Using data from a large surveillance program in South Africa, we estimated the longitudinal change in the prevalence of detectable HIV viremia. Results show the need to report the prevalence of detectable viremia among all adults, irrespective of HIV status.
Keywords: HIV, viral load, detectable viremia, prevalence, South Africa
Abstract
Background
The prevalence of detectable viremia has previously been used to infer the potential for ongoing human immunodeficiency virus (HIV) transmission. To date, no study has evaluated the longitudinal change in the prevalence of detectable viremia within the HIV-positive community (PDV+) and the entire population (PDVP) using data from a sub-Saharan African setting.
Methods
In 2011, 2013, and 2014, we obtained 6752 HIV-positive and 15415 HIV-negative test results from a population-based surveillance system in the KwaZulu-Natal province of South Africa. We quantified the PDV+ as the proportion of the 6752 HIV-positive results with a viral load >1550 copies/mL and the PDVP as the proportion of the 6752 HIV-positive and 15415 HIV-negative results with a viral load >1550 copies/mL.
Results
Between 2011 and 2014, the PDV+ decreased by 16.5 percentage points (pp) for women (from 71.8% to 55.3%) and 10.6 pp for men (from 77.8% to 67.2%). However, a steady rise in the overall HIV prevalence, from 26.7% to 32.4%, offset the declines in the PDV+ for both sexes. For women, the PDVP decreased by only 2.1 pp, from 21.3% to 19.2%, but for men, the PDVP actually increased by 1.6 pp, from 14.6% to 16.2%, over the survey period.
Conclusions
The PDV+, which is currently being tracked under the UNAIDS 90-90-90 targets, may not be an accurate indicator of the potential for ongoing HIV transmission. There is a critical need for countries to monitor and report the prevalence of detectable viremia among all adults, irrespective of HIV status.
By 2015, almost half of the 36.7 million people living with human immunodeficiency virus (HIV) were on combination antiretroviral therapy (ART) [1]. ART is expected to prevent the onward transmission of HIV by reducing the number of infected persons with detectable viremia [2, 3]. For this reason, the HIV-positive prevalence of detectable viremia (PDV+), which is the proportion of all infected persons with a recent viral load above a copies/mL threshold, has been promoted as a sensitive biological index of ART program effectiveness. The PDV+ has previously been used to monitor a community’s uptake of ART [4, 5], and is central to the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets to have 90% of all ART-initiated patients achieve undetectable viremia by the year 2020 [6]. In addition, the PDV+ has been used to quantify the potential for ongoing HIV transmission within a well-defined community or geographic area [4, 5, 7–9]. An assumption underlying the use of this measure is that higher levels of ART coverage will lower the PDV+ and thus reduce the incidence of HIV infection within the general population.
However, one key limitation of the PDV+ is that it does not account for the relative sizes of the HIV-infected and HIV-uninfected populations [10]. This information is important because the risk of acquiring HIV will depend not only on the number of infected persons with detectable viremia (ie, PDV+) but also on the number of infected persons in the general population (ie, HIV prevalence), and the rate of sexual contact between them [10]. Thus, an improved biological index, which we call the population prevalence of detectable viremia (PDVP) [11], can be obtained by multiplying the PDV+ with the HIV prevalence (see Supplementary Figure 1). Aggregated viral load indices that account for the HIV prevalence have gained traction in the literature [12–15], and we recently showed that the PDVP is significantly better than the PDV+ at predicting the prospective risk of HIV infection [11].
As far as we know, time trends in both the PDV+ and the PDVP have not been evaluated and compared using data from a sub-Saharan African population. In 2011, 2013, and 2014, we obtained 6752 HIV-positive and 15415 HIV-negative test results from a population-based surveillance system in the KwaZulu-Natal province of South Africa. We quantified the PDV+ as the proportion of the HIV-positive test results with a viral load >1550 copies/mL and then quantified the PDVP as the proportion of the HIV-positive and HIV-negative test results with a viral load >1550 copies/mL. Using this population-based data, we had a unique opportunity to empirically estimate and compare the changes in both the PDV+ and PDVP measures over time.
