own intervention compliance data, a facility could determine customized results on intervention comparative effectiveness at its institution. Future evaluations of predicative validity are needed to provide additional evidence for the applicability of outcomes to real-world settings.
Despite its complexity, this model relies on many simplifications and assumptions that allow the model to be computationally tractable and reflect the availability of parameter estimate data in the literature. For example, the model does not incorporate patient heterogeneity beyond age and antibiotic usage. Yet, known risk factors such as immunocompromised status, history of hospitalization, and prior C. difficile infection result in underlying variability in C. difficile susceptibility to colonization and infection.
Infection and colonization are also simulated by a generic C. difficile strain. Thus, the model does not account for inherent differences in transmission and health outcomes across strains such as Bl/NAP1/027. Furthermore, the hospital layout is defined as a series of identical patient rooms and wards. This does not allow for investigation of potentially unique transmission dynamics in an intensive care unit or bone marrow transplant ward, or for evaluation of the impact of these high-risk units on hospital-wide C. difficile transmission.
Finally, we did not evaluate an antibiotic stewardship intervention. While recent evidence has shown that fluoroquinolone restrictions may be particularly effective at reducing CDI rates [48], proper modeling of this intervention requires more robust consideration of patient heterogeneity than is possible using currently available data in the literature. Thus, the effectiveness of an antibiotic stewardship intervention has not been evaluated by any existing agent-based C. difficile models to date [18, 21, 47].
CONCLUSIONS
This C. difficile agent-based model is the first to compare patient-centered interventions with hospital-centered strategies. Our results provide much-needed direction to HCWs and infection control leadership regarding which interventions to prioritize to optimally control disease transmission. The findings also highlight the importance of patients’ own hand hygiene, which has historically been overlooked. Many interventions we found to be highly effective are horizontal approaches to infection control that are not pathogen-specific [22, 39, 49, 50]. These strategies are key to the prevention of countless infectious diseases and our results have implications well beyond prevention of C. difficile.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We acknowledge Josh Koscher, UW Health, for facilitating extraction of institutional patient transfer data.
Financial support. This work was supported by a predoctoral traineeship from the National Institutes of Health (grant number TL1TR000429) to A. K. B. The traineeship is administered by the University of Wisconsin–Madison, Institute for Clinical and Translational Research, funded by the National Institutes of Health (grant number UL1TR000427). N. S. is supported by a Veterans Affairs–funded patient safety center of inquiry.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 24 April 2017. [Google Scholar]
- 2. Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist 2014; 7:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuya-Kanamori L, Yakob L, Riley TV et al. Community-acquired Clostridium difficile infection, Queensland, Australia. Emerg Infect Dis 2016; 22:1659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vindigni SM, Surawicz CM. C. difficile infection: changing epidemiology and management paradigms. Clin Transl Gastroenterol 2015; 6:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghose C. Clostridium difficile infection in the twenty-first century. Emerg Microbes Infect 2013; 2:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ilchmann C, Zaiss NH, Speicher A, Christner M, Ackermann G, Rohde H. Comparison of resistance against erythromycin and moxifloxacin, presence of binary toxin gene and PCR ribotypes in Clostridium difficile isolates from 1990 and 2008. Eur J Clin Microbiol Infect Dis 2010; 29:1571–3. [DOI] [PubMed] [Google Scholar]
- 9. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011; 32:387–90. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Medicare and Medicaid Service. CMS to improve quality of care during hospital inpatient stays 2014. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2014-Fact-sheets-items/2014-08-04-2.html. Accessed 15 December 2016.
