Abstract
Background
The PROPKD score has been proposed to stratify the risk of progression to end-stage renal disease in autosomal dominant polycystic kidney disease (ADPKD) subjects. We aimed to assess its prognostic value in a genotyped subgroup of subjects from the Tolvaptan Phase 3 Efficacy and Safety Study in Autosomal Dominant Polycystic Kidney Disease (TEMPO3/4) trial.
Methods
In the post hoc analysis, PKD1 and PKD2 were screened in 770 subjects and the PROPKD score was calculated in mutation-positive subjects (male: 1 point; hypertension <35 years: 2 points; first urologic event <35 years: 2 points; nontruncating PKD1 mutation: 2 points; truncating PKD1 mutation: 4 points). Subjects were classified into low-risk (LR; 0–3 points), intermediate-risk (IR; 4–6 points) and high-risk (HR; 7–9 points) groups.
Results
The PROPKD score was calculated in 749 subjects (LR = 132, IR = 344 and HR = 273); age was inversely related to risk (LR = 43.6 years, IR = 39.5 years, HR = 36.2 years; P < 0.001). Subjects from the HR group had significantly higher height-adjusted total kidney volume (TKV) and rates of TKV growth. While baseline renal function was similar across all risk groups, the rate of estimated glomerular filtration rate (eGFR) decline significantly increased from LR to HR in the placebo group. Tolvaptan treatment effectiveness to reduce TKV growth was similar in all three risk categories. While tolvaptan significantly slowed eGFR decline in the IR (tolvaptan = −2.34 versus placebo = −3.33 mL/min/1.73 m2/year; P = 0.008) and HR groups (tolvaptan = −2.74 versus placebo = −3.94 mL/min/1.73 m2/year; P = 0.002), there was no difference in the LR group (tolvaptan = −2.35 versus placebo = −2.50 mL/min/1.73 m2/year; P = 0.72). Excluding the LR subjects from the analysis improved the apparent treatment effect of tolvaptan on eGFR decline.
Conclusion
This study confirms the prognostic value of the PROPKD score and suggests that it could reduce costs and enhance endpoint sensitivity by enriching future study populations for rapidly progressing ADPKD subjects.
Keywords: autosomal dominant polycystic kidney disease, genetics, PKD1, PKD2, TEMPO 3/4
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the fourth leading cause of end-stage renal disease (ESRD) worldwide [1], with a prevalence of renal replacement therapy (RRT) calculated at 91.1 per million in Europe [2]. The course of ADPKD varies considerably among individuals, with some reaching ESRD before 40 years of age and others living a normal lifespan without requiring RRT. Two principal genes, PKD1 and PKD2, are involved in ∼72–77% and ∼13–18% of cases, respectively [3–8]. A third gene, GANAB, has recently been described, which causes milder polycystic kidney disease but in some cases severe polycystic liver disease [9]. Genetic variability strongly influences the severity of ADPKD, with PKD1 truncating mutations typically associated with an earlier age at ESRD (median age ∼58 years) than PKD1 nontruncating mutations (∼67 years) and PKD2 mutations (∼79 years) [3].
Substantial progress in understanding the pathogenesis of ADPKD has triggered the development of new therapeutic strategies [10]. Tolvaptan, a vasopressin 2 receptor antagonist, was demonstrated to slow the rate of total kidney volume (TKV) growth and the rate of kidney function decline in the Tolvaptan Efficacy and Safety in Autosomal Dominant Polycystic Kidney Disease (TEMPO) trial [11]. A post hoc analysis suggested clinically similar beneficial effects of tolvaptan in ADPKD across chronic kidney disease (CKD) Stages 1–3, as defined by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12].
Two major factors have made the design of clinical trials in ADPKD particularly challenging. First, the significant variability of renal disease severity complicates the evaluation of candidate drugs, as nonselected cohorts are highly heterogeneous. Second, the loss of kidney function [evaluated by estimated glomerular filtration rate (eGFR)] usually happens gradually, when irreversible structural damage has occurred and intervention is unlikely to be successful. Consequently, classic clinical endpoints such as doubling of serum creatinine or onset of ESRD are difficult to study in a placebo-controlled trial of reasonable length. Ideally, patients at risk of rapid progression should be selected and treatment should be initiated early to maximize the chance of detecting therapeutic effects in a limited population size [13].
Several approaches have been developed to assess the severity and the prognosis of ADPKD. Previous studies from the Consortium for Radiologic Imaging Study of PKD (CRISP) provided a strong rationale for the prognostic value of TKV, including height adjusted (HtTKV), in ADPKD [14–19]. The Mayo imaging classification (MIC) was developed to predict the rate of decline of eGFR according to the HtTKV at a given age [20]. The authors recommended enriching clinical trials with patients who present with typical imaging presentations and higher HtTKV/age, specifically imaging categories 1C–1E. Aside from the imaging-based prognostic strategies, a different approach was developed in the French cohort Genkyst, which aims to include all the consenting ADPKD patients from the western part of France, irrespective of their disease severity. The Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score, based on clinical and genetic data, was shown to stratify the risk of progression to ESRD [4]. The authors suggested enriching clinical trials with subjects classified as high risk by the PROPKD score.