METHODS
Setting
The Africa Health Research Institute (AHRI) maintains a population-based surveillance system in the Umkhanyakude district of the northern KwaZulu-Natal province. Most of the surveillance area is poor and rural, with several informal periurban settlements and a single urban township [16]. The area is 438 km2 in size with a population of approximately 90000 people and 11000 households.
HIV Surveillance Survey
AHRI has collected longitudinal data on households and individuals within the surveillance area since 2000. Every 6 months, trained field workers visit a key informant within the household to collect information on both resident and nonresident members. Biannual participation rates for household data collection are typically >95%. Nested within the AHRI cohort is the population-based HIV cohort. Field workers have visited households every 12 months since 2004 and identified eligible participants >15 years of age for HIV testing. After obtaining consent, the field workers extract blood according to the UNAIDS and World Health Organization Guidelines for Using HIV Testing Technologies in Surveillance. Of the eligible participants contacted, 78% agreed to be tested for HIV at least once in the 3 survey years. Participants from the AHRI and HIV cohorts were linked across the survey years and the data were stored in a SQL database server. The AHRI and HIV cohorts are described in greater detail elsewhere [16].
HIV Incidence and ART Usage
The AHRI surveillance area is situated at the epicenter of the global AIDS epidemic. Between 2004 and 2011, the crude HIV incidence was 2.6 new infections per 100 person-years (95% confidence interval [CI], 2.50–2.77) [17]. Incidence peaked at 6.6 new infections per 100 person-years in women aged 24 years and at 4.1 new infections per 100 person-years in males aged 29 years [17]. Since 2005, the HIV prevalence among men and women aged 15–54 years has increased steadily from 21.7% in 2005 to 28.7% in 2010 [18]. The increase in HIV prevalence has been attributed to ART-associated reductions in mortality [19].
ART can be accessed for free at any of the 17 primary healthcare clinics within or adjacent to the surveillance area [20]. When ART was first made available in 2004, the CD4+ T-cell count eligibility criteria was <200 cells/μL. In 2010, treatment eligibility was extended to pregnant women with CD4+ T-cell counts <350 cells/μL and patients with active tuberculosis. All patients with CD4+ T-cell counts <350 cells/μL became eligible for ART in 2011. Approximately 32 of the HIV-participants in our study area were on ART in 2011.
Viral Load Measurements
All of the 5368 participants, aged 15–64 years, who tested HIV positive in 2011 (n = 2401), 2013 (n = 2510), and 2014 (n = 2611), provided dried blood spot (DBS) samples. The total number of DBS samples was 7522 as 32.4% of the 5368 participants tested HIV-positive in >1 survey year. From all 7522 DBS samples, we extracted nucleic acid with NucliSENS EasyMag (Bordeaux, France) and used the Generic HIV Viral Load kit (Biocentric) to quantify the viral load levels. As described in greater detail elsewhere [21], the quantification method has a lower detection limit of 1550 copies/mL. Due to insufficient specimens, we had to exclude 770 (10.24%) viral load samples. For the final analysis, we therefore used a total of 6752 viral load measurements from 4991 unique participants who tested HIV positive in 2011 (n = 2366), 2013 (n = 2135), and 2014 (n = 2251).
Prevalence of Detectable Viremia Measures
We calculated the PDV+ for each survey year as follows (we drop the subscript as it is implicit throughout). Letdenote the th viral load measurement for where is the number of HIV-positive test results, and let if1550 copies/mL otherwise Then, the PDV+ which is the number of viral loads >1550 copies/mL divided by the number of HIV-positive test results. This PDV+ measure is a true population estimator because the viral load measurements come from a representative sample of HIV-positive participants. For this reason, our analysis avoids the sampling biases typically associated with facility-based studies in which patients self-select into care [10].
We calculated the PDVP for each survey year as follows: let denote the number of HIV-negative test results and let denote the total number of HIV-positive and HIV-negative test results, with For all HIV-negative test results we denote Then, the PDVP , which is the number of viral loads >1550 copies/mL divided by the total number of HIV-positive and HIV-negative test results.
We note that the number of HIV-negative test results for each survey year was determined with where is the HIV prevalence and the subscripts and denote the age group and sex, respectively. Overall, 15415 HIV-negative test results were sampled from 11522 unique participants. We used this proportional allocation approach [22] to determine because 770 HIV-positive samples were excluded from the analysis due to insufficient specimens (as described in the previous section). Otherwise, we would underestimate the PDVP if we did not sample the correct using this approach.