- 11. Dubberke ER. Prevention of healthcare‐associated Clostridium difficile infection: what works?Infect Control Hosp Epidemiol 2010; 31(Suppl 1):S38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yakob L, Riley TV, Paterson DL, Marquess J, Clements AC. Assessing control bundles for Clostridium difficile: a review and mathematical model. Emerg Microbes Infect 2014; 3:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubberke ER, Carling P, Carrico R et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(Suppl 2):S48–65. [DOI] [PubMed] [Google Scholar]
- 14. Abbett SK, Yokoe DS, Lipsitz SR et al. Proposed checklist of hospital interventions to decrease the incidence of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol 2009; 30:1062–9. [DOI] [PubMed] [Google Scholar]
- 15. Barker AK, Ngam C, Musuuza JS, Vaughn VM, Safdar N. Reducing Clostridium difficile in the inpatient setting: a systematic review of the adherence to and effectiveness of C. difficile prevention bundles. Infect Control Hosp Epidemiol 2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gingras G, Guertin MH, Laprise JF, Drolet M, Brisson M. Mathematical modeling of the transmission dynamics of Clostridium difficile infection and colonization in healthcare settings: a systematic review. PLoS One 2016; 11:e0163880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci U S A 2002; 99(Suppl 3):7280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Codella J, Safdar N, Heffernan R, Alagoz O. An agent-based simulation model for Clostridium difficile infection control. Med Decis Making 2015; 35:211–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Troisi A, Wong V, Ratner MA. An agent-based approach for modeling molecular self-organization. Proc Natl Acad Sci U S A 2005; 102:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parunak HVD, Savit R, Riolo RL. Agent-based modeling vs. equation-based modeling: a case study and users’ guide. In: Sichman JS, Conte R, Gilbert N, eds. Multi-agent systems and agent-based simulation. Springer Berlin Heidelberg, 1998: 10–25. [Google Scholar]
- 21. Rubin MA, Jones M, Leecaster M et al. A simulation-based assessment of strategies to control Clostridium difficile transmission and infection. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009; 73:305–15. [DOI] [PubMed] [Google Scholar]
- 23. Friedman ND, Pollard J, Stupart D et al. Prevalence of Clostridium difficile colonization among healthcare workers. BMC Infect Dis 2013; 13:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Multidrug-resistant organism and Clostridium difficile infection (MDRO/CDI) module 2017. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual /12pscmdro_cdadcurrent.pdf. Accessed 18 April 2017.
- 25. Wilensky U. Netlogo 1999. Available at: http://ccl.northwestern.edu/netlogo/. Accessed 30 April 2016.
- 26. Stout NK, Goldie SJ. Keeping the noise down: common random numbers for disease simulation modeling. Health Care Manag Sci 2008; 11:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eyre DW, Cule ML, Wilson DJ et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013; 369:1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orenstein R, Aronhalt KC, McManus JE Jr, Fedraw LA. A targeted strategy to wipe out Clostridium difficile. Infect Control Hosp Epidemiol 2011; 32:1137–9. [DOI] [PubMed] [Google Scholar]
- 29. Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. An environmental disinfection odyssey: evaluation of sequential interventions to improve disinfection of Clostridium difficile isolation rooms. Infect Control Hosp Epidemiol 2013; 34:459–65. [DOI] [PubMed] [Google Scholar]
- 30. Goodman ER, Platt R, Bass R, Onderdonk AB, Yokoe DS, Huang SS. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008; 29:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayden MK, Bonten MJ, Blom DW, Lyle EA, van de Vijver DA, Weinstein RA. Reduction in acquisition of vancomycin-resistant Enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006; 42:1552–60. [DOI] [PubMed] [Google Scholar]
- 32. Boyce JM, Havill NL, Dumigan DG, Golebiewski M, Balogun O, Rizvani R. Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect Control Hosp Epidemiol 2009; 30:678–84. [DOI] [PubMed] [Google Scholar]