In this post hoc analysis involving a subgroup of subjects from the TEMPO 3/4 trial with genetic data available, we first aimed to assess the prognostic value of the PROPKD score. Our second objective was to investigate whether risk stratification using the PROPKD score in the TEMPO 3/4 trial, by excluding subjects from the low-risk group, where progression of TKV and eGFR would be expected to be slowest, may have further enriched the population for subjects with rapidly progressing ADPKD enhancing discriminative ability.
MATERIALS AND METHODS
Study design
This is a post hoc exploratory analysis of TEMPO 3/4, a prospective, randomized, double-blinded trial in 1445 ADPKD adult patients (18–50 years) with an estimated creatinine clearance (Cockroft and Gault) >60 mL/min and a TKV >750 mL/min. The participants were randomized in a 2:1 ratio to receive tolvaptan or placebo [11, 21].
Study participants
TEMPO 3/4 participants with available genetic analysis were included in this study, namely subjects enrolled in the open-label extension trial TEMPO 4/4 who consented to provide a blood sample for DNA analysis [22]. The PROPKD score was calculated in all the subjects in whom a mutation of PKD1 or PKD2 was identified (n = 749).
Molecular analysis of the PKD1 and PKD2 genes
The entire coding regions of the PKD1 and PKD2 genes and their flanking intronic regions were screened by Sanger sequencing, followed if negative by the detection of gross rearrangements using multiplex ligation-dependent probe amplification [23, 24].
Calculation of the PROPKD
The PROPKD score, ranging from 0 to 9 points, was calculated in the mutation-positive subjects as the sum of the following factors: being a male: 1 point; hypertension onset before age 35 years: 2 points; first urologic event before age 35 years (including cyst infection, gross hematuria and/or flank pain related to cysts): 2 points; PKD2 mutation: 0 points; nontruncating mutation of PKD1: 2 points; truncating mutation of PKD1: 4 points (Supplementary data, Table S1). Subjects were classified into low-risk (LR; 0–3 points), intermediate-risk (IR; 4–6 points) and high-risk (HR; 7–9 points) groups (Supplementary data, Table S1) [4]. For subjects <35 years of age who had not developed hypertension and/or urological events, a score of 0 was allocated to these clinical variables and the score calculated as the sum of the remaining factors.
Outcome measure
Two endpoints of the TEMPO 3/4 trial were considered in this analysis: the primary outcome measure, which was the annual rate of change in TKV over time, and the secondary outcome measure, the rate of kidney function decline.
Statistical analyses
Annualized TKV growth rate was calculated in each risk subgroup by regressing logarithm-transformed kidney volume data against time and then displaying regression slope exponentials. All eGFR values presented were calculated using the CKD-EPI formula [25]. The rate of eGFR decline was obtained in each risk subgroup by regressing eGFR from steady-state after baseline (i.e. Week 3 and beyond) against time by subject. Treatment effects for both endpoints corresponded to the difference between the slopes of tolvaptan and placebo.
RESULTS
Description of the study population and comparison of risk groups defined by the PROPKD score at baseline
Molecular analysis of PKD1 and PKD2 was conducted in 770 subjects. The mutation detection rate was high, with mutations identified in 749 subjects (97.3%; 583 different mutations), of whom 61.3% had a truncating PKD1 mutation, 26.3% had a nontruncating PKD1 mutation and 12.4% had a PKD2 mutation. A majority of the mutations identified were private, the two most frequent variants were the missense c.8311G>A (p.Glu2771Lys) and the frameshifting deletion c.5014_5015delAG (p.Arg1672fs97X), each identified in 2% of the subjects (n = 15). Baseline and demographic characteristics in these 749 tolvaptan- and placebo-treated subjects were well-balanced overall and similar to baseline characteristics in the TEMPO 3/4 trial (Supplementary data, Table S2). After calculation of the PROPKD score in the 749 mutation-positive patients, most subjects were categorized to the more severe risk groups [n = 132 (17.6%) in LR, 344 (45.9%) in IR and 273 (36.5%) in HR], with the mean age inversely related to risk (LR = 43.6 years, IR = 39.5 years, HR = 36.3 years; P < 0.001) (Table 1).
Table 1.