Statistical Analysis
We performed summary statistics for the unadjusted and age- and sex-adjusted PDV+, PDVP, and HIV prevalence measures by year. To statistically assess the change in the PDV+ and PDVP measures over time, we used a generalized estimating equation (GEE) model with a logit link function. We chose a GEE model because 32.4% of the participants tested HIV positive in >1 survey year. We fitted 4 regression models using data from the HIV-positive participants only (ie, PDV+) and from the HIV-positive and HIV-negative participants (ie, PDVP). For model 1, we included a variable indicating the year of the HIV-positive (ie, viral load measurement) or HIV-negative test result. For model 2, we added a sex variable to the year variable of model 1, and for model 3 we added an age variable (>25 years) to the model 2 variables. For model 4, we added a sex-year interaction term to the model 3 variables to determine if the PDV+ and PDVP measures changed significantly for men and women over time.
RESULTS
For all participants with a viral load measurement, the median age was 35 (interquartile range [IQR], 27–45) years, and 79% were female. For the HIV-positive and HIV-negative participants, the median age was 31 (IQR, 21–47) years and 69% were female, as shown in Table 1.
Table 1.
Summary Statistics for the Human Immunodeficiency Virus (HIV)–Positive Population Only and the Entire Population (HIV-Positive and HIV-Negative Participants) for the 2011, 2013, and 2014 Survey Years
| Year | ||||||
|---|---|---|---|---|---|---|
| Characteristic | 2011 | 2013 | 2014 | |||
| HIV-positive population | ||||||
| Dried blood spot samples, No. | 2401 | 2510 | 2611 | |||
| Successful viral load measurements, No. (%) | 2366 | 98.54 | 2135 | 85.06 | 2251 | 86.21 |
| Viral load >1550 copies/mL | 1663 | 1304 | 1237 | |||
| HIV-positive prevalence of detectable viremia (PDV+) | ||||||
| Unadjusted, mean (95% CI) | 70.29 | (66.95–73.75) | 61.08 | (57.81–64.48) | 54.95 | (51.93–58.1) |
| Age- and sex-adjusted, mean (95% CI) | 73.76 | (68.77–79.26) | 64.38 | (59.63–69.64) | 59.90 | (54.98–65.37) |
| Female sex, No. (%) | 1877 | 79.33 | 1690 | 79.16 | 1794 | 79.70 |
| Age, y, median (IQR) | 35 | (27–45) | 35 | (27–44) | 35 | (28–45) |
| HIV-positive and HIV-negative population | ||||||
| Observations, No. | 8626 | 6881 | 6660 | |||
| Population prevalence of detectable viremia (PDVP) | ||||||
| Unadjusted, mean (95% CI) | 19.28 | (18.36–20.23) | 18.95 | (17.94–20.01) | 18.57 | (17.55–19.64) |
| Age-sex adjusted, mean (95% CI) | 18.83 | (17.94–19.76) | 18.80 | (17.79–19.85) | 17.91 | (16.92–18.95) |
| HIV prevalence | ||||||
| Unadjusted, mean (95% CI) | 27.43 | (26.33–28.56) | 31.03 | (29.73–32.37) | 33.80 | (32.42–35.22) |
| Age- and sex-adjusted, mean (95% CI) | 26.73 | (25.66–27.83) | 30.64 | (29.35–31.97) | 32.36 | (31.03–33.73) |
| Female, No. (%) | 5832 | 67.61 | 4775 | 69.39 | 4730 | 71.02 |
| Age, y, median (IQR) | 31 | (21–47) | 30 | (20–47) | 31 | (21–47) |
Unadjusted and age-sex adjusted results for the HIV-positive prevalence of detectable viremia (PDV+), population prevalence of detectable viremia (PDVP), and HIV prevalence measures are shown. The unadjusted PDVP is obtained by multiplying the PDV+ by the HIV prevalence. For example, in 2011, there were 1663 HIV-positive participants with a viral load >1550 copies/mL. Therefore, the unadjusted PDV+ = 1663/2366 (70.29%), the HIV prevalence = 2366/8626 (27.43%), and the PDVP = 1663/8626 (19.28%). Multiplying the PDV+ by the HIV prevalence (H) returns the PDVP: PDV+H = 70.29% 27.43% = 19.28%. We also report the age- and sex-adjusted PDV+, PDVP, and HIV prevalence measures.