- 33. Hess AS, Shardell M, Johnson JK et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infect Control Hosp Epidemiol 2013; 34:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grabsch EA, Mahony AA, Cameron DR et al. Significant reduction in vancomycin-resistant Enterococcus colonization and bacteraemia after introduction of a bleach-based cleaning-disinfection programme. J Hosp Infect 2012; 82:234–42. [DOI] [PubMed] [Google Scholar]
- 35. Eiamsitrakoon T, Apisarnthanarak A, Nuallaong W, Khawcharoenporn T, Mundy LM. Hand hygiene behavior: translating behavioral research into infection control practice. Infect Control Hosp Epidemiol 2013; 34:1137–45. [DOI] [PubMed] [Google Scholar]
- 36. Whitby M, McLaws ML, Ross MW. Why healthcare workers don’t wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol 2006; 27:484–92. [DOI] [PubMed] [Google Scholar]
- 37. O’Boyle CA, Henly SJ, Larson E. Understanding adherence to hand hygiene recommendations: the theory of planned behavior. Am J Infect Control 2001; 29:352–60. [DOI] [PubMed] [Google Scholar]
- 38. Jain R, Kralovic SM, Evans ME et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011; 364:1419–30. [DOI] [PubMed] [Google Scholar]
- 39. Cohen SH, Gerding DN, Johnson S et al. Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 40. Longtin Y, Paquet-Bolduc B, Gilca R et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med 2016; 176:796–804. [DOI] [PubMed] [Google Scholar]
- 41. Landers T, Abusalem S, Coty MB, Bingham J. Patient-centered hand hygiene: the next step in infection prevention. Am J Infect Control 2012; 40:S11–7. [DOI] [PubMed] [Google Scholar]
- 42. Barker A, Sethi A, Shulkin E, Caniza R, Zerbel S, Safdar N. Patients’ hand hygiene at home predicts their hand hygiene practices in the hospital. Infect Control Hosp Epidemiol 2014; 35:585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abad C, Fearday A, Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J Hosp Infect 2010; 76:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tarzi S, Kennedy P, Stone S, Evans M. Methicillin-resistant Staphylococcus aureus: psychological impact of hospitalization and isolation in an older adult population. J Hosp Infect 2001; 49:250–4. [DOI] [PubMed] [Google Scholar]
- 45. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA 2003; 290:1899–905. [DOI] [PubMed] [Google Scholar]
- 46. Munoz-Price LS, Banach DB, Bearman G et al. Isolation precautions for visitors. Infect Control Hosp Epidemiol 2015; 36:747–58. [DOI] [PubMed] [Google Scholar]
- 47. Lanzas C, Dubberke ER. Effectiveness of screening hospital admissions to detect asymptomatic carriers of Clostridium difficile: a modeling evaluation. Infect Control Hosp Epidemiol 2014; 35:1043–50. [DOI] [PubMed] [Google Scholar]
- 48. Dingle KE, Didelot X, Quan TP et al. Modernising Medical Microbiology Informatics Group Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ellingson K, Haas JP, Aiello AE et al. Society for Healthcare Epidemiology of America Strategies to prevent healthcare-associated infections through hand hygiene. Infect Control Hosp Epidemiol 2014; 35:937–60. [DOI] [PubMed] [Google Scholar]
- 50. Calfee DP, Salgado CD, Milstone AM et al. Society for Healthcare Epidemiology of America Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35:772–96. [DOI] [PubMed] [Google Scholar]
- 51. American Hospital Association. AHA Hospital Statistics. Chicago, IL: Healthcare InfoSource, 2016. [Google Scholar]
- 52. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project statistical brief #180: overview of hospital stays in the United States, 2012 Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed 24 April 2017.
- 53. Witt W, Weiss A, Elixhauser A. Overview of hospital stays for children in the United States, 2012 Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.pdf. Accessed 24 April 2017. [PubMed]
- 54. Kaboli PJ, Go JT, Hockenberry J et al. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med 2012; 157:837–45. [DOI] [PubMed] [Google Scholar]
- 55. Stevens VW, Khader K, Nelson RE et al. Excess length of stay attributable to Clostridium difficile infection (CDI) in the acute care setting: a multistate model. Infect Control Hosp Epidemiol 2015; 36:1024–30. [DOI] [PubMed] [Google Scholar]
- 56. Centers for Disease Control and Prevention. Table 89. Hospitals, beds, and occupancy rates, by type of ownership and size of hospital: United States, selected years 1975–2013 Available at: https://www.cdc.gov/nchs/data/hus/2015/089.pdf. Accessed 24 April 2017.