Baseline characteristics
| (A) Comparison of the baseline characteristics in the three PROPKD risk groups | ||||||
|---|---|---|---|---|---|---|
| LR PROPKD 1–3 | IR PROPKD 4–6 | HR PROPKD 7–9 | P-values | |||
| (n = 132) | (n = 344) | (n = 273) | LR to IR | IR to HR | LR to HR | |
| Age (years), mean (SD) | 43.6 (5.3) | 39.5 (6.6) | 36.3 (6.8) | <0.0001 | <0.0001 | <0.0001 |
| Caucasian, n (%) | 124 (93.9) | 339 (98.5) | 259 (94.9) | 0.01 | 0.7 | 0.003 |
| Male, n (%) | 80 (60.6) | 101 (29.3) | 209 (76.6) | <0.0001 | <0.0001 | 0.002 |
| Age at diagnosis of ADPKD (years), mean (SD) | 35.4 (8.6) | 26.7 (8.9) | 23.6 (7.7) | <0.0001 | <0.0001 | <0.0001 |
| HTN, n (%) | 108 (81.8%) | 277 (80.5%) | 252 (92.3%) | 0.80 | <0.0001 | 0.003 |
| Age at diagnosis of HTN, (years), mean (SD) | 38.3 (5.77) | 33.1 (7.9) | 26.5 (6.5) | <0.0001 | <0.0001 | <0.0001 |
| Median TKV (mL) (IQR) | 1367 (838–1896) | 1338 (916–1759) | 1682 (1181–2182) | 0.15 | <0.0001 | 0.002 |
| Median HtTKV (mL/m), (IQR) | 785 (492–1077) | 784 (545–1022) | 947 (663–1231) | 0.28 | <0.0001 | 0.005 |
| eGFRCKD-EPI (mL/min/1.73 m2, mean (SD) | 84.8 (21.1) | 83.4 (20.9) | 82.3 (23.1) | 0.56 | 0.49 | 0.32 |
| Genotype, n (%) | ||||||
| PKD1 truncating | 0 (0.0) | 219 (63.7) | 240 (87.9) | <0.0001 | <0.0001 | <0.0001 |
| PKD1 nontruncating | 53 (40.2) | 111 (32.2) | 33 (12.1) | 0.11 | <0.0001 | <0.0001 |
| PKD2 | 79 (59.8) | 14 (4.1) | 0 (0.0) | <0.0001 | 0.304 | <0.0001 |
| Mayo imaging class, n (%) | ||||||
| Class 2 | 10 (7.6) | 7 (2) | 1 (0.4) | 0.097 | 0.0831 | <0.0001 |
| Class 1B | 17 (12.9) | 35 (10.2) | 6 (2.2) | 0.41 | <0.0001 | <0.0001 |
| Class 1C | 62 (47) | 151 (43.9) | 70 (25.6) | 0.61 | 0.0119 | 0.003 |
| Class 1D | 36 (27.2) | 113 (32.8) | 117 (42.9) | 0.27 | <0.0001 | <0.0001 |
| Class 1E | 7 (5.3) | 36 (10.5) | 76 (27.8) | 0.11 | <0.0001 | <0.0001 |
| Missing | 0 | 2 (0.6) | 3 (1.1) | |||
| (B) Comparison of the baseline characteristics in the tolvaptan-(T) and placebo (P)-treated patients within each PROPKD risk group | ||||||
|---|---|---|---|---|---|---|
| LR PROPKD 1–3 | IR PROPKD 4–6 | HR PROPKD 7–9 | ||||
| T (n = 79) | P (n = 53) | T (n = 226) | P (n = 118) | T (n = 167) | P (n = 106) | |
| Age (years), mean (SD) | 43.7 (5.0) | 43.5 (5.8) | 39.2 (6.7) | 40.2 (6.5) | 36.3 (6.4) | 36.1 (7.3) |
| Caucasian, n (%) | 75 (94.9) | 49 (92.5) | 222 (98.2) | 117(99.2) | 157 (94) | 102 (96.2) |
| Male, n (%) | 50 (63.3) | 30 (56.6) | 74 (32.7) | 27 (22.9) | 135* (80.8) | 74* (69.8) |
| TKV (mL), median (IQR) | 1574* | 1241* | 1330 | 1352 | 1699 | 1677 |
| (1009–2138) | (943–1538) | (905–1754) | (950–1754) | (1163–2235) | (1216–2138) | |
| HtTKV (mL/m), median (IQR) | 917* | 705* | 756 | 793 | 952 | 939 |
| (596–1238) | (538–871) | (520–991) | (553–1032) | (666–1238) | (663–1215) | |
| eGFRCKD-EPI, mean mL/min/1.73 m2 (SD) | 81.1* (18.5) | 90.3* (23.6) | 83.7 (20.7) | 82.9 (21.4) | 82.0 (22.5) | 82.8 (24.1) |
HTN, hypertension; IQR, interquartile range.
P-value between T and P < 0.05.
At baseline, while HtTKV was significantly higher in the HR group than in the IR and LR groups (respective median values of 947, 784 and 785 mL/m; P < 0.005), eGFR was similar in the three groups (Table 1, panel A).
Patients with no mutation detected
While age and eGFR at baseline were similar in the 21 patients with no mutation detected (NMD) and the 749 mutation-positive subjects, median baseline TKV (1167 mL) and HtTKV (648 mL/m) were lower (P-values 0.015 and 0.018, respectively) and these subjects were more frequently classified at lower risk by the MIC (Class 2 or 1B) (38.1% versus 10.2%; P = 0.009).