Abbreviations: CI, confidence interval; HIV, Human Immunodeficiency Virus; IQR, interquartile range.
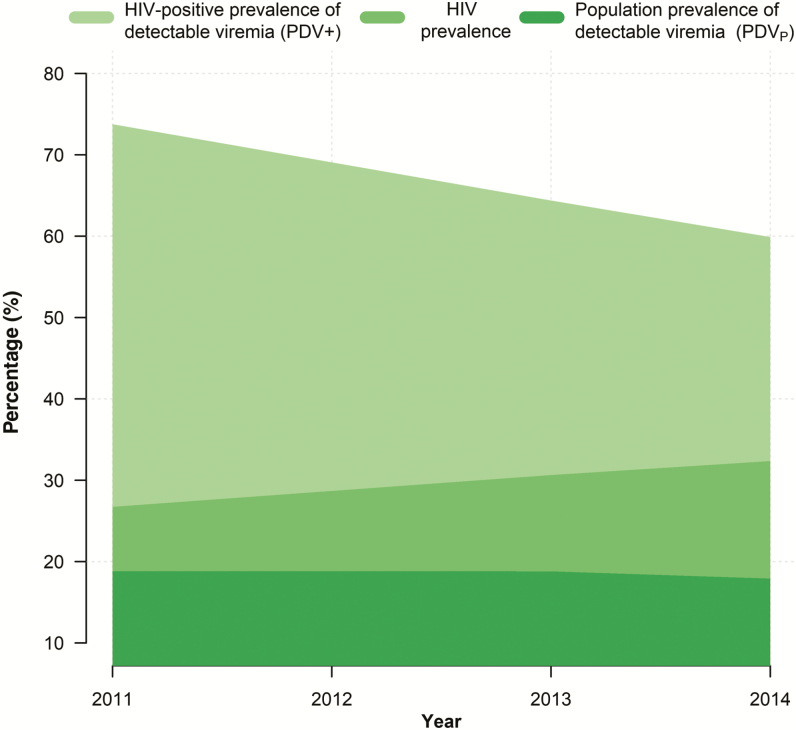
Results show that the adjusted PDV+ decreased by 13.86 percentage points (pp), from 73.76% in 2011 to 64.38% in 2013, and then to 59.90% in 2014 (Table 1 and Figure 1). During this time, the adjusted HIV prevalence increased from 26.73% in 2011 to 30.64% in 2013 and then to 32.36% in 2014. Thus, when we accounted for the HIV prevalence, the adjusted PDVP decreased by only 0.92 pp, from 18.83% in 2011 to 18.80% in 2013 and then to 17.91% in 2014.
Figure 1.
Time trends in the human immunodeficiency virus (HIV)–positive prevalence of detectable viremia (PDV+), the population prevalence of detectable viremia (PDVP), and the HIV prevalence over the 2011–2014 survey period.
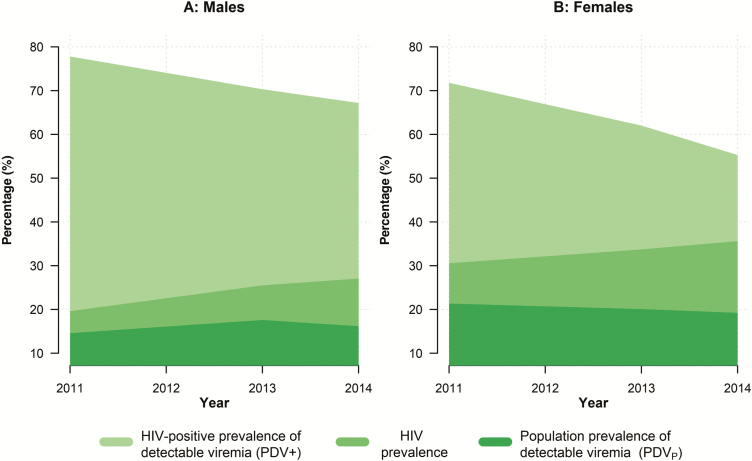
We observed marked differences in the adjusted PDV+ and PDVP measures by sex over time, as shown in Figure 2. Between 2011 and 2014, the PDV+ for women decreased by 16.5 pp, from 71.8% to 55.3%, compared with a 10.6 pp decrease in the PDV+ for men, from 77.80% to 67.18% (Supplementary Table 1). However, women had a higher HIV prevalence, 30.56% in 2011 and 35.61% in 2014, and therefore a higher PDVP, which decreased by 2.1 pp, from 21.35% to 19.23% over the survey period. For men, the HIV prevalence rose sharply from 19.63% in 2011 to 27.05% in 2014, which offset the decline in their PDV+. Thus, the PDVP for men actually increased by 1.6 pp over the survey period, from 14.58% to 16.18%.