- 57. McArdle FI, Lee RJ, Gibb AP, Walsh TS. How much time is needed for hand hygiene in intensive care? A prospective trained observer study of rates of contact between healthcare workers and intensive care patients. J Hosp Infect 2006; 62:304–10. [DOI] [PubMed] [Google Scholar]
- 58. Cohen B, Hyman S, Rosenberg L, Larson E. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt Comm J Qual Patient Saf 2012; 38:560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morgan DJ, Pineles L, Shardell M et al. The effect of contact precautions on healthcare worker activity in acute care hospitals. Infect Control Hosp Epidemiol 2013; 34:69–73. [DOI] [PubMed] [Google Scholar]
- 60. Magill SS, Edwards JR, Beldavs ZG et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fridkin S, Baggs J, Fagan R et al. Centers for Disease Control and Prevention Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 63. Louie TJ, Miller MA, Mullane KM et al. OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 64. Cornely OA, Crook DW, Esposito R et al. OPT-80-004 Clinical Study Group Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 65. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis 2009; 48:e41–6. [DOI] [PubMed] [Google Scholar]
- 66. Johnson S, Louie TJ, Gerding DN et al. Polymer Alternative for CDI Treatment (PACT) Investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 67. California Code of Regulations. Nurse service staff 2003. 22 C.C.R., Sec. 70217 2003. Available at: https://govt.westlaw.com/calregs/Document/I8612C410941 F11E29091E6B951DDF6CE?viewType=FullText&originationContext= documenttoc&transitionType=CategoryPageItem&contextData= (sc.Default&bhcp=1. Accessed 14 April 2017.
- 68. Spetz J, Donaldson N, Aydin C, Brown DS. How many nurses per patient? Measurements of nurse staffing in health services research. Health Serv Res 2008; 43:1674–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA 2002; 288:1987–93. [DOI] [PubMed] [Google Scholar]
- 70. Evans HL, Shaffer MM, Hughes MG et al. Contact isolation in surgical patients: a barrier to care?Surgery 2003; 134:180–8. [DOI] [PubMed] [Google Scholar]
- 71. Barker AK, Codella J, Ewers T, Dundon A, Alagoz O, Safdar N. Changes to physician and nurse time burdens when caring for patients under contact precautions. Am J Infect Control 2017; 45:542–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cross KW, Turner RD. Factors affecting the visiting pattern of geriatric patients in a rural area. Br J Prev Soc Med 1974; 28:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eriksson T, Bergbom I. Visits to intensive care unit patients—frequency, duration and impact on outcome. Nurs Crit Care 2007; 12:20–6. [DOI] [PubMed] [Google Scholar]
- 74. Gonzalez CE, Carroll DL, Elliott JS, Fitzgerald PA, Vallent HJ. Visiting preferences of patients in the intensive care unit and in a complex care medical unit. Am J Crit Care 2004; 13:194–8. [PubMed] [Google Scholar]
- 75. Fumagalli S, Boncinelli L, Lo Nostro A et al. Reduced cardiocirculatory complications with unrestrictive visiting policy in an intensive care unit: results from a pilot, randomized trial. Circulation 2006; 113:946–52. [DOI] [PubMed] [Google Scholar]
- 76. Centers for Disease Control and Prevention. Number, rate, and average length of stay for discharges from short-stay hospitals, by age, region, and sex: United States, 2010 Available at: https://www.cdc.gov/nchs/data/nhds/1general/2010gen1_agesexalos.pdf. Accessed 12 April 2017.