Rate of TKV growth and PROPKD risk categories
Rate of TKV growth was significantly higher in subjects classified in the HR group than those in the IR and LR groups for both treatment arms. Indeed, in placebo-treated subjects, the rate of TKV growth in the HR group was 32–43% higher than in the LR and IR groups, whereas in the tolvaptan-treated subjects, TKV growth in the HR group was 48–80% higher than in the LR and IR groups (Table 2, panel A). In the LR group, TKV at baseline was significantly higher in the tolvaptan- versus placebo-treated subjects (Table 1, panel B). However, TKV growth was significantly lower in tolvaptan- versus placebo-treated subjects in each of the risk groups. Treatment effect was similar in the three risk groups (Table 2, panel A).
Table 2.
Rate of change in TKV and eGFR by PROPKD risk categories
| (A) Analysis in the 749 individuals included in the post hoc analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | LR | IR | HR | |||
| T | P | T | P | T | P | |
| (n = 79) | (n = 53) | (n = 226) | (n = 118) | (n = 167) | (n = 106) | |
| Rate of TKV growth (%/year) | 2.80 | 5.11 | 2.30 | 4.72 | 4.15 | 6.75 |
| P-value | 0.0022 | <0.0001 | <0.0001 | |||
| Relative treatment effect (%) | 45.8 | 51.8 | 38.2 | |||
| T | P | T | P | T | P | |
|---|---|---|---|---|---|---|
| (n = 79) | (n = 52) | (n = 214) | (n = 115) | (n = 159) | (n = 103) | |
| Rate of eGFRCKD-EPI decline (mL/min/1.73 m2) | −2.35 | −2.50 | −2.34 | −3.33 | −2.74 | −3.94 |
| P-value | 0.72 | 0.008 | 0.002 | |||
| Relative treatment effect (%) | 6.9 | 30.3 | 30.6 | |||
| (B) Subgroup analysis in patients from MICs C, D and E (n = 668) | ||||||
|---|---|---|---|---|---|---|
| Variable | LR | IR | HR | |||
| T | P | T | P | T | P | |
| (n = 65) | (n = 40) | (n = 196) | (n = 104) | (n = 160) | (n = 103) | |
| Rate of TKV growth (%/year) | 2.71 | 5.36 | 2.47 | 5.08 | 4.27 | 6.9 |
| P-value | 0.0010 | <0.0001 | <0.0001 | |||
| Relative treatment effect (%) | 50.4 | 52.2 | 38.1 | |||
| T | P | T | P | T | P | |
|---|---|---|---|---|---|---|
| (n = 65) | (n = 39) | (n = 186) | (n = 101) | (n = 152) | (n = 100) | |
| Rate of eGFRCKD-EPI decline (mL/min/1.73 m2) | −2.55 | −2.58 | −2.47 | −3.53 | −2.74 | −4.11 |
| P-value | 0.91 | 0.009 | 0.0003 | |||
| Relative treatment effect (%) | 2.3 | 30.5 | 33.7 | |||
P, placebo; T, tolvaptan.
Rate of eGFR decline and PROPKD risk categories