Figure 2.
Time trends in the human immunodeficiency virus (HIV)–positive prevalence of detectable viremia (PDV+), the population prevalence of detectable viremia (PDVP), and the HIV prevalence over the 2011–2014 survey period for males (A) and females (B).
The GEE model results show that the odds of detectable viremia within the HIV-positive population (PDV+) was significantly lower in 2013 (0.647 [95% CI, .575–.727]; P < .001) and 2014 (0.490 [95% CI, .436–.551]; P < .001) compared with 2011 (Table 2). In addition, the odds of detectable viremia was significantly lower in women than men, but there was no difference between men and women over time, as shown by the 2 interaction terms in Table 2 (P > .266).
Table 2.
Regression Results Showing the Relative Odds of a Detectable Viral Load for the Human Immunodeficiency Virus (HIV)–Positive Population, Adjusting for Year, Age, and Sex
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | |
| Year (Ref: 2011) | ||||||||||||
| 2013 | 0.647 | (.575–.727) | <.001 | 0.646 | (.575–.726) | <.001 | 0.649 | (.577–.729) | <.001 | 0.749 | (.565–.993) | .044 |
| 2014 | 0.490 | (.436–.551) | <.001 | 0.49 | (.436–.551) | <.001 | 0.495 | (.44–.556) | <.001 | 0.498 | (.379–.654) | <.001 |
| Female sex | 0.700 | (.611–.801) | <.001 | 0.680 | (.594–.779) | <.001 | 0.721 | (.573–.908) | .005 | |||
| Age (>25 y) | 0.605 | (.521–.702) | <.001 | 0.605 | (.521–.702) | <.001 | ||||||
| 2013 * Female | 0.839 | (.616–1.144) | .266 | |||||||||
| 2014 * Female | 0.993 | (.735–1.343) | .966 | |||||||||
| Constant | 2.508 | (2.294–2.741) | <.001 | 3.33 | (2.89–3.837) | <.001 | 5.167 | (4.26–6.268) | <.001 | 4.922 | (3.848–6.295) | <.001 |
| HIV tests, No. | 6752 | 6752 | 6752 | 6752 | ||||||||
| Participants, No. | 4991 | 4991 | 4991 | 4991 | ||||||||
Abbreviations: CI, confidence interval; HIV, Human Immunodeficiency Virus; OR, odds ratio.
The odds of detectable viremia within the entire population (PDVP) was slightly lower in 2014 (0.911 [95% CI, .850–.977]; P = .009), but not in 2013 (0.968 [95% CI, .908–1.031]; P = .31), compared with 2011 (Table 3). Although the odd of detectable viremia was higher for women, these odds declined significantly over time when compared with men. We found a similar result when we stratified our analysis by sex (Supplementary Table 2).
Table 3.