- 77. Hicks LA, Bartoces MG, Roberts RM et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60:1308–16. [DOI] [PubMed] [Google Scholar]
- 78. Frenk SM, Kit BK, Lukacs SL, Hicks LA, Gu Q. Trends in the use of prescription antibiotics: NHANES 1999–2012. J Antimicrob Chemother 2016; 71:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dantes R, Mu Y, Hicks LA et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficile infection. Open Forum Infect Dis 2015; 2:ofv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Koo HL, Van JN, Zhao M et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol 2014; 35:667–73. [DOI] [PubMed] [Google Scholar]
- 81. Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 2014; 59:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leekha S, Aronhalt KC, Sloan LM, Patel R, Orenstein R. Asymptomatic Clostridium difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control 2013; 41:390–3. [DOI] [PubMed] [Google Scholar]
- 83. Loo VG, Bourgault AM, Poirier L et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011; 365:1693–703. [DOI] [PubMed] [Google Scholar]
- 84. Eyre DW, Griffiths D, Vaughan A et al. Asymptomatic Clostridium difficile colonisation and onward transmission. PLoS One 2013; 8:e78445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nissle K, Kopf D, Rösler A. Asymptomatic and yet C. difficile-toxin positive? Prevalence and risk factors of carriers of toxigenic Clostridium difficile among geriatric in-patients. BMC Geriatr 2016; 16:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kagan S, Wiener-Well Y, Ben-Chetrit E et al. The risk for Clostridium difficile colitis during hospitalization in asymptomatic carriers. J Hosp Infect 2017; 95:442–3. [DOI] [PubMed] [Google Scholar]
- 87. Gupta S, Mehta V, Herring T et al. A large prospective North American epidemiologic study of hospital-associated Clostridium difficile colonization & infection. In: International Clostridium difficile Symposium, Bled, Slovenia, 2012. Abstract 020. [Google Scholar]
- 88. Hung YP, Lin HJ, Wu TC et al. Risk factors of fecal toxigenic or non-toxigenic Clostridium difficile colonization: impact of Toll-like receptor polymorphisms and prior antibiotic exposure. PLoS One 2013; 8:e69577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dubberke ER, Burnham CA. Diagnosis of Clostridium difficile infection: treat the patient, not the test. JAMA Intern Med 2015; 175:1801–2. [DOI] [PubMed] [Google Scholar]
- 90. Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009: statistical brief 124. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2012. Available at: http://www.ncbi.nlm.nih.gov/books/NBK92613/. Accessed 19 February 2017. [PubMed] [Google Scholar]
- 91. Evans ME, Simbartl LA, Kralovic SM, Jain R, Roselle GA. Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol 2014; 35:1037–42. [DOI] [PubMed] [Google Scholar]
- 92. Jabbar U, Leischner J, Kasper D et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol 2010; 31:565–70. [DOI] [PubMed] [Google Scholar]
- 93. Lopez GU, Gerba CP, Tamimi AH, Kitajima M, Maxwell SL, Rose JB. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol 2013; 79:5728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barker AK, Zellmer C, Tischendorf J et al. On the hands of patients with Clostridium difficile: a study of spore prevalence and the effect of hand hygiene on C difficile removal. Am J Infect Control 2017; 45:1154–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis 2008; 46:447–50. [DOI] [PubMed] [Google Scholar]
- 96. Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol 2010; 31:21–7. [DOI] [PubMed] [Google Scholar]
- 97. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 1989; 320:204–10. [DOI] [PubMed] [Google Scholar]
- 98. Struelens MJ, Maas A, Nonhoff C et al. Control of nosocomial transmission of Clostridium difficile based on sporadic case surveillance. Am J Med 1991; 91:S138–44. [DOI] [PubMed] [Google Scholar]
- 99. Eckstein BC, Adams DA, Eckstein EC et al. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis 2007; 7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Faires MC, Pearl DL, Berke O, Reid-Smith RJ, Weese JS. The identification and epidemiology of meticillin-resistant Staphylococcus aureus and Clostridium difficile in patient rooms and the ward environment. BMC Infect Dis 2013; 13:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dubberke ER, Reske KA, Noble-Wang J et al. Prevalence of Clostridium difficile environmental contamination and strain variability in multiple health care facilities. Am J Infect Control 2007; 35:315–8. [DOI] [PubMed] [Google Scholar]