In the placebo-treated subjects, the eGFR decline was greater from the HR to the LR groups. While tolvaptan significantly reduced the rate of renal function decline in the IR and HR groups, with relative treatment effects of 30.3% and 30.6%, there was no significant difference between the tolvaptan- and placebo-treated subjects in the LR group (Table 2, panel A). In the latter group, however, the eGFR at baseline was significantly higher in the placebo-treated group (Table 1, panel B).
Effect of the exclusion of subjects from the LR group on the outcome measures
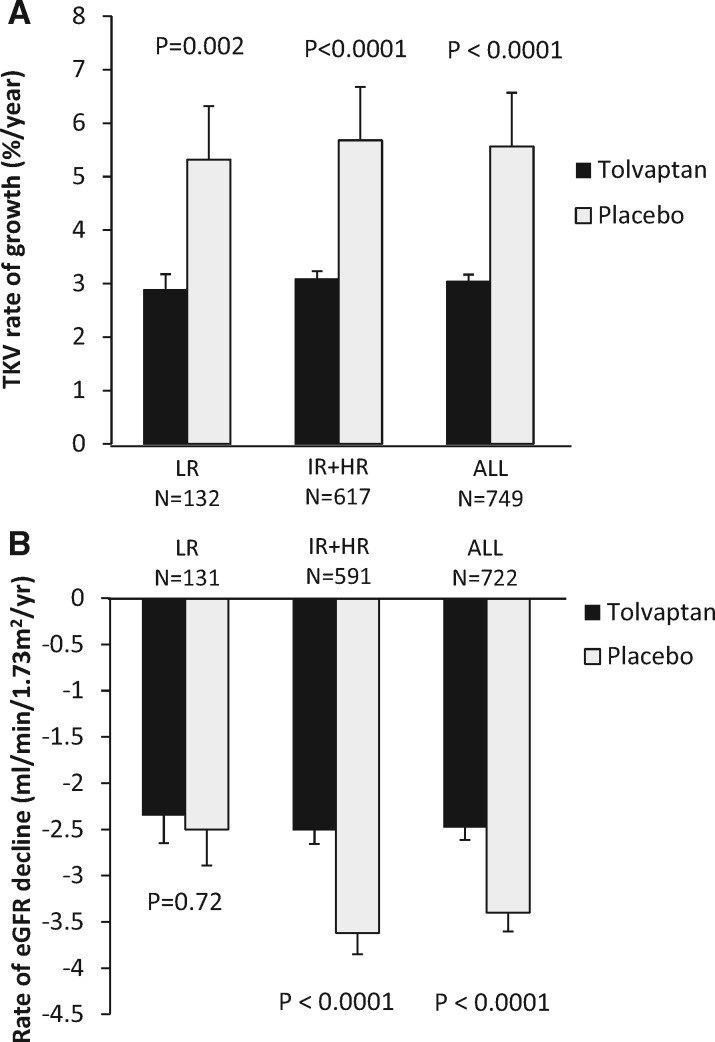
We investigated the effect of excluding subjects from the LR group [n = 132 (17.6%)]. While this exclusion did not change the tolvaptan-mediated decrease in TKV growth rate (Figure 1A), there was a nonsignificant trend of increased treatment effect on the rate of eGFR decline (treatment effect 30.6% after exclusion of LR versus 27.1%). In the three combined groups, tolvaptan reduced the rate of eGFR decline from −3.40 to −2.48 mL/min/1.73 m2/year (P = 0.0001). Excluding subjects in the LR group from the analysis increased this difference (−3.62 to −2.51 mL/min/1.73 m2/year; P < 0.0001) (Figure 1B).
FIGURE 1.
(A) Rate of TKV growth in the LR group, in the combined HR and IR group and in the three combined groups. (B) Rate of eGFR decline in the LR group, in the combined HR and IR group and in the three combined groups. Error bars represent the standard error of the mean.
Stability of PROPKD risk groups in subjects <35 years of age during the trial follow-up
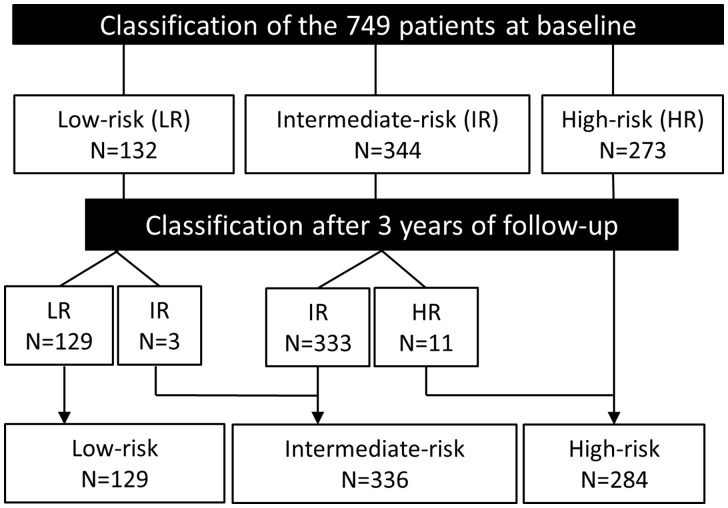
The clinical variables included in the PROPKD score, i.e. hypertension and urological events, are set as binary variables occurring before 35 years (2 points) or not (0 points). Among the 749 mutation-positive subjects, 168 were <35 years of age, almost exclusively in the more severe groups (7 in LR, 61 in IR, 100 in HR). Fourteen of these subjects changed risk category after 3 years of follow-up: 3 from LR to IR and 11 from IR to HR (Figure 2).
FIGURE 2.
Classification remains stable in most patients over time. Flowchart representing the classification of the 749 subjects in the three PROPKD risk groups at baseline and after 3 years of follow-up. Fourteen of these subjects changed risk category after 3 years of follow-up: 3 from LR to IR and 11 from IR to HR as a consequence of a diagnosis of hypertension in 10 subjects and the occurrence of a first urological event in 6 subjects.