Regression Results Showing the Relative Odds of a Detectable Viral Load for the Human Immunodeficiency Virus (HIV)–Positive and HIV-Negative Population by Year, Adjusting for Sex and Age
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | |
| Year (Ref: 2011) | ||||||||||||
| 2013 | 0.968 | (.908–1.031) | .310 | 0.964 | (.905–1.026) | .248 | 0.962 | (.903–1.024) | .222 | 1.163 | (1.018–1.328) | .026 |
| 2014 | 0.911 | (.850–.977) | .009 | 0.904 | (.844–.968) | .004 | 0.878 | (.82–.94) | <.001 | 1.049 | (.903–1.219) | .532 |
| Female sex | 1.708 | (1.568–1.861) | <.001 | 1.461 | (1.337–1.595) | <.001 | 1.674 | (1.487–1.884) | <.001 | |||
| Age (>25 y) | 3.029 | (2.761–3.322) | <.001 | 3.036 | (2.767–3.33) | <.001 | ||||||
| 2013 * Female | 0.785 | (.675–.912) | .002 | |||||||||
| 2014 * Female | 0.798 | (.674–.944) | .009 | |||||||||
| Constant | 0.246 | (.234–.258) | <.001 | 0.169 | (.156–.183) | <.001 | 0.089 | (.081–.097) | <.001 | 0.080 | (.071–.089) | <.001 |
| HIV tests, No. | 22167 | 22167 | 22167 | 22167 | ||||||||
| Participants, No. | 16319 | 16319 | 16319 | 16319 | ||||||||
Abbreviations: CI, confidence interval; HIV, Human Immunodeficiency Virus; OR, odds ratio.
DISCUSSION
Our study has quantified the temporal change in the HIV-positive prevalence of detectable viremia (PDV+) and the population prevalence of detectable viremia (PDVP) using data from a sub-Saharan African population. The results show that the PDV+ decreased by almost 14 percentage points (PP), from 73.8% to 59.9%, over the 2011–2014 survey period. In this regard, the 17 healthcare clinics within or adjacent to our surveillance area have been effective in getting HIV-infected persons onto ART and then reducing their viral load levels over time. This is positive news for the global HIV treatment-as-prevention initiative as well as for our study community, which is considered to be at the epicentre of the global AIDS epidemic.
We compare our 40.1% prevalence of undetectable viremia in the HIV-positive community (ie, 100 PDV+) in 2014 with population-based studies undertaken in Malawi [23], Zambia [24], and Zimbabwe [25] in 2015–2016. In Malawi, the prevalence of undetectable viremia (<1000 copies/mL) in the HIV-positive community was 67.6% (95% CI, 65.0%–70.2%) among 15- to 64-year-olds, 59.8% (95% CI, 57.4%–62.2%) among 15- to 59-year-olds in Zambia, and 60.4% (95% CI, 58.3%–62.5%) among 15- to 64-year-olds in Zimbabwe. These estimates are markedly higher than our PDV+ result, despite a lower detection level. It is likely that these differences would be slightly smaller in 2015–2016, if our PDV+ continued to decrease as it did over the survey period. Nevertheless, we acknowledge that our 40.1% estimate is well below the UNAIDS target of 73% (ie, to be achieved by 2020.
In addition to quantifying a community’s exposure to ART, the PDV+ has also been used to infer the potential for ongoing HIV transmission at the population level [2–5, 7]. However, measures such as the PDV+ have been criticized by Miller et al [10] and others [11–15] because they do not account for the relative sizes of the infected and uninfected populations (ie, HIV prevalence). Following this work, we multiplied the PDV+ by the HIV prevalence to construct a measure called the population prevalence of detectable viremia (PDVP) [11]. This measure enabled us to account for the high HIV prevalence in the AHRI study area, which increased from 26.7% to 32.4% over the 2011–2014 period. Our results show that the steady rise in the HIV prevalence offset the gains made by the declining PDV+. Thus, the PDVP only decreased by <1 pp, from 18.8% in 2011 to 17.9% in 2014.
We also observed significant differences in the PDV+ and PDVP measures by sex over time. For example, the PDV+ for women decreased by 16.5 pp between 2011 and 2014, from 71.8% to 55.3%, when compared with a decrease of 10.6 pp for men, from 77.8% to 67.2%. Previous research has shown that women have more frequent contact with the healthcare system, due in large part to their antenatal treatment and care needs, where they can initiate ART early and have their viral loads monitored [26, 27]. However, because women had a higher HIV prevalence, they also had a higher overall PDVP, which decreased by 2.1 pp, from 21.3% to 19.2%, over the survey period. Importantly, we found that men had a greater increase in their HIV prevalence over time, which offset the decline in their PDV+. Thus, the PDVP for men actually increased by 1.6 pp, from 14.6% in 2011 to 16.2% in 2014.