- 102. Bettin K, Clabots C, Mathie P, Willard K, Gerding DN. Effectiveness of liquid soap vs. chlorhexidine gluconate for the removal of Clostridium difficile from bare hands and gloved hands. Infect Control Hosp Epidemiol 1994; 15:697–702. [DOI] [PubMed] [Google Scholar]
- 103. Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol 2009; 30:939–44. [DOI] [PubMed] [Google Scholar]
- 104. Edmonds SL, Zapka C, Kasper D et al. Effectiveness of hand hygiene for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol 2013; 34:302–5. [DOI] [PubMed] [Google Scholar]
- 105. Dierssen-Sotos T, Brugos-Llamazares V, Robles-García M et al. Evaluating the impact of a hand hygiene campaign on improving adherence. Am J Infect Control 2010; 38:240–3. [DOI] [PubMed] [Google Scholar]
- 106. Randle J, Firth J, Vaughan N. An observational study of hand hygiene compliance in paediatric wards. J Clin Nurs 2013; 22:2586–92. [DOI] [PubMed] [Google Scholar]
- 107. Monistrol O, Calbo E, Riera M et al. Impact of a hand hygiene educational programme on hospital-acquired infections in medical wards. Clin Microbiol Infect 2012; 18:1212–8. [DOI] [PubMed] [Google Scholar]
- 108. Tromp M, Huis A, de Guchteneire I et al. The short-term and long-term effectiveness of a multidisciplinary hand hygiene improvement program. Am J Infect Control 2012; 40:732–6. [DOI] [PubMed] [Google Scholar]
- 109. Kowitt B, Jefferson J, Mermel LA. Factors associated with hand hygiene compliance at a tertiary care teaching hospital. Infect Control Hosp Epidemiol 2013; 34:1146–52. [DOI] [PubMed] [Google Scholar]
- 110. Mestre G, Berbel C, Tortajada P et al. “The 3/3 strategy”: a successful multifaceted hospital wide hand hygiene intervention based on WHO and continuous quality improvement methodology. PLoS One 2012; 7:e47200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Eldridge NE, Woods SS, Bonello RS et al. Using the six sigma process to implement the Centers for Disease Control and Prevention guideline for hand hygiene in 4 intensive care units. J Gen Intern Med 2006; 21(Suppl 2):S35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zerr DM, Allpress AL, Heath J, Bornemann R, Bennett E. Decreasing hospital-associated rotavirus infection: a multidisciplinary hand hygiene campaign in a children’s hospital. Pediatr Infect Dis J 2005; 24:397–403. [DOI] [PubMed] [Google Scholar]
- 113. Mayer J, Mooney B, Gundlapalli A et al. Dissemination and sustainability of a hospital-wide hand hygiene program emphasizing positive reinforcement. Infect Control Hosp Epidemiol 2011; 32:59–66. [DOI] [PubMed] [Google Scholar]
- 114. Muto CA, Blank MK, Marsh JW et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis 2007; 45:1266–73. [DOI] [PubMed] [Google Scholar]
- 115. Grant AM, Hofmann DA. It’s not all about me: motivating hand hygiene among health care professionals by focusing on patients. Psychol Sci 2011; 22:1494–9. [DOI] [PubMed] [Google Scholar]
- 116. Grayson ML, Russo PL, Cruickshank M et al. Outcomes from the first 2 years of the Australian National Hand Hygiene Initiative. Med J Aust 2011; 195:615–9. [DOI] [PubMed] [Google Scholar]
- 117. Pittet D, Simon A, Hugonnet S, Pessoa-Silva CL, Sauvan V, Perneger TV. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004; 141:1–8. [DOI] [PubMed] [Google Scholar]
- 118. Clock SA, Cohen B, Behta M, Ross B, Larson EL. Contact precautions for multidrug-resistant organisms: current recommendations and actual practice. Am J Infect Control 2010; 38:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Birnbach DJ, Rosen LF, Fitzpatrick M, Arheart KL, Munoz-Price LS. An evaluation of hand hygiene in an intensive care unit: are visitors a potential vector for pathogens?J Infect Public Health 2015; 8:570–4. [DOI] [PubMed] [Google Scholar]
- 120. Randle J, Arthur A, Vaughan N, Wharrad H, Windle R. An observational study of hand hygiene adherence following the introduction of an education intervention. J Infect Prev 2014; 15:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Birnbach DJ, Nevo I, Barnes S et al. Do hospital visitors wash their hands? Assessing the use of alcohol-based hand sanitizer in a hospital lobby. Am J Infect Control 2012; 40:340–3. [DOI] [PubMed] [Google Scholar]
- 122. Caroe Aarestrup S, Moesgaard F, Schuldt-Jensen J. Nudging hospital visitors’ hand hygiene compliance 2016. Available at: http://inudgeyou.com/wp-content/uploads/2016/05/Hand_Hygiene.pdf. Accessed 25 March 2017.