Combination of the MIC and PROPKD approaches
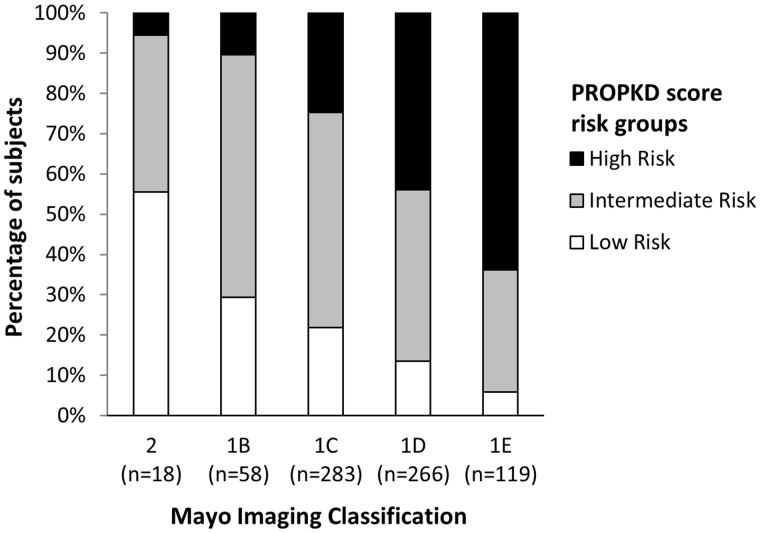
To investigate whether the PROPKD score and the MIC could be used as complementary enrichment strategies, we studied the distribution of LR, IR and HR subjects in the different classes of the MIC. As expected, HR subjects were more represented in the more severe classes (1C, 1D and 1E; Table 1, panel A and Figure 3). While 75.7% of subjects were defined at higher risk of significant progression by both methods (Group 1, i.e. IR and HR by the PROPKD and MIC groups 1C, 1D and 1E), only 3.6% were defined at low risk of progression by both methods (Group 2), 14.1% were considered at higher risk only by the MIC (Group 3) and 6.6% only by the PROPKD score (Group 4). Consistent with the entry criteria of the TEMPO trial, a majority of the subjects classified at lower risk by each method were >35 years of age (96% for the MIC and 94% for the PROPKD). Inversely, subjects from Group 1 had similar baseline kidney function but were significantly younger than patients from Group 3 and Group 4 [mean age 37 versus 43 and 43 years, respectively (P < 0.001)]. The proportion of subjects from MIC Class 1C was significantly higher in the discordant Group 3 than in the concordant Group 1 (59% versus 39.3%; P < 0.001). Similarly, the proportion of subjects from the PROPKD IR group was higher in the discordant Group 4 than in the concordant Group 1 (85.7% versus 40%; P < 0.001). This suggests an overall milder disease in these two discordant subgroups. The most frequent missense variant, p.Glu2771Lys, was more frequently identified in the discordant Group 3 than in the rest of the cohort (5.7% versus 1.4%; P = 0.004).
FIGURE 3.
Distribution of LR, IR and HR groups in each category of the MIC model.
To evaluate whether the PROPKD further improved the imaging stratification in Classes 1C, 1D and 1E, we excluded subjects classified as Class 2 (n = 18) and 1B (n = 58). In subjects from Classes 1C, 1D and 1E, while tolvaptan reduced TKV growth in each of the three PROPKD risk groups, it was associated with significantly slower renal function decline in the IR and HR groups, but not in the LR group (Table 2, panel B).
DISCUSSION
Designing optimal clinical trials in ADPKD is a difficult task given the lifelong progression of the disease and the high variability of its severity. Inclusion criteria in the TEMPO trial combined volume and age thresholds in individuals with preserved kidney function, thus enriching for patients with rapidly progressive ADPKD. Reflecting this enrichment, when compared with the Genkyst cohort, PKD1 truncating mutations were more frequent in TEMPO patients (61.3% versus 53%) and PKD2 mutations less frequent (12.4% versus 20.2%).
The PROPKD score was developed in a population-based cohort representative of the wide spectrum of disease severity in adult ADPKD. Herein, we confirm the prognostic value of the PROPKD score in a genotyped subgroup of subjects from the TEMPO trial. Indeed, subjects from the HR group, although younger, had higher HtTKVs at baseline and higher rates of TKV growth. While subjects from the three risks groups had similar average eGFR at baseline, we observed increasingly steeper rates of eGFR decline from the LR to the HR group. This study provides strong validation of the prognostic value of the PROPKD score for two reasons. First, it demonstrates that the PROPKD score stratifies the risk for disease progression even in ADPKD patients selected to have rapidly progressive disease. Second, although the PROPKD score was developed to predict age at ESRD, we show here that it also predicts rates of eGFR decline and TKV growth.
Our second objective was to investigate whether excluding subjects from the LR group defined by the PROPKD score may have enhanced the discriminative ability of the TEMPO trial. As a result of the TEMPO entry criteria, a higher proportion of subjects was classified in the HR (36.5%) and IR groups (45.9%) when compared with the Genkyst cohort, where the HR and IR groups represented 14% and 46.7% of the subjects, respectively [4]. In this post hoc analysis, exclusion of the 132 LR group subjects (17.6%) slightly maximized the difference in the rate of eGFR decline between the tolvaptan- and the placebo-treated subjects. The treatment effect on the rate of TKV growth was similar in the three risk groups.
This analysis demonstrates that the PROPKD score can be used to enrich clinical trial cohorts for rapidly progressive patients, and complement enrichment from TKV criteria, to increase the chances of observing significant differences in the rate of kidney function decline. Such strategies in future trials may allow cost reductions by decreasing the number of subjects to recruit while maximizing the chance of positive results. While the high cost of a comprehensive analysis of PKD genes has long been a disincentive, the current widespread use of next-generation sequencing allows significant cost reductions and is likely to facilitate access to genetic testing [26–30].