We have previously exploited the substantial space-time heterogeneity in ART scale-up over 8 years to demonstrate independent reductions in the individual risk of HIV acquisition with increasing ART exposure [17, 28, 29]. In more recent work, we used viral load survey data from 2011 to show that the prospective risk of HIV acquisition (5 years of follow-up) was independently associated with the PDVP (adjusted hazard ratio [aHR], 1.07, P < .001) but not the PDV+ (aHR, 1.005, P = .4) [11]. Barring substantial changes in sexual behavior, one might expect that the minimal change in the PDVP would translate into a minimal change in the crude HIV incidence rate. In this regard, we report elsewhere that the crude HIV incidence rate has been relatively stable in the AHRI study population between 2008 and 2016 [30, 31]. Thus, at an ecological level, the HIV incidence rate corresponds with the PDVP, rather than declining in relation to the marked decrease in the PDV+. These findings, and the results from our earlier work [11], provide further empirical support for the PDVP’s utility as a measure of the potential for HIV transmission.
The PDVP will not capture all the fundamental phenomena that underlie HIV transmission dynamics within a population. To better quantify the potential for HIV transmission, it would be ideal to use population-based surveillance systems to collect information on the number and patterns of condomless sex acts. But reliable self-report data is often difficult to obtain, and not all countries will have population-based surveillance systems, which are costly to establish and maintain. Public healthcare facilities can be a more affordable and convenient source of data. However, 2 recent studies have shown that facility-based PDV+ measures are poor indicators of the incidence of HIV infection [11, 12].
One potential limitation of the study is that 22% of the participants refused to take an HIV test during the survey period. In a previous study, Larmarange et al [32] found that HIV-infected participants were significantly less likely than HIV-uninfected participants to consent to an HIV test during a single survey round. This refusal rate could potentially bias both the HIV prevalence and PDVP measures downward. However, 2 recent studies have confirmed that survey nonparticipation in this community did not lead to large biases in the cross-sectional estimation of the HIV prevalence [33, 34]. Furthermore, it is unlikely that the 22% refusal rate would bias the PDV+ measure, as viral load measurements were obtained from all of the HIV-positive test results.
The PDV+ has been promoted as a proxy for ART program effectiveness. In recent years, it has gained traction in light of the UNAIDS target to have 90% of all ART-initiated persons achieve and maintain undetectable viremia by the year 2020 [6]. But while the PDV+ may reflect an infected community’s exposure to ART, it may not tell us enough about the potential for HIV transmission within the general population. Recent work has therefore begun to promote the PDVP as a more sensitive biological measure for this purpose, primarily because it accounts for the underlying prevalence of HIV [10–15]. We therefore highlight the need for countries to monitor and report the prevalence of detectable viremia among all adults, irrespective of HIV status.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers R01HD084233 and R01AI124389) , T.d.O., A.T., and a South African Medical Research Council flagship grant (grant number MRC-RFA-UFSP-01-2013/UKZN HIVEPI). Funding for the Africa Health Research Institute’s Demographic Surveillance Information System and Population-Based HIV Survey was received from the Wellcome Trust. F. T. was was supported by a UK Academy of Medical Sciences Newton Advanced Fellowship (award number NA150161). J. H. was supported by the NIH (grant numbers R01AI108490 and R01AI127232). T. B. was supported by the Alexander von Humboldt Foundation through the endowed Alexander von Humboldt Professorship funded by the German Federal Ministry of Education and Research, as well as by the Wellcome Trust, the European Commission, the Clinton Health Access Initiative, and by the NIH (grant numbers D43-TW009775 and R01-AI112339) .
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. Global AIDS update. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 2. The White House. National HIV/AIDS strategy for the United States 2011 Available at: https://obamawhitehouse.archives.gov/files/documents/nhas-implementation.pdf. Accessed 21 November 2017.