- 123. Nishimura S, Kagehira M, Kono F, Nishimura M, Taenaka N. Handwashing before entering the intensive care unit: what we learned from continuous video-camera surveillance. Am J Infect Control 1999; 27:367–9. [DOI] [PubMed] [Google Scholar]
- 124. Randle J, Arthur A, Vaughan N. Twenty-four-hour observational study of hospital hand hygiene compliance. J Hosp Infect 2010; 76:252–5. [DOI] [PubMed] [Google Scholar]
- 125. Davis CR. Infection-free surgery: how to improve hand-hygiene compliance and eradicate methicillin-resistant Staphylococcus aureus from surgical wards. Ann R Coll Surg Engl 2010; 92:316–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Srigley JA, Furness CD, Gardam M. Measurement of patient hand hygiene in multiorgan transplant units using a novel technology: an observational study. Infect Control Hosp Epidemiol 2014; 35:1336–41. [DOI] [PubMed] [Google Scholar]
- 127. Cheng VCC, Wu AKL, Cheung CHY et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect 2007; 67:336–43. [DOI] [PubMed] [Google Scholar]
- 128. Hedin G, Blomkvist A, Janson M, Lindblom A. Occurrence of potentially pathogenic bacteria on the hands of hospital patients before and after the introduction of patient hand disinfection. APMIS 2012; 120:802–7. [DOI] [PubMed] [Google Scholar]
- 129. Gagné D, Bédard G, Maziade PJ. Systematic patients’ hand disinfection: impact on meticillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect 2010; 75:269–72. [DOI] [PubMed] [Google Scholar]
- 130. Stone PW, Hasan S, Quiros D, Larson EL. Effect of guideline implementation on costs of hand hygiene. Nurs Econ 2007; 25:279–84. [PMC free article] [PubMed] [Google Scholar]
- 131. Golan Y, Doron S, Griffith J et al. The impact of gown-use requirement on hand hygiene compliance. Clin Infect Dis 2006; 42:370–6. [DOI] [PubMed] [Google Scholar]
- 132. Swoboda SM, Earsing K, Strauss K, Lane S, Lipsett PA. Isolation status and voice prompts improve hand hygiene. Am J Infect Control 2007; 35:470–6. [DOI] [PubMed] [Google Scholar]
- 133. Almaguer-Leyva M, Mendoza-Flores L, Medina-Torres AG et al. Hand hygiene compliance in patients under contact precautions and in the general hospital population. Am J Infect Control 2013; 41:976–8. [DOI] [PubMed] [Google Scholar]
- 134. Zellmer C, Blakney R, Van Hoof S, Safdar N. Impact of sink location on hand hygiene compliance for Clostridium difficile infection. Am J Infect Control 2015; 43:387–9. [DOI] [PubMed] [Google Scholar]
- 135. Morgan DJ, Rogawski E, Thom KA et al. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med 2012; 40:1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Landelle C, Verachten M, Legrand P et al. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol 2014; 35:10–5. [DOI] [PubMed] [Google Scholar]
- 137. Tomas ME, Kundrapu S, Thota P et al. Contamination of health care personnel during removal of personal protective equipment. JAMA Intern Med 2015; 175:1904–10. [DOI] [PubMed] [Google Scholar]
- 138. Weber DJ, Sickbert-Bennett EE, Brown VM et al. Compliance with isolation precautions at a university hospital. Infect Control Hosp Epidemiol 2007; 28:358–61. [DOI] [PubMed] [Google Scholar]
- 139. Manian FA, Ponzillo JJ. Compliance with routine use of gowns by healthcare workers (HCWs) and non-HCW visitors on entry into the rooms of patients under contact precautions. Infect Control Hosp Epidemiol 2007; 28:337–40. [DOI] [PubMed] [Google Scholar]