Two recent studies evaluating an enrichment strategy using the MIC have been published [31, 32]. The first post hoc analysis was conducted in early disease in the HALT-PKD study, a randomized controlled trial that studied the effect of rigorous versus standard blood pressure control on rates of TKV increase and eGFR decline in ADPKD subjects ages 15–49 years with preserved renal function (eGFR > 60 mL/min/1.73 m2) at inclusion [33]. Treatment was more beneficial in subjects from Classes 1D and 1E, both in terms of TKV increase and eGFR decrease. This cohort of subjects was more heterogeneous in terms of disease severity than in the TEMPO 3/4 trial. Analysis of the PROPKD score performance in that population would be interesting, but age at the first urological event was not systematically collected. The second post hoc analysis was conducted in the TEMPO trial [32]. Exclusion of Class 2A and 1B subjects resulted in a slightly higher treatment effect on TKV and eGFR slopes, although nonsignificant. Interestingly in our study, 105 subjects from MICs 1C–1E were categorized in the PROPKD LR group. Subjects in this subgroup were significantly older and more frequently classified as MIC 1C, suggesting an overall milder disease in this discordant subgroup. In subgroup analysis including only the 668 subjects from MICs 1C–1E, the rate of eGFR decline was lower in the LR than in the IR and HR groups. And while treatment was associated with a slower renal function decline in the HR and IR groups, there was no difference from placebo in the LR group. This suggests that combining imaging, genetics and clinical criteria in a single scoring system may be of interest to develop future prognostic tools in ADPKD. Such an approach will allow evaluation of the relative contributions of the different predictors and provide accurate prognostic information earlier: before the occurrence of significant volume enlargement and/or hypertension or a urological event. Meanwhile, the use of both tools seems particularly interesting in patients at intermediate risk, for instance using PROPKD to reclassify subjects from MIC 1C and MIC to reclassify subjects from the PROPKD IR group, depending on which prognosis tool was used first.
Tolvaptan is now available in Canada, Japan, Europe, South Korea and Switzerland and a position statement for the use of tolvaptan has recently been issued by a European group of experts [34]. One of the objectives of this group was to define the definition of ‘evidence of rapid disease progression’. Besides historical kidney growth and eGFR decline, demonstrated by sequential imaging or creatinine measurements, the authors suggested that subjects from MICs 1C–1E or from the PROPKD HR group were likely to have rapid progression. Taking into account the results of the present study, subjects from the PROPKD IR group should also be considered at risk for rapid progression, although we must keep in mind that these subjects met the inclusion criteria for the TEMPO trial and so potentially had more rapid progression than nonpreselected IR group subjects.
This study has some limitations. First, this is a post hoc analysis, which was run in a subgroup of the TEMPO trial, and a sample for genetic analysis was collected on only ∼53% of subjects. As a consequence, the LR group was quite small and baseline characteristics between placebo- and tolvaptan-treated patients differed significantly in this subgroup. Reassuringly, baseline characteristics in the genotyped subgroup were similar to the full TEMPO 3/4 cohort [11]. Second, while disease severity is overall milder in patients with nontruncating variants, a handful of missense variants has been shown in vitro to be fully penetrant, including p.Glu2771Lys, which disrupts cleavage of polycystin 1 at the G-protein coupled receptor proteolytic site [35, 36], and was more frequent in subjects considered at higher risk only by the MIC. Attributing four points (truncating mutation) rather than two points (nontruncating mutation) for the genetic component of the PROPKD score in the ∼2% of ADPKD patients harboring this mutation would improve their prognostic assessment. In the future, the development of functional assays will allow more refined variant classification. Moreover, in 21 patients no mutation of PKD1 or PKD2 was identified and thus the PROPKD score could not been calculated. Aside from missed mutations in the complex PKD1 gene, or in the intronic portions of both genes, missense variants of unknown significance or mosaic cases may explain some of these genetically unresolved cases, which is suggested by the higher proportion of subjects from MIC 2, i.e. with segmental, asymmetric or lopsided imaging presentation. Mutations in GANAB are unlikely to be involved here, as none of the 20 GANAB patients reported so far would have met the age and TKV inclusion criteria of the TEMPO 3/4 trial [9]. Nevertheless, the mutation detection rate was particularly high in this cohort, with only 2.7% of the subjects having NMD, compared with 7–10% in other recent ADPKD cohorts [3, 4, 7, 24]. A potential explanation is that subjects with NMD tend to have milder disease [7] and were hence less likely to be included in the TEMPO trial. Last, due to the scoring criteria, the PROPKD score can increase in subjects <35 years of age if they develop hypertension or experience a first urological event. Therefore, excluding young LR group subjects from studies and treatment has the risk of removing a few subjects with rapidly progressive disease. The follow-up analysis in the TEMPO trial timeframe is reassuring, as only three subjects moved from the LR to the IR group.
In conclusion, the PROPKD score is an efficient strategy to enrich future randomized control trials cohorts for rapidly progressive patients. Ultimately, the combination of imaging and genetic-based approaches will likely enhance our capacity to predict renal outcomes and tailor therapeutic approaches to individual ADPKD patients.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients involved in the TEMPO 3/4 trial for their participation and contribution.