- 3. Centers for Disease Control and Prevention. Guidance on community viral load: a family of measures, definitions, and method for calculation. 2011:1–46. Available at: https://stacks.cdc.gov/view/cdc/28147. Accessed 20 October 2011. [Google Scholar]
- 4. Das M, Chu PL, Santos GM et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montaner JS, Lima VD, Barrios R et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joint United Nations Programme on HIV/AIDS. 90–90–90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 7. Castel AD, Befus M, Willis S et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS 2012; 26:345–53. [DOI] [PubMed] [Google Scholar]
- 8. Wood E, Kerr T, Marshall BD et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ 2009; 338:b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henard S, Jeanmaire E, Nguyen Y et al. ICONE Group Is total community viral load a robust predictive marker of the efficacy of the TasP strategy?J Acquir Immune Defic Syndr 2012; 61:400–2. [DOI] [PubMed] [Google Scholar]
- 10. Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis 2013; 13:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanser F, Vandormael A, Cuadros D et al. Effect of population viral load on prospective HIV incidence in a hyper-endemic rural South African community: a population-based cohort study. Sci Transl Med 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon SS, Mehta SH, McFall AM et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. Lancet HIV 2016; 3:e183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain V, Byonanebye DM, Liegler T et al. SEARCH Collaboration Changes in population HIV RNA levels in Mbarara, Uganda, during scale-up of HIV antiretroviral therapy access. J Acquir Immune Defic Syndr 2014; 65:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain V, Liegler T, Kabami J et al. SEARCH Collaboration Assessment of population-based HIV RNA levels in a rural East African setting using a fingerprick-based blood collection method. Clin Infect Dis 2013; 56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelley CF, Rosenberg ES, O’Hara BM et al. Measuring population transmission risk for HIV: an alternative metric of exposure risk in men who have sex with men (MSM) in the US. PLoS One 2012; 7:e53284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanser F, Hosegood V, Bärnighausen T et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol 2008; 37:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vandormael A, de Oliveira T, Tanser F, Bärnighausen T, Herbeck J. A high percentage of undiagnosed HIV cases in a rural and hyper-endemic South African community. J Epidemiol Community Health 2017. In press. [DOI] [PMC free article] [PubMed]
- 19. Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339:961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houlihan CF, Bland RM, Mutevedzi PC et al. Cohort profile: Hlabisa HIV treatment and care programme. Int J Epidemiol 2011; 40:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viljoen J, Gampini S, Danaviah S et al. World Health Organization/ANRS 1289 Kesho Bora Study Group Dried blood spot HIV-1 RNA quantification using open real-time systems in South Africa and Burkina Faso. J Acquir Immune Defic Syndr 2010; 55:290–8. [DOI] [PubMed] [Google Scholar]
- 22. Lohr S. Sampling: design and analysis. Boston: Brooks/Cole, 2010. [Google Scholar]
- 23. Malawi Population-Based HIV Impact Assessment (MPHIA). Summary sheet: preliminary findings. New York: PHIA Project;2016. [Google Scholar]
- 24. Zambia Population-Based HIV Impact Assessment (ZAMPHIA). Summary sheet: preliminary findings. New York: PHIA Project;2016. [Google Scholar]
- 25. Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA). Summary sheet: preliminary findings. New York: PHIA Project;2016. [Google Scholar]
- 26. Bor J, Rosen S, Chimbindi N et al. Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med 2015; 12:e1001905; discussion e1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandormael A, Newell ML, Bärnighausen T, Tanser F. Use of antiretroviral therapy in households and risk of HIV acquisition in rural KwaZulu-Natal, South Africa, 2004–12: a prospective cohort study. Lancet Glob Health 2014; 2:e209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oldenburg CE, Bärnighausen T, Tanser F et al. Antiretroviral therapy to prevent HIV acquisition in serodiscordant couples in a hyperendemic community in rural South Africa. Clin Infect Dis 2016; 63:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandormael A, Dobra A, Bärnighausen T, de Oliveira T, Tanser F. Incidence rate estimation, periodic testing and the limitations of the mid-point imputation approach. Int J Epidemiol 2017. doi:10.1093/ije/dyx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanser F, Bärnighausen T, Dobra A, Sartorius B. Identifying ‘corridors of HIV transmission’ in a severely affected rural South African population: a case for a shift toward targeted prevention strategies. Int J Epidemiol 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larmarange J, Mossong J, Bärnighausen T, Newell ML. Participation dynamics in population-based longitudinal HIV surveillance in rural South Africa. PLoS One 2015; 10:e0123345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS 2013; 27:2301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGovern ME, Bärnighausen T, Salomon JA, Canning D. Using interviewer random effects to remove selection bias from HIV prevalence estimates. BMC Med Res Methodol 2015; 15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.