- 140. Bearman GM, Marra AR, Sessler CN et al. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. Am J Infect Control 2007; 35:650–5. [DOI] [PubMed] [Google Scholar]
- 141. Bearman G, Rosato AE, Duane TM et al. Trial of universal gloving with emollient-impregnated gloves to promote skin health and prevent the transmission of multidrug-resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol 2010; 31:491–7. [DOI] [PubMed] [Google Scholar]
- 142. Deyneko A, Cordeiro F, Berlin L, Ben-David D, Perna S, Longtin Y. Impact of sink location on hand hygiene compliance after care of patients with Clostridium difficile infection: a cross-sectional study. BMC Infect Dis 2016; 16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ramphal L, Suzuki S, McCracken IM, Addai A. Improving hospital staff compliance with environmental cleaning behavior. Proc (Bayl Univ Med Cent) 2014; 27:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Anderson DJ, Chen LF, Weber DJ et al. CDC Prevention Epicenters Program Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet 2017; 389:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Clifford R, Sparks M, Hosford E et al. Correlating cleaning thoroughness with effectiveness and briefly intervening to affect cleaning outcomes: how clean is cleaned?PLoS One 2016; 11:e0155779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Carling PC, Parry MM, Rupp ME, Po JL, Dick B, Von Beheren S; Healthcare Environmental Hygiene Study Group Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect Control Hosp Epidemiol 2008; 29:1035–41. [DOI] [PubMed] [Google Scholar]
- 147. Nerandzic MM, Donskey CJ. A quaternary ammonium disinfectant containing germinants reduces Clostridium difficile spores on surfaces by inducing susceptibility to environmental stressors. Open Forum Infect Dis 2016; 3:ofw196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wullt M, Odenholt I, Walder M. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect Control Hosp Epidemiol 2003; 24:765–8. [DOI] [PubMed] [Google Scholar]
- 149. Perez J, Springthorpe VS, Sattar SA. Activity of selected oxidizing microbicides against the spores of Clostridium difficile: relevance to environmental control. Am J Infect Control 2005; 33:320–5. [DOI] [PubMed] [Google Scholar]
- 150. Deshpande A, Mana TS, Cadnum JL et al. Evaluation of a sporicidal peracetic acid/hydrogen peroxide-based daily disinfectant cleaner. Infect Control Hosp Epidemiol 2014; 35:1414–6. [DOI] [PubMed] [Google Scholar]
- 151. Block C. The effect of Perasafe and sodium dichloroisocyanurate (NaDCC) against spores of Clostridium difficile and Bacillus atrophaeus on stainless steel and polyvinyl chloride surfaces. J Hosp Infect 2004; 57:144–8. [DOI] [PubMed] [Google Scholar]
- 152. Harbarth S, Fankhauser C, Schrenzel J et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008; 299:1149–57. [DOI] [PubMed] [Google Scholar]
- 153. Deshpande A, Pasupuleti V, Rolston DD et al. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis 2011; 53:e81–90. [DOI] [PubMed] [Google Scholar]
- 154. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. O’Horo JC, Jones A, Sternke M, Harper C, Safdar N. Molecular techniques for diagnosis of Clostridium difficile infection: systematic review and meta-analysis. Mayo Clin Proc 2012; 87:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.