FUNDING
The trial was funded by Otsuka Pharmaceutical, Tokyo, Japan and Otsuka Pharmaceutical Development and Commercialization, Rockville, MD, USA. An Otsuka grant also funded mutation analysis of the TEMPO population at the Mayo Translational PKD Center. E.C.-L.G. was funded by an American Society of Nephrology Foundation Kidney Research Fellowship.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Chebib FT, Torres VE.. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis 2016; 67: 792–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spithoven EM, Kramer A, Meijer E. et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 2014; 29(Suppl 4): iv15–iv25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornec-Le Gall E, Audrezet MP, Chen JM. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 2013; 24: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornec-Le Gall E, Audrezet MP, Rousseau A. et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2016; 27: 942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornec-Le Gall EC-L, Audrézet M-P, Le Meur Y. et al. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: twenty years on. Hum Mutat 2014; 35: 1393–1406 [DOI] [PubMed] [Google Scholar]
- 6. Harris PC, Rossetti S.. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2010; 6: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heyer CM, Sundsbak JL, Abebe KZ. et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype–phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2016; 27: 2872–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang YH, Conklin J, Chan W. et al. Refining genotype–phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2016; 27: 1861–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porath B, Gainullin VG, Cornec-Le Gall E. et al. Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 2016; 98: 1193–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ong AC, Devuyst O, Knebelmann B. et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015; 385: 1993–2002 [DOI] [PubMed] [Google Scholar]
- 11. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres VE, Higashihara E, Devuyst O. et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 trial. Clin J Am Soc Nephrol 2016; 11: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grantham JJ. Rationale for early treatment of polycystic kidney disease. Pediatr Nephrol 2015; 30: 1053–1062 [DOI] [PubMed] [Google Scholar]
- 14. Bhutani H, Smith V, Rahbari-Oskoui F. et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 2015; 88: 146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chapman AB, Bost JE, Torres VE. et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2012; 7: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Medicines Agency. Total Kidney Volume (TKV) as a prognostic biomarker for use in clinical trials evaluating patients with autosomal dominant polycystic kidney disease (ADPKD). 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2015/11/WC500196569.pdf.
- 17. US Food and Drug Administration. biomarker qualification review for total kidney volume. 2015. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/UCM458496.pdf (6 April 2017, date last accessed).
- 18. Grantham JJ, Torres VE.. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 2016; 12: 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grantham JJ, Torres VE, Chapman AB. et al. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354: 2122–2130 [DOI] [PubMed] [Google Scholar]
- 20. Irazabal MV, Rangel LJ, Bergstralh EJ. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2015; 26: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Torres VE, Meijer E, Bae KT. et al. Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis 2011; 57: 692–699 [DOI] [PubMed] [Google Scholar]
- 22. Torres VE, Chapman AB, Devuyst O. et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2017; https://clinicaltrials.gov/ct2/show/NCT01214421 (6 April 2017, date last accessed) [DOI] [PubMed]
- 23. Consugar MB, Wong WC, Lundquist PA. et al. Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int 2008; 74: 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossetti S, Consugar MB, Chapman AB. et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18: 2143–2160 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eisenberger T, Decker C, Hiersche M. et al. An efficient and comprehensive strategy for genetic diagnostics of polycystic kidney disease. PLoS One 2015; 10: e0116680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossetti S, Hopp K, Sikkink RA. et al. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol 2012; 23: 915–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan Y-C, Blumenfeld JD, Anghel R. et al. Novel method for genomic analysis of PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease. Hum Mutat 2009; 30: 264–273 [DOI] [PubMed] [Google Scholar]
- 29. Trujillano D, Bullich G, Ossowski S. et al. Diagnosis of autosomal dominant polycystic kidney disease using efficient PKD1 and PKD2 targeted next-generation sequencing. Mol Genet Genomic Med 2014; 2: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T, Meng Y, Wei X. et al. Identification of novel mutations of PKD1 gene in Chinese patients with autosomal dominant polycystic kidney disease by targeted next-generation sequencing. Clin Chim Acta 2014; 433: 12–19 [DOI] [PubMed] [Google Scholar]
- 31. Irazabal MV, Abebe KZ, Bae KT. et al. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial. Nephrol Dial Transplant 2017; 32: 1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irazabal MV, Blais JD, Perrone RD. et al. Prognostic enrichment design in clinical trials for ADPKD: the TEMPO 3:4 clinical trial. Kidney Int Rep 2016; 1: 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schrier RW, Abebe KZ, Perrone RD. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gansevoort RT, Arici M, Benzing T. et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 2016; 31: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Gonzalez MA, Jones JG, Allen SK. et al. Evaluating the clinical utility of a molecular genetic test for polycystic kidney disease. Mol Genet Metab 2007; 92: 160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qian F, Boletta A, Bhunia AK. et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci USA 2002; 99: 16981–16986 